

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D-3-phosphoglycerate dehydrogenase (Homo sapiens (Human)) | BDBM50512575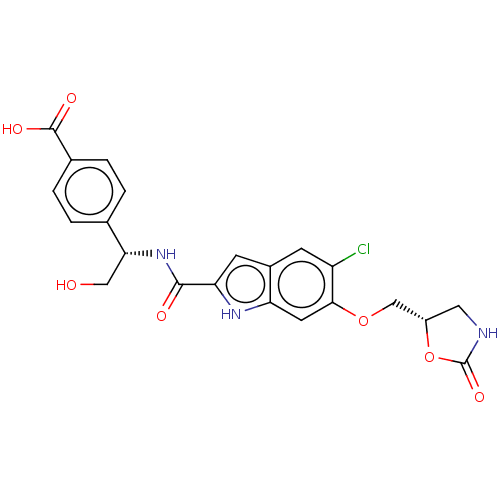 (CHEMBL4554985) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 214 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meyer Cancer Center Curated by ChEMBL | Assay Description Mixed type inhibition of PHGDH (unknown origin) using 3-PG as substrate incubated for 20 mins in presence of , PSAT1, PSPH and varying NAD+ by diapho... | Bioorg Med Chem Lett 29: 2503-2510 (2019) Article DOI: 10.1016/j.bmcl.2019.07.011 BindingDB Entry DOI: 10.7270/Q27D2ZGJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50586159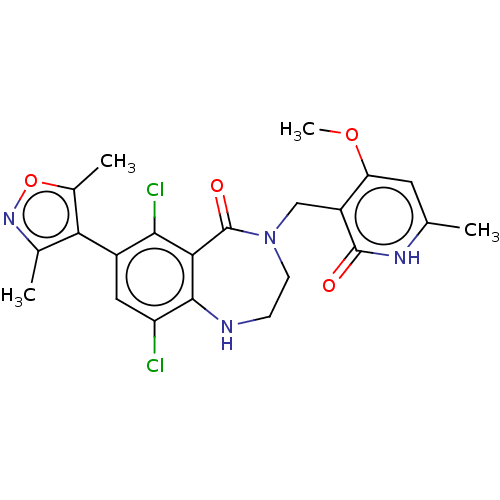 (CHEMBL5087917) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of EZH2 (unknown origin) using 3H-SAM as substrate incubated for 1 hour by filter binding method | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00448 BindingDB Entry DOI: 10.7270/Q2J10735 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50247130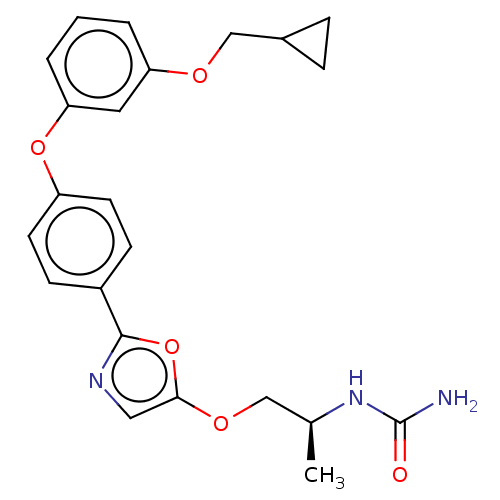 (CHEMBL4073202) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of recombinant human ACC1 expressed in SF-9 cells preincubated for 60 mins followed by addition of substrate solution containing acetyl-Co... | J Med Chem 61: 1098-1117 (2018) Article DOI: 10.1021/acs.jmedchem.7b01547 BindingDB Entry DOI: 10.7270/Q2HQ429W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50247081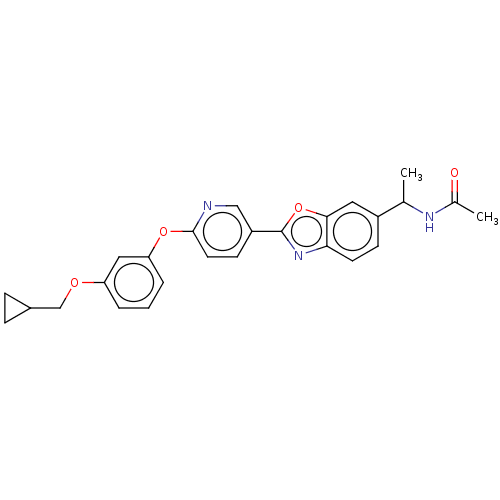 (CHEMBL4060253) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of ACC1 in human HCT116 cells assessed as reduction of [14C]acetate uptake preincubated for 60 mins followed by addition of [14C]acetate a... | J Med Chem 61: 1098-1117 (2018) Article DOI: 10.1021/acs.jmedchem.7b01547 BindingDB Entry DOI: 10.7270/Q2HQ429W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50586160 (CHEMBL5085993) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of EZH2 (unknown origin) using 3H-SAM as substrate incubated for 1 hour by filter binding method | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00448 BindingDB Entry DOI: 10.7270/Q2J10735 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50586173 (CHEMBL5087318) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of EZH2 (unknown origin) using 3H-SAM as substrate incubated for 1 hour by filter binding method | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00448 BindingDB Entry DOI: 10.7270/Q2J10735 