 Found 93 hits with Last Name = 'tomabechi' and Initial = 'y'
Found 93 hits with Last Name = 'tomabechi' and Initial = 'y' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein kinase HCK
(Homo sapiens (Human)) | BDBM50268924
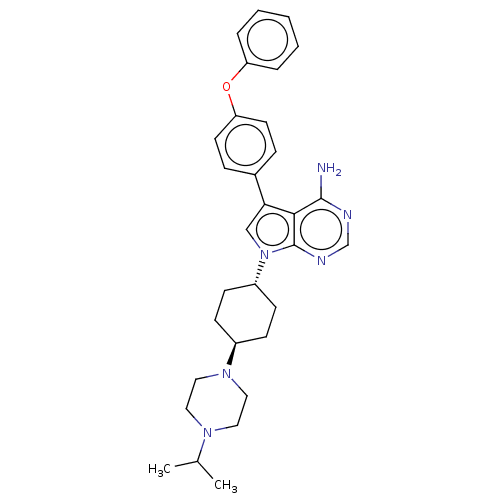
(CHEMBL4075002)Show SMILES CC(C)N1CCN(CC1)[C@H]1CC[C@@H](CC1)n1cc(-c2ccc(Oc3ccccc3)cc2)c2c(N)ncnc12 |r,wU:12.16,wD:9.9,(9.09,-24.29,;10.12,-23.15,;11.63,-23.47,;9.65,-21.68,;8.15,-21.36,;7.68,-19.91,;8.71,-18.78,;10.2,-19.09,;10.67,-20.55,;8.23,-17.32,;9.26,-16.18,;8.78,-14.71,;7.27,-14.4,;6.24,-15.55,;6.72,-17.01,;6.79,-12.95,;7.69,-11.68,;6.77,-10.43,;7.24,-8.96,;8.75,-8.65,;9.21,-7.18,;8.17,-6.04,;8.64,-4.57,;10.14,-4.24,;11.18,-5.38,;12.68,-5.04,;13.15,-3.57,;12.1,-2.44,;10.6,-2.77,;6.66,-6.37,;6.2,-7.84,;5.3,-10.92,;3.96,-10.16,;3.96,-8.62,;2.64,-10.92,;2.64,-12.47,;3.96,-13.24,;5.31,-12.47,)| Show InChI InChI=1S/C31H38N6O/c1-22(2)35-16-18-36(19-17-35)24-10-12-25(13-11-24)37-20-28(29-30(32)33-21-34-31(29)37)23-8-14-27(15-9-23)38-26-6-4-3-5-7-26/h3-9,14-15,20-22,24-25H,10-13,16-19H2,1-2H3,(H2,32,33,34)/t24-,25- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.257 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN Center for Life Science Technologies
Curated by ChEMBL
| Assay Description
Inhibition of HCK (unknown origin) |
Bioorg Med Chem 25: 4259-4264 (2017)
Article DOI: 10.1016/j.bmc.2017.05.053
BindingDB Entry DOI: 10.7270/Q2SB4875 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase HCK
(Homo sapiens (Human)) | BDBM50268924

(CHEMBL4075002)Show SMILES CC(C)N1CCN(CC1)[C@H]1CC[C@@H](CC1)n1cc(-c2ccc(Oc3ccccc3)cc2)c2c(N)ncnc12 |r,wU:12.16,wD:9.9,(9.09,-24.29,;10.12,-23.15,;11.63,-23.47,;9.65,-21.68,;8.15,-21.36,;7.68,-19.91,;8.71,-18.78,;10.2,-19.09,;10.67,-20.55,;8.23,-17.32,;9.26,-16.18,;8.78,-14.71,;7.27,-14.4,;6.24,-15.55,;6.72,-17.01,;6.79,-12.95,;7.69,-11.68,;6.77,-10.43,;7.24,-8.96,;8.75,-8.65,;9.21,-7.18,;8.17,-6.04,;8.64,-4.57,;10.14,-4.24,;11.18,-5.38,;12.68,-5.04,;13.15,-3.57,;12.1,-2.44,;10.6,-2.77,;6.66,-6.37,;6.2,-7.84,;5.3,-10.92,;3.96,-10.16,;3.96,-8.62,;2.64,-10.92,;2.64,-12.47,;3.96,-13.24,;5.31,-12.47,)| Show InChI InChI=1S/C31H38N6O/c1-22(2)35-16-18-36(19-17-35)24-10-12-25(13-11-24)37-20-28(29-30(32)33-21-34-31(29)37)23-8-14-27(15-9-23)38-26-6-4-3-5-7-26/h3-9,14-15,20-22,24-25H,10-13,16-19H2,1-2H3,(H2,32,33,34)/t24-,25- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN Center for Life Science Technologies
Curated by ChEMBL
| Assay Description
Inhibition of HCK (unknown origin) |
Bioorg Med Chem 25: 4259-4264 (2017)
Article DOI: 10.1016/j.bmc.2017.05.053
BindingDB Entry DOI: 10.7270/Q2SB4875 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase HCK
(Homo sapiens (Human)) | BDBM8793

(7-[4-(4-methylpiperazin-1-yl)cyclohexyl]-5-(4-phen...)Show SMILES CN1CCN(CC1)[C@H]1CC[C@@H](CC1)n1cc(-c2ccc(Oc3ccccc3)cc2)c2c(N)ncnc12 |r,wU:10.14,wD:7.7,(-.91,-10.51,;-1.37,-9.04,;-2.87,-8.71,;-3.34,-7.24,;-2.3,-6.11,;-.8,-6.44,;-.33,-7.91,;-2.72,-4.63,;-1.64,-3.52,;-2.06,-2.04,;-3.55,-1.66,;-4.63,-2.76,;-4.21,-4.25,;-4.03,-.2,;-3.12,1.05,;-4.03,2.3,;-3.55,3.76,;-4.69,4.8,;-4.36,6.3,;-2.89,6.77,;-2.56,8.27,;-1.1,8.74,;-.48,10.15,;1.05,10.33,;1.96,9.09,;1.35,7.68,;-.18,7.5,;-1.75,5.73,;-2.08,4.23,;-5.49,1.82,;-6.82,2.59,;-6.82,4.13,;-8.16,1.82,;-8.16,.28,;-6.82,-.49,;-5.49,.28,)| Show InChI InChI=1S/C29H34N6O/c1-33-15-17-34(18-16-33)22-9-11-23(12-10-22)35-19-26(27-28(30)31-20-32-29(27)35)21-7-13-25(14-8-21)36-24-5-3-2-4-6-24/h2-8,13-14,19-20,22-23H,9-12,15-18H2,1H3,(H2,30,31,32)/t22-,23- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN Center for Life Science Technologies
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HCK SH3-SH2-KD (75 to 526 residues) after 20 mins by mobility shift assay |
Bioorg Med Chem Lett 27: 4994-4998 (2017)
Article DOI: 10.1016/j.bmcl.2017.10.012
BindingDB Entry DOI: 10.7270/Q2X92DV1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase HCK
(Homo sapiens (Human)) | BDBM8793

(7-[4-(4-methylpiperazin-1-yl)cyclohexyl]-5-(4-phen...)Show SMILES CN1CCN(CC1)[C@H]1CC[C@@H](CC1)n1cc(-c2ccc(Oc3ccccc3)cc2)c2c(N)ncnc12 |r,wU:10.14,wD:7.7,(-.91,-10.51,;-1.37,-9.04,;-2.87,-8.71,;-3.34,-7.24,;-2.3,-6.11,;-.8,-6.44,;-.33,-7.91,;-2.72,-4.63,;-1.64,-3.52,;-2.06,-2.04,;-3.55,-1.66,;-4.63,-2.76,;-4.21,-4.25,;-4.03,-.2,;-3.12,1.05,;-4.03,2.3,;-3.55,3.76,;-4.69,4.8,;-4.36,6.3,;-2.89,6.77,;-2.56,8.27,;-1.1,8.74,;-.48,10.15,;1.05,10.33,;1.96,9.09,;1.35,7.68,;-.18,7.5,;-1.75,5.73,;-2.08,4.23,;-5.49,1.82,;-6.82,2.59,;-6.82,4.13,;-8.16,1.82,;-8.16,.28,;-6.82,-.49,;-5.49,.28,)| Show InChI InChI=1S/C29H34N6O/c1-33-15-17-34(18-16-33)22-9-11-23(12-10-22)35-19-26(27-28(30)31-20-32-29(27)35)21-7-13-25(14-8-21)36-24-5-3-2-4-6-24/h2-8,13-14,19-20,22-23H,9-12,15-18H2,1H3,(H2,30,31,32)/t22-,23- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.427 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN Center for Life Science Technologies
Curated by ChEMBL
| Assay Description
Inhibition of HCK (75 to 526 residues) (unknown origin) expressed in Sf9 insect cells after 120 mins |
Bioorg Med Chem 25: 4259-4264 (2017)
Article DOI: 10.1016/j.bmc.2017.05.053
BindingDB Entry DOI: 10.7270/Q2SB4875 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase HCK
(Homo sapiens (Human)) | BDBM8793

(7-[4-(4-methylpiperazin-1-yl)cyclohexyl]-5-(4-phen...)Show SMILES CN1CCN(CC1)[C@H]1CC[C@@H](CC1)n1cc(-c2ccc(Oc3ccccc3)cc2)c2c(N)ncnc12 |r,wU:10.14,wD:7.7,(-.91,-10.51,;-1.37,-9.04,;-2.87,-8.71,;-3.34,-7.24,;-2.3,-6.11,;-.8,-6.44,;-.33,-7.91,;-2.72,-4.63,;-1.64,-3.52,;-2.06,-2.04,;-3.55,-1.66,;-4.63,-2.76,;-4.21,-4.25,;-4.03,-.2,;-3.12,1.05,;-4.03,2.3,;-3.55,3.76,;-4.69,4.8,;-4.36,6.3,;-2.89,6.77,;-2.56,8.27,;-1.1,8.74,;-.48,10.15,;1.05,10.33,;1.96,9.09,;1.35,7.68,;-.18,7.5,;-1.75,5.73,;-2.08,4.23,;-5.49,1.82,;-6.82,2.59,;-6.82,4.13,;-8.16,1.82,;-8.16,.28,;-6.82,-.49,;-5.49,.28,)| Show InChI InChI=1S/C29H34N6O/c1-33-15-17-34(18-16-33)22-9-11-23(12-10-22)35-19-26(27-28(30)31-20-32-29(27)35)21-7-13-25(14-8-21)36-24-5-3-2-4-6-24/h2-8,13-14,19-20,22-23H,9-12,15-18H2,1H3,(H2,30,31,32)/t22-,23- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN Center for Life Science Technologies
Curated by ChEMBL
| Assay Description
Inhibition of human HCK (81 to 526 residues) expressed in Sf9 insect cells after 120 mins |
Bioorg Med Chem 25: 4259-4264 (2017)
Article DOI: 10.1016/j.bmc.2017.05.053
BindingDB Entry DOI: 10.7270/Q2SB4875 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase HCK
(Homo sapiens (Human)) | BDBM50451482

