 Found 977 hits with Last Name = 'tom' and Initial = 'l'
Found 977 hits with Last Name = 'tom' and Initial = 'l' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cannabinoid receptor 1
(Mus musculus (Mouse)) | BDBM50176988
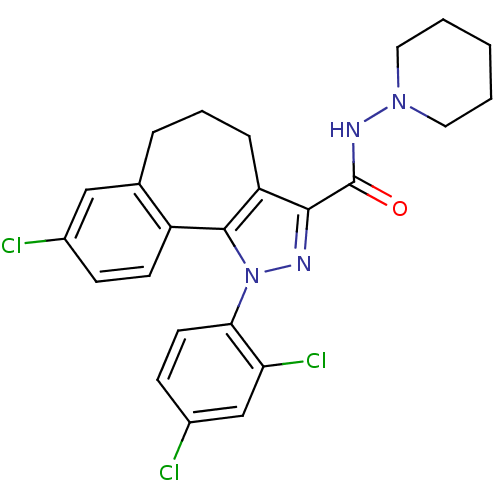
(8-Chloro-1-(2,4-dichloro-phenyl)-1,3a,4,5,6,10b-he...)Show SMILES Clc1ccc(c(Cl)c1)-n1nc(C(=O)NN2CCCCC2)c2CCCc3cc(Cl)ccc3-c12 Show InChI InChI=1S/C24H23Cl3N4O/c25-16-7-9-18-15(13-16)5-4-6-19-22(24(32)29-30-11-2-1-3-12-30)28-31(23(18)19)21-10-8-17(26)14-20(21)27/h7-10,13-14H,1-6,11-12H2,(H,29,32) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.000350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Sassari
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from CB1 receptor in CD1 mouse brain |
J Med Chem 48: 7351-62 (2005)
Article DOI: 10.1021/jm050317f
BindingDB Entry DOI: 10.7270/Q2CF9PNQ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Mus musculus (Mouse)) | BDBM50176980
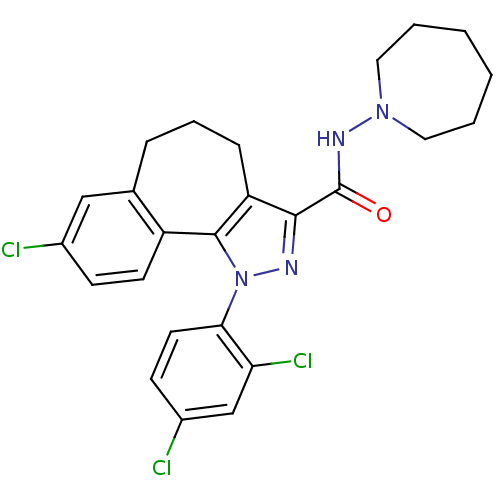
(8-chloro-1-(2',4'-dichlorophenyl)-N-homopiperidin-...)Show SMILES Clc1ccc(c(Cl)c1)-n1nc(C(=O)NN2CCCCCC2)c2CCCc3cc(Cl)ccc3-c12 Show InChI InChI=1S/C25H25Cl3N4O/c26-17-8-10-19-16(14-17)6-5-7-20-23(25(33)30-31-12-3-1-2-4-13-31)29-32(24(19)20)22-11-9-18(27)15-21(22)28/h8-11,14-15H,1-7,12-13H2,(H,30,33) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Sassari
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from CB1 receptor in CD1 mouse brain |
J Med Chem 48: 7351-62 (2005)
Article DOI: 10.1021/jm050317f
BindingDB Entry DOI: 10.7270/Q2CF9PNQ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Mus musculus (Mouse)) | BDBM50176989
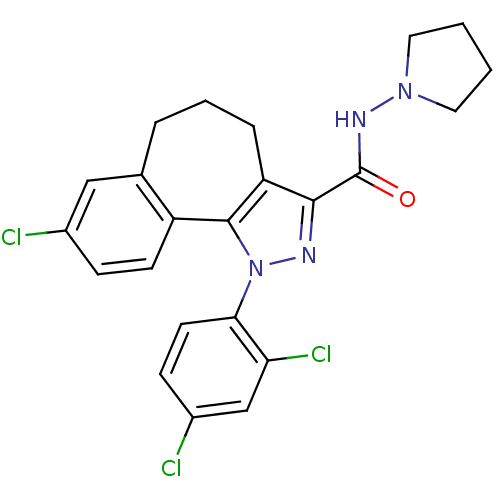
(8-chloro-1-(2',4'-dichlorophenyl)-N-pyrrolidin-1-y...)Show SMILES Clc1ccc(c(Cl)c1)-n1nc(C(=O)NN2CCCC2)c2CCCc3cc(Cl)ccc3-c12 Show InChI InChI=1S/C23H21Cl3N4O/c24-15-6-8-17-14(12-15)4-3-5-18-21(23(31)28-29-10-1-2-11-29)27-30(22(17)18)20-9-7-16(25)13-19(20)26/h6-9,12-13H,1-5,10-11H2,(H,28,31) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Sassari
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from CB1 receptor in CD1 mouse brain |
J Med Chem 48: 7351-62 (2005)
Article DOI: 10.1021/jm050317f
BindingDB Entry DOI: 10.7270/Q2CF9PNQ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Mus musculus (Mouse)) | BDBM50176979
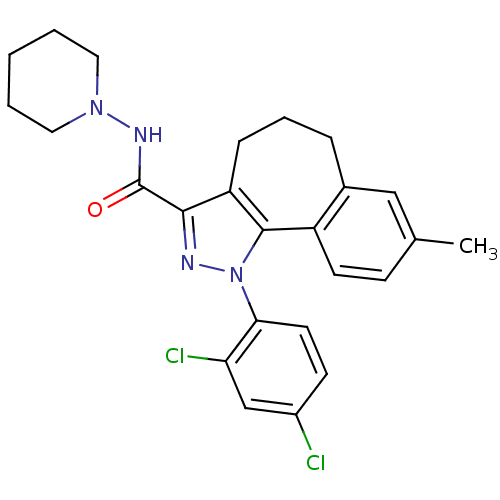
(8-methyl-1-(2',4'-dichlorophenyl)-N-piperidin-1-yl...)Show SMILES Cc1ccc2-c3c(CCCc2c1)c(nn3-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCC1 Show InChI InChI=1S/C25H26Cl2N4O/c1-16-8-10-19-17(14-16)6-5-7-20-23(25(32)29-30-12-3-2-4-13-30)28-31(24(19)20)22-11-9-18(26)15-21(22)27/h8-11,14-15H,2-7,12-13H2,1H3,(H,29,32) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Sassari
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from CB1 receptor in CD1 mouse brain |
J Med Chem 48: 7351-62 (2005)
Article DOI: 10.1021/jm050317f
BindingDB Entry DOI: 10.7270/Q2CF9PNQ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Mus musculus (Mouse)) | BDBM50176986
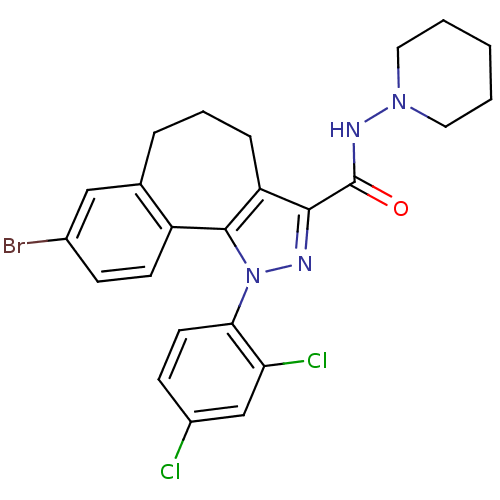
(8-bromo-1-(2',4'-dichlorophenyl)-N-piperidin-1-yl-...)Show SMILES Clc1ccc(c(Cl)c1)-n1nc(C(=O)NN2CCCCC2)c2CCCc3cc(Br)ccc3-c12 Show InChI InChI=1S/C24H23BrCl2N4O/c25-16-7-9-18-15(13-16)5-4-6-19-22(24(32)29-30-11-2-1-3-12-30)28-31(23(18)19)21-10-8-17(26)14-20(21)27/h7-10,13-14H,1-6,11-12H2,(H,29,32) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Sassari
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from CB1 receptor in CD1 mouse brain |
J Med Chem 48: 7351-62 (2005)
