 Found 168 hits with Last Name = 'thorn' and Initial = 'k'
Found 168 hits with Last Name = 'thorn' and Initial = 'k' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50384444

(CHEMBL2035510)Show SMILES CC(C)C1(OC(=O)NC1=O)c1ccc([nH]c1=O)-c1ccc2cccc(F)c2c1 Show InChI InChI=1S/C21H17FN2O4/c1-11(2)21(19(26)24-20(27)28-21)15-8-9-17(23-18(15)25)13-7-6-12-4-3-5-16(22)14(12)10-13/h3-11H,1-2H3,(H,23,25)(H,24,26,27) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at human EP3c receptor expressed in human U2OS cells assessed as inhibition of PGE2-induced calcium mobilization after 24 hrs by ... |
ACS Med Chem Lett 1: 316-320 (2010)
Article DOI: 10.1021/ml100077x
BindingDB Entry DOI: 10.7270/Q2BG2Q1F |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(RAT) | BDBM50384444

(CHEMBL2035510)Show SMILES CC(C)C1(OC(=O)NC1=O)c1ccc([nH]c1=O)-c1ccc2cccc(F)c2c1 Show InChI InChI=1S/C21H17FN2O4/c1-11(2)21(19(26)24-20(27)28-21)15-8-9-17(23-18(15)25)13-7-6-12-4-3-5-16(22)14(12)10-13/h3-11H,1-2H3,(H,23,25)(H,24,26,27) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at rat EP3 receptor expressed in human U2OS cells co-expressing Gqi5 assessed as inhibition of PGE2-induced response after 24 hrs... |
ACS Med Chem Lett 1: 316-320 (2010)
Article DOI: 10.1021/ml100077x
BindingDB Entry DOI: 10.7270/Q2BG2Q1F |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50384443

(CHEMBL1770317)Show SMILES CC(C)C1(OC(O)=NC1=O)c1ccc([nH]c1=O)-c1ccc2ccccc2c1 |c:6| Show InChI InChI=1S/C21H18N2O4/c1-12(2)21(19(25)23-20(26)27-21)16-9-10-17(22-18(16)24)15-8-7-13-5-3-4-6-14(13)11-15/h3-12H,1-2H3,(H,22,24)(H,23,25,26) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.01 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human EP3 |
ACS Med Chem Lett 1: 316-320 (2010)
Article DOI: 10.1021/ml100077x
BindingDB Entry DOI: 10.7270/Q2BG2Q1F |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50384445
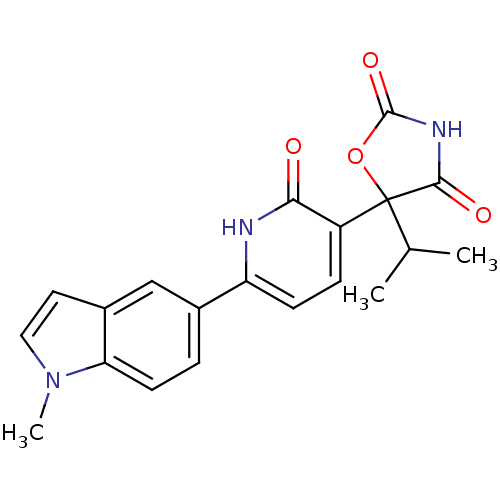
(CHEMBL2035508)Show SMILES CC(C)C1(OC(=O)NC1=O)c1ccc([nH]c1=O)-c1ccc2n(C)ccc2c1 Show InChI InChI=1S/C20H19N3O4/c1-11(2)20(18(25)22-19(26)27-20)14-5-6-15(21-17(14)24)12-4-7-16-13(10-12)8-9-23(16)3/h4-11H,1-3H3,(H,21,24)(H,22,25,26) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at human EP3c receptor expressed in human U2OS cells assessed as inhibition of PGE2-induced calcium mobilization after 24 hrs by ... |
ACS Med Chem Lett 1: 316-320 (2010)
Article DOI: 10.1021/ml100077x
BindingDB Entry DOI: 10.7270/Q2BG2Q1F |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(RAT) | BDBM50384445

(CHEMBL2035508)Show SMILES CC(C)C1(OC(=O)NC1=O)c1ccc([nH]c1=O)-c1ccc2n(C)ccc2c1 Show InChI InChI=1S/C20H19N3O4/c1-11(2)20(18(25)22-19(26)27-20)14-5-6-15(21-17(14)24)12-4-7-16-13(10-12)8-9-23(16)3/h4-11H,1-3H3,(H,21,24)(H,22,25,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at rat EP3 receptor expressed in human U2OS cells co-expressing Gqi5 assessed as inhibition of PGE2-induced response after 24 hrs... |
ACS Med Chem Lett 1: 316-320 (2010)
Article DOI: 10.1021/ml100077x
BindingDB Entry DOI: 10.7270/Q2BG2Q1F |
More data for this
Ligand-Target Pair | |
Arginase-2, mitochondrial
(Homo sapiens (Human)) | BDBM50350311
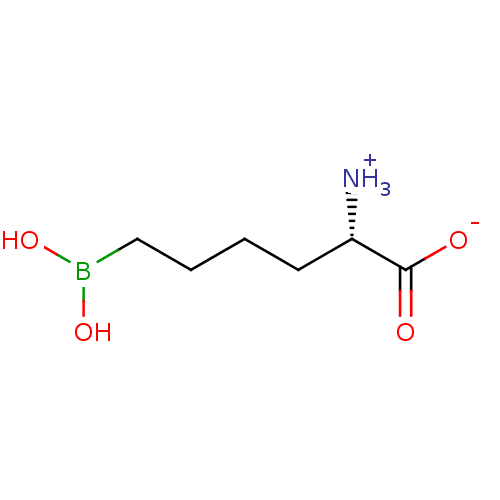
(CHEMBL1812661)Show InChI InChI=1S/C6H14BNO4/c8-5(6(9)10)3-1-2-4-7(11)12/h5,11-12H,1-4,8H2,(H,9,10)/t5-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Drexel University
Curated by ChEMBL
| Assay Description
Binding affinity to human arginase 2 |
J Med Chem 54: 5432-43 (2011)
Article DOI: 10.1021/jm200443b
BindingDB Entry DOI: 10.7270/Q2TH8N21 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50384443

(CHEMBL1770317)Show SMILES CC(C)C1(OC(O)=NC1=O)c1ccc([nH]c1=O)-c1ccc2ccccc2c1 |c:6| Show InChI InChI=1S/C21H18N2O4/c1-12(2)21(19(25)23-20(26)27-21)16-9-10-17(22-18(16)24)15-8-7-13-5-3-4-6-14(13)11-15/h3-12H,1-2H3,(H,22,24)(H,23,25,26) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at human EP3c receptor expressed in human U2OS cells assessed as inhibition of PGE2-induced calcium mobilization after 24 hrs by ... |
ACS Med Chem Lett 1: 316-320 (2010)
Article DOI: 10.1021/ml100077x
BindingDB Entry DOI: 10.7270/Q2BG2Q1F |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(RAT) | BDBM50384443

(CHEMBL1770317)Show SMILES CC(C)C1(OC(O)=NC1=O)c1ccc([nH]c1=O)-c1ccc2ccccc2c1 |c:6| Show InChI InChI=1S/C21H18N2O4/c1-12(2)21(19(25)23-20(26)27-21)16-9-10-17(22-18(16)24)15-8-7-13-5-3-4-6-14(13)11-15/h3-12H,1-2H3,(H,22,24)(H,23,25,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at rat EP3 receptor expressed in human U2OS cells co-expressing Gqi5 assessed as inhibition of PGE2-induced response after 24 hrs... |
ACS Med Chem Lett 1: 316-320 (2010)
Article DOI: 10.1021/ml100077x
BindingDB Entry DOI: 10.7270/Q2BG2Q1F |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(RAT) | BDBM50384446

(CHEMBL2035509)Show SMILES CC(C)C1(OC(=O)NC1=O)c1ccc([nH]c1=O)-c1ccc(C)c(C)c1 Show InChI InChI=1S/C19H20N2O4/c1-10(2)19(17(23)21-18(24)25-19)14-7-8-15(20-16(14)22)13-6-5-11(3)12(4)9-13/h5-10H,1-4H3,(H,20,22)(H,21,23,24) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at rat EP3 receptor expressed in human U2OS cells co-expressing Gqi5 assessed as inhibition of PGE2-induced response after 24 hrs... |
ACS Med Chem Lett 1: 316-320 (2010)
Article DOI: 10.1021/ml100077x
BindingDB Entry DOI: 10.7270/Q2BG2Q1F |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50384446