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50247114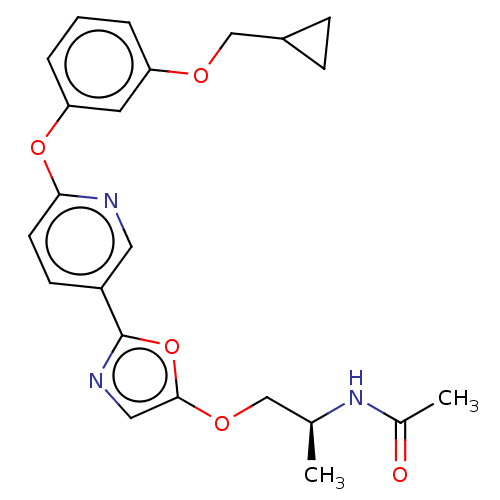 (CHEMBL4086127) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.960 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of recombinant human ACC1 expressed in SF-9 cells preincubated for 60 mins followed by addition of substrate solution containing acetyl-Co... | J Med Chem 61: 1098-1117 (2018) Article DOI: 10.1021/acs.jmedchem.7b01547 BindingDB Entry DOI: 10.7270/Q2HQ429W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50247112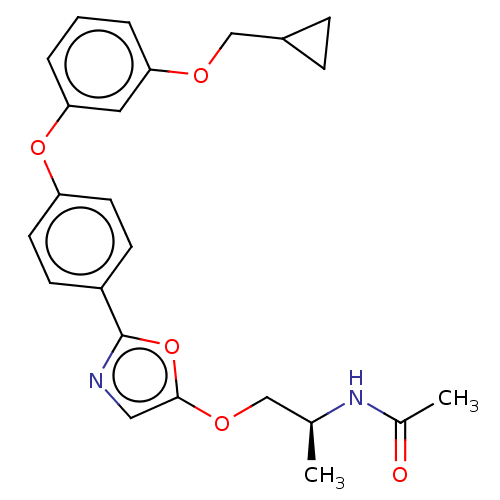 (CHEMBL4085633) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.960 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of recombinant human ACC1 expressed in SF-9 cells preincubated for 60 mins followed by addition of substrate solution containing acetyl-Co... | J Med Chem 61: 1098-1117 (2018) Article DOI: 10.1021/acs.jmedchem.7b01547 BindingDB Entry DOI: 10.7270/Q2HQ429W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50193709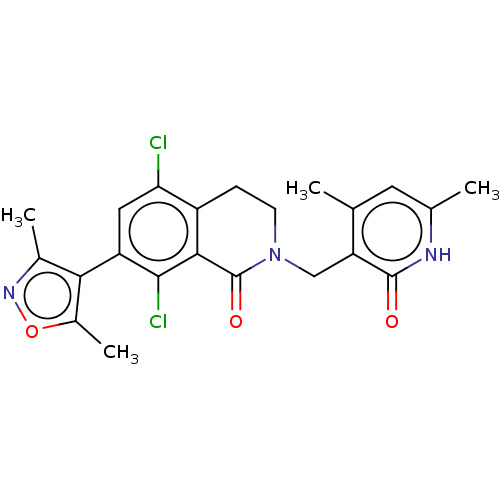 (CHEMBL3911017) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of EZH2 (unknown origin) using 3H-SAM as substrate incubated for 1 hour by filter binding method | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00448 BindingDB Entry DOI: 10.7270/Q2J10735 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50586158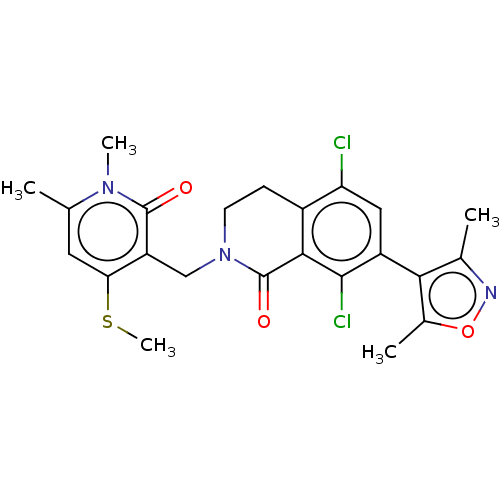 (CHEMBL5088217) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of EZH2 (unknown origin) using 3H-SAM as substrate incubated for 1 hour by filter binding method | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00448 BindingDB Entry DOI: 10.7270/Q2J10735 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 3 (Homo sapiens (Human)) | BDBM50425719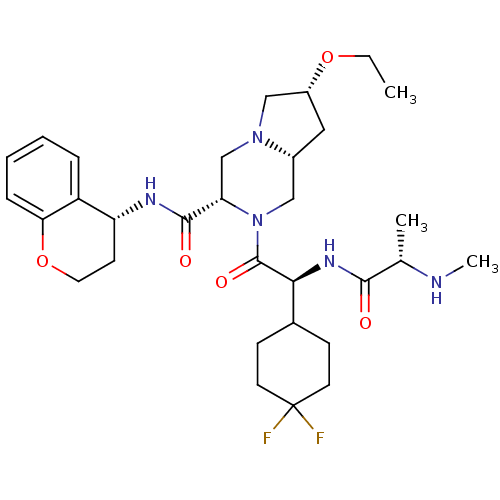 (CHEMBL2316217) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human recombinant His-tagged cIAP1 protein BIR3 domain (250 to 350 amino acid residues) using biotinylated-Smac as substrate after over... | J Med Chem 56: 1228-46 (2013) Article DOI: 10.1021/jm301674z BindingDB Entry DOI: 10.7270/Q23N24QX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 3 (Homo sapiens (Human)) | BDBM50425721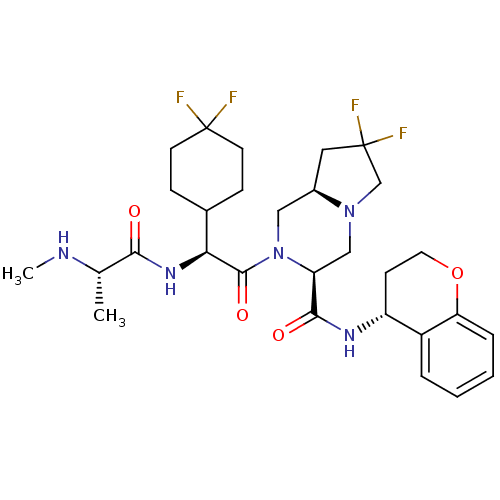 (CHEMBL2311586) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human recombinant His-tagged cIAP1 protein BIR3 domain (250 to 350 amino acid residues) using biotinylated-Smac as substrate after over... | J Med Chem 56: 1228-46 (2013) Article