 Found 2758 hits with Last Name = 'thompson' and Initial = 'm'
Found 2758 hits with Last Name = 'thompson' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Nicotinic acetylcholine receptor
(RAT) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University
Curated by PDSP Ki Database
| |
Mol Pharmacol 54: 322-33 (1998)
Article DOI: 10.1124/mol.54.2.322
BindingDB Entry DOI: 10.7270/Q2ZW1JGJ |
More data for this
Ligand-Target Pair | |
Nicotinic acetylcholine receptor
(RAT) | BDBM50049750

((S)-3-(azetidin-2-ylmethoxy)pyridine | 3-((S)-1-Az...)Show InChI InChI=1S/C9H12N2O/c1-2-9(6-10-4-1)12-7-8-3-5-11-8/h1-2,4,6,8,11H,3,5,7H2/t8-/m0/s1 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University
Curated by PDSP Ki Database
| |
Mol Pharmacol 54: 322-33 (1998)
Article DOI: 10.1124/mol.54.2.322
BindingDB Entry DOI: 10.7270/Q2ZW1JGJ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475465
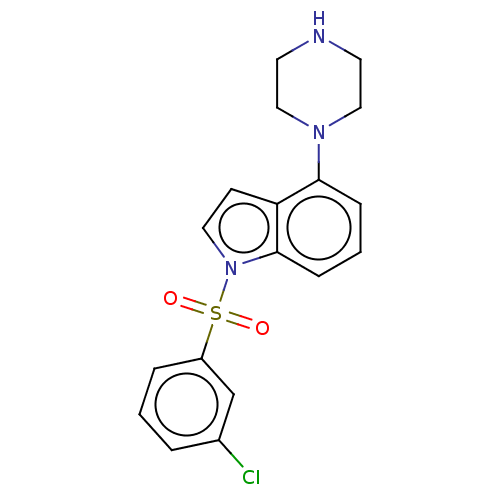
(CHEMBL196410)Show SMILES Clc1cccc(c1)S(=O)(=O)n1ccc2c(cccc12)N1CCNCC1 Show InChI InChI=1S/C18H18ClN3O2S/c19-14-3-1-4-15(13-14)25(23,24)22-10-7-16-17(5-2-6-18(16)22)21-11-8-20-9-12-21/h1-7,10,13,20H,8-9,11-12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475480
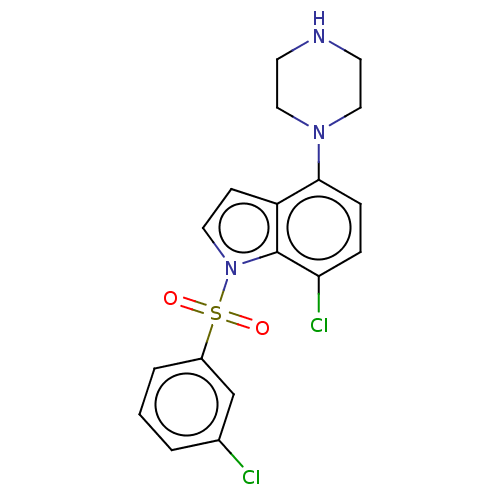
(CHEMBL193629)Show SMILES Clc1cccc(c1)S(=O)(=O)n1ccc2c(ccc(Cl)c12)N1CCNCC1 Show InChI InChI=1S/C18H17Cl2N3O2S/c19-13-2-1-3-14(12-13)26(24,25)23-9-6-15-17(5-4-16(20)18(15)23)22-10-7-21-8-11-22/h1-6,9,12,21H,7-8,10-11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475462
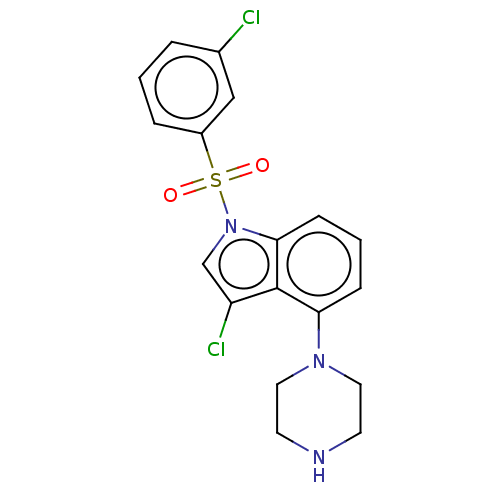
(CHEMBL371375)Show SMILES Clc1cn(c2cccc(N3CCNCC3)c12)S(=O)(=O)c1cccc(Cl)c1 Show InChI InChI=1S/C18H17Cl2N3O2S/c19-13-3-1-4-14(11-13)26(24,25)23-12-15(20)18-16(5-2-6-17(18)23)22-9-7-21-8-10-22/h1-6,11-12,21H,7-10H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475467

(CHEMBL425015)Show SMILES Clc1cccc(c1)S(=O)(=O)c1c[nH]c2c(cccc12)N1CCNCC1 Show InChI InChI=1S/C18H18ClN3O2S/c19-13-3-1-4-14(11-13)25(23,24)17-12-21-18-15(17)5-2-6-16(18)22-9-7-20-8-10-22/h1-6,11-12,20-21H,7-10H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50174269

