 Found 44 hits with Last Name = 'thress' and Initial = 'k'
Found 44 hits with Last Name = 'thress' and Initial = 'k' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50392791

(CHEMBL2151321 | US8486966, 1)Show SMILES CC(C)Oc1cc(n[nH]1)-n1cnc2ccc(N[C@@H](C)c3ccc(F)cn3)nc12 |r| Show InChI InChI=1S/C19H20FN7O/c1-11(2)28-18-8-17(25-26-18)27-10-22-15-6-7-16(24-19(15)27)23-12(3)14-5-4-13(20)9-21-14/h4-12H,1-3H3,(H,23,24)(H,25,26)/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Abl |
ACS Med Chem Lett 3: 705-709 (2012)
Article DOI: 10.1021/ml300074j
BindingDB Entry DOI: 10.7270/Q2GH9K2G |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50392791

(CHEMBL2151321 | US8486966, 1)Show SMILES CC(C)Oc1cc(n[nH]1)-n1cnc2ccc(N[C@@H](C)c3ccc(F)cn3)nc12 |r| Show InChI InChI=1S/C19H20FN7O/c1-11(2)28-18-8-17(25-26-18)27-10-22-15-6-7-16(24-19(15)27)23-12(3)14-5-4-13(20)9-21-14/h4-12H,1-3H3,(H,23,24)(H,25,26)/t12-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of TrkA |
ACS Med Chem Lett 3: 705-709 (2012)
Article DOI: 10.1021/ml300074j
BindingDB Entry DOI: 10.7270/Q2GH9K2G |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50392791

(CHEMBL2151321 | US8486966, 1)Show SMILES CC(C)Oc1cc(n[nH]1)-n1cnc2ccc(N[C@@H](C)c3ccc(F)cn3)nc12 |r| Show InChI InChI=1S/C19H20FN7O/c1-11(2)28-18-8-17(25-26-18)27-10-22-15-6-7-16(24-19(15)27)23-12(3)14-5-4-13(20)9-21-14/h4-12H,1-3H3,(H,23,24)(H,25,26)/t12-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Flt3 |
ACS Med Chem Lett 3: 705-709 (2012)
Article DOI: 10.1021/ml300074j
BindingDB Entry DOI: 10.7270/Q2GH9K2G |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Fgr
(Homo sapiens (Human)) | BDBM50392791

(CHEMBL2151321 | US8486966, 1)Show SMILES CC(C)Oc1cc(n[nH]1)-n1cnc2ccc(N[C@@H](C)c3ccc(F)cn3)nc12 |r| Show InChI InChI=1S/C19H20FN7O/c1-11(2)28-18-8-17(25-26-18)27-10-22-15-6-7-16(24-19(15)27)23-12(3)14-5-4-13(20)9-21-14/h4-12H,1-3H3,(H,23,24)(H,25,26)/t12-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Fgr |
ACS Med Chem Lett 3: 705-709 (2012)
Article DOI: 10.1021/ml300074j
BindingDB Entry DOI: 10.7270/Q2GH9K2G |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50392791

(CHEMBL2151321 | US8486966, 1)Show SMILES CC(C)Oc1cc(n[nH]1)-n1cnc2ccc(N[C@@H](C)c3ccc(F)cn3)nc12 |r| Show InChI InChI=1S/C19H20FN7O/c1-11(2)28-18-8-17(25-26-18)27-10-22-15-6-7-16(24-19(15)27)23-12(3)14-5-4-13(20)9-21-14/h4-12H,1-3H3,(H,23,24)(H,25,26)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of BTK |
ACS Med Chem Lett 3: 705-709 (2012)
Article DOI: 10.1021/ml300074j
BindingDB Entry DOI: 10.7270/Q2GH9K2G |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase SIK1
(Homo sapiens (Human)) | BDBM50392791

(CHEMBL2151321 | US8486966, 1)Show SMILES CC(C)Oc1cc(n[nH]1)-n1cnc2ccc(N[C@@H](C)c3ccc(F)cn3)nc12 |r| Show InChI InChI=1S/C19H20FN7O/c1-11(2)28-18-8-17(25-26-18)27-10-22-15-6-7-16(24-19(15)27)23-12(3)14-5-4-13(20)9-21-14/h4-12H,1-3H3,(H,23,24)(H,25,26)/t12-/m0/s1 | NCI pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of SIK |
ACS Med Chem Lett 3: 705-709 (2012)
Article DOI: 10.1021/ml300074j
BindingDB Entry DOI: 10.7270/Q2GH9K2G |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM24705

(4-aminopyrazolylpyrimidine analogue, 10k | 5-bromo...)Show SMILES C[C@H](Nc1ncc(Br)c(Nc2cc(n[nH]2)C2CC2)n1)c1ccc(F)cc1 |r| Show InChI InChI=1S/C18H18BrFN6/c1-10(11-4-6-13(20)7-5-11)22-18-21-9-14(19)17(24-18)23-16-8-15(25-26-16)12-2-3-12/h4-10,12H,2-3H2,1H3,(H3,21,22,23,24,25,26)/t10-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | 7.4 | 22 |
AstraZeneca
| Assay Description
TrkA kinase activity was determined by measuring the kinase ability to phosphorylate synthetic tyrosine residues within a generic polypeptide substra... |
J Med Chem 51: 4672-84 (2008)
Article DOI: 10.1021/jm800343j
BindingDB Entry DOI: 10.7270/Q2RN365V |
More data for this
Ligand-Target Pair | |
Phosphorylase b kinase gamma catalytic chain, liver/testis isoform
(Homo sapiens (Human)) | BDBM50392791

(CHEMBL2151321 | US8486966, 1)Show SMILES CC(C)Oc1cc(n[nH]1)-n1cnc2ccc(N[C@@H](C)c3ccc(F)cn3)nc12 |r| Show InChI InChI=1S/C19H20FN7O/c1-11(2)28-18-8-17(25-26-18)27-10-22-15-6-7-16(24-19(15)27)23-12(3)14-5-4-13(20)9-21-14/h4-12H,1-3H3,(H,23,24)(H,25,26)/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PhKg2 |
ACS Med Chem Lett 3: 705-709 (2012)
Article DOI: 10.1021/ml300074j
BindingDB Entry DOI: 10.7270/Q2GH9K2G |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM50392791

