 Found 826 hits with Last Name = 'tilley' and Initial = 'jw'
Found 826 hits with Last Name = 'tilley' and Initial = 'jw' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50371874
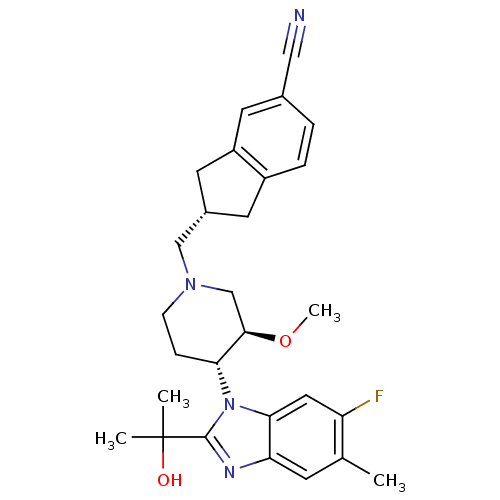
(CHEMBL257733)Show SMILES CO[C@@H]1CN(C[C@H]2Cc3ccc(cc3C2)C#N)CC[C@H]1n1c(nc2cc(C)c(F)cc12)C(C)(C)O Show InChI InChI=1S/C28H33FN4O2/c1-17-9-23-25(13-22(17)29)33(27(31-23)28(2,3)34)24-7-8-32(16-26(24)35-4)15-19-11-20-6-5-18(14-30)10-21(20)12-19/h5-6,9-10,13,19,24,26,34H,7-8,11-12,15-16H2,1-4H3/t19-,24+,26+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to MCHR1 |
Bioorg Med Chem Lett 18: 1402-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.010
BindingDB Entry DOI: 10.7270/Q2M909HR |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50371872
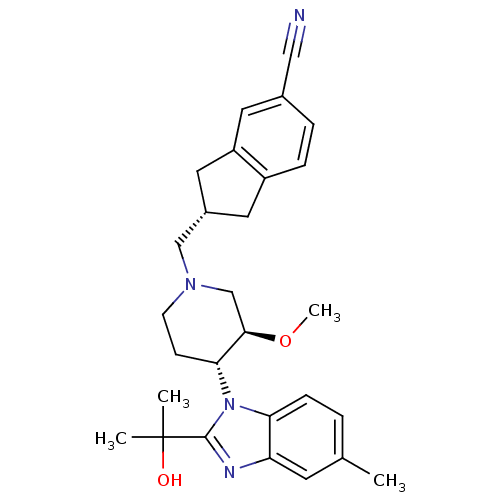
(CHEMBL257280)Show SMILES CO[C@@H]1CN(C[C@H]2Cc3ccc(cc3C2)C#N)CC[C@H]1n1c(nc2cc(C)ccc12)C(C)(C)O Show InChI InChI=1S/C28H34N4O2/c1-18-5-8-24-23(11-18)30-27(28(2,3)33)32(24)25-9-10-31(17-26(25)34-4)16-20-13-21-7-6-19(15-29)12-22(21)14-20/h5-8,11-12,20,25-26,33H,9-10,13-14,16-17H2,1-4H3/t20-,25+,26+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to MCHR1 |
Bioorg Med Chem Lett 18: 1402-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.010
BindingDB Entry DOI: 10.7270/Q2M909HR |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50371868

(CHEMBL269939)Show SMILES CO[C@@H]1CN(C[C@H]2Cc3ccc(Br)cc3C2)CC[C@H]1n1c(C)nc2cc(C)ccc12 Show InChI InChI=1S/C25H30BrN3O/c1-16-4-7-23-22(10-16)27-17(2)29(23)24-8-9-28(15-25(24)30-3)14-18-11-19-5-6-21(26)13-20(19)12-18/h4-7,10,13,18,24-25H,8-9,11-12,14-15H2,1-3H3/t18-,24+,25+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to MCHR1 |
Bioorg Med Chem Lett 18: 1402-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.010
BindingDB Entry DOI: 10.7270/Q2M909HR |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50371871
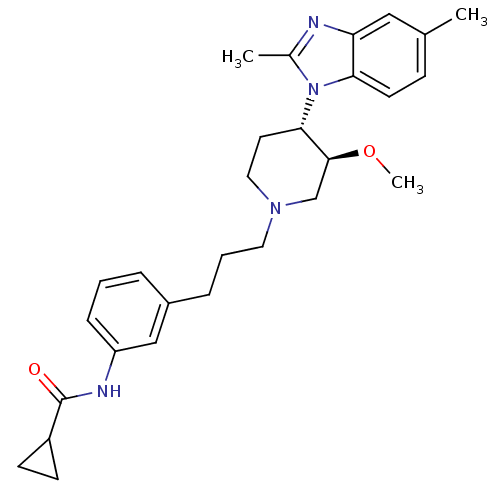
(CHEMBL255112)Show SMILES CO[C@H]1CN(CCCc2cccc(NC(=O)C3CC3)c2)CC[C@@H]1n1c(C)nc2cc(C)ccc12 Show InChI InChI=1S/C28H36N4O2/c1-19-9-12-25-24(16-19)29-20(2)32(25)26-13-15-31(18-27(26)34-3)14-5-7-21-6-4-8-23(17-21)30-28(33)22-10-11-22/h4,6,8-9,12,16-17,22,26-27H,5,7,10-11,13-15,18H2,1-3H3,(H,30,33)/t26-,27-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to MCHR1 |
Bioorg Med Chem Lett 18: 1402-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.010
BindingDB Entry DOI: 10.7270/Q2M909HR |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50371866
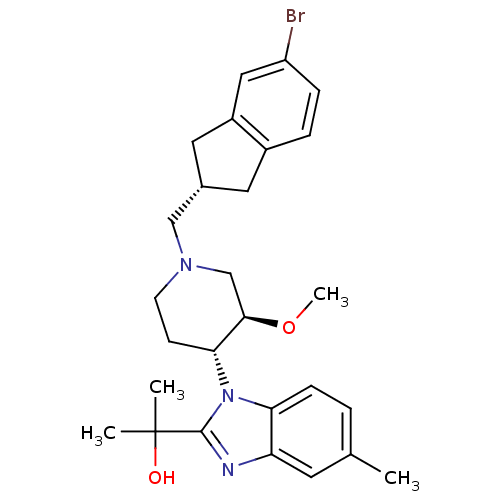
(CHEMBL257906)Show SMILES CO[C@@H]1CN(C[C@H]2Cc3ccc(Br)cc3C2)CC[C@H]1n1c(nc2cc(C)ccc12)C(C)(C)O Show InChI InChI=1S/C27H34BrN3O2/c1-17-5-8-23-22(11-17)29-26(27(2,3)32)31(23)24-9-10-30(16-25(24)33-4)15-18-12-19-6-7-21(28)14-20(19)13-18/h5-8,11,14,18,24-25,32H,9-10,12-13,15-16H2,1-4H3/t18-,24+,25+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to MCHR1 |
Bioorg Med Chem Lett 18: 1402-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.010
BindingDB Entry DOI: 10.7270/Q2M909HR |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50371869
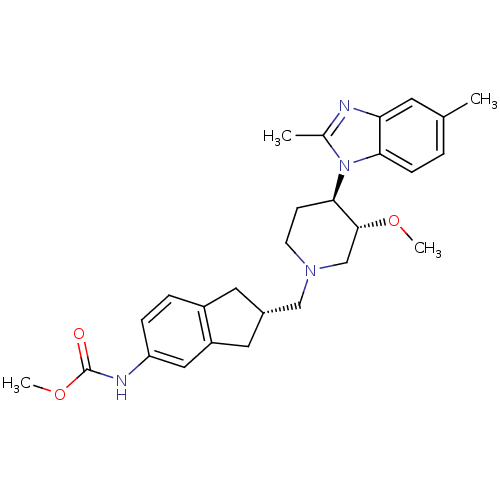
(CHEMBL270151)Show SMILES CO[C@@H]1CN(C[C@H]2Cc3ccc(NC(=O)OC)cc3C2)CC[C@H]1n1c(C)nc2cc(C)ccc12 Show InChI InChI=1S/C27H34N4O3/c1-17-5-8-24-23(11-17)28-18(2)31(24)25-9-10-30(16-26(25)33-3)15-19-12-20-6-7-22(14-21(20)13-19)29-27(32)34-4/h5-8,11,14,19,25-26H,9-10,12-13,15-16H2,1-4H3,(H,29,32)/t19-,25+,26+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to MCHR1 |
Bioorg Med Chem Lett 18: 1402-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.010
BindingDB Entry DOI: 10.7270/Q2M909HR |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50371873
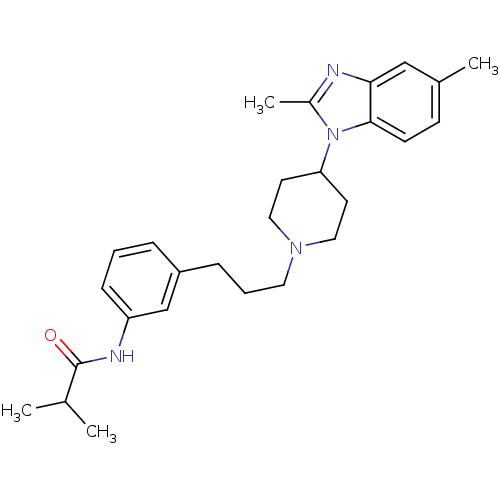
(CHEMBL403730)Show SMILES CC(C)C(=O)Nc1cccc(CCCN2CCC(CC2)n2c(C)nc3cc(C)ccc23)c1 Show InChI InChI=1S/C27H36N4O/c1-19(2)27(32)29-23-9-5-7-22(18-23)8-6-14-30-15-12-24(13-16-30)31-21(4)28-25-17-20(3)10-11-26(25)31/h5,7,9-11,17-19,24H,6,8,12-16H2,1-4H3,(H,29,32) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to MCHR1 |
Bioorg Med Chem Lett 18: 1402-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.010
BindingDB Entry DOI: 10.7270/Q2M909HR |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50371870
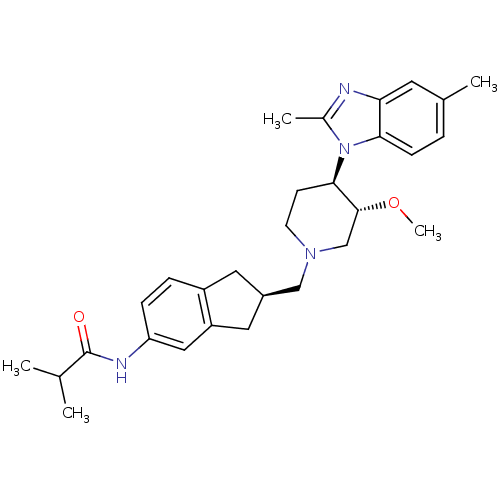