Article DOI: 10.1021/jm050317f
BindingDB Entry DOI: 10.7270/Q2CF9PNQ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Mus musculus (Mouse)) | BDBM50176990
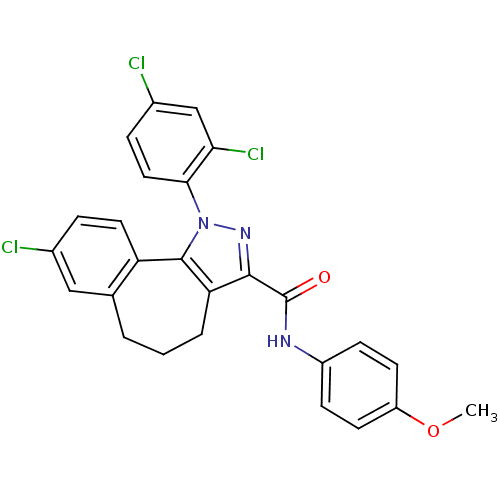
(8-chloro-1-(2',4'-dichlorophenyl)-N-p-methoxylphen...)Show SMILES COc1ccc(NC(=O)c2nn(c-3c2CCCc2cc(Cl)ccc-32)-c2ccc(Cl)cc2Cl)cc1 Show InChI InChI=1S/C26H20Cl3N3O2/c1-34-19-9-7-18(8-10-19)30-26(33)24-21-4-2-3-15-13-16(27)5-11-20(15)25(21)32(31-24)23-12-6-17(28)14-22(23)29/h5-14H,2-4H2,1H3,(H,30,33) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Sassari
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from CB1 receptor in CD1 mouse brain |
J Med Chem 48: 7351-62 (2005)
Article DOI: 10.1021/jm050317f
BindingDB Entry DOI: 10.7270/Q2CF9PNQ |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Rattus norvegicus (Rat)) | BDBM50216315
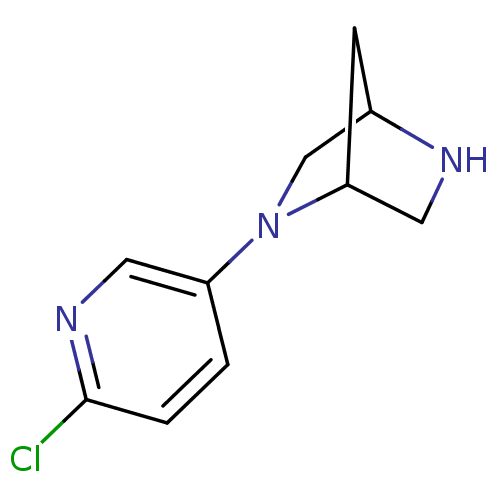
((1R,4R)-2-(6-chloro-3-pyridinyl)-2,5-diazabicyclo[...)Show InChI InChI=1S/C10H12ClN3/c11-10-2-1-8(4-13-10)14-6-7-3-9(14)5-12-7/h1-2,4,7,9,12H,3,5-6H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Sassari
Curated by ChEMBL
| Assay Description
Displacement of [3H]cytosine form alpha4beta2 nAChR in rat striatum |
Bioorg Med Chem Lett 18: 6147-50 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.002
BindingDB Entry DOI: 10.7270/Q29G5MPJ |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Rattus norvegicus (Rat)) | BDBM50275878
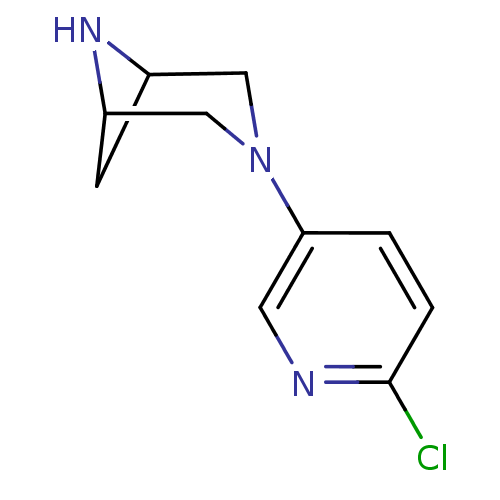
(3-(6-chloropyridin-3-yl)-3,6-diazabicyclo[3.1.1]he...)Show InChI InChI=1S/C10H12ClN3/c11-10-2-1-9(4-12-10)14-5-7-3-8(6-14)13-7/h1-2,4,7-8,13H,3,5-6H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Sassari
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine form alpha4beta2 nAChR in rat cortex |
Bioorg Med Chem Lett 18: 6147-50 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.002
BindingDB Entry DOI: 10.7270/Q29G5MPJ |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Rattus norvegicus (Rat)) | BDBM50275880
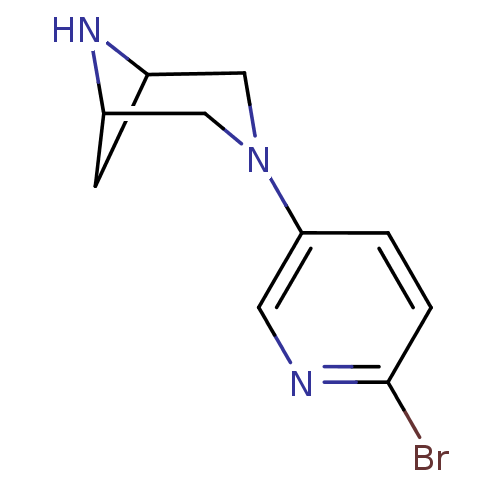
(3-(6-bromopyridin-3-yl)-3,6-diazabicyclo[3.1.1]hep...)Show InChI InChI=1S/C10H12BrN3/c11-10-2-1-9(4-12-10)14-5-7-3-8(6-14)13-7/h1-2,4,7-8,13H,3,5-6H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Sassari
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine form alpha4beta2 nAChR in rat cortex |
Bioorg Med Chem Lett 18: 6147-50 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.002
BindingDB Entry DOI: 10.7270/Q29G5MPJ |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Rattus norvegicus (Rat)) | BDBM50275879
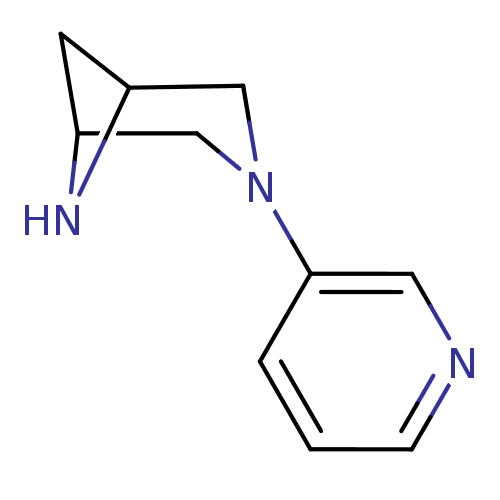
(3-(pyridin-3-yl)-3,6-diazabicyclo[3.1.1]heptane | ...)Show InChI InChI=1S/C10H13N3/c1-2-10(5-11-3-1)13-6-8-4-9(7-13)12-8/h1-3,5,8-9,12H,4,6-7H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Sassari
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine form alpha4beta2 nAChR in rat cortex |
Bioorg Med Chem Lett 18: 6147-50 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.002
BindingDB Entry DOI: 10.7270/Q29G5MPJ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50463758
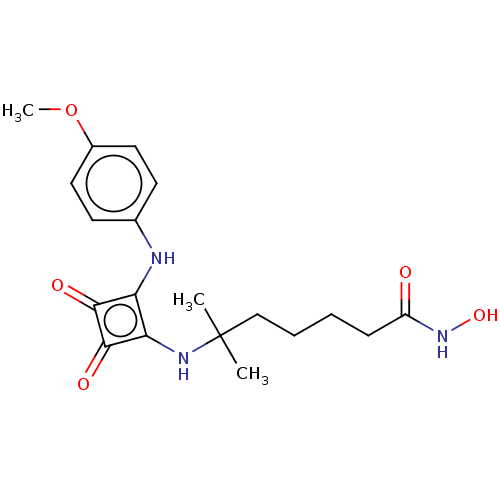
(CHEMBL4250302)Show SMILES COc1ccc(Nc2c(NC(C)(C)CCCCC(=O)NO)c(=O)c2=O)cc1 Show InChI InChI=1S/C19H25N3O5/c1-19(2,11-5-4-6-14(23)22-26)21-16-15(17(24)18(16)25)20-12-7-9-13(27-3)10-8-12/h7-10,20-21,26H,4-6,11H2,1-3H3,(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestle Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 using Fluor de Lys as substrate after 2 hrs by fluorescence method |
