 Found 196 hits with Last Name = 'tonelli' and Initial = 'm'
Found 196 hits with Last Name = 'tonelli' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM66082

((2S)-2-[[4-[(2,4-diaminopteridin-6-yl)methyl-methy...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O Show InChI InChI=1S/C20H22N8O5/c1-28(9-11-8-23-17-15(24-11)16(21)26-20(22)27-17)12-4-2-10(3-5-12)18(31)25-13(19(32)33)6-7-14(29)30/h2-5,8,13H,6-7,9H2,1H3,(H,25,31)(H,29,30)(H,32,33)(H4,21,22,23,26,27)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PDB
Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dipartimento di Farmacia, UniversitÓ di Genova, Viale Benedetto XV 3, 16132 Genova, Italy. Electronic address: tonelli@difar.unige.it.
Curated by ChEMBL
| Assay Description
Inhibition of DHFR (unknown origin) |
Eur J Med Chem 135: 467-478 (2017)
Article DOI: 10.1016/j.ejmech.2017.04.070
BindingDB Entry DOI: 10.7270/Q2057JD0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50219206
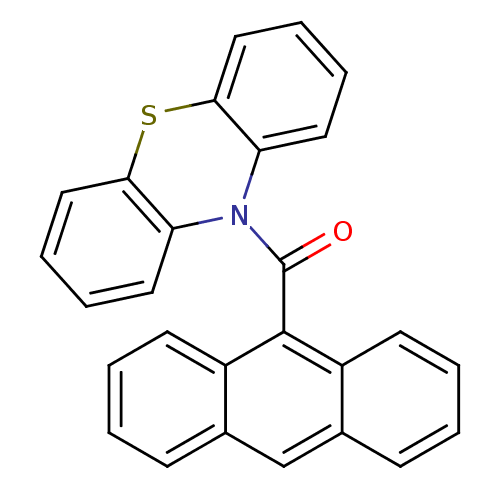
(Anthracen-9-yl (10H-phenothiazine-10yl) methanone,...)Show SMILES O=C(N1c2ccccc2Sc2ccccc12)c1c2ccccc2cc2ccccc12 Show InChI InChI=1S/C27H17NOS/c29-27(26-20-11-3-1-9-18(20)17-19-10-2-4-12-21(19)26)28-22-13-5-7-15-24(22)30-25-16-8-6-14-23(25)28/h1-17H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Genova
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocytes BChE |
Eur J Med Chem 46: 2170-84 (2011)
Article DOI: 10.1016/j.ejmech.2011.02.071
BindingDB Entry DOI: 10.7270/Q2BK1CPG |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50219206