(CHEMBL258260)Show SMILES CO[C@@H]1CN(C[C@@H]2Cc3ccc(NC(=O)C(C)C)cc3C2)CC[C@H]1n1c(C)nc2cc(C)ccc12 Show InChI InChI=1S/C29H38N4O2/c1-18(2)29(34)31-24-8-7-22-13-21(14-23(22)15-24)16-32-11-10-27(28(17-32)35-5)33-20(4)30-25-12-19(3)6-9-26(25)33/h6-9,12,15,18,21,27-28H,10-11,13-14,16-17H2,1-5H3,(H,31,34)/t21-,27-,28-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to MCHR1 |
Bioorg Med Chem Lett 18: 1402-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.010
BindingDB Entry DOI: 10.7270/Q2M909HR |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50371867
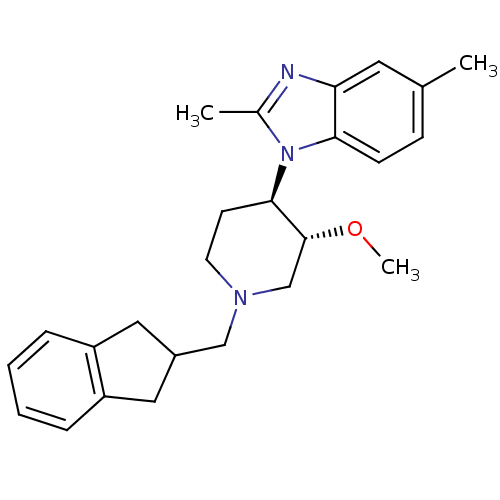
(CHEMBL257905)Show SMILES CO[C@@H]1CN(CC2Cc3ccccc3C2)CC[C@H]1n1c(C)nc2cc(C)ccc12 Show InChI InChI=1S/C25H31N3O/c1-17-8-9-23-22(12-17)26-18(2)28(23)24-10-11-27(16-25(24)29-3)15-19-13-20-6-4-5-7-21(20)14-19/h4-9,12,19,24-25H,10-11,13-16H2,1-3H3/t24-,25-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to MCHR1 |
Bioorg Med Chem Lett 18: 1402-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.010
BindingDB Entry DOI: 10.7270/Q2M909HR |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Bos taurus) | BDBM50003204
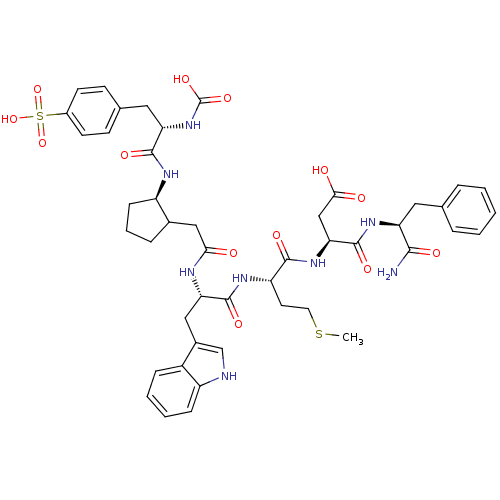
(CHEMBL122438 | N-(1-Carbamoyl-2-phenyl-ethyl)-3-{2...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)CC1CCC[C@H]1NC(=O)[C@H](Cc1ccc(cc1)S(O)(=O)=O)NC(O)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C46H56N8O13S2/c1-68-19-18-34(42(59)53-38(24-40(56)57)45(62)52-35(41(47)58)20-26-8-3-2-4-9-26)51-44(61)37(22-29-25-48-33-12-6-5-11-31(29)33)49-39(55)23-28-10-7-13-32(28)50-43(60)36(54-46(63)64)21-27-14-16-30(17-15-27)69(65,66)67/h2-6,8-9,11-12,14-17,25,28,32,34-38,48,54H,7,10,13,18-24H2,1H3,(H2,47,58)(H,49,55)(H,50,60)(H,51,61)(H,52,62)(H,53,59)(H,56,57)(H,63,64)(H,65,66,67)/t28?,32-,34+,35+,36+,37+,38+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.0290 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-propanoyl-CCK-8 binding to Cholecystokinin type B receptor in bovine striatum |
J Med Chem 35: 3774-83 (1992)
BindingDB Entry DOI: 10.7270/Q2CC0ZMG |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(RAT) | BDBM50003666

(3-[2-[2-tert-Butoxycarbonylamino-3-(1H-indol-3-yl)...)Show SMILES CN([C@@H](Cc1ccccc1)C(N)=O)C(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCCCNC(=O)Nc1ccccc1C)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)OC(C)(C)C Show InChI InChI=1S/C44H56N8O9/c1-27-15-9-11-19-31(27)50-42(59)46-22-14-13-21-33(39(56)49-35(25-37(53)54)41(58)52(5)36(38(45)55)23-28-16-7-6-8-17-28)48-40(57)34(51-43(60)61-44(2,3)4)24-29-26-47-32-20-12-10-18-30(29)32/h6-12,15-20,26,33-36,47H,13-14,21-25H2,1-5H3,(H2,45,55)(H,48,57)(H,49,56)(H,51,60)(H,53,54)(H2,46,50,59)/t33?,34-,35?,36?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibitory activity against cholecystokinin type A receptor from rat pancreas binding assay |
J Med Chem 35: 4249-52 (1992)
BindingDB Entry DOI: 10.7270/Q2Z60N07 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(RAT) | BDBM50003669

(Ac-Tyr(SO3H)-Met-Gly-Trp-Met-R-Dtc-Phe-NH2 | CHEMB...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccc(cc1)S(O)(=O)=O)NC(C)=O)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCSC)C(=O)N1CSC(C)(C)[C@@H]1C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C49H63N9O11S4/c1-29(59)53-39(24-31-15-17-33(18-16-31)73(67,68)69)45(63)55-36(19-21-70-4)44(62)52-27-41(60)54-40(25-32-26-51-35-14-10-9-13-34(32)35)46(64)56-37(20-22-71-5)48(66)58-28-72-49(2,3)42(58)47(65)57-38(43(50)61)23-30-11-7-6-8-12-30/h6-18,26,36-40,42,51H,19-25,27-28H2,1-5H3,(H2,50,61)(H,52,62)(H,53,59)(H,54,60)(H,55,63)(H,56,64)(H,57,65)(H,67,68,69)/t36-,37-,38-,39-,40-,42-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Concentration inhibiting [3H]-propanoyl-CCK-8 binding to cholecystokinin type A receptor of rat pancreatic membranes. |
J Med Chem 35: 4249-52 (1992)
BindingDB Entry DOI: 10.7270/Q2Z60N07 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Bos taurus) | BDBM50003199
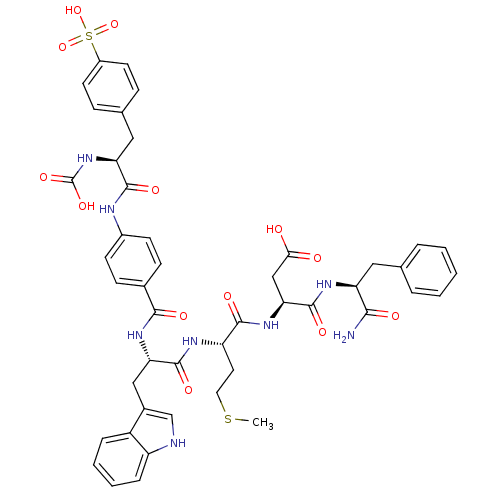
(CHEMBL126008 | N-(1-Carbamoyl-2-phenyl-ethyl)-3-{2...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)c1ccc(NC(=O)[C@H](Cc2ccc(cc2)S(O)(=O)=O)NC(O)=O)cc1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C46H50N8O13S2/c1-68-20-19-34(42(59)53-38(24-39(55)56)45(62)51-35(40(47)57)21-26-7-3-2-4-8-26)50-44(61)37(23-29-25-48-33-10-6-5-9-32(29)33)52-41(58)28-13-15-30(16-14-28)49-43(60)36(54-46(63)64)22-27-11-17-31(18-12-27)69(65,66)67/h2-18,25,34-38,48,54H,19-24H2,1H3,(H2,47,57)(H,49,60)(H,50,61)(H,51,62)(H,52,58)(H,53,59)(H,55,56)(H,63,64)(H,65,66,67)/t34-,35-,36-,37-,38-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.0850 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-propanoyl-CCK-8 binding to Cholecystokinin type B receptor in bovine striatum |