Bioorg Med Chem Lett 28: 2985-2992 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.029
BindingDB Entry DOI: 10.7270/Q2M0484T |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50463741
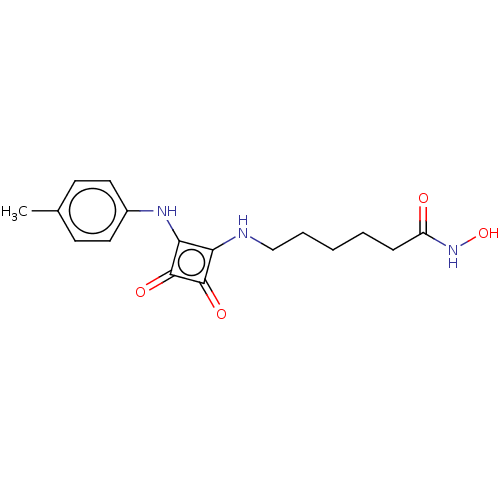
(CHEMBL4239232)Show InChI InChI=1S/C17H21N3O4/c1-11-6-8-12(9-7-11)19-15-14(16(22)17(15)23)18-10-4-2-3-5-13(21)20-24/h6-9,18-19,24H,2-5,10H2,1H3,(H,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestle Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 using Fluor de Lys as substrate after 2 hrs by fluorescence method |
Bioorg Med Chem Lett 28: 2985-2992 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.029
BindingDB Entry DOI: 10.7270/Q2M0484T |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50463759
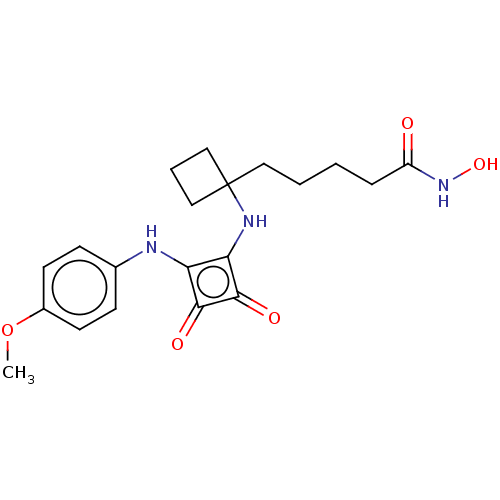
(CHEMBL4237636)Show SMILES COc1ccc(Nc2c(NC3(CCCCC(=O)NO)CCC3)c(=O)c2=O)cc1 Show InChI InChI=1S/C20H25N3O5/c1-28-14-8-6-13(7-9-14)21-16-17(19(26)18(16)25)22-20(11-4-12-20)10-3-2-5-15(24)23-27/h6-9,21-22,27H,2-5,10-12H2,1H3,(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestle Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 using Fluor de Lys as substrate after 2 hrs by fluorescence method |
Bioorg Med Chem Lett 28: 2985-2992 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.029
BindingDB Entry DOI: 10.7270/Q2M0484T |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50463739
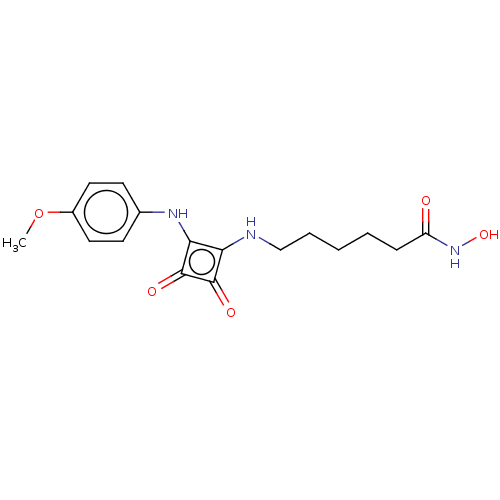
(CHEMBL4237803)Show InChI InChI=1S/C17H21N3O5/c1-25-12-8-6-11(7-9-12)19-15-14(16(22)17(15)23)18-10-4-2-3-5-13(21)20-24/h6-9,18-19,24H,2-5,10H2,1H3,(H,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0750 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestle Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 using Fluor de Lys as substrate after 2 hrs by fluorescence method |
Bioorg Med Chem Lett 28: 2985-2992 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.029
BindingDB Entry DOI: 10.7270/Q2M0484T |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50463736
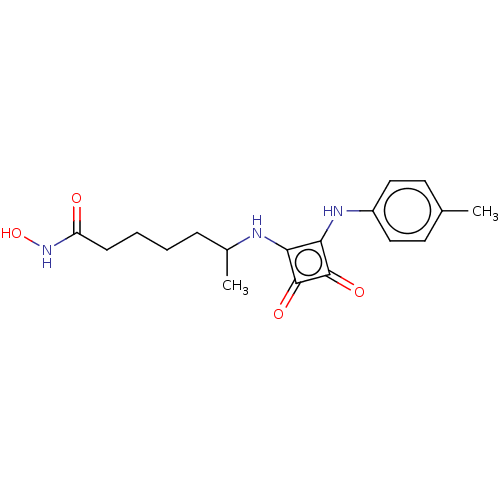
(CHEMBL4251203)Show InChI InChI=1S/C18H23N3O4/c1-11-7-9-13(10-8-11)20-16-15(17(23)18(16)24)19-12(2)5-3-4-6-14(22)21-25/h7-10,12,19-20,25H,3-6H2,1-2H3,(H,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestle Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 using Fluor de Lys as substrate after 2 hrs by fluorescence method |
Bioorg Med Chem Lett 28: 2985-2992 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.029
BindingDB Entry DOI: 10.7270/Q2M0484T |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Mus musculus (Mouse)) | BDBM50176987
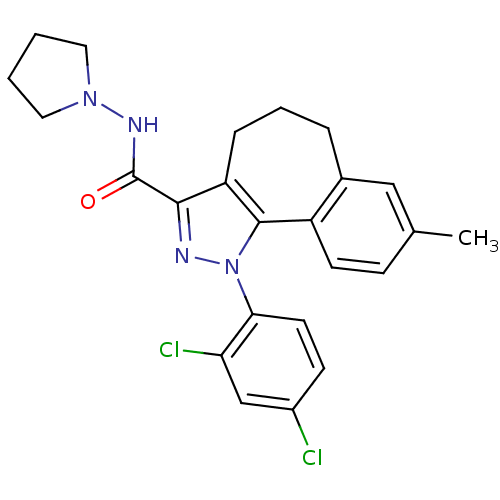
(8-methyl-1-(2',4'-dichlorophenyl)-N-pyrrolidin-1-y...)Show SMILES Cc1ccc2-c3c(CCCc2c1)c(nn3-c1ccc(Cl)cc1Cl)C(=O)NN1CCCC1 Show InChI InChI=1S/C24H24Cl2N4O/c1-15-7-9-18-16(13-15)5-4-6-19-22(24(31)28-29-11-2-3-12-29)27-30(23(18)19)21-10-8-17(25)14-20(21)26/h7-10,13-14H,2-6,11-12H2,1H3,(H,28,31) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Sassari
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from CB1 receptor in CD1 mouse brain |
J Med Chem 48: 7351-62 (2005)
Article DOI: 10.1021/jm050317f
BindingDB Entry DOI: 10.7270/Q2CF9PNQ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50463756
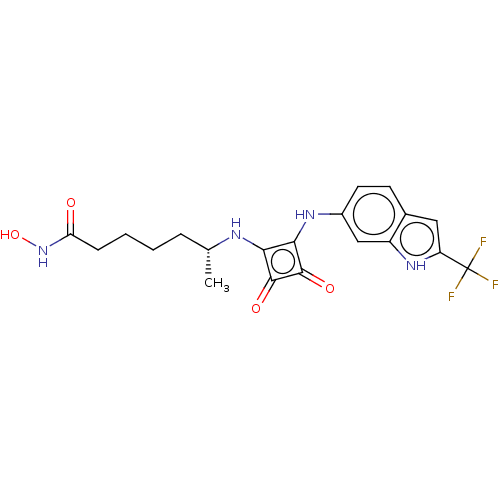
(CHEMBL4246561)Show SMILES C[C@H](CCCCC(=O)NO)Nc1c(Nc2ccc3cc([nH]c3c2)C(F)(F)F)c(=O)c1=O |r| Show InChI InChI=1S/C20H21F3N4O4/c1-10(4-2-3-5-15(28)27-31)24-16-17(19(30)18(16)29)25-12-7-6-11-8-14(20(21,22)23)26-13(11)9-12/h6-10,24-26,31H,2-5H2,1H3,(H,27,28)/t10-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestle Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 using Fluor de Lys as substrate after 2 hrs by fluorescence method |