(CHEMBL4208645)Show SMILES Nc1ncnc2n(cc(-c3ccc(Oc4ccccc4)cc3)c12)[C@H]1CC[C@@H](CC1)N[C@@H](CO)C(O)=O |r,wU:23.26,wD:26.33,30.35,(20.08,-15.89,;20.08,-17.43,;18.76,-18.2,;18.76,-19.75,;20.08,-20.51,;21.43,-19.74,;22.89,-20.2,;23.8,-18.95,;22.88,-17.71,;23.35,-16.24,;24.86,-15.92,;25.33,-14.46,;24.29,-13.32,;24.75,-11.85,;26.25,-11.52,;27.29,-12.66,;28.79,-12.33,;29.25,-10.86,;28.21,-9.72,;26.71,-10.05,;22.77,-13.66,;22.32,-15.12,;21.42,-18.2,;23.38,-21.67,;24.89,-21.97,;25.37,-23.43,;24.34,-24.58,;22.83,-24.27,;22.35,-22.81,;24.81,-26.04,;26.31,-26.36,;26.79,-27.81,;25.77,-28.95,;27.34,-25.21,;28.83,-25.53,;26.86,-23.76,)| Show InChI InChI=1S/C27H29N5O4/c28-25-24-22(17-6-12-21(13-7-17)36-20-4-2-1-3-5-20)14-32(26(24)30-16-29-25)19-10-8-18(9-11-19)31-23(15-33)27(34)35/h1-7,12-14,16,18-19,23,31,33H,8-11,15H2,(H,34,35)(H2,28,29,30)/t18-,19-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN Center for Life Science Technologies
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HCK SH3-SH2-KD (75 to 526 residues) after 20 mins by mobility shift assay |
Bioorg Med Chem Lett 27: 4994-4998 (2017)
Article DOI: 10.1016/j.bmcl.2017.10.012
BindingDB Entry DOI: 10.7270/Q2X92DV1 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50451491
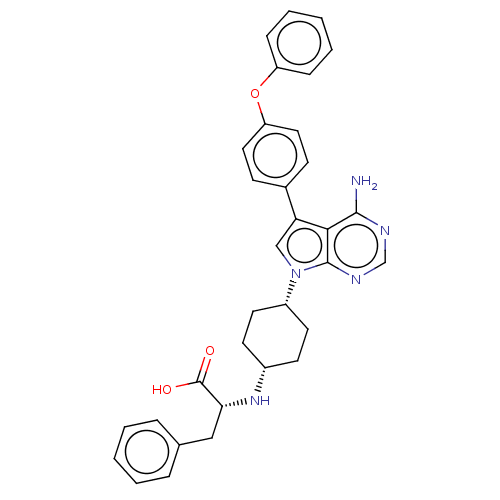
(CHEMBL4210294)Show SMILES Nc1ncnc2n(cc(-c3ccc(Oc4ccccc4)cc3)c12)[C@@H]1CC[C@@H](CC1)N[C@H](Cc1ccccc1)C(O)=O |r,wU:23.26,26.33,30.35,(20.09,-15.9,;20.09,-17.44,;18.77,-18.22,;18.77,-19.76,;20.1,-20.53,;21.44,-19.75,;22.91,-20.22,;23.82,-18.96,;22.9,-17.72,;23.37,-16.25,;24.88,-15.93,;25.34,-14.47,;24.3,-13.33,;24.77,-11.86,;26.27,-11.52,;27.31,-12.67,;28.81,-12.34,;29.27,-10.87,;28.23,-9.73,;26.73,-10.06,;22.79,-13.67,;22.33,-15.13,;21.43,-18.21,;23.4,-21.68,;22.37,-22.83,;22.85,-24.29,;24.36,-24.6,;25.39,-23.45,;24.91,-21.98,;24.83,-26.06,;26.33,-26.37,;26.81,-27.83,;28.31,-28.15,;28.78,-29.6,;30.28,-29.92,;31.3,-28.78,;30.82,-27.32,;29.32,-27.01,;27.36,-25.23,;28.86,-25.55,;26.88,-23.78,)| Show InChI InChI=1S/C33H33N5O3/c34-31-30-28(23-11-17-27(18-12-23)41-26-9-5-2-6-10-26)20-38(32(30)36-21-35-31)25-15-13-24(14-16-25)37-29(33(39)40)19-22-7-3-1-4-8-22/h1-12,17-18,20-21,24-25,29,37H,13-16,19H2,(H,39,40)(H2,34,35,36)/t24-,25+,29-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN Center for Life Science Technologies
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human C-terminal FLT3 ITD mutant (571 to 993 residues) after 90 mins by mobility shift assay |
Bioorg Med Chem Lett 27: 4994-4998 (2017)
Article DOI: 10.1016/j.bmcl.2017.10.012
BindingDB Entry DOI: 10.7270/Q2X92DV1 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50451490
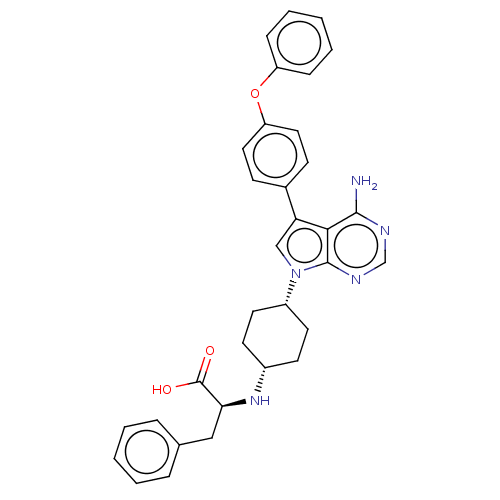
(CHEMBL4204499)Show SMILES Nc1ncnc2n(cc(-c3ccc(Oc4ccccc4)cc3)c12)[C@@H]1CC[C@@H](CC1)N[C@@H](Cc1ccccc1)C(O)=O |r,wU:23.26,26.33,wD:30.35,(20.09,-15.9,;20.09,-17.44,;18.77,-18.22,;18.77,-19.76,;20.1,-20.53,;21.44,-19.75,;22.91,-20.22,;23.82,-18.96,;22.9,-17.72,;23.37,-16.25,;24.88,-15.93,;25.34,-14.47,;24.3,-13.33,;24.77,-11.86,;26.27,-11.52,;27.31,-12.67,;28.81,-12.34,;29.27,-10.87,;28.23,-9.73,;26.73,-10.06,;22.79,-13.67,;22.33,-15.13,;21.43,-18.21,;23.4,-21.68,;22.37,-22.83,;22.85,-24.29,;24.36,-24.6,;25.39,-23.45,;24.91,-21.98,;24.83,-26.06,;26.33,-26.37,;26.81,-27.83,;28.31,-28.15,;28.78,-29.6,;30.28,-29.92,;31.3,-28.78,;30.82,-27.32,;29.32,-27.01,;27.36,-25.23,;28.86,-25.55,;26.88,-23.78,)| Show InChI InChI=1S/C33H33N5O3/c34-31-30-28(23-11-17-27(18-12-23)41-26-9-5-2-6-10-26)20-38(32(30)36-21-35-31)25-15-13-24(14-16-25)37-29(33(39)40)19-22-7-3-1-4-8-22/h1-12,17-18,20-21,24-25,29,37H,13-16,19H2,(H,39,40)(H2,34,35,36)/t24-,25+,29-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN Center for Life Science Technologies
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human C-terminal FLT3 ITD mutant (571 to 993 residues) after 90 mins by mobility shift assay |
Bioorg Med Chem Lett 27: 4994-4998 (2017)
Article DOI: 10.1016/j.bmcl.2017.10.012
BindingDB Entry DOI: 10.7270/Q2X92DV1 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase HCK
(Homo sapiens (Human)) | BDBM50268923

(CHEMBL4095434)Show SMILES Nc1ncnc2n(cc(-c3ccc(Oc4ccccc4)cc3)c12)[C@H]1CC[C@@H](CC1)N1CCN(CCO)CC1 |r,wU:23.26,wD:26.33,(17.06,-10.23,;17.07,-11.77,;15.74,-12.54,;15.74,-14.09,;17.07,-14.85,;18.41,-14.09,;19.89,-14.55,;20.8,-13.29,;19.88,-12.05,;20.35,-10.58,;21.85,-10.26,;22.32,-8.79,;21.28,-7.65,;21.74,-6.18,;23.24,-5.85,;24.28,-6.99,;25.79,-6.66,;26.25,-5.19,;25.21,-4.05,;23.71,-4.39,;19.76,-7.99,;19.31,-9.45,;18.4,-12.53,;20.38,-16.01,;21.88,-16.32,;22.36,-17.79,;21.33,-18.94,;19.82,-18.62,;19.35,-17.16,;21.81,-20.39,;20.78,-21.53,;21.25,-22.98,;22.75,-23.3,;23.22,-24.76,;22.2,-25.91,;22.67,-27.37,;23.78,-22.16,;23.31,-20.69,)| Show InChI InChI=1S/C30H36N6O2/c31-29-28-27(22-6-12-26(13-7-22)38-25-4-2-1-3-5-25)20-36(30(28)33-21-32-29)24-10-8-23(9-11-24)35-16-14-34(15-17-35)18-19-37/h1-7,12-13,20-21,23-24,37H,8-11,14-19H2,(H2,31,32,33)/t23-,24- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN Center for Life Science Technologies
Curated by ChEMBL
| Assay Description
Inhibition of HCK (unknown origin) |
Bioorg Med Chem 25: 4259-4264 (2017)
Article DOI: 10.1016/j.bmc.2017.05.053
BindingDB Entry DOI: 10.7270/Q2SB4875 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase HCK
(Homo sapiens (Human)) | BDBM50268923

(CHEMBL4095434)Show SMILES Nc1ncnc2n(cc(-c3ccc(Oc4ccccc4)cc3)c12)[C@H]1CC[C@@H](CC1)N1CCN(CCO)CC1 |r,wU:23.26,wD:26.33,(17.06,-10.23,;17.07,-11.77,;15.74,-12.54,;15.74,-14.09,;17.07,-14.85,;18.41,-14.09,;19.89,-14.55,;20.8,-13.29,;19.88,-12.05,;20.35,-10.58,;21.85,-10.26,;22.32,-8.79,;21.28,-7.65,;21.74,-6.18,;23.24,-5.85,;24.28,-6.99,;25.79,-6.66,;26.25,-5.19,;25.21,-4.05,;23.71,-4.39,;19.76,-7.99,;19.31,-9.45,;18.4,-12.53,;20.38,-16.01,;21.88,-16.32,;22.36,-17.79,;21.33,-18.94,;19.82,-18.62,;19.35,-17.16,;21.81,-20.39,;20.78,-21.53,;21.25,-22.98,;22.75,-23.3,;23.22,-24.76,;22.2,-25.91,;22.67,-27.37,;23.78,-22.16,;23.31,-20.69,)| Show InChI InChI=1S/C30H36N6O2/c31-29-28-27(22-6-12-26(13-7-22)38-25-4-2-1-3-5-25)20-36(30(28)33-21-32-29)24-10-8-23(9-11-24)35-16-14-34(15-17-35)18-19-37/h1-7,12-13,20-21,23-24,37H,8-11,14-19H2,(H2,31,32,33)/t23-,24- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN Center for Life Science Technologies
Curated by ChEMBL
| Assay Description
Inhibition of HCK (unknown origin) |
Bioorg Med Chem 25: 4259-4264 (2017)
Article DOI: 10.1016/j.bmc.2017.05.053
BindingDB Entry DOI: 10.7270/Q2SB4875 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase HCK
(Homo sapiens (Human)) | BDBM50268922

(CHEMBL4066401)Show SMILES Nc1ncnc2n(cc(-c3ccc(Oc4ccccc4)cc3)c12)[C@H]1CC[C@@H](CC1)NCC(O)=O |r,wU:23.26,wD:26.33,(28.73,-10.35,;28.73,-11.89,;27.41,-12.66,;27.4,-14.21,;28.74,-14.98,;30.08,-14.21,;31.56,-14.68,;32.46,-13.42,;31.54,-12.17,;32.01,-10.7,;33.51,-10.38,;33.98,-8.92,;32.94,-7.78,;33.41,-6.31,;34.91,-5.98,;35.95,-7.11,;37.45,-6.78,;37.92,-5.32,;36.87,-4.18,;35.37,-4.51,;31.43,-8.11,;30.97,-9.58,;30.07,-12.66,;32.04,-16.13,;33.54,-16.44,;34.02,-17.91,;33,-19.06,;31.49,-18.74,;31.01,-17.28,;33.47,-20.51,;34.95,-20.91,;35.35,-22.39,;34.27,-23.48,;36.84,-22.79,)| Show InChI InChI=1S/C26H27N5O3/c27-25-24-22(17-6-12-21(13-7-17)34-20-4-2-1-3-5-20)15-31(26(24)30-16-29-25)19-10-8-18(9-11-19)28-14-23(32)33/h1-7,12-13,15-16,18-19,28H,8-11,14H2,(H,32,33)(H2,27,29,30)/t18-,19- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN Center for Life Science Technologies
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HCK SH3-SH2-KD (75 to 526 residues) after 20 mins by mobility shift assay |
Bioorg Med Chem Lett 27: 4994-4998 (2017)
Article DOI: 10.1016/j.bmcl.2017.10.012
BindingDB Entry DOI: 10.7270/Q2X92DV1 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase HCK
(Homo sapiens (Human)) | BDBM50268922

(CHEMBL4066401)Show SMILES Nc1ncnc2n(cc(-c3ccc(Oc4ccccc4)cc3)c12)[C@H]1CC[C@@H](CC1)NCC(O)=O |r,wU:23.26,wD:26.33,(28.73,-10.35,;28.73,-11.89,;27.41,-12.66,;27.4,-14.21,;28.74,-14.98,;30.08,-14.21,;31.56,-14.68,;32.46,-13.42,;31.54,-12.17,;32.01,-10.7,;33.51,-10.38,;33.98,-8.92,;32.94,-7.78,;33.41,-6.31,;34.91,-5.98,;35.95,-7.11,;37.45,-6.78,;37.92,-5.32,;36.87,-4.18,;35.37,-4.51,;31.43,-8.11,;30.97,-9.58,;30.07,-12.66,;32.04,-16.13,;33.54,-16.44,;34.02,-17.91,;33,-19.06,;31.49,-18.74,;31.01,-17.28,;33.47,-20.51,;34.95,-20.91,;35.35,-22.39,;34.27,-23.48,;36.84,-22.79,)| Show InChI InChI=1S/C26H27N5O3/c27-25-24-22(17-6-12-21(13-7-17)34-20-4-2-1-3-5-20)15-31(26(24)30-16-29-25)19-10-8-18(9-11-19)28-14-23(32)33/h1-7,12-13,15-16,18-19,28H,8-11,14H2,(H,32,33)(H2,27,29,30)/t18-,19- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN Center for Life Science Technologies
Curated by ChEMBL
| Assay Description
Inhibition of HCK (unknown origin) |
Bioorg Med Chem 25: 4259-4264 (2017)
Article DOI: 10.1016/j.bmc.2017.05.053
BindingDB Entry DOI: 10.7270/Q2SB4875 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase HCK
(Homo sapiens (Human)) | BDBM50268922