J Med Chem 35: 3774-83 (1992)
BindingDB Entry DOI: 10.7270/Q2CC0ZMG |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
(Homo sapiens (Human)) | BDBM155041
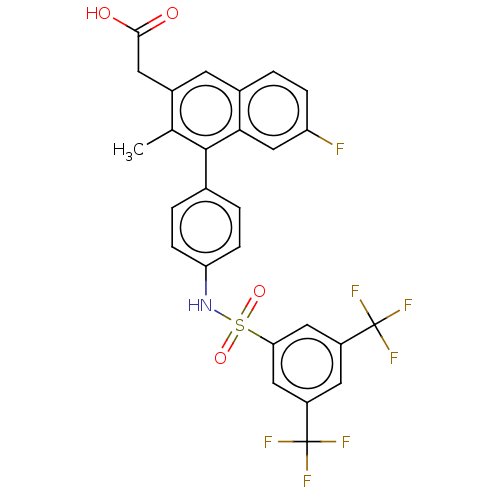
(US9000044, 60)Show SMILES Cc1c(CC(O)=O)cc2ccc(F)cc2c1-c1ccc(NS(=O)(=O)c2cc(cc(c2)C(F)(F)F)C(F)(F)F)cc1 Show InChI InChI=1S/C27H18F7NO4S/c1-14-17(9-24(36)37)8-16-2-5-20(28)13-23(16)25(14)15-3-6-21(7-4-15)35-40(38,39)22-11-18(26(29,30)31)10-19(12-22)27(32,33)34/h2-8,10-13,35H,9H2,1H3,(H,36,37) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
US Patent
| Assay Description
Cell Culture Conditions: CHO-K1 cells previously transfected with G-alpha 16 were subsequently transfected with the human CRTH2 receptor and the neom... |
US Patent US9000044 (2015)
BindingDB Entry DOI: 10.7270/Q2319TM9 |
More data for this
Ligand-Target Pair | |
Integrin alpha-4
(Homo sapiens (Human)) | BDBM50089021
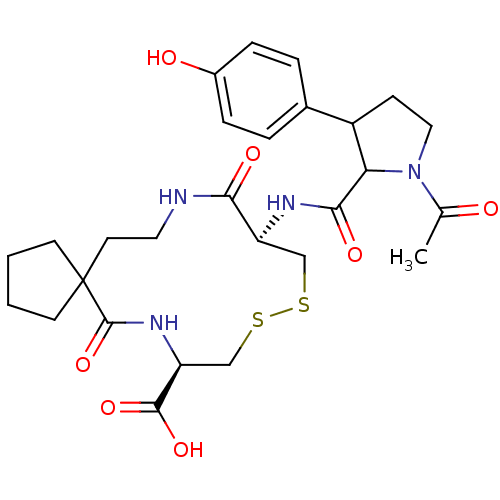
((8R,13R)-13-{[1-Acetyl-3-(4-hydroxy-phenyl)-pyrrol...)Show SMILES CC(=O)N1CCC(C1C(=O)N[C@H]1CSSC[C@H](NC(=O)C2(CCCC2)CCNC1=O)C(O)=O)c1ccc(O)cc1 Show InChI InChI=1S/C27H36N4O7S2/c1-16(32)31-13-8-19(17-4-6-18(33)7-5-17)22(31)24(35)29-20-14-39-40-15-21(25(36)37)30-26(38)27(9-2-3-10-27)11-12-28-23(20)34/h4-7,19-22,33H,2-3,8-15H2,1H3,(H,28,34)(H,29,35)(H,30,38)(H,36,37)/t19?,20-,21-,22?/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Compound was evaluated for VCAM-VLA-4 antagonistic activity using solid phase assay |
Bioorg Med Chem Lett 10: 1167-9 (2000)
BindingDB Entry DOI: 10.7270/Q2V1241K |
More data for this
Ligand-Target Pair | |
Integrin alpha-4/beta-1
(Homo sapiens (Human)) | BDBM50117037
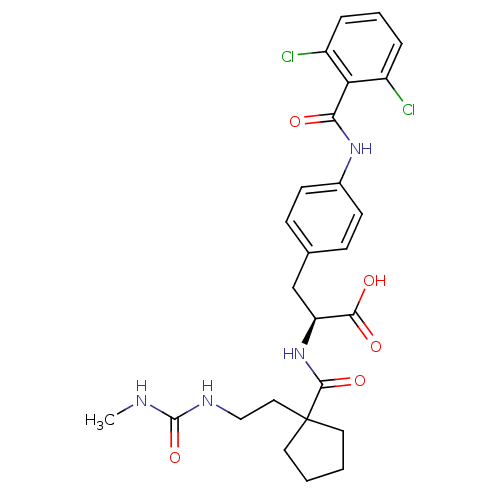
((S)-3-[4-(2,6-Dichloro-benzoylamino)-phenyl]-2-({1...)Show SMILES CNC(=O)NCCC1(CCCC1)C(=O)N[C@@H](Cc1ccc(NC(=O)c2c(Cl)cccc2Cl)cc1)C(O)=O Show InChI InChI=1S/C26H30Cl2N4O5/c1-29-25(37)30-14-13-26(11-2-3-12-26)24(36)32-20(23(34)35)15-16-7-9-17(10-8-16)31-22(33)21-18(27)5-4-6-19(21)28/h4-10,20H,2-3,11-15H2,1H3,(H,31,33)(H,32,36)(H,34,35)(H2,29,30,37)/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibitory binding concentration determined against VCAM/VLA-4 in ELISA |
Bioorg Med Chem Lett 12: 2475-8 (2002)
BindingDB Entry DOI: 10.7270/Q2251HH8 |
More data for this
Ligand-Target Pair | |
Integrin alpha-4/beta-1
(Homo sapiens (Human)) | BDBM50117004
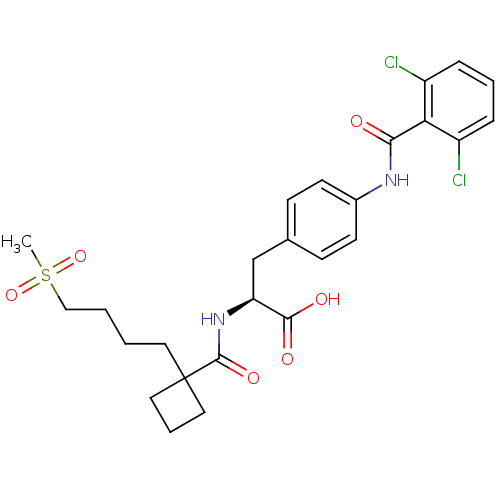
((S)-3-[4-(2,6-Dichloro-benzoylamino)-phenyl]-2-{[1...)Show SMILES CS(=O)(=O)CCCCC1(CCC1)C(=O)N[C@@H](Cc1ccc(NC(=O)c2c(Cl)cccc2Cl)cc1)C(O)=O Show InChI InChI=1S/C26H30Cl2N2O6S/c1-37(35,36)15-3-2-12-26(13-5-14-26)25(34)30-21(24(32)33)16-17-8-10-18(11-9-17)29-23(31)22-19(27)6-4-7-20(22)28/h4,6-11,21H,2-3,5,12-16H2,1H3,(H,29,31)(H,30,34)(H,32,33)/t21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of Very late antigen 4/vascular cell adhesion molecule 1 interaction in ELISA |
Bioorg Med Chem Lett 12: 2475-8 (2002)
BindingDB Entry DOI: 10.7270/Q2251HH8 |
More data for this
Ligand-Target Pair | |
Integrin alpha-4/beta-1
(Homo sapiens (Human)) | BDBM50117041
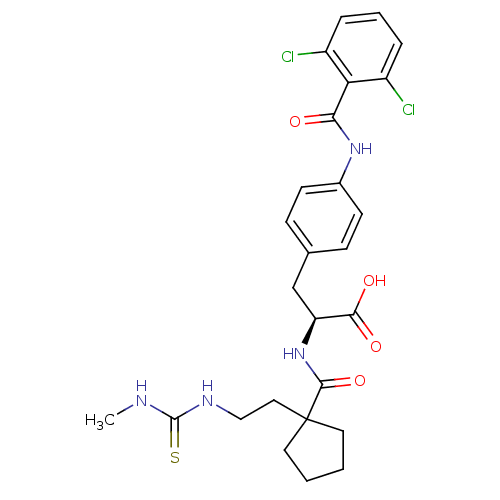
((S)-3-[4-(2,6-Dichloro-benzoylamino)-phenyl]-2-({1...)Show SMILES CNC(=S)NCCC1(CCCC1)C(=O)N[C@@H](Cc1ccc(NC(=O)c2c(Cl)cccc2Cl)cc1)C(O)=O Show InChI InChI=1S/C26H30Cl2N4O4S/c1-29-25(37)30-14-13-26(11-2-3-12-26)24(36)32-20(23(34)35)15-16-7-9-17(10-8-16)31-22(33)21-18(27)5-4-6-19(21)28/h4-10,20H,2-3,11-15H2,1H3,(H,31,33)(H,32,36)(H,34,35)(H2,29,30,37)/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibitory binding concentration determined against VCAM/VLA-4 in ELISA |
Bioorg Med Chem Lett 12: 2475-8 (2002)
BindingDB Entry DOI: 10.7270/Q2251HH8 |
More data for this
Ligand-Target Pair | |
Integrin alpha-4/beta-1
(Homo sapiens (Human)) | BDBM50117036
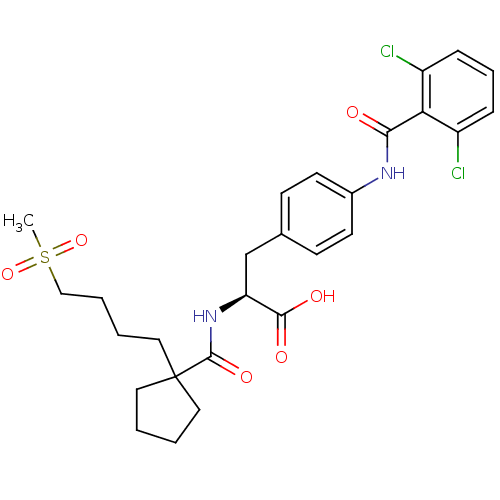