DOI: 10.1021/jm301674z BindingDB Entry DOI: 10.7270/Q23N24QX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50247081 (CHEMBL4060253) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of recombinant human ACC1 expressed in SF-9 cells preincubated for 60 mins followed by addition of substrate solution containing acetyl-Co... | J Med Chem 61: 1098-1117 (2018) Article DOI: 10.1021/acs.jmedchem.7b01547 BindingDB Entry DOI: 10.7270/Q2HQ429W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50586171 (CHEMBL5071153) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of EZH2 (unknown origin) using 3H-SAM as substrate incubated for 1 hour by filter binding method | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00448 BindingDB Entry DOI: 10.7270/Q2J10735 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50586167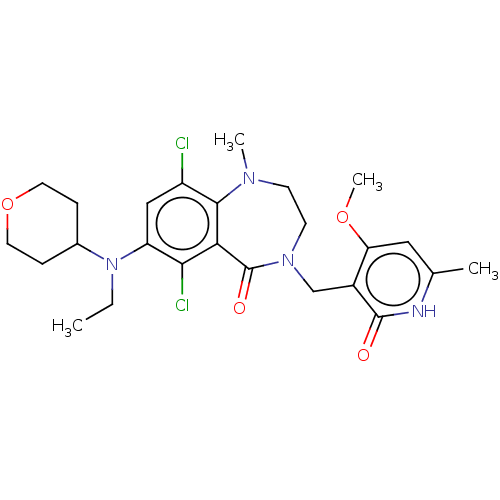 (CHEMBL5074786) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of EZH2 (unknown origin) using 3H-SAM as substrate incubated for 1 hour by filter binding method | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00448 BindingDB Entry DOI: 10.7270/Q2J10735 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50586165 (CHEMBL5093442) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of EZH2 (unknown origin) using 3H-SAM as substrate incubated for 1 hour by filter binding method | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00448 BindingDB Entry DOI: 10.7270/Q2J10735 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50586175 (CHEMBL5088474) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of EZH2 (unknown origin) using 3H-SAM as substrate incubated for 1 hour by filter binding method | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00448 BindingDB Entry DOI: 10.7270/Q2J10735 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 3 (Homo sapiens (Human)) | BDBM50425728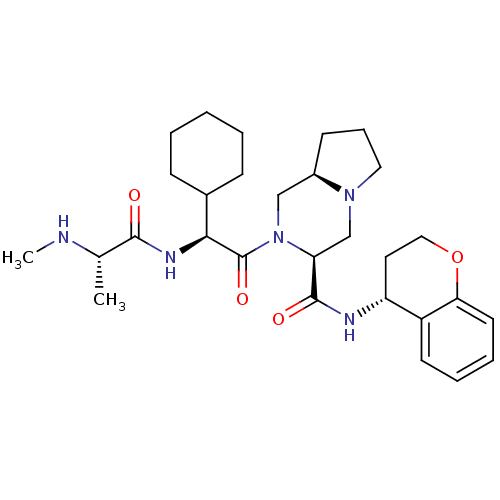 (CHEMBL2365533) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human recombinant His-tagged cIAP1 protein BIR3 domain (250 to 350 amino acid residues) using biotinylated-Smac as substrate after over... | J Med Chem 56: 1228-46 (2013) Article DOI: 10.1021/jm301674z BindingDB Entry DOI: 10.7270/Q23N24QX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50247080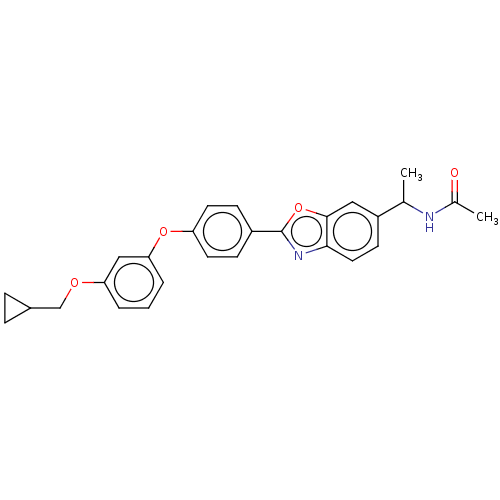 (CHEMBL4098647) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of ACC1 in human HCT116 cells assessed as reduction of [14C]acetate uptake preincubated for 60 mins