 Found 6358 hits with Last Name = 'tino' and Initial = 'j'
Found 6358 hits with Last Name = 'tino' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM18050

(2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C20H22N8O5/c1-28(9-11-8-23-17-15(24-11)16(21)26-20(22)27-17)12-4-2-10(3-5-12)18(31)25-13(19(32)33)6-7-14(29)30/h2-5,8,13H,6-7,9H2,1H3,(H,25,31)(H,29,30)(H,32,33)(H4,21,22,23,26,27)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
PubMed
| 0.00120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory activity against Wild-type human DHFR |
J Med Chem 38: 745-52 (1995)
BindingDB Entry DOI: 10.7270/Q2JQ11PK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50026273
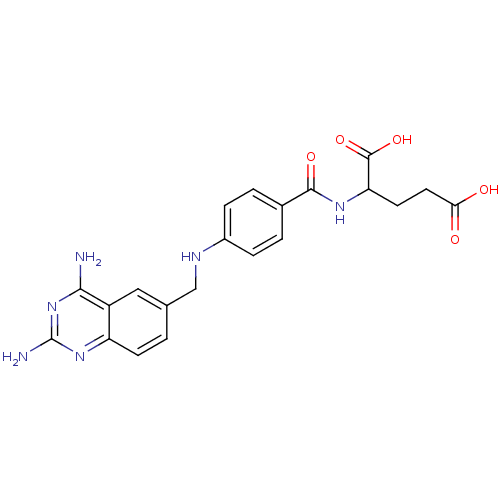
(2-{4-[(2,4-Diamino-quinazolin-6-ylmethyl)-amino]-b...)Show SMILES Nc1nc(N)c2cc(CNc3ccc(cc3)C(=O)NC(CCC(O)=O)C(O)=O)ccc2n1 Show InChI InChI=1S/C21H22N6O5/c22-18-14-9-11(1-6-15(14)26-21(23)27-18)10-24-13-4-2-12(3-5-13)19(30)25-16(20(31)32)7-8-17(28)29/h1-6,9,16,24H,7-8,10H2,(H,25,30)(H,28,29)(H,31,32)(H4,22,23,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory activity against Leu22-Phe mutant human Dihydrofolate reductase |
J Med Chem 38: 745-52 (1995)
BindingDB Entry DOI: 10.7270/Q2JQ11PK |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50026273

(2-{4-[(2,4-Diamino-quinazolin-6-ylmethyl)-amino]-b...)Show SMILES Nc1nc(N)c2cc(CNc3ccc(cc3)C(=O)NC(CCC(O)=O)C(O)=O)ccc2n1 Show InChI InChI=1S/C21H22N6O5/c22-18-14-9-11(1-6-15(14)26-21(23)27-18)10-24-13-4-2-12(3-5-13)19(30)25-16(20(31)32)7-8-17(28)29/h1-6,9,16,24H,7-8,10H2,(H,25,30)(H,28,29)(H,31,32)(H4,22,23,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory activity against Leu22-Phe mutant human Dihydrofolate reductase |
J Med Chem 38: 745-52 (1995)
BindingDB Entry DOI: 10.7270/Q2JQ11PK |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(BOVINE) | BDBM21173

(1,3-dipropyl-8-cyclopentylxanthine | 8-cyclopentyl...)Show InChI InChI=1S/C16H24N4O2/c1-3-9-19-14-12(15(21)20(10-4-2)16(19)22)17-13(18-14)11-7-5-6-8-11/h11H,3-10H2,1-2H3,(H,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Heidelberg
Curated by PDSP Ki Database
| |
Naunyn Schmiedebergs Arch Pharmacol 336: 204-10 (1987)
Article DOI: 10.1007/bf00165806
BindingDB Entry DOI: 10.7270/Q26H4FXN |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(BOVINE) | BDBM50207816
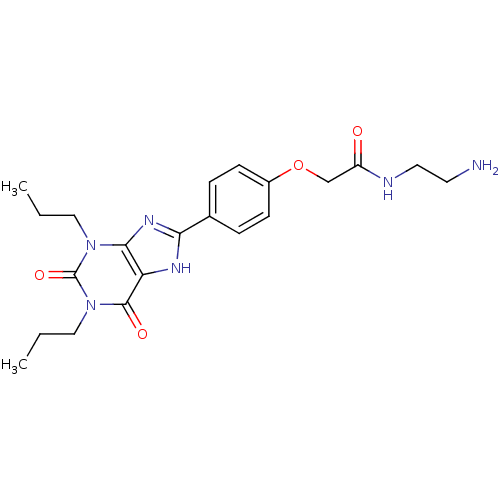
(CHEMBL273094 | N-(2-Amino-ethyl)-2-[4-(2,6-dioxo-1...)Show SMILES CCCn1c2nc([nH]c2c(=O)n(CCC)c1=O)-c1ccc(OCC(=O)NCCN)cc1 Show InChI InChI=1S/C21H28N6O4/c1-3-11-26-19-17(20(29)27(12-4-2)21(26)30)24-18(25-19)14-5-7-15(8-6-14)31-13-16(28)23-10-9-22/h5-8H,3-4,9-13,22H2,1-2H3,(H,23,28)(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Heidelberg
Curated by PDSP Ki Database
| |
Naunyn Schmiedebergs Arch Pharmacol 336: 204-10 (1987)
Article DOI: 10.1007/bf00165806
BindingDB Entry DOI: 10.7270/Q26H4FXN |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM18050

(2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C20H22N8O5/c1-28(9-11-8-23-17-15(24-11)16(21)26-20(22)27-17)12-4-2-10(3-5-12)18(31)25-13(19(32)33)6-7-14(29)30/h2-5,8,13H,6-7,9H2,1H3,(H,25,31)(H,29,30)(H,32,33)(H4,21,22,23,26,27)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory activity against Leu22-Phe mutant human Dihydrofolate reductase |
J Med Chem 38: 745-52 (1995)
BindingDB Entry DOI: 10.7270/Q2JQ11PK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenosine receptor A1
(BOVINE) | BDBM50207816

(CHEMBL273094 | N-(2-Amino-ethyl)-2-[4-(2,6-dioxo-1...)Show SMILES CCCn1c2nc([nH]c2c(=O)n(CCC)c1=O)-c1ccc(OCC(=O)NCCN)cc1 Show InChI InChI=1S/C21H28N6O4/c1-3-11-26-19-17(20(29)27(12-4-2)21(26)30)24-18(25-19)14-5-7-15(8-6-14)31-13-16(28)23-10-9-22/h5-8H,3-4,9-13,22H2,1-2H3,(H,23,28)(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Heidelberg
Curated by PDSP Ki Database
| |
Naunyn Schmiedebergs Arch Pharmacol 336: 204-10 (1987)
Article DOI: 10.1007/bf00165806
BindingDB Entry DOI: 10.7270/Q26H4FXN |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50367055