((S)-3-[4-(2,6-Dichloro-benzoylamino)-phenyl]-2-{[1...)Show SMILES CS(=O)(=O)CCCCC1(CCCC1)C(=O)N[C@@H](Cc1ccc(NC(=O)c2c(Cl)cccc2Cl)cc1)C(O)=O Show InChI InChI=1S/C27H32Cl2N2O6S/c1-38(36,37)16-5-4-15-27(13-2-3-14-27)26(35)31-22(25(33)34)17-18-9-11-19(12-10-18)30-24(32)23-20(28)7-6-8-21(23)29/h6-12,22H,2-5,13-17H2,1H3,(H,30,32)(H,31,35)(H,33,34)/t22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of Very late antigen 4/vascular cell adhesion molecule 1 interaction in ELISA |
Bioorg Med Chem Lett 12: 2475-8 (2002)
BindingDB Entry DOI: 10.7270/Q2251HH8 |
More data for this
Ligand-Target Pair | |
Integrin alpha-4/beta-1
(Homo sapiens (Human)) | BDBM50117056

((S)-2-(2-Bromo-6-methyl-benzoylamino)-3-[4-(2,6-di...)Show SMILES Cc1cccc(Br)c1C(=O)N[C@@H](Cc1ccc(NC(=O)c2c(Cl)cccc2Cl)cc1)C(O)=O Show InChI InChI=1S/C24H19BrCl2N2O4/c1-13-4-2-5-16(25)20(13)22(30)29-19(24(32)33)12-14-8-10-15(11-9-14)28-23(31)21-17(26)6-3-7-18(21)27/h2-11,19H,12H2,1H3,(H,28,31)(H,29,30)(H,32,33)/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibitory binding concentration against VCAM/VLA-4 in ELISA. |
Bioorg Med Chem Lett 12: 2479-82 (2002)
BindingDB Entry DOI: 10.7270/Q2V989C5 |
More data for this
Ligand-Target Pair | |
Integrin alpha-4/beta-1
(Homo sapiens (Human)) | BDBM50117016

((S)-3-[4-(2,6-Dichloro-benzoylamino)-phenyl]-2-{[1...)Show SMILES CS(=O)CCC1(CCCC1)C(=O)N[C@@H](Cc1ccc(NC(=O)c2c(Cl)cccc2Cl)cc1)C(O)=O Show InChI InChI=1S/C25H28Cl2N2O5S/c1-35(34)14-13-25(11-2-3-12-25)24(33)29-20(23(31)32)15-16-7-9-17(10-8-16)28-22(30)21-18(26)5-4-6-19(21)27/h4-10,20H,2-3,11-15H2,1H3,(H,28,30)(H,29,33)(H,31,32)/t20-,35?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of Very late antigen 4/vascular cell adhesion molecule 1 interaction in ELISA |
Bioorg Med Chem Lett 12: 2475-8 (2002)
BindingDB Entry DOI: 10.7270/Q2251HH8 |
More data for this
Ligand-Target Pair | |
Integrin alpha-4/beta-1
(Homo sapiens (Human)) | BDBM50117073
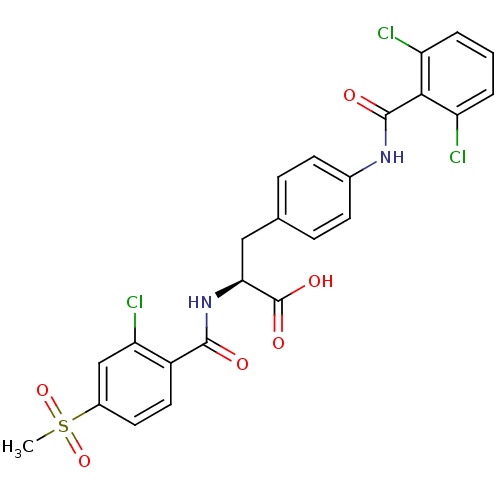
((S)-2-(2-Chloro-4-methanesulfonyl-benzoylamino)-3-...)Show SMILES CS(=O)(=O)c1ccc(C(=O)N[C@@H](Cc2ccc(NC(=O)c3c(Cl)cccc3Cl)cc2)C(O)=O)c(Cl)c1 Show InChI InChI=1S/C24H19Cl3N2O6S/c1-36(34,35)15-9-10-16(19(27)12-15)22(30)29-20(24(32)33)11-13-5-7-14(8-6-13)28-23(31)21-17(25)3-2-4-18(21)26/h2-10,12,20H,11H2,1H3,(H,28,31)(H,29,30)(H,32,33)/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibitory binding concentration against VCAM/VLA-4 in ELISA. |
Bioorg Med Chem Lett 12: 2479-82 (2002)
BindingDB Entry DOI: 10.7270/Q2V989C5 |
More data for this
Ligand-Target Pair | |
Integrin alpha-4/beta-1
(Homo sapiens (Human)) | BDBM50117050
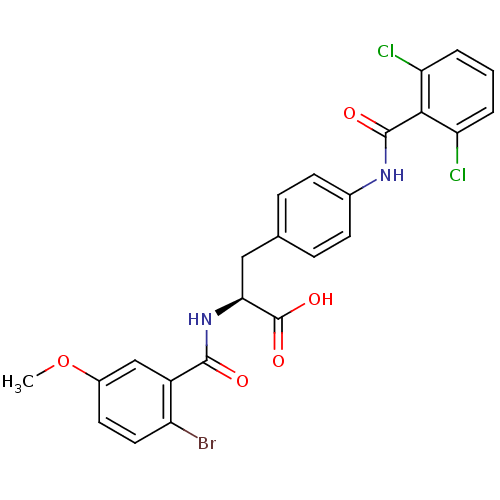
((S)-2-(2-Bromo-5-methoxy-benzoylamino)-3-[4-(2,6-d...)Show SMILES COc1ccc(Br)c(c1)C(=O)N[C@@H](Cc1ccc(NC(=O)c2c(Cl)cccc2Cl)cc1)C(O)=O Show InChI InChI=1S/C24H19BrCl2N2O5/c1-34-15-9-10-17(25)16(12-15)22(30)29-20(24(32)33)11-13-5-7-14(8-6-13)28-23(31)21-18(26)3-2-4-19(21)27/h2-10,12,20H,11H2,1H3,(H,28,31)(H,29,30)(H,32,33)/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibitory binding concentration against VCAM/VLA-4 in ELISA. |
Bioorg Med Chem Lett 12: 2479-82 (2002)
BindingDB Entry DOI: 10.7270/Q2V989C5 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(RAT) | BDBM50011545

(CHEMBL430906 | Desamino-Tyr(SO3H)-Met-Gly-Trp-Met-...)Show SMILES CSCC[C@H](NC(=O)CCc1ccc(OS(O)(=O)=O)cc1)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C45H56N8O13S3/c1-67-20-18-33(49-38(54)17-14-27-12-15-30(16-13-27)66-69(63,64)65)42(59)48-26-39(55)50-36(23-29-25-47-32-11-7-6-10-31(29)32)44(61)51-34(19-21-68-2)43(60)53-37(24-40(56)57)45(62)52-35(41(46)58)22-28-8-4-3-5-9-28/h3-13,15-16,25,33-37,47H,14,17-24,26H2,1-2H3,(H2,46,58)(H,48,59)(H,49,54)(H,50,55)(H,51,61)(H,52,62)(H,53,60)(H,56,57)(H,63,64,65)/t33-,34-,35-,36-,37-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-propanoyl binding to cholecystokinin type A receptor was determined in fresh rat pancreatic tissue membranes |
J Med Chem 34: 1125-36 (1991)
BindingDB Entry DOI: 10.7270/Q27P8XCT |
More data for this
Ligand-Target Pair | |
Integrin alpha-4/beta-1
(Homo sapiens (Human)) | BDBM50117026
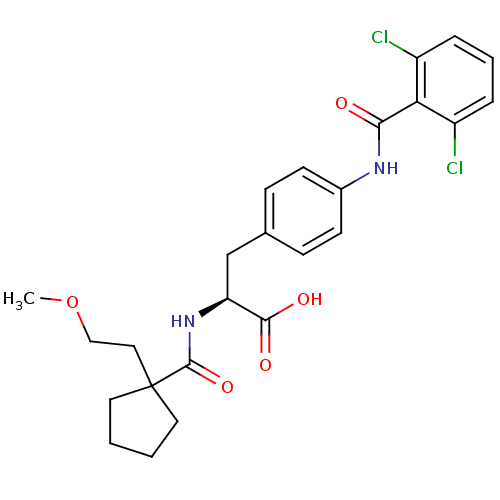
((S)-3-[4-(2,6-Dichloro-benzoylamino)-phenyl]-2-{[1...)Show SMILES COCCC1(CCCC1)C(=O)N[C@@H](Cc1ccc(NC(=O)c2c(Cl)cccc2Cl)cc1)C(O)=O Show InChI InChI=1S/C25H28Cl2N2O5/c1-34-14-13-25(11-2-3-12-25)24(33)29-20(23(31)32)15-16-7-9-17(10-8-16)28-22(30)21-18(26)5-4-6-19(21)27/h4-10,20H,2-3,11-15H2,1H3,(H,28,30)(H,29,33)(H,31,32)/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibitory binding concentration determined against VCAM/VLA-4 in ELISA |