(CHEMBL4066401)Show SMILES Nc1ncnc2n(cc(-c3ccc(Oc4ccccc4)cc3)c12)[C@H]1CC[C@@H](CC1)NCC(O)=O |r,wU:23.26,wD:26.33,(28.73,-10.35,;28.73,-11.89,;27.41,-12.66,;27.4,-14.21,;28.74,-14.98,;30.08,-14.21,;31.56,-14.68,;32.46,-13.42,;31.54,-12.17,;32.01,-10.7,;33.51,-10.38,;33.98,-8.92,;32.94,-7.78,;33.41,-6.31,;34.91,-5.98,;35.95,-7.11,;37.45,-6.78,;37.92,-5.32,;36.87,-4.18,;35.37,-4.51,;31.43,-8.11,;30.97,-9.58,;30.07,-12.66,;32.04,-16.13,;33.54,-16.44,;34.02,-17.91,;33,-19.06,;31.49,-18.74,;31.01,-17.28,;33.47,-20.51,;34.95,-20.91,;35.35,-22.39,;34.27,-23.48,;36.84,-22.79,)| Show InChI InChI=1S/C26H27N5O3/c27-25-24-22(17-6-12-21(13-7-17)34-20-4-2-1-3-5-20)15-31(26(24)30-16-29-25)19-10-8-18(9-11-19)28-14-23(32)33/h1-7,12-13,15-16,18-19,28H,8-11,14H2,(H,32,33)(H2,27,29,30)/t18-,19- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN Center for Life Science Technologies
Curated by ChEMBL
| Assay Description
Inhibition of HCK (unknown origin) |
Bioorg Med Chem 25: 4259-4264 (2017)
Article DOI: 10.1016/j.bmc.2017.05.053
BindingDB Entry DOI: 10.7270/Q2SB4875 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase HCK
(Homo sapiens (Human)) | BDBM50268912

(CHEMBL4093003)Show SMILES CC(C)C[C@H](N[C@H]1CC[C@@H](CC1)n1cc(-c2ccc(Oc3ccccc3)cc2)c2c(N)ncnc12)C(O)=O |r,wU:9.12,wD:6.5,4.3,(47.66,-23.12,;47.18,-21.66,;45.67,-21.34,;48.21,-20.51,;47.73,-19.05,;46.22,-18.73,;45.74,-17.28,;46.77,-16.13,;46.29,-14.66,;44.78,-14.35,;43.76,-15.5,;44.24,-16.96,;44.3,-12.9,;45.21,-11.64,;44.29,-10.39,;44.76,-8.92,;46.26,-8.6,;46.73,-7.13,;45.69,-5.99,;46.16,-4.53,;47.66,-4.2,;48.69,-5.33,;50.2,-5,;50.66,-3.53,;49.62,-2.39,;48.12,-2.73,;44.18,-6.33,;43.72,-7.8,;42.82,-10.87,;41.48,-10.11,;41.48,-8.57,;40.15,-10.88,;40.15,-12.43,;41.49,-13.2,;42.83,-12.43,;48.75,-17.9,;50.26,-18.21,;48.27,-16.44,)| Show InChI InChI=1S/C30H35N5O3/c1-19(2)16-26(30(36)37)34-21-10-12-22(13-11-21)35-17-25(27-28(31)32-18-33-29(27)35)20-8-14-24(15-9-20)38-23-6-4-3-5-7-23/h3-9,14-15,17-19,21-22,26,34H,10-13,16H2,1-2H3,(H,36,37)(H2,31,32,33)/t21-,22-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN Center for Life Science Technologies
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HCK SH3-SH2-KD (75 to 526 residues) after 20 mins by mobility shift assay |
Bioorg Med Chem Lett 27: 4994-4998 (2017)
Article DOI: 10.1016/j.bmcl.2017.10.012
BindingDB Entry DOI: 10.7270/Q2X92DV1 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50451504
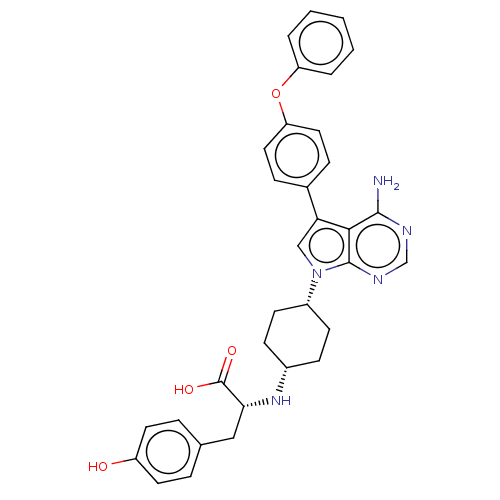
(CHEMBL4218162)Show SMILES Nc1ncnc2n(cc(-c3ccc(Oc4ccccc4)cc3)c12)[C@@H]1CC[C@@H](CC1)N[C@H](Cc1ccc(O)cc1)C(O)=O |r,wU:23.26,26.33,30.35,(20.09,-15.9,;20.09,-17.44,;18.77,-18.22,;18.77,-19.76,;20.1,-20.53,;21.44,-19.75,;22.91,-20.22,;23.82,-18.96,;22.9,-17.72,;23.37,-16.25,;24.88,-15.93,;25.34,-14.47,;24.3,-13.33,;24.77,-11.86,;26.27,-11.52,;27.31,-12.67,;28.81,-12.34,;29.27,-10.87,;28.23,-9.73,;26.73,-10.06,;22.79,-13.67,;22.33,-15.13,;21.43,-18.21,;23.4,-21.68,;22.37,-22.83,;22.85,-24.29,;24.36,-24.6,;25.39,-23.45,;24.91,-21.98,;24.83,-26.06,;26.33,-26.37,;26.81,-27.83,;28.31,-28.15,;28.78,-29.6,;30.28,-29.92,;31.3,-28.78,;32.8,-29.09,;30.82,-27.32,;29.32,-27.01,;27.36,-25.23,;28.86,-25.55,;26.88,-23.78,)| Show InChI InChI=1S/C33H33N5O4/c34-31-30-28(22-8-16-27(17-9-22)42-26-4-2-1-3-5-26)19-38(32(30)36-20-35-31)24-12-10-23(11-13-24)37-29(33(40)41)18-21-6-14-25(39)15-7-21/h1-9,14-17,19-20,23-24,29,37,39H,10-13,18H2,(H,40,41)(H2,34,35,36)/t23-,24+,29-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN Center for Life Science Technologies
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human C-terminal FLT3 ITD mutant (571 to 993 residues) after 90 mins by mobility shift assay |
Bioorg Med Chem Lett 27: 4994-4998 (2017)
Article DOI: 10.1016/j.bmcl.2017.10.012
BindingDB Entry DOI: 10.7270/Q2X92DV1 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50451501

(CHEMBL4214689)Show SMILES Nc1ncnc2n(cc(-c3ccc(Oc4ccccc4)cc3)c12)[C@@H]1CC[C@@H](CC1)N[C@@H](Cc1ccc(O)cc1)C(O)=O |r,wU:23.26,26.33,wD:30.35,(20.09,-15.9,;20.09,-17.44,;18.77,-18.22,;18.77,-19.76,;20.1,-20.53,;21.44,-19.75,;22.91,-20.22,;23.82,-18.96,;22.9,-17.72,;23.37,-16.25,;24.88,-15.93,;25.34,-14.47,;24.3,-13.33,;24.77,-11.86,;26.27,-11.52,;27.31,-12.67,;28.81,-12.34,;29.27,-10.87,;28.23,-9.73,;26.73,-10.06,;22.79,-13.67,;22.33,-15.13,;21.43,-18.21,;23.4,-21.68,;22.37,-22.83,;22.85,-24.29,;24.36,-24.6,;25.39,-23.45,;24.91,-21.98,;24.83,-26.06,;26.33,-26.37,;26.81,-27.83,;28.31,-28.15,;28.78,-29.6,;30.28,-29.92,;31.3,-28.78,;32.8,-29.09,;30.82,-27.32,;29.32,-27.01,;27.36,-25.23,;28.86,-25.55,;26.88,-23.78,)| Show InChI InChI=1S/C33H33N5O4/c34-31-30-28(22-8-16-27(17-9-22)42-26-4-2-1-3-5-26)19-38(32(30)36-20-35-31)24-12-10-23(11-13-24)37-29(33(40)41)18-21-6-14-25(39)15-7-21/h1-9,14-17,19-20,23-24,29,37,39H,10-13,18H2,(H,40,41)(H2,34,35,36)/t23-,24+,29-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN Center for Life Science Technologies
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human C-terminal FLT3 ITD mutant (571 to 993 residues) after 90 mins by mobility shift assay |
Bioorg Med Chem Lett 27: 4994-4998 (2017)
Article DOI: 10.1016/j.bmcl.2017.10.012
BindingDB Entry DOI: 10.7270/Q2X92DV1 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase HCK
(Homo sapiens (Human)) | BDBM50451468
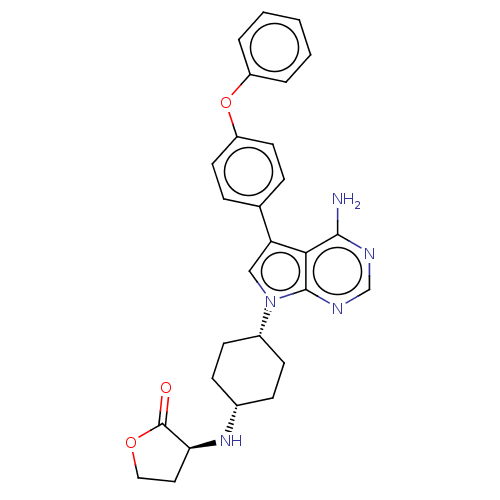
(CHEMBL4207885)Show SMILES [H][C@@]1(CCOC1=O)N[C@H]1CC[C@H](CC1)n1cc(-c2ccc(Oc3ccccc3)cc2)c2c(N)ncnc12 |r,wU:11.15,8.8,1.0,(25.23,-27.44,;26.32,-26.36,;26.95,-27.76,;28.48,-27.6,;28.79,-26.1,;27.46,-25.33,;27.3,-23.81,;24.82,-26.05,;24.35,-24.59,;22.84,-24.28,;22.36,-22.82,;23.39,-21.67,;24.9,-21.97,;25.38,-23.44,;22.9,-20.21,;23.81,-18.96,;22.89,-17.71,;23.36,-16.25,;24.87,-15.93,;25.33,-14.46,;24.29,-13.33,;24.76,-11.86,;26.26,-11.52,;27.3,-12.66,;28.8,-12.33,;29.26,-10.86,;28.22,-9.72,;26.72,-10.06,;22.78,-13.66,;22.32,-15.13,;21.42,-18.2,;20.09,-17.44,;20.08,-15.9,;18.76,-18.21,;18.76,-19.75,;20.09,-20.52,;21.43,-19.75,)| Show InChI InChI=1S/C28H29N5O3/c29-26-25-23(18-6-12-22(13-7-18)36-21-4-2-1-3-5-21)16-33(27(25)31-17-30-26)20-10-8-19(9-11-20)32-24-14-15-35-28(24)34/h1-7,12-13,16-17,19-20,24,32H,8-11,14-15H2,(H2,29,30,31)/t19-,20+,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN Center for Life Science Technologies
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HCK SH3-SH2-KD (75 to 526 residues) after 20 mins by mobility shift assay |
Bioorg Med Chem Lett 27: 4994-4998 (2017)
Article DOI: 10.1016/j.bmcl.2017.10.012
BindingDB Entry DOI: 10.7270/Q2X92DV1 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase HCK
(Homo sapiens (Human)) | BDBM50451483
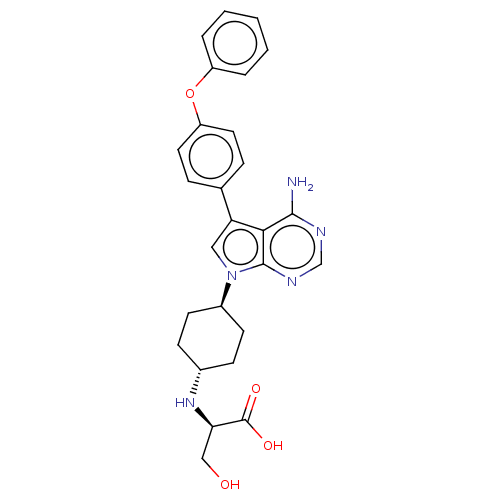
(CHEMBL4215201)Show SMILES Nc1ncnc2n(cc(-c3ccc(Oc4ccccc4)cc3)c12)[C@H]1CC[C@@H](CC1)N[C@H](CO)C(O)=O |r,wU:23.26,30.35,wD:26.33,(20.08,-15.89,;20.08,-17.43,;18.76,-18.2,;18.76,-19.75,;20.08,-20.51,;21.43,-19.74,;22.89,-20.2,;23.8,-18.95,;22.88,-17.71,;23.35,-16.24,;24.86,-15.92,;25.33,-14.46,;24.29,-13.32,;24.75,-11.85,;26.25,-11.52,;27.29,-12.66,;28.79,-12.33,;29.25,-10.86,;28.21,-9.72,;26.71,-10.05,;22.77,-13.66,;22.32,-15.12,;21.42,-18.2,;23.38,-21.67,;24.89,-21.97,;25.37,-23.43,;24.34,-24.58,;22.83,-24.27,;22.35,-22.81,;24.81,-26.04,;26.31,-26.36,;26.79,-27.81,;25.77,-28.95,;27.34,-25.21,;28.83,-25.53,;26.86,-23.76,)| Show InChI InChI=1S/C27H29N5O4/c28-25-24-22(17-6-12-21(13-7-17)36-20-4-2-1-3-5-20)14-32(26(24)30-16-29-25)19-10-8-18(9-11-19)31-23(15-33)27(34)35/h1-7,12-14,16,18-19,23,31,33H,8-11,15H2,(H,34,35)(H2,28,29,30)/t18-,19-,23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN Center for Life Science Technologies
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HCK SH3-SH2-KD (75 to 526 residues) after 20 mins by mobility shift assay |
Bioorg Med Chem Lett 27: 4994-4998 (2017)
Article DOI: 10.1016/j.bmcl.2017.10.012
BindingDB Entry DOI: 10.7270/Q2X92DV1 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase HCK
(Homo sapiens (Human)) | BDBM50451477

