 Found 156 hits with Last Name = 'thuong' and Initial = 'pt'
Found 156 hits with Last Name = 'thuong' and Initial = 'pt' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Neuraminidase
(Influenza A virus) | BDBM50482873
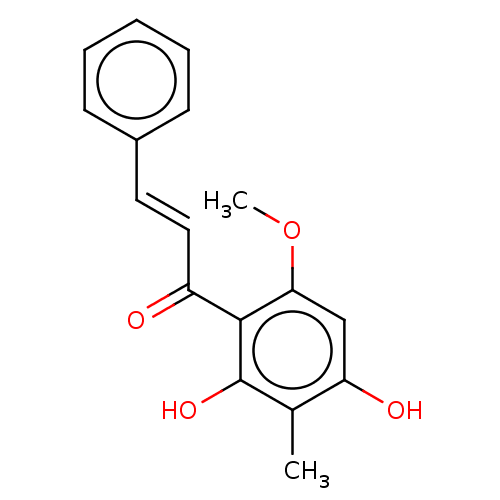
(CHEBI:70659 | CHEMBL1271362)Show InChI InChI=1S/C17H16O4/c1-11-14(19)10-15(21-2)16(17(11)20)13(18)9-8-12-6-4-3-5-7-12/h3-10,19-20H,1-2H3/b9-8+ | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University
Curated by ChEMBL
| Assay Description
Inhibition of Influenza A virus (A/California/08/2009(H1N1)) neuraminidase expressed in human 293T cells by Dixon plot analysis |
J Nat Prod 73: 1636-42 (2010)
Article DOI: 10.1021/np1002753
BindingDB Entry DOI: 10.7270/Q2DZ0C4B |
More data for this
Ligand-Target Pair | |
Neuraminidase
(Influenza A virus) | BDBM50482872
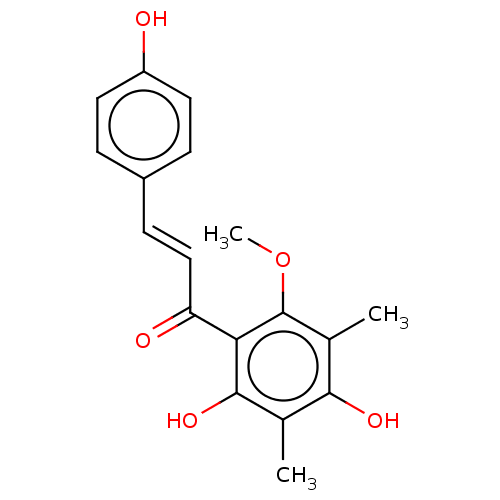
(CHEBI:70655 | CHEMBL1271157)Show SMILES COc1c(C)c(O)c(C)c(O)c1C(=O)\C=C\c1ccc(O)cc1 Show InChI InChI=1S/C18H18O5/c1-10-16(21)11(2)18(23-3)15(17(10)22)14(20)9-6-12-4-7-13(19)8-5-12/h4-9,19,21-22H,1-3H3/b9-6+ | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.49E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University
Curated by ChEMBL
| Assay Description
Inhibition of Influenza A virus (A/California/08/2009(H1N1)) neuraminidase expressed in human 293T cells by Dixon plot analysis |
J Nat Prod 73: 1636-42 (2010)
Article DOI: 10.1021/np1002753
BindingDB Entry DOI: 10.7270/Q2DZ0C4B |
More data for this
Ligand-Target Pair | |
Neuraminidase
(Influenza A virus) | BDBM50482871
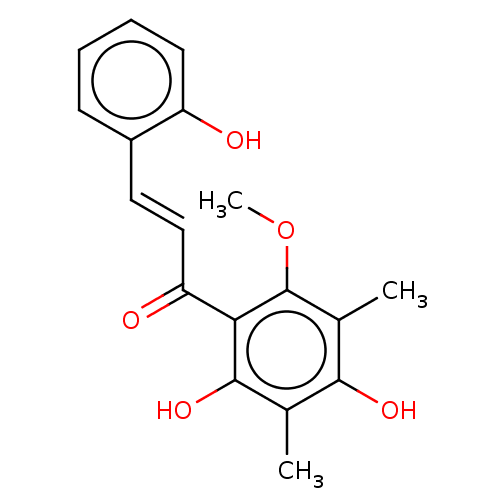
(CHEBI:66265 | CHEMBL509947)Show InChI InChI=1S/C18H18O5/c1-10-16(21)11(2)18(23-3)15(17(10)22)14(20)9-8-12-6-4-5-7-13(12)19/h4-9,19,21-22H,1-3H3/b9-8+ | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.41E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University
Curated by ChEMBL
| Assay Description
Inhibition of Influenza A virus (A/California/08/2009(H1N1)) neuraminidase expressed in human 293T cells by Dixon plot analysis |
J Nat Prod 73: 1636-42 (2010)
Article DOI: 10.1021/np1002753
BindingDB Entry DOI: 10.7270/Q2DZ0C4B |
More data for this
Ligand-Target Pair | |
Neuraminidase
(Influenza A virus) | BDBM50482882
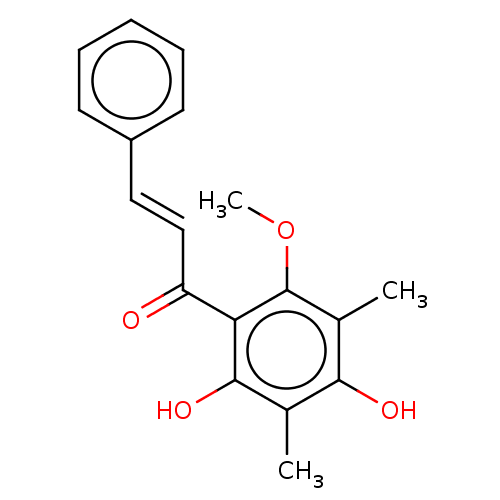
(CHEBI:70658 | CHEMBL463095)Show InChI InChI=1S/C18H18O4/c1-11-16(20)12(2)18(22-3)15(17(11)21)14(19)10-9-13-7-5-4-6-8-13/h4-10,20-21H,1-3H3/b10-9+ | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.14E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University
Curated by ChEMBL
| Assay Description
Inhibition of Influenza A virus (A/California/08/2009(H1N1)) neuraminidase expressed in human 293T cells by Dixon plot analysis |
J Nat Prod 73: 1636-42 (2010)
Article DOI: 10.1021/np1002753
BindingDB Entry DOI: 10.7270/Q2DZ0C4B |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM25737
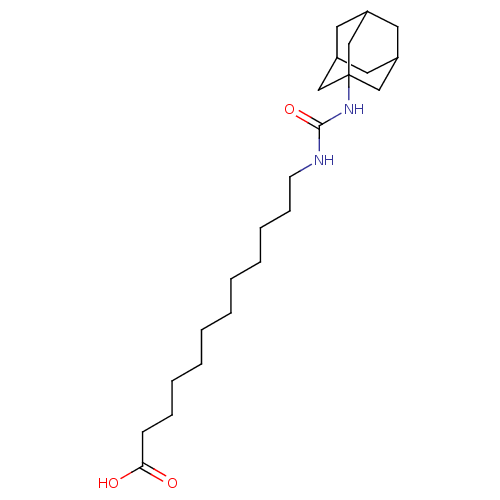
(12-[(adamantan-1-ylcarbamoyl)amino]dodecanoic acid...)Show SMILES OC(=O)CCCCCCCCCCCNC(=O)NC12CC3CC(CC(C3)C1)C2 |TLB:25:20:27:24.23.26,25:24:20.21.19:27,THB:23:22:19:24.25.26,23:24:19:22.21.27| Show InChI InChI=1S/C23H40N2O3/c26-21(27)10-8-6-4-2-1-3-5-7-9-11-24-22(28)25-23-15-18-12-19(16-23)14-20(13-18)17-23/h18-20H,1-17H2,(H,26,27)(H2,24,25,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of sEH (unknown origin) expressed in baculovirus expression system incubated for 1 hr |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.1c00037
BindingDB Entry DOI: 10.7270/Q2DF6W2R |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM25737