(1-(phenylsulfonyl)-4-(piperazin-1-yl)-1H-indole | ...)Show InChI InChI=1S/C18H19N3O2S/c22-24(23,15-5-2-1-3-6-15)21-12-9-16-17(7-4-8-18(16)21)20-13-10-19-11-14-20/h1-9,12,19H,10-11,13-14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50412114
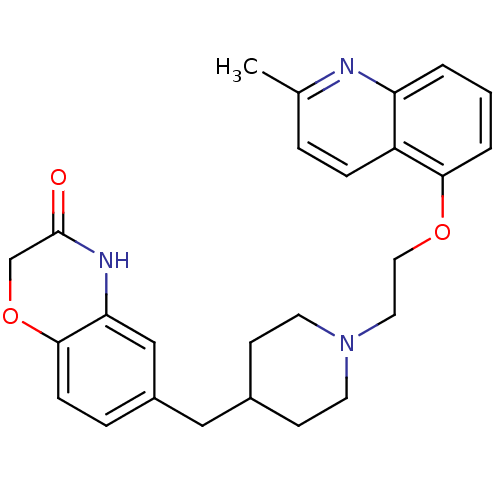
(CHEMBL183460 | SB-649915)Show SMILES Cc1ccc2c(OCCN3CCC(Cc4ccc5OCC(=O)Nc5c4)CC3)cccc2n1 Show InChI InChI=1S/C26H29N3O3/c1-18-5-7-21-22(27-18)3-2-4-24(21)31-14-13-29-11-9-19(10-12-29)15-20-6-8-25-23(16-20)28-26(30)17-32-25/h2-8,16,19H,9-15,17H2,1H3,(H,28,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Mean negative logarithim of binding affinity was measured for the human 5-hydroxytryptamine 1A receptor; n>/=3 |
Bioorg Med Chem Lett 15: 737-41 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.030
BindingDB Entry DOI: 10.7270/Q2RR220M |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-3
(Rattus norvegicus (Rat)) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University
Curated by PDSP Ki Database
| |
Mol Pharmacol 54: 322-33 (1998)
Article DOI: 10.1124/mol.54.2.322
BindingDB Entry DOI: 10.7270/Q2ZW1JGJ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475475
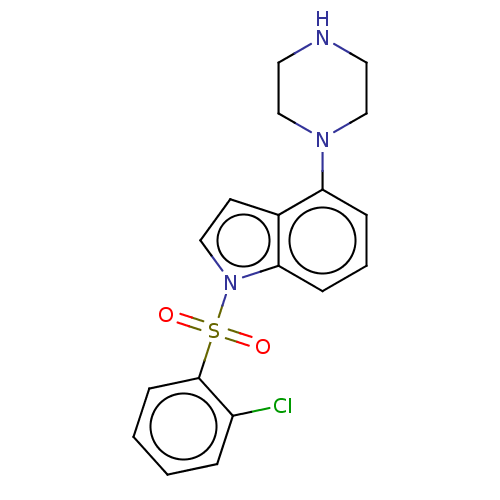
(CHEMBL372513)Show InChI InChI=1S/C18H18ClN3O2S/c19-15-4-1-2-7-18(15)25(23,24)22-11-8-14-16(5-3-6-17(14)22)21-12-9-20-10-13-21/h1-8,11,20H,9-10,12-13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475477
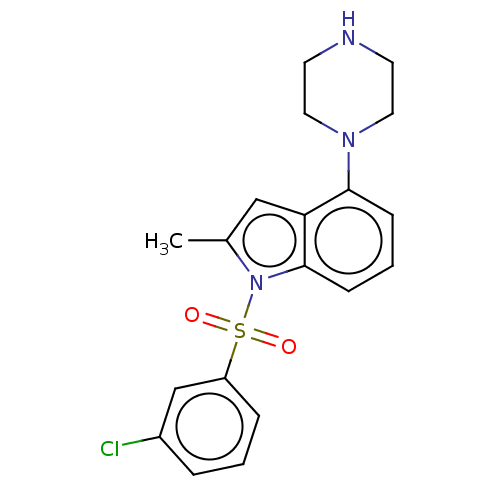
(CHEMBL372929)Show SMILES Cc1cc2c(cccc2n1S(=O)(=O)c1cccc(Cl)c1)N1CCNCC1 Show InChI InChI=1S/C19H20ClN3O2S/c1-14-12-17-18(22-10-8-21-9-11-22)6-3-7-19(17)23(14)26(24,25)16-5-2-4-15(20)13-16/h2-7,12-13,21H,8-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475463
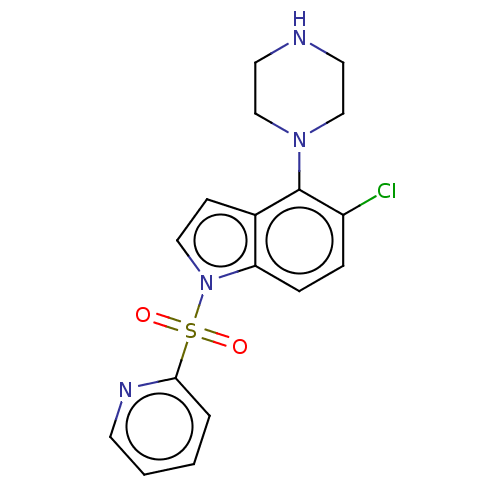
(CHEMBL194915)Show InChI InChI=1S/C17H17ClN4O2S/c18-14-4-5-15-13(17(14)21-11-8-19-9-12-21)6-10-22(15)25(23,24)16-3-1-2-7-20-16/h1-7,10,19H,8-9,11-12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50044607
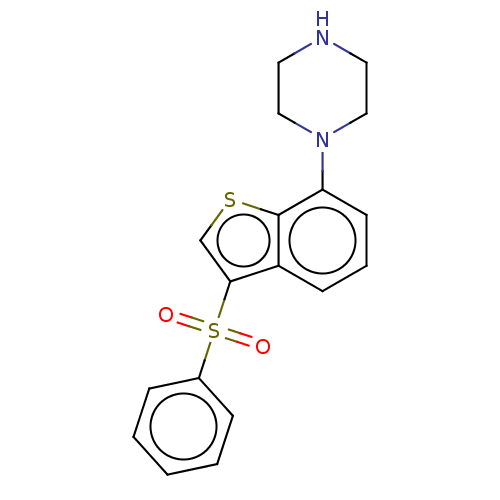
(CHEMBL372537)Show InChI InChI=1S/C18H18N2O2S2/c21-24(22,14-5-2-1-3-6-14)17-13-23-18-15(17)7-4-8-16(18)20-11-9-19-10-12-20/h1-8,13,19H,9-12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475473
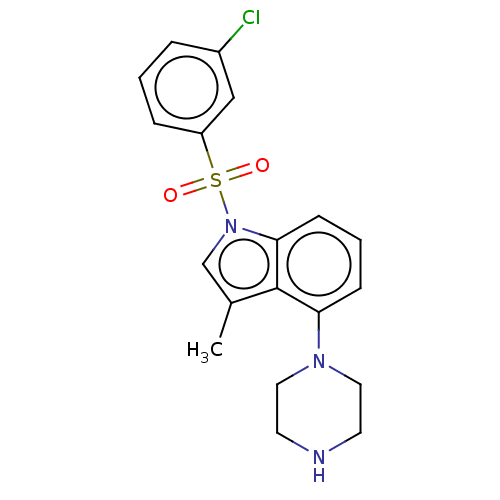
(CHEMBL194039)Show SMILES Cc1cn(c2cccc(N3CCNCC3)c12)S(=O)(=O)c1cccc(Cl)c1 Show InChI InChI=1S/C19H20ClN3O2S/c1-14-13-23(26(24,25)16-5-2-4-15(20)12-16)18-7-3-6-17(19(14)18)22-10-8-21-9-11-22/h2-7,12-13,21H,8-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Homo sapiens (Human)) | BDBM50475428
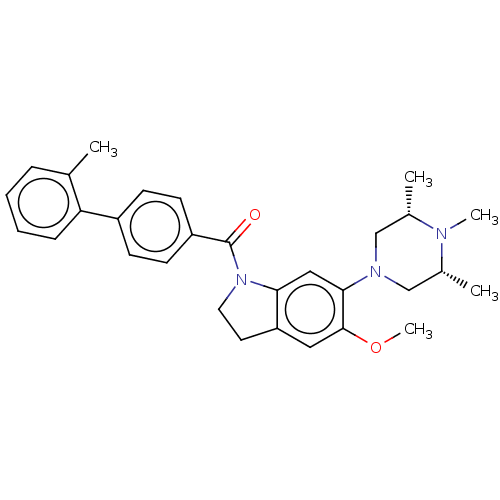
(CHEMBL196848)Show SMILES COc1cc2CCN(C(=O)c3ccc(cc3)-c3ccccc3C)c2cc1N1C[C@H](C)N(C)[C@H](C)C1 Show InChI InChI=1S/C30H35N3O2/c1-20-8-6-7-9-26(20)23-10-12-24(13-11-23)30(34)33-15-14-25-16-29(35-5)28(17-27(25)33)32-18-21(2)31(4)22(3)19-32/h6-13,16-17,21-22H,14-15,18-19H2,1-5H3/t21-,22+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity against cloned human 5-hydroxytryptamine 1B receptor |
Bioorg Med Chem Lett 15: 4708-12 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.085
BindingDB Entry DOI: 10.7270/Q2CC13F8 |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50027185