Bioorg Med Chem Lett 28: 2985-2992 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.029
BindingDB Entry DOI: 10.7270/Q2M0484T |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Rattus norvegicus (Rat)) | BDBM50275932
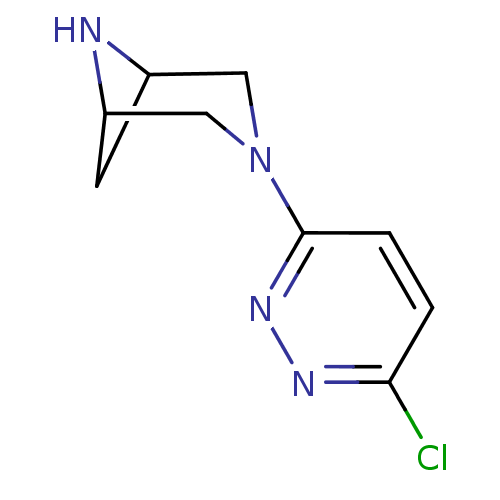
(3-(6-chloropyridazin-3-yl)-3,6-diazabicyclo[3.1.1]...)Show InChI InChI=1S/C9H11ClN4/c10-8-1-2-9(13-12-8)14-4-6-3-7(5-14)11-6/h1-2,6-7,11H,3-5H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.168 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Sassari
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine form alpha4beta2 nAChR in rat cortex |
Bioorg Med Chem Lett 18: 6147-50 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.002
BindingDB Entry DOI: 10.7270/Q29G5MPJ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50463750
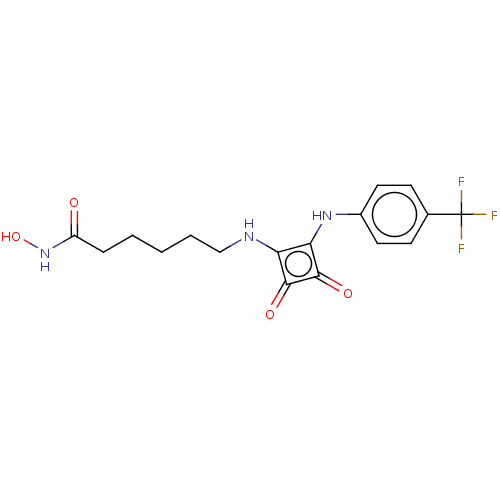
(CHEMBL4241807)Show SMILES ONC(=O)CCCCCNc1c(Nc2ccc(cc2)C(F)(F)F)c(=O)c1=O Show InChI InChI=1S/C17H18F3N3O4/c18-17(19,20)10-5-7-11(8-6-10)22-14-13(15(25)16(14)26)21-9-3-1-2-4-12(24)23-27/h5-8,21-22,27H,1-4,9H2,(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestle Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 using Fluor de Lys as substrate after 2 hrs by fluorescence method |
Bioorg Med Chem Lett 28: 2985-2992 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.029
BindingDB Entry DOI: 10.7270/Q2M0484T |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50463743
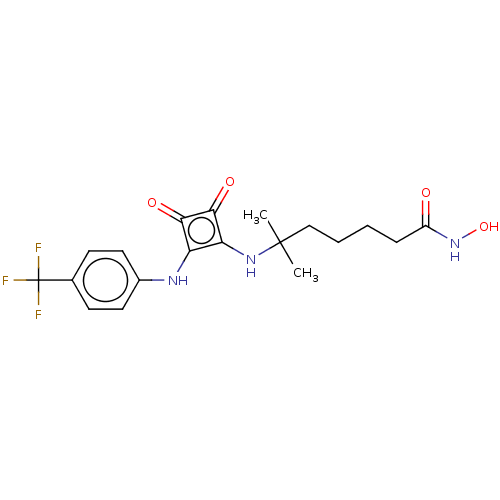
(CHEMBL4241370)Show SMILES CC(C)(CCCCC(=O)NO)Nc1c(Nc2ccc(cc2)C(F)(F)F)c(=O)c1=O Show InChI InChI=1S/C19H22F3N3O4/c1-18(2,10-4-3-5-13(26)25-29)24-15-14(16(27)17(15)28)23-12-8-6-11(7-9-12)19(20,21)22/h6-9,23-24,29H,3-5,10H2,1-2H3,(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestle Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 using Fluor de Lys as substrate after 2 hrs by fluorescence method |
Bioorg Med Chem Lett 28: 2985-2992 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.029
BindingDB Entry DOI: 10.7270/Q2M0484T |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50463753
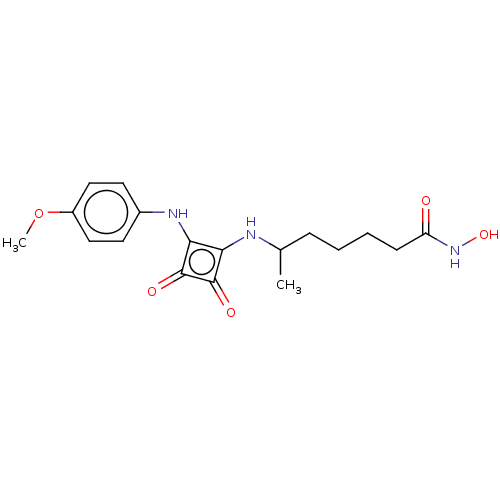
(CHEMBL4250739)Show SMILES COc1ccc(Nc2c(NC(C)CCCCC(=O)NO)c(=O)c2=O)cc1 Show InChI InChI=1S/C18H23N3O5/c1-11(5-3-4-6-14(22)21-25)19-15-16(18(24)17(15)23)20-12-7-9-13(26-2)10-8-12/h7-11,19-20,25H,3-6H2,1-2H3,(H,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestle Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 using Fluor de Lys as substrate after 2 hrs by fluorescence method |
Bioorg Med Chem Lett 28: 2985-2992 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.029
BindingDB Entry DOI: 10.7270/Q2M0484T |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50463752
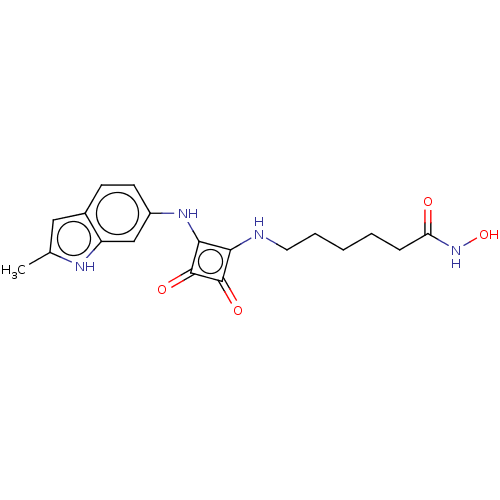
(CHEMBL4247370)Show SMILES Cc1cc2ccc(Nc3c(NCCCCCC(=O)NO)c(=O)c3=O)cc2[nH]1 Show InChI InChI=1S/C19H22N4O4/c1-11-9-12-6-7-13(10-14(12)21-11)22-17-16(18(25)19(17)26)20-8-4-2-3-5-15(24)23-27/h6-7,9-10,20-22,27H,2-5,8H2,1H3,(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestle Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 using Fluor de Lys as substrate after 2 hrs by fluorescence method |
Bioorg Med Chem Lett 28: 2985-2992 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.029
BindingDB Entry DOI: 10.7270/Q2M0484T |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50463754
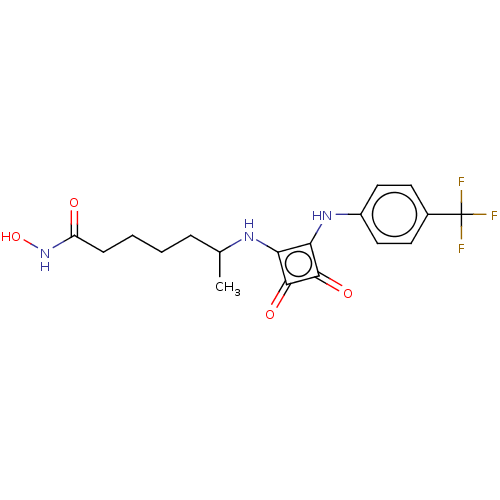