(4-Aminofolic acid | 4-Aminopteroic acid | AMINOPTE...)Show SMILES Nc1nc(N)c2nc(CNc3ccc(cc3)C(=O)N[C@@H](CCC(O)=O)C(O)=O)cnc2n1 |r| Show InChI InChI=1S/C19H20N8O5/c20-15-14-16(27-19(21)26-15)23-8-11(24-14)7-22-10-3-1-9(2-4-10)17(30)25-12(18(31)32)5-6-13(28)29/h1-4,8,12,22H,5-7H2,(H,25,30)(H,28,29)(H,31,32)(H4,20,21,23,26,27)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory activity against Leu22-Phe mutant human Dihydrofolate reductase |
J Med Chem 38: 745-52 (1995)
BindingDB Entry DOI: 10.7270/Q2JQ11PK |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50367055

(4-Aminofolic acid | 4-Aminopteroic acid | AMINOPTE...)Show SMILES Nc1nc(N)c2nc(CNc3ccc(cc3)C(=O)N[C@@H](CCC(O)=O)C(O)=O)cnc2n1 |r| Show InChI InChI=1S/C19H20N8O5/c20-15-14-16(27-19(21)26-15)23-8-11(24-14)7-22-10-3-1-9(2-4-10)17(30)25-12(18(31)32)5-6-13(28)29/h1-4,8,12,22H,5-7H2,(H,25,30)(H,28,29)(H,31,32)(H4,20,21,23,26,27)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory activity against Leu22-Phe mutant human Dihydrofolate reductase |
J Med Chem 38: 745-52 (1995)
BindingDB Entry DOI: 10.7270/Q2JQ11PK |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Rattus norvegicus (rat)) | BDBM21173

(1,3-dipropyl-8-cyclopentylxanthine | 8-cyclopentyl...)Show InChI InChI=1S/C16H24N4O2/c1-3-9-19-14-12(15(21)20(10-4-2)16(19)22)17-13(18-14)11-7-5-6-8-11/h11H,3-10H2,1-2H3,(H,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Heidelberg
Curated by PDSP Ki Database
| |
Naunyn Schmiedebergs Arch Pharmacol 336: 204-10 (1987)
Article DOI: 10.1007/bf00165806
BindingDB Entry DOI: 10.7270/Q26H4FXN |
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1 [30-579]
(Rattus norvegicus (rat)) | BDBM50204512
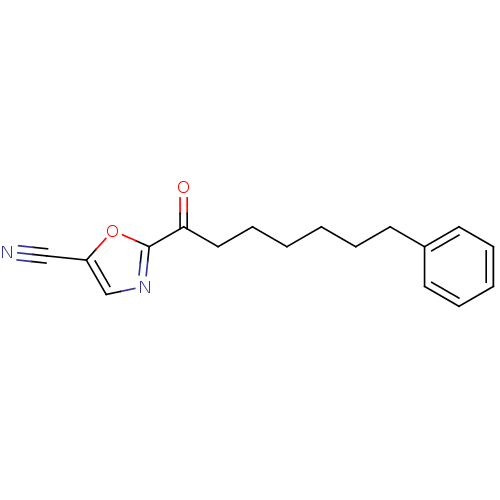
(2-(7-phenylheptanoyl)oxazole-5-carbonitrile | CHEM...)Show InChI InChI=1S/C17H18N2O2/c18-12-15-13-19-17(21-15)16(20)11-7-2-1-4-8-14-9-5-3-6-10-14/h3,5-6,9-10,13H,1-2,4,7-8,11H2 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of rat FAAH |
Bioorg Med Chem Lett 18: 5842-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.084
BindingDB Entry DOI: 10.7270/Q2DN44V8 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Rattus norvegicus (rat)) | BDBM21173

(1,3-dipropyl-8-cyclopentylxanthine | 8-cyclopentyl...)Show InChI InChI=1S/C16H24N4O2/c1-3-9-19-14-12(15(21)20(10-4-2)16(19)22)17-13(18-14)11-7-5-6-8-11/h11H,3-10H2,1-2H3,(H,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Heidelberg
Curated by PDSP Ki Database
| |
Naunyn Schmiedebergs Arch Pharmacol 336: 204-10 (1987)
Article DOI: 10.1007/bf00165806
BindingDB Entry DOI: 10.7270/Q26H4FXN |
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1 [30-579]
(Rattus norvegicus (rat)) | BDBM50247136

(2-(7-phenylheptanoyl)oxazole-4-carbonitrile | CHEM...)Show InChI InChI=1S/C17H18N2O2/c18-12-15-13-21-17(19-15)16(20)11-7-2-1-4-8-14-9-5-3-6-10-14/h3,5-6,9-10,13H,1-2,4,7-8,11H2 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of rat FAAH |
Bioorg Med Chem Lett 18: 5842-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.084
BindingDB Entry DOI: 10.7270/Q2DN44V8 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50036495
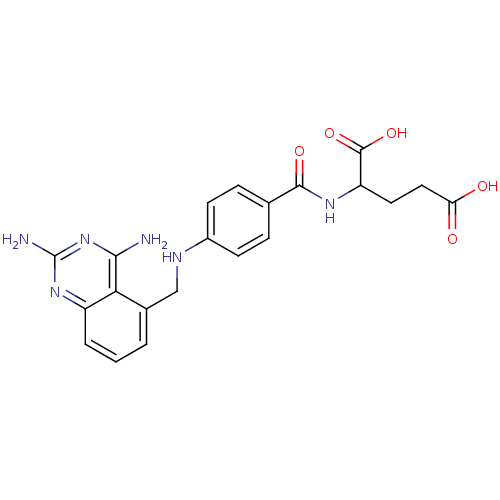
(2-{4-[(2,4-Diamino-quinazolin-5-ylmethyl)-amino]-b...)Show SMILES Nc1nc(N)c2c(CNc3ccc(cc3)C(=O)NC(CCC(O)=O)C(O)=O)cccc2n1 Show InChI InChI=1S/C21H22N6O5/c22-18-17-12(2-1-3-14(17)26-21(23)27-18)10-24-13-6-4-11(5-7-13)19(30)25-15(20(31)32)8-9-16(28)29/h1-7,15,24H,8-10H2,(H,25,30)(H,28,29)(H,31,32)(H4,22,23,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory activity against Wild-type human DHFR |
J Med Chem 38: 745-52 (1995)
BindingDB Entry DOI: 10.7270/Q2JQ11PK |
More data for this
Ligand-Target Pair | |
Motilin receptor
(Homo sapiens (Human)) | BDBM50143028