(CHEMBL2151321 | US8486966, 1)Show SMILES CC(C)Oc1cc(n[nH]1)-n1cnc2ccc(N[C@@H](C)c3ccc(F)cn3)nc12 |r| Show InChI InChI=1S/C19H20FN7O/c1-11(2)28-18-8-17(25-26-18)27-10-22-15-6-7-16(24-19(15)27)23-12(3)14-5-4-13(20)9-21-14/h4-12H,1-3H3,(H,23,24)(H,25,26)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of FGFR1 |
ACS Med Chem Lett 3: 705-709 (2012)
Article DOI: 10.1021/ml300074j
BindingDB Entry DOI: 10.7270/Q2GH9K2G |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM24706
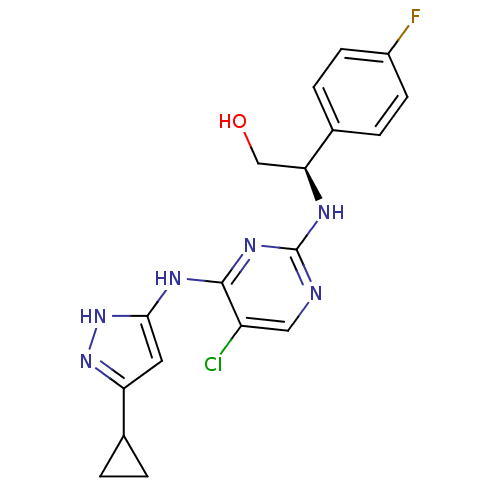
((2R)-2-({5-chloro-4-[(5-cyclopropyl-1H-pyrazol-3-y...)Show SMILES OC[C@H](Nc1ncc(Cl)c(Nc2cc(n[nH]2)C2CC2)n1)c1ccc(F)cc1 |r| Show InChI InChI=1S/C18H18ClFN6O/c19-13-8-21-18(22-15(9-27)11-3-5-12(20)6-4-11)24-17(13)23-16-7-14(25-26-16)10-1-2-10/h3-8,10,15,27H,1-2,9H2,(H3,21,22,23,24,25,26)/t15-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | 7.4 | 22 |
AstraZeneca
| Assay Description
TrkA kinase activity was determined by measuring the kinase ability to phosphorylate synthetic tyrosine residues within a generic polypeptide substra... |
J Med Chem 51: 4672-84 (2008)
Article DOI: 10.1021/jm800343j
BindingDB Entry DOI: 10.7270/Q2RN365V |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM24707

((3S)-3-({5-chloro-4-[(5-cyclopropyl-1H-pyrazol-3-y...)Show SMILES CN(C)C(=O)C[C@H](Nc1ncc(Cl)c(Nc2cc(n[nH]2)C2CC2)n1)c1ccc(F)cc1 |r| Show InChI InChI=1S/C21H23ClFN7O/c1-30(2)19(31)10-16(12-5-7-14(23)8-6-12)25-21-24-11-15(22)20(27-21)26-18-9-17(28-29-18)13-3-4-13/h5-9,11,13,16H,3-4,10H2,1-2H3,(H3,24,25,26,27,28,29)/t16-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | 7.4 | 22 |
AstraZeneca
| Assay Description
TrkA kinase activity was determined by measuring the kinase ability to phosphorylate synthetic tyrosine residues within a generic polypeptide substra... |
J Med Chem 51: 4672-84 (2008)
Article DOI: 10.1021/jm800343j
BindingDB Entry DOI: 10.7270/Q2RN365V |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50392791

(CHEMBL2151321 | US8486966, 1)Show SMILES CC(C)Oc1cc(n[nH]1)-n1cnc2ccc(N[C@@H](C)c3ccc(F)cn3)nc12 |r| Show InChI InChI=1S/C19H20FN7O/c1-11(2)28-18-8-17(25-26-18)27-10-22-15-6-7-16(24-19(15)27)23-12(3)14-5-4-13(20)9-21-14/h4-12H,1-3H3,(H,23,24)(H,25,26)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13.4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of SAPK2a |
ACS Med Chem Lett 3: 705-709 (2012)
Article DOI: 10.1021/ml300074j
BindingDB Entry DOI: 10.7270/Q2GH9K2G |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM24708
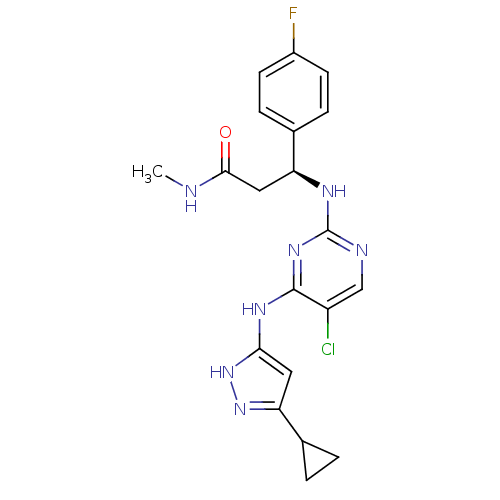
((3S)-3-({5-chloro-4-[(5-cyclopropyl-1H-pyrazol-3-y...)Show SMILES CNC(=O)C[C@H](Nc1ncc(Cl)c(Nc2cc(n[nH]2)C2CC2)n1)c1ccc(F)cc1 |r| Show InChI InChI=1S/C20H21ClFN7O/c1-23-18(30)9-15(11-4-6-13(22)7-5-11)25-20-24-10-14(21)19(27-20)26-17-8-16(28-29-17)12-2-3-12/h4-8,10,12,15H,2-3,9H2,1H3,(H,23,30)(H3,24,25,26,27,28,29)/t15-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | 7.4 | 22 |
AstraZeneca
| Assay Description
TrkA kinase activity was determined by measuring the kinase ability to phosphorylate synthetic tyrosine residues within a generic polypeptide substra... |
J Med Chem 51: 4672-84 (2008)
Article DOI: 10.1021/jm800343j
BindingDB Entry DOI: 10.7270/Q2RN365V |
More data for this
Ligand-Target Pair | |
Focal adhesion kinase 1
(Homo sapiens (Human)) | BDBM50392791