(CHEMBL4240635)Show SMILES CC(CCCCC(=O)NO)Nc1c(Nc2ccc(cc2)C(F)(F)F)c(=O)c1=O Show InChI InChI=1S/C18H20F3N3O4/c1-10(4-2-3-5-13(25)24-28)22-14-15(17(27)16(14)26)23-12-8-6-11(7-9-12)18(19,20)21/h6-10,22-23,28H,2-5H2,1H3,(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestle Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 using Fluor de Lys as substrate after 2 hrs by fluorescence method |
Bioorg Med Chem Lett 28: 2985-2992 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.029
BindingDB Entry DOI: 10.7270/Q2M0484T |
More data for this
Ligand-Target Pair | |
Alpha-1B adrenergic receptor
(Homo sapiens (Human)) | BDBM29568

(CHEMBL2 | PRAZOSIN | PRAZOSIN HYDROCHLORIDE | [3H]...)Show SMILES COc1cc2nc(nc(N)c2cc1OC)N1CCN(CC1)C(=O)c1ccco1 Show InChI InChI=1S/C19H21N5O4/c1-26-15-10-12-13(11-16(15)27-2)21-19(22-17(12)20)24-7-5-23(6-8-24)18(25)14-4-3-9-28-14/h3-4,9-11H,5-8H2,1-2H3,(H2,20,21,22) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Milano
Curated by ChEMBL
| Assay Description
The compound was tested for binding affinity on [3H]- prazosin as specific ligand on Human cloned alpha-1B adrenergic receptor in CHO cells |
J Med Chem 42: 173-7 (1999)
Article DOI: 10.1021/jm981006q
BindingDB Entry DOI: 10.7270/Q22J6B05 |
More data for this
Ligand-Target Pair | |
Alpha-1D adrenergic receptor
(Homo sapiens (Human)) | BDBM29568

(CHEMBL2 | PRAZOSIN | PRAZOSIN HYDROCHLORIDE | [3H]...)Show SMILES COc1cc2nc(nc(N)c2cc1OC)N1CCN(CC1)C(=O)c1ccco1 Show InChI InChI=1S/C19H21N5O4/c1-26-15-10-12-13(11-16(15)27-2)21-19(22-17(12)20)24-7-5-23(6-8-24)18(25)14-4-3-9-28-14/h3-4,9-11H,5-8H2,1-2H3,(H2,20,21,22) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Milano
Curated by ChEMBL
| Assay Description
The compound was tested for binding affinity on [3H]- prazosin as specific ligand on Human cloned alpha-1D adrenergic receptor in CHO cells |
J Med Chem 42: 173-7 (1999)
Article DOI: 10.1021/jm981006q
BindingDB Entry DOI: 10.7270/Q22J6B05 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50463740
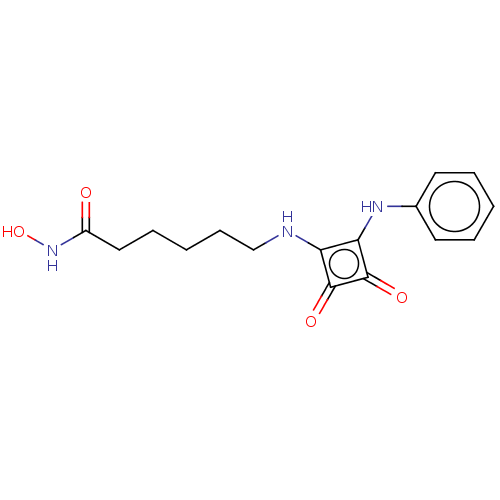
(CHEMBL4251365)Show InChI InChI=1S/C16H19N3O4/c20-12(19-23)9-5-2-6-10-17-13-14(16(22)15(13)21)18-11-7-3-1-4-8-11/h1,3-4,7-8,17-18,23H,2,5-6,9-10H2,(H,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestle Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 using Fluor de Lys as substrate after 2 hrs by fluorescence method |
Bioorg Med Chem Lett 28: 2985-2992 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.029
BindingDB Entry DOI: 10.7270/Q2M0484T |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Mus musculus (Mouse)) | BDBM50176976

(8-chloro-1-(2',4'-dichlorophenyl)-N-cyclohexyl-1,4...)Show SMILES Clc1ccc(c(Cl)c1)-n1nc(C(=O)NC2CCCCC2)c2CCCc3cc(Cl)ccc3-c12 Show InChI InChI=1S/C25H24Cl3N3O/c26-16-9-11-19-15(13-16)5-4-8-20-23(25(32)29-18-6-2-1-3-7-18)30-31(24(19)20)22-12-10-17(27)14-21(22)28/h9-14,18H,1-8H2,(H,29,32) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Sassari
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from CB1 receptor in CD1 mouse brain |
J Med Chem 48: 7351-62 (2005)
Article DOI: 10.1021/jm050317f
BindingDB Entry DOI: 10.7270/Q2CF9PNQ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50606735
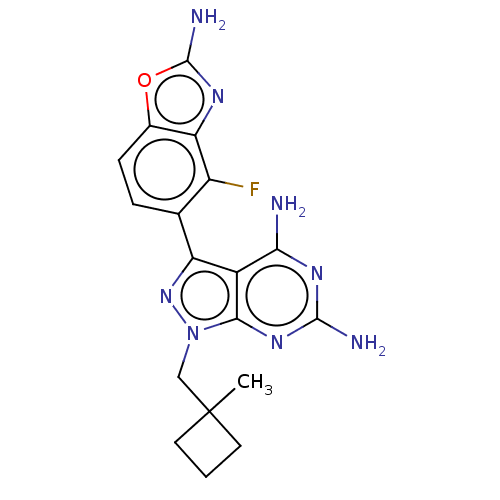
(CHEMBL5219718 | US11731973, Example 3)Show SMILES CC1(Cn2nc(-c3ccc4oc(N)nc4c3F)c3c(N)nc(N)nc23)CCC1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q26W9G6B |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50463739