(12-[(adamantan-1-ylcarbamoyl)amino]dodecanoic acid...)Show SMILES OC(=O)CCCCCCCCCCCNC(=O)NC12CC3CC(CC(C3)C1)C2 |TLB:25:20:27:24.23.26,25:24:20.21.19:27,THB:23:22:19:24.25.26,23:24:19:22.21.27| Show InChI InChI=1S/C23H40N2O3/c26-21(27)10-8-6-4-2-1-3-5-7-9-11-24-22(28)25-23-15-18-12-19(16-23)14-20(13-18)17-23/h18-20H,1-17H2,(H,26,27)(H2,24,25,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Inhibition of soluble epoxide hydrolase (unknown origin) using NEPC as substrate after 1 hr by fluorescence assay |
J Nat Prod 81: 2429-2435 (2018)
Article DOI: 10.1021/acs.jnatprod.8b00441
BindingDB Entry DOI: 10.7270/Q2HQ42KG |
More data for this
Ligand-Target Pair | |
Neuraminidase
(Influenza A virus) | BDBM5025
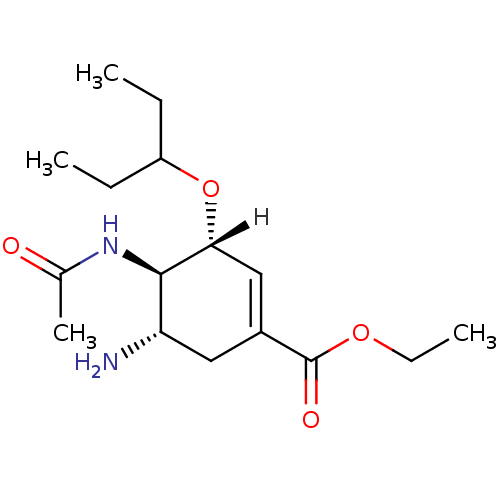
(Oseltamivir | US10919856, POSITIVE CONTROL | ethyl...)Show SMILES [H][C@@]1(OC(CC)CC)C=C(C[C@H](N)[C@H]1NC(C)=O)C(=O)OCC |r,c:8| Show InChI InChI=1S/C16H28N2O4/c1-5-12(6-2)22-14-9-11(16(20)21-7-3)8-13(17)15(14)18-10(4)19/h9,12-15H,5-8,17H2,1-4H3,(H,18,19)/t13-,14+,15+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University
Curated by ChEMBL
| Assay Description
Inhibition of Influenza A virus H9N2 neuraminidase using 4-MU-NANA as substrate by fluorescence assay |
Bioorg Med Chem Lett 22: 3688-92 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.028
BindingDB Entry DOI: 10.7270/Q27947JG |
More data for this
Ligand-Target Pair | |
Neuraminidase
(Influenza A virus) | BDBM5025

(Oseltamivir | US10919856, POSITIVE CONTROL | ethyl...)Show SMILES [H][C@@]1(OC(CC)CC)C=C(C[C@H](N)[C@H]1NC(C)=O)C(=O)OCC |r,c:8| Show InChI InChI=1S/C16H28N2O4/c1-5-12(6-2)22-14-9-11(16(20)21-7-3)8-13(17)15(14)18-10(4)19/h9,12-15H,5-8,17H2,1-4H3,(H,18,19)/t13-,14+,15+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 67.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University
Curated by ChEMBL
| Assay Description
Inhibition of wild type H1N1 swine influenza virus neuraminidase using 4-MU-NANA as substrate by fluorescence assay |
Bioorg Med Chem Lett 22: 3688-92 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.028
BindingDB Entry DOI: 10.7270/Q27947JG |
More data for this
Ligand-Target Pair | |
Neuraminidase
(Influenza A virus) | BDBM5025

(Oseltamivir | US10919856, POSITIVE CONTROL | ethyl...)Show SMILES [H][C@@]1(OC(CC)CC)C=C(C[C@H](N)[C@H]1NC(C)=O)C(=O)OCC |r,c:8| Show InChI InChI=1S/C16H28N2O4/c1-5-12(6-2)22-14-9-11(16(20)21-7-3)8-13(17)15(14)18-10(4)19/h9,12-15H,5-8,17H2,1-4H3,(H,18,19)/t13-,14+,15+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 127 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University
Curated by ChEMBL
| Assay Description
Inhibition of Influenza A virus H1N1 neuraminidase using 4-MU-NANA as substrate by fluorescence assay |
Bioorg Med Chem Lett 22: 3688-92 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.028
BindingDB Entry DOI: 10.7270/Q27947JG |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50468031
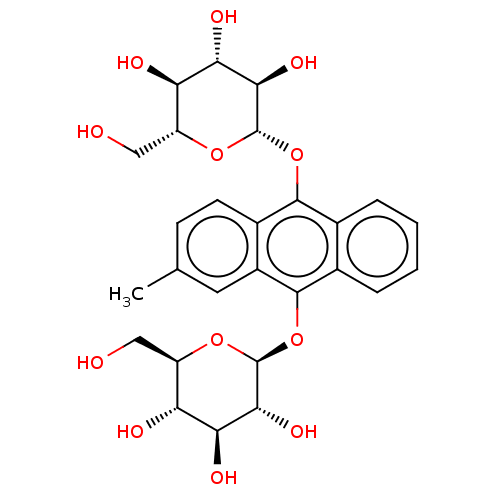
(CHEMBL4282716)Show SMILES Cc1ccc2c(O[C@@H]3O[C@H](CO)[C@@H](O)[C@H](O)[C@H]3O)c3ccccc3c(O[C@@H]3O[C@H](CO)[C@@H](O)[C@H](O)[C@H]3O)c2c1 |r| Show InChI InChI=1S/C27H32O12/c1-11-6-7-14-15(8-11)25(39-27-23(35)21(33)19(31)17(10-29)37-27)13-5-3-2-4-12(13)24(14)38-26-22(34)20(32)18(30)16(9-28)36-26/h2-8,16-23,26-35H,9-10H2,1H3/t16-,17-,18-,19-,20+,21+,22-,23-,26+,27+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Inhibition of soluble epoxide hydrolase (unknown origin) using NEPC as substrate after 1 hr by fluorescence assay |
J Nat Prod 81: 2429-2435 (2018)
Article DOI: 10.1021/acs.jnatprod.8b00441
BindingDB Entry DOI: 10.7270/Q2HQ42KG |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50577986
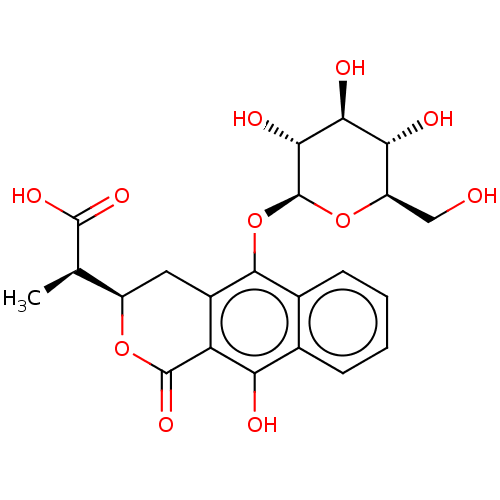
(CHEMBL4873654)Show SMILES [H][C@@]1(Cc2c(O[C@@H]3O[C@H](CO)[C@@H](O)[C@H](O)[C@H]3O)c3ccccc3c(O)c2C(=O)O1)[C@@H](C)C(O)=O |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of sEH (unknown origin) expressed in baculovirus expression system incubated for 1 hr |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.1c00037
BindingDB Entry DOI: 10.7270/Q2DF6W2R |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50468030
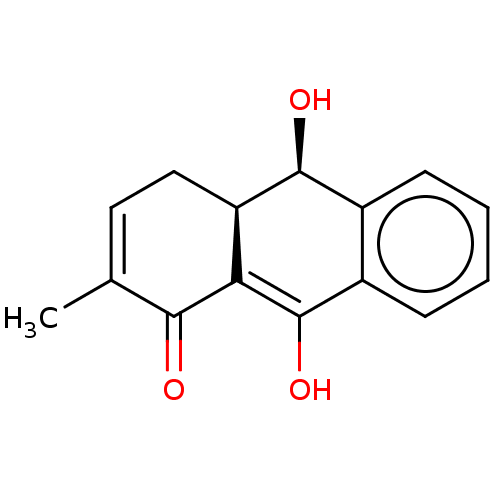
(CHEMBL4282083)Show SMILES [H][C@@]12CC=C(C)C(=O)C1=C(O)c1ccccc1[C@@H]2O |r,t:3,9| Show InChI InChI=1S/C15H14O3/c1-8-6-7-11-12(13(8)16)15(18)10-5-3-2-4-9(10)14(11)17/h2-6,11,14,17-18H,7H2,1H3/t11-,14+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Inhibition of soluble epoxide hydrolase (unknown origin) using NEPC as substrate after 1 hr by fluorescence assay |
J Nat Prod 81: 2429-2435 (2018)
Article DOI: 10.1021/acs.jnatprod.8b00441
BindingDB Entry DOI: 10.7270/Q2HQ42KG |
More data for this
Ligand-Target Pair | |
Neuraminidase
(Influenza A virus) | BDBM50482871