(CHEMBL183921)Show SMILES CC(COc1ccc2OCC(=O)Nc2c1)NCC(O)COc1cccc2[nH]ccc12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity for human beta-2 adrenergic receptor by displacing [125I]iodocyanopindolol expressed in hamster CHO cells |
Bioorg Med Chem Lett 15: 737-41 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.030
BindingDB Entry DOI: 10.7270/Q2RR220M |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50027185

(CHEMBL183921)Show SMILES CC(COc1ccc2OCC(=O)Nc2c1)NCC(O)COc1cccc2[nH]ccc12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity for human beta-2 adrenergic receptor by displacing [125I]iodocyanopindolol expressed in hamster CHO cells |
Bioorg Med Chem Lett 15: 737-41 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.030
BindingDB Entry DOI: 10.7270/Q2RR220M |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50027185

(CHEMBL183921)Show SMILES CC(COc1ccc2OCC(=O)Nc2c1)NCC(O)COc1cccc2[nH]ccc12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity for human beta-2 adrenergic receptor by displacing [125I]iodocyanopindolol expressed in hamster CHO cells |
Bioorg Med Chem Lett 15: 737-41 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.030
BindingDB Entry DOI: 10.7270/Q2RR220M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50027185

(CHEMBL183921)Show SMILES CC(COc1ccc2OCC(=O)Nc2c1)NCC(O)COc1cccc2[nH]ccc12 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Mean negative logarithim of binding affinity was measured for the human 5-hydroxytryptamine 1A receptor; n>/=3 |
Bioorg Med Chem Lett 15: 737-41 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.030
BindingDB Entry DOI: 10.7270/Q2RR220M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50027185

(CHEMBL183921)Show SMILES CC(COc1ccc2OCC(=O)Nc2c1)NCC(O)COc1cccc2[nH]ccc12 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Mean negative logarithim of binding affinity was measured for the human 5-hydroxytryptamine 1A receptor; n>/=3 |
Bioorg Med Chem Lett 15: 737-41 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.030
BindingDB Entry DOI: 10.7270/Q2RR220M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475470