(Anthracen-9-yl (10H-phenothiazine-10yl) methanone,...)Show SMILES O=C(N1c2ccccc2Sc2ccccc12)c1c2ccccc2cc2ccccc12 Show InChI InChI=1S/C27H17NOS/c29-27(26-20-11-3-1-9-18(20)17-19-10-2-4-12-21(19)26)28-22-13-5-7-15-24(22)30-25-16-8-6-14-23(25)28/h1-17H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Genova
Curated by ChEMBL
| Assay Description
Inhibition of human plasma BChE |
Eur J Med Chem 46: 2170-84 (2011)
Article DOI: 10.1016/j.ejmech.2011.02.071
BindingDB Entry DOI: 10.7270/Q2BK1CPG |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50433811
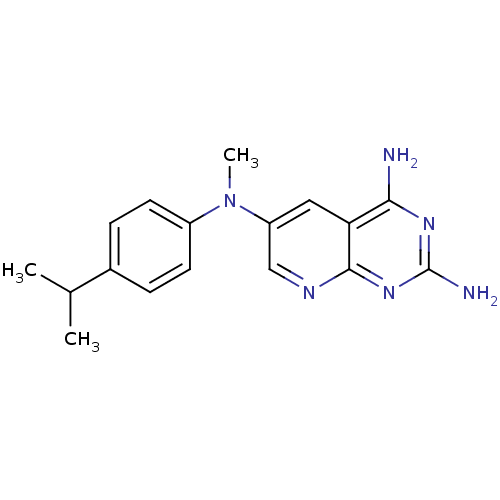
(CHEMBL2382332)Show InChI InChI=1S/C17H20N6/c1-10(2)11-4-6-12(7-5-11)23(3)13-8-14-15(18)21-17(19)22-16(14)20-9-13/h4-10H,1-3H3,(H4,18,19,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dipartimento di Farmacia, UniversitÓ di Genova, Viale Benedetto XV 3, 16132 Genova, Italy. Electronic address: tonelli@difar.unige.it.
Curated by ChEMBL
| Assay Description
Inhibition of human DHFR using dihydrofolate as substrate after 180 secs by spectrophotometric analysis |
Eur J Med Chem 135: 467-478 (2017)
Article DOI: 10.1016/j.ejmech.2017.04.070
BindingDB Entry DOI: 10.7270/Q2057JD0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50405066
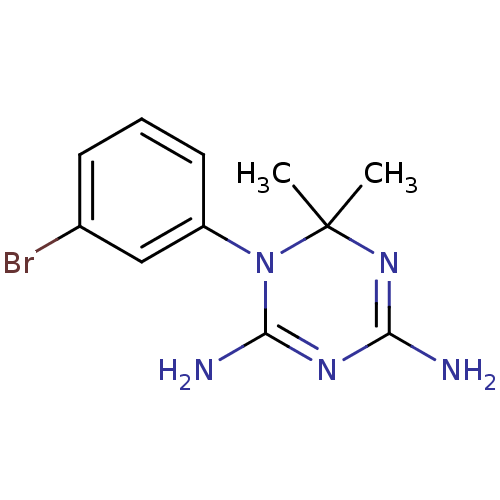
(CHEMBL268088)Show InChI InChI=1S/C11H14BrN5/c1-11(2)16-9(13)15-10(14)17(11)8-5-3-4-7(12)6-8/h3-6H,1-2H3,(H4,13,14,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dipartimento di Farmacia, UniversitÓ di Genova, Viale Benedetto XV 3, 16132 Genova, Italy. Electronic address: tonelli@difar.unige.it.
Curated by ChEMBL
| Assay Description
Inhibition of human DHFR using dihydrofolate as substrate after 180 secs by spectrophotometric analysis |
Eur J Med Chem 135: 467-478 (2017)
Article DOI: 10.1016/j.ejmech.2017.04.070
BindingDB Entry DOI: 10.7270/Q2057JD0 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50090054
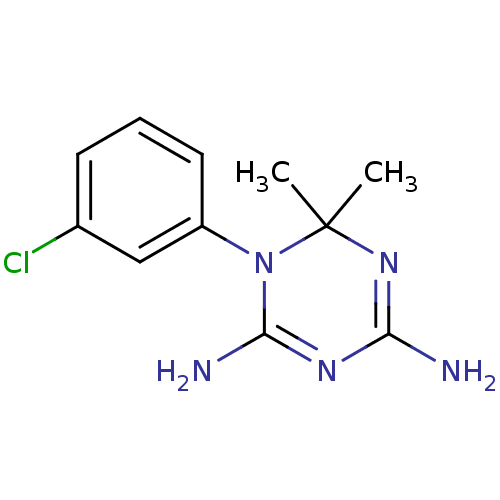
(1-(3-Chloro-phenyl)-6,6-dimethyl-1,6-dihydro-[1,3,...)Show InChI InChI=1S/C11H14ClN5/c1-11(2)16-9(13)15-10(14)17(11)8-5-3-4-7(12)6-8/h3-6H,1-2H3,(H4,13,14,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dipartimento di Farmacia, UniversitÓ di Genova, Viale Benedetto XV 3, 16132 Genova, Italy. Electronic address: tonelli@difar.unige.it.
Curated by ChEMBL
| Assay Description
Inhibition of human DHFR using dihydrofolate as substrate after 180 secs by spectrophotometric analysis |
Eur J Med Chem 135: 467-478 (2017)
Article DOI: 10.1016/j.ejmech.2017.04.070
BindingDB Entry DOI: 10.7270/Q2057JD0 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50090069