(CHEMBL4206460)Show SMILES C[C@H](N[C@H]1CC[C@@H](CC1)n1cc(-c2ccc(Oc3ccccc3)cc2)c2c(N)ncnc12)C(O)=O |r,wU:6.9,wD:3.2,1.0,(26.79,-27.81,;26.31,-26.36,;24.81,-26.04,;24.34,-24.58,;25.37,-23.43,;24.89,-21.97,;23.38,-21.67,;22.35,-22.81,;22.83,-24.27,;22.89,-20.2,;23.8,-18.95,;22.88,-17.71,;23.35,-16.24,;24.86,-15.92,;25.33,-14.46,;24.29,-13.32,;24.75,-11.85,;26.25,-11.52,;27.29,-12.66,;28.79,-12.33,;29.25,-10.86,;28.21,-9.72,;26.71,-10.05,;22.77,-13.66,;22.32,-15.12,;21.42,-18.2,;20.08,-17.43,;20.08,-15.89,;18.76,-18.2,;18.76,-19.75,;20.08,-20.51,;21.43,-19.74,;27.34,-25.21,;28.83,-25.53,;26.86,-23.76,)| Show InChI InChI=1S/C27H29N5O3/c1-17(27(33)34)31-19-9-11-20(12-10-19)32-15-23(24-25(28)29-16-30-26(24)32)18-7-13-22(14-8-18)35-21-5-3-2-4-6-21/h2-8,13-17,19-20,31H,9-12H2,1H3,(H,33,34)(H2,28,29,30)/t17-,19-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN Center for Life Science Technologies
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HCK SH3-SH2-KD (75 to 526 residues) after 20 mins by mobility shift assay |
Bioorg Med Chem Lett 27: 4994-4998 (2017)
Article DOI: 10.1016/j.bmcl.2017.10.012
BindingDB Entry DOI: 10.7270/Q2X92DV1 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase HCK
(Homo sapiens (Human)) | BDBM50451499
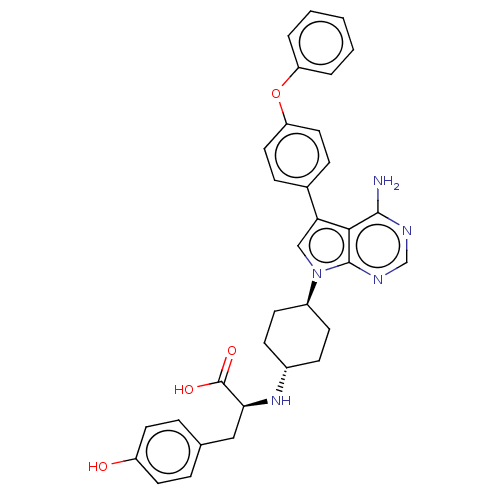
(CHEMBL4209781)Show SMILES Nc1ncnc2n(cc(-c3ccc(Oc4ccccc4)cc3)c12)[C@H]1CC[C@@H](CC1)N[C@@H](Cc1ccc(O)cc1)C(O)=O |r,wU:23.26,wD:26.33,30.35,(20.09,-15.9,;20.09,-17.44,;18.77,-18.22,;18.77,-19.76,;20.1,-20.53,;21.44,-19.75,;22.91,-20.22,;23.82,-18.96,;22.9,-17.72,;23.37,-16.25,;24.88,-15.93,;25.34,-14.47,;24.3,-13.33,;24.77,-11.86,;26.27,-11.52,;27.31,-12.67,;28.81,-12.34,;29.27,-10.87,;28.23,-9.73,;26.73,-10.06,;22.79,-13.67,;22.33,-15.13,;21.43,-18.21,;23.4,-21.68,;24.91,-21.98,;25.39,-23.45,;24.36,-24.6,;22.85,-24.29,;22.37,-22.83,;24.83,-26.06,;26.33,-26.37,;26.81,-27.83,;28.31,-28.15,;28.78,-29.6,;30.28,-29.92,;31.3,-28.78,;32.82,-29.09,;30.82,-27.32,;29.32,-27.01,;27.36,-25.23,;28.86,-25.55,;26.88,-23.78,)| Show InChI InChI=1S/C33H33N5O4/c34-31-30-28(22-8-16-27(17-9-22)42-26-4-2-1-3-5-26)19-38(32(30)36-20-35-31)24-12-10-23(11-13-24)37-29(33(40)41)18-21-6-14-25(39)15-7-21/h1-9,14-17,19-20,23-24,29,37,39H,10-13,18H2,(H,40,41)(H2,34,35,36)/t23-,24-,29-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN Center for Life Science Technologies
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HCK SH3-SH2-KD (75 to 526 residues) after 20 mins by mobility shift assay |
Bioorg Med Chem Lett 27: 4994-4998 (2017)
Article DOI: 10.1016/j.bmcl.2017.10.012
BindingDB Entry DOI: 10.7270/Q2X92DV1 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase HCK
(Homo sapiens (Human)) | BDBM50451489
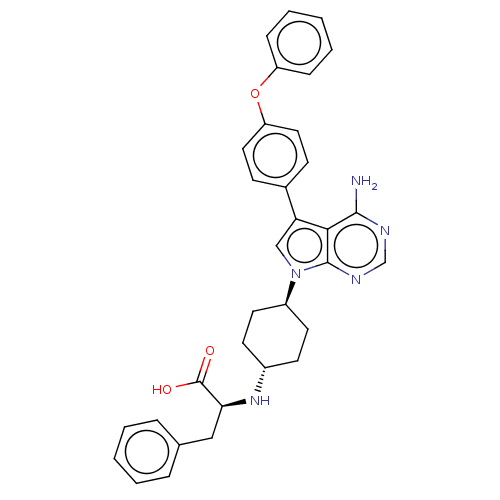
(CHEMBL4216163)Show SMILES Nc1ncnc2n(cc(-c3ccc(Oc4ccccc4)cc3)c12)[C@H]1CC[C@@H](CC1)N[C@@H](Cc1ccccc1)C(O)=O |r,wU:23.26,wD:26.33,30.35,(20.09,-15.9,;20.09,-17.44,;18.77,-18.22,;18.77,-19.76,;20.1,-20.53,;21.44,-19.75,;22.91,-20.22,;23.82,-18.96,;22.9,-17.72,;23.37,-16.25,;24.88,-15.93,;25.34,-14.47,;24.3,-13.33,;24.77,-11.86,;26.27,-11.52,;27.31,-12.67,;28.81,-12.34,;29.27,-10.87,;28.23,-9.73,;26.73,-10.06,;22.79,-13.67,;22.33,-15.13,;21.43,-18.21,;23.4,-21.68,;24.91,-21.98,;25.39,-23.45,;24.36,-24.6,;22.85,-24.29,;22.37,-22.83,;24.83,-26.06,;26.33,-26.37,;26.81,-27.83,;28.31,-28.15,;28.78,-29.6,;30.28,-29.92,;31.3,-28.78,;30.82,-27.32,;29.32,-27.01,;27.36,-25.23,;28.86,-25.55,;26.88,-23.78,)| Show InChI InChI=1S/C33H33N5O3/c34-31-30-28(23-11-17-27(18-12-23)41-26-9-5-2-6-10-26)20-38(32(30)36-21-35-31)25-15-13-24(14-16-25)37-29(33(39)40)19-22-7-3-1-4-8-22/h1-12,17-18,20-21,24-25,29,37H,13-16,19H2,(H,39,40)(H2,34,35,36)/t24-,25-,29-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN Center for Life Science Technologies
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HCK SH3-SH2-KD (75 to 526 residues) after 20 mins by mobility shift assay |
Bioorg Med Chem Lett 27: 4994-4998 (2017)
Article DOI: 10.1016/j.bmcl.2017.10.012
BindingDB Entry DOI: 10.7270/Q2X92DV1 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase HCK
(Homo sapiens (Human)) | BDBM50268925

(CHEMBL4087733)Show SMILES NC(=O)CN[C@H]1CC[C@@H](CC1)n1cc(-c2ccc(Oc3ccccc3)cc2)c2c(N)ncnc12 |r,wU:8.11,wD:5.4,(48.2,-23.66,;49.28,-22.57,;50.77,-22.97,;48.88,-21.09,;47.4,-20.69,;46.93,-19.24,;47.95,-18.09,;47.47,-16.62,;45.97,-16.31,;44.94,-17.46,;45.42,-18.92,;45.49,-14.86,;46.39,-13.6,;45.47,-12.35,;45.94,-10.88,;47.44,-10.56,;47.91,-9.09,;46.87,-7.95,;47.34,-6.49,;48.84,-6.16,;49.88,-7.29,;51.38,-6.96,;51.85,-5.49,;50.8,-4.35,;49.3,-4.69,;45.36,-8.29,;44.9,-9.76,;44,-12.83,;42.66,-12.07,;42.66,-10.53,;41.34,-12.84,;41.33,-14.39,;42.67,-15.16,;44.01,-14.39,)| Show InChI InChI=1S/C26H28N6O2/c27-23(33)14-29-18-8-10-19(11-9-18)32-15-22(24-25(28)30-16-31-26(24)32)17-6-12-21(13-7-17)34-20-4-2-1-3-5-20/h1-7,12-13,15-16,18-19,29H,8-11,14H2,(H2,27,33)(H2,28,30,31)/t18-,19- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN Center for Life Science Technologies
Curated by ChEMBL
| Assay Description
Inhibition of HCK (unknown origin) |
Bioorg Med Chem 25: 4259-4264 (2017)
Article DOI: 10.1016/j.bmc.2017.05.053
BindingDB Entry DOI: 10.7270/Q2SB4875 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase HCK
(Homo sapiens (Human)) | BDBM50268925

(CHEMBL4087733)Show SMILES NC(=O)CN[C@H]1CC[C@@H](CC1)n1cc(-c2ccc(Oc3ccccc3)cc2)c2c(N)ncnc12 |r,wU:8.11,wD:5.4,(48.2,-23.66,;49.28,-22.57,;50.77,-22.97,;48.88,-21.09,;47.4,-20.69,;46.93,-19.24,;47.95,-18.09,;47.47,-16.62,;45.97,-16.31,;44.94,-17.46,;45.42,-18.92,;45.49,-14.86,;46.39,-13.6,;45.47,-12.35,;45.94,-10.88,;47.44,-10.56,;47.91,-9.09,;46.87,-7.95,;47.34,-6.49,;48.84,-6.16,;49.88,-7.29,;51.38,-6.96,;51.85,-5.49,;50.8,-4.35,;49.3,-4.69,;45.36,-8.29,;44.9,-9.76,;44,-12.83,;42.66,-12.07,;42.66,-10.53,;41.34,-12.84,;41.33,-14.39,;42.67,-15.16,;44.01,-14.39,)| Show InChI InChI=1S/C26H28N6O2/c27-23(33)14-29-18-8-10-19(11-9-18)32-15-22(24-25(28)30-16-31-26(24)32)17-6-12-21(13-7-17)34-20-4-2-1-3-5-20/h1-7,12-13,15-16,18-19,29H,8-11,14H2,(H2,27,33)(H2,28,30,31)/t18-,19- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN Center for Life Science Technologies
Curated by ChEMBL
| Assay Description
Inhibition of HCK (unknown origin) |
Bioorg Med Chem 25: 4259-4264 (2017)
Article DOI: 10.1016/j.bmc.2017.05.053
BindingDB Entry DOI: 10.7270/Q2SB4875 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase HCK
(Homo sapiens (Human)) | BDBM50268921

(CHEMBL4071932)Show SMILES Nc1ncnc2n(cc(-c3ccc(Oc4ccccc4)cc3)c12)[C@H]1CC[C@@H](CC1)NCc1ccncc1 |r,wU:23.26,wD:26.33,(55.45,-12.2,;55.46,-13.74,;54.13,-14.51,;54.13,-16.06,;55.46,-16.83,;56.8,-16.06,;58.28,-16.53,;59.19,-15.27,;58.27,-14.02,;58.73,-12.55,;60.24,-12.23,;60.7,-10.77,;59.67,-9.63,;60.13,-8.16,;61.64,-7.83,;62.67,-8.97,;64.17,-8.64,;64.64,-7.17,;63.59,-6.03,;62.09,-6.36,;58.16,-9.96,;57.7,-11.43,;56.79,-14.51,;58.76,-17.99,;60.27,-18.29,;60.75,-19.76,;59.72,-20.91,;58.21,-20.59,;57.73,-19.14,;60.2,-22.36,;61.68,-22.76,;62.08,-24.24,;60.98,-25.33,;61.39,-26.81,;62.87,-27.21,;63.96,-26.11,;63.56,-24.63,)| Show InChI InChI=1S/C30H30N6O/c31-29-28-27(22-6-12-26(13-7-22)37-25-4-2-1-3-5-25)19-36(30(28)35-20-34-29)24-10-8-23(9-11-24)33-18-21-14-16-32-17-15-21/h1-7,12-17,19-20,23-24,33H,8-11,18H2,(H2,31,34,35)/t23-,24- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN Center for Life Science Technologies
Curated by ChEMBL
| Assay Description
Inhibition of HCK (unknown origin) |
Bioorg Med Chem 25: 4259-4264 (2017)
Article DOI: 10.1016/j.bmc.2017.05.053
BindingDB Entry DOI: 10.7270/Q2SB4875 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase HCK
(Homo sapiens (Human)) | BDBM50268921