(8'N-[1-[1-carbamoyl-3-phenyl-(1S)-propylcarbamoyl]...)Show SMILES NC(=O)[C@H](CCc1ccccc1)NC(=O)[C@H](CCc1ccccc1)NC(=O)C1CCC2(CCNCC2)n2n1c(=O)n(Cc1ccc3OCOc3c1)c2=O Show InChI InChI=1S/C39H45N7O7/c40-34(47)29(14-11-26-7-3-1-4-8-26)42-35(48)30(15-12-27-9-5-2-6-10-27)43-36(49)31-17-18-39(19-21-41-22-20-39)46-38(51)44(37(50)45(31)46)24-28-13-16-32-33(23-28)53-25-52-32/h1-10,13,16,23,29-31,41H,11-12,14-15,17-22,24-25H2,(H2,40,47)(H,42,48)(H,43,49)/t29-,30-,31?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards human motilin receptor |
J Med Chem 47: 1704-8 (2004)
Article DOI: 10.1021/jm0304865
BindingDB Entry DOI: 10.7270/Q2571CRC |
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1 [30-579]
(Rattus norvegicus (rat)) | BDBM50204483
(7-phenyl-1-(5-(trifluoromethyl)oxazol-2-yl)heptan-...)Show InChI InChI=1S/C17H18F3NO2/c18-17(19,20)15-12-21-16(23-15)14(22)11-7-2-1-4-8-13-9-5-3-6-10-13/h3,5-6,9-10,12H,1-2,4,7-8,11H2 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of rat FAAH |
Bioorg Med Chem Lett 18: 5842-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.084
BindingDB Entry DOI: 10.7270/Q2DN44V8 |
More data for this
Ligand-Target Pair | |
Motilin receptor
(Homo sapiens (Human)) | BDBM50143032
(8'N-[1-(1-carbamoyl-3-phenylpropylcarbamoyl)-3-phe...)Show SMILES NC(=O)C(CCc1ccccc1)NC(=O)C(CCc1ccccc1)NC(=O)C1CCC2(CCNCC2)n2n1c(=O)n(Cc1ccc3OCOc3c1)c2=O Show InChI InChI=1S/C39H45N7O7/c40-34(47)29(14-11-26-7-3-1-4-8-26)42-35(48)30(15-12-27-9-5-2-6-10-27)43-36(49)31-17-18-39(19-21-41-22-20-39)46-38(51)44(37(50)45(31)46)24-28-13-16-32-33(23-28)53-25-52-32/h1-10,13,16,23,29-31,41H,11-12,14-15,17-22,24-25H2,(H2,40,47)(H,42,48)(H,43,49) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards human motilin receptor |
J Med Chem 47: 1704-8 (2004)
Article DOI: 10.1021/jm0304865
BindingDB Entry DOI: 10.7270/Q2571CRC |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50088301

((E)-N,N-dimethyl-N-(4-(2-p-tolyl-6,7-dihydro-5H-be...)Show SMILES Cc1ccc(cc1)-c1ccc2CCCC(=Cc2c1)C(=O)Nc1ccc(C[N+](C)(C)C2CCOCC2)cc1 |c:15| Show InChI InChI=1S/C33H38N2O2/c1-24-7-11-27(12-8-24)28-14-13-26-5-4-6-29(22-30(26)21-28)33(36)34-31-15-9-25(10-16-31)23-35(2,3)32-17-19-37-20-18-32/h7-16,21-22,32H,4-6,17-20,23H2,1-3H3/p+1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against specific binding of [125I]-MIP-1 alpha to human CCR5 receptor |
Bioorg Med Chem Lett 11: 265-70 (2001)
BindingDB Entry DOI: 10.7270/Q2668CFZ |
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1 [30-579]
(Rattus norvegicus (rat)) | BDBM50247103

(7-phenyl-1-(4-(pyridin-4-yl)oxazol-2-yl)heptan-1-o...)Show InChI InChI=1S/C21H22N2O2/c24-20(11-7-2-1-4-8-17-9-5-3-6-10-17)21-23-19(16-25-21)18-12-14-22-15-13-18/h3,5-6,9-10,12-16H,1-2,4,7-8,11H2 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of rat FAAH |
Bioorg Med Chem Lett 18: 5842-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.084
BindingDB Entry DOI: 10.7270/Q2DN44V8 |
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1 [30-579]
(Rattus norvegicus (rat)) | BDBM50247106

(2-(7-phenylheptanoyl)oxazole-4-carboxamide | CHEMB...)Show InChI InChI=1S/C17H20N2O3/c18-16(21)14-12-22-17(19-14)15(20)11-7-2-1-4-8-13-9-5-3-6-10-13/h3,5-6,9-10,12H,1-2,4,7-8,11H2,(H2,18,21) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of rat FAAH |
Bioorg Med Chem Lett 18: 5842-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.084
BindingDB Entry DOI: 10.7270/Q2DN44V8 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(BOVINE) | BDBM81971
(1,3-Diethyl-8-phenyl-3,7-dihydro-purine-2,6-dione ...)Show InChI InChI=1S/C15H16N4O2/c1-3-18-13-11(14(20)19(4-2)15(18)21)16-12(17-13)10-8-6-5-7-9-10/h5-9H,3-4H2,1-2H3,(H,16,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Heidelberg
Curated by PDSP Ki Database
| |
Naunyn Schmiedebergs Arch Pharmacol 336: 204-10 (1987)
Article DOI: 10.1007/bf00165806
BindingDB Entry DOI: 10.7270/Q26H4FXN |
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1 [30-579]
(Rattus norvegicus (rat)) | BDBM50247107