(CHEBI:66265 | CHEMBL509947)Show InChI InChI=1S/C18H18O5/c1-10-16(21)11(2)18(23-3)15(17(10)22)14(20)9-8-12-6-4-5-7-13(12)19/h4-9,19,21-22H,1-3H3/b9-8+ | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University
Curated by ChEMBL
| Assay Description
Inhibition of oseltamivir-resistant Influenza A virus (A/California/08/2009(H1N1)) neuraminidase H274Y mutant expressed in human 293T cells after 30 ... |
J Nat Prod 73: 1636-42 (2010)
Article DOI: 10.1021/np1002753
BindingDB Entry DOI: 10.7270/Q2DZ0C4B |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50148911
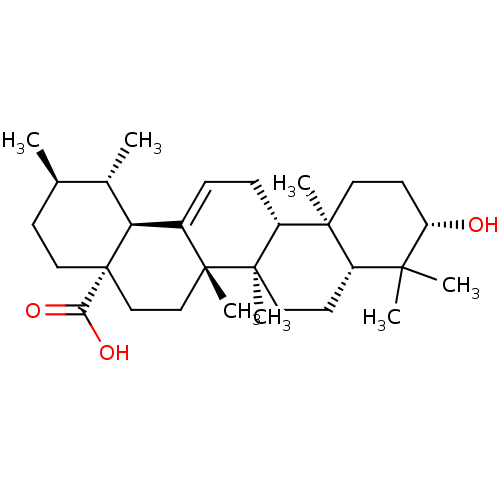
((3beta)-3-hydroxyurs-12-en-28-oic acid | 3beta-hyd...)Show SMILES C[C@@H]1CC[C@@]2(CC[C@]3(C)C(=CC[C@@H]4[C@@]5(C)CC[C@H](O)C(C)(C)[C@@H]5CC[C@@]34C)[C@@H]2[C@H]1C)C(O)=O |r,c:9| Show InChI InChI=1S/C30H48O3/c1-18-10-15-30(25(32)33)17-16-28(6)20(24(30)19(18)2)8-9-22-27(5)13-12-23(31)26(3,4)21(27)11-14-29(22,28)7/h8,18-19,21-24,31H,9-17H2,1-7H3,(H,32,33)/t18-,19+,21+,22-,23+,24+,27+,28-,29-,30+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B after 30 mins |
J Nat Prod 71: 1775-8 (2008)
Article DOI: 10.1021/np800298w
BindingDB Entry DOI: 10.7270/Q2TQ62F7 |
More data for this
Ligand-Target Pair | |
Neuraminidase
(Influenza A virus) | BDBM50482872

(CHEBI:70655 | CHEMBL1271157)Show SMILES COc1c(C)c(O)c(C)c(O)c1C(=O)\C=C\c1ccc(O)cc1 Show InChI InChI=1S/C18H18O5/c1-10-16(21)11(2)18(23-3)15(17(10)22)14(20)9-6-12-4-7-13(19)8-5-12/h4-9,19,21-22H,1-3H3/b9-6+ | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University
Curated by ChEMBL
| Assay Description
Inhibition of oseltamivir-resistant Influenza A virus (A/California/08/2009(H1N1)) neuraminidase H274Y mutant expressed in human 293T cells after 30 ... |
J Nat Prod 73: 1636-42 (2010)
Article DOI: 10.1021/np1002753
BindingDB Entry DOI: 10.7270/Q2DZ0C4B |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50346613
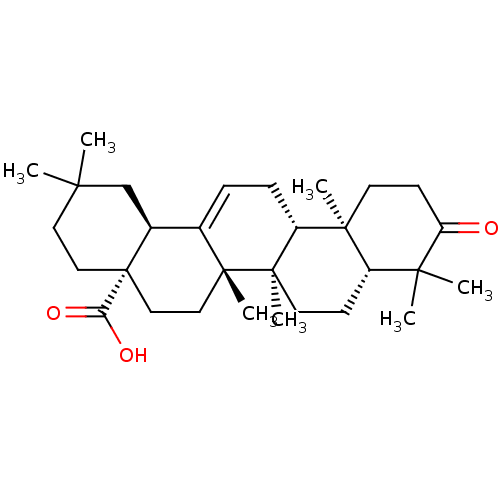
(CHEMBL470029 | Oleanonic Acid)Show SMILES CC1(C)CC[C@@]2(CC[C@]3(C)C(=CC[C@@H]4[C@@]5(C)CCC(=O)C(C)(C)[C@@H]5CC[C@@]34C)[C@@H]2C1)C(O)=O |r,c:10| Show InChI InChI=1S/C30H46O3/c1-25(2)14-16-30(24(32)33)17-15-28(6)19(20(30)18-25)8-9-22-27(5)12-11-23(31)26(3,4)21(27)10-13-29(22,28)7/h8,20-22H,9-18H2,1-7H3,(H,32,33)/t20-,21-,22+,27-,28+,29+,30-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B after 30 mins |
J Nat Prod 71: 1775-8 (2008)
Article DOI: 10.1021/np800298w
BindingDB Entry DOI: 10.7270/Q2TQ62F7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50148911

((3beta)-3-hydroxyurs-12-en-28-oic acid | 3beta-hyd...)Show SMILES C[C@@H]1CC[C@@]2(CC[C@]3(C)C(=CC[C@@H]4[C@@]5(C)CC[C@H](O)C(C)(C)[C@@H]5CC[C@@]34C)[C@@H]2[C@H]1C)C(O)=O |r,c:9| Show InChI InChI=1S/C30H48O3/c1-18-10-15-30(25(32)33)17-16-28(6)20(24(30)19(18)2)8-9-22-27(5)13-12-23(31)26(3,4)21(27)11-14-29(22,28)7/h8,18-19,21-24,31H,9-17H2,1-7H3,(H,32,33)/t18-,19+,21+,22-,23+,24+,27+,28-,29-,30+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B assessed as p-nitrophenol production |
Bioorg Med Chem Lett 19: 6745-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.108
BindingDB Entry DOI: 10.7270/Q24B328V |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50148911