(CHEMBL4071932)Show SMILES Nc1ncnc2n(cc(-c3ccc(Oc4ccccc4)cc3)c12)[C@H]1CC[C@@H](CC1)NCc1ccncc1 |r,wU:23.26,wD:26.33,(55.45,-12.2,;55.46,-13.74,;54.13,-14.51,;54.13,-16.06,;55.46,-16.83,;56.8,-16.06,;58.28,-16.53,;59.19,-15.27,;58.27,-14.02,;58.73,-12.55,;60.24,-12.23,;60.7,-10.77,;59.67,-9.63,;60.13,-8.16,;61.64,-7.83,;62.67,-8.97,;64.17,-8.64,;64.64,-7.17,;63.59,-6.03,;62.09,-6.36,;58.16,-9.96,;57.7,-11.43,;56.79,-14.51,;58.76,-17.99,;60.27,-18.29,;60.75,-19.76,;59.72,-20.91,;58.21,-20.59,;57.73,-19.14,;60.2,-22.36,;61.68,-22.76,;62.08,-24.24,;60.98,-25.33,;61.39,-26.81,;62.87,-27.21,;63.96,-26.11,;63.56,-24.63,)| Show InChI InChI=1S/C30H30N6O/c31-29-28-27(22-6-12-26(13-7-22)37-25-4-2-1-3-5-25)19-36(30(28)35-20-34-29)24-10-8-23(9-11-24)33-18-21-14-16-32-17-15-21/h1-7,12-17,19-20,23-24,33H,8-11,18H2,(H2,31,34,35)/t23-,24- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN Center for Life Science Technologies
Curated by ChEMBL
| Assay Description
Inhibition of HCK (unknown origin) |
Bioorg Med Chem 25: 4259-4264 (2017)
Article DOI: 10.1016/j.bmc.2017.05.053
BindingDB Entry DOI: 10.7270/Q2SB4875 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase HCK
(Homo sapiens (Human)) | BDBM50268912

(CHEMBL4093003)Show SMILES CC(C)C[C@H](N[C@H]1CC[C@@H](CC1)n1cc(-c2ccc(Oc3ccccc3)cc2)c2c(N)ncnc12)C(O)=O |r,wU:9.12,wD:6.5,4.3,(47.66,-23.12,;47.18,-21.66,;45.67,-21.34,;48.21,-20.51,;47.73,-19.05,;46.22,-18.73,;45.74,-17.28,;46.77,-16.13,;46.29,-14.66,;44.78,-14.35,;43.76,-15.5,;44.24,-16.96,;44.3,-12.9,;45.21,-11.64,;44.29,-10.39,;44.76,-8.92,;46.26,-8.6,;46.73,-7.13,;45.69,-5.99,;46.16,-4.53,;47.66,-4.2,;48.69,-5.33,;50.2,-5,;50.66,-3.53,;49.62,-2.39,;48.12,-2.73,;44.18,-6.33,;43.72,-7.8,;42.82,-10.87,;41.48,-10.11,;41.48,-8.57,;40.15,-10.88,;40.15,-12.43,;41.49,-13.2,;42.83,-12.43,;48.75,-17.9,;50.26,-18.21,;48.27,-16.44,)| Show InChI InChI=1S/C30H35N5O3/c1-19(2)16-26(30(36)37)34-21-10-12-22(13-11-21)35-17-25(27-28(31)32-18-33-29(27)35)20-8-14-24(15-9-20)38-23-6-4-3-5-7-23/h3-9,14-15,17-19,21-22,26,34H,10-13,16H2,1-2H3,(H,36,37)(H2,31,32,33)/t21-,22-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN Center for Life Science Technologies
Curated by ChEMBL
| Assay Description
Inhibition of HCK (75 to 526 residues) (unknown origin) expressed in Sf9 insect cells after 120 mins |
Bioorg Med Chem 25: 4259-4264 (2017)
Article DOI: 10.1016/j.bmc.2017.05.053
BindingDB Entry DOI: 10.7270/Q2SB4875 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50451502

(CHEMBL4213227)Show SMILES Nc1ncnc2n(cc(-c3ccc(Oc4ccccc4)cc3)c12)[C@H]1CC[C@@H](CC1)N[C@H](Cc1ccc(O)cc1)C(O)=O |r,wU:23.26,30.35,wD:26.33,(20.09,-15.9,;20.09,-17.44,;18.77,-18.22,;18.77,-19.76,;20.1,-20.53,;21.44,-19.75,;22.91,-20.22,;23.82,-18.96,;22.9,-17.72,;23.37,-16.25,;24.88,-15.93,;25.34,-14.47,;24.3,-13.33,;24.77,-11.86,;26.27,-11.52,;27.31,-12.67,;28.81,-12.34,;29.27,-10.87,;28.23,-9.73,;26.73,-10.06,;22.79,-13.67,;22.33,-15.13,;21.43,-18.21,;23.4,-21.68,;24.91,-21.98,;25.39,-23.45,;24.36,-24.6,;22.85,-24.29,;22.37,-22.83,;24.83,-26.06,;26.33,-26.37,;26.81,-27.83,;28.31,-28.15,;28.78,-29.6,;30.28,-29.92,;31.3,-28.78,;32.8,-29.09,;30.82,-27.32,;29.32,-27.01,;27.36,-25.23,;28.86,-25.55,;26.88,-23.78,)| Show InChI InChI=1S/C33H33N5O4/c34-31-30-28(22-8-16-27(17-9-22)42-26-4-2-1-3-5-26)19-38(32(30)36-20-35-31)24-12-10-23(11-13-24)37-29(33(40)41)18-21-6-14-25(39)15-7-21/h1-9,14-17,19-20,23-24,29,37,39H,10-13,18H2,(H,40,41)(H2,34,35,36)/t23-,24-,29-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN Center for Life Science Technologies
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human C-terminal FLT3 ITD mutant (571 to 993 residues) after 90 mins by mobility shift assay |
Bioorg Med Chem Lett 27: 4994-4998 (2017)
Article DOI: 10.1016/j.bmcl.2017.10.012
BindingDB Entry DOI: 10.7270/Q2X92DV1 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase HCK
(Homo sapiens (Human)) | BDBM50268912

(CHEMBL4093003)Show SMILES CC(C)C[C@H](N[C@H]1CC[C@@H](CC1)n1cc(-c2ccc(Oc3ccccc3)cc2)c2c(N)ncnc12)C(O)=O |r,wU:9.12,wD:6.5,4.3,(47.66,-23.12,;47.18,-21.66,;45.67,-21.34,;48.21,-20.51,;47.73,-19.05,;46.22,-18.73,;45.74,-17.28,;46.77,-16.13,;46.29,-14.66,;44.78,-14.35,;43.76,-15.5,;44.24,-16.96,;44.3,-12.9,;45.21,-11.64,;44.29,-10.39,;44.76,-8.92,;46.26,-8.6,;46.73,-7.13,;45.69,-5.99,;46.16,-4.53,;47.66,-4.2,;48.69,-5.33,;50.2,-5,;50.66,-3.53,;49.62,-2.39,;48.12,-2.73,;44.18,-6.33,;43.72,-7.8,;42.82,-10.87,;41.48,-10.11,;41.48,-8.57,;40.15,-10.88,;40.15,-12.43,;41.49,-13.2,;42.83,-12.43,;48.75,-17.9,;50.26,-18.21,;48.27,-16.44,)| Show InChI InChI=1S/C30H35N5O3/c1-19(2)16-26(30(36)37)34-21-10-12-22(13-11-21)35-17-25(27-28(31)32-18-33-29(27)35)20-8-14-24(15-9-20)38-23-6-4-3-5-7-23/h3-9,14-15,17-19,21-22,26,34H,10-13,16H2,1-2H3,(H,36,37)(H2,31,32,33)/t21-,22-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN Center for Life Science Technologies
Curated by ChEMBL
| Assay Description
Inhibition of HCK (75 to 526 residues) (unknown origin) expressed in Sf9 insect cells after 120 mins |
Bioorg Med Chem 25: 4259-4264 (2017)
Article DOI: 10.1016/j.bmc.2017.05.053
BindingDB Entry DOI: 10.7270/Q2SB4875 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase HCK
(Homo sapiens (Human)) | BDBM50451476

(CHEMBL4202763)Show SMILES Nc1ncnc2n(cc(-c3ccc(Oc4ccccc4)cc3)c12)[C@@H]1CC[C@@H](CC1)NCC(O)=O |r,wU:23.26,26.33,(20.08,-15.89,;20.08,-17.43,;18.76,-18.2,;18.76,-19.75,;20.08,-20.51,;21.43,-19.74,;22.89,-20.2,;23.8,-18.95,;22.88,-17.71,;23.35,-16.24,;24.86,-15.92,;25.33,-14.46,;24.29,-13.32,;24.75,-11.85,;26.25,-11.52,;27.29,-12.66,;28.79,-12.33,;29.25,-10.86,;28.21,-9.72,;26.71,-10.05,;22.77,-13.66,;22.32,-15.12,;21.42,-18.2,;23.38,-21.67,;22.35,-22.81,;22.83,-24.27,;24.34,-24.58,;25.37,-23.43,;24.89,-21.97,;24.81,-26.04,;26.31,-26.36,;27.34,-25.21,;28.83,-25.53,;26.86,-23.76,)| Show InChI InChI=1S/C26H27N5O3/c27-25-24-22(17-6-12-21(13-7-17)34-20-4-2-1-3-5-20)15-31(26(24)30-16-29-25)19-10-8-18(9-11-19)28-14-23(32)33/h1-7,12-13,15-16,18-19,28H,8-11,14H2,(H,32,33)(H2,27,29,30)/t18-,19+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN Center for Life Science Technologies
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HCK SH3-SH2-KD (75 to 526 residues) after 20 mins by mobility shift assay |
Bioorg Med Chem Lett 27: 4994-4998 (2017)
Article DOI: 10.1016/j.bmcl.2017.10.012
BindingDB Entry DOI: 10.7270/Q2X92DV1 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50451484

(CHEMBL4203527)Show SMILES Nc1ncnc2n(cc(-c3ccc(Oc4ccccc4)cc3)c12)[C@@H]1CC[C@@H](CC1)N[C@H](CO)C(O)=O |r,wU:23.26,26.33,30.35,(20.08,-15.89,;20.08,-17.43,;18.76,-18.2,;18.76,-19.75,;20.08,-20.51,;21.43,-19.74,;22.89,-20.2,;23.8,-18.95,;22.88,-17.71,;23.35,-16.24,;24.86,-15.92,;25.33,-14.46,;24.29,-13.32,;24.75,-11.85,;26.25,-11.52,;27.29,-12.66,;28.79,-12.33,;29.25,-10.86,;28.21,-9.72,;26.71,-10.05,;22.77,-13.66,;22.32,-15.12,;21.42,-18.2,;23.38,-21.67,;22.35,-22.81,;22.83,-24.27,;24.34,-24.58,;25.37,-23.43,;24.89,-21.97,;24.81,-26.04,;26.31,-26.36,;26.79,-27.81,;25.77,-28.95,;27.34,-25.21,;28.83,-25.53,;26.86,-23.76,)| Show InChI InChI=1S/C27H29N5O4/c28-25-24-22(17-6-12-21(13-7-17)36-20-4-2-1-3-5-20)14-32(26(24)30-16-29-25)19-10-8-18(9-11-19)31-23(15-33)27(34)35/h1-7,12-14,16,18-19,23,31,33H,8-11,15H2,(H,34,35)(H2,28,29,30)/t18-,19+,23-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN Center for Life Science Technologies
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human C-terminal FLT3 ITD mutant (571 to 993 residues) after 90 mins by mobility shift assay |
Bioorg Med Chem Lett 27: 4994-4998 (2017)
Article DOI: 10.1016/j.bmcl.2017.10.012
BindingDB Entry DOI: 10.7270/Q2X92DV1 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase HCK
(Homo sapiens (Human)) | BDBM50268915