(CHEMBL2151321 | US8486966, 1)Show SMILES CC(C)Oc1cc(n[nH]1)-n1cnc2ccc(N[C@@H](C)c3ccc(F)cn3)nc12 |r| Show InChI InChI=1S/C19H20FN7O/c1-11(2)28-18-8-17(25-26-18)27-10-22-15-6-7-16(24-19(15)27)23-12(3)14-5-4-13(20)9-21-14/h4-12H,1-3H3,(H,23,24)(H,25,26)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17.2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of FAK |
ACS Med Chem Lett 3: 705-709 (2012)
Article DOI: 10.1021/ml300074j
BindingDB Entry DOI: 10.7270/Q2GH9K2G |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50392791

(CHEMBL2151321 | US8486966, 1)Show SMILES CC(C)Oc1cc(n[nH]1)-n1cnc2ccc(N[C@@H](C)c3ccc(F)cn3)nc12 |r| Show InChI InChI=1S/C19H20FN7O/c1-11(2)28-18-8-17(25-26-18)27-10-22-15-6-7-16(24-19(15)27)23-12(3)14-5-4-13(20)9-21-14/h4-12H,1-3H3,(H,23,24)(H,25,26)/t12-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18.2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of cSrc |
ACS Med Chem Lett 3: 705-709 (2012)
Article DOI: 10.1021/ml300074j
BindingDB Entry DOI: 10.7270/Q2GH9K2G |
More data for this
Ligand-Target Pair | |
Testis-specific serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50392791

(CHEMBL2151321 | US8486966, 1)Show SMILES CC(C)Oc1cc(n[nH]1)-n1cnc2ccc(N[C@@H](C)c3ccc(F)cn3)nc12 |r| Show InChI InChI=1S/C19H20FN7O/c1-11(2)28-18-8-17(25-26-18)27-10-22-15-6-7-16(24-19(15)27)23-12(3)14-5-4-13(20)9-21-14/h4-12H,1-3H3,(H,23,24)(H,25,26)/t12-/m0/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30.6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of TSSK2 |
ACS Med Chem Lett 3: 705-709 (2012)
Article DOI: 10.1021/ml300074j
BindingDB Entry DOI: 10.7270/Q2GH9K2G |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50392791

(CHEMBL2151321 | US8486966, 1)Show SMILES CC(C)Oc1cc(n[nH]1)-n1cnc2ccc(N[C@@H](C)c3ccc(F)cn3)nc12 |r| Show InChI InChI=1S/C19H20FN7O/c1-11(2)28-18-8-17(25-26-18)27-10-22-15-6-7-16(24-19(15)27)23-12(3)14-5-4-13(20)9-21-14/h4-12H,1-3H3,(H,23,24)(H,25,26)/t12-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 34.1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of ALK |
ACS Med Chem Lett 3: 705-709 (2012)
Article DOI: 10.1021/ml300074j
BindingDB Entry DOI: 10.7270/Q2GH9K2G |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase 9
(Homo sapiens (Human)) | BDBM50392791

(CHEMBL2151321 | US8486966, 1)Show SMILES CC(C)Oc1cc(n[nH]1)-n1cnc2ccc(N[C@@H](C)c3ccc(F)cn3)nc12 |r| Show InChI InChI=1S/C19H20FN7O/c1-11(2)28-18-8-17(25-26-18)27-10-22-15-6-7-16(24-19(15)27)23-12(3)14-5-4-13(20)9-21-14/h4-12H,1-3H3,(H,23,24)(H,25,26)/t12-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 44.3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of MLK1 |
ACS Med Chem Lett 3: 705-709 (2012)
Article DOI: 10.1021/ml300074j
BindingDB Entry DOI: 10.7270/Q2GH9K2G |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 3
(Homo sapiens (Human)) | BDBM50392791

(CHEMBL2151321 | US8486966, 1)Show SMILES CC(C)Oc1cc(n[nH]1)-n1cnc2ccc(N[C@@H](C)c3ccc(F)cn3)nc12 |r| Show InChI InChI=1S/C19H20FN7O/c1-11(2)28-18-8-17(25-26-18)27-10-22-15-6-7-16(24-19(15)27)23-12(3)14-5-4-13(20)9-21-14/h4-12H,1-3H3,(H,23,24)(H,25,26)/t12-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of FLT4 |
ACS Med Chem Lett 3: 705-709 (2012)
Article DOI: 10.1021/ml300074j
BindingDB Entry DOI: 10.7270/Q2GH9K2G |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50392791

(CHEMBL2151321 | US8486966, 1)Show SMILES CC(C)Oc1cc(n[nH]1)-n1cnc2ccc(N[C@@H](C)c3ccc(F)cn3)nc12 |r| Show InChI InChI=1S/C19H20FN7O/c1-11(2)28-18-8-17(25-26-18)27-10-22-15-6-7-16(24-19(15)27)23-12(3)14-5-4-13(20)9-21-14/h4-12H,1-3H3,(H,23,24)(H,25,26)/t12-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 68.3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 |
ACS Med Chem Lett 3: 705-709 (2012)
Article DOI: 10.1021/ml300074j
BindingDB Entry DOI: 10.7270/Q2GH9K2G |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50392791