(1-(3,4-Dichloro-phenyl)-6,6-dimethyl-1,6-dihydro-[...)Show SMILES CC1(C)N=C(N)N=C(N)N1c1ccc(Cl)c(Cl)c1 |t:3,6| Show InChI InChI=1S/C11H13Cl2N5/c1-11(2)17-9(14)16-10(15)18(11)6-3-4-7(12)8(13)5-6/h3-5H,1-2H3,(H4,14,15,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dipartimento di Farmacia, UniversitÓ di Genova, Viale Benedetto XV 3, 16132 Genova, Italy. Electronic address: tonelli@difar.unige.it.
Curated by ChEMBL
| Assay Description
Inhibition of human DHFR using dihydrofolate as substrate after 180 secs by spectrophotometric analysis |
Eur J Med Chem 135: 467-478 (2017)
Article DOI: 10.1016/j.ejmech.2017.04.070
BindingDB Entry DOI: 10.7270/Q2057JD0 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50291793
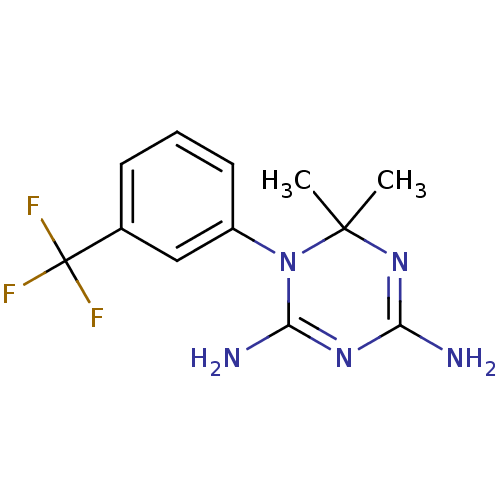
(6,6-Dimethyl-1-(3-trifluoromethyl-phenyl)-1,6-dihy...)Show SMILES CC1(C)N=C(N)N=C(N)N1c1cccc(c1)C(F)(F)F |t:3,6| Show InChI InChI=1S/C12H14F3N5/c1-11(2)19-9(16)18-10(17)20(11)8-5-3-4-7(6-8)12(13,14)15/h3-6H,1-2H3,(H4,16,17,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dipartimento di Farmacia, UniversitÓ di Genova, Viale Benedetto XV 3, 16132 Genova, Italy. Electronic address: tonelli@difar.unige.it.
Curated by ChEMBL
| Assay Description
Inhibition of human DHFR using dihydrofolate as substrate after 180 secs by spectrophotometric analysis |
Eur J Med Chem 135: 467-478 (2017)
Article DOI: 10.1016/j.ejmech.2017.04.070
BindingDB Entry DOI: 10.7270/Q2057JD0 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM18792
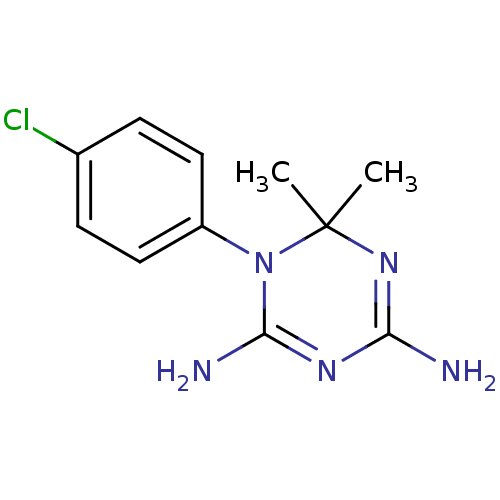
(1-(4-chlorophenyl)-6,6-dimethyl-1,6-dihydro-1,3,5-...)Show InChI InChI=1S/C11H14ClN5/c1-11(2)16-9(13)15-10(14)17(11)8-5-3-7(12)4-6-8/h3-6H,1-2H3,(H4,13,14,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dipartimento di Farmacia, UniversitÓ di Genova, Viale Benedetto XV 3, 16132 Genova, Italy. Electronic address: tonelli@difar.unige.it.
Curated by ChEMBL
| Assay Description
Inhibition of human DHFR using dihydrofolate as substrate after 180 secs by spectrophotometric analysis |
Eur J Med Chem 135: 467-478 (2017)
Article DOI: 10.1016/j.ejmech.2017.04.070
BindingDB Entry DOI: 10.7270/Q2057JD0 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM18512