(CHEMBL4061078)Show SMILES [H][C@@]1(CCNC1=O)N[C@H]1CC[C@@H](CC1)n1cc(-c2ccc(Oc3ccccc3)cc2)c2c(N)ncnc12 |r,wU:11.15,1.0,wD:8.8,(75.41,-22.35,;76.19,-21.03,;76.83,-22.43,;78.36,-22.26,;78.68,-20.75,;77.34,-19.99,;77.17,-18.46,;74.69,-20.72,;74.21,-19.26,;75.24,-18.11,;74.76,-16.64,;73.25,-16.34,;72.22,-17.49,;72.7,-18.94,;72.77,-14.88,;73.68,-13.62,;72.76,-12.37,;73.22,-10.9,;74.73,-10.58,;75.19,-9.12,;74.16,-7.98,;74.62,-6.51,;76.13,-6.18,;77.16,-7.32,;78.66,-6.99,;79.13,-5.52,;78.08,-4.38,;76.58,-4.71,;72.64,-8.31,;72.18,-9.78,;71.28,-12.86,;69.95,-12.09,;69.94,-10.55,;68.62,-12.86,;68.62,-14.41,;69.95,-15.18,;71.29,-14.41,)| Show InChI InChI=1S/C28H30N6O2/c29-26-25-23(18-6-12-22(13-7-18)36-21-4-2-1-3-5-21)16-34(27(25)32-17-31-26)20-10-8-19(9-11-20)33-24-14-15-30-28(24)35/h1-7,12-13,16-17,19-20,24,33H,8-11,14-15H2,(H,30,35)(H2,29,31,32)/t19-,20-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN Center for Life Science Technologies
Curated by ChEMBL
| Assay Description
Inhibition of HCK (unknown origin) |
Bioorg Med Chem 25: 4259-4264 (2017)
Article DOI: 10.1016/j.bmc.2017.05.053
BindingDB Entry DOI: 10.7270/Q2SB4875 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50451467

(CHEMBL4203767)Show SMILES Nc1ncnc2n(cc(-c3ccc(Oc4ccccc4)cc3)c12)[C@@H]1CC[C@@H](CC1)N[C@@H](CO)C(O)=O |r,wU:23.26,26.33,wD:30.35,(20.08,-15.89,;20.08,-17.43,;18.76,-18.2,;18.76,-19.75,;20.08,-20.51,;21.43,-19.74,;22.89,-20.2,;23.8,-18.95,;22.88,-17.71,;23.35,-16.24,;24.86,-15.92,;25.33,-14.46,;24.29,-13.32,;24.75,-11.85,;26.25,-11.52,;27.29,-12.66,;28.79,-12.33,;29.25,-10.86,;28.21,-9.72,;26.71,-10.05,;22.77,-13.66,;22.32,-15.12,;21.42,-18.2,;23.38,-21.67,;22.35,-22.81,;22.83,-24.27,;24.34,-24.58,;25.37,-23.43,;24.89,-21.97,;24.81,-26.04,;26.31,-26.36,;26.79,-27.81,;25.77,-28.95,;27.34,-25.21,;28.83,-25.53,;26.86,-23.76,)| Show InChI InChI=1S/C27H29N5O4/c28-25-24-22(17-6-12-21(13-7-17)36-20-4-2-1-3-5-20)14-32(26(24)30-16-29-25)19-10-8-18(9-11-19)31-23(15-33)27(34)35/h1-7,12-14,16,18-19,23,31,33H,8-11,15H2,(H,34,35)(H2,28,29,30)/t18-,19+,23-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN Center for Life Science Technologies
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human C-terminal FLT3 ITD mutant (571 to 993 residues) after 90 mins by mobility shift assay |
Bioorg Med Chem Lett 27: 4994-4998 (2017)
Article DOI: 10.1016/j.bmcl.2017.10.012
BindingDB Entry DOI: 10.7270/Q2X92DV1 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase HCK
(Homo sapiens (Human)) | BDBM50451504

(CHEMBL4218162)Show SMILES Nc1ncnc2n(cc(-c3ccc(Oc4ccccc4)cc3)c12)[C@@H]1CC[C@@H](CC1)N[C@H](Cc1ccc(O)cc1)C(O)=O |r,wU:23.26,26.33,30.35,(20.09,-15.9,;20.09,-17.44,;18.77,-18.22,;18.77,-19.76,;20.1,-20.53,;21.44,-19.75,;22.91,-20.22,;23.82,-18.96,;22.9,-17.72,;23.37,-16.25,;24.88,-15.93,;25.34,-14.47,;24.3,-13.33,;24.77,-11.86,;26.27,-11.52,;27.31,-12.67,;28.81,-12.34,;29.27,-10.87,;28.23,-9.73,;26.73,-10.06,;22.79,-13.67,;22.33,-15.13,;21.43,-18.21,;23.4,-21.68,;22.37,-22.83,;22.85,-24.29,;24.36,-24.6,;25.39,-23.45,;24.91,-21.98,;24.83,-26.06,;26.33,-26.37,;26.81,-27.83,;28.31,-28.15,;28.78,-29.6,;30.28,-29.92,;31.3,-28.78,;32.8,-29.09,;30.82,-27.32,;29.32,-27.01,;27.36,-25.23,;28.86,-25.55,;26.88,-23.78,)| Show InChI InChI=1S/C33H33N5O4/c34-31-30-28(22-8-16-27(17-9-22)42-26-4-2-1-3-5-26)19-38(32(30)36-20-35-31)24-12-10-23(11-13-24)37-29(33(40)41)18-21-6-14-25(39)15-7-21/h1-9,14-17,19-20,23-24,29,37,39H,10-13,18H2,(H,40,41)(H2,34,35,36)/t23-,24+,29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN Center for Life Science Technologies
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HCK SH3-SH2-KD (75 to 526 residues) after 20 mins by mobility shift assay |
Bioorg Med Chem Lett 27: 4994-4998 (2017)
Article DOI: 10.1016/j.bmcl.2017.10.012
BindingDB Entry DOI: 10.7270/Q2X92DV1 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50268912

(CHEMBL4093003)Show SMILES CC(C)C[C@H](N[C@H]1CC[C@@H](CC1)n1cc(-c2ccc(Oc3ccccc3)cc2)c2c(N)ncnc12)C(O)=O |r,wU:9.12,wD:6.5,4.3,(47.66,-23.12,;47.18,-21.66,;45.67,-21.34,;48.21,-20.51,;47.73,-19.05,;46.22,-18.73,;45.74,-17.28,;46.77,-16.13,;46.29,-14.66,;44.78,-14.35,;43.76,-15.5,;44.24,-16.96,;44.3,-12.9,;45.21,-11.64,;44.29,-10.39,;44.76,-8.92,;46.26,-8.6,;46.73,-7.13,;45.69,-5.99,;46.16,-4.53,;47.66,-4.2,;48.69,-5.33,;50.2,-5,;50.66,-3.53,;49.62,-2.39,;48.12,-2.73,;44.18,-6.33,;43.72,-7.8,;42.82,-10.87,;41.48,-10.11,;41.48,-8.57,;40.15,-10.88,;40.15,-12.43,;41.49,-13.2,;42.83,-12.43,;48.75,-17.9,;50.26,-18.21,;48.27,-16.44,)| Show InChI InChI=1S/C30H35N5O3/c1-19(2)16-26(30(36)37)34-21-10-12-22(13-11-21)35-17-25(27-28(31)32-18-33-29(27)35)20-8-14-24(15-9-20)38-23-6-4-3-5-7-23/h3-9,14-15,17-19,21-22,26,34H,10-13,16H2,1-2H3,(H,36,37)(H2,31,32,33)/t21-,22-,26-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN Center for Life Science Technologies
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human C-terminal FLT3 ITD mutant (571 to 993 residues) after 90 mins by mobility shift assay |
Bioorg Med Chem Lett 27: 4994-4998 (2017)
Article DOI: 10.1016/j.bmcl.2017.10.012
BindingDB Entry DOI: 10.7270/Q2X92DV1 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase HCK
(Homo sapiens (Human)) | BDBM50268915

(CHEMBL4061078)Show SMILES [H][C@@]1(CCNC1=O)N[C@H]1CC[C@@H](CC1)n1cc(-c2ccc(Oc3ccccc3)cc2)c2c(N)ncnc12 |r,wU:11.15,1.0,wD:8.8,(75.41,-22.35,;76.19,-21.03,;76.83,-22.43,;78.36,-22.26,;78.68,-20.75,;77.34,-19.99,;77.17,-18.46,;74.69,-20.72,;74.21,-19.26,;75.24,-18.11,;74.76,-16.64,;73.25,-16.34,;72.22,-17.49,;72.7,-18.94,;72.77,-14.88,;73.68,-13.62,;72.76,-12.37,;73.22,-10.9,;74.73,-10.58,;75.19,-9.12,;74.16,-7.98,;74.62,-6.51,;76.13,-6.18,;77.16,-7.32,;78.66,-6.99,;79.13,-5.52,;78.08,-4.38,;76.58,-4.71,;72.64,-8.31,;72.18,-9.78,;71.28,-12.86,;69.95,-12.09,;69.94,-10.55,;68.62,-12.86,;68.62,-14.41,;69.95,-15.18,;71.29,-14.41,)| Show InChI InChI=1S/C28H30N6O2/c29-26-25-23(18-6-12-22(13-7-18)36-21-4-2-1-3-5-21)16-34(27(25)32-17-31-26)20-10-8-19(9-11-20)33-24-14-15-30-28(24)35/h1-7,12-13,16-17,19-20,24,33H,8-11,14-15H2,(H,30,35)(H2,29,31,32)/t19-,20-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN Center for Life Science Technologies
Curated by ChEMBL
| Assay Description
Inhibition of HCK (unknown origin) |
Bioorg Med Chem 25: 4259-4264 (2017)
Article DOI: 10.1016/j.bmc.2017.05.053
BindingDB Entry DOI: 10.7270/Q2SB4875 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase HCK
(Homo sapiens (Human)) | BDBM50451481

(CHEMBL4203665)Show SMILES Nc1ncnc2n(cc(-c3ccc(Oc4ccccc4)cc3)c12)[C@H]1CC[C@@H](CC1)NCCC(O)=O |r,wU:23.26,wD:26.33,(20.08,-15.89,;20.08,-17.43,;18.76,-18.2,;18.76,-19.75,;20.08,-20.51,;21.43,-19.74,;22.89,-20.2,;23.8,-18.95,;22.88,-17.71,;23.35,-16.24,;24.86,-15.92,;25.33,-14.46,;24.29,-13.32,;24.75,-11.85,;26.25,-11.52,;27.29,-12.66,;28.79,-12.33,;29.25,-10.86,;28.21,-9.72,;26.71,-10.05,;22.77,-13.66,;22.32,-15.12,;21.42,-18.2,;23.38,-21.67,;24.89,-21.97,;25.37,-23.43,;24.34,-24.58,;22.83,-24.27,;22.35,-22.81,;24.81,-26.04,;26.31,-26.36,;27.34,-25.21,;28.83,-25.53,;29.86,-24.39,;29.31,-26.99,)| Show InChI InChI=1S/C27H29N5O3/c28-26-25-23(18-6-12-22(13-7-18)35-21-4-2-1-3-5-21)16-32(27(25)31-17-30-26)20-10-8-19(9-11-20)29-15-14-24(33)34/h1-7,12-13,16-17,19-20,29H,8-11,14-15H2,(H,33,34)(H2,28,30,31)/t19-,20- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN Center for Life Science Technologies
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HCK SH3-SH2-KD (75 to 526 residues) after 20 mins by mobility shift assay |
Bioorg Med Chem Lett 27: 4994-4998 (2017)
Article DOI: 10.1016/j.bmcl.2017.10.012
BindingDB Entry DOI: 10.7270/Q2X92DV1 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase HCK
(Homo sapiens (Human)) | BDBM50451502

(CHEMBL4213227)Show SMILES Nc1ncnc2n(cc(-c3ccc(Oc4ccccc4)cc3)c12)[C@H]1CC[C@@H](CC1)N[C@H](Cc1ccc(O)cc1)C(O)=O |r,wU:23.26,30.35,wD:26.33,(20.09,-15.9,;20.09,-17.44,;18.77,-18.22,;18.77,-19.76,;20.1,-20.53,;21.44,-19.75,;22.91,-20.22,;23.82,-18.96,;22.9,-17.72,;23.37,-16.25,;24.88,-15.93,;25.34,-14.47,;24.3,-13.33,;24.77,-11.86,;26.27,-11.52,;27.31,-12.67,;28.81,-12.34,;29.27,-10.87,;28.23,-9.73,;26.73,-10.06,;22.79,-13.67,;22.33,-15.13,;21.43,-18.21,;23.4,-21.68,;24.91,-21.98,;25.39,-23.45,;24.36,-24.6,;22.85,-24.29,;22.37,-22.83,;24.83,-26.06,;26.33,-26.37,;26.81,-27.83,;28.31,-28.15,;28.78,-29.6,;30.28,-29.92,;31.3,-28.78,;32.8,-29.09,;30.82,-27.32,;29.32,-27.01,;27.36,-25.23,;28.86,-25.55,;26.88,-23.78,)| Show InChI InChI=1S/C33H33N5O4/c34-31-30-28(22-8-16-27(17-9-22)42-26-4-2-1-3-5-26)19-38(32(30)36-20-35-31)24-12-10-23(11-13-24)37-29(33(40)41)18-21-6-14-25(39)15-7-21/h1-9,14-17,19-20,23-24,29,37,39H,10-13,18H2,(H,40,41)(H2,34,35,36)/t23-,24-,29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN Center for Life Science Technologies
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HCK SH3-SH2-KD (75 to 526 residues) after 20 mins by mobility shift assay |
Bioorg Med Chem Lett 27: 4994-4998 (2017)
Article DOI: 10.1016/j.bmcl.2017.10.012
BindingDB Entry DOI: 10.7270/Q2X92DV1 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50451488