(CHEMBL2151321 | US8486966, 1)Show SMILES CC(C)Oc1cc(n[nH]1)-n1cnc2ccc(N[C@@H](C)c3ccc(F)cn3)nc12 |r| Show InChI InChI=1S/C19H20FN7O/c1-11(2)28-18-8-17(25-26-18)27-10-22-15-6-7-16(24-19(15)27)23-12(3)14-5-4-13(20)9-21-14/h4-12H,1-3H3,(H,23,24)(H,25,26)/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 72.2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Itk |
ACS Med Chem Lett 3: 705-709 (2012)
Article DOI: 10.1021/ml300074j
BindingDB Entry DOI: 10.7270/Q2GH9K2G |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase WNK3
(Homo sapiens (Human)) | BDBM50392791

(CHEMBL2151321 | US8486966, 1)Show SMILES CC(C)Oc1cc(n[nH]1)-n1cnc2ccc(N[C@@H](C)c3ccc(F)cn3)nc12 |r| Show InChI InChI=1S/C19H20FN7O/c1-11(2)28-18-8-17(25-26-18)27-10-22-15-6-7-16(24-19(15)27)23-12(3)14-5-4-13(20)9-21-14/h4-12H,1-3H3,(H,23,24)(H,25,26)/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 99.8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of WNK3 |
ACS Med Chem Lett 3: 705-709 (2012)
Article DOI: 10.1021/ml300074j
BindingDB Entry DOI: 10.7270/Q2GH9K2G |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM24701
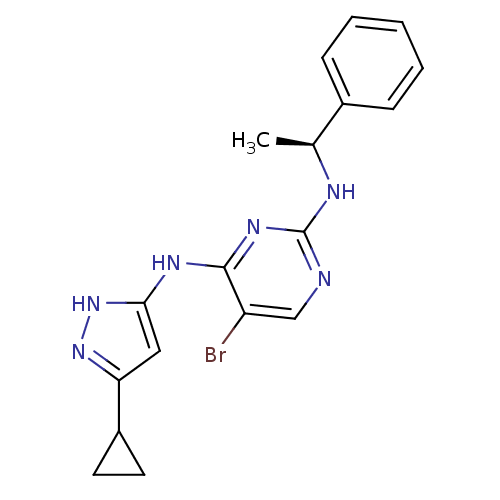
(4-aminopyrazolylpyrimidine analogue, 10g | 5-bromo...)Show SMILES C[C@H](Nc1ncc(Br)c(Nc2cc(n[nH]2)C2CC2)n1)c1ccccc1 |r| Show InChI InChI=1S/C18H19BrN6/c1-11(12-5-3-2-4-6-12)21-18-20-10-14(19)17(23-18)22-16-9-15(24-25-16)13-7-8-13/h2-6,9-11,13H,7-8H2,1H3,(H3,20,21,22,23,24,25)/t11-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | 7.4 | 22 |
AstraZeneca
| Assay Description
TrkA kinase activity was determined by measuring the kinase ability to phosphorylate synthetic tyrosine residues within a generic polypeptide substra... |
J Med Chem 51: 4672-84 (2008)
Article DOI: 10.1021/jm800343j
BindingDB Entry DOI: 10.7270/Q2RN365V |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM24699

(4-aminopyrazolylpyrimidine analogue, 10e | 5-bromo...)Show SMILES Fc1ccc(CNc2ncc(Br)c(Nc3cc(n[nH]3)C3CC3)n2)cc1 Show InChI InChI=1S/C17H16BrFN6/c18-13-9-21-17(20-8-10-1-5-12(19)6-2-10)23-16(13)22-15-7-14(24-25-15)11-3-4-11/h1-2,5-7,9,11H,3-4,8H2,(H3,20,21,22,23,24,25) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | 7.4 | 22 |
AstraZeneca
| Assay Description
TrkA kinase activity was determined by measuring the kinase ability to phosphorylate synthetic tyrosine residues within a generic polypeptide substra... |
J Med Chem 51: 4672-84 (2008)
Article DOI: 10.1021/jm800343j
BindingDB Entry DOI: 10.7270/Q2RN365V |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM24698
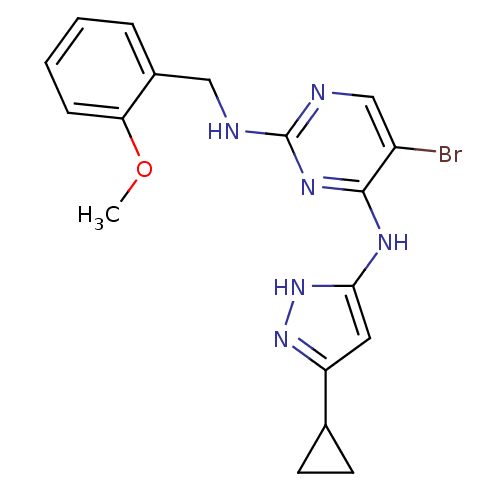
(4-aminopyrazolylpyrimidine analogue, 10d | 5-bromo...)Show SMILES COc1ccccc1CNc1ncc(Br)c(Nc2cc(n[nH]2)C2CC2)n1 Show InChI InChI=1S/C18H19BrN6O/c1-26-15-5-3-2-4-12(15)9-20-18-21-10-13(19)17(23-18)22-16-8-14(24-25-16)11-6-7-11/h2-5,8,10-11H,6-7,9H2,1H3,(H3,20,21,22,23,24,25) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | 7.4 | 22 |
AstraZeneca
| Assay Description
TrkA kinase activity was determined by measuring the kinase ability to phosphorylate synthetic tyrosine residues within a generic polypeptide substra... |
J Med Chem 51: 4672-84 (2008)
Article DOI: 10.1021/jm800343j
BindingDB Entry DOI: 10.7270/Q2RN365V |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM24704
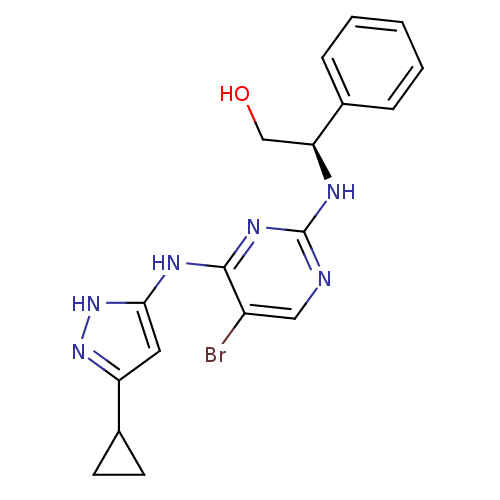
((2R)-2-({5-bromo-4-[(5-cyclopropyl-1H-pyrazol-3-yl...)Show SMILES OC[C@H](Nc1ncc(Br)c(Nc2cc(n[nH]2)C2CC2)n1)c1ccccc1 |r| Show InChI InChI=1S/C18H19BrN6O/c19-13-9-20-18(21-15(10-26)11-4-2-1-3-5-11)23-17(13)22-16-8-14(24-25-16)12-6-7-12/h1-5,8-9,12,15,26H,6-7,10H2,(H3,20,21,22,23,24,25)/t15-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | 7.4 | 22 |
AstraZeneca
| Assay Description
TrkA kinase activity was determined by measuring the kinase ability to phosphorylate synthetic tyrosine residues within a generic polypeptide substra... |
J Med Chem 51: 4672-84 (2008)
Article DOI: 10.1021/jm800343j
BindingDB Entry DOI: 10.7270/Q2RN365V |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM24694
(4-aminopyrazolylpyrimidine analogue, 5 | 5-bromo-4...)Show SMILES Cc1cc(CNc2ncc(Br)c(Nc3cc(n[nH]3)C3CC3)n2)on1 Show InChI InChI=1S/C15H16BrN7O/c1-8-4-10(24-23-8)6-17-15-18-7-11(16)14(20-15)19-13-5-12(21-22-13)9-2-3-9/h4-5,7,9H,2-3,6H2,1H3,(H3,17,18,19,20,21,22) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 270 | n/a | n/a | n/a | n/a | 7.4 | 22 |
AstraZeneca
| Assay Description
TrkA kinase activity was determined by measuring the kinase ability to phosphorylate synthetic tyrosine residues within a generic polypeptide substra... |
J Med Chem 51: 4672-84 (2008)
Article DOI: 10.1021/jm800343j
BindingDB Entry DOI: 10.7270/Q2RN365V |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM24700