(CHEMBL370209)Show InChI InChI=1S/C17H18N4O2S/c22-24(23,17-6-1-2-8-19-17)21-11-7-14-15(4-3-5-16(14)21)20-12-9-18-10-13-20/h1-8,11,18H,9-10,12-13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50410435
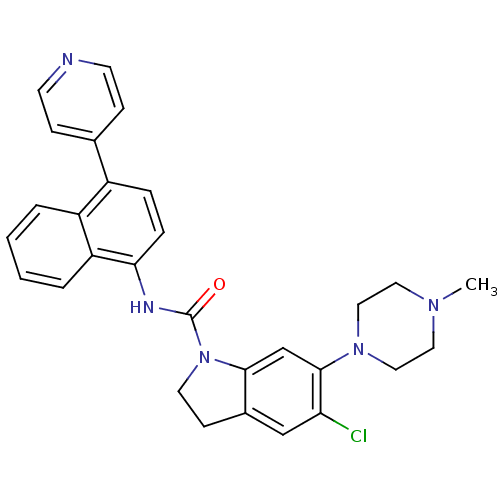
(CHEMBL191971 | SB-272183)Show SMILES CN1CCN(CC1)c1cc2N(CCc2cc1Cl)C(=O)Nc1ccc(-c2ccncc2)c2ccccc12 Show InChI InChI=1S/C29H28ClN5O/c1-33-14-16-34(17-15-33)28-19-27-21(18-25(28)30)10-13-35(27)29(36)32-26-7-6-22(20-8-11-31-12-9-20)23-4-2-3-5-24(23)26/h2-9,11-12,18-19H,10,13-17H2,1H3,(H,32,36) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity against cloned human 5-hydroxytryptamine 1D receptor |
Bioorg Med Chem Lett 15: 4708-12 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.085
BindingDB Entry DOI: 10.7270/Q2CC13F8 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475479
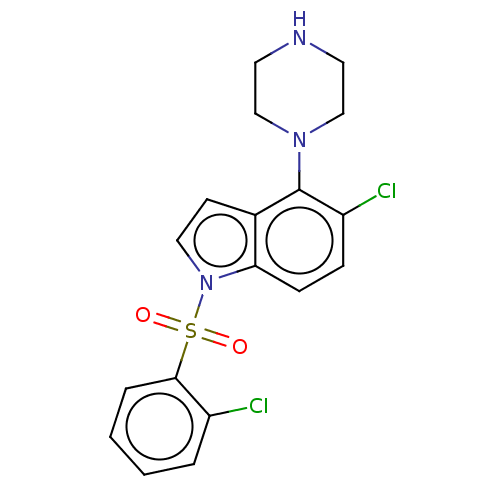
(CHEMBL371176)Show SMILES Clc1ccccc1S(=O)(=O)n1ccc2c(N3CCNCC3)c(Cl)ccc12 Show InChI InChI=1S/C18H17Cl2N3O2S/c19-14-3-1-2-4-17(14)26(24,25)23-10-7-13-16(23)6-5-15(20)18(13)22-11-8-21-9-12-22/h1-7,10,21H,8-9,11-12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
Nicotinic acetylcholine receptor
(RAT) | BDBM50107863
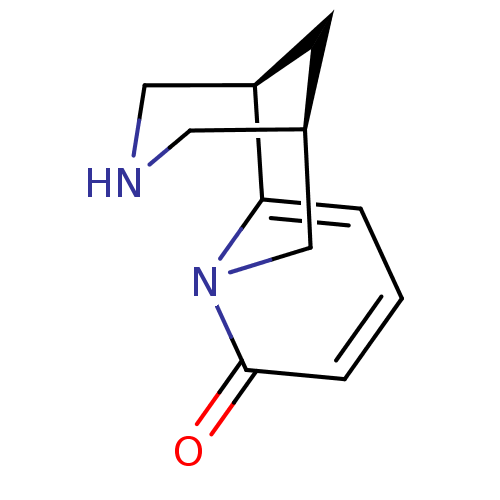
((-)-cytisine | (1R,9R)-7,11-diazatricyclo[7.3.1.0~...)Show InChI InChI=1S/C11H14N2O/c14-11-3-1-2-10-9-4-8(5-12-6-9)7-13(10)11/h1-3,8-9,12H,4-7H2 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.03 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University
Curated by PDSP Ki Database
| |
Mol Pharmacol 54: 322-33 (1998)
Article DOI: 10.1124/mol.54.2.322
BindingDB Entry DOI: 10.7270/Q2ZW1JGJ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475464
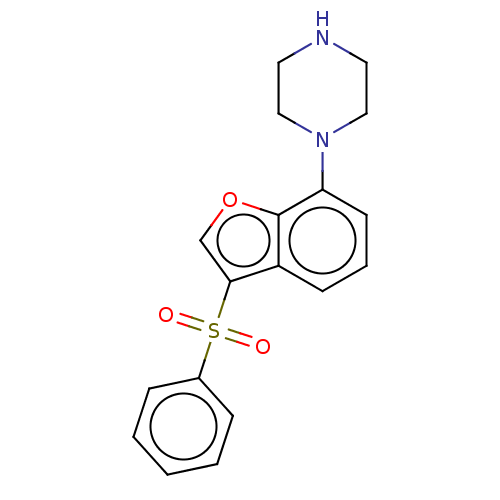
(CHEMBL197574)Show InChI InChI=1S/C18H18N2O3S/c21-24(22,14-5-2-1-3-6-14)17-13-23-18-15(17)7-4-8-16(18)20-11-9-19-10-12-20/h1-8,13,19H,9-12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM28583
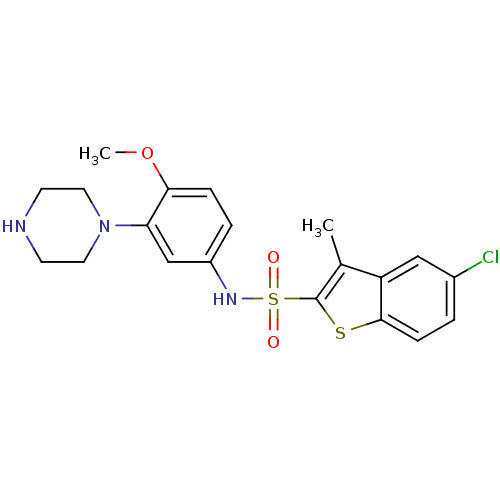
(5-chloro-N-[4-methoxy-3-(piperazin-1-yl)phenyl]-3-...)Show SMILES COc1ccc(NS(=O)(=O)c2sc3ccc(Cl)cc3c2C)cc1N1CCNCC1 Show InChI InChI=1S/C20H22ClN3O3S2/c1-13-16-11-14(21)3-6-19(16)28-20(13)29(25,26)23-15-4-5-18(27-2)17(12-15)24-9-7-22-8-10-24/h3-6,11-12,22-23H,7-10H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50474976