Bioorg Med Chem Lett 12: 2475-8 (2002)
BindingDB Entry DOI: 10.7270/Q2251HH8 |
More data for this
Ligand-Target Pair | |
Integrin alpha-4/beta-1
(Homo sapiens (Human)) | BDBM50116998

((S)-3-[4-(2,6-Dichloro-benzoylamino)-phenyl]-2-{[1...)Show SMILES CSCCC1(CCCC1)C(=O)N[C@@H](Cc1ccc(NC(=O)c2c(Cl)cccc2Cl)cc1)C(O)=O Show InChI InChI=1S/C25H28Cl2N2O4S/c1-34-14-13-25(11-2-3-12-25)24(33)29-20(23(31)32)15-16-7-9-17(10-8-16)28-22(30)21-18(26)5-4-6-19(21)27/h4-10,20H,2-3,11-15H2,1H3,(H,28,30)(H,29,33)(H,31,32)/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of Very late antigen 4/vascular cell adhesion molecule 1 interaction in ELISA |
Bioorg Med Chem Lett 12: 2475-8 (2002)
BindingDB Entry DOI: 10.7270/Q2251HH8 |
More data for this
Ligand-Target Pair | |
Integrin alpha-4/beta-1
(Homo sapiens (Human)) | BDBM50117053

((S)-3-[4-(2,6-Dichloro-benzoylamino)-phenyl]-2-(2-...)Show SMILES CCc1cccc(C)c1C(=O)N[C@@H](Cc1ccc(NC(=O)c2c(Cl)cccc2Cl)cc1)C(O)=O Show InChI InChI=1S/C26H24Cl2N2O4/c1-3-17-7-4-6-15(2)22(17)24(31)30-21(26(33)34)14-16-10-12-18(13-11-16)29-25(32)23-19(27)8-5-9-20(23)28/h4-13,21H,3,14H2,1-2H3,(H,29,32)(H,30,31)(H,33,34)/t21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibitory binding concentration against VCAM/VLA-4 in ELISA. |
Bioorg Med Chem Lett 12: 2479-82 (2002)
BindingDB Entry DOI: 10.7270/Q2V989C5 |
More data for this
Ligand-Target Pair | |
Integrin alpha-4/beta-1
(Homo sapiens (Human)) | BDBM50117069
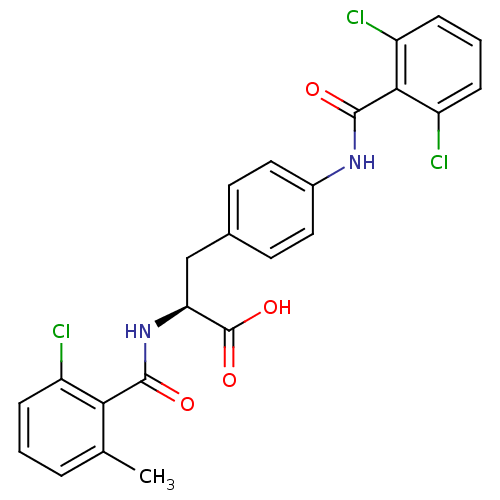
((S)-2-(2-Chloro-6-methyl-benzoylamino)-3-[4-(2,6-d...)Show SMILES Cc1cccc(Cl)c1C(=O)N[C@@H](Cc1ccc(NC(=O)c2c(Cl)cccc2Cl)cc1)C(O)=O Show InChI InChI=1S/C24H19Cl3N2O4/c1-13-4-2-5-16(25)20(13)22(30)29-19(24(32)33)12-14-8-10-15(11-9-14)28-23(31)21-17(26)6-3-7-18(21)27/h2-11,19H,12H2,1H3,(H,28,31)(H,29,30)(H,32,33)/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibitory binding concentration against VCAM/VLA-4 in ELISA. |
Bioorg Med Chem Lett 12: 2479-82 (2002)
BindingDB Entry DOI: 10.7270/Q2V989C5 |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
(Homo sapiens (Human)) | BDBM154982
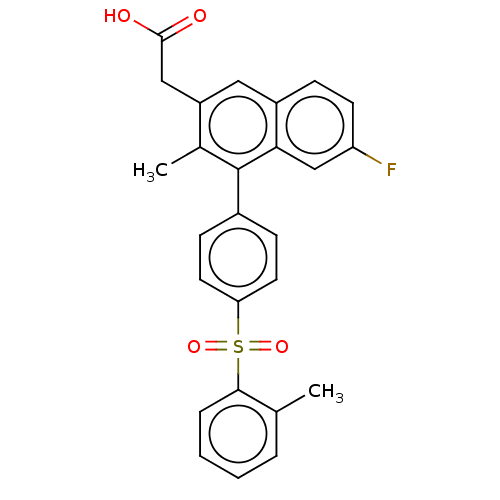
(US9000044, 1)Show SMILES Cc1ccccc1S(=O)(=O)c1ccc(cc1)-c1c(C)c(CC(O)=O)cc2ccc(F)cc12 Show InChI InChI=1S/C26H21FO4S/c1-16-5-3-4-6-24(16)32(30,31)22-11-8-18(9-12-22)26-17(2)20(14-25(28)29)13-19-7-10-21(27)15-23(19)26/h3-13,15H,14H2,1-2H3,(H,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
US Patent
| Assay Description
Cell Culture Conditions: CHO-K1 cells previously transfected with G-alpha 16 were subsequently transfected with the human CRTH2 receptor and the neom... |
US Patent US9000044 (2015)
BindingDB Entry DOI: 10.7270/Q2319TM9 |
More data for this
Ligand-Target Pair | |
Integrin alpha-4/beta-1
(Homo sapiens (Human)) | BDBM50117011

((S)-3-[4-(2,6-Dichloro-benzoylamino)-phenyl]-2-{[1...)Show SMILES COCCCCC1(CCCC1)C(=O)N[C@@H](Cc1ccc(NC(=O)c2c(Cl)cccc2Cl)cc1)C(O)=O Show InChI InChI=1S/C27H32Cl2N2O5/c1-36-16-5-4-15-27(13-2-3-14-27)26(35)31-22(25(33)34)17-18-9-11-19(12-10-18)30-24(32)23-20(28)7-6-8-21(23)29/h6-12,22H,2-5,13-17H2,1H3,(H,30,32)(H,31,35)(H,33,34)/t22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibitory binding concentration determined against VCAM/VLA-4 in Ramos |
Bioorg Med Chem Lett 12: 2475-8 (2002)
BindingDB Entry DOI: 10.7270/Q2251HH8 |
More data for this
Ligand-Target Pair | |
Integrin alpha-4/beta-1
(Homo sapiens (Human)) | BDBM50117028
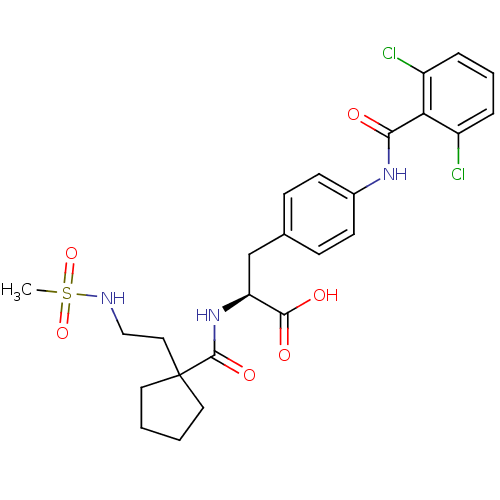
((S)-3-[4-(2,6-Dichloro-benzoylamino)-phenyl]-2-{[1...)Show SMILES CS(=O)(=O)NCCC1(CCCC1)C(=O)N[C@@H](Cc1ccc(NC(=O)c2c(Cl)cccc2Cl)cc1)C(O)=O Show InChI InChI=1S/C25H29Cl2N3O6S/c1-37(35,36)28-14-13-25(11-2-3-12-25)24(34)30-20(23(32)33)15-16-7-9-17(10-8-16)29-22(31)21-18(26)5-4-6-19(21)27/h4-10,20,28H,2-3,11-15H2,1H3,(H,29,31)(H,30,34)(H,32,33)/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibitory binding concentration determined against VCAM/VLA-4 in ELISA |
Bioorg Med Chem Lett 12: 2475-8 (2002)
BindingDB Entry DOI: 10.7270/Q2251HH8 |
More data for this
Ligand-Target Pair | |
Integrin alpha-4/beta-1
(Homo sapiens (Human)) | BDBM50095252
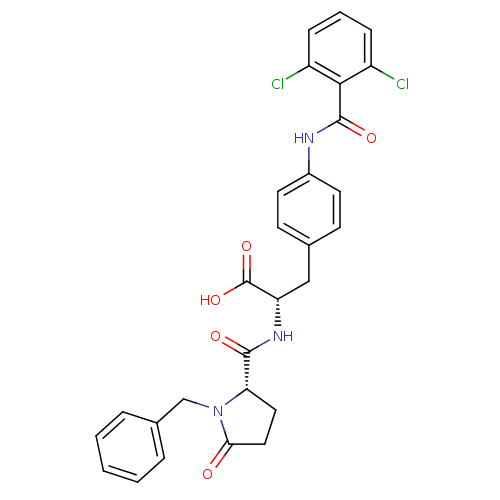