followed by addition of [14C]acetate a... | J Med Chem 61: 1098-1117 (2018) Article DOI: 10.1021/acs.jmedchem.7b01547 BindingDB Entry DOI: 10.7270/Q2HQ429W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 3 (Homo sapiens (Human)) | BDBM50425722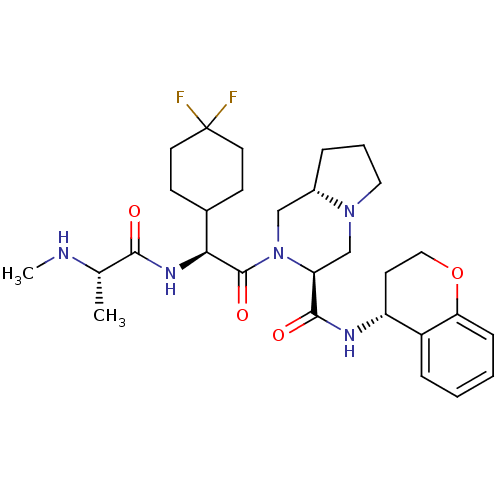 (CHEMBL2316215) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human recombinant His-tagged cIAP1 protein BIR3 domain (250 to 350 amino acid residues) using biotinylated-Smac as substrate after over... | J Med Chem 56: 1228-46 (2013) Article DOI: 10.1021/jm301674z BindingDB Entry DOI: 10.7270/Q23N24QX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 3 (Homo sapiens (Human)) | BDBM50425725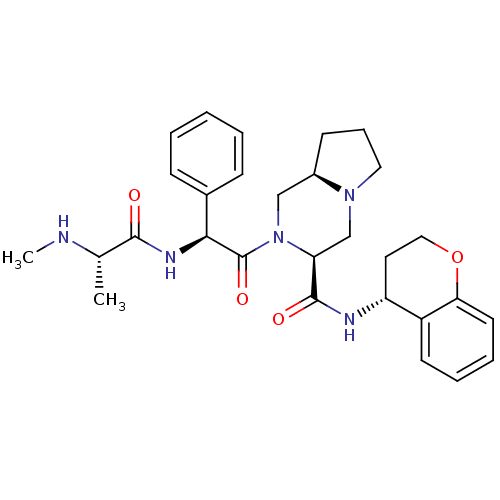 (CHEMBL2316224) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human recombinant His-tagged cIAP1 protein BIR3 domain (250 to 350 amino acid residues) using biotinylated-Smac as substrate after over... | J Med Chem 56: 1228-46 (2013) Article DOI: 10.1021/jm301674z BindingDB Entry DOI: 10.7270/Q23N24QX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 3 (Homo sapiens (Human)) | BDBM50425730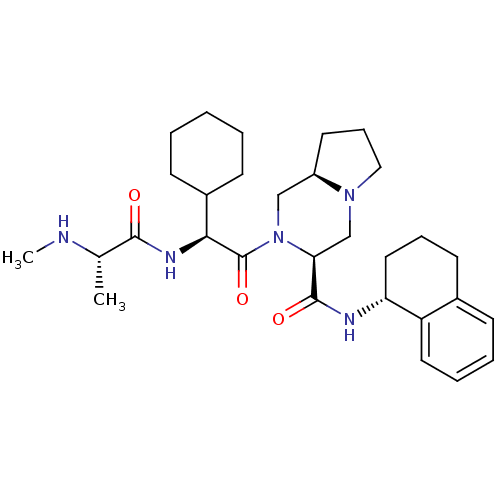 (CHEMBL2316219) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human recombinant His-tagged cIAP1 protein BIR3 domain (250 to 350 amino acid residues) using biotinylated-Smac as substrate after over... | J Med Chem 56: 1228-46 (2013) Article DOI: 10.1021/jm301674z BindingDB Entry DOI: 10.7270/Q23N24QX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50247114 (CHEMBL4086127) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of ACC1 in human HCT116 cells assessed as reduction of [14C]acetate uptake preincubated for 60 mins followed by addition of [14C]acetate a... | J Med Chem 61: 1098-1117 (2018) Article DOI: 10.1021/acs.jmedchem.7b01547 BindingDB Entry DOI: 10.7270/Q2HQ429W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50586162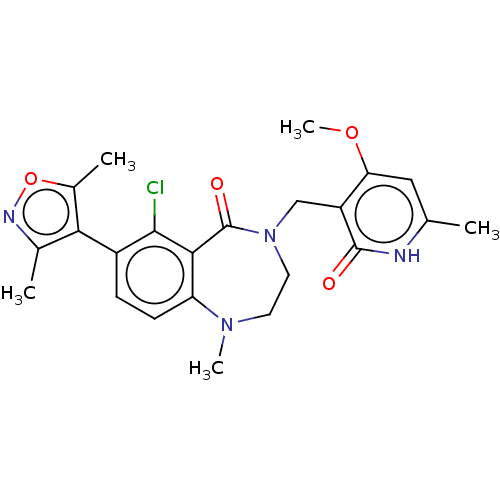 (CHEMBL5079717) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of EZH2 (unknown origin) using 3H-SAM as substrate incubated for 1 hour by filter binding method | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00448 BindingDB Entry DOI: 10.7270/Q2J10735 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 3 (Homo sapiens (Human)) | BDBM50425724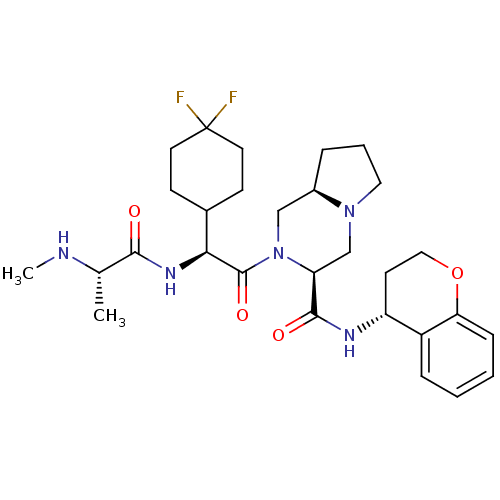 (CHEMBL2316213) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human recombinant His-tagged cIAP1 protein BIR3 domain (250 to 350 amino