(CHEMBL180557)Show SMILES O=C1COc2ccc(CC3CCN(CCOc4cccc5ncccc45)CC3)cc2N1 Show InChI InChI=1S/C25H27N3O3/c29-25-17-31-24-7-6-19(16-22(24)27-25)15-18-8-11-28(12-9-18)13-14-30-23-5-1-4-21-20(23)3-2-10-26-21/h1-7,10,16,18H,8-9,11-15,17H2,(H,27,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Mean negative logarithim of binding affinity was measured for the human 5-hydroxytryptamine 1A receptor; n>/=3 |
Bioorg Med Chem Lett 15: 737-41 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.030
BindingDB Entry DOI: 10.7270/Q2RR220M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50474983
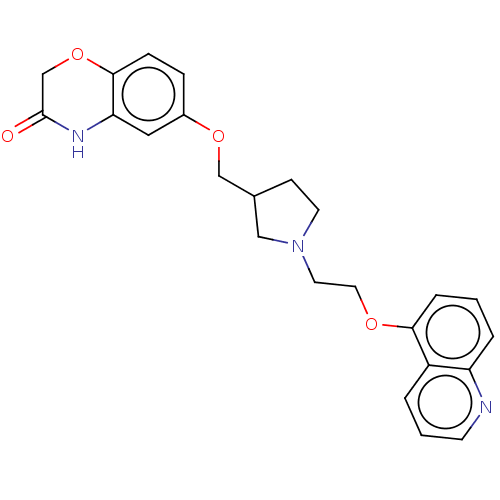
(CHEMBL183034)Show SMILES O=C1COc2ccc(OCC3CCN(CCOc4cccc5ncccc45)C3)cc2N1 Show InChI InChI=1S/C24H25N3O4/c28-24-16-31-23-7-6-18(13-21(23)26-24)30-15-17-8-10-27(14-17)11-12-29-22-5-1-4-20-19(22)3-2-9-25-20/h1-7,9,13,17H,8,10-12,14-16H2,(H,26,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Mean negative logarithim of binding affinity was measured for the human 5-hydroxytryptamine 1A receptor; n>/=3 |
Bioorg Med Chem Lett 15: 737-41 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.030
BindingDB Entry DOI: 10.7270/Q2RR220M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Homo sapiens (Human)) | BDBM50475427

(CHEMBL196600)Show SMILES COc1cc2CCN(C(=O)Cc3cccc(c3F)C(F)(F)F)c2cc1N1C[C@H](C)N(C)[C@H](C)C1 Show InChI InChI=1S/C25H29F4N3O2/c1-15-13-31(14-16(2)30(15)3)21-12-20-17(10-22(21)34-4)8-9-32(20)23(33)11-18-6-5-7-19(24(18)26)25(27,28)29/h5-7,10,12,15-16H,8-9,11,13-14H2,1-4H3/t15-,16+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity against cloned human 5-hydroxytryptamine 1B receptor |
Bioorg Med Chem Lett 15: 4708-12 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.085
BindingDB Entry DOI: 10.7270/Q2CC13F8 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50474975
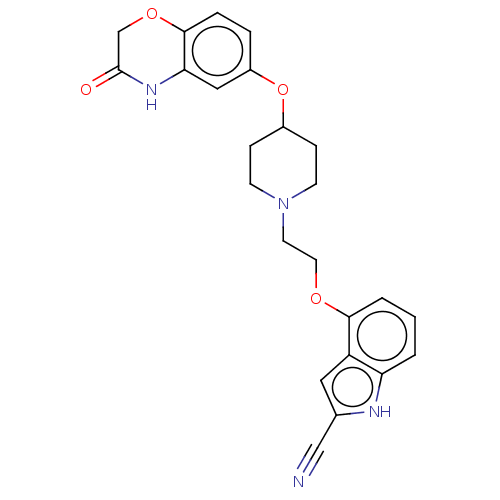
(CHEMBL3706797)Show SMILES O=C1COc2ccc(OC3CCN(CCOc4cccc5[nH]c(cc45)C#N)CC3)cc2N1 Show InChI InChI=1S/C24H24N4O4/c25-14-16-12-19-20(26-16)2-1-3-22(19)30-11-10-28-8-6-17(7-9-28)32-18-4-5-23-21(13-18)27-24(29)15-31-23/h1-5,12-13,17,26H,6-11,15H2,(H,27,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Mean negative logarithim of binding affinity was measured for the human 5-hydroxytryptamine 1A receptor; n>/=3 |
Bioorg Med Chem Lett 15: 737-41 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.030
BindingDB Entry DOI: 10.7270/Q2RR220M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475466
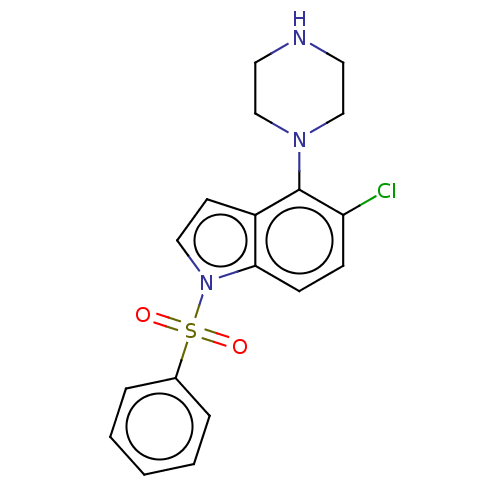
(CHEMBL193665)Show InChI InChI=1S/C18H18ClN3O2S/c19-16-6-7-17-15(18(16)21-12-9-20-10-13-21)8-11-22(17)25(23,24)14-4-2-1-3-5-14/h1-8,11,20H,9-10,12-13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50027184