(4-aminopyrazolylpyrimidine analogue, 10f | 5-bromo...)Show SMILES Clc1ccc(CNc2ncc(Br)c(Nc3cc(n[nH]3)C3CC3)n2)cc1 Show InChI InChI=1S/C17H16BrClN6/c18-13-9-21-17(20-8-10-1-5-12(19)6-2-10)23-16(13)22-15-7-14(24-25-15)11-3-4-11/h1-2,5-7,9,11H,3-4,8H2,(H3,20,21,22,23,24,25) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 360 | n/a | n/a | n/a | n/a | 7.4 | 22 |
AstraZeneca
| Assay Description
TrkA kinase activity was determined by measuring the kinase ability to phosphorylate synthetic tyrosine residues within a generic polypeptide substra... |
J Med Chem 51: 4672-84 (2008)
Article DOI: 10.1021/jm800343j
BindingDB Entry DOI: 10.7270/Q2RN365V |
More data for this
Ligand-Target Pair | |
BDNF/NT-3 growth factors receptor
(Homo sapiens (Human)) | BDBM14824
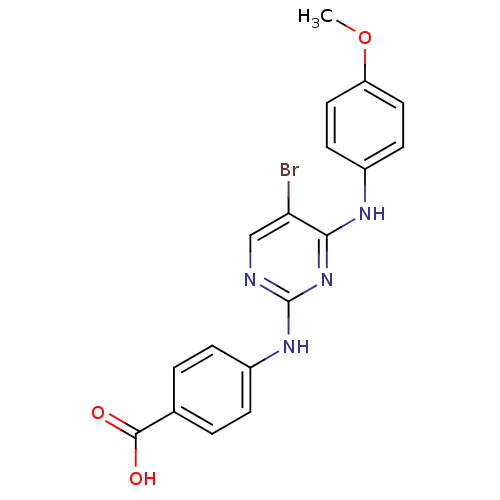
(4-({5-bromo-4-[(4-methoxyphenyl)amino]pyrimidin-2-...)Show InChI InChI=1S/C18H15BrN4O3/c1-26-14-8-6-12(7-9-14)21-16-15(19)10-20-18(23-16)22-13-4-2-11(3-5-13)17(24)25/h2-10H,1H3,(H,24,25)(H2,20,21,22,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 646 | n/a | n/a | n/a | n/a | 7.4 | 22 |
AstraZeneca
| Assay Description
TrkB kinase activity was measured against its ability to phosphorylate synthetic tyrosine residues within a generic polypeptide substrate using homog... |
J Med Chem 51: 4672-84 (2008)
Article DOI: 10.1021/jm800343j
BindingDB Entry DOI: 10.7270/Q2RN365V |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM24702
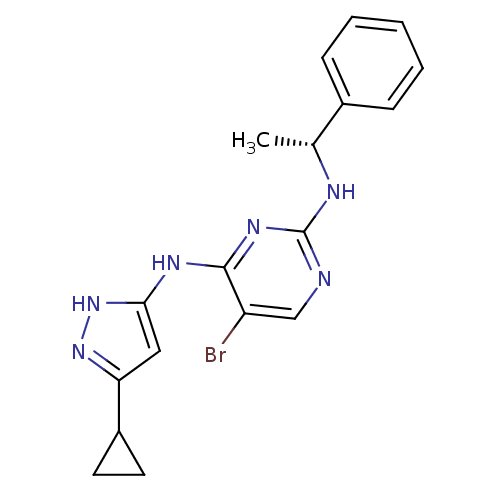
(4-aminopyrazolylpyrimidine analogue, 10h | 5-bromo...)Show SMILES C[C@@H](Nc1ncc(Br)c(Nc2cc(n[nH]2)C2CC2)n1)c1ccccc1 |r| Show InChI InChI=1S/C18H19BrN6/c1-11(12-5-3-2-4-6-12)21-18-20-10-14(19)17(23-18)22-16-9-15(24-25-16)13-7-8-13/h2-6,9-11,13H,7-8H2,1H3,(H3,20,21,22,23,24,25)/t11-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 650 | n/a | n/a | n/a | n/a | 7.4 | 22 |
AstraZeneca
| Assay Description
TrkA kinase activity was determined by measuring the kinase ability to phosphorylate synthetic tyrosine residues within a generic polypeptide substra... |
J Med Chem 51: 4672-84 (2008)
Article DOI: 10.1021/jm800343j
BindingDB Entry DOI: 10.7270/Q2RN365V |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM24696
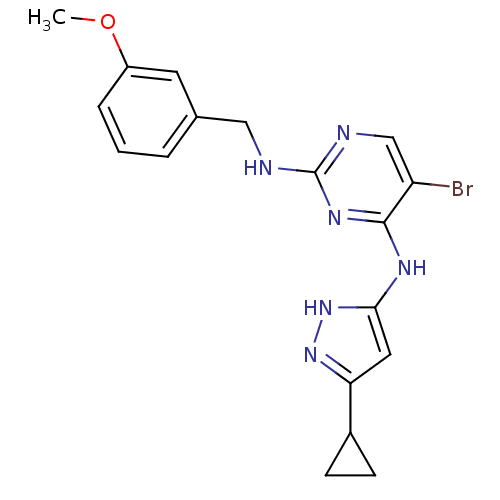
(4-aminopyrazolylpyrimidine analogue, 10b | 5-bromo...)Show SMILES COc1cccc(CNc2ncc(Br)c(Nc3cc(n[nH]3)C3CC3)n2)c1 Show InChI InChI=1S/C18H19BrN6O/c1-26-13-4-2-3-11(7-13)9-20-18-21-10-14(19)17(23-18)22-16-8-15(24-25-16)12-5-6-12/h2-4,7-8,10,12H,5-6,9H2,1H3,(H3,20,21,22,23,24,25) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 720 | n/a | n/a | n/a | n/a | 7.4 | 22 |
AstraZeneca
| Assay Description
TrkA kinase activity was determined by measuring the kinase ability to phosphorylate synthetic tyrosine residues within a generic polypeptide substra... |
J Med Chem 51: 4672-84 (2008)
Article DOI: 10.1021/jm800343j
BindingDB Entry DOI: 10.7270/Q2RN365V |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM24695