(CHEMBL4237803)Show InChI InChI=1S/C17H21N3O5/c1-25-12-8-6-11(7-9-12)19-15-14(16(22)17(15)23)18-10-4-2-3-5-13(21)20-24/h6-9,18-19,24H,2-5,10H2,1H3,(H,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestle Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 using fluorogenic HDAC substrate after 15 mins by fluorimetrc method |
Bioorg Med Chem Lett 28: 2985-2992 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.029
BindingDB Entry DOI: 10.7270/Q2M0484T |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(MOUSE) | BDBM50176977
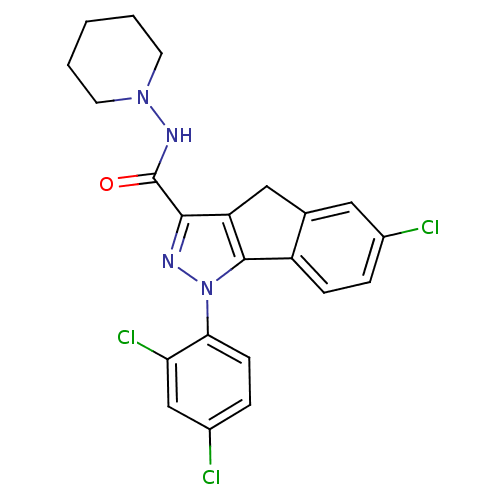
(1,4-dihydroindeno[1,2-c]-pyrazole | 6-chloro-1-(2'...)Show SMILES Clc1ccc-2c(Cc3c(nn(c-23)-c2ccc(Cl)cc2Cl)C(=O)NN2CCCCC2)c1 Show InChI InChI=1S/C22H19Cl3N4O/c23-14-4-6-16-13(10-14)11-17-20(22(30)27-28-8-2-1-3-9-28)26-29(21(16)17)19-7-5-15(24)12-18(19)25/h4-7,10,12H,1-3,8-9,11H2,(H,27,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Sassari
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from CB2 receptor in CD1 mouse spleen |
J Med Chem 48: 7351-62 (2005)
Article DOI: 10.1021/jm050317f
BindingDB Entry DOI: 10.7270/Q2CF9PNQ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50463763
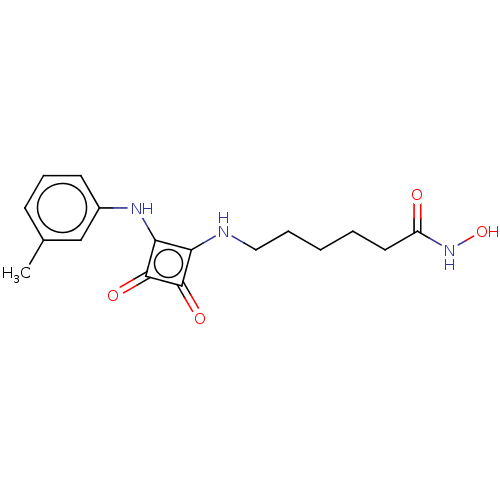
(CHEMBL4248374)Show InChI InChI=1S/C17H21N3O4/c1-11-6-5-7-12(10-11)19-15-14(16(22)17(15)23)18-9-4-2-3-8-13(21)20-24/h5-7,10,18-19,24H,2-4,8-9H2,1H3,(H,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestle Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 using Fluor de Lys as substrate after 2 hrs by fluorescence method |
Bioorg Med Chem Lett 28: 2985-2992 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.029
BindingDB Entry DOI: 10.7270/Q2M0484T |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50606737
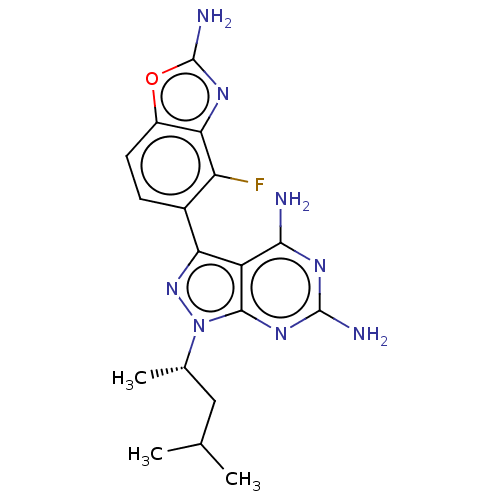
(CHEMBL5218727 | US11731973, Example 5)Show SMILES CC(C)C[C@H](C)n1nc(-c2ccc3oc(N)nc3c2F)c2c(N)nc(N)nc12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q26W9G6B |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Rattus norvegicus (Rat)) | BDBM50275933
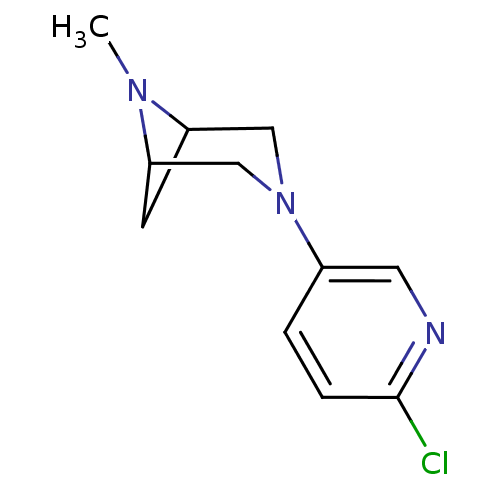
(3-(6-chloropyridin-3-yl)-6-methyl-3,6-diazabicyclo...)Show InChI InChI=1S/C11H14ClN3/c1-14-9-4-10(14)7-15(6-9)8-2-3-11(12)13-5-8/h2-3,5,9-10H,4,6-7H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Sassari
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine form alpha4beta2 nAChR in rat cortex |
Bioorg Med Chem Lett 18: 6147-50 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.002
BindingDB Entry DOI: 10.7270/Q29G5MPJ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(MOUSE) | BDBM50176979

(8-methyl-1-(2',4'-dichlorophenyl)-N-piperidin-1-yl...)Show SMILES Cc1ccc2-c3c(CCCc2c1)c(nn3-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCC1 Show InChI InChI=1S/C25H26Cl2N4O/c1-16-8-10-19-17(14-16)6-5-7-20-23(25(32)29-30-12-3-2-4-13-30)28-31(24(19)20)22-11-9-18(26)15-21(22)27/h8-11,14-15H,2-7,12-13H2,1H3,(H,29,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Sassari
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from CB2 receptor in CD1 mouse spleen |
J Med Chem 48: 7351-62 (2005)
Article DOI: 10.1021/jm050317f
BindingDB Entry DOI: 10.7270/Q2CF9PNQ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50105327

(JNJ-26481585 | Quisinostat)Show SMILES Cn1cc(CNCC2CCN(CC2)c2ncc(cn2)C(=O)NO)c2ccccc12 Show InChI InChI=1S/C21H26N6O2/c1-26-14-17(18-4-2-3-5-19(18)26)11-22-10-15-6-8-27(9-7-15)21-23-12-16(13-24-21)20(28)25-29/h2-5,12-15,22,29H,6-11H2,1H3,(H,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestle Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 using Fluor de Lys as substrate after 2 hrs by fluorescence method |