(5-(4-chlorophenyl)-6-ethylpyrimidine-2,4-diamine |...)Show InChI InChI=1S/C12H13ClN4/c1-2-9-10(11(14)17-12(15)16-9)7-3-5-8(13)6-4-7/h3-6H,2H2,1H3,(H4,14,15,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dipartimento di Farmacia, UniversitÓ di Genova, Viale Benedetto XV 3, 16132 Genova, Italy. Electronic address: tonelli@difar.unige.it.
Curated by ChEMBL
| Assay Description
Inhibition of human DHFR using dihydrofolate as substrate after 180 secs by spectrophotometric analysis |
Eur J Med Chem 135: 467-478 (2017)
Article DOI: 10.1016/j.ejmech.2017.04.070
BindingDB Entry DOI: 10.7270/Q2057JD0 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50449542

(CHEMBL4170787)Show SMILES NC1=NC2(CCN(CC2)C(=O)Nc2ccc(F)cc2)N(C(N)=N1)c1cccc(Cl)c1 |c:23,t:1| Show InChI InChI=1S/C20H21ClFN7O/c21-13-2-1-3-16(12-13)29-18(24)26-17(23)27-20(29)8-10-28(11-9-20)19(30)25-15-6-4-14(22)5-7-15/h1-7,12H,8-11H2,(H,25,30)(H4,23,24,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Genoa
Curated by ChEMBL
| Assay Description
Binding affinity to human recombinant DHFR using dihydrofolate as substrate after 180 secs by spectrophotometric method |
Eur J Med Chem 155: 229-243 (2018)
Article DOI: 10.1016/j.ejmech.2018.05.059
BindingDB Entry DOI: 10.7270/Q29Z97GN |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50449541

(CHEMBL4167391)Show SMILES Cc1ccc(cc1)C(=O)N1CCC2(CC1)N=C(N)N=C(N)N2c1cccc(Cl)c1 |t:17,20| Show InChI InChI=1S/C21H23ClN6O/c1-14-5-7-15(8-6-14)18(29)27-11-9-21(10-12-27)26-19(23)25-20(24)28(21)17-4-2-3-16(22)13-17/h2-8,13H,9-12H2,1H3,(H4,23,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Genoa
Curated by ChEMBL
| Assay Description
Binding affinity to human recombinant DHFR using dihydrofolate as substrate after 180 secs by spectrophotometric method |
Eur J Med Chem 155: 229-243 (2018)
Article DOI: 10.1016/j.ejmech.2018.05.059
BindingDB Entry DOI: 10.7270/Q29Z97GN |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50449543

(CHEMBL4169854)Show SMILES CCS(=O)(=O)N1CCC2(CC1)N=C(N)N=C(N)N2CCOC |t:12,15| Show InChI InChI=1S/C12H24N6O3S/c1-3-22(19,20)17-6-4-12(5-7-17)16-10(13)15-11(14)18(12)8-9-21-2/h3-9H2,1-2H3,(H4,13,14,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Genoa
Curated by ChEMBL
| Assay Description
Binding affinity to human recombinant DHFR using dihydrofolate as substrate after 180 secs by spectrophotometric method |
Eur J Med Chem 155: 229-243 (2018)
Article DOI: 10.1016/j.ejmech.2018.05.059
BindingDB Entry DOI: 10.7270/Q29Z97GN |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50267663