(CHEMBL460493 | N-methyl-2-(7-phenylheptanoyl)oxazo...)Show InChI InChI=1S/C18H22N2O3/c1-19-17(22)15-13-23-18(20-15)16(21)12-8-3-2-5-9-14-10-6-4-7-11-14/h4,6-7,10-11,13H,2-3,5,8-9,12H2,1H3,(H,19,22) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of rat FAAH |
Bioorg Med Chem Lett 18: 5842-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.084
BindingDB Entry DOI: 10.7270/Q2DN44V8 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50036484
(5-((E)-Styryl)-quinazoline-2,4-diamine | CHEMBL164...)Show InChI InChI=1S/C16H14N4/c17-15-14-12(10-9-11-5-2-1-3-6-11)7-4-8-13(14)19-16(18)20-15/h1-10H,(H4,17,18,19,20)/b10-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory activity against Leu22-Phe mutant human Dihydrofolate reductase |
J Med Chem 38: 745-52 (1995)
BindingDB Entry DOI: 10.7270/Q2JQ11PK |
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1 [30-579]
(Rattus norvegicus (rat)) | BDBM50247074
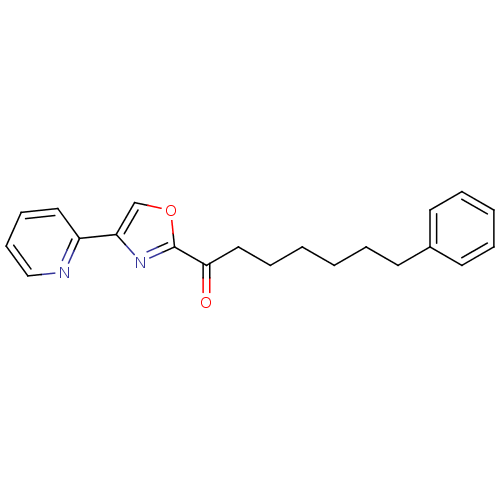
(7-phenyl-1-(4-(pyridin-2-yl)oxazol-2-yl)heptan-1-o...)Show InChI InChI=1S/C21H22N2O2/c24-20(14-7-2-1-4-10-17-11-5-3-6-12-17)21-23-19(16-25-21)18-13-8-9-15-22-18/h3,5-6,8-9,11-13,15-16H,1-2,4,7,10,14H2 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of rat FAAH |
Bioorg Med Chem Lett 18: 5842-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.084
BindingDB Entry DOI: 10.7270/Q2DN44V8 |
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1 [30-579]
(Rattus norvegicus (rat)) | BDBM50247139
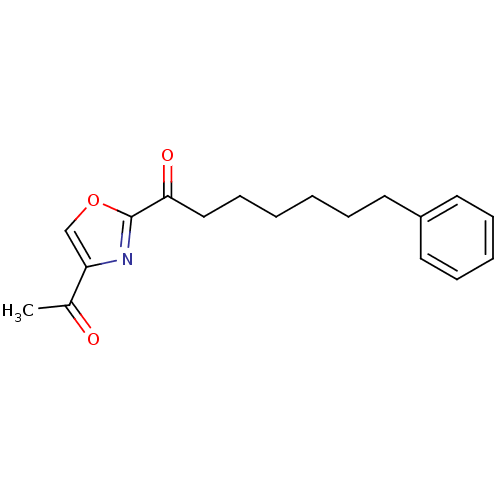
(1-(4-acetyloxazol-2-yl)-7-phenylheptan-1-one | CHE...)Show InChI InChI=1S/C18H21NO3/c1-14(20)16-13-22-18(19-16)17(21)12-8-3-2-5-9-15-10-6-4-7-11-15/h4,6-7,10-11,13H,2-3,5,8-9,12H2,1H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of rat FAAH |
Bioorg Med Chem Lett 18: 5842-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.084
BindingDB Entry DOI: 10.7270/Q2DN44V8 |
More data for this
Ligand-Target Pair | |
Motilin receptor
(Homo sapiens (Human)) | BDBM50143038
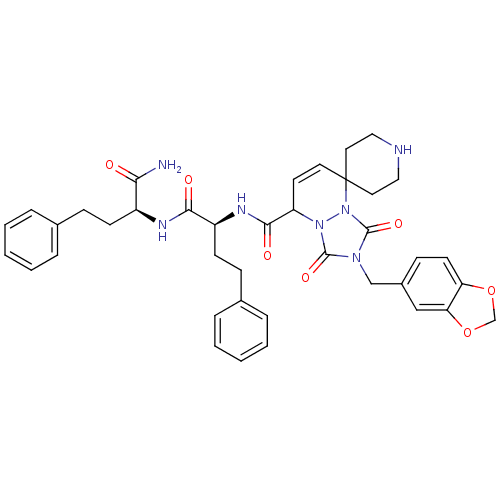
(8'N-[1-[1-carbamoyl-3-phenyl-(1S)-propylcarbamoyl]...)Show SMILES NC(=O)[C@H](CCc1ccccc1)NC(=O)[C@H](CCc1ccccc1)NC(=O)C1C=CC2(CCNCC2)n2n1c(=O)n(Cc1ccc3OCOc3c1)c2=O |c:30| Show InChI InChI=1S/C39H43N7O7/c40-34(47)29(14-11-26-7-3-1-4-8-26)42-35(48)30(15-12-27-9-5-2-6-10-27)43-36(49)31-17-18-39(19-21-41-22-20-39)46-38(51)44(37(50)45(31)46)24-28-13-16-32-33(23-28)53-25-52-32/h1-10,13,16-18,23,29-31,41H,11-12,14-15,19-22,24-25H2,(H2,40,47)(H,42,48)(H,43,49)/t29-,30-,31?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards human motilin receptor |
J Med Chem 47: 1704-8 (2004)
Article DOI: 10.1021/jm0304865
BindingDB Entry DOI: 10.7270/Q2571CRC |
More data for this
Ligand-Target Pair | |
Motilin receptor
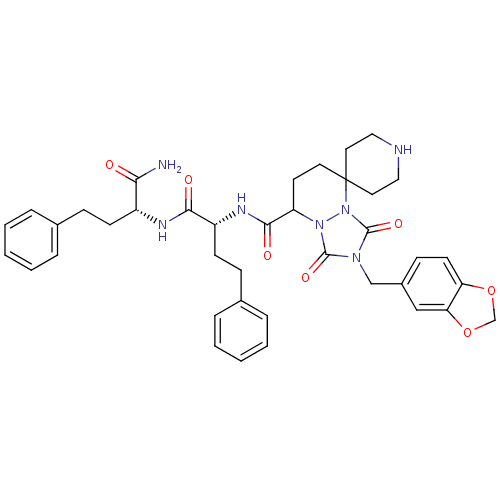
(Homo sapiens (Human)) | BDBM50143039
(8'N-[1-[1-carbamoyl-3-phenyl-(1R)-propylcarbamoyl]...)Show SMILES NC(=O)[C@@H](CCc1ccccc1)NC(=O)[C@@H](CCc1ccccc1)NC(=O)C1CCC2(CCNCC2)n2n1c(=O)n(Cc1ccc3OCOc3c1)c2=O Show InChI InChI=1S/C39H45N7O7/c40-34(47)29(14-11-26-7-3-1-4-8-26)42-35(48)30(15-12-27-9-5-2-6-10-27)43-36(49)31-17-18-39(19-21-41-22-20-39)46-38(51)44(37(50)45(31)46)24-28-13-16-32-33(23-28)53-25-52-32/h1-10,13,16,23,29-31,41H,11-12,14-15,17-22,24-25H2,(H2,40,47)(H,42,48)(H,43,49)/t29-,30-,31?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards human motilin receptor |
J Med Chem 47: 1704-8 (2004)
Article DOI: 10.1021/jm0304865
BindingDB Entry DOI: 10.7270/Q2571CRC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50036495