acid residues) using biotinylated-Smac as substrate after over... | J Med Chem 56: 1228-46 (2013) Article DOI: 10.1021/jm301674z BindingDB Entry DOI: 10.7270/Q23N24QX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50247112 (CHEMBL4085633) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of ACC1 in human HCT116 cells assessed as reduction of [14C]acetate uptake preincubated for 60 mins followed by addition of [14C]acetate a... | J Med Chem 61: 1098-1117 (2018) Article DOI: 10.1021/acs.jmedchem.7b01547 BindingDB Entry DOI: 10.7270/Q2HQ429W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 3 (Homo sapiens (Human)) | BDBM50425723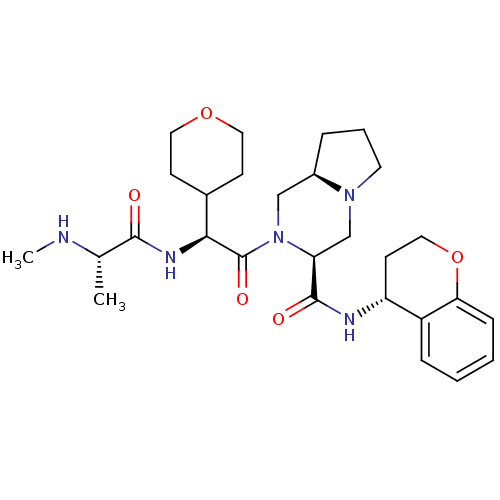 (CHEMBL2316214) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human recombinant His-tagged cIAP1 protein BIR3 domain (250 to 350 amino acid residues) using biotinylated-Smac as substrate after over... | J Med Chem 56: 1228-46 (2013) Article DOI: 10.1021/jm301674z BindingDB Entry DOI: 10.7270/Q23N24QX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 3 (Homo sapiens (Human)) | BDBM50425727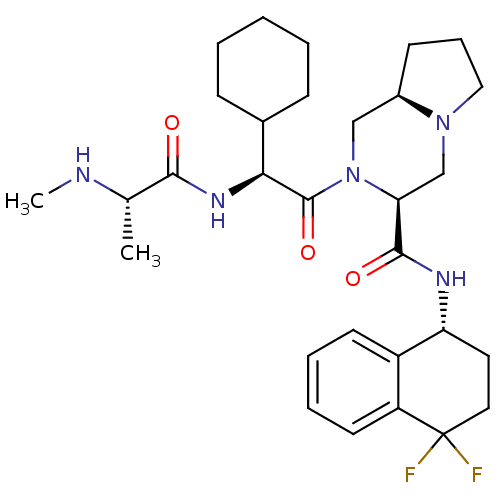 (CHEMBL2316222) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human recombinant His-tagged cIAP1 protein BIR3 domain (250 to 350 amino acid residues) using biotinylated-Smac as substrate after over... | J Med Chem 56: 1228-46 (2013) Article DOI: 10.1021/jm301674z BindingDB Entry DOI: 10.7270/Q23N24QX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 3 (Homo sapiens (Human)) | BDBM50425720 (CHEMBL2316216) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human recombinant His-tagged cIAP1 protein BIR3 domain (250 to 350 amino acid residues) using biotinylated-Smac as substrate after over... | J Med Chem 56: 1228-46 (2013) Article DOI: 10.1021/jm301674z BindingDB Entry DOI: 10.7270/Q23N24QX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50247111 (CHEMBL4090919) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of recombinant human ACC1 expressed in SF-9 cells preincubated for 60 mins followed by addition of substrate solution containing acetyl-Co... | J Med Chem 61: 1098-1117 (2018) Article DOI: 10.1021/acs.jmedchem.7b01547 BindingDB Entry DOI: 10.7270/Q2HQ429W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50247080 (CHEMBL4098647) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of recombinant human ACC1 expressed in SF-9 cells preincubated for 60 mins followed by addition of substrate solution containing acetyl-Co... | J Med