(CHEMBL4105317)Show SMILES NC1=NC2(CCCCC2)N(C(N)=N1)c1cccc(c1)C(F)(F)F |c:12,t:1| Show InChI InChI=1S/C15H18F3N5/c16-15(17,18)10-5-4-6-11(9-10)23-13(20)21-12(19)22-14(23)7-2-1-3-8-14/h4-6,9H,1-3,7-8H2,(H4,19,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.32E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dipartimento di Farmacia, UniversitÓ di Genova, Viale Benedetto XV 3, 16132 Genova, Italy. Electronic address: tonelli@difar.unige.it.
Curated by ChEMBL
| Assay Description
Inhibition of human DHFR using dihydrofolate as substrate after 180 secs by spectrophotometric analysis |
Eur J Med Chem 135: 467-478 (2017)
Article DOI: 10.1016/j.ejmech.2017.04.070
BindingDB Entry DOI: 10.7270/Q2057JD0 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM18792

(1-(4-chlorophenyl)-6,6-dimethyl-1,6-dihydro-1,3,5-...)Show InChI InChI=1S/C11H14ClN5/c1-11(2)16-9(13)15-10(14)17(11)8-5-3-7(12)4-6-8/h3-6H,1-2H3,(H4,13,14,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 4.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dipartimento di Farmacia, UniversitÓ di Genova, Viale Benedetto XV 3, 16132 Genova, Italy. Electronic address: tonelli@difar.unige.it.
Curated by ChEMBL
| Assay Description
Inhibition of human DHFR |
Eur J Med Chem 135: 467-478 (2017)
Article DOI: 10.1016/j.ejmech.2017.04.070
BindingDB Entry DOI: 10.7270/Q2057JD0 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM18069

(5-[(3,4,5-trimethoxyphenyl)methyl]pyrimidine-2,4-d...)Show InChI InChI=1S/C14H18N4O3/c1-19-10-5-8(6-11(20-2)12(10)21-3)4-9-7-17-14(16)18-13(9)15/h5-7H,4H2,1-3H3,(H4,15,16,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 4.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dipartimento di Farmacia, UniversitÓ di Genova, Viale Benedetto XV 3, 16132 Genova, Italy. Electronic address: tonelli@difar.unige.it.
Curated by ChEMBL
| Assay Description
Inhibition of human DHFR |
Eur J Med Chem 135: 467-478 (2017)
Article DOI: 10.1016/j.ejmech.2017.04.070
BindingDB Entry DOI: 10.7270/Q2057JD0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dihydrofolate reductase
(Pneumocystis carinii) | BDBM18792

(1-(4-chlorophenyl)-6,6-dimethyl-1,6-dihydro-1,3,5-...)Show InChI InChI=1S/C11H14ClN5/c1-11(2)16-9(13)15-10(14)17(11)8-5-3-7(12)4-6-8/h3-6H,1-2H3,(H4,13,14,15,16) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.09E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dipartimento di Farmacia, UniversitÓ di Genova, Viale Benedetto XV 3, 16132 Genova, Italy. Electronic address: tonelli@difar.unige.it.
Curated by ChEMBL
| Assay Description
Inhibition of Pneumocystis carinii DHFR |
Eur J Med Chem 135: 467-478 (2017)
Article DOI: 10.1016/j.ejmech.2017.04.070
BindingDB Entry DOI: 10.7270/Q2057JD0 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Pneumocystis carinii) | BDBM18069