(2-{4-[(2,4-Diamino-quinazolin-5-ylmethyl)-amino]-b...)Show SMILES Nc1nc(N)c2c(CNc3ccc(cc3)C(=O)NC(CCC(O)=O)C(O)=O)cccc2n1 Show InChI InChI=1S/C21H22N6O5/c22-18-17-12(2-1-3-14(17)26-21(23)27-18)10-24-13-6-4-11(5-7-13)19(30)25-15(20(31)32)8-9-16(28)29/h1-7,15,24H,8-10H2,(H,25,30)(H,28,29)(H,31,32)(H4,22,23,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory activity against Leu22-Phe mutant human Dihydrofolate reductase |
J Med Chem 38: 745-52 (1995)
BindingDB Entry DOI: 10.7270/Q2JQ11PK |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50036483
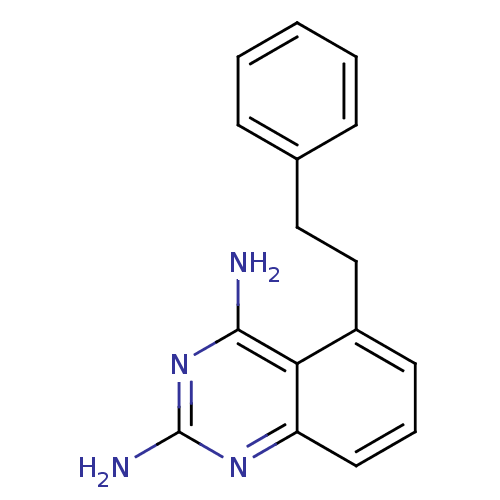
(5-Phenethyl-quinazoline-2,4-diamine | CHEMBL341703)Show InChI InChI=1S/C16H16N4/c17-15-14-12(10-9-11-5-2-1-3-6-11)7-4-8-13(14)19-16(18)20-15/h1-8H,9-10H2,(H4,17,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory activity against Leu22-Phe mutant human Dihydrofolate reductase |
J Med Chem 38: 745-52 (1995)
BindingDB Entry DOI: 10.7270/Q2JQ11PK |
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1 [30-579]
(Rattus norvegicus (rat)) | BDBM50247035

(1-(4-bromooxazol-2-yl)-7-phenylheptan-1-one | CHEM...)Show InChI InChI=1S/C16H18BrNO2/c17-15-12-20-16(18-15)14(19)11-7-2-1-4-8-13-9-5-3-6-10-13/h3,5-6,9-10,12H,1-2,4,7-8,11H2 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of rat FAAH |
Bioorg Med Chem Lett 18: 5842-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.084
BindingDB Entry DOI: 10.7270/Q2DN44V8 |
More data for this
Ligand-Target Pair | |
Motilin receptor
(Homo sapiens (Human)) | BDBM50143037
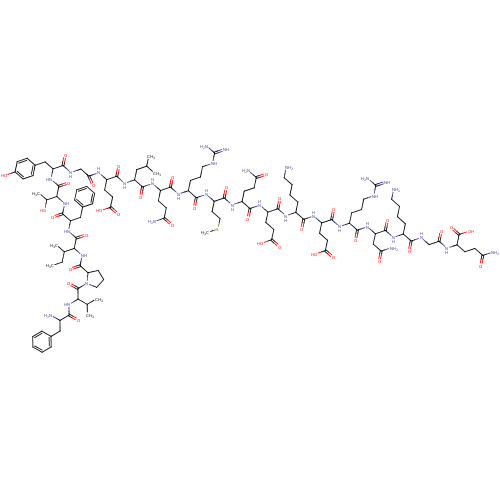
(CHEMBL411576 | MOTILIN)Show SMILES CCC(C)C(NC(=O)C1CCCN1C(=O)C(NC(=O)C(N)Cc1ccccc1)C(C)C)C(=O)NC(Cc1ccccc1)C(=O)NC(C(C)O)C(=O)NC(Cc1ccc(O)cc1)C(=O)NCC(=O)NC(CCC(O)=O)C(=O)NC(CC(C)C)C(=O)NC(CCC(N)=O)C(=O)NC(CCCNC(N)=N)C(=O)NC(CCSC)C(=O)NC(CCC(N)=O)C(=O)NC(CCC(O)=O)C(=O)NC(CCCCN)C(=O)NC(CCC(O)=O)C(=O)NC(CCCNC(N)=N)C(=O)NC(CC(N)=O)C(=O)NC(CCCCN)C(=O)NCC(=O)NC(CCC(N)=O)C(O)=O Show InChI InChI=1S/C120H188N34O35S/c1-9-64(6)97(152-114(184)86-31-22-53-154(86)117(187)96(63(4)5)151-99(169)70(123)56-66-23-12-10-13-24-66)115(185)150-84(57-67-25-14-11-15-26-67)113(183)153-98(65(7)155)116(186)149-83(58-68-32-34-69(156)35-33-68)101(171)135-60-91(161)136-75(39-45-93(163)164)105(175)147-82(55-62(2)3)111(181)145-77(37-43-88(125)158)106(176)140-73(29-20-51-132-119(128)129)103(173)146-80(48-54-190-8)110(180)142-76(36-42-87(124)157)107(177)144-79(41-47-95(167)168)108(178)139-72(28-17-19-50-122)102(172)143-78(40-46-94(165)166)109(179)141-74(30-21-52-133-120(130)131)104(174)148-85(59-90(127)160)112(182)138-71(27-16-18-49-121)100(170)134-61-92(162)137-81(118(188)189)38-44-89(126)159/h10-15,23-26,32-35,62-65,70-86,96-98,155-156H,9,16-22,27-31,36-61,121-123H2,1-8H3,(H2,124,157)(H2,125,158)(H2,126,159)(H2,127,160)(H,134,170)(H,135,171)(H,136,161)(H,137,162)(H,138,182)(H,139,178)(H,140,176)(H,141,179)(H,142,180)(H,143,172)(H,144,177)(H,145,181)(H,146,173)(H,147,175)(H,148,174)(H,149,186)(H,150,185)(H,151,169)(H,152,184)(H,153,183)(H,163,164)(H,165,166)(H,167,168)(H,188,189)(H4,128,129,132)(H4,130,131,133) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards human motilin receptor |
J Med Chem 47: 1704-8 (2004)
Article DOI: 10.1021/jm0304865
BindingDB Entry DOI: 10.7270/Q2571CRC |
More data for this
Ligand-Target Pair | |
Motilin receptor
(Homo sapiens (Human)) | BDBM50344952
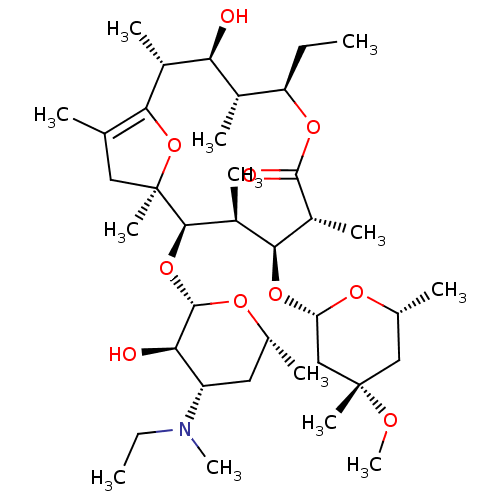
((2R,3S,4R,5R,8R,9S,10S,11R,12S)-5-ethyl-11-((2S,3R...)Show SMILES CC[C@H]1OC(=O)[C@H](C)[C@@H](O[C@H]2C[C@](C)(C[C@@H](C)O2)OC)[C@H](C)[C@@H](O[C@@H]2O[C@H](C)C[C@@H]([C@H]2O)N(C)CC)[C@@]2(C)CC(C)=C(O2)[C@H](C)[C@@H](O)[C@H]1C |wU:24.24,8.8,29.33,36.38,6.6,20.21,2.1,12.12,26.27,wD:22.23,10.9,30.32,45.48,12.18,43.46,47.51,15.15,c:42,(6.35,-6.68,;6.37,-5.14,;7.71,-4.39,;7.7,-5.93,;9.03,-6.7,;9.01,-8.24,;10.37,-5.93,;11.7,-6.7,;10.37,-4.39,;11.7,-5.16,;13.03,-5.93,;13.03,-7.47,;14.35,-8.24,;13.56,-9.57,;15.69,-7.47,;15.69,-5.95,;17.02,-5.16,;14.36,-5.16,;15.1,-9.57,;16.64,-9.57,;11.71,-3.64,;13.04,-4.42,;11.72,-2.1,;13.05,-1.34,;14.38,-.56,;15.71,-1.31,;17.04,-.56,;18.37,-1.33,;17.04,.98,;15.71,1.77,;14.38,.98,;13.05,1.75,;15.71,3.31,;17.04,4.08,;14.38,4.08,;14.38,5.62,;10.39,-1.31,;11.72,-.54,;10.39,.23,;9.06,1,;9.08,2.54,;7.73,.23,;8.82,-.85,;7.72,-1.31,;6.38,-.54,;6.39,-2.08,;5.06,-1.29,;6.38,-3.62,;5.04,-4.35,)| Show InChI InChI=1S/C38H67NO10/c1-14-28-23(6)30(40)24(7)32-20(3)17-38(11,49-32)34(48-36-31(41)27(39(12)15-2)16-21(4)45-36)25(8)33(26(9)35(42)46-28)47-29-19-37(10,43-13)18-22(5)44-29/h21-31,33-34,36,40-41H,14-19H2,1-13H3/t21-,22-,23+,24-,25+,26-,27+,28-,29+,30+,31-,33+,34-,36+,37+,38-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards human motilin receptor |
J Med Chem 47: 1704-8 (2004)
Article DOI: 10.1021/jm0304865
BindingDB Entry DOI: 10.7270/Q2571CRC |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(BOVINE) | BDBM81971