(CHEMBL4216680)Show SMILES Nc1ncnc2n(cc(-c3ccc(Oc4ccccc4)cc3)c12)[C@@H]1CC[C@@H](CC1)N1CCC[C@@H]1C(O)=O |r,wU:23.26,26.33,33.39,(20.08,-15.9,;20.09,-17.44,;18.77,-18.21,;18.76,-19.76,;20.09,-20.52,;21.43,-19.75,;22.9,-20.21,;23.81,-18.96,;22.89,-17.71,;23.36,-16.25,;24.87,-15.93,;25.34,-14.46,;24.3,-13.33,;24.76,-11.86,;26.26,-11.52,;27.3,-12.66,;28.8,-12.34,;29.27,-10.87,;28.22,-9.72,;26.72,-10.06,;22.78,-13.66,;22.33,-15.13,;21.43,-18.2,;23.39,-21.68,;22.36,-22.82,;22.84,-24.28,;24.35,-24.6,;25.38,-23.44,;24.9,-21.97,;24.82,-26.05,;23.93,-27.29,;24.84,-28.53,;26.3,-28.06,;26.29,-26.52,;27.53,-25.62,;28.93,-26.24,;27.36,-24.1,)| Show InChI InChI=1S/C29H31N5O3/c30-27-26-24(19-8-14-23(15-9-19)37-22-5-2-1-3-6-22)17-34(28(26)32-18-31-27)21-12-10-20(11-13-21)33-16-4-7-25(33)29(35)36/h1-3,5-6,8-9,14-15,17-18,20-21,25H,4,7,10-13,16H2,(H,35,36)(H2,30,31,32)/t20-,21+,25-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN Center for Life Science Technologies
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human C-terminal FLT3 ITD mutant (571 to 993 residues) after 90 mins by mobility shift assay |
Bioorg Med Chem Lett 27: 4994-4998 (2017)
Article DOI: 10.1016/j.bmcl.2017.10.012
BindingDB Entry DOI: 10.7270/Q2X92DV1 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50451483

(CHEMBL4215201)Show SMILES Nc1ncnc2n(cc(-c3ccc(Oc4ccccc4)cc3)c12)[C@H]1CC[C@@H](CC1)N[C@H](CO)C(O)=O |r,wU:23.26,30.35,wD:26.33,(20.08,-15.89,;20.08,-17.43,;18.76,-18.2,;18.76,-19.75,;20.08,-20.51,;21.43,-19.74,;22.89,-20.2,;23.8,-18.95,;22.88,-17.71,;23.35,-16.24,;24.86,-15.92,;25.33,-14.46,;24.29,-13.32,;24.75,-11.85,;26.25,-11.52,;27.29,-12.66,;28.79,-12.33,;29.25,-10.86,;28.21,-9.72,;26.71,-10.05,;22.77,-13.66,;22.32,-15.12,;21.42,-18.2,;23.38,-21.67,;24.89,-21.97,;25.37,-23.43,;24.34,-24.58,;22.83,-24.27,;22.35,-22.81,;24.81,-26.04,;26.31,-26.36,;26.79,-27.81,;25.77,-28.95,;27.34,-25.21,;28.83,-25.53,;26.86,-23.76,)| Show InChI InChI=1S/C27H29N5O4/c28-25-24-22(17-6-12-21(13-7-17)36-20-4-2-1-3-5-20)14-32(26(24)30-16-29-25)19-10-8-18(9-11-19)31-23(15-33)27(34)35/h1-7,12-14,16,18-19,23,31,33H,8-11,15H2,(H,34,35)(H2,28,29,30)/t18-,19-,23-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN Center for Life Science Technologies
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human C-terminal FLT3 ITD mutant (571 to 993 residues) after 90 mins by mobility shift assay |
Bioorg Med Chem Lett 27: 4994-4998 (2017)
Article DOI: 10.1016/j.bmcl.2017.10.012
BindingDB Entry DOI: 10.7270/Q2X92DV1 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase HCK
(Homo sapiens (Human)) | BDBM50451491

(CHEMBL4210294)Show SMILES Nc1ncnc2n(cc(-c3ccc(Oc4ccccc4)cc3)c12)[C@@H]1CC[C@@H](CC1)N[C@H](Cc1ccccc1)C(O)=O |r,wU:23.26,26.33,30.35,(20.09,-15.9,;20.09,-17.44,;18.77,-18.22,;18.77,-19.76,;20.1,-20.53,;21.44,-19.75,;22.91,-20.22,;23.82,-18.96,;22.9,-17.72,;23.37,-16.25,;24.88,-15.93,;25.34,-14.47,;24.3,-13.33,;24.77,-11.86,;26.27,-11.52,;27.31,-12.67,;28.81,-12.34,;29.27,-10.87,;28.23,-9.73,;26.73,-10.06,;22.79,-13.67,;22.33,-15.13,;21.43,-18.21,;23.4,-21.68,;22.37,-22.83,;22.85,-24.29,;24.36,-24.6,;25.39,-23.45,;24.91,-21.98,;24.83,-26.06,;26.33,-26.37,;26.81,-27.83,;28.31,-28.15,;28.78,-29.6,;30.28,-29.92,;31.3,-28.78,;30.82,-27.32,;29.32,-27.01,;27.36,-25.23,;28.86,-25.55,;26.88,-23.78,)| Show InChI InChI=1S/C33H33N5O3/c34-31-30-28(23-11-17-27(18-12-23)41-26-9-5-2-6-10-26)20-38(32(30)36-21-35-31)25-15-13-24(14-16-25)37-29(33(39)40)19-22-7-3-1-4-8-22/h1-12,17-18,20-21,24-25,29,37H,13-16,19H2,(H,39,40)(H2,34,35,36)/t24-,25+,29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN Center for Life Science Technologies
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HCK SH3-SH2-KD (75 to 526 residues) after 20 mins by mobility shift assay |
Bioorg Med Chem Lett 27: 4994-4998 (2017)
Article DOI: 10.1016/j.bmcl.2017.10.012
BindingDB Entry DOI: 10.7270/Q2X92DV1 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50451482

(CHEMBL4208645)Show SMILES Nc1ncnc2n(cc(-c3ccc(Oc4ccccc4)cc3)c12)[C@H]1CC[C@@H](CC1)N[C@@H](CO)C(O)=O |r,wU:23.26,wD:26.33,30.35,(20.08,-15.89,;20.08,-17.43,;18.76,-18.2,;18.76,-19.75,;20.08,-20.51,;21.43,-19.74,;22.89,-20.2,;23.8,-18.95,;22.88,-17.71,;23.35,-16.24,;24.86,-15.92,;25.33,-14.46,;24.29,-13.32,;24.75,-11.85,;26.25,-11.52,;27.29,-12.66,;28.79,-12.33,;29.25,-10.86,;28.21,-9.72,;26.71,-10.05,;22.77,-13.66,;22.32,-15.12,;21.42,-18.2,;23.38,-21.67,;24.89,-21.97,;25.37,-23.43,;24.34,-24.58,;22.83,-24.27,;22.35,-22.81,;24.81,-26.04,;26.31,-26.36,;26.79,-27.81,;25.77,-28.95,;27.34,-25.21,;28.83,-25.53,;26.86,-23.76,)| Show InChI InChI=1S/C27H29N5O4/c28-25-24-22(17-6-12-21(13-7-17)36-20-4-2-1-3-5-20)14-32(26(24)30-16-29-25)19-10-8-18(9-11-19)31-23(15-33)27(34)35/h1-7,12-14,16,18-19,23,31,33H,8-11,15H2,(H,34,35)(H2,28,29,30)/t18-,19-,23-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN Center for Life Science Technologies
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human C-terminal FLT3 ITD mutant (571 to 993 residues) after 90 mins by mobility shift assay |
Bioorg Med Chem Lett 27: 4994-4998 (2017)
Article DOI: 10.1016/j.bmcl.2017.10.012
BindingDB Entry DOI: 10.7270/Q2X92DV1 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50451478

(CHEMBL4209614)Show SMILES C[C@H](N[C@H]1CC[C@H](CC1)n1cc(-c2ccc(Oc3ccccc3)cc2)c2c(N)ncnc12)C(O)=O |r,wU:6.9,3.2,wD:1.0,(26.79,-27.81,;26.31,-26.36,;24.81,-26.04,;24.34,-24.58,;22.83,-24.27,;22.35,-22.81,;23.38,-21.67,;24.89,-21.97,;25.37,-23.43,;22.89,-20.2,;23.8,-18.95,;22.88,-17.71,;23.35,-16.24,;24.86,-15.92,;25.33,-14.46,;24.29,-13.32,;24.75,-11.85,;26.25,-11.52,;27.29,-12.66,;28.79,-12.33,;29.25,-10.86,;28.21,-9.72,;26.71,-10.05,;22.77,-13.66,;22.32,-15.12,;21.42,-18.2,;20.08,-17.43,;20.08,-15.89,;18.76,-18.2,;18.76,-19.75,;20.08,-20.51,;21.43,-19.74,;27.34,-25.21,;28.83,-25.53,;26.86,-23.76,)| Show InChI InChI=1S/C27H29N5O3/c1-17(27(33)34)31-19-9-11-20(12-10-19)32-15-23(24-25(28)29-16-30-26(24)32)18-7-13-22(14-8-18)35-21-5-3-2-4-6-21/h2-8,13-17,19-20,31H,9-12H2,1H3,(H,33,34)(H2,28,29,30)/t17-,19-,20+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN Center for Life Science Technologies
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human C-terminal FLT3 ITD mutant (571 to 993 residues) after 90 mins by mobility shift assay |
Bioorg Med Chem Lett 27: 4994-4998 (2017)
Article DOI: 10.1016/j.bmcl.2017.10.012
BindingDB Entry DOI: 10.7270/Q2X92DV1 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50451487

(CHEMBL4212241)Show SMILES Nc1ncnc2n(cc(-c3ccc(Oc4ccccc4)cc3)c12)[C@@H]1CC[C@@H](CC1)N1CCC[C@H]1C(O)=O |r,wU:23.26,26.33,wD:33.39,(20.08,-15.9,;20.09,-17.44,;18.77,-18.21,;18.76,-19.76,;20.09,-20.52,;21.43,-19.75,;22.9,-20.21,;23.81,-18.96,;22.89,-17.71,;23.36,-16.25,;24.87,-15.93,;25.34,-14.46,;24.3,-13.33,;24.76,-11.86,;26.26,-11.52,;27.3,-12.66,;28.8,-12.34,;29.27,-10.87,;28.22,-9.72,;26.72,-10.06,;22.78,-13.66,;22.33,-15.13,;21.43,-18.2,;23.39,-21.68,;22.36,-22.82,;22.84,-24.28,;24.35,-24.6,;25.38,-23.44,;24.9,-21.97,;24.82,-26.05,;23.93,-27.29,;24.84,-28.53,;26.3,-28.06,;26.29,-26.52,;27.53,-25.62,;28.93,-26.24,;27.36,-24.1,)| Show InChI InChI=1S/C29H31N5O3/c30-27-26-24(19-8-14-23(15-9-19)37-22-5-2-1-3-6-22)17-34(28(26)32-18-31-27)21-12-10-20(11-13-21)33-16-4-7-25(33)29(35)36/h1-3,5-6,8-9,14-15,17-18,20-21,25H,4,7,10-13,16H2,(H,35,36)(H2,30,31,32)/t20-,21+,25-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN Center for Life Science Technologies
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human C-terminal FLT3 ITD mutant (571 to 993 residues) after 90 mins by mobility shift assay |
Bioorg Med Chem Lett 27: 4994-4998 (2017)
Article DOI: 10.1016/j.bmcl.2017.10.012
BindingDB Entry DOI: 10.7270/Q2X92DV1 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50451473

(CHEMBL4208669)Show SMILES CC(C)C[C@H](N[C@H]1CC[C@H](CC1)n1cc(-c2ccc(Oc3ccccc3)cc2)c2c(N)ncnc12)C(O)=O |r,wU:9.12,6.5,wD:4.3,(28.78,-29.6,;28.3,-28.14,;29.32,-27,;26.8,-27.82,;26.33,-26.37,;24.83,-26.05,;24.35,-24.6,;22.84,-24.28,;22.36,-22.82,;23.4,-21.68,;24.9,-21.98,;25.38,-23.44,;22.91,-20.21,;23.81,-18.96,;22.89,-17.72,;23.36,-16.25,;24.87,-15.93,;25.34,-14.46,;24.3,-13.33,;24.76,-11.86,;26.26,-11.52,;27.3,-12.66,;28.8,-12.34,;29.27,-10.87,;28.22,-9.72,;26.72,-10.06,;22.79,-13.66,;22.33,-15.13,;21.43,-18.21,;20.09,-17.44,;20.08,-15.9,;18.77,-18.21,;18.77,-19.76,;20.09,-20.52,;21.44,-19.75,;27.35,-25.23,;28.85,-25.54,;26.87,-23.77,)| Show InChI InChI=1S/C30H35N5O3/c1-19(2)16-26(30(36)37)34-21-10-12-22(13-11-21)35-17-25(27-28(31)32-18-33-29(27)35)20-8-14-24(15-9-20)38-23-6-4-3-5-7-23/h3-9,14-15,17-19,21-22,26,34H,10-13,16H2,1-2H3,(H,36,37)(H2,31,32,33)/t21-,22+,26-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN Center for Life Science Technologies
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human C-terminal FLT3 ITD mutant (571 to 993 residues) after 90 mins by mobility shift assay |
Bioorg Med Chem Lett 27: 4994-4998 (2017)
Article DOI: 10.1016/j.bmcl.2017.10.012
BindingDB Entry DOI: 10.7270/Q2X92DV1 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase HCK
(Homo sapiens (Human)) | BDBM50451484