(CHEMBL2035509)Show SMILES CC(C)C1(OC(=O)NC1=O)c1ccc([nH]c1=O)-c1ccc(C)c(C)c1 Show InChI InChI=1S/C19H20N2O4/c1-10(2)19(17(23)21-18(24)25-19)14-7-8-15(20-16(14)22)13-6-5-11(3)12(4)9-13/h5-10H,1-4H3,(H,20,22)(H,21,23,24) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 19.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at human EP3c receptor expressed in human U2OS cells assessed as inhibition of PGE2-induced calcium mobilization after 24 hrs by ... |
ACS Med Chem Lett 1: 316-320 (2010)
Article DOI: 10.1021/ml100077x
BindingDB Entry DOI: 10.7270/Q2BG2Q1F |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50384442
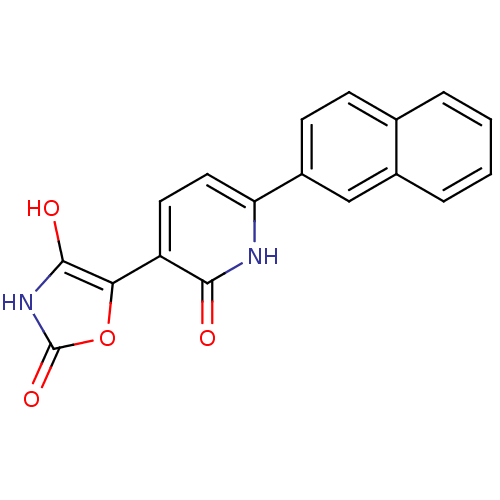
(CHEMBL2035507)Show SMILES Oc1[nH]c(=O)oc1-c1ccc([nH]c1=O)-c1ccc2ccccc2c1 Show InChI InChI=1S/C18H12N2O4/c21-16-13(15-17(22)20-18(23)24-15)7-8-14(19-16)12-6-5-10-3-1-2-4-11(10)9-12/h1-9,22H,(H,19,21)(H,20,23) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at human EP3c receptor expressed in human U2OS cells assessed as inhibition of PGE2-induced calcium mobilization after 24 hrs by ... |
ACS Med Chem Lett 1: 316-320 (2010)
Article DOI: 10.1021/ml100077x
BindingDB Entry DOI: 10.7270/Q2BG2Q1F |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(RAT) | BDBM50384442

(CHEMBL2035507)Show SMILES Oc1[nH]c(=O)oc1-c1ccc([nH]c1=O)-c1ccc2ccccc2c1 Show InChI InChI=1S/C18H12N2O4/c21-16-13(15-17(22)20-18(23)24-15)7-8-14(19-16)12-6-5-10-3-1-2-4-11(10)9-12/h1-9,22H,(H,19,21)(H,20,23) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at rat EP3 receptor expressed in human U2OS cells co-expressing Gqi5 assessed as inhibition of PGE2-induced response after 24 hrs... |
ACS Med Chem Lett 1: 316-320 (2010)
Article DOI: 10.1021/ml100077x
BindingDB Entry DOI: 10.7270/Q2BG2Q1F |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50384442

(CHEMBL2035507)Show SMILES Oc1[nH]c(=O)oc1-c1ccc([nH]c1=O)-c1ccc2ccccc2c1 Show InChI InChI=1S/C18H12N2O4/c21-16-13(15-17(22)20-18(23)24-15)7-8-14(19-16)12-6-5-10-3-1-2-4-11(10)9-12/h1-9,22H,(H,19,21)(H,20,23) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >5.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at EP1 receptor in human U2OS cells expressing Gqi5 assessed as inhibition of PGE2-induced response after 24 hrs by FLIPR assay |
ACS Med Chem Lett 1: 316-320 (2010)
Article DOI: 10.1021/ml100077x
BindingDB Entry DOI: 10.7270/Q2BG2Q1F |
More data for this
Ligand-Target Pair | |
Arginase
(Plasmodium falciparum) | BDBM50350311

(CHEMBL1812661)Show InChI InChI=1S/C6H14BNO4/c8-5(6(9)10)3-1-2-4-7(11)12/h5,11-12H,1-4,8H2,(H,9,10)/t5-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Drexel University
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum arginase using L-arginine as substrate by colorimetric assay |
J Med Chem 54: 5432-43 (2011)
Article DOI: 10.1021/jm200443b
BindingDB Entry DOI: 10.7270/Q2TH8N21 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50384443