(1,3-Diethyl-8-phenyl-3,7-dihydro-purine-2,6-dione ...)Show InChI InChI=1S/C15H16N4O2/c1-3-18-13-11(14(20)19(4-2)15(18)21)16-12(17-13)10-8-6-5-7-9-10/h5-9H,3-4H2,1-2H3,(H,16,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Heidelberg
Curated by PDSP Ki Database
| |
Naunyn Schmiedebergs Arch Pharmacol 336: 204-10 (1987)
Article DOI: 10.1007/bf00165806
BindingDB Entry DOI: 10.7270/Q26H4FXN |
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1 [30-579]
(Rattus norvegicus (rat)) | BDBM50247073
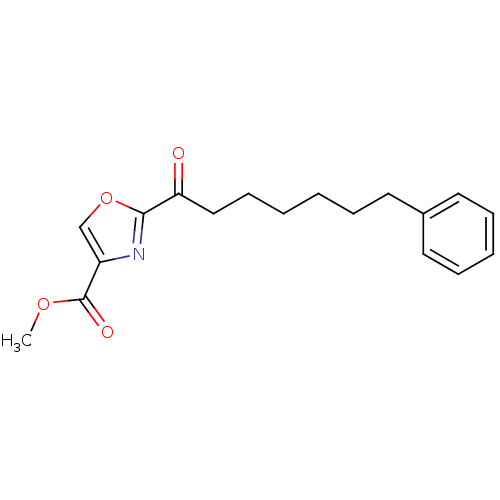
(CHEMBL461539 | methyl 2-(7-phenylheptanoyl)oxazole...)Show InChI InChI=1S/C18H21NO4/c1-22-18(21)15-13-23-17(19-15)16(20)12-8-3-2-5-9-14-10-6-4-7-11-14/h4,6-7,10-11,13H,2-3,5,8-9,12H2,1H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of rat FAAH |
Bioorg Med Chem Lett 18: 5842-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.084
BindingDB Entry DOI: 10.7270/Q2DN44V8 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Rattus norvegicus (rat)) | BDBM50207816