(5-[(3,4,5-trimethoxyphenyl)methyl]pyrimidine-2,4-d...)Show InChI InChI=1S/C14H18N4O3/c1-19-10-5-8(6-11(20-2)12(10)21-3)4-9-7-17-14(16)18-13(9)15/h5-7H,4H2,1-3H3,(H4,15,16,17,18) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 2.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dipartimento di Farmacia, UniversitÓ di Genova, Viale Benedetto XV 3, 16132 Genova, Italy. Electronic address: tonelli@difar.unige.it.
Curated by ChEMBL
| Assay Description
Inhibition of Pneumocystis carinii DHFR |
Eur J Med Chem 135: 467-478 (2017)
Article DOI: 10.1016/j.ejmech.2017.04.070
BindingDB Entry DOI: 10.7270/Q2057JD0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM10972
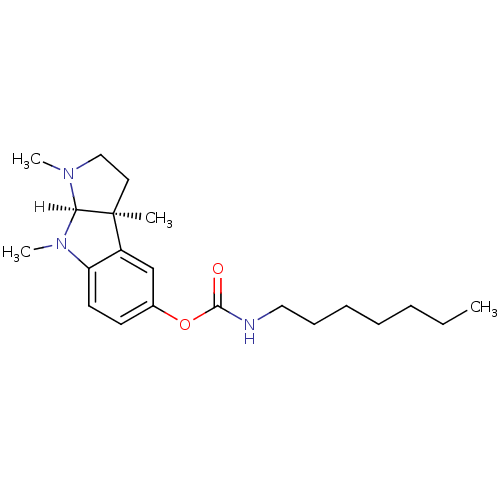
((3aS,8aR)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyr...)Show SMILES [H][C@]12N(C)CC[C@@]1(C)c1cc(OC(=O)NCCCCCCC)ccc1N2C Show InChI InChI=1S/C21H33N3O2/c1-5-6-7-8-9-13-22-20(25)26-16-10-11-18-17(15-16)21(2)12-14-23(3)19(21)24(18)4/h10-11,15,19H,5-9,12-14H2,1-4H3,(H,22,25)/t19-,21+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Genova
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocytes BChE |
Eur J Med Chem 46: 2170-84 (2011)
Article DOI: 10.1016/j.ejmech.2011.02.071
BindingDB Entry DOI: 10.7270/Q2BK1CPG |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM10972

((3aS,8aR)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyr...)Show SMILES [H][C@]12N(C)CC[C@@]1(C)c1cc(OC(=O)NCCCCCCC)ccc1N2C Show InChI InChI=1S/C21H33N3O2/c1-5-6-7-8-9-13-22-20(25)26-16-10-11-18-17(15-16)21(2)12-14-23(3)19(21)24(18)4/h10-11,15,19H,5-9,12-14H2,1-4H3,(H,22,25)/t19-,21+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Genova
Curated by ChEMBL
| Assay Description
Inhibition of human plasma BChE |
Eur J Med Chem 46: 2170-84 (2011)
Article DOI: 10.1016/j.ejmech.2011.02.071
BindingDB Entry DOI: 10.7270/Q2BK1CPG |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50312803
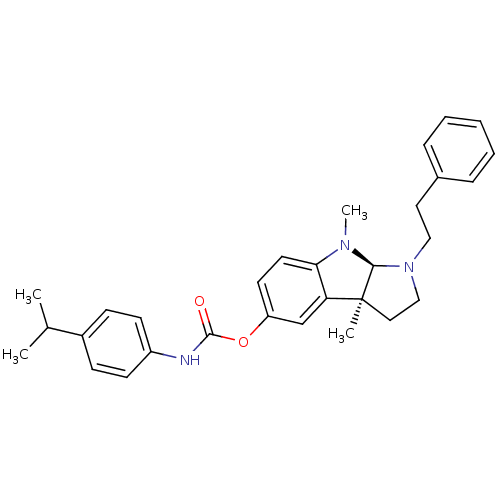
((3aS,8aR)-3a,8-dimethyl-1-phenethyl-1,2,3,3a,8,8a-...)Show SMILES CC(C)c1ccc(NC(=O)Oc2ccc3N(C)[C@H]4N(CCc5ccccc5)CC[C@@]4(C)c3c2)cc1 |r| Show InChI InChI=1S/C30H35N3O2/c1-21(2)23-10-12-24(13-11-23)31-29(34)35-25-14-15-27-26(20-25)30(3)17-19-33(28(30)32(27)4)18-16-22-8-6-5-7-9-22/h5-15,20-21,28H,16-19H2,1-4H3,(H,31,34)/t28-,30-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Genova
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocytes BChE |
Eur J Med Chem 46: 2170-84 (2011)
Article DOI: 10.1016/j.ejmech.2011.02.071
BindingDB Entry DOI: 10.7270/Q2BK1CPG |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50312803