(2-N-benzyl-5-bromo-4-N-(5-cyclopropyl-1H-pyrazol-3...)Show InChI InChI=1S/C17H17BrN6/c18-13-10-20-17(19-9-11-4-2-1-3-5-11)22-16(13)21-15-8-14(23-24-15)12-6-7-12/h1-5,8,10,12H,6-7,9H2,(H3,19,20,21,22,23,24) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 750 | n/a | n/a | n/a | n/a | 7.4 | 22 |
AstraZeneca
| Assay Description
TrkA kinase activity was determined by measuring the kinase ability to phosphorylate synthetic tyrosine residues within a generic polypeptide substra... |
J Med Chem 51: 4672-84 (2008)
Article DOI: 10.1021/jm800343j
BindingDB Entry DOI: 10.7270/Q2RN365V |
More data for this
Ligand-Target Pair | |
BDNF/NT-3 growth factors receptor
(Homo sapiens (Human)) | BDBM24694

(4-aminopyrazolylpyrimidine analogue, 5 | 5-bromo-4...)Show SMILES Cc1cc(CNc2ncc(Br)c(Nc3cc(n[nH]3)C3CC3)n2)on1 Show InChI InChI=1S/C15H16BrN7O/c1-8-4-10(24-23-8)6-17-15-18-7-11(16)14(20-15)19-13-5-12(21-22-13)9-2-3-9/h4-5,7,9H,2-3,6H2,1H3,(H3,17,18,19,20,21,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | 7.4 | 22 |
AstraZeneca
| Assay Description
TrkB kinase activity was measured against its ability to phosphorylate synthetic tyrosine residues within a generic polypeptide substrate using homog... |
J Med Chem 51: 4672-84 (2008)
Article DOI: 10.1021/jm800343j
BindingDB Entry DOI: 10.7270/Q2RN365V |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM24697

(4-aminopyrazolylpyrimidine analogue, 10c | 5-bromo...)Show SMILES Clc1ccccc1CNc1ncc(Br)c(Nc2cc(n[nH]2)C2CC2)n1 Show InChI InChI=1S/C17H16BrClN6/c18-12-9-21-17(20-8-11-3-1-2-4-13(11)19)23-16(12)22-15-7-14(24-25-15)10-5-6-10/h1-4,7,9-10H,5-6,8H2,(H3,20,21,22,23,24,25) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.17E+3 | n/a | n/a | n/a | n/a | 7.4 | 22 |
AstraZeneca
| Assay Description
TrkA kinase activity was determined by measuring the kinase ability to phosphorylate synthetic tyrosine residues within a generic polypeptide substra... |
J Med Chem 51: 4672-84 (2008)
Article DOI: 10.1021/jm800343j
BindingDB Entry DOI: 10.7270/Q2RN365V |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM24703
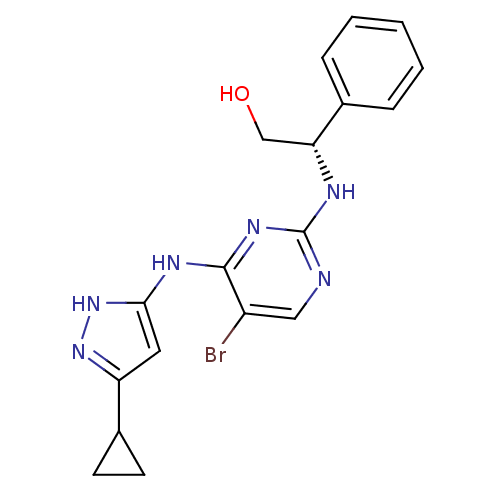
((2S)-2-({5-bromo-4-[(5-cyclopropyl-1H-pyrazol-3-yl...)Show SMILES OC[C@@H](Nc1ncc(Br)c(Nc2cc(n[nH]2)C2CC2)n1)c1ccccc1 |r| Show InChI InChI=1S/C18H19BrN6O/c19-13-9-20-18(21-15(10-26)11-4-2-1-3-5-11)23-17(13)22-16-8-14(24-25-16)12-6-7-12/h1-5,8-9,12,15,26H,6-7,10H2,(H3,20,21,22,23,24,25)/t15-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | 7.4 | 22 |
AstraZeneca
| Assay Description
TrkA kinase activity was determined by measuring the kinase ability to phosphorylate synthetic tyrosine residues within a generic polypeptide substra... |
J Med Chem 51: 4672-84 (2008)
Article DOI: 10.1021/jm800343j
BindingDB Entry DOI: 10.7270/Q2RN365V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50392788