(CHEMBL273094 | N-(2-Amino-ethyl)-2-[4-(2,6-dioxo-1...)Show SMILES CCCn1c2nc([nH]c2c(=O)n(CCC)c1=O)-c1ccc(OCC(=O)NCCN)cc1 Show InChI InChI=1S/C21H28N6O4/c1-3-11-26-19-17(20(29)27(12-4-2)21(26)30)24-18(25-19)14-5-7-15(8-6-14)31-13-16(28)23-10-9-22/h5-8H,3-4,9-13,22H2,1-2H3,(H,23,28)(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Heidelberg
Curated by PDSP Ki Database
| |
Naunyn Schmiedebergs Arch Pharmacol 336: 204-10 (1987)
Article DOI: 10.1007/bf00165806
BindingDB Entry DOI: 10.7270/Q26H4FXN |
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1 [30-579]
(Rattus norvegicus (rat)) | BDBM50247137
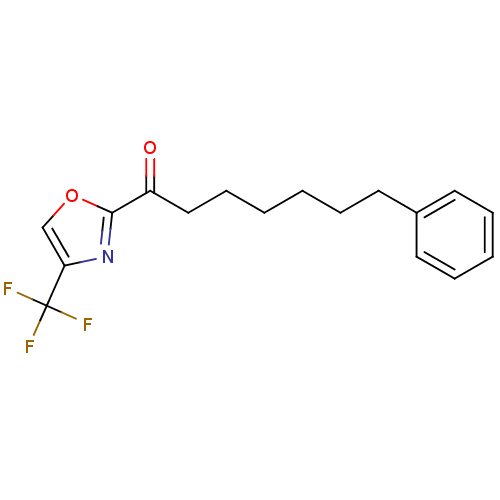
(7-phenyl-1-(4-(trifluoromethyl)oxazol-2-yl)heptan-...)Show InChI InChI=1S/C17H18F3NO2/c18-17(19,20)15-12-23-16(21-15)14(22)11-7-2-1-4-8-13-9-5-3-6-10-13/h3,5-6,9-10,12H,1-2,4,7-8,11H2 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of rat FAAH |
Bioorg Med Chem Lett 18: 5842-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.084
BindingDB Entry DOI: 10.7270/Q2DN44V8 |
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1 [30-579]
(Rattus norvegicus (rat)) | BDBM50247036
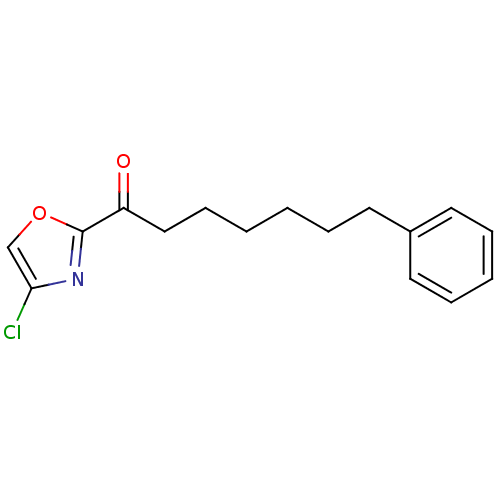
(1-(4-chlorooxazol-2-yl)-7-phenylheptan-1-one | CHE...)Show InChI InChI=1S/C16H18ClNO2/c17-15-12-20-16(18-15)14(19)11-7-2-1-4-8-13-9-5-3-6-10-13/h3,5-6,9-10,12H,1-2,4,7-8,11H2 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of rat FAAH |
Bioorg Med Chem Lett 18: 5842-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.084
BindingDB Entry DOI: 10.7270/Q2DN44V8 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50036491
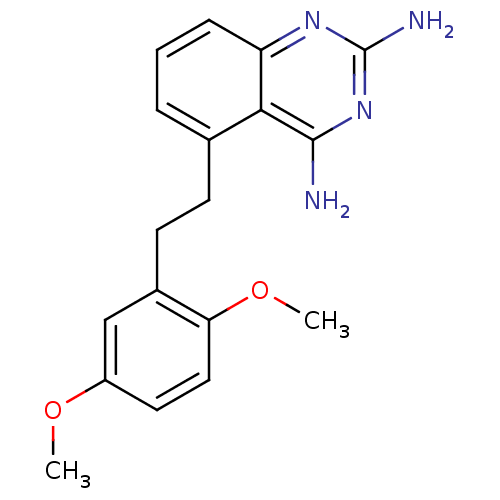
(5-[2-(2,5-Dimethoxy-phenyl)-ethyl]-quinazoline-2,4...)Show InChI InChI=1S/C18H20N4O2/c1-23-13-8-9-15(24-2)12(10-13)7-6-11-4-3-5-14-16(11)17(19)22-18(20)21-14/h3-5,8-10H,6-7H2,1-2H3,(H4,19,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory activity against Leu22-Phe mutant human Dihydrofolate reductase |
J Med Chem 38: 745-52 (1995)
BindingDB Entry DOI: 10.7270/Q2JQ11PK |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50036486
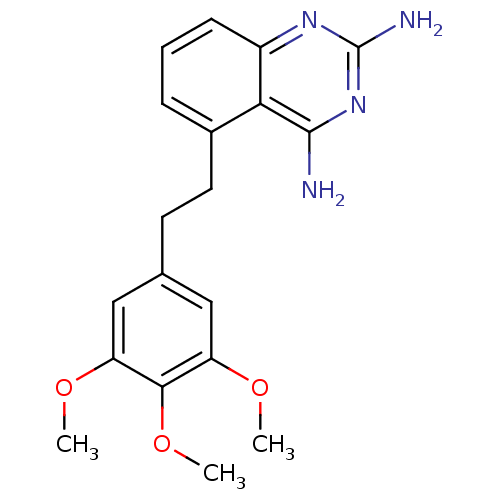
(5-[2-(3,4,5-Trimethoxy-phenyl)-ethyl]-quinazoline-...)Show InChI InChI=1S/C19H22N4O3/c1-24-14-9-11(10-15(25-2)17(14)26-3)7-8-12-5-4-6-13-16(12)18(20)23-19(21)22-13/h4-6,9-10H,7-8H2,1-3H3,(H4,20,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory activity against Leu22-Phe mutant human Dihydrofolate reductase |
J Med Chem 38: 745-52 (1995)
BindingDB Entry DOI: 10.7270/Q2JQ11PK |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Rattus norvegicus (rat)) | BDBM50207816

(CHEMBL273094 | N-(2-Amino-ethyl)-2-[4-(2,6-dioxo-1...)Show SMILES CCCn1c2nc([nH]c2c(=O)n(CCC)c1=O)-c1ccc(OCC(=O)NCCN)cc1 Show InChI InChI=1S/C21H28N6O4/c1-3-11-26-19-17(20(29)27(12-4-2)21(26)30)24-18(25-19)14-5-7-15(8-6-14)31-13-16(28)23-10-9-22/h5-8H,3-4,9-13,22H2,1-2H3,(H,23,28)(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Heidelberg
Curated by PDSP Ki Database
| |
Naunyn Schmiedebergs Arch Pharmacol 336: 204-10 (1987)
Article DOI: 10.1007/bf00165806
BindingDB Entry DOI: 10.7270/Q26H4FXN |
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1 [30-579]
(Rattus norvegicus (rat)) | BDBM23120
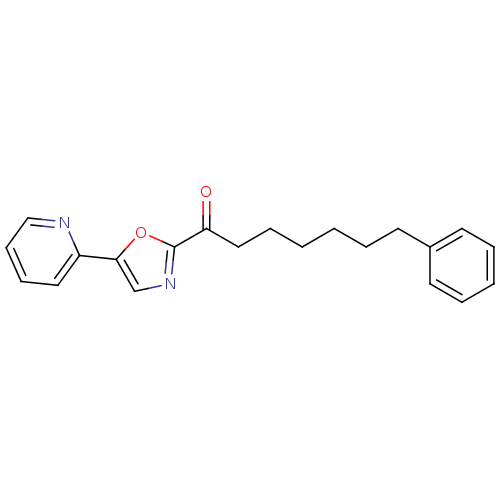
(7-phenyl-1-[5-(pyridin-2-yl)-1,3-oxazol-2-yl]hepta...)Show InChI InChI=1S/C21H22N2O2/c24-19(14-7-2-1-4-10-17-11-5-3-6-12-17)21-23-16-20(25-21)18-13-8-9-15-22-18/h3,5-6,8-9,11-13,15-16H,1-2,4,7,10,14H2 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of rat FAAH |
Bioorg Med Chem Lett 18: 5842-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.084
BindingDB Entry DOI: 10.7270/Q2DN44V8 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50036484