(CHEMBL1770317)Show SMILES CC(C)C1(OC(O)=NC1=O)c1ccc([nH]c1=O)-c1ccc2ccccc2c1 |c:6| Show InChI InChI=1S/C21H18N2O4/c1-12(2)21(19(25)23-20(26)27-21)16-9-10-17(22-18(16)24)15-8-7-13-5-3-4-6-14(13)11-15/h3-12H,1-2H3,(H,22,24)(H,23,25,26) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >5.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at EP1 receptor in human U2OS cells expressing Gqi5 assessed as inhibition of PGE2-induced response after 24 hrs by FLIPR assay |
ACS Med Chem Lett 1: 316-320 (2010)
Article DOI: 10.1021/ml100077x
BindingDB Entry DOI: 10.7270/Q2BG2Q1F |
More data for this
Ligand-Target Pair | |
Arginase
(Plasmodium falciparum) | BDBM50350309
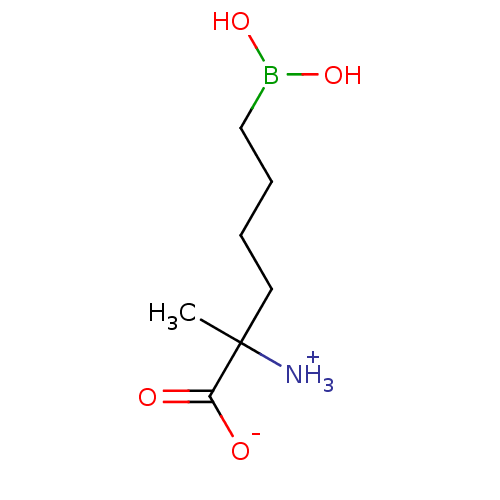
(CHEMBL1812662)Show InChI InChI=1S/C7H16BNO4/c1-7(9,6(10)11)4-2-3-5-8(12)13/h12-13H,2-5,9H2,1H3,(H,10,11) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Drexel University
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum arginase using L-arginine as substrate by colorimetric assay |
J Med Chem 54: 5432-43 (2011)
Article DOI: 10.1021/jm200443b
BindingDB Entry DOI: 10.7270/Q2TH8N21 |
More data for this
Ligand-Target Pair | |
Arginase
(Plasmodium falciparum) | BDBM50350310
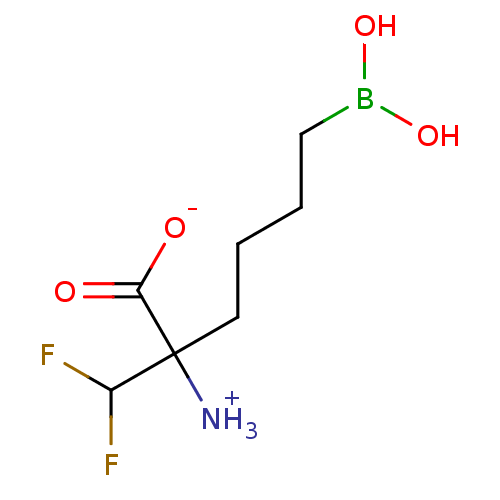
(CHEMBL1812663)Show InChI InChI=1S/C7H14BF2NO4/c9-5(10)7(11,6(12)13)3-1-2-4-8(14)15/h5,14-15H,1-4,11H2,(H,12,13) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 2.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Drexel University
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum arginase using L-arginine as substrate by colorimetric assay |
J Med Chem 54: 5432-43 (2011)
Article DOI: 10.1021/jm200443b
BindingDB Entry DOI: 10.7270/Q2TH8N21 |
More data for this
Ligand-Target Pair | |
Solute carrier family 15 member 1
(Homo sapiens (Human)) | BDBM50418932
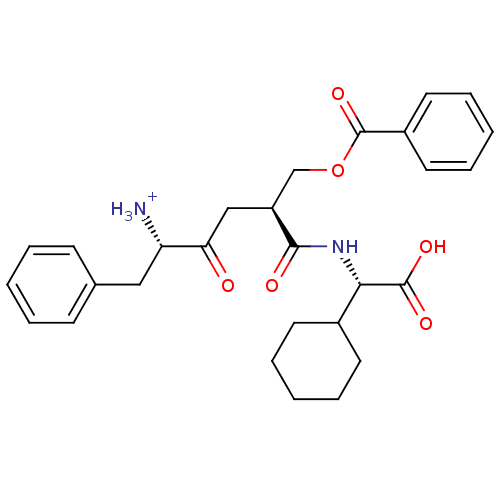
(CHEMBL1807354)Show SMILES [NH3+][C@@H](Cc1ccccc1)C(=O)C[C@@H](COC(=O)c1ccccc1)C(=O)N[C@@H](C1CCCCC1)C(O)=O |r| Show InChI InChI=1S/C28H34N2O6/c29-23(16-19-10-4-1-5-11-19)24(31)17-22(18-36-28(35)21-14-8-3-9-15-21)26(32)30-25(27(33)34)20-12-6-2-7-13-20/h1,3-5,8-11,14-15,20,22-23,25H,2,6-7,12-13,16-18,29H2,(H,30,32)(H,33,34)/p+1/t22-,23-,25-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.68E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to PEPT1 in human Caco2 cells assessed as inhibition of [14C]Gly-Sar apical uptake after 5 mins by liquid scintillation counting |
Bioorg Med Chem Lett 21: 4597-601 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.108
BindingDB Entry DOI: 10.7270/Q2VM4DHS |
More data for this
Ligand-Target Pair | |
Solute carrier family 15 member 1
(Homo sapiens (Human)) | BDBM50418933
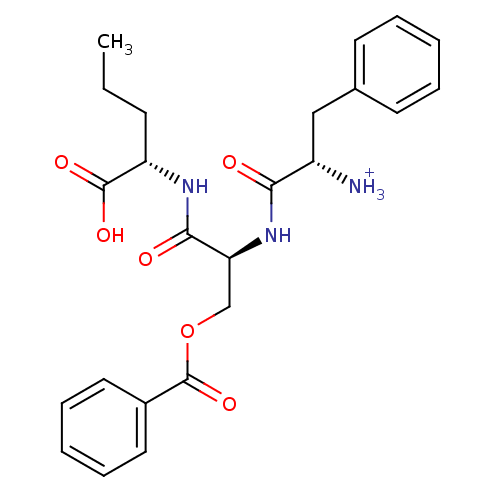
(CHEMBL1807356)Show SMILES CCC[C@H](NC(=O)[C@H](COC(=O)c1ccccc1)NC(=O)[C@@H]([NH3+])Cc1ccccc1)C(O)=O |r| Show InChI InChI=1S/C24H29N3O6/c1-2-9-19(23(30)31)26-22(29)20(15-33-24(32)17-12-7-4-8-13-17)27-21(28)18(25)14-16-10-5-3-6-11-16/h3-8,10-13,18-20H,2,9,14-15,25H2,1H3,(H,26,29)(H,27,28)(H,30,31)/p+1/t18-,19-,20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.23E+8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to PEPT1 in human Caco2 cells assessed as inhibition of [14C]Gly-Sar apical uptake after 5 mins by liquid scintillation counting |
Bioorg Med Chem Lett 21: 4597-601 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.108
BindingDB Entry DOI: 10.7270/Q2VM4DHS |
More data for this
Ligand-Target Pair | |
Solute carrier family 15 member 1
(Homo sapiens (Human)) | BDBM50418934
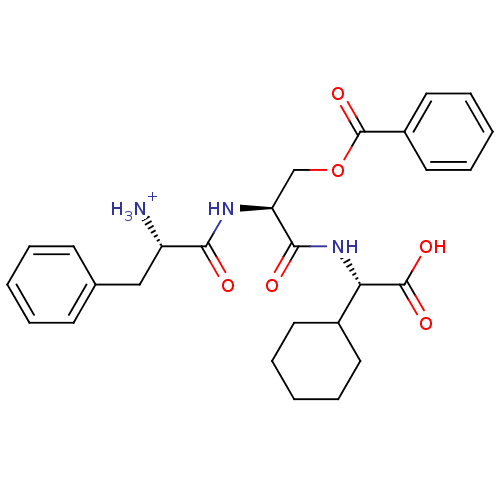
(CHEMBL1807357)Show SMILES [NH3+][C@@H](Cc1ccccc1)C(=O)N[C@@H](COC(=O)c1ccccc1)C(=O)N[C@@H](C1CCCCC1)C(O)=O |r| Show InChI InChI=1S/C27H33N3O6/c28-21(16-18-10-4-1-5-11-18)24(31)29-22(17-36-27(35)20-14-8-3-9-15-20)25(32)30-23(26(33)34)19-12-6-2-7-13-19/h1,3-5,8-11,14-15,19,21-23H,2,6-7,12-13,16-17,28H2,(H,29,31)(H,30,32)(H,33,34)/p+1/t21-,22-,23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.45E+8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to PEPT1 in human Caco2 cells assessed as inhibition of [14C]Gly-Sar apical uptake after 5 mins by liquid scintillation counting |
Bioorg Med Chem Lett 21: 4597-601 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.108
BindingDB Entry DOI: 10.7270/Q2VM4DHS |
More data for this
Ligand-Target Pair | |
Solute carrier family 15 member 1
(Homo sapiens (Human)) | BDBM50418935
(CHEMBL1807353)Show SMILES CCC[C@H](NC(=O)[C@H](COC(=O)c1ccccc1)CC(=O)[C@@H]([NH3+])Cc1ccccc1)C(O)=O |r| Show InChI InChI=1S/C25H30N2O6/c1-2-9-21(24(30)31)27-23(29)19(16-33-25(32)18-12-7-4-8-13-18)15-22(28)20(26)14-17-10-5-3-6-11-17/h3-8,10-13,19-21H,2,9,14-16,26H2,1H3,(H,27,29)(H,30,31)/p+1/t19-,20-,21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.51E+8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to PEPT1 in human Caco2 cells assessed as inhibition of [14C]Gly-Sar apical uptake after 5 mins by liquid scintillation counting |
Bioorg Med Chem Lett 21: 4597-601 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.108
BindingDB Entry DOI: 10.7270/Q2VM4DHS |
More data for this
Ligand-Target Pair | |
Solute carrier family 15 member 1
(Homo sapiens (Human)) | BDBM50418931
(CHEMBL1807351)Show SMILES C[C@H](NC(=O)[C@H](CO)NC(=O)[C@@H](N)Cc1ccccc1)C(O)=O |r| Show InChI InChI=1S/C15H21N3O5/c1-9(15(22)23)17-14(21)12(8-19)18-13(20)11(16)7-10-5-3-2-4-6-10/h2-6,9,11-12,19H,7-8,16H2,1H3,(H,17,21)(H,18,20)(H,22,23)/t9-,11-,12-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.57E+8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to PEPT1 in human Caco2 cells assessed as inhibition of [14C]Gly-Sar apical uptake after 5 mins by liquid scintillation counting |
Bioorg Med Chem Lett 21: 4597-601 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.108
BindingDB Entry DOI: 10.7270/Q2VM4DHS |
More data for this
Ligand-Target Pair | |
Solute carrier family 15 member 1
(Homo sapiens (Human)) | BDBM50418936
(CHEMBL1807352)Show SMILES C[C@H](NC(=O)[C@H](COC(=O)c1ccccc1)CC(=O)[C@@H]([NH3+])Cc1ccccc1)C(O)=O |r| Show InChI InChI=1S/C23H26N2O6/c1-15(22(28)29)25-21(27)18(14-31-23(30)17-10-6-3-7-11-17)13-20(26)19(24)12-16-8-4-2-5-9-16/h2-11,15,18-19H,12-14,24H2,1H3,(H,25,27)(H,28,29)/p+1/t15-,18-,19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.24E+8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to PEPT1 in human Caco2 cells assessed as inhibition of [14C]Gly-Sar apical uptake after 5 mins by liquid scintillation counting |
Bioorg Med Chem Lett 21: 4597-601 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.108
BindingDB Entry DOI: 10.7270/Q2VM4DHS |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 4
(Homo sapiens (Human)) | BDBM50502628