(CHEMBL457614)Show SMILES CC(C)Oc1cc(Nc2nc(N[C@@H](C)c3ccc(F)cn3)ncc2Cl)[nH]n1 |r| Show InChI InChI=1S/C17H19ClFN7O/c1-9(2)27-15-6-14(25-26-15)23-16-12(18)8-21-17(24-16)22-10(3)13-5-4-11(19)7-20-13/h4-10H,1-3H3,(H3,21,22,23,24,25,26)/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
ACS Med Chem Lett 3: 705-709 (2012)
Article DOI: 10.1021/ml300074j
BindingDB Entry DOI: 10.7270/Q2GH9K2G |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50392794
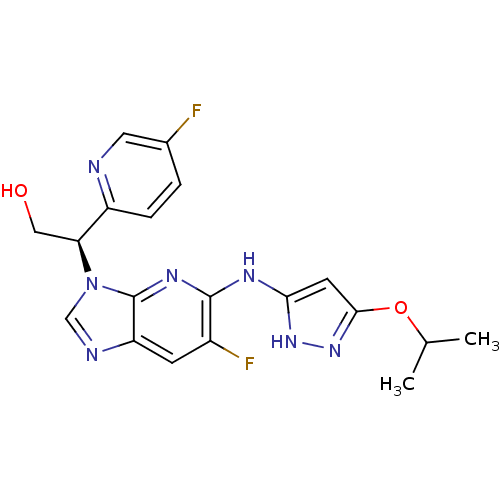
(CHEMBL2151324)Show SMILES CC(C)Oc1cc(Nc2nc3n(cnc3cc2F)[C@@H](CO)c2ccc(F)cn2)[nH]n1 |r| Show InChI InChI=1S/C19H19F2N7O2/c1-10(2)30-17-6-16(26-27-17)24-18-12(21)5-14-19(25-18)28(9-23-14)15(8-29)13-4-3-11(20)7-22-13/h3-7,9-10,15,29H,8H2,1-2H3,(H2,24,25,26,27)/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
ACS Med Chem Lett 3: 705-709 (2012)
Article DOI: 10.1021/ml300074j
BindingDB Entry DOI: 10.7270/Q2GH9K2G |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50392796
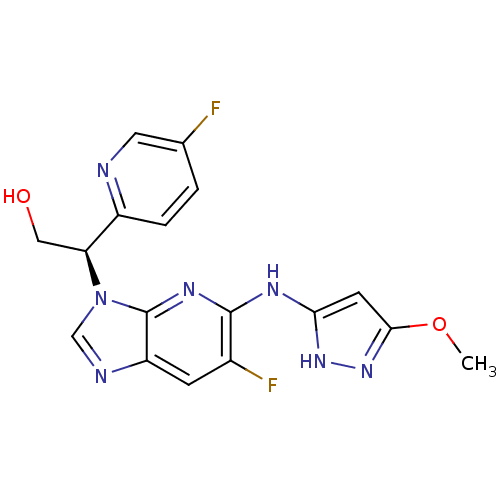
(CHEMBL2151326)Show SMILES COc1cc(Nc2nc3n(cnc3cc2F)[C@@H](CO)c2ccc(F)cn2)[nH]n1 |r| Show InChI InChI=1S/C17H15F2N7O2/c1-28-15-5-14(24-25-15)22-16-10(19)4-12-17(23-16)26(8-21-12)13(7-27)11-3-2-9(18)6-20-11/h2-6,8,13,27H,7H2,1H3,(H2,22,23,24,25)/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
ACS Med Chem Lett 3: 705-709 (2012)
Article DOI: 10.1021/ml300074j
BindingDB Entry DOI: 10.7270/Q2GH9K2G |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50392790
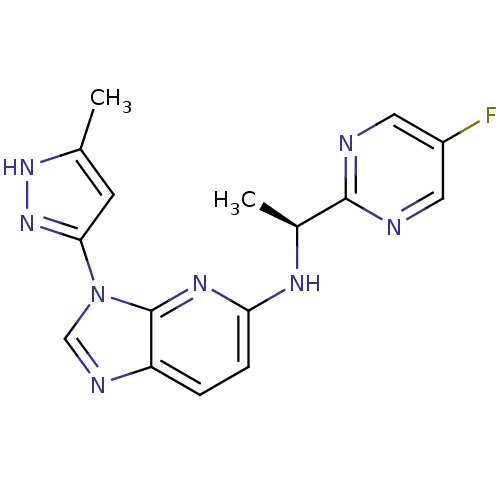
(CHEMBL2151320 | US8486966, 16)Show SMILES C[C@H](Nc1ccc2ncn(-c3cc(C)[nH]n3)c2n1)c1ncc(F)cn1 |r| Show InChI InChI=1S/C16H15FN8/c1-9-5-14(24-23-9)25-8-20-12-3-4-13(22-16(12)25)21-10(2)15-18-6-11(17)7-19-15/h3-8,10H,1-2H3,(H,21,22)(H,23,24)/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
ACS Med Chem Lett 3: 705-709 (2012)
Article DOI: 10.1021/ml300074j
BindingDB Entry DOI: 10.7270/Q2GH9K2G |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50392791