((3beta)-3-hydroxyurs-12-en-28-oic acid | 3beta-hyd...)Show SMILES C[C@@H]1CC[C@@]2(CC[C@]3(C)C(=CC[C@@H]4[C@@]5(C)CC[C@H](O)C(C)(C)[C@@H]5CC[C@@]34C)[C@@H]2[C@H]1C)C(O)=O |r,c:9| Show InChI InChI=1S/C30H48O3/c1-18-10-15-30(25(32)33)17-16-28(6)20(24(30)19(18)2)8-9-22-27(5)13-12-23(31)26(3,4)21(27)11-14-29(22,28)7/h8,18-19,21-24,31H,9-17H2,1-7H3,(H,32,33)/t18-,19+,21+,22-,23+,24+,27+,28-,29-,30+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B |
J Nat Prod 70: 1039-42 (2007)
Article DOI: 10.1021/np060477+
BindingDB Entry DOI: 10.7270/Q2KH0P5K |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50148911

((3beta)-3-hydroxyurs-12-en-28-oic acid | 3beta-hyd...)Show SMILES C[C@@H]1CC[C@@]2(CC[C@]3(C)C(=CC[C@@H]4[C@@]5(C)CC[C@H](O)C(C)(C)[C@@H]5CC[C@@]34C)[C@@H]2[C@H]1C)C(O)=O |r,c:9| Show InChI InChI=1S/C30H48O3/c1-18-10-15-30(25(32)33)17-16-28(6)20(24(30)19(18)2)8-9-22-27(5)13-12-23(31)26(3,4)21(27)11-14-29(22,28)7/h8,18-19,21-24,31H,9-17H2,1-7H3,(H,32,33)/t18-,19+,21+,22-,23+,24+,27+,28-,29-,30+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology (KRIBB)
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B |
Bioorg Med Chem 16: 10356-62 (2008)
Article DOI: 10.1016/j.bmc.2008.10.012
BindingDB Entry DOI: 10.7270/Q251404T |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50241553
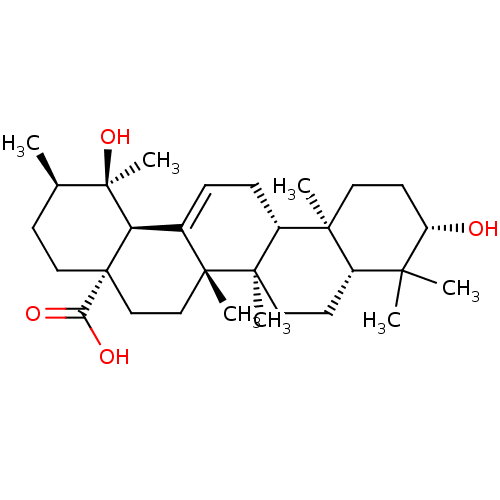
(CHEMBL486986 | pomolic acid)Show SMILES C[C@@H]1CC[C@@]2(CC[C@]3(C)C(=CC[C@@H]4[C@@]5(C)CC[C@H](O)C(C)(C)[C@@H]5CC[C@@]34C)[C@@H]2[C@]1(C)O)C(O)=O |r,c:9| Show InChI InChI=1S/C30H48O4/c1-18-10-15-30(24(32)33)17-16-27(5)19(23(30)29(18,7)34)8-9-21-26(4)13-12-22(31)25(2,3)20(26)11-14-28(21,27)6/h8,18,20-23,31,34H,9-17H2,1-7H3,(H,32,33)/t18-,20+,21-,22+,23-,26+,27-,28-,29-,30+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B after 30 mins |
J Nat Prod 71: 1775-8 (2008)
Article DOI: 10.1021/np800298w
BindingDB Entry DOI: 10.7270/Q2TQ62F7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50104694
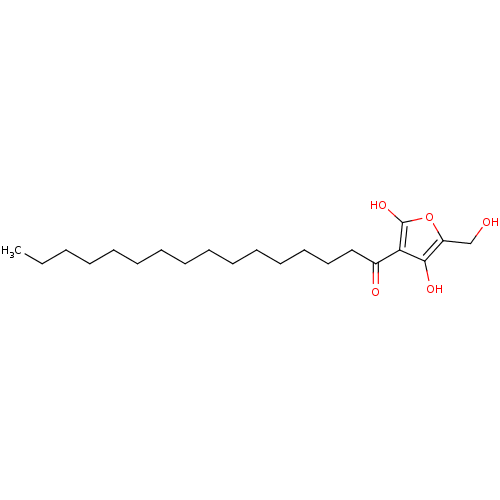
((R)-4-hydroxy-5-(hydroxymethyl)-3-palmitoylfuran-2...)Show InChI InChI=1S/C21H36O5/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-17(23)19-20(24)18(16-22)26-21(19)25/h22,24-25H,2-16H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B after 30 mins |
J Nat Prod 71: 1775-8 (2008)
Article DOI: 10.1021/np800298w
BindingDB Entry DOI: 10.7270/Q2TQ62F7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50311577
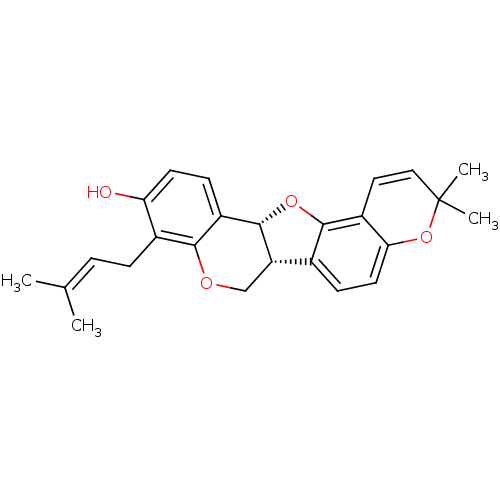
(CHEMBL561967 | Erybreadin B | erybraedin B)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-c1c(-[#8])ccc2-[#6@@H]-3-[#8]-c4c(ccc5-[#8]C([#6])([#6])[#6]=[#6]-c45)-[#6@@H]-3-[#6]-[#8]-c12 |r,c:22| Show InChI InChI=1S/C25H26O4/c1-14(2)5-6-16-20(26)9-7-18-22(16)27-13-19-15-8-10-21-17(23(15)28-24(18)19)11-12-25(3,4)29-21/h5,7-12,19,24,26H,6,13H2,1-4H3/t19-,24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B assessed as p-nitrophenol production |
Bioorg Med Chem Lett 19: 6745-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.108
BindingDB Entry DOI: 10.7270/Q24B328V |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50104694

((R)-4-hydroxy-5-(hydroxymethyl)-3-palmitoylfuran-2...)Show InChI InChI=1S/C21H36O5/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-17(23)19-20(24)18(16-22)26-21(19)25/h22,24-25H,2-16H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B assessed as p-nitrophenol production |
Bioorg Med Chem Lett 19: 6745-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.108
BindingDB Entry DOI: 10.7270/Q24B328V |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50104694

((R)-4-hydroxy-5-(hydroxymethyl)-3-palmitoylfuran-2...)Show InChI InChI=1S/C21H36O5/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-17(23)19-20(24)18(16-22)26-21(19)25/h22,24-25H,2-16H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B |
J Nat Prod 70: 1039-42 (2007)
Article DOI: 10.1021/np060477+
BindingDB Entry DOI: 10.7270/Q2KH0P5K |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50104694

((R)-4-hydroxy-5-(hydroxymethyl)-3-palmitoylfuran-2...)Show InChI InChI=1S/C21H36O5/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-17(23)19-20(24)18(16-22)26-21(19)25/h22,24-25H,2-16H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology (KRIBB)
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B |
Bioorg Med Chem 16: 10356-62 (2008)
Article DOI: 10.1016/j.bmc.2008.10.012
BindingDB Entry DOI: 10.7270/Q251404T |
More data for this
Ligand-Target Pair | |
Neuraminidase
(Influenza A virus) | BDBM50482882