(5-((E)-Styryl)-quinazoline-2,4-diamine | CHEMBL164...)Show InChI InChI=1S/C16H14N4/c17-15-14-12(10-9-11-5-2-1-3-6-11)7-4-8-13(14)19-16(18)20-15/h1-10H,(H4,17,18,19,20)/b10-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory compound against Wild-type human DHFR. |
J Med Chem 38: 745-52 (1995)
BindingDB Entry DOI: 10.7270/Q2JQ11PK |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50371684
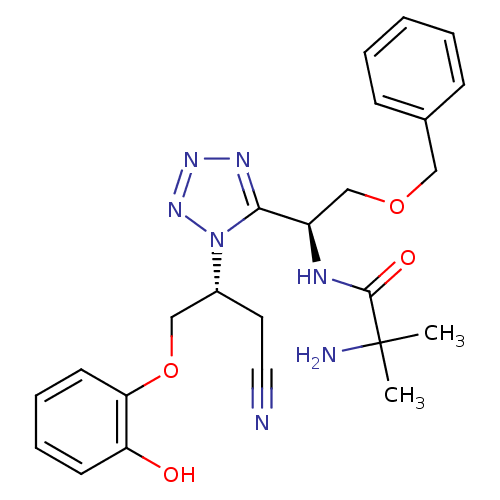
(CHEMBL270666)Show SMILES CC(C)(N)C(=O)N[C@H](COCc1ccccc1)c1nnnn1[C@@H](COc1ccccc1O)CC#N Show InChI InChI=1S/C24H29N7O4/c1-24(2,26)23(33)27-19(16-34-14-17-8-4-3-5-9-17)22-28-29-30-31(22)18(12-13-25)15-35-21-11-7-6-10-20(21)32/h3-11,18-19,32H,12,14-16,26H2,1-2H3,(H,27,33)/t18-,19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Agonist activity at human GHS receptor expressed in H4 glioma cells |
Bioorg Med Chem Lett 18: 2067-72 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.100
BindingDB Entry DOI: 10.7270/Q21G0N4D |
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1 [30-579]
(Rattus norvegicus (rat)) | BDBM50247037
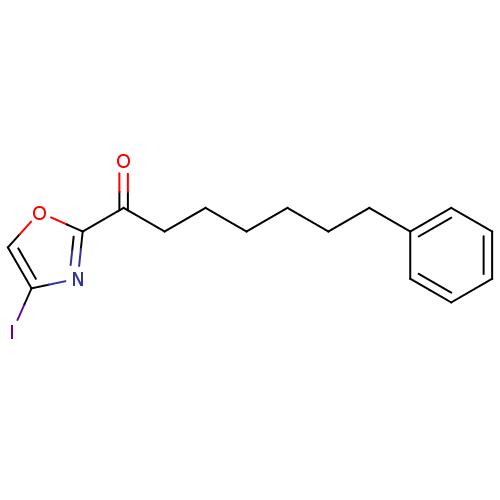
(1-(4-iodooxazol-2-yl)-7-phenylheptan-1-one | CHEMB...)Show InChI InChI=1S/C16H18INO2/c17-15-12-20-16(18-15)14(19)11-7-2-1-4-8-13-9-5-3-6-10-13/h3,5-6,9-10,12H,1-2,4,7-8,11H2 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of rat FAAH |
Bioorg Med Chem Lett 18: 5842-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.084
BindingDB Entry DOI: 10.7270/Q2DN44V8 |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50371688

(CHEMBL404545)Show SMILES CC(C)(N)C(=O)N[C@H](COCc1ccccc1)c1nnnn1[C@@H](COc1ccccc1)CC#N Show InChI InChI=1S/C24H29N7O3/c1-24(2,26)23(32)27-21(17-33-15-18-9-5-3-6-10-18)22-28-29-30-31(22)19(13-14-25)16-34-20-11-7-4-8-12-20/h3-12,19,21H,13,15-17,26H2,1-2H3,(H,27,32)/t19-,21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Agonist activity at human GHS receptor expressed in H4 glioma cells |
Bioorg Med Chem Lett 18: 2067-72 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.100
BindingDB Entry DOI: 10.7270/Q21G0N4D |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM18268

(5-methyl-6-{[(3,4,5-trimethoxyphenyl)amino]methyl}...)Show InChI InChI=1S/C19H23N5O3/c1-10-11(5-6-13-16(10)18(20)24-19(21)23-13)9-22-12-7-14(25-2)17(27-4)15(8-12)26-3/h5-8,22H,9H2,1-4H3,(H4,20,21,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory compound against Wild-type human DHFR. |
J Med Chem 38: 745-52 (1995)
BindingDB Entry DOI: 10.7270/Q2JQ11PK |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM213826

(Dextromethorphan | US11535596, Compound Dextrometh...)Show SMILES COc1ccc2C[C@@H]3[C@H]4CCCC[C@]4(CCN3C)c2c1 |THB:17:16:8:18.6.5| Show InChI InChI=1S/C18H25NO/c1-19-10-9-18-8-4-3-5-15(18)17(19)11-13-6-7-14(20-2)12-16(13)18/h6-7,12,15,17H,3-5,8-11H2,1-2H3/t15-,17-,18-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-Citalopram from SERT receptor (unknown origin) expressed in HEK cell membrane incubated for 2 hrs by liquid scintillation counte... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00055
BindingDB Entry DOI: 10.7270/Q2WS8Z41 |
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1 [30-579]
(Rattus norvegicus (rat)) | BDBM50247075
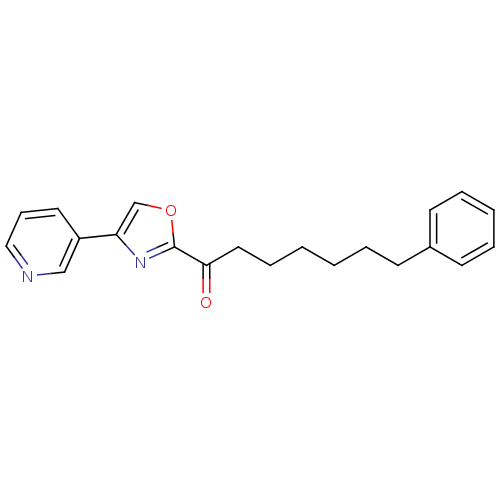
(7-phenyl-1-(4-(pyridin-3-yl)oxazol-2-yl)heptan-1-o...)Show InChI InChI=1S/C21H22N2O2/c24-20(13-7-2-1-4-9-17-10-5-3-6-11-17)21-23-19(16-25-21)18-12-8-14-22-15-18/h3,5-6,8,10-12,14-16H,1-2,4,7,9,13H2 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of rat FAAH |
Bioorg Med Chem Lett 18: 5842-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.084
BindingDB Entry DOI: 10.7270/Q2DN44V8 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50036483

(5-Phenethyl-quinazoline-2,4-diamine | CHEMBL341703)Show InChI InChI=1S/C16H16N4/c17-15-14-12(10-9-11-5-2-1-3-6-11)7-4-8-13(14)19-16(18)20-15/h1-8H,9-10H2,(H4,17,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory compound against Wild-type human DHFR. |
J Med Chem 38: 745-52 (1995)
BindingDB Entry DOI: 10.7270/Q2JQ11PK |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50284925

(CHEMBL288759 | {(1S,2R)-1-Benzyl-2-hydroxy-3-[(2R,...)Show SMILES CC(C)(C)OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CNC[C@@H](O)[C@H](Cc1ccccc1)NC(=O)[C@@](C)(O)C(C)(C)C Show InChI InChI=1S/C32H49N3O6/c1-30(2,3)32(7,40)28(38)34-24(18-22-14-10-8-11-15-22)26(36)20-33-21-27(37)25(19-23-16-12-9-13-17-23)35-29(39)41-31(4,5)6/h8-17,24-27,33,36-37,40H,18-21H2,1-7H3,(H,34,38)(H,35,39)/t24-,25-,26+,27+,32+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition constant against HIV protease was determined |
Bioorg Med Chem Lett 5: 1729-1734 (1995)
Article DOI: 10.1016/0960-894X(95)00293-3
BindingDB Entry DOI: 10.7270/Q2RN37TS |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data