(CHEMBL4469630)Show SMILES CC(C)(C)CN1C[C@@]2(CCC[C@](C)(Cn3cnc4ccc(cc34)C#N)C2)OC1=O |r| Show InChI InChI=1S/C23H30N4O2/c1-21(2,3)13-26-15-23(29-20(26)28)9-5-8-22(4,12-23)14-27-16-25-18-7-6-17(11-24)10-19(18)27/h6-7,10,16H,5,8-9,12-15H2,1-4H3/t22-,23-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human TRPV4 expressed in baculovirus infected HEK/MSR 2 cells pre-incubated for 10 mins followed by GSK634775A addition by Fura-2 dye b... |
ACS Med Chem Lett 10: 1228-1233 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00274
BindingDB Entry DOI: 10.7270/Q2RX9GBF |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 4
(Homo sapiens (Human)) | BDBM50502648
(CHEMBL4588831)Show SMILES CC(C)(C)c1cnc(cn1)N1C[C@@]2(CCC[C@](C)(Cn3cnc4ccc(cc34)C#N)C2)OC1=O |r| Show InChI InChI=1S/C26H30N6O2/c1-24(2,3)21-12-29-22(13-28-21)32-16-26(34-23(32)33)9-5-8-25(4,14-26)15-31-17-30-19-7-6-18(11-27)10-20(19)31/h6-7,10,12-13,17H,5,8-9,14-16H2,1-4H3/t25-,26-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human TRPV4 expressed in baculovirus infected HEK/MSR 2 cells pre-incubated for 10 mins followed by GSK634775A addition by Fura-2 dye b... |
ACS Med Chem Lett 10: 1228-1233 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00274
BindingDB Entry DOI: 10.7270/Q2RX9GBF |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 4
(Homo sapiens (Human)) | BDBM50502635
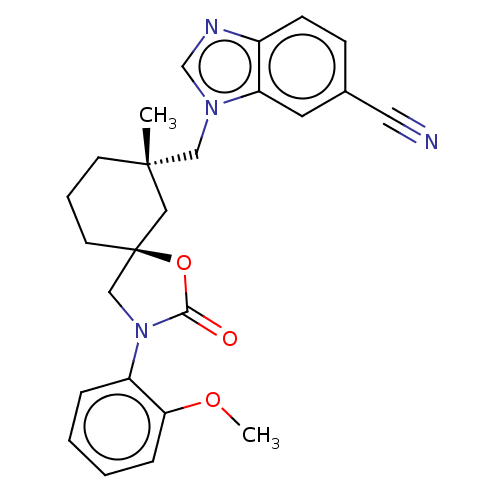
(CHEMBL4521512)Show SMILES COc1ccccc1N1C[C@@]2(CCC[C@](C)(Cn3cnc4ccc(cc34)C#N)C2)OC1=O |r| Show InChI InChI=1S/C25H26N4O3/c1-24(15-28-17-27-19-9-8-18(13-26)12-21(19)28)10-5-11-25(14-24)16-29(23(30)32-25)20-6-3-4-7-22(20)31-2/h3-4,6-9,12,17H,5,10-11,14-16H2,1-2H3/t24-,25-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human TRPV4 expressed in baculovirus infected HEK/MSR 2 cells pre-incubated for 10 mins followed by GSK634775A addition by Fura-2 dye b... |
ACS Med Chem Lett 10: 1228-1233 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00274
BindingDB Entry DOI: 10.7270/Q2RX9GBF |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 4
(Homo sapiens (Human)) | BDBM50502626
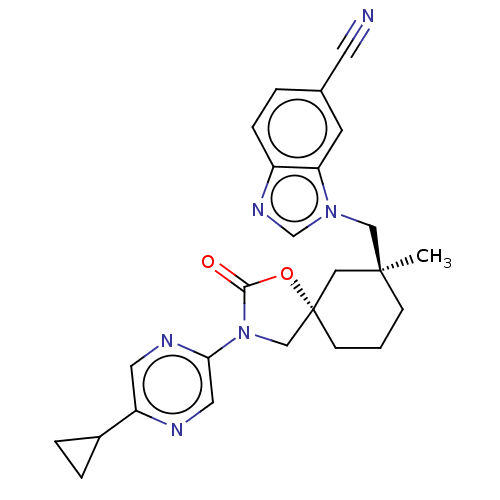
(CHEMBL4536058)Show SMILES C[C@]1(Cn2cnc3ccc(cc23)C#N)CCC[C@@]2(CN(C(=O)O2)c2cnc(cn2)C2CC2)C1 |r| Show InChI InChI=1S/C25H26N6O2/c1-24(14-30-16-29-19-6-3-17(10-26)9-21(19)30)7-2-8-25(13-24)15-31(23(32)33-25)22-12-27-20(11-28-22)18-4-5-18/h3,6,9,11-12,16,18H,2,4-5,7-8,13-15H2,1H3/t24-,25-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human TRPV4 expressed in baculovirus infected HEK/MSR 2 cells pre-incubated for 10 mins followed by GSK634775A addition by Fura-2 dye b... |
ACS Med Chem Lett 10: 1228-1233 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00274
BindingDB Entry DOI: 10.7270/Q2RX9GBF |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 4
(Homo sapiens (Human)) | BDBM50502649
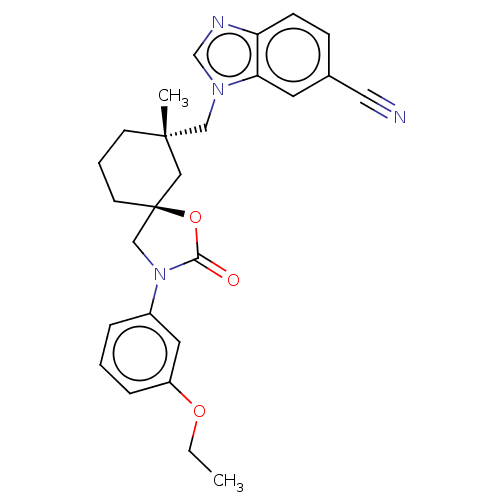
(CHEMBL4532369)Show SMILES CCOc1cccc(c1)N1C[C@@]2(CCC[C@](C)(Cn3cnc4ccc(cc34)C#N)C2)OC1=O |r| Show InChI InChI=1S/C26H28N4O3/c1-3-32-21-7-4-6-20(13-21)30-17-26(33-24(30)31)11-5-10-25(2,15-26)16-29-18-28-22-9-8-19(14-27)12-23(22)29/h4,6-9,12-13,18H,3,5,10-11,15-17H2,1-2H3/t25-,26-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human TRPV4 expressed in baculovirus infected HEK/MSR 2 cells pre-incubated for 10 mins followed by GSK634775A addition by Fura-2 dye b... |
ACS Med Chem Lett 10: 1228-1233 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00274
BindingDB Entry DOI: 10.7270/Q2RX9GBF |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 4
(Rattus norvegicus) | BDBM50502640

(CHEMBL4470585)Show SMILES CC(C)(O)c1cnc(cn1)N1C[C@@]2(CCC[C@](C)(Cn3cnc4ccc(cc34)C#N)C2)OC1=O |r| Show InChI InChI=1S/C25H28N6O3/c1-23(2,33)20-11-28-21(12-27-20)31-15-25(34-22(31)32)8-4-7-24(3,13-25)14-30-16-29-18-6-5-17(10-26)9-19(18)30/h5-6,9,11-12,16,33H,4,7-8,13-15H2,1-3H3/t24-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of rat TRPV4 expressed in baculovirus infected HEK/MSR 2 cells pre-incubated for 10 mins followed by GSK634775A addition by Fura-2 dye bas... |
ACS Med Chem Lett 10: 1228-1233 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00274
BindingDB Entry DOI: 10.7270/Q2RX9GBF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Transient receptor potential cation channel subfamily V member 4
(Rattus norvegicus) | BDBM50232815