Chem 61: 1098-1117 (2018) Article DOI: 10.1021/acs.jmedchem.7b01547 BindingDB Entry DOI: 10.7270/Q2HQ429W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50247113 (CHEMBL4064621) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of recombinant human ACC1 expressed in SF-9 cells preincubated for 60 mins followed by addition of substrate solution containing acetyl-Co... | J Med Chem 61: 1098-1117 (2018) Article DOI: 10.1021/acs.jmedchem.7b01547 BindingDB Entry DOI: 10.7270/Q2HQ429W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50247130 (CHEMBL4073202) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of ACC1 in human HCT116 cells assessed as reduction of [14C]acetate uptake preincubated for 60 mins followed by addition of [14C]acetate a... | J Med Chem 61: 1098-1117 (2018) Article DOI: 10.1021/acs.jmedchem.7b01547 BindingDB Entry DOI: 10.7270/Q2HQ429W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50247153 (CHEMBL4082153) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of recombinant human ACC1 expressed in SF-9 cells preincubated for 60 mins followed by addition of substrate solution containing acetyl-Co... | J Med Chem 61: 1098-1117 (2018) Article DOI: 10.1021/acs.jmedchem.7b01547 BindingDB Entry DOI: 10.7270/Q2HQ429W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50247094 (CHEMBL4101119) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of recombinant human ACC1 expressed in SF-9 cells preincubated for 60 mins followed by addition of substrate solution containing acetyl-Co... | J Med Chem 61: 1098-1117 (2018) Article DOI: 10.1021/acs.jmedchem.7b01547 BindingDB Entry DOI: 10.7270/Q2HQ429W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 3 (Homo sapiens (Human)) | BDBM50425726 (CHEMBL2316223) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human recombinant His-tagged cIAP1 protein BIR3 domain (250 to 350 amino acid residues) using biotinylated-Smac as substrate after over... | J Med Chem 56: 1228-46 (2013) Article DOI: 10.1021/jm301674z BindingDB Entry DOI: 10.7270/Q23N24QX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50247111 (CHEMBL4090919) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of ACC1 in human HCT116 cells assessed as reduction of [14C]acetate uptake preincubated for 60 mins followed by addition of [14C]acetate a... | J Med Chem 61: 1098-1117 (2018) Article DOI: 10.1021/acs.jmedchem.7b01547 BindingDB Entry DOI: 10.7270/Q2HQ429W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50586166 (CHEMBL5091527) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of EZH2 (unknown origin) using 3H-SAM as substrate incubated for 1 hour by filter binding method | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00448 BindingDB Entry DOI: 10.7270/Q2J10735 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50247153 (CHEMBL4082153) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of ACC1 in human HCT116 cells assessed as reduction of [14C]acetate uptake preincubated for 60 mins followed by addition of [14C]acetate a... | J Med Chem 61: 1098-1117 (2018) Article DOI: 10.1021/acs.jmedchem.7b01547 BindingDB Entry DOI: 10.7270/Q2HQ429W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50247110 (CHEMBL4083131) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of ACC1 in human HCT116 cells assessed as reduction of [14C]acetate uptake preincubated for 60 mins followed by addition of [14C]acetate a... | J Med Chem 61: 1098-1117 (2018) Article DOI: 10.1021/acs.jmedchem.7b01547 BindingDB Entry DOI: 10.7270/Q2HQ429W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50586174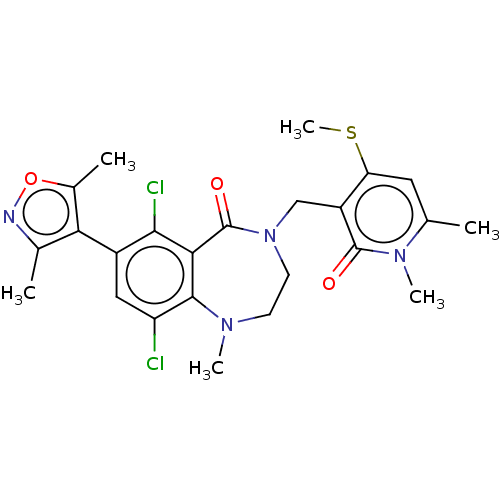 (CHEMBL5081019) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of EZH2 (unknown origin) using 3H-SAM as substrate incubated for 1 hour by filter binding method | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00448 BindingDB Entry DOI: 10.7270/Q2J10735 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50586157 (CHEMBL5082529) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of EZH2 (unknown origin) using 3H-SAM as substrate incubated for 1 hour by filter binding method | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00448 BindingDB Entry DOI: 10.7270/Q2J10735 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 3 (Homo sapiens (Human)) | BDBM50425731 (CHEMBL2316218) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human recombinant His-tagged cIAP1 protein BIR3 domain (250 to 350 amino acid residues) using biotinylated-Smac as substrate after over... | J Med Chem 56: 1228-46 (2013) Article DOI: 10.1021/jm301674z BindingDB Entry DOI: 10.7270/Q23N24QX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50586153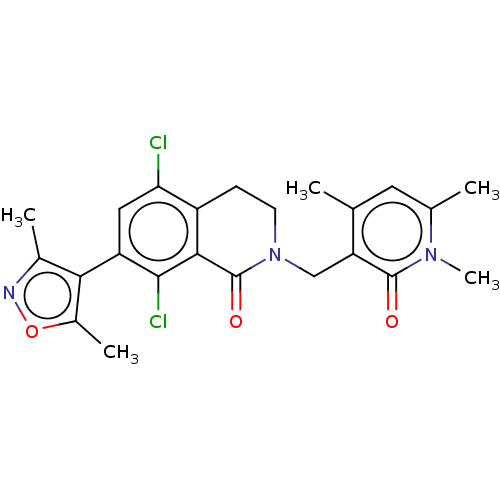 (CHEMBL5078476) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of EZH2 (unknown origin) using 3H-SAM as substrate incubated for 1 hour by filter binding method | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00448 BindingDB Entry DOI: 10.7270/Q2J10735 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50302337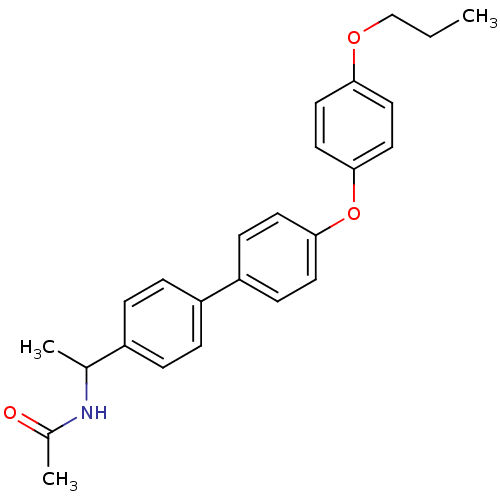 (CHEMBL567935 | N-(1-(4'-(4-propoxyphenoxy)biphenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human N-terminal His6-tagged ACC2 expressed in baculovirus infected Sf9 insect cells using acetyl-CoA as substrate preincub... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116056 BindingDB Entry DOI: 10.7270/Q29C7231 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50247094 (CHEMBL4101119) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of ACC1 in human HCT116 cells assessed as reduction of [14C]acetate uptake preincubated for 60 mins followed by addition of [14C]acetate a... | J Med Chem 61: 1098-1117 (2018) Article DOI: 10.1021/acs.jmedchem.7b01547 BindingDB Entry DOI: 10.7270/Q2HQ429W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-3-phosphoglycerate dehydrogenase (Homo sapiens (Human)) | BDBM50512584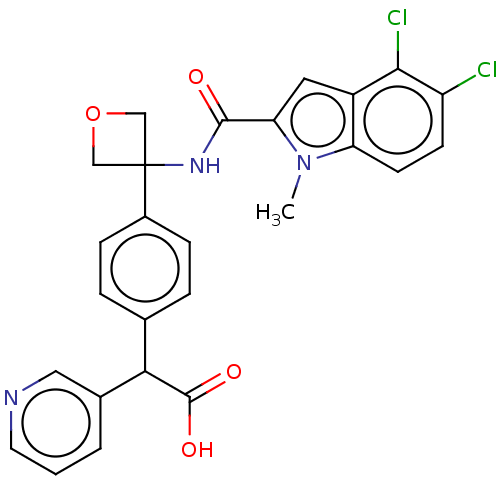 (CHEMBL4443265) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Meyer Cancer Center Curated by ChEMBL | Assay Description Inhibition of PHGDH (unknown origin) using 11 nM of PHGDH and 3-PG as substrate incubated for 20 mins in presence of NAD+, PSAT1, PSPH by diaphorase ... | Bioorg Med Chem Lett 29: 2503-2510 (2019) Article DOI: 10.1016/j.bmcl.2019.07.011 BindingDB Entry DOI: 10.7270/Q27D2ZGJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50557465 (CHEMBL4742115) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human N-terminal His6-tagged ACC1 expressed in baculovirus infected Sf9 insect cells using acetyl-CoA as substrate preincub... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116056 BindingDB Entry DOI: 10.7270/Q29C7231 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50193709 (CHEMBL3911017) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of EZH2 in HEK293T cells assessed as H3K27me3 levels incubated for 48 hrs by flow cytometry | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00448 BindingDB Entry DOI: 10.7270/Q2J10735 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50302337 (CHEMBL567935 | N-(1-(4'-(4-propoxyphenoxy)biphenyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human N-terminal His6-tagged ACC1 expressed in baculovirus infected Sf9 insect cells using acetyl-CoA as substrate preincub... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116056 BindingDB Entry DOI: 10.7270/Q29C7231 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 969 total ) | Next | Last >> |