(CHEMBL180700)Show InChI InChI=1S/C21H23N3O4/c25-21-14-28-20-6-5-15(13-18(20)24-21)26-12-10-22-8-2-11-27-19-4-1-3-17-16(19)7-9-23-17/h1,3-7,9,13,22-23H,2,8,10-12,14H2,(H,24,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Mean negative logarithim of binding affinity was measured for the human 5-hydroxytryptamine 1A receptor; n>/=3 |
Bioorg Med Chem Lett 15: 737-41 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.030
BindingDB Entry DOI: 10.7270/Q2RR220M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50474977

(CHEMBL183901)Show SMILES O=C1COc2ccc(OCC3CCCN(CCOc4cccc5ncccc45)C3)cc2N1 Show InChI InChI=1S/C25H27N3O4/c29-25-17-32-24-9-8-19(14-22(24)27-25)31-16-18-4-3-11-28(15-18)12-13-30-23-7-1-6-21-20(23)5-2-10-26-21/h1-2,5-10,14,18H,3-4,11-13,15-17H2,(H,27,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Mean negative logarithim of binding affinity was measured for the human 5-hydroxytryptamine 1A receptor; n>/=3 |
Bioorg Med Chem Lett 15: 737-41 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.030
BindingDB Entry DOI: 10.7270/Q2RR220M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Homo sapiens (Human)) | BDBM50475421
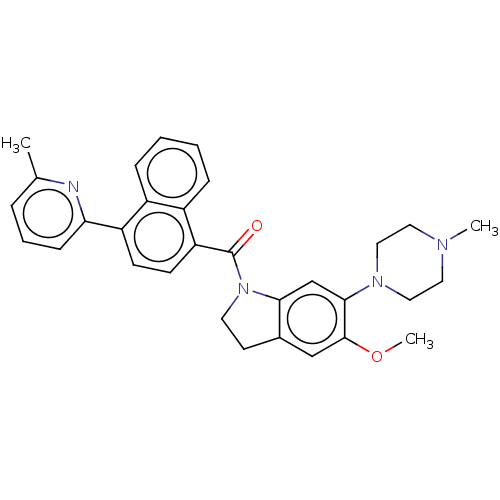
(CHEMBL195114)Show SMILES COc1cc2CCN(C(=O)c3ccc(-c4cccc(C)n4)c4ccccc34)c2cc1N1CCN(C)CC1 Show InChI InChI=1S/C31H32N4O2/c1-21-7-6-10-27(32-21)25-11-12-26(24-9-5-4-8-23(24)25)31(36)35-14-13-22-19-30(37-3)29(20-28(22)35)34-17-15-33(2)16-18-34/h4-12,19-20H,13-18H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity against cloned human 5-hydroxytryptamine 1B receptor |
Bioorg Med Chem Lett 15: 4708-12 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.085
BindingDB Entry DOI: 10.7270/Q2CC13F8 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50027185

(CHEMBL183921)Show SMILES CC(COc1ccc2OCC(=O)Nc2c1)NCC(O)COc1cccc2[nH]ccc12 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Mean negative logarithim of binding affinity was measured for the human 5-hydroxytryptamine 1A receptor; n>/=3 |
Bioorg Med Chem Lett 15: 737-41 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.030
BindingDB Entry DOI: 10.7270/Q2RR220M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50474980

(CHEMBL180405)Show SMILES CC1(C)OC2C=CC=CC2=C1OCCN1CCC(CC1)Oc1ccc2OCC(=O)Nc2c1 |c:5,7,10| Show InChI InChI=1S/C25H30N2O5/c1-25(2)24(19-5-3-4-6-21(19)32-25)29-14-13-27-11-9-17(10-12-27)31-18-7-8-22-20(15-18)26-23(28)16-30-22/h3-8,15,17,21H,9-14,16H2,1-2H3,(H,26,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Mean negative logarithim of binding affinity was measured for the human 5-hydroxytryptamine 1A receptor; n>/=3 |
Bioorg Med Chem Lett 15: 737-41 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.030
BindingDB Entry DOI: 10.7270/Q2RR220M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Homo sapiens (Human)) | BDBM50475435

(CHEMBL195898)Show SMILES COc1cc2CCN(C(=O)Cc3cccc(Cl)c3F)c2cc1N1C[C@H](C)N(C)[C@H](C)C1 Show InChI InChI=1S/C24H29ClFN3O2/c1-15-13-28(14-16(2)27(15)3)21-12-20-17(10-22(21)31-4)8-9-29(20)23(30)11-18-6-5-7-19(25)24(18)26/h5-7,10,12,15-16H,8-9,11,13-14H2,1-4H3/t15-,16+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity against cloned human 5-hydroxytryptamine 1B receptor |
Bioorg Med Chem Lett 15: 4708-12 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.085
BindingDB Entry DOI: 10.7270/Q2CC13F8 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475471
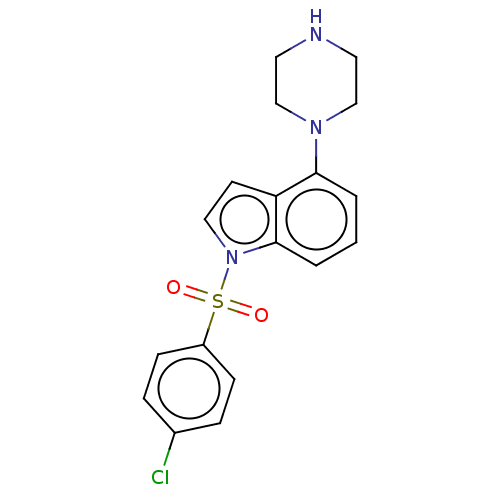
(CHEMBL371876)Show SMILES Clc1ccc(cc1)S(=O)(=O)n1ccc2c(cccc12)N1CCNCC1 Show InChI InChI=1S/C18H18ClN3O2S/c19-14-4-6-15(7-5-14)25(23,24)22-11-8-16-17(2-1-3-18(16)22)21-12-9-20-10-13-21/h1-8,11,20H,9-10,12-13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Homo sapiens (Human)) | BDBM50475422
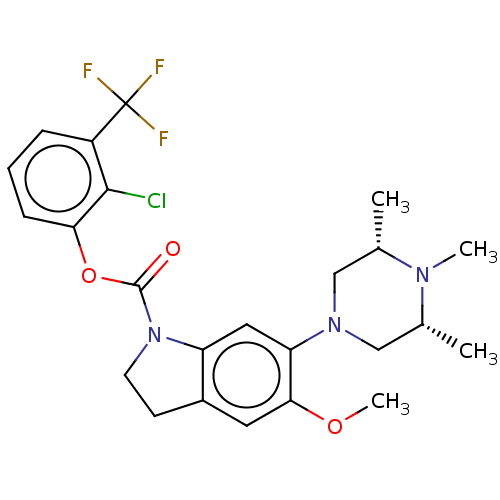
(CHEMBL372731)Show SMILES COc1cc2CCN(C(=O)Oc3cccc(c3Cl)C(F)(F)F)c2cc1N1C[C@H](C)N(C)[C@H](C)C1 Show InChI InChI=1S/C24H27ClF3N3O3/c1-14-12-30(13-15(2)29(14)3)19-11-18-16(10-21(19)33-4)8-9-31(18)23(32)34-20-7-5-6-17(22(20)25)24(26,27)28/h5-7,10-11,14-15H,8-9,12-13H2,1-4H3/t14-,15+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity against cloned human 5-hydroxytryptamine 1B receptor |
Bioorg Med Chem Lett 15: 4708-12 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.085
BindingDB Entry DOI: 10.7270/Q2CC13F8 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Homo sapiens (Human)) | BDBM50475425