(CHEMBL4104743)Show SMILES CCOc1ccc2c(C(=O)NC3(CC3)c3ccccc3)c(CN3CCC(CC3)N3CCCCC3)c(nc2c1)-c1cccc(c1)C(F)(F)F Show InChI InChI=1S/C39H43F3N4O2/c1-2-48-31-14-15-32-34(25-31)43-36(27-10-9-13-29(24-27)39(40,41)42)33(26-45-22-16-30(17-23-45)46-20-7-4-8-21-46)35(32)37(47)44-38(18-19-38)28-11-5-3-6-12-28/h3,5-6,9-15,24-25,30H,2,4,7-8,16-23,26H2,1H3,(H,44,47) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at rat TRPV4 expressed in HEK293 cells assessed as inhibition of GSK634775A-induced calcium influx preincubated for 10 mins follo... |
ACS Med Chem Lett 8: 549-554 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00094
BindingDB Entry DOI: 10.7270/Q2HD7XWW |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 4
(Homo sapiens (Human)) | BDBM50502643
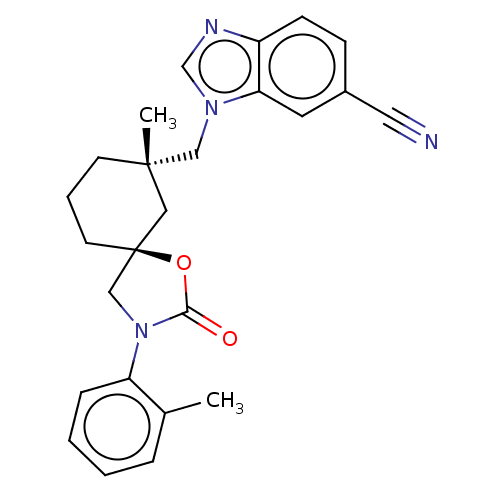
(CHEMBL4541827)Show SMILES Cc1ccccc1N1C[C@@]2(CCC[C@](C)(Cn3cnc4ccc(cc34)C#N)C2)OC1=O |r| Show InChI InChI=1S/C25H26N4O2/c1-18-6-3-4-7-21(18)29-16-25(31-23(29)30)11-5-10-24(2,14-25)15-28-17-27-20-9-8-19(13-26)12-22(20)28/h3-4,6-9,12,17H,5,10-11,14-16H2,1-2H3/t24-,25-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human TRPV4 expressed in baculovirus infected HEK/MSR 2 cells pre-incubated for 10 mins followed by GSK634775A addition by Fura-2 dye b... |
ACS Med Chem Lett 10: 1228-1233 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00274
BindingDB Entry DOI: 10.7270/Q2RX9GBF |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 4
(Homo sapiens (Human)) | BDBM50502640

(CHEMBL4470585)Show SMILES CC(C)(O)c1cnc(cn1)N1C[C@@]2(CCC[C@](C)(Cn3cnc4ccc(cc34)C#N)C2)OC1=O |r| Show InChI InChI=1S/C25H28N6O3/c1-23(2,33)20-11-28-21(12-27-20)31-15-25(34-22(31)32)8-4-7-24(3,13-25)14-30-16-29-18-6-5-17(10-26)9-19(18)30/h5-6,9,11-12,16,33H,4,7-8,13-15H2,1-3H3/t24-,25-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human TRPV4 expressed in baculovirus infected HEK/MSR 2 cells pre-incubated for 10 mins followed by GSK634775A addition by Fura-2 dye b... |
ACS Med Chem Lett 10: 1228-1233 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00274
BindingDB Entry DOI: 10.7270/Q2RX9GBF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Transient receptor potential cation channel subfamily V member 4
(Rattus norvegicus) | BDBM50232797
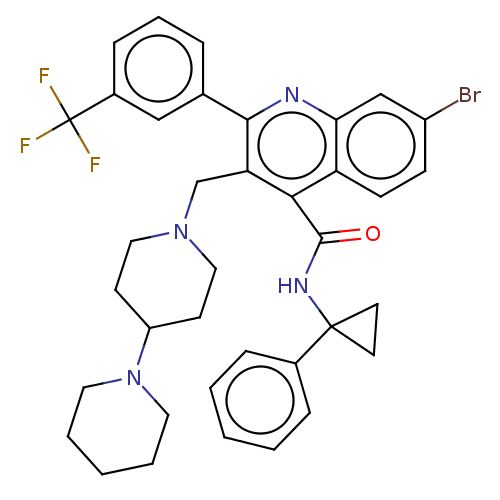
(CHEMBL4073922)Show SMILES FC(F)(F)c1cccc(c1)-c1nc2cc(Br)ccc2c(C(=O)NC2(CC2)c2ccccc2)c1CN1CCC(CC1)N1CCCCC1 Show InChI InChI=1S/C37H38BrF3N4O/c38-28-12-13-30-32(23-28)42-34(25-8-7-11-27(22-25)37(39,40)41)31(24-44-20-14-29(15-21-44)45-18-5-2-6-19-45)33(30)35(46)43-36(16-17-36)26-9-3-1-4-10-26/h1,3-4,7-13,22-23,29H,2,5-6,14-21,24H2,(H,43,46) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at rat TRPV4 expressed in HEK293 cells assessed as inhibition of GSK634775A-induced calcium influx preincubated for 10 mins follo... |
ACS Med Chem Lett 8: 549-554 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00094
BindingDB Entry DOI: 10.7270/Q2HD7XWW |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 4
(Rattus norvegicus) | BDBM50232804
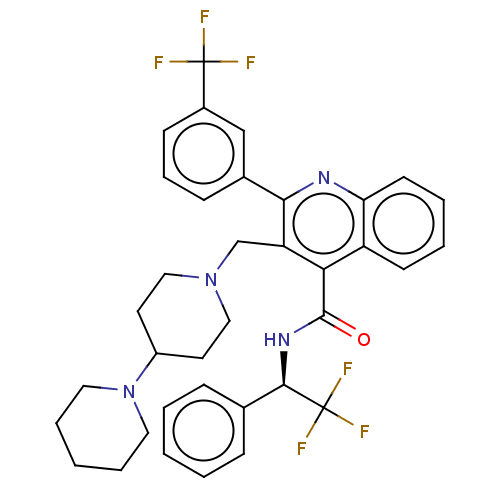
(CHEMBL4087716)Show SMILES FC(F)(F)[C@H](NC(=O)c1c(CN2CCC(CC2)N2CCCCC2)c(nc2ccccc12)-c1cccc(c1)C(F)(F)F)c1ccccc1 |r| Show InChI InChI=1S/C36H36F6N4O/c37-35(38,39)26-13-9-12-25(22-26)32-29(23-45-20-16-27(17-21-45)46-18-7-2-8-19-46)31(28-14-5-6-15-30(28)43-32)34(47)44-33(36(40,41)42)24-10-3-1-4-11-24/h1,3-6,9-15,22,27,33H,2,7-8,16-21,23H2,(H,44,47)/t33-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at rat TRPV4 expressed in HEK293 cells assessed as inhibition of GSK634775A-induced calcium influx preincubated for 10 mins follo... |
ACS Med Chem Lett 8: 549-554 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00094
BindingDB Entry DOI: 10.7270/Q2HD7XWW |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 4
(Homo sapiens (Human)) | BDBM50502653