((S)-2-[((S)-1-Benzyl-5-oxo-pyrrolidine-2-carbonyl)...)Show SMILES OC(=O)[C@H](Cc1ccc(NC(=O)c2c(Cl)cccc2Cl)cc1)NC(=O)[C@@H]1CCC(=O)N1Cc1ccccc1 Show InChI InChI=1S/C28H25Cl2N3O5/c29-20-7-4-8-21(30)25(20)27(36)31-19-11-9-17(10-12-19)15-22(28(37)38)32-26(35)23-13-14-24(34)33(23)16-18-5-2-1-3-6-18/h1-12,22-23H,13-16H2,(H,31,36)(H,32,35)(H,37,38)/t22-,23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Antagonistic activity against VLA-4 (derived from ramos cells) binding to recombinant human VCAM by ELISA assay |
Bioorg Med Chem Lett 11: 1-4 (2001)
BindingDB Entry DOI: 10.7270/Q2MS3V2H |
More data for this
Ligand-Target Pair | |
Integrin alpha-4/beta-1
(Homo sapiens (Human)) | BDBM50087594
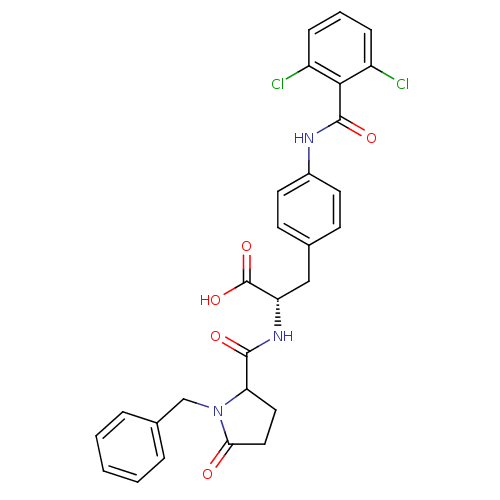
((S)-2-[(1-Benzyl-5-oxo-pyrrolidine-2-carbonyl)-ami...)Show SMILES OC(=O)[C@H](Cc1ccc(NC(=O)c2c(Cl)cccc2Cl)cc1)NC(=O)C1CCC(=O)N1Cc1ccccc1 Show InChI InChI=1S/C28H25Cl2N3O5/c29-20-7-4-8-21(30)25(20)27(36)31-19-11-9-17(10-12-19)15-22(28(37)38)32-26(35)23-13-14-24(34)33(23)16-18-5-2-1-3-6-18/h1-12,22-23H,13-16H2,(H,31,36)(H,32,35)(H,37,38)/t22-,23?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Antagonistic activity against VLA-4 (derived from ramos cells) binding to recombinant human VCAM by ELISA assay |
Bioorg Med Chem Lett 10: 729-33 (2000)
BindingDB Entry DOI: 10.7270/Q2125TXJ |
More data for this
Ligand-Target Pair | |
Integrin alpha-4/beta-1
(Homo sapiens (Human)) | BDBM50117006
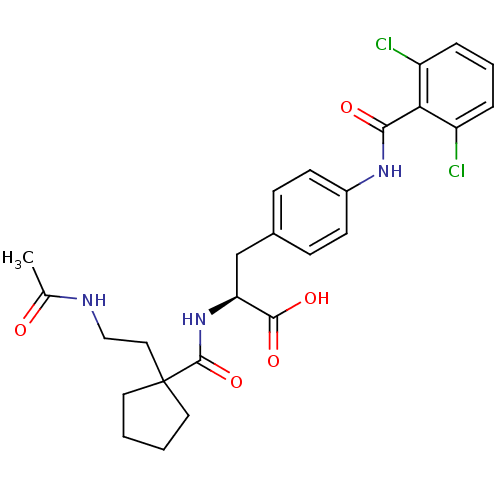
((S)-2-{[1-(2-Acetylamino-ethyl)-cyclopentanecarbon...)Show SMILES CC(=O)NCCC1(CCCC1)C(=O)N[C@@H](Cc1ccc(NC(=O)c2c(Cl)cccc2Cl)cc1)C(O)=O Show InChI InChI=1S/C26H29Cl2N3O5/c1-16(32)29-14-13-26(11-2-3-12-26)25(36)31-21(24(34)35)15-17-7-9-18(10-8-17)30-23(33)22-19(27)5-4-6-20(22)28/h4-10,21H,2-3,11-15H2,1H3,(H,29,32)(H,30,33)(H,31,36)(H,34,35)/t21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibitory binding concentration determined against VCAM/VLA-4 in ELISA |
Bioorg Med Chem Lett 12: 2475-8 (2002)
BindingDB Entry DOI: 10.7270/Q2251HH8 |
More data for this
Ligand-Target Pair | |
Integrin alpha-4/beta-1
(Homo sapiens (Human)) | BDBM50117031
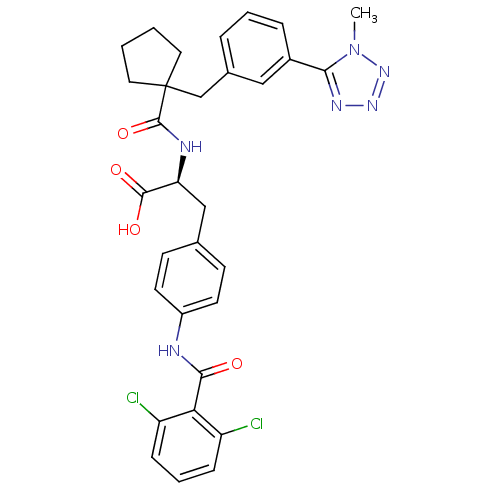
((S)-3-[4-(2,6-Dichloro-benzoylamino)-phenyl]-2-({1...)Show SMILES Cn1nnnc1-c1cccc(CC2(CCCC2)C(=O)N[C@@H](Cc2ccc(NC(=O)c3c(Cl)cccc3Cl)cc2)C(O)=O)c1 Show InChI InChI=1S/C31H30Cl2N6O4/c1-39-27(36-37-38-39)21-7-4-6-20(16-21)18-31(14-2-3-15-31)30(43)35-25(29(41)42)17-19-10-12-22(13-11-19)34-28(40)26-23(32)8-5-9-24(26)33/h4-13,16,25H,2-3,14-15,17-18H2,1H3,(H,34,40)(H,35,43)(H,41,42)/t25-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibitory binding concentration determined against VCAM/VLA-4 in ELISA |
Bioorg Med Chem Lett 12: 2475-8 (2002)
BindingDB Entry DOI: 10.7270/Q2251HH8 |
More data for this
Ligand-Target Pair | |
Integrin alpha-4/beta-1
(Homo sapiens (Human)) | BDBM50117066
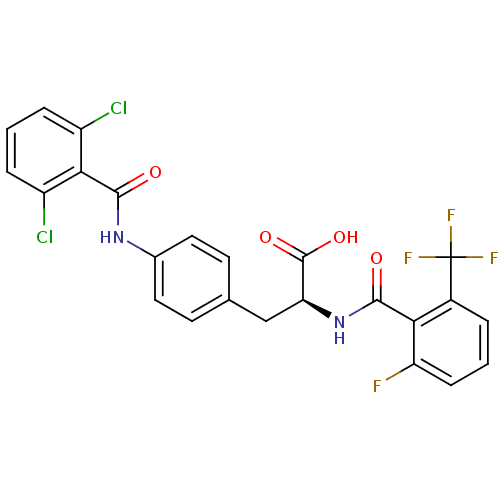
((S)-3-[4-(2,6-Dichloro-benzoylamino)-phenyl]-2-(2-...)Show SMILES OC(=O)[C@H](Cc1ccc(NC(=O)c2c(Cl)cccc2Cl)cc1)NC(=O)c1c(F)cccc1C(F)(F)F Show InChI InChI=1S/C24H16Cl2F4N2O4/c25-15-4-2-5-16(26)20(15)22(34)31-13-9-7-12(8-10-13)11-18(23(35)36)32-21(33)19-14(24(28,29)30)3-1-6-17(19)27/h1-10,18H,11H2,(H,31,34)(H,32,33)(H,35,36)/t18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibitory binding concentration against VCAM/VLA-4 in Ramos. |
Bioorg Med Chem Lett 12: 2479-82 (2002)
BindingDB Entry DOI: 10.7270/Q2V989C5 |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
(Homo sapiens (Human)) | BDBM155032

(US9000044, 51)Show SMILES Cc1c(CC(O)=O)cc2ccc(F)cc2c1-c1ccc(cc1)S(=O)(=O)NCc1ccccc1 Show InChI InChI=1S/C26H22FNO4S/c1-17-21(14-25(29)30)13-20-7-10-22(27)15-24(20)26(17)19-8-11-23(12-9-19)33(31,32)28-16-18-5-3-2-4-6-18/h2-13,15,28H,14,16H2,1H3,(H,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
US Patent
| Assay Description
Cell Culture Conditions: CHO-K1 cells previously transfected with G-alpha 16 were subsequently transfected with the human CRTH2 receptor and the neom... |
US Patent US9000044 (2015)
BindingDB Entry DOI: 10.7270/Q2319TM9 |
More data for this
Ligand-Target Pair | |
Integrin alpha-4/beta-1
(Homo sapiens (Human)) | BDBM50117048
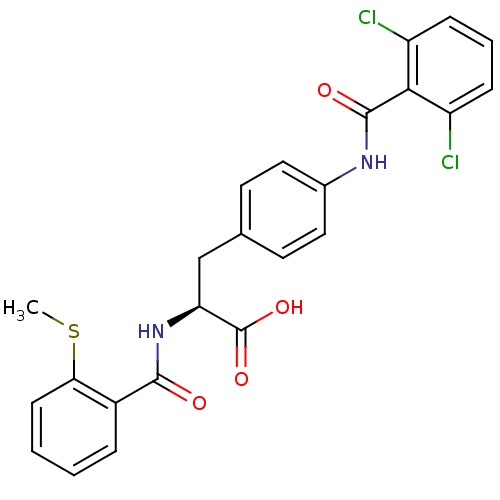
((S)-3-[4-(2,6-Dichloro-benzoylamino)-phenyl]-2-(2-...)Show SMILES CSc1ccccc1C(=O)N[C@@H](Cc1ccc(NC(=O)c2c(Cl)cccc2Cl)cc1)C(O)=O Show InChI InChI=1S/C24H20Cl2N2O4S/c1-33-20-8-3-2-5-16(20)22(29)28-19(24(31)32)13-14-9-11-15(12-10-14)27-23(30)21-17(25)6-4-7-18(21)26/h2-12,19H,13H2,1H3,(H,27,30)(H,28,29)(H,31,32)/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibitory binding concentration against VCAM/VLA-4 in Ramos. |