(CHEMBL197505)Show SMILES COc1cc2CCN(C(=O)Oc3cccc(c3F)C(F)(F)F)c2cc1N1C[C@H](C)N(C)[C@H](C)C1 Show InChI InChI=1S/C24H27F4N3O3/c1-14-12-30(13-15(2)29(14)3)19-11-18-16(10-21(19)33-4)8-9-31(18)23(32)34-20-7-5-6-17(22(20)25)24(26,27)28/h5-7,10-11,14-15H,8-9,12-13H2,1-4H3/t14-,15+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity against cloned human 5-hydroxytryptamine 1B receptor |
Bioorg Med Chem Lett 15: 4708-12 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.085
BindingDB Entry DOI: 10.7270/Q2CC13F8 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Homo sapiens (Human)) | BDBM50475430
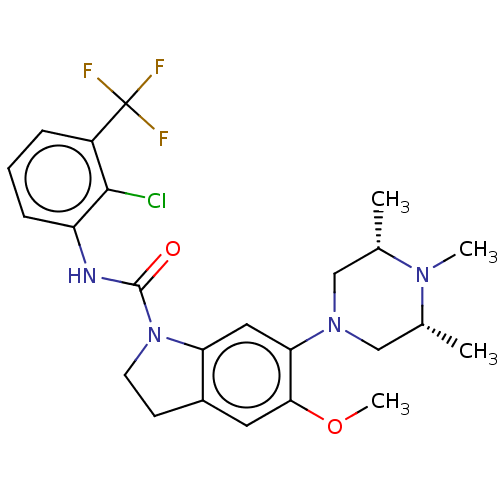
(CHEMBL370214)Show SMILES COc1cc2CCN(C(=O)Nc3cccc(c3Cl)C(F)(F)F)c2cc1N1C[C@H](C)N(C)[C@H](C)C1 Show InChI InChI=1S/C24H28ClF3N4O2/c1-14-12-31(13-15(2)30(14)3)20-11-19-16(10-21(20)34-4)8-9-32(19)23(33)29-18-7-5-6-17(22(18)25)24(26,27)28/h5-7,10-11,14-15H,8-9,12-13H2,1-4H3,(H,29,33)/t14-,15+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity against cloned human 5-hydroxytryptamine 1B receptor |
Bioorg Med Chem Lett 15: 4708-12 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.085
BindingDB Entry DOI: 10.7270/Q2CC13F8 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50474979

(CHEMBL180445)Show SMILES O=C1COc2ccc(OC3CCN(CCOc4cccc5ccccc45)CC3)cc2N1 Show InChI InChI=1S/C25H26N2O4/c28-25-17-30-24-9-8-20(16-22(24)26-25)31-19-10-12-27(13-11-19)14-15-29-23-7-3-5-18-4-1-2-6-21(18)23/h1-9,16,19H,10-15,17H2,(H,26,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Mean negative logarithim of binding affinity was measured for the human 5-hydroxytryptamine 1A receptor; n>/=3 |
Bioorg Med Chem Lett 15: 737-41 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.030
BindingDB Entry DOI: 10.7270/Q2RR220M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475481

(CHEMBL197297)Show SMILES Cn1cc(c2cccc(N3CCNCC3)c12)S(=O)(=O)c1cccc(Cl)c1 Show InChI InChI=1S/C19H20ClN3O2S/c1-22-13-18(26(24,25)15-5-2-4-14(20)12-15)16-6-3-7-17(19(16)22)23-10-8-21-9-11-23/h2-7,12-13,21H,8-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50044623

(CHEMBL193400)Show SMILES Clc1cccc(c1)S(=O)(=O)n1ccc2c(N3CCNCC3)c(Cl)ccc12 Show InChI InChI=1S/C18H17Cl2N3O2S/c19-13-2-1-3-14(12-13)26(24,25)23-9-6-15-17(23)5-4-16(20)18(15)22-10-7-21-8-11-22/h1-6,9,12,21H,7-8,10-11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50067499