(CHEMBL4514491)Show SMILES C[C@]1(Cn2cnc3ccc(cc23)C#N)CCC[C@@]2(CN(C(=O)O2)c2ccccc2Cl)C1 |r| Show InChI InChI=1S/C24H23ClN4O2/c1-23(14-28-16-27-19-8-7-17(12-26)11-21(19)28)9-4-10-24(13-23)15-29(22(30)31-24)20-6-3-2-5-18(20)25/h2-3,5-8,11,16H,4,9-10,13-15H2,1H3/t23-,24-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human TRPV4 expressed in baculovirus infected HEK/MSR 2 cells pre-incubated for 10 mins followed by GSK634775A addition by Fura-2 dye b... |
ACS Med Chem Lett 10: 1228-1233 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00274
BindingDB Entry DOI: 10.7270/Q2RX9GBF |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 4
(Rattus norvegicus) | BDBM50232800
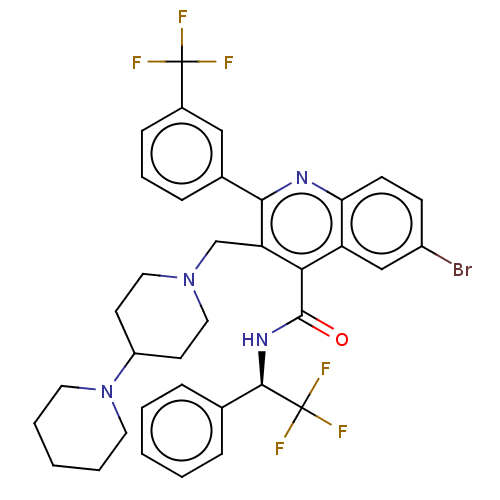
(CHEMBL4103166)Show SMILES FC(F)(F)[C@H](NC(=O)c1c(CN2CCC(CC2)N2CCCCC2)c(nc2ccc(Br)cc12)-c1cccc(c1)C(F)(F)F)c1ccccc1 |r| Show InChI InChI=1S/C36H35BrF6N4O/c37-26-12-13-30-28(21-26)31(34(48)45-33(36(41,42)43)23-8-3-1-4-9-23)29(32(44-30)24-10-7-11-25(20-24)35(38,39)40)22-46-18-14-27(15-19-46)47-16-5-2-6-17-47/h1,3-4,7-13,20-21,27,33H,2,5-6,14-19,22H2,(H,45,48)/t33-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at rat TRPV4 expressed in HEK293 cells assessed as inhibition of GSK634775A-induced calcium influx preincubated for 10 mins follo... |
ACS Med Chem Lett 8: 549-554 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00094
BindingDB Entry DOI: 10.7270/Q2HD7XWW |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 4
(Rattus norvegicus) | BDBM50232813
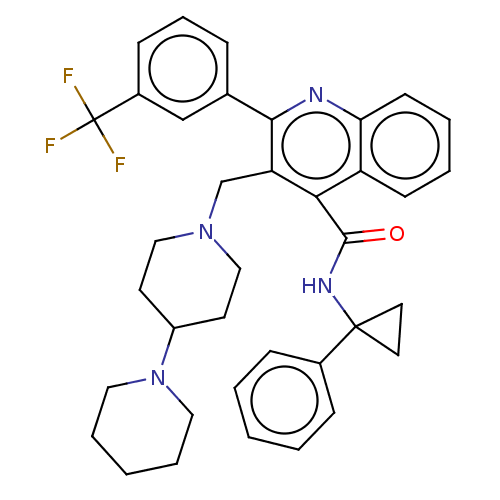
(CHEMBL4097859)Show SMILES FC(F)(F)c1cccc(c1)-c1nc2ccccc2c(C(=O)NC2(CC2)c2ccccc2)c1CN1CCC(CC1)N1CCCCC1 Show InChI InChI=1S/C37H39F3N4O/c38-37(39,40)28-13-9-10-26(24-28)34-31(25-43-22-16-29(17-23-43)44-20-7-2-8-21-44)33(30-14-5-6-15-32(30)41-34)35(45)42-36(18-19-36)27-11-3-1-4-12-27/h1,3-6,9-15,24,29H,2,7-8,16-23,25H2,(H,42,45) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at rat TRPV4 expressed in HEK293 cells assessed as inhibition of GSK634775A-induced calcium influx preincubated for 10 mins follo... |
ACS Med Chem Lett 8: 549-554 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00094
BindingDB Entry DOI: 10.7270/Q2HD7XWW |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 4
(Rattus norvegicus) | BDBM50232790
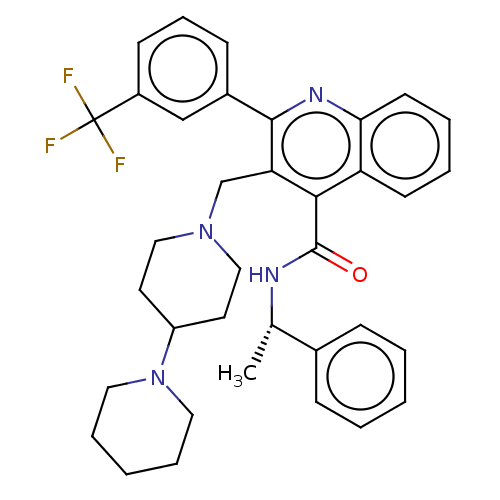
(CHEMBL4084542)Show SMILES C[C@H](NC(=O)c1c(CN2CCC(CC2)N2CCCCC2)c(nc2ccccc12)-c1cccc(c1)C(F)(F)F)c1ccccc1 |r| Show InChI InChI=1S/C36H39F3N4O/c1-25(26-11-4-2-5-12-26)40-35(44)33-30-15-6-7-16-32(30)41-34(27-13-10-14-28(23-27)36(37,38)39)31(33)24-42-21-17-29(18-22-42)43-19-8-3-9-20-43/h2,4-7,10-16,23,25,29H,3,8-9,17-22,24H2,1H3,(H,40,44)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at rat TRPV4 expressed in HEK293 cells assessed as inhibition of GSK634775A-induced calcium influx preincubated for 10 mins follo... |
ACS Med Chem Lett 8: 549-554 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00094
BindingDB Entry DOI: 10.7270/Q2HD7XWW |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 4
(Rattus norvegicus) | BDBM50232796