Bioorg Med Chem Lett 28: 2985-2992 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.029
BindingDB Entry DOI: 10.7270/Q2M0484T |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50463751
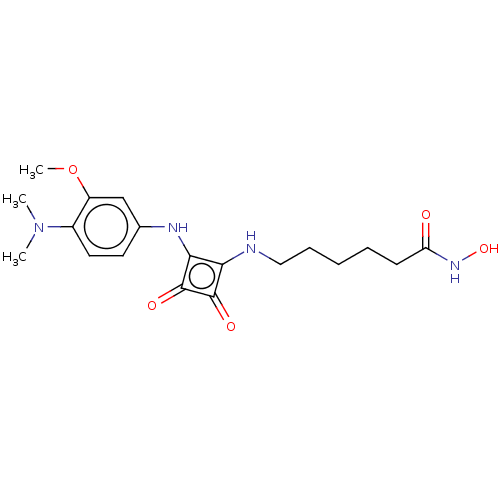
(CHEMBL4244350)Show SMILES COc1cc(Nc2c(NCCCCCC(=O)NO)c(=O)c2=O)ccc1N(C)C Show InChI InChI=1S/C19H26N4O5/c1-23(2)13-9-8-12(11-14(13)28-3)21-17-16(18(25)19(17)26)20-10-6-4-5-7-15(24)22-27/h8-9,11,20-21,27H,4-7,10H2,1-3H3,(H,22,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestle Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 using Fluor de Lys as substrate after 2 hrs by fluorescence method |
Bioorg Med Chem Lett 28: 2985-2992 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.029
BindingDB Entry DOI: 10.7270/Q2M0484T |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50606738
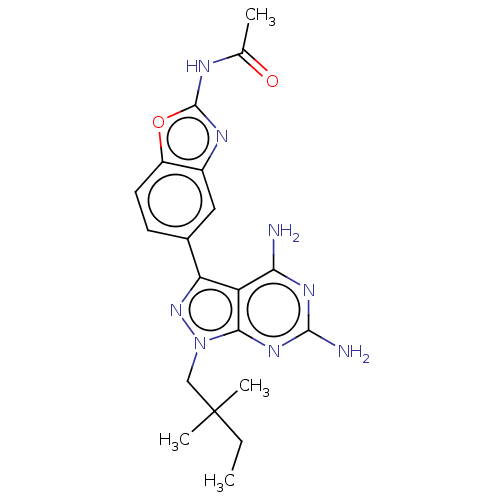
(CHEMBL5218590 | US11731973, Example 6)Show SMILES CCC(C)(C)Cn1nc(-c2ccc3oc(NC(C)=O)nc3c2)c2c(N)nc(N)nc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q26W9G6B |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50463757
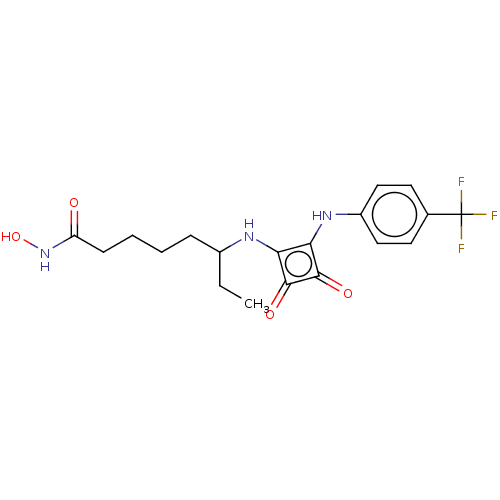
(CHEMBL4249385)Show SMILES CCC(CCCCC(=O)NO)Nc1c(Nc2ccc(cc2)C(F)(F)F)c(=O)c1=O Show InChI InChI=1S/C19H22F3N3O4/c1-2-12(5-3-4-6-14(26)25-29)23-15-16(18(28)17(15)27)24-13-9-7-11(8-10-13)19(20,21)22/h7-10,12,23-24,29H,2-6H2,1H3,(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestle Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 using Fluor de Lys as substrate after 2 hrs by fluorescence method |
Bioorg Med Chem Lett 28: 2985-2992 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.029
BindingDB Entry DOI: 10.7270/Q2M0484T |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM29589

(Faridak | LBH-589 | LBH-589B | Panobinostat | US10...)Show SMILES Cc1[nH]c2ccccc2c1CCNCc1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C21H23N3O2/c1-15-18(19-4-2-3-5-20(19)23-15)12-13-22-14-17-8-6-16(7-9-17)10-11-21(25)24-26/h2-11,22-23,26H,12-14H2,1H3,(H,24,25)/b11-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestle Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 using Fluor de Lys as substrate after 2 hrs by fluorescence method |
Bioorg Med Chem Lett 28: 2985-2992 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.029
BindingDB Entry DOI: 10.7270/Q2M0484T |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50463764
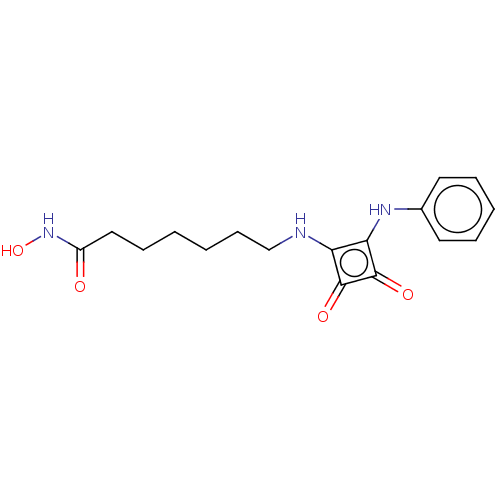
(CHEMBL4245007)Show InChI InChI=1S/C17H21N3O4/c21-13(20-24)10-6-1-2-7-11-18-14-15(17(23)16(14)22)19-12-8-4-3-5-9-12/h3-5,8-9,18-19,24H,1-2,6-7,10-11H2,(H,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestle Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 using Fluor de Lys as substrate after 2 hrs by fluorescence method |
Bioorg Med Chem Lett 28: 2985-2992 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.029
BindingDB Entry DOI: 10.7270/Q2M0484T |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50463742
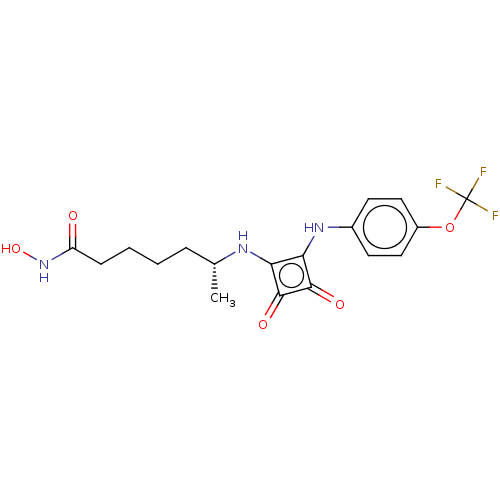
(CHEMBL4242292)Show SMILES C[C@H](CCCCC(=O)NO)Nc1c(Nc2ccc(OC(F)(F)F)cc2)c(=O)c1=O |r| Show InChI InChI=1S/C18H20F3N3O5/c1-10(4-2-3-5-13(25)24-28)22-14-15(17(27)16(14)26)23-11-6-8-12(9-7-11)29-18(19,20)21/h6-10,22-23,28H,2-5H2,1H3,(H,24,25)/t10-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestle Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 using Fluor de Lys as substrate after 2 hrs by fluorescence method |
Bioorg Med Chem Lett 28: 2985-2992 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.029