((6aR,10aR)-3(1,1-dimethylheptyl)-9-hydroxymethyl)-...)Show SMILES CCCCCCC(C)(C)c1cc(O)c2[C@@H]3CC(CO)=CC[C@H]3C(C)(C)Oc2c1 |r,c:18| Show InChI InChI=1S/C25H38O3/c1-6-7-8-9-12-24(2,3)18-14-21(27)23-19-13-17(16-26)10-11-20(19)25(4,5)28-22(23)15-18/h10,14-15,19-20,26-27H,6-9,11-13,16H2,1-5H3/t19-,20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Montana
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55940 from human recombinant cannabinoid CB2 receptor expressed in CHO-K1 cells |
Bioorg Med Chem Lett 21: 5859-62 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.099
BindingDB Entry DOI: 10.7270/Q2K074P5 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475478
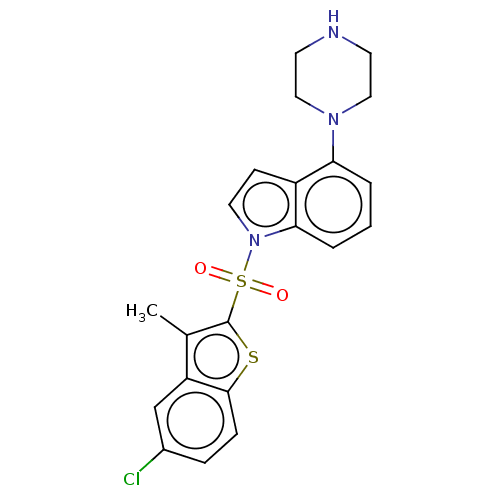
(CHEMBL196644)Show SMILES Cc1c(sc2ccc(Cl)cc12)S(=O)(=O)n1ccc2c(cccc12)N1CCNCC1 Show InChI InChI=1S/C21H20ClN3O2S2/c1-14-17-13-15(22)5-6-20(17)28-21(14)29(26,27)25-10-7-16-18(3-2-4-19(16)25)24-11-8-23-9-12-24/h2-7,10,13,23H,8-9,11-12H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Homo sapiens (Human)) | BDBM50475424

(CHEMBL196666)Show SMILES COc1cc2CCN(C(=O)c3ccc(cc3)-c3ccc(cc3C)-c3noc(C)n3)c2cc1N1C[C@H](C)N[C@H](C)C1 Show InChI InChI=1S/C32H35N5O3/c1-19-14-26(31-34-22(4)40-35-31)10-11-27(19)23-6-8-24(9-7-23)32(38)37-13-12-25-15-30(39-5)29(16-28(25)37)36-17-20(2)33-21(3)18-36/h6-11,14-16,20-21,33H,12-13,17-18H2,1-5H3/t20-,21+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity against cloned human 5-hydroxytryptamine 1B receptor |
Bioorg Med Chem Lett 15: 4708-12 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.085
BindingDB Entry DOI: 10.7270/Q2CC13F8 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Homo sapiens (Human)) | BDBM50475426

(CHEMBL196951)Show SMILES COc1cc2CCN(C(=O)c3ccc(cc3)-c3ccc(cc3C)C(C)=O)c2cc1N1C[C@H](C)N[C@H](C)C1 Show InChI InChI=1S/C31H35N3O3/c1-19-14-25(22(4)35)10-11-27(19)23-6-8-24(9-7-23)31(36)34-13-12-26-15-30(37-5)29(16-28(26)34)33-17-20(2)32-21(3)18-33/h6-11,14-16,20-21,32H,12-13,17-18H2,1-5H3/t20-,21+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity against cloned human 5-hydroxytryptamine 1B receptor |
Bioorg Med Chem Lett 15: 4708-12 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.085
BindingDB Entry DOI: 10.7270/Q2CC13F8 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Homo sapiens (Human)) | BDBM50475432

(CHEMBL195651)Show SMILES COc1cc2CCN(C(=O)Cc3cccc(F)c3C(F)(F)F)c2cc1N1C[C@H](C)N(C)[C@H](C)C1 Show InChI InChI=1S/C25H29F4N3O2/c1-15-13-31(14-16(2)30(15)3)21-12-20-17(10-22(21)34-4)8-9-32(20)23(33)11-18-6-5-7-19(26)24(18)25(27,28)29/h5-7,10,12,15-16H,8-9,11,13-14H2,1-4H3/t15-,16+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity against cloned human 5-hydroxytryptamine 1B receptor |
Bioorg Med Chem Lett 15: 4708-12 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.085
BindingDB Entry DOI: 10.7270/Q2CC13F8 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Homo sapiens (Human)) | BDBM50475415
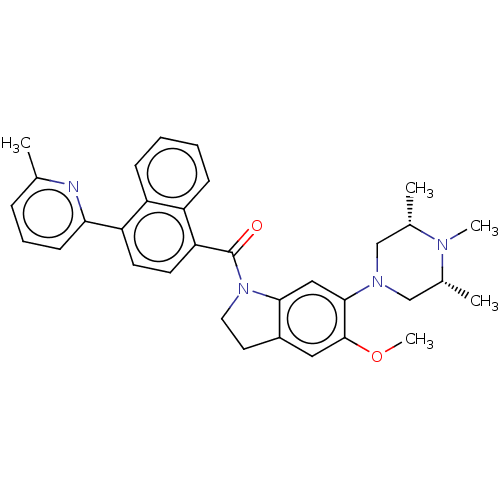
(CHEMBL195497)Show SMILES COc1cc2CCN(C(=O)c3ccc(-c4cccc(C)n4)c4ccccc34)c2cc1N1C[C@H](C)N(C)[C@H](C)C1 Show InChI InChI=1S/C33H36N4O2/c1-21-9-8-12-29(34-21)27-13-14-28(26-11-7-6-10-25(26)27)33(38)37-16-15-24-17-32(39-5)31(18-30(24)37)36-19-22(2)35(4)23(3)20-36/h6-14,17-18,22-23H,15-16,19-20H2,1-5H3/t22-,23+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity against cloned human 5-hydroxytryptamine 1B receptor |
Bioorg Med Chem Lett 15: 4708-12 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.085
BindingDB Entry DOI: 10.7270/Q2CC13F8 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data