(CHEMBL4094483)Show SMILES COc1ccc2c(C(=O)NC3(CC3)c3ccccc3)c(CN3CCC(CC3)N3CCCCC3)c(nc2c1)-c1cccc(c1)C(F)(F)F Show InChI InChI=1S/C38H41F3N4O2/c1-47-30-13-14-31-33(24-30)42-35(26-9-8-12-28(23-26)38(39,40)41)32(25-44-21-15-29(16-22-44)45-19-6-3-7-20-45)34(31)36(46)43-37(17-18-37)27-10-4-2-5-11-27/h2,4-5,8-14,23-24,29H,3,6-7,15-22,25H2,1H3,(H,43,46) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at rat TRPV4 expressed in HEK293 cells assessed as inhibition of GSK634775A-induced calcium influx preincubated for 10 mins follo... |
ACS Med Chem Lett 8: 549-554 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00094
BindingDB Entry DOI: 10.7270/Q2HD7XWW |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 4
(Homo sapiens (Human)) | BDBM50502636
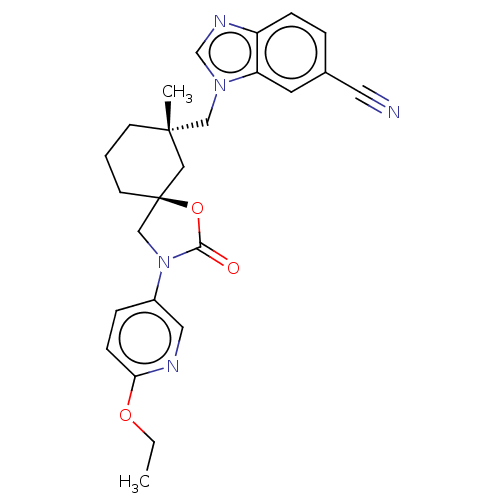
(CHEMBL4588158)Show SMILES CCOc1ccc(cn1)N1C[C@@]2(CCC[C@](C)(Cn3cnc4ccc(cc34)C#N)C2)OC1=O |r| Show InChI InChI=1S/C25H27N5O3/c1-3-32-22-8-6-19(13-27-22)30-16-25(33-23(30)31)10-4-9-24(2,14-25)15-29-17-28-20-7-5-18(12-26)11-21(20)29/h5-8,11,13,17H,3-4,9-10,14-16H2,1-2H3/t24-,25-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human TRPV4 expressed in baculovirus infected HEK/MSR 2 cells pre-incubated for 10 mins followed by GSK634775A addition by Fura-2 dye b... |
ACS Med Chem Lett 10: 1228-1233 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00274
BindingDB Entry DOI: 10.7270/Q2RX9GBF |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 4
(Rattus norvegicus) | BDBM50232803
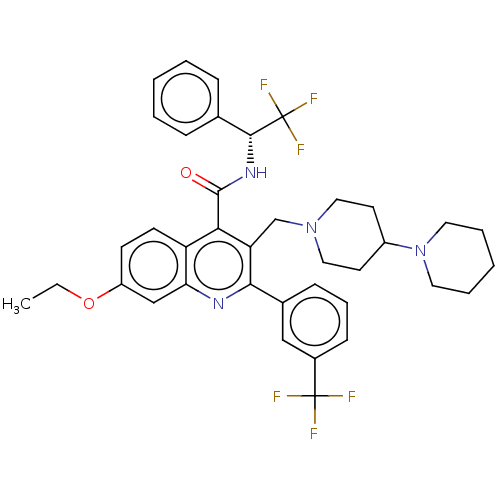
(CHEMBL4068023)Show SMILES CCOc1ccc2c(C(=O)N[C@H](c3ccccc3)C(F)(F)F)c(CN3CCC(CC3)N3CCCCC3)c(nc2c1)-c1cccc(c1)C(F)(F)F |r| Show InChI InChI=1S/C38H40F6N4O2/c1-2-50-29-14-15-30-32(23-29)45-34(26-12-9-13-27(22-26)37(39,40)41)31(24-47-20-16-28(17-21-47)48-18-7-4-8-19-48)33(30)36(49)46-35(38(42,43)44)25-10-5-3-6-11-25/h3,5-6,9-15,22-23,28,35H,2,4,7-8,16-21,24H2,1H3,(H,46,49)/t35-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at rat TRPV4 expressed in HEK293 cells assessed as inhibition of GSK634775A-induced calcium influx preincubated for 10 mins follo... |
ACS Med Chem Lett 8: 549-554 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00094
BindingDB Entry DOI: 10.7270/Q2HD7XWW |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 4
(Homo sapiens (Human)) | BDBM50426532
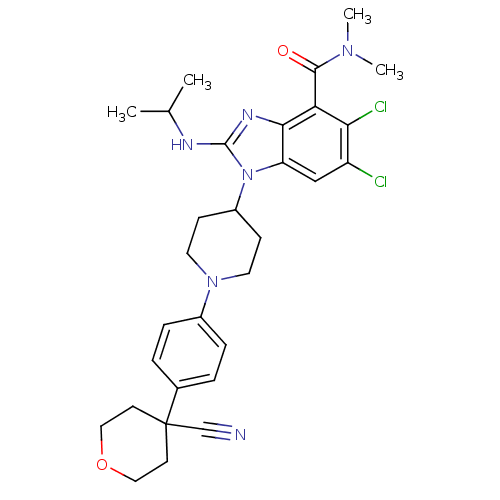
(CHEMBL2323936)Show SMILES CC(C)Nc1nc2c(C(=O)N(C)C)c(Cl)c(Cl)cc2n1C1CCN(CC1)c1ccc(cc1)C1(CCOCC1)C#N Show InChI InChI=1S/C30H36Cl2N6O2/c1-19(2)34-29-35-27-24(17-23(31)26(32)25(27)28(39)36(3)4)38(29)22-9-13-37(14-10-22)21-7-5-20(6-8-21)30(18-33)11-15-40-16-12-30/h5-8,17,19,22H,9-16H2,1-4H3,(H,34,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant TRPV4 expressed in BacMam virus infected HEK MSRII cells assessed as inhibition of GSK1016790A-induced calci... |
ACS Med Chem Lett 4: 293-6 (2013)
Article DOI: 10.1021/ml300449k
BindingDB Entry DOI: 10.7270/Q2VT1TDB |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 4
(Rattus norvegicus) | BDBM50232799
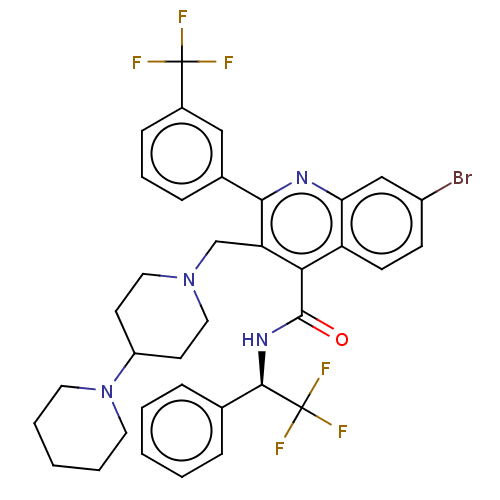
(CHEMBL4078886)Show SMILES FC(F)(F)[C@H](NC(=O)c1c(CN2CCC(CC2)N2CCCCC2)c(nc2cc(Br)ccc12)-c1cccc(c1)C(F)(F)F)c1ccccc1 |r| Show InChI InChI=1S/C36H35BrF6N4O/c37-26-12-13-28-30(21-26)44-32(24-10-7-11-25(20-24)35(38,39)40)29(22-46-18-14-27(15-19-46)47-16-5-2-6-17-47)31(28)34(48)45-33(36(41,42)43)23-8-3-1-4-9-23/h1,3-4,7-13,20-21,27,33H,2,5-6,14-19,22H2,(H,45,48)/t33-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at rat TRPV4 expressed in HEK293 cells assessed as inhibition of GSK634775A-induced calcium influx preincubated for 10 mins follo... |
ACS Med Chem Lett 8: 549-554 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00094
BindingDB Entry DOI: 10.7270/Q2HD7XWW |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 4
(Rattus norvegicus) | BDBM50232798
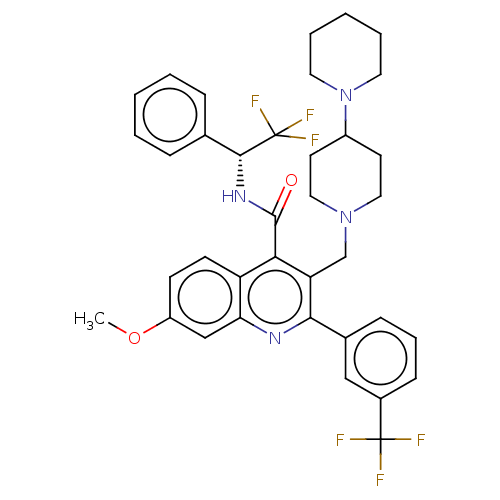
(CHEMBL4095413)Show SMILES COc1ccc2c(C(=O)N[C@H](c3ccccc3)C(F)(F)F)c(CN3CCC(CC3)N3CCCCC3)c(nc2c1)-c1cccc(c1)C(F)(F)F |r| Show InChI InChI=1S/C37H38F6N4O2/c1-49-28-13-14-29-31(22-28)44-33(25-11-8-12-26(21-25)36(38,39)40)30(23-46-19-15-27(16-20-46)47-17-6-3-7-18-47)32(29)35(48)45-34(37(41,42)43)24-9-4-2-5-10-24/h2,4-5,8-14,21-22,27,34H,3,6-7,15-20,23H2,1H3,(H,45,48)/t34-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at rat TRPV4 expressed in HEK293 cells assessed as inhibition of GSK634775A-induced calcium influx preincubated for 10 mins follo... |
ACS Med Chem Lett 8: 549-554 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00094
BindingDB Entry DOI: 10.7270/Q2HD7XWW |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 4
(Rattus norvegicus) | BDBM50232809
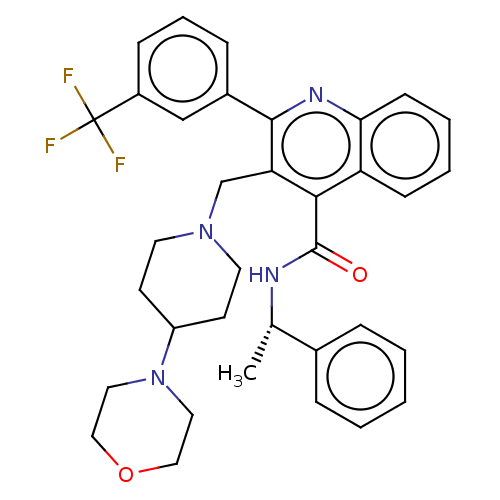
(CHEMBL4065154)Show SMILES C[C@H](NC(=O)c1c(CN2CCC(CC2)N2CCOCC2)c(nc2ccccc12)-c1cccc(c1)C(F)(F)F)c1ccccc1 |r| Show InChI InChI=1S/C35H37F3N4O2/c1-24(25-8-3-2-4-9-25)39-34(43)32-29-12-5-6-13-31(29)40-33(26-10-7-11-27(22-26)35(36,37)38)30(32)23-41-16-14-28(15-17-41)42-18-20-44-21-19-42/h2-13,22,24,28H,14-21,23H2,1H3,(H,39,43)/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at rat TRPV4 expressed in HEK293 cells assessed as inhibition of GSK634775A-induced calcium influx preincubated for 10 mins follo... |
ACS Med Chem Lett 8: 549-554 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00094
BindingDB Entry DOI: 10.7270/Q2HD7XWW |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 4
(Homo sapiens (Human)) | BDBM50502637