BindingDB Entry DOI: 10.7270/Q2M0484T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50606740
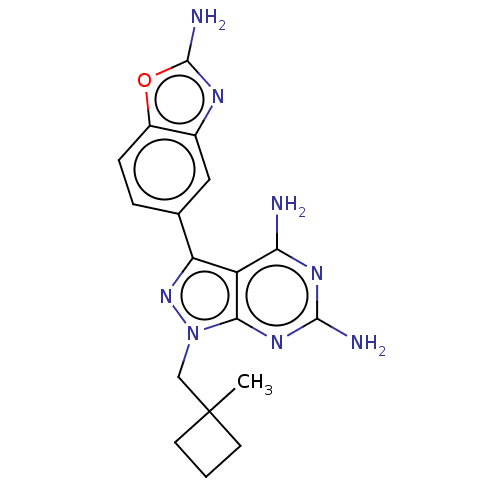
(CHEMBL5220536 | US11731973, Example 30)Show SMILES CC1(Cn2nc(-c3ccc4oc(N)nc4c3)c3c(N)nc(N)nc23)CCC1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q26W9G6B |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Homo sapiens (Human)) | BDBM29568

(CHEMBL2 | PRAZOSIN | PRAZOSIN HYDROCHLORIDE | [3H]...)Show SMILES COc1cc2nc(nc(N)c2cc1OC)N1CCN(CC1)C(=O)c1ccco1 Show InChI InChI=1S/C19H21N5O4/c1-26-15-10-12-13(11-16(15)27-2)21-19(22-17(12)20)24-7-5-23(6-8-24)18(25)14-4-3-9-28-14/h3-4,9-11H,5-8H2,1-2H3,(H2,20,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Milano
Curated by ChEMBL
| Assay Description
The compound was tested for binding affinity on [3H]- prazosin as specific ligand on Human cloned alpha-1A adrenergic receptor expressed in CHO cells |
J Med Chem 42: 173-7 (1999)
Article DOI: 10.1021/jm981006q
BindingDB Entry DOI: 10.7270/Q22J6B05 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50606739
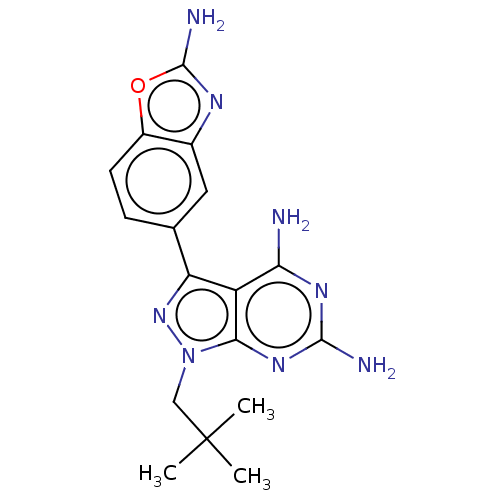
(CHEMBL5220152 | US11731973, Example 7)Show SMILES CC(C)(C)Cn1nc(-c2ccc3oc(N)nc3c2)c2c(N)nc(N)nc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q26W9G6B |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM613740
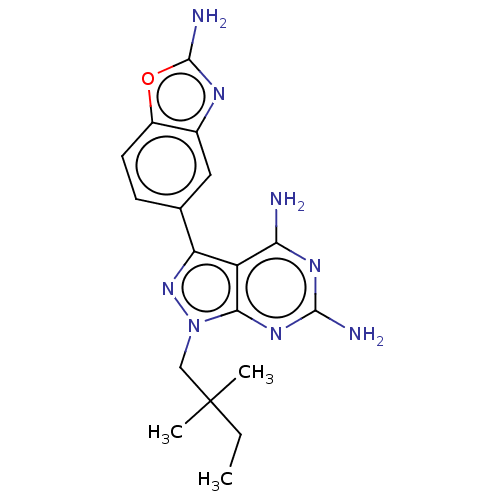
(3-(2-aminobenzoxazol-5-yl)-1-(2,2- dimethylbutyl)-...)Show SMILES CCC(C)(C)Cn1nc(-c2ccc3oc(N)nc3c2)c2c(N)nc(N)nc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q26W9G6B |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50606742
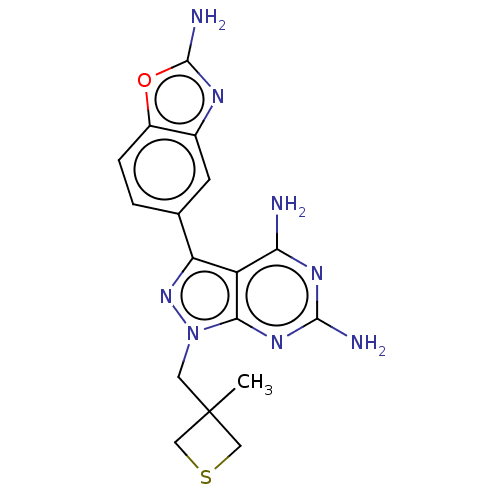
(CHEMBL5220948 | US11731973, Example 9)Show SMILES CC1(Cn2nc(-c3ccc4oc(N)nc4c3)c3c(N)nc(N)nc23)CSC1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q26W9G6B |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(MOUSE) | BDBM50176976

(8-chloro-1-(2',4'-dichlorophenyl)-N-cyclohexyl-1,4...)Show SMILES Clc1ccc(c(Cl)c1)-n1nc(C(=O)NC2CCCCC2)c2CCCc3cc(Cl)ccc3-c12 Show InChI InChI=1S/C25H24Cl3N3O/c26-16-9-11-19-15(13-16)5-4-8-20-23(25(32)29-18-6-2-1-3-7-18)30-31(24(19)20)22-12-10-17(27)14-21(22)28/h9-14,18H,1-8H2,(H,29,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Sassari
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from CB2 receptor in CD1 mouse spleen |
J Med Chem 48: 7351-62 (2005)
Article DOI: 10.1021/jm050317f
BindingDB Entry DOI: 10.7270/Q2CF9PNQ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50463767
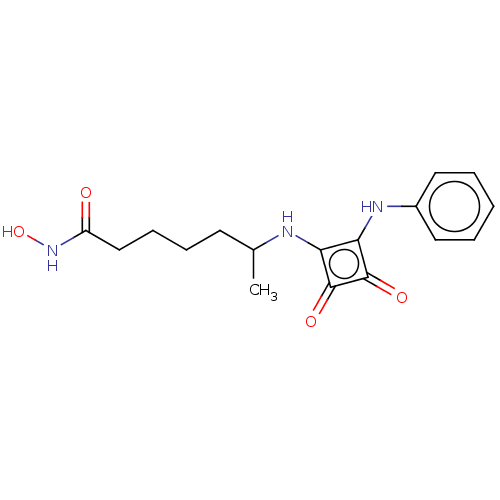
(CHEMBL4246919)Show InChI InChI=1S/C17H21N3O4/c1-11(7-5-6-10-13(21)20-24)18-14-15(17(23)16(14)22)19-12-8-3-2-4-9-12/h2-4,8-9,11,18-19,24H,5-7,10H2,1H3,(H,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestle Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 using Fluor de Lys as substrate after 2 hrs by fluorescence method |
Bioorg Med Chem Lett 28: 2985-2992 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.029
BindingDB Entry DOI: 10.7270/Q2M0484T |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50606736
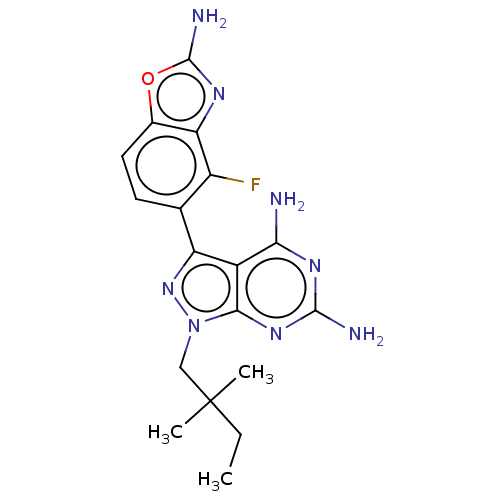
(CHEMBL5218916 | US11731973, Example 10)Show SMILES CCC(C)(C)Cn1nc(-c2ccc3oc(N)nc3c2F)c2c(N)nc(N)nc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q26W9G6B |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50606741
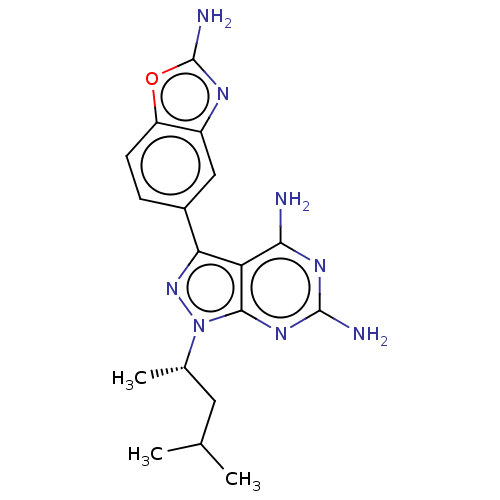
(CHEMBL5219710 | US11731973, Example 1)Show SMILES CC(C)C[C@H](C)n1nc(-c2ccc3oc(N)nc3c2)c2c(N)nc(N)nc12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q26W9G6B |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data