((3aS,8aR)-3a,8-dimethyl-1-phenethyl-1,2,3,3a,8,8a-...)Show SMILES CC(C)c1ccc(NC(=O)Oc2ccc3N(C)[C@H]4N(CCc5ccccc5)CC[C@@]4(C)c3c2)cc1 |r| Show InChI InChI=1S/C30H35N3O2/c1-21(2)23-10-12-24(13-11-23)31-29(34)35-25-14-15-27-26(20-25)30(3)17-19-33(28(30)32(27)4)18-16-22-8-6-5-7-9-22/h5-15,20-21,28H,16-19H2,1-4H3,(H,31,34)/t28-,30-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Genova
Curated by ChEMBL
| Assay Description
Inhibition of human plasma BChE |
Eur J Med Chem 46: 2170-84 (2011)
Article DOI: 10.1016/j.ejmech.2011.02.071
BindingDB Entry DOI: 10.7270/Q2BK1CPG |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50346444
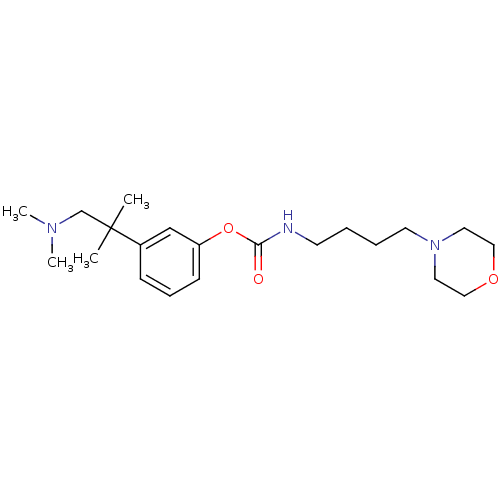
(CHEMBL1782707 | [4-(4-morpholinyl)butyl]carbamic a...)Show InChI InChI=1S/C21H35N3O3/c1-21(2,17-23(3)4)18-8-7-9-19(16-18)27-20(25)22-10-5-6-11-24-12-14-26-15-13-24/h7-9,16H,5-6,10-15,17H2,1-4H3,(H,22,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Genova
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocytes BChE |
Eur J Med Chem 46: 2170-84 (2011)
Article DOI: 10.1016/j.ejmech.2011.02.071
BindingDB Entry DOI: 10.7270/Q2BK1CPG |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50346444

(CHEMBL1782707 | [4-(4-morpholinyl)butyl]carbamic a...)Show InChI InChI=1S/C21H35N3O3/c1-21(2,17-23(3)4)18-8-7-9-19(16-18)27-20(25)22-10-5-6-11-24-12-14-26-15-13-24/h7-9,16H,5-6,10-15,17H2,1-4H3,(H,22,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Genova
Curated by ChEMBL
| Assay Description
Inhibition of human plasma BChE |
Eur J Med Chem 46: 2170-84 (2011)
Article DOI: 10.1016/j.ejmech.2011.02.071
BindingDB Entry DOI: 10.7270/Q2BK1CPG |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM8960

((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...)Show SMILES COc1cc2CC(CC3CCN(Cc4ccccc4)CC3)C(=O)c2cc1OC Show InChI InChI=1S/C24H29NO3/c1-27-22-14-19-13-20(24(26)21(19)15-23(22)28-2)12-17-8-10-25(11-9-17)16-18-6-4-3-5-7-18/h3-7,14-15,17,20H,8-13,16H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Genova
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocytes AChE |
Eur J Med Chem 46: 2170-84 (2011)
Article DOI: 10.1016/j.ejmech.2011.02.071
BindingDB Entry DOI: 10.7270/Q2BK1CPG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM8960

((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...)Show SMILES COc1cc2CC(CC3CCN(Cc4ccccc4)CC3)C(=O)c2cc1OC Show InChI InChI=1S/C24H29NO3/c1-27-22-14-19-13-20(24(26)21(19)15-23(22)28-2)12-17-8-10-25(11-9-17)16-18-6-4-3-5-7-18/h3-7,14-15,17,20H,8-13,16H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Genova
Curated by ChEMBL
| Assay Description
Inhibition of human plasma AChE |
Eur J Med Chem 46: 2170-84 (2011)
Article DOI: 10.1016/j.ejmech.2011.02.071
BindingDB Entry DOI: 10.7270/Q2BK1CPG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM10972