(CHEMBL4469982)Show SMILES CCOc1cnc(cn1)N1C[C@@]2(CCC[C@](C)(Cn3cnc4ccc(cc34)C#N)C2)OC1=O |r| Show InChI InChI=1S/C24H26N6O3/c1-3-32-21-12-26-20(11-27-21)30-15-24(33-22(30)31)8-4-7-23(2,13-24)14-29-16-28-18-6-5-17(10-25)9-19(18)29/h5-6,9,11-12,16H,3-4,7-8,13-15H2,1-2H3/t23-,24-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human TRPV4 expressed in baculovirus infected HEK/MSR 2 cells pre-incubated for 10 mins followed by GSK634775A addition by Fura-2 dye b... |
ACS Med Chem Lett 10: 1228-1233 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00274
BindingDB Entry DOI: 10.7270/Q2RX9GBF |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 4
(Homo sapiens (Human)) | BDBM50502646
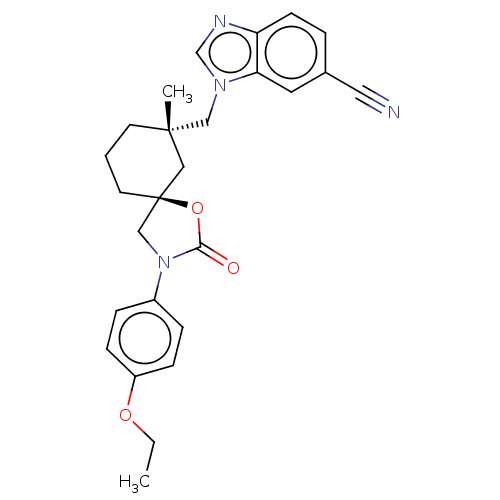
(CHEMBL4579838)Show SMILES CCOc1ccc(cc1)N1C[C@@]2(CCC[C@](C)(Cn3cnc4ccc(cc34)C#N)C2)OC1=O |r| Show InChI InChI=1S/C26H28N4O3/c1-3-32-21-8-6-20(7-9-21)30-17-26(33-24(30)31)12-4-11-25(2,15-26)16-29-18-28-22-10-5-19(14-27)13-23(22)29/h5-10,13,18H,3-4,11-12,15-17H2,1-2H3/t25-,26-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human TRPV4 expressed in baculovirus infected HEK/MSR 2 cells pre-incubated for 10 mins followed by GSK634775A addition by Fura-2 dye b... |
ACS Med Chem Lett 10: 1228-1233 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00274
BindingDB Entry DOI: 10.7270/Q2RX9GBF |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 4
(Homo sapiens (Human)) | BDBM50502642
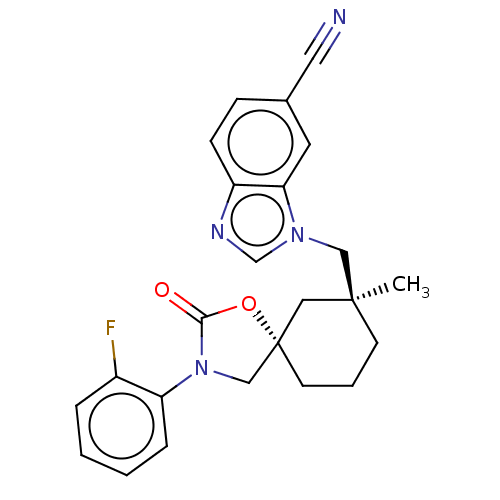
(CHEMBL4560266)Show SMILES C[C@]1(Cn2cnc3ccc(cc23)C#N)CCC[C@@]2(CN(C(=O)O2)c2ccccc2F)C1 |r| Show InChI InChI=1S/C24H23FN4O2/c1-23(14-28-16-27-19-8-7-17(12-26)11-21(19)28)9-4-10-24(13-23)15-29(22(30)31-24)20-6-3-2-5-18(20)25/h2-3,5-8,11,16H,4,9-10,13-15H2,1H3/t23-,24-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human TRPV4 expressed in baculovirus infected HEK/MSR 2 cells pre-incubated for 10 mins followed by GSK634775A addition by Fura-2 dye b... |
ACS Med Chem Lett 10: 1228-1233 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00274
BindingDB Entry DOI: 10.7270/Q2RX9GBF |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 4
(Rattus norvegicus) | BDBM50502650
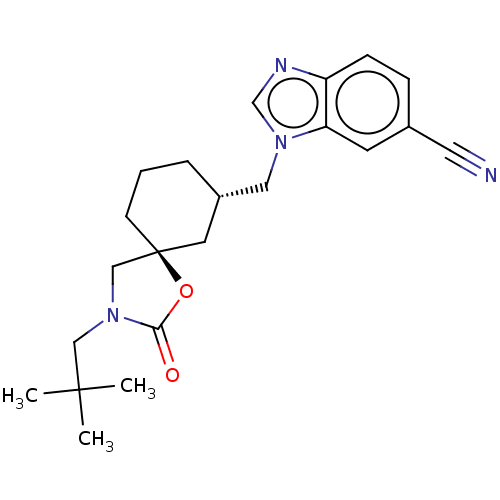
(CHEMBL4549613)Show SMILES CC(C)(C)CN1C[C@@]2(CCC[C@H](Cn3cnc4ccc(cc34)C#N)C2)OC1=O |r| Show InChI InChI=1S/C22H28N4O2/c1-21(2,3)13-26-14-22(28-20(26)27)8-4-5-17(10-22)12-25-15-24-18-7-6-16(11-23)9-19(18)25/h6-7,9,15,17H,4-5,8,10,12-14H2,1-3H3/t17-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of rat TRPV4 expressed in baculovirus infected HEK/MSR 2 cells pre-incubated for 10 mins followed by GSK634775A addition by Fura-2 dye bas... |
ACS Med Chem Lett 10: 1228-1233 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00274
BindingDB Entry DOI: 10.7270/Q2RX9GBF |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 4
(Rattus norvegicus) | BDBM50232805
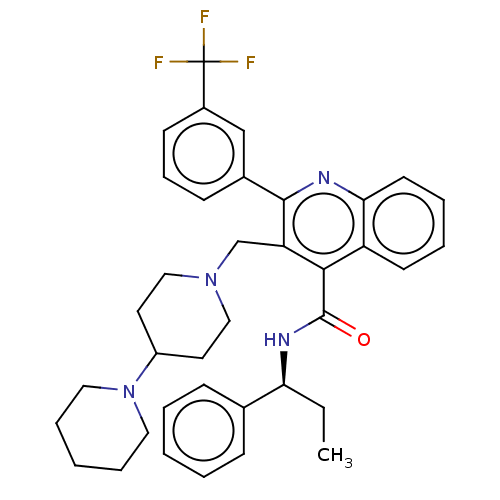
(CHEMBL4069979)Show SMILES CC[C@H](NC(=O)c1c(CN2CCC(CC2)N2CCCCC2)c(nc2ccccc12)-c1cccc(c1)C(F)(F)F)c1ccccc1 |r| Show InChI InChI=1S/C37H41F3N4O/c1-2-32(26-12-5-3-6-13-26)42-36(45)34-30-16-7-8-17-33(30)41-35(27-14-11-15-28(24-27)37(38,39)40)31(34)25-43-22-18-29(19-23-43)44-20-9-4-10-21-44/h3,5-8,11-17,24,29,32H,2,4,9-10,18-23,25H2,1H3,(H,42,45)/t32-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at rat TRPV4 expressed in HEK293 cells assessed as inhibition of GSK634775A-induced calcium influx preincubated for 10 mins follo... |
ACS Med Chem Lett 8: 549-554 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00094
BindingDB Entry DOI: 10.7270/Q2HD7XWW |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data