Bioorg Med Chem Lett 12: 2479-82 (2002)
BindingDB Entry DOI: 10.7270/Q2V989C5 |
More data for this
Ligand-Target Pair | |
Integrin alpha-4/beta-1
(Homo sapiens (Human)) | BDBM50117065
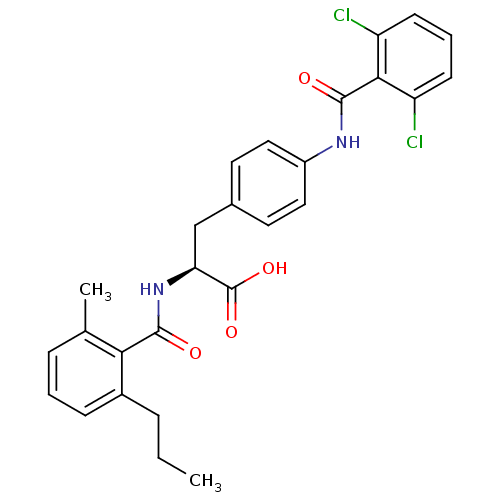
((S)-3-[4-(2,6-Dichloro-benzoylamino)-phenyl]-2-(2-...)Show SMILES CCCc1cccc(C)c1C(=O)N[C@@H](Cc1ccc(NC(=O)c2c(Cl)cccc2Cl)cc1)C(O)=O Show InChI InChI=1S/C27H26Cl2N2O4/c1-3-6-18-8-4-7-16(2)23(18)25(32)31-22(27(34)35)15-17-11-13-19(14-12-17)30-26(33)24-20(28)9-5-10-21(24)29/h4-5,7-14,22H,3,6,15H2,1-2H3,(H,30,33)(H,31,32)(H,34,35)/t22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibitory binding concentration against VCAM/VLA-4 in Ramos. |
Bioorg Med Chem Lett 12: 2479-82 (2002)
BindingDB Entry DOI: 10.7270/Q2V989C5 |
More data for this
Ligand-Target Pair | |
Integrin alpha-4/beta-1
(Homo sapiens (Human)) | BDBM50117000
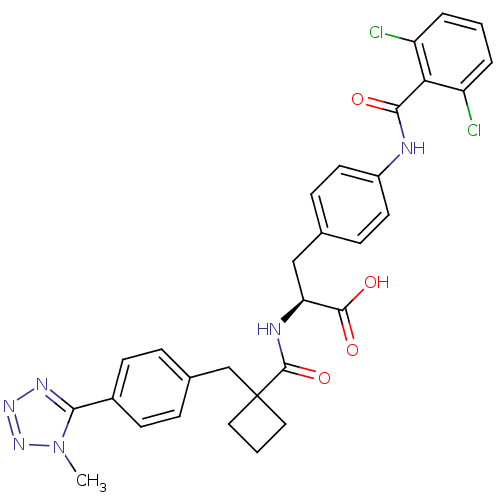
((S)-3-[4-(2,6-Dichloro-benzoylamino)-phenyl]-2-({1...)Show SMILES Cn1nnnc1-c1ccc(CC2(CCC2)C(=O)N[C@@H](Cc2ccc(NC(=O)c3c(Cl)cccc3Cl)cc2)C(O)=O)cc1 Show InChI InChI=1S/C30H28Cl2N6O4/c1-38-26(35-36-37-38)20-10-6-19(7-11-20)17-30(14-3-15-30)29(42)34-24(28(40)41)16-18-8-12-21(13-9-18)33-27(39)25-22(31)4-2-5-23(25)32/h2,4-13,24H,3,14-17H2,1H3,(H,33,39)(H,34,42)(H,40,41)/t24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibitory binding concentration determined against VCAM/VLA-4 in ELISA |
Bioorg Med Chem Lett 12: 2475-8 (2002)
BindingDB Entry DOI: 10.7270/Q2251HH8 |
More data for this
Ligand-Target Pair | |
Integrin alpha-4/beta-1
(Homo sapiens (Human)) | BDBM50117047
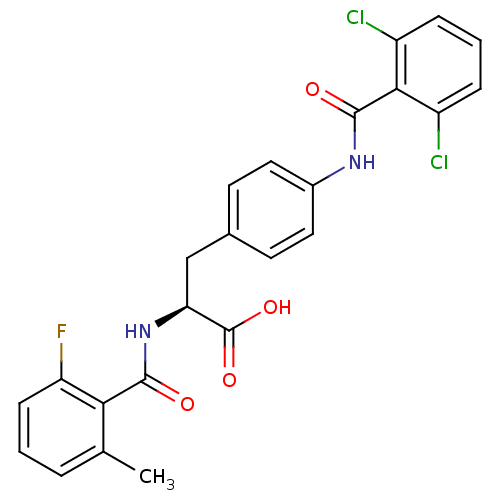
((S)-3-[4-(2,6-Dichloro-benzoylamino)-phenyl]-2-(2-...)Show SMILES Cc1cccc(F)c1C(=O)N[C@@H](Cc1ccc(NC(=O)c2c(Cl)cccc2Cl)cc1)C(O)=O Show InChI InChI=1S/C24H19Cl2FN2O4/c1-13-4-2-7-18(27)20(13)22(30)29-19(24(32)33)12-14-8-10-15(11-9-14)28-23(31)21-16(25)5-3-6-17(21)26/h2-11,19H,12H2,1H3,(H,28,31)(H,29,30)(H,32,33)/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibitory binding concentration against VCAM/VLA-4 in ELISA. |
Bioorg Med Chem Lett 12: 2479-82 (2002)
BindingDB Entry DOI: 10.7270/Q2V989C5 |
More data for this
Ligand-Target Pair | |
Integrin alpha-4/beta-1
(Homo sapiens (Human)) | BDBM50117074
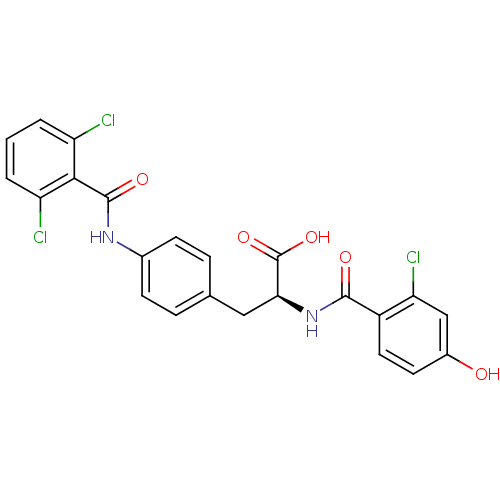
((S)-2-(2-Chloro-4-hydroxy-benzoylamino)-3-[4-(2,6-...)Show SMILES OC(=O)[C@H](Cc1ccc(NC(=O)c2c(Cl)cccc2Cl)cc1)NC(=O)c1ccc(O)cc1Cl Show InChI InChI=1S/C23H17Cl3N2O5/c24-16-2-1-3-17(25)20(16)22(31)27-13-6-4-12(5-7-13)10-19(23(32)33)28-21(30)15-9-8-14(29)11-18(15)26/h1-9,11,19,29H,10H2,(H,27,31)(H,28,30)(H,32,33)/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibitory binding concentration against VCAM/VLA-4 in ELISA. |
Bioorg Med Chem Lett 12: 2479-82 (2002)
BindingDB Entry DOI: 10.7270/Q2V989C5 |
More data for this
Ligand-Target Pair | |
Integrin alpha-4/beta-1
(Homo sapiens (Human)) | BDBM50117007
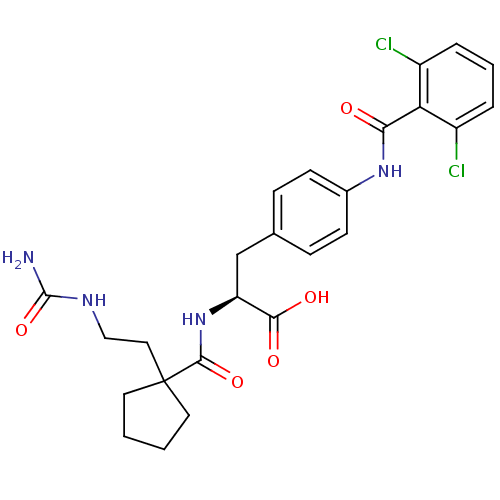
((S)-3-[4-(2,6-Dichloro-benzoylamino)-phenyl]-2-{[1...)Show SMILES NC(=O)NCCC1(CCCC1)C(=O)N[C@@H](Cc1ccc(NC(=O)c2c(Cl)cccc2Cl)cc1)C(O)=O Show InChI InChI=1S/C25H28Cl2N4O5/c26-17-4-3-5-18(27)20(17)21(32)30-16-8-6-15(7-9-16)14-19(22(33)34)31-23(35)25(10-1-2-11-25)12-13-29-24(28)36/h3-9,19H,1-2,10-14H2,(H,30,32)(H,31,35)(H,33,34)(H3,28,29,36)/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibitory binding concentration determined against VCAM/VLA-4 in ELISA |
Bioorg Med Chem Lett 12: 2475-8 (2002)
BindingDB Entry DOI: 10.7270/Q2251HH8 |
More data for this
Ligand-Target Pair | |
Integrin alpha-4/beta-1
(Homo sapiens (Human)) | BDBM50117034
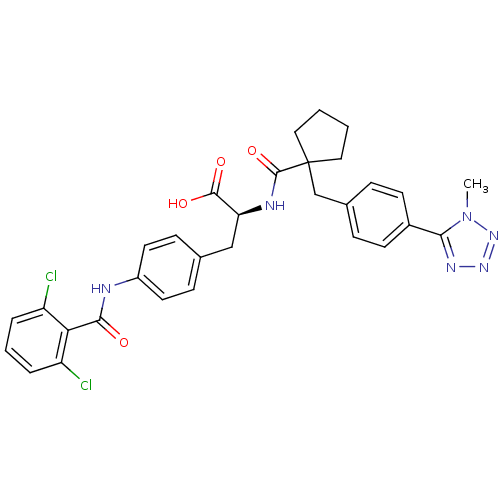
((S)-3-[4-(2,6-Dichloro-benzoylamino)-phenyl]-2-({1...)Show SMILES Cn1nnnc1-c1ccc(CC2(CCCC2)C(=O)N[C@@H](Cc2ccc(NC(=O)c3c(Cl)cccc3Cl)cc2)C(O)=O)cc1 Show InChI InChI=1S/C31H30Cl2N6O4/c1-39-27(36-37-38-39)21-11-7-20(8-12-21)18-31(15-2-3-16-31)30(43)35-25(29(41)42)17-19-9-13-22(14-10-19)34-28(40)26-23(32)5-4-6-24(26)33/h4-14,25H,2-3,15-18H2,1H3,(H,34,40)(H,35,43)(H,41,42)/t25-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibitory binding concentration determined against VCAM/VLA-4 in ELISA |
Bioorg Med Chem Lett 12: 2475-8 (2002)