(CHEBI:70658 | CHEMBL463095)Show InChI InChI=1S/C18H18O4/c1-11-16(20)12(2)18(22-3)15(17(11)21)14(19)10-9-13-7-5-4-6-8-13/h4-10,20-21H,1-3H3/b10-9+ | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University
Curated by ChEMBL
| Assay Description
Inhibition of oseltamivir-resistant Influenza A virus (A/California/08/2009(H1N1)) neuraminidase H274Y mutant expressed in human 293T cells after 30 ... |
J Nat Prod 73: 1636-42 (2010)
Article DOI: 10.1021/np1002753
BindingDB Entry DOI: 10.7270/Q2DZ0C4B |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50311580
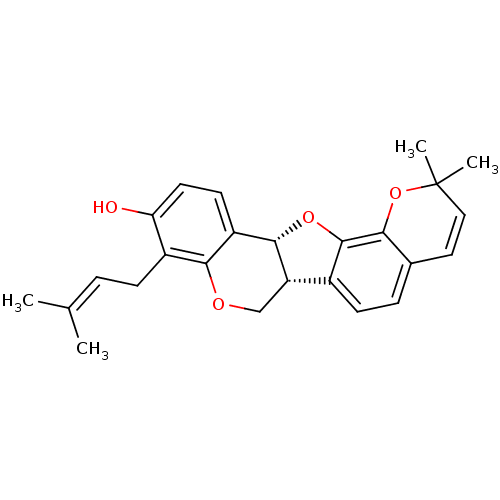
(CHEMBL1087148 | Erybreadin D)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-c1c(-[#8])ccc2-[#6@@H]-3-[#8]-c4c(ccc5-[#6]=[#6]C([#6])([#6])[#8]-c45)-[#6@@H]-3-[#6]-[#8]-c12 |r,c:18| Show InChI InChI=1S/C25H26O4/c1-14(2)5-7-17-20(26)10-9-18-22(17)27-13-19-16-8-6-15-11-12-25(3,4)29-21(15)24(16)28-23(18)19/h5-6,8-12,19,23,26H,7,13H2,1-4H3/t19-,23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B assessed as p-nitrophenol production |
Bioorg Med Chem Lett 19: 6745-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.108
BindingDB Entry DOI: 10.7270/Q24B328V |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50311582
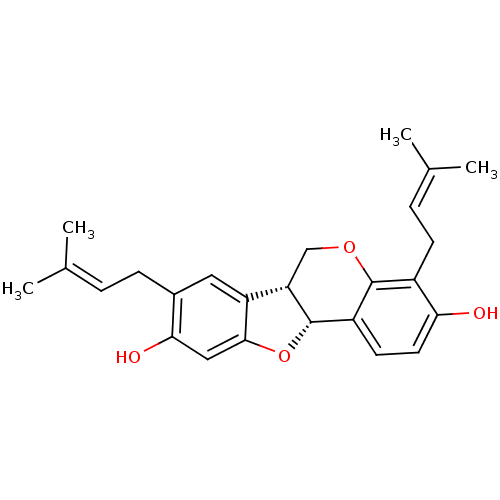
(CHEMBL1086765 | Erybreadin C)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-c1cc2-[#6@@H]-3-[#6]-[#8]-c4c(-[#6]\[#6]=[#6](\[#6])-[#6])c(-[#8])ccc4-[#6@@H]-3-[#8]-c2cc1-[#8] |r| Show InChI InChI=1S/C25H28O4/c1-14(2)5-7-16-11-19-20-13-28-24-17(8-6-15(3)4)21(26)10-9-18(24)25(20)29-23(19)12-22(16)27/h5-6,9-12,20,25-27H,7-8,13H2,1-4H3/t20-,25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B assessed as p-nitrophenol production |
Bioorg Med Chem Lett 19: 6745-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.108
BindingDB Entry DOI: 10.7270/Q24B328V |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50346601
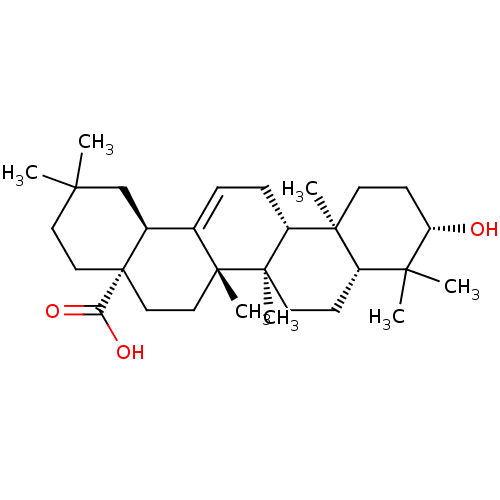
(NSC-114945 | OLEANOLIC_ACID | Oleanolic acid | Ole...)Show SMILES CC1(C)CC[C@@]2(CC[C@]3(C)C(=CC[C@@H]4[C@@]5(C)CC[C@H](O)C(C)(C)[C@@H]5CC[C@@]34C)[C@@H]2C1)C(O)=O |r,c:10| Show InChI InChI=1S/C30H48O3/c1-25(2)14-16-30(24(32)33)17-15-28(6)19(20(30)18-25)8-9-22-27(5)12-11-23(31)26(3,4)21(27)10-13-29(22,28)7/h8,20-23,31H,9-18H2,1-7H3,(H,32,33)/t20-,21-,22+,23-,27-,28+,29+,30-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B after 30 mins |
J Nat Prod 71: 1775-8 (2008)
Article DOI: 10.1021/np800298w
BindingDB Entry DOI: 10.7270/Q2TQ62F7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50317432
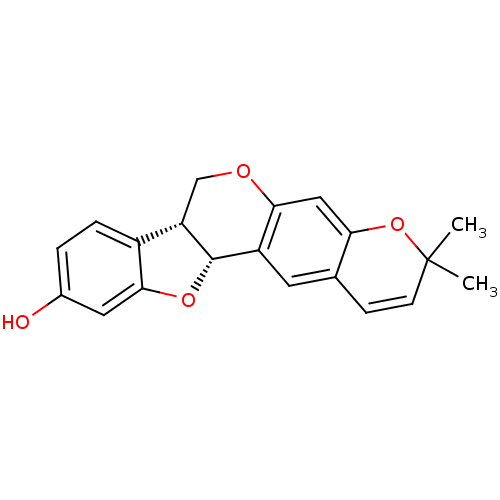
(CHEMBL1098728 | NEORAUTENOL)Show SMILES CC1(C)Oc2cc3OC[C@@H]4[C@@H](Oc5cc(O)ccc45)c3cc2C=C1 |r,c:26| Show InChI InChI=1S/C20H18O4/c1-20(2)6-5-11-7-14-17(9-16(11)24-20)22-10-15-13-4-3-12(21)8-18(13)23-19(14)15/h3-9,15,19,21H,10H2,1-2H3/t15-,19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B assessed as p-nitrophenol production |
Bioorg Med Chem Lett 19: 6745-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.108
BindingDB Entry DOI: 10.7270/Q24B328V |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50311579
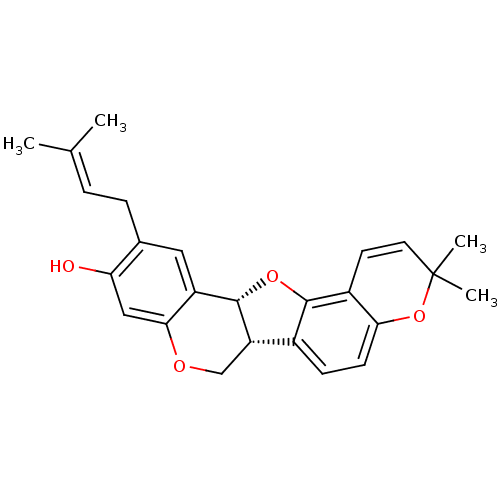
(CHEMBL551155 | folitenol)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-c1cc2-[#6@@H]-3-[#8]-c4c(ccc5-[#8]C([#6])([#6])[#6]=[#6]-c45)-[#6@@H]-3-[#6]-[#8]-c2cc1-[#8] |r,c:19| Show InChI InChI=1S/C25H26O4/c1-14(2)5-6-15-11-18-22(12-20(15)26)27-13-19-16-7-8-21-17(23(16)28-24(18)19)9-10-25(3,4)29-21/h5,7-12,19,24,26H,6,13H2,1-4H3/t19-,24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B assessed as p-nitrophenol production |
Bioorg Med Chem Lett 19: 6745-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.108
BindingDB Entry DOI: 10.7270/Q24B328V |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50253117
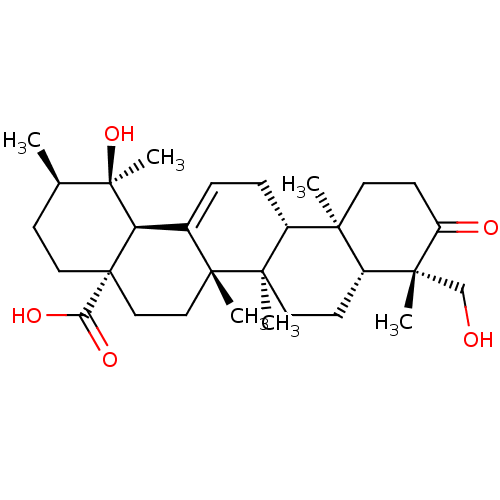
(19alpha,24-dihydroxyurs-12-en-3-on-28-oic acid | C...)Show SMILES C[C@@H]1CC[C@@]2(CC[C@]3(C)C(=CC[C@@H]4[C@@]5(C)CCC(=O)[C@](C)(CO)[C@@H]5CC[C@@]34C)[C@@H]2[C@]1(C)O)C(O)=O |r,c:9| Show InChI InChI=1S/C30H46O5/c1-18-9-14-30(24(33)34)16-15-27(4)19(23(30)29(18,6)35)7-8-21-25(2)12-11-22(32)26(3,17-31)20(25)10-13-28(21,27)5/h7,18,20-21,23,31,35H,8-17H2,1-6H3,(H,33,34)/t18-,20-,21-,23-,25+,26-,27-,28-,29-,30+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B after 30 mins |
J Nat Prod 71: 1775-8 (2008)
Article DOI: 10.1021/np800298w
BindingDB Entry DOI: 10.7270/Q2TQ62F7 |
More data for this
Ligand-Target Pair | |
Neuraminidase
(Influenza A virus) | BDBM50482872