(CHEMBL2151321 | US8486966, 1)Show SMILES CC(C)Oc1cc(n[nH]1)-n1cnc2ccc(N[C@@H](C)c3ccc(F)cn3)nc12 |r| Show InChI InChI=1S/C19H20FN7O/c1-11(2)28-18-8-17(25-26-18)27-10-22-15-6-7-16(24-19(15)27)23-12(3)14-5-4-13(20)9-21-14/h4-12H,1-3H3,(H,23,24)(H,25,26)/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
ACS Med Chem Lett 3: 705-709 (2012)
Article DOI: 10.1021/ml300074j
BindingDB Entry DOI: 10.7270/Q2GH9K2G |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50392793

(CHEMBL2151323 | US8486966, 12)Show SMILES CC(C)Oc1cc(n[nH]1)-n1cnc2cnc(N[C@@H](C)c3ncc(F)cn3)nc12 |r| Show InChI InChI=1S/C17H18FN9O/c1-9(2)28-14-4-13(25-26-14)27-8-22-12-7-21-17(24-16(12)27)23-10(3)15-19-5-11(18)6-20-15/h4-10H,1-3H3,(H,25,26)(H,21,23,24)/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
ACS Med Chem Lett 3: 705-709 (2012)
Article DOI: 10.1021/ml300074j
BindingDB Entry DOI: 10.7270/Q2GH9K2G |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50392795
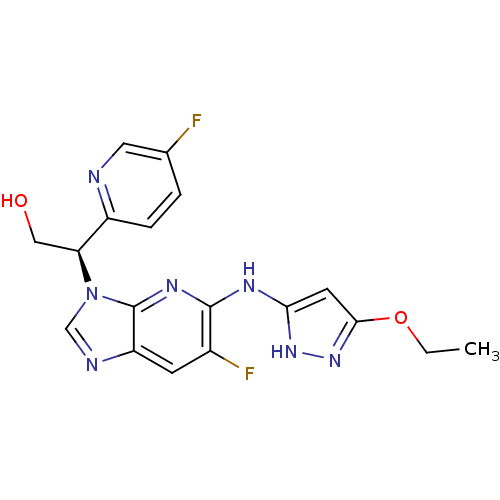
(CHEMBL2151325)Show SMILES CCOc1cc(Nc2nc3n(cnc3cc2F)[C@@H](CO)c2ccc(F)cn2)[nH]n1 |r| Show InChI InChI=1S/C18H17F2N7O2/c1-2-29-16-6-15(25-26-16)23-17-11(20)5-13-18(24-17)27(9-22-13)14(8-28)12-4-3-10(19)7-21-12/h3-7,9,14,28H,2,8H2,1H3,(H2,23,24,25,26)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
ACS Med Chem Lett 3: 705-709 (2012)
Article DOI: 10.1021/ml300074j
BindingDB Entry DOI: 10.7270/Q2GH9K2G |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50392789
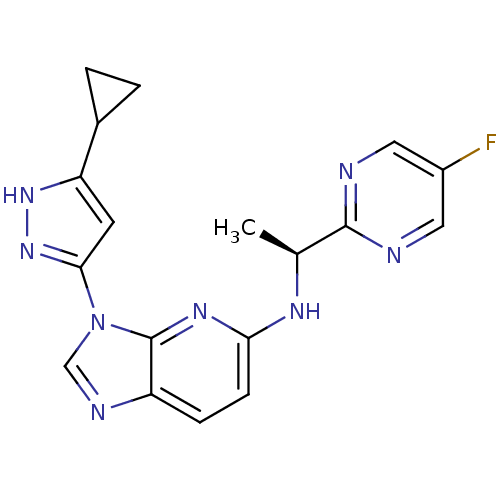
(CHEMBL2151319 | US8486966, 3)Show SMILES C[C@H](Nc1ccc2ncn(-c3cc([nH]n3)C3CC3)c2n1)c1ncc(F)cn1 |r| Show InChI InChI=1S/C18H17FN8/c1-10(17-20-7-12(19)8-21-17)23-15-5-4-13-18(24-15)27(9-22-13)16-6-14(25-26-16)11-2-3-11/h4-11H,2-3H2,1H3,(H,23,24)(H,25,26)/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
ACS Med Chem Lett 3: 705-709 (2012)
Article DOI: 10.1021/ml300074j
BindingDB Entry DOI: 10.7270/Q2GH9K2G |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50392792
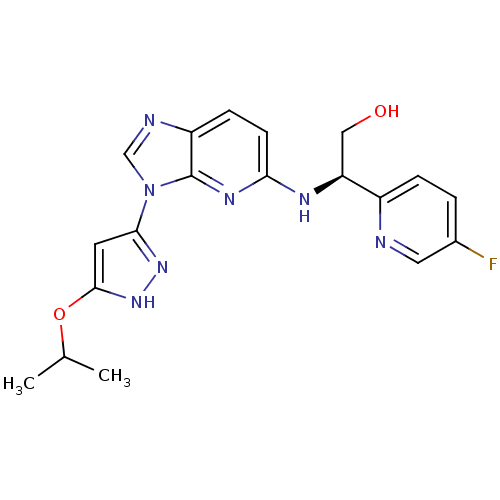
(CHEMBL2151322 | US8486966, 7)Show SMILES CC(C)Oc1cc(n[nH]1)-n1cnc2ccc(N[C@@H](CO)c3ccc(F)cn3)nc12 |r| Show InChI InChI=1S/C19H20FN7O2/c1-11(2)29-18-7-17(25-26-18)27-10-22-14-5-6-16(24-19(14)27)23-15(9-28)13-4-3-12(20)8-21-13/h3-8,10-11,15,28H,9H2,1-2H3,(H,23,24)(H,25,26)/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
ACS Med Chem Lett 3: 705-709 (2012)
Article DOI: 10.1021/ml300074j
BindingDB Entry DOI: 10.7270/Q2GH9K2G |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data