BindingDB Entry DOI: 10.7270/Q2251HH8 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Bos taurus) | BDBM50003202
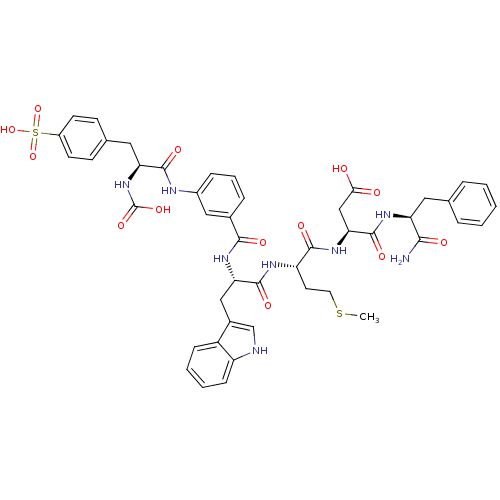
(CHEMBL331408 | N-(1-Carbamoyl-2-phenyl-ethyl)-3-{2...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)c1cccc(NC(=O)[C@H](Cc2ccc(cc2)S(O)(=O)=O)NC(O)=O)c1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C46H50N8O13S2/c1-68-19-18-34(42(59)53-38(24-39(55)56)45(62)51-35(40(47)57)20-26-8-3-2-4-9-26)50-44(61)37(23-29-25-48-33-13-6-5-12-32(29)33)52-41(58)28-10-7-11-30(22-28)49-43(60)36(54-46(63)64)21-27-14-16-31(17-15-27)69(65,66)67/h2-17,22,25,34-38,48,54H,18-21,23-24H2,1H3,(H2,47,57)(H,49,60)(H,50,61)(H,51,62)(H,52,58)(H,53,59)(H,55,56)(H,63,64)(H,65,66,67)/t34-,35-,36-,37-,38-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-propanoyl-CCK-8 binding to Cholecystokinin type B receptor in bovine striatum |
J Med Chem 35: 3774-83 (1992)
BindingDB Entry DOI: 10.7270/Q2CC0ZMG |
More data for this
Ligand-Target Pair | |
Integrin alpha-4/beta-1
(Homo sapiens (Human)) | BDBM50117044
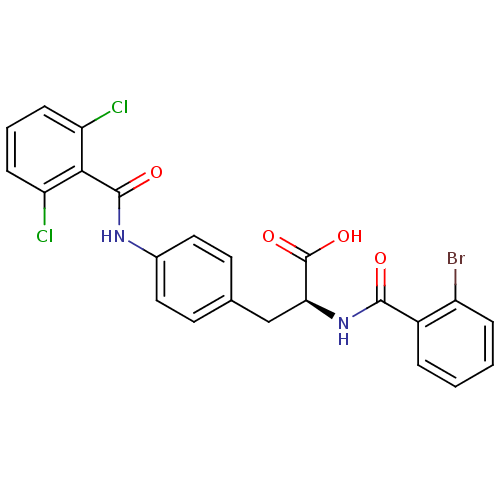
((S)-2-(2-Bromo-benzoylamino)-3-[4-(2,6-dichloro-be...)Show SMILES OC(=O)[C@H](Cc1ccc(NC(=O)c2c(Cl)cccc2Cl)cc1)NC(=O)c1ccccc1Br Show InChI InChI=1S/C23H17BrCl2N2O4/c24-16-5-2-1-4-15(16)21(29)28-19(23(31)32)12-13-8-10-14(11-9-13)27-22(30)20-17(25)6-3-7-18(20)26/h1-11,19H,12H2,(H,27,30)(H,28,29)(H,31,32)/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibitory binding concentration against VCAM/VLA-4 in Ramos. |
Bioorg Med Chem Lett 12: 2479-82 (2002)
BindingDB Entry DOI: 10.7270/Q2V989C5 |
More data for this
Ligand-Target Pair | |
Integrin alpha-4
(Homo sapiens (Human)) | BDBM50089019
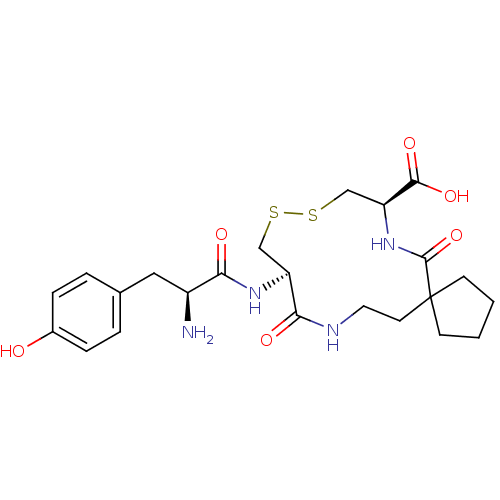
((8R,13R)-13-[(S)-2-Amino-3-(4-hydroxy-phenyl)-prop...)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@H]1CSSC[C@H](NC(=O)C2(CCCC2)CCNC1=O)C(O)=O Show InChI InChI=1S/C23H32N4O6S2/c24-16(11-14-3-5-15(28)6-4-14)19(29)26-17-12-34-35-13-18(21(31)32)27-22(33)23(7-1-2-8-23)9-10-25-20(17)30/h3-6,16-18,28H,1-2,7-13,24H2,(H,25,30)(H,26,29)(H,27,33)(H,31,32)/t16-,17-,18-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of VLA-4 binding to recombinant human VCAM using Solid phase assay |
Bioorg Med Chem Lett 10: 1171-3 (2000)
BindingDB Entry DOI: 10.7270/Q2Q81C9V |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
(Homo sapiens (Human)) | BDBM155016

(US9000044, 35)Show SMILES CCN(CC)S(=O)(=O)c1ccc(cc1)-c1c(C)c(CC(O)=O)cc2ccc(Cl)cc12 Show InChI InChI=1S/C23H24ClNO4S/c1-4-25(5-2)30(28,29)20-10-7-16(8-11-20)23-15(3)18(13-22(26)27)12-17-6-9-19(24)14-21(17)23/h6-12,14H,4-5,13H2,1-3H3,(H,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
US Patent
| Assay Description
Cell Culture Conditions: CHO-K1 cells previously transfected with G-alpha 16 were subsequently transfected with the human CRTH2 receptor and the neom... |
US Patent US9000044 (2015)
BindingDB Entry DOI: 10.7270/Q2319TM9 |
More data for this
Ligand-Target Pair | |
Integrin alpha-4/beta-1
(Homo sapiens (Human)) | BDBM50117017
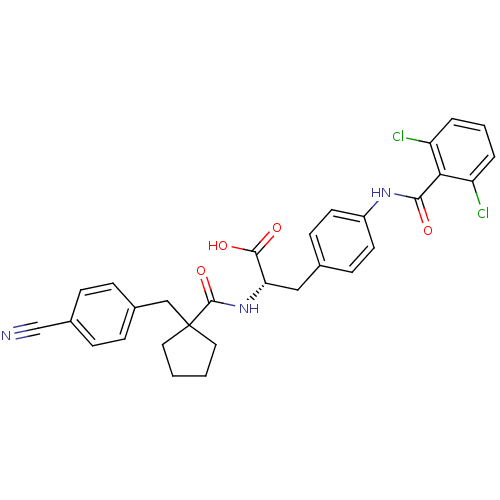
((S)-2-{[1-(4-Cyano-benzyl)-cyclopentanecarbonyl]-a...)Show SMILES OC(=O)[C@H](Cc1ccc(NC(=O)c2c(Cl)cccc2Cl)cc1)NC(=O)C1(Cc2ccc(cc2)C#N)CCCC1 Show InChI InChI=1S/C30H27Cl2N3O4/c31-23-4-3-5-24(32)26(23)27(36)34-22-12-10-19(11-13-22)16-25(28(37)38)35-29(39)30(14-1-2-15-30)17-20-6-8-21(18-33)9-7-20/h3-13,25H,1-2,14-17H2,(H,34,36)(H,35,39)(H,37,38)/t25-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibitory binding concentration determined against VCAM/VLA-4 in Ramos |
Bioorg Med Chem Lett 12: 2475-8 (2002)
BindingDB Entry DOI: 10.7270/Q2251HH8 |
More data for this
Ligand-Target Pair | |
Integrin alpha-4/beta-1
(Homo sapiens (Human)) | BDBM50117042
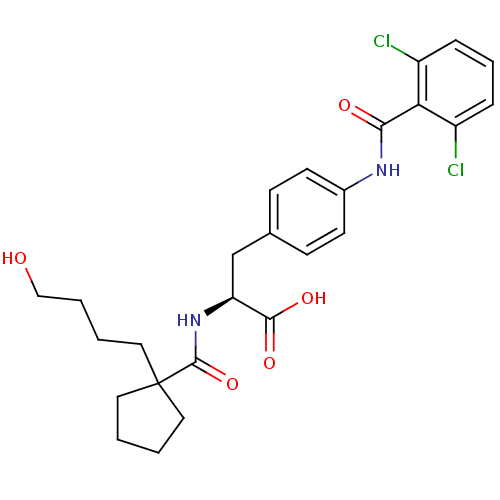
((S)-3-[4-(2,6-Dichloro-benzoylamino)-phenyl]-2-{[1...)Show SMILES OCCCCC1(CCCC1)C(=O)N[C@@H](Cc1ccc(NC(=O)c2c(Cl)cccc2Cl)cc1)C(O)=O Show InChI InChI=1S/C26H30Cl2N2O5/c27-19-6-5-7-20(28)22(19)23(32)29-18-10-8-17(9-11-18)16-21(24(33)34)30-25(35)26(12-1-2-13-26)14-3-4-15-31/h5-11,21,31H,1-4,12-16H2,(H,29,32)(H,30,35)(H,33,34)/t21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibitory binding concentration determined against VCAM/VLA-4 in ELISA |
Bioorg Med Chem Lett 12: 2475-8 (2002)
BindingDB Entry DOI: 10.7270/Q2251HH8 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data