(CHEBI:70655 | CHEMBL1271157)Show SMILES COc1c(C)c(O)c(C)c(O)c1C(=O)\C=C\c1ccc(O)cc1 Show InChI InChI=1S/C18H18O5/c1-10-16(21)11(2)18(23-3)15(17(10)22)14(20)9-6-12-4-7-13(19)8-5-12/h4-9,19,21-22H,1-3H3/b9-6+ | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University
Curated by ChEMBL
| Assay Description
Inhibition of Influenza A virus (A/California/08/2009(H1N1)) wild type neuraminidase expressed in human 293T cells after 30 mins by spectrofluorimetr... |
J Nat Prod 73: 1636-42 (2010)
Article DOI: 10.1021/np1002753
BindingDB Entry DOI: 10.7270/Q2DZ0C4B |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50311581
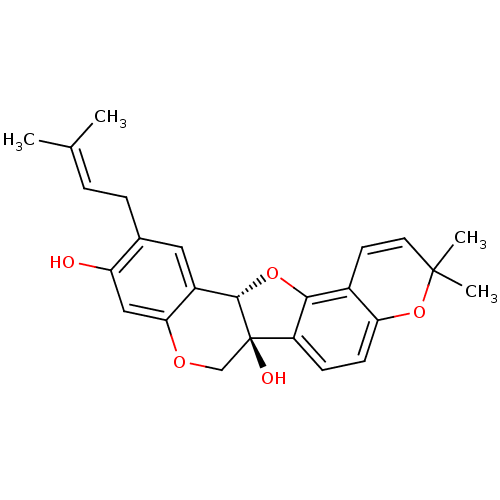
(CHEMBL1086764 | erysubin E)Show SMILES [#6]\[#6](-[#6])=[#6]/[#6]-c1cc2-[#6@@H]3-[#8]-c4c(ccc5-[#8]C([#6])([#6])[#6]=[#6]-c45)[C@]3([#8])[#6]-[#8]-c2cc1-[#8] |r,c:19| Show InChI InChI=1S/C25H26O5/c1-14(2)5-6-15-11-17-21(12-19(15)26)28-13-25(27)18-7-8-20-16(22(18)29-23(17)25)9-10-24(3,4)30-20/h5,7-12,23,26-27H,6,13H2,1-4H3/t23-,25+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B assessed as p-nitrophenol production |
Bioorg Med Chem Lett 19: 6745-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.108
BindingDB Entry DOI: 10.7270/Q24B328V |
More data for this
Ligand-Target Pair | |
Neuraminidase
(Influenza A virus) | BDBM50482871

(CHEBI:66265 | CHEMBL509947)Show InChI InChI=1S/C18H18O5/c1-10-16(21)11(2)18(23-3)15(17(10)22)14(20)9-8-12-6-4-5-7-13(12)19/h4-9,19,21-22H,1-3H3/b9-8+ | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University
Curated by ChEMBL
| Assay Description
Inhibition of Influenza A virus (A/California/08/2009(H1N1)) wild type neuraminidase expressed in human 293T cells after 30 mins by spectrofluorimetr... |
J Nat Prod 73: 1636-42 (2010)
Article DOI: 10.1021/np1002753
BindingDB Entry DOI: 10.7270/Q2DZ0C4B |
More data for this
Ligand-Target Pair | |
Neuraminidase
(Influenza A virus) | BDBM5025

(Oseltamivir | US10919856, POSITIVE CONTROL | ethyl...)Show SMILES [H][C@@]1(OC(CC)CC)C=C(C[C@H](N)[C@H]1NC(C)=O)C(=O)OCC |r,c:8| Show InChI InChI=1S/C16H28N2O4/c1-5-12(6-2)22-14-9-11(16(20)21-7-3)8-13(17)15(14)18-10(4)19/h9,12-15H,5-8,17H2,1-4H3,(H,18,19)/t13-,14+,15+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.03E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University
Curated by ChEMBL
| Assay Description
Inhibition of Influenza A virus (A/chicken/Korea/01310/2001 (H9N2)) neuraminidase after 30 mins by spectrofluorimetric analysis |
J Nat Prod 73: 1636-42 (2010)
Article DOI: 10.1021/np1002753
BindingDB Entry DOI: 10.7270/Q2DZ0C4B |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50253053
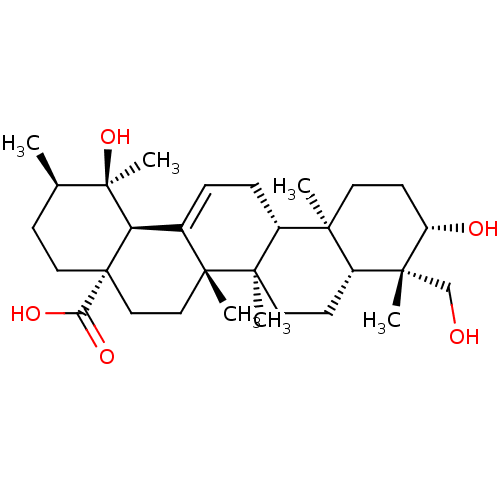
(CHEMBL493908 | rotungenic acid)Show SMILES C[C@@H]1CC[C@@]2(CC[C@]3(C)C(=CC[C@@H]4[C@@]5(C)CC[C@H](O)[C@](C)(CO)[C@@H]5CC[C@@]34C)[C@@H]2[C@]1(C)O)C(O)=O |r,c:9| Show InChI InChI=1S/C30H48O5/c1-18-9-14-30(24(33)34)16-15-27(4)19(23(30)29(18,6)35)7-8-21-25(2)12-11-22(32)26(3,17-31)20(25)10-13-28(21,27)5/h7,18,20-23,31-32,35H,8-17H2,1-6H3,(H,33,34)/t18-,20-,21-,22+,23-,25+,26-,27-,28-,29-,30+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.09E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B after 30 mins |
J Nat Prod 71: 1775-8 (2008)
Article DOI: 10.1021/np800298w
BindingDB Entry DOI: 10.7270/Q2TQ62F7 |
More data for this
Ligand-Target Pair | |
Neuraminidase
(Influenza A virus) | BDBM5025