(CHEMBL4203527)Show SMILES Nc1ncnc2n(cc(-c3ccc(Oc4ccccc4)cc3)c12)[C@@H]1CC[C@@H](CC1)N[C@H](CO)C(O)=O |r,wU:23.26,26.33,30.35,(20.08,-15.89,;20.08,-17.43,;18.76,-18.2,;18.76,-19.75,;20.08,-20.51,;21.43,-19.74,;22.89,-20.2,;23.8,-18.95,;22.88,-17.71,;23.35,-16.24,;24.86,-15.92,;25.33,-14.46,;24.29,-13.32,;24.75,-11.85,;26.25,-11.52,;27.29,-12.66,;28.79,-12.33,;29.25,-10.86,;28.21,-9.72,;26.71,-10.05,;22.77,-13.66,;22.32,-15.12,;21.42,-18.2,;23.38,-21.67,;22.35,-22.81,;22.83,-24.27,;24.34,-24.58,;25.37,-23.43,;24.89,-21.97,;24.81,-26.04,;26.31,-26.36,;26.79,-27.81,;25.77,-28.95,;27.34,-25.21,;28.83,-25.53,;26.86,-23.76,)| Show InChI InChI=1S/C27H29N5O4/c28-25-24-22(17-6-12-21(13-7-17)36-20-4-2-1-3-5-20)14-32(26(24)30-16-29-25)19-10-8-18(9-11-19)31-23(15-33)27(34)35/h1-7,12-14,16,18-19,23,31,33H,8-11,15H2,(H,34,35)(H2,28,29,30)/t18-,19+,23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN Center for Life Science Technologies
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HCK SH3-SH2-KD (75 to 526 residues) after 20 mins by mobility shift assay |
Bioorg Med Chem Lett 27: 4994-4998 (2017)
Article DOI: 10.1016/j.bmcl.2017.10.012
BindingDB Entry DOI: 10.7270/Q2X92DV1 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50451479
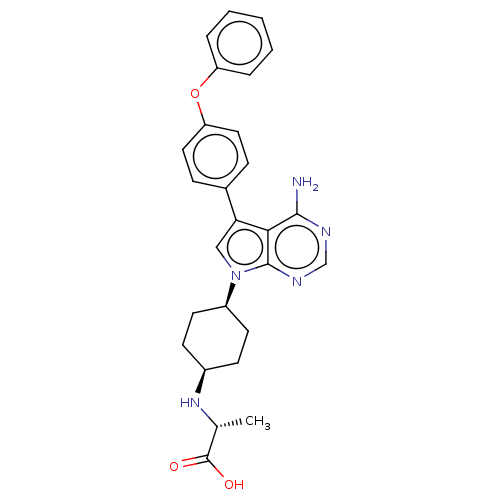
(CHEMBL4205815)Show SMILES C[C@@H](N[C@H]1CC[C@H](CC1)n1cc(-c2ccc(Oc3ccccc3)cc2)c2c(N)ncnc12)C(O)=O |r,wU:6.9,3.2,1.0,(26.79,-27.81,;26.31,-26.36,;24.81,-26.04,;24.34,-24.58,;22.83,-24.27,;22.35,-22.81,;23.38,-21.67,;24.89,-21.97,;25.37,-23.43,;22.89,-20.2,;23.8,-18.95,;22.88,-17.71,;23.35,-16.24,;24.86,-15.92,;25.33,-14.46,;24.29,-13.32,;24.75,-11.85,;26.25,-11.52,;27.29,-12.66,;28.79,-12.33,;29.25,-10.86,;28.21,-9.72,;26.71,-10.05,;22.77,-13.66,;22.32,-15.12,;21.42,-18.2,;20.08,-17.43,;20.08,-15.89,;18.76,-18.2,;18.76,-19.75,;20.08,-20.51,;21.43,-19.74,;27.34,-25.21,;28.83,-25.53,;26.86,-23.76,)| Show InChI InChI=1S/C27H29N5O3/c1-17(27(33)34)31-19-9-11-20(12-10-19)32-15-23(24-25(28)29-16-30-26(24)32)18-7-13-22(14-8-18)35-21-5-3-2-4-6-21/h2-8,13-17,19-20,31H,9-12H2,1H3,(H,33,34)(H2,28,29,30)/t17-,19-,20+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN Center for Life Science Technologies
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human C-terminal FLT3 ITD mutant (571 to 993 residues) after 90 mins by mobility shift assay |
Bioorg Med Chem Lett 27: 4994-4998 (2017)
Article DOI: 10.1016/j.bmcl.2017.10.012
BindingDB Entry DOI: 10.7270/Q2X92DV1 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase HCK
(Homo sapiens (Human)) | BDBM50451470

(CHEMBL4205400)Show SMILES Nc1ncnc2n(cc(-c3ccc(Oc4ccccc4)cc3)c12)[C@H]1CC[C@@H](CC1)N[C@H](Cc1ccccc1)C(O)=O |r,wU:23.26,30.35,wD:26.33,(20.09,-15.9,;20.09,-17.44,;18.77,-18.22,;18.77,-19.76,;20.1,-20.53,;21.44,-19.75,;22.91,-20.22,;23.82,-18.96,;22.9,-17.72,;23.37,-16.25,;24.88,-15.93,;25.34,-14.47,;24.3,-13.33,;24.77,-11.86,;26.27,-11.52,;27.31,-12.67,;28.81,-12.34,;29.27,-10.87,;28.23,-9.73,;26.73,-10.06,;22.79,-13.67,;22.33,-15.13,;21.43,-18.21,;23.4,-21.68,;24.91,-21.98,;25.39,-23.45,;24.36,-24.6,;22.85,-24.29,;22.37,-22.83,;24.83,-26.06,;26.33,-26.37,;26.81,-27.83,;28.31,-28.15,;28.78,-29.6,;30.28,-29.92,;31.3,-28.78,;30.82,-27.32,;29.32,-27.01,;27.36,-25.23,;28.86,-25.55,;26.88,-23.78,)| Show InChI InChI=1S/C33H33N5O3/c34-31-30-28(23-11-17-27(18-12-23)41-26-9-5-2-6-10-26)20-38(32(30)36-21-35-31)25-15-13-24(14-16-25)37-29(33(39)40)19-22-7-3-1-4-8-22/h1-12,17-18,20-21,24-25,29,37H,13-16,19H2,(H,39,40)(H2,34,35,36)/t24-,25-,29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN Center for Life Science Technologies
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HCK SH3-SH2-KD (75 to 526 residues) after 20 mins by mobility shift assay |
Bioorg Med Chem Lett 27: 4994-4998 (2017)
Article DOI: 10.1016/j.bmcl.2017.10.012
BindingDB Entry DOI: 10.7270/Q2X92DV1 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM8793

(7-[4-(4-methylpiperazin-1-yl)cyclohexyl]-5-(4-phen...)Show SMILES CN1CCN(CC1)[C@H]1CC[C@@H](CC1)n1cc(-c2ccc(Oc3ccccc3)cc2)c2c(N)ncnc12 |r,wU:10.14,wD:7.7,(-.91,-10.51,;-1.37,-9.04,;-2.87,-8.71,;-3.34,-7.24,;-2.3,-6.11,;-.8,-6.44,;-.33,-7.91,;-2.72,-4.63,;-1.64,-3.52,;-2.06,-2.04,;-3.55,-1.66,;-4.63,-2.76,;-4.21,-4.25,;-4.03,-.2,;-3.12,1.05,;-4.03,2.3,;-3.55,3.76,;-4.69,4.8,;-4.36,6.3,;-2.89,6.77,;-2.56,8.27,;-1.1,8.74,;-.48,10.15,;1.05,10.33,;1.96,9.09,;1.35,7.68,;-.18,7.5,;-1.75,5.73,;-2.08,4.23,;-5.49,1.82,;-6.82,2.59,;-6.82,4.13,;-8.16,1.82,;-8.16,.28,;-6.82,-.49,;-5.49,.28,)| Show InChI InChI=1S/C29H34N6O/c1-33-15-17-34(18-16-33)22-9-11-23(12-10-22)35-19-26(27-28(30)31-20-32-29(27)35)21-7-13-25(14-8-21)36-24-5-3-2-4-6-24/h2-8,13-14,19-20,22-23H,9-12,15-18H2,1H3,(H2,30,31,32)/t22-,23- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN Center for Life Science Technologies
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human C-terminal FLT3 ITD mutant (571 to 993 residues) after 90 mins by mobility shift assay |
Bioorg Med Chem Lett 27: 4994-4998 (2017)
Article DOI: 10.1016/j.bmcl.2017.10.012
BindingDB Entry DOI: 10.7270/Q2X92DV1 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50451489

(CHEMBL4216163)Show SMILES Nc1ncnc2n(cc(-c3ccc(Oc4ccccc4)cc3)c12)[C@H]1CC[C@@H](CC1)N[C@@H](Cc1ccccc1)C(O)=O |r,wU:23.26,wD:26.33,30.35,(20.09,-15.9,;20.09,-17.44,;18.77,-18.22,;18.77,-19.76,;20.1,-20.53,;21.44,-19.75,;22.91,-20.22,;23.82,-18.96,;22.9,-17.72,;23.37,-16.25,;24.88,-15.93,;25.34,-14.47,;24.3,-13.33,;24.77,-11.86,;26.27,-11.52,;27.31,-12.67,;28.81,-12.34,;29.27,-10.87,;28.23,-9.73,;26.73,-10.06,;22.79,-13.67,;22.33,-15.13,;21.43,-18.21,;23.4,-21.68,;24.91,-21.98,;25.39,-23.45,;24.36,-24.6,;22.85,-24.29,;22.37,-22.83,;24.83,-26.06,;26.33,-26.37,;26.81,-27.83,;28.31,-28.15,;28.78,-29.6,;30.28,-29.92,;31.3,-28.78,;30.82,-27.32,;29.32,-27.01,;27.36,-25.23,;28.86,-25.55,;26.88,-23.78,)| Show InChI InChI=1S/C33H33N5O3/c34-31-30-28(23-11-17-27(18-12-23)41-26-9-5-2-6-10-26)20-38(32(30)36-21-35-31)25-15-13-24(14-16-25)37-29(33(39)40)19-22-7-3-1-4-8-22/h1-12,17-18,20-21,24-25,29,37H,13-16,19H2,(H,39,40)(H2,34,35,36)/t24-,25-,29-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN Center for Life Science Technologies
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human C-terminal FLT3 ITD mutant (571 to 993 residues) after 90 mins by mobility shift assay |
Bioorg Med Chem Lett 27: 4994-4998 (2017)
Article DOI: 10.1016/j.bmcl.2017.10.012
BindingDB Entry DOI: 10.7270/Q2X92DV1 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50451485

(CHEMBL4202997)Show SMILES [H][C@@]1(CCOC1=O)N[C@H]1CC[C@@H](CC1)n1cc(-c2ccc(Oc3ccccc3)cc2)c2c(N)ncnc12 |r,wU:11.15,1.0,wD:8.8,(25.23,-27.44,;26.32,-26.36,;26.95,-27.76,;28.48,-27.6,;28.79,-26.1,;27.46,-25.33,;27.3,-23.81,;24.82,-26.05,;24.35,-24.59,;25.38,-23.44,;24.9,-21.97,;23.39,-21.67,;22.36,-22.82,;22.84,-24.28,;22.9,-20.21,;23.81,-18.96,;22.89,-17.71,;23.36,-16.25,;24.87,-15.93,;25.33,-14.46,;24.29,-13.33,;24.76,-11.86,;26.26,-11.52,;27.3,-12.66,;28.8,-12.33,;29.26,-10.86,;28.22,-9.72,;26.72,-10.06,;22.78,-13.66,;22.32,-15.13,;21.42,-18.2,;20.09,-17.44,;20.08,-15.9,;18.76,-18.21,;18.76,-19.75,;20.09,-20.52,;21.43,-19.75,)| Show InChI InChI=1S/C28H29N5O3/c29-26-25-23(18-6-12-22(13-7-18)36-21-4-2-1-3-5-21)16-33(27(25)31-17-30-26)20-10-8-19(9-11-20)32-24-14-15-35-28(24)34/h1-7,12-13,16-17,19-20,24,32H,8-11,14-15H2,(H2,29,30,31)/t19-,20-,24-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN Center for Life Science Technologies
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human C-terminal FLT3 ITD mutant (571 to 993 residues) after 90 mins by mobility shift assay |
Bioorg Med Chem Lett 27: 4994-4998 (2017)
Article DOI: 10.1016/j.bmcl.2017.10.012
BindingDB Entry DOI: 10.7270/Q2X92DV1 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data