(Oseltamivir | US10919856, POSITIVE CONTROL | ethyl...)Show SMILES [H][C@@]1(OC(CC)CC)C=C(C[C@H](N)[C@H]1NC(C)=O)C(=O)OCC |r,c:8| Show InChI InChI=1S/C16H28N2O4/c1-5-12(6-2)22-14-9-11(16(20)21-7-3)8-13(17)15(14)18-10(4)19/h9,12-15H,5-8,17H2,1-4H3,(H,18,19)/t13-,14+,15+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.23E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University
Curated by ChEMBL
| Assay Description
Inhibition of oseltamivir-resistant Influenza A virus (A/California/08/2009(H1N1)) neuraminidase H274Y mutant expressed in human 293T cells after 30 ... |
J Nat Prod 73: 1636-42 (2010)
Article DOI: 10.1021/np1002753
BindingDB Entry DOI: 10.7270/Q2DZ0C4B |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50253075
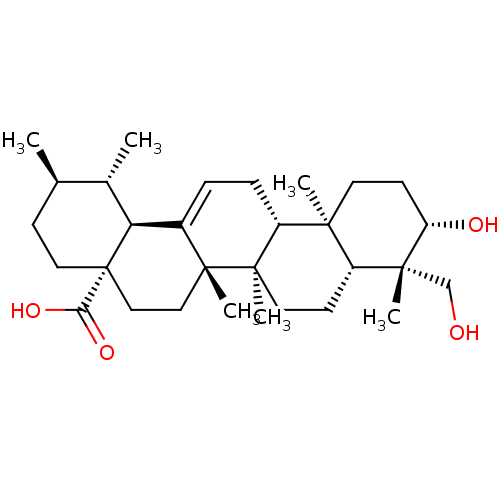
(24-hydroxyursolic acid | CHEMBL522373)Show SMILES C[C@@H]1CC[C@@]2(CC[C@]3(C)C(=CC[C@@H]4[C@@]5(C)CC[C@H](O)[C@](C)(CO)[C@@H]5CC[C@@]34C)[C@@H]2[C@H]1C)C(O)=O |r,c:9| Show InChI InChI=1S/C30H48O4/c1-18-9-14-30(25(33)34)16-15-28(5)20(24(30)19(18)2)7-8-22-26(3)12-11-23(32)27(4,17-31)21(26)10-13-29(22,28)6/h7,18-19,21-24,31-32H,8-17H2,1-6H3,(H,33,34)/t18-,19+,21-,22-,23+,24+,26+,27-,28-,29-,30+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.28E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B after 30 mins |
J Nat Prod 71: 1775-8 (2008)
Article DOI: 10.1021/np800298w
BindingDB Entry DOI: 10.7270/Q2TQ62F7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50213492
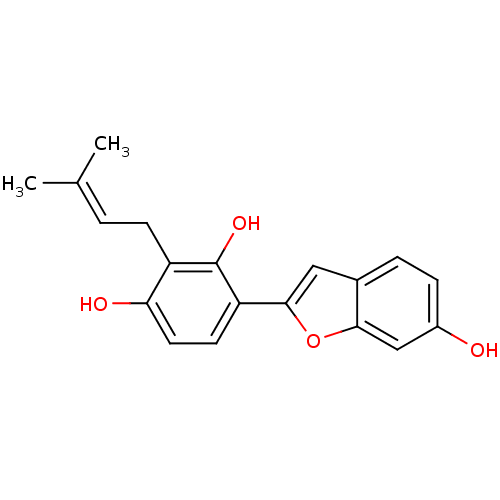
(2-[2',4'-dihydroxy-3'-(3-methylbut-2-enyl)phenyl]-...)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-c1c(-[#8])ccc(-c2cc3ccc(-[#8])cc3o2)c1-[#8] Show InChI InChI=1S/C19H18O4/c1-11(2)3-6-14-16(21)8-7-15(19(14)22)18-9-12-4-5-13(20)10-17(12)23-18/h3-5,7-10,20-22H,6H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.36E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B |
Bioorg Med Chem Lett 17: 3868-71 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.005
BindingDB Entry DOI: 10.7270/Q29W0F6F |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50274831
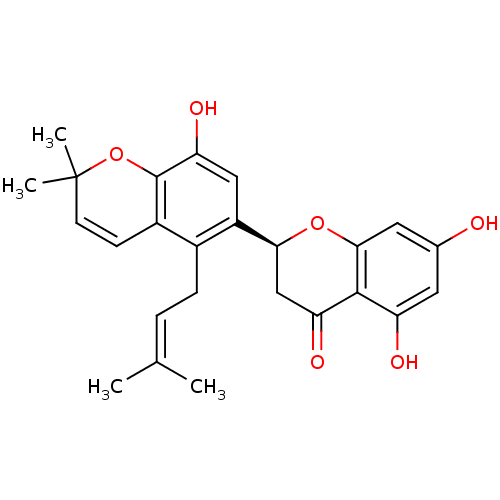
((S)-5,7,8'-trihydroxy-2',2'-dimethyl-5'-(3-methylb...)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-c1c(cc(-[#8])c2-[#8]C([#6])([#6])[#6]=[#6]-c12)-[#6@@H]-1-[#6]-[#6](=O)-c2c(-[#8])cc(-[#8])cc2-[#8]-1 |r,c:15| Show InChI InChI=1S/C25H26O6/c1-13(2)5-6-15-16-7-8-25(3,4)31-24(16)20(29)11-17(15)21-12-19(28)23-18(27)9-14(26)10-22(23)30-21/h5,7-11,21,26-27,29H,6,12H2,1-4H3/t21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.39E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology (KRIBB)
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B |
Bioorg Med Chem 16: 10356-62 (2008)
Article DOI: 10.1016/j.bmc.2008.10.012
BindingDB Entry DOI: 10.7270/Q251404T |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50212398

(CHEMBL229222 | sigmoidin F)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-c1c(-[#8])c2-[#8]C([#6])([#6])[#6]=[#6]-c2cc1-[#6@H]-1-[#6]-[#6](=O)-c2c(-[#8])cc(-[#8])cc2-[#8]-1 |c:13| Show InChI InChI=1S/C25H26O6/c1-13(2)5-6-16-17(9-14-7-8-25(3,4)31-24(14)23(16)29)20-12-19(28)22-18(27)10-15(26)11-21(22)30-20/h5,7-11,20,26-27,29H,6,12H2,1-4H3/t20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.42E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B |
J Nat Prod 70: 1039-42 (2007)
Article DOI: 10.1021/np060477+
BindingDB Entry DOI: 10.7270/Q2KH0P5K |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50212392

(CHEMBL229506 | sigmoidin A)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-c1cc(-[#6@@H]-2-[#6]-[#6](=O)-c3c(-[#8])cc(-[#8])cc3-[#8]-2)c(-[#6]\[#6]=[#6](\[#6])-[#6])c(-[#8])c1-[#8] Show InChI InChI=1S/C25H28O6/c1-13(2)5-7-15-9-18(17(8-6-14(3)4)25(30)24(15)29)21-12-20(28)23-19(27)10-16(26)11-22(23)31-21/h5-6,9-11,21,26-27,29-30H,7-8,12H2,1-4H3/t21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.44E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B |
J Nat Prod 70: 1039-42 (2007)
Article DOI: 10.1021/np060477+
BindingDB Entry DOI: 10.7270/Q2KH0P5K |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50274874
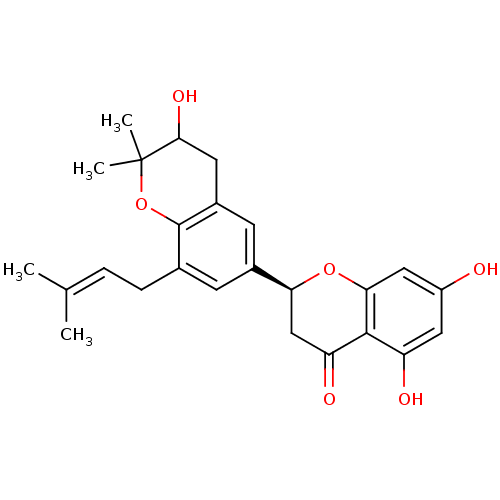
((2S)-3',5,7-trihydroxy-2',2'-dimethyl-8'-(3-methyl...)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-c1cc(cc2-[#6]-[#6](-[#8])C([#6])([#6])[#8]-c12)-[#6@@H]-1-[#6]-[#6](=O)-c2c(-[#8])cc(-[#8])cc2-[#8]-1 |r| Show InChI InChI=1S/C25H28O6/c1-13(2)5-6-14-7-15(8-16-9-22(29)25(3,4)31-24(14)16)20-12-19(28)23-18(27)10-17(26)11-21(23)30-20/h5,7-8,10-11,20,22,26-27,29H,6,9,12H2,1-4H3/t20-,22?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.49E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology (KRIBB)
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B |
Bioorg Med Chem 16: 10356-62 (2008)
Article DOI: 10.1016/j.bmc.2008.10.012
BindingDB Entry DOI: 10.7270/Q251404T |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50218196
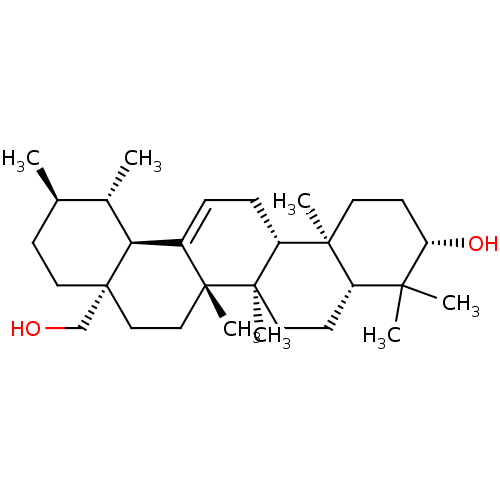
(3-beta,28-Dihydroxy-urs-12-ene | CHEMBL399873 | US...)Show SMILES C[C@@H]1CC[C@]2(CO)CC[C@]3(C)C(=CC[C@@H]4[C@@]5(C)CC[C@H](O)C(C)(C)[C@@H]5CC[C@@]34C)[C@@H]2[C@H]1C |r,c:11| Show InChI InChI=1S/C30H50O2/c1-19-10-15-30(18-31)17-16-28(6)21(25(30)20(19)2)8-9-23-27(5)13-12-24(32)26(3,4)22(27)11-14-29(23,28)7/h8,19-20,22-25,31-32H,9-18H2,1-7H3/t19-,20+,22+,23-,24+,25+,27+,28-,29-,30-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.56E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B after 30 mins |
J Nat Prod 71: 1775-8 (2008)
Article DOI: 10.1021/np800298w
BindingDB Entry DOI: 10.7270/Q2TQ62F7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50212393

(CHEMBL229220 | abyssinoflavanone VII)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-c1cc(cc(-[#6]-[#6](-[#8])-[#6](-[#6])=[#6])c1-[#8])-[#6@@H]-1-[#6]-[#6](=O)-c2c(-[#8])cc(-[#8])cc2-[#8]-1 |r| Show InChI InChI=1S/C25H28O6/c1-13(2)5-6-15-7-16(8-17(25(15)30)9-19(27)14(3)4)22-12-21(29)24-20(28)10-18(26)11-23(24)31-22/h5,7-8,10-11,19,22,26-28,30H,3,6,9,12H2,1-2,4H3/t19?,22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.57E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B |
J Nat Prod 70: 1039-42 (2007)
Article DOI: 10.1021/np060477+
BindingDB Entry DOI: 10.7270/Q2KH0P5K |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50213493

(2-(2'-methoxy-4'-hydroxyphenyl)-5-(3-methylbut-2-e...)Show SMILES [#6]-[#8]-c1cc(-[#8])ccc1-c1cc2cc(-[#6]\[#6]=[#6](/[#6])-[#6])c(-[#8])cc2o1 Show InChI InChI=1S/C20H20O4/c1-12(2)4-5-13-8-14-9-20(24-18(14)11-17(13)22)16-7-6-15(21)10-19(16)23-3/h4,6-11,21-22H,5H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.57E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B |
Bioorg Med Chem Lett 17: 3868-71 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.005
BindingDB Entry DOI: 10.7270/Q29W0F6F |
More data for this
Ligand-Target Pair | |
Neuraminidase
(Influenza A virus) | BDBM5025

(Oseltamivir | US10919856, POSITIVE CONTROL | ethyl...)Show SMILES [H][C@@]1(OC(CC)CC)C=C(C[C@H](N)[C@H]1NC(C)=O)C(=O)OCC |r,c:8| Show InChI InChI=1S/C16H28N2O4/c1-5-12(6-2)22-14-9-11(16(20)21-7-3)8-13(17)15(14)18-10(4)19/h9,12-15H,5-8,17H2,1-4H3,(H,18,19)/t13-,14+,15+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.63E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University
Curated by ChEMBL
| Assay Description
Inhibition of oseltamivir-resistant H1N1 swine influenza virus neuraminidase H274Y mutant using 4-MU-NANA as substrate by fluorescence assay |
Bioorg Med Chem Lett 22: 3688-92 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.028
BindingDB Entry DOI: 10.7270/Q27947JG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50212400
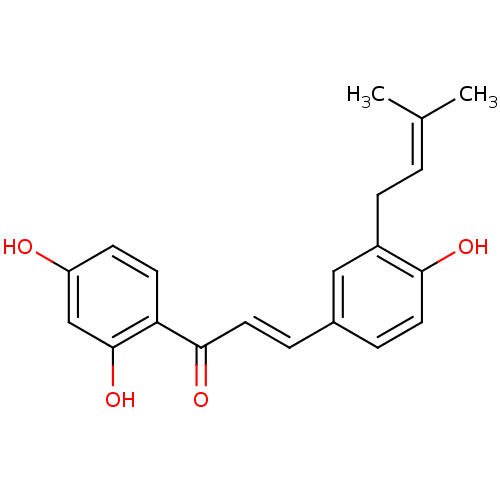
(1-(2,4-Dihydroxyphenyl)-3-[4-hydroxy-3-(3-methylbu...)Show SMILES [#6]\[#6](-[#6])=[#6]/[#6]-c1cc(\[#6]=[#6]\[#6](=O)-c2ccc(-[#8])cc2-[#8])ccc1-[#8] Show InChI InChI=1S/C20H20O4/c1-13(2)3-6-15-11-14(4-9-18(15)22)5-10-19(23)17-8-7-16(21)12-20(17)24/h3-5,7-12,21-22,24H,6H2,1-2H3/b10-5+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.69E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B |
J Nat Prod 70: 1039-42 (2007)
Article DOI: 10.1021/np060477+
BindingDB Entry DOI: 10.7270/Q2KH0P5K |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50212397

(CHEMBL388722 | abyssinin II)Show SMILES [#6]-[#8]-c1cc(cc(-[#6]\[#6]=[#6](\[#6])-[#6])c1-[#8])-[#6@@H]-1-[#6]-[#6](=O)-c2c(-[#8])cc(-[#8])cc2-[#8]-1 Show InChI InChI=1S/C21H22O6/c1-11(2)4-5-12-6-13(7-19(26-3)21(12)25)17-10-16(24)20-15(23)8-14(22)9-18(20)27-17/h4,6-9,17,22-23,25H,5,10H2,1-3H3/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.73E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B |
J Nat Prod 70: 1039-42 (2007)
Article DOI: 10.1021/np060477+
BindingDB Entry DOI: 10.7270/Q2KH0P5K |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data