((3aS,8aR)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyr...)Show SMILES [H][C@]12N(C)CC[C@@]1(C)c1cc(OC(=O)NCCCCCCC)ccc1N2C Show InChI InChI=1S/C21H33N3O2/c1-5-6-7-8-9-13-22-20(25)26-16-10-11-18-17(15-16)21(2)12-14-23(3)19(21)24(18)4/h10-11,15,19H,5-9,12-14H2,1-4H3,(H,22,25)/t19-,21+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Genova
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocytes AChE |
Eur J Med Chem 46: 2170-84 (2011)
Article DOI: 10.1016/j.ejmech.2011.02.071
BindingDB Entry DOI: 10.7270/Q2BK1CPG |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM10620

((S)-3-(1-(dimethylamino)ethyl)phenyl ethyl(methyl)...)Show InChI InChI=1S/C14H22N2O2/c1-6-16(5)14(17)18-13-9-7-8-12(10-13)11(2)15(3)4/h7-11H,6H2,1-5H3/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Genova
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocytes BChE |
Eur J Med Chem 46: 2170-84 (2011)
Article DOI: 10.1016/j.ejmech.2011.02.071
BindingDB Entry DOI: 10.7270/Q2BK1CPG |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM10620

((S)-3-(1-(dimethylamino)ethyl)phenyl ethyl(methyl)...)Show InChI InChI=1S/C14H22N2O2/c1-6-16(5)14(17)18-13-9-7-8-12(10-13)11(2)15(3)4/h7-11H,6H2,1-5H3/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Genova
Curated by ChEMBL
| Assay Description
Inhibition of human plasma BChE |
Eur J Med Chem 46: 2170-84 (2011)
Article DOI: 10.1016/j.ejmech.2011.02.071
BindingDB Entry DOI: 10.7270/Q2BK1CPG |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Genova
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocytes BChE |
Eur J Med Chem 46: 2170-84 (2011)
Article DOI: 10.1016/j.ejmech.2011.02.071
BindingDB Entry DOI: 10.7270/Q2BK1CPG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Genova
Curated by ChEMBL
| Assay Description
Inhibition of human plasma BChE |
Eur J Med Chem 46: 2170-84 (2011)
Article DOI: 10.1016/j.ejmech.2011.02.071
BindingDB Entry DOI: 10.7270/Q2BK1CPG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cholinesterase
(Equus caballus (Horse)) | BDBM50346435
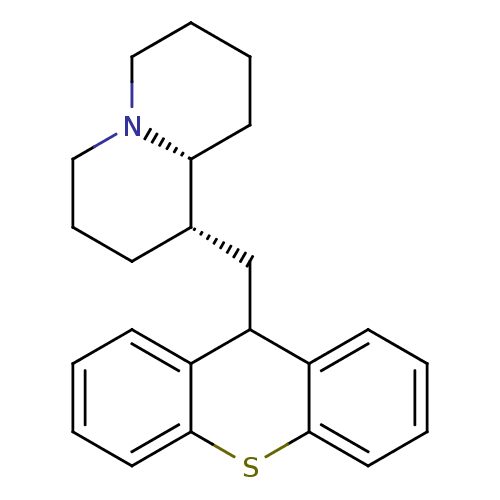
((1S,9aR)-1-((9H-thioxanthen-9-yl)methyl)octahydro-...)Show SMILES C([C@@H]1CCCN2CCCC[C@H]12)C1c2ccccc2Sc2ccccc12 |r| Show InChI InChI=1S/C23H27NS/c1-3-12-22-18(9-1)20(19-10-2-4-13-23(19)25-22)16-17-8-7-15-24-14-6-5-11-21(17)24/h1-4,9-10,12-13,17,20-21H,5-8,11,14-16H2/t17-,21+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Genova
Curated by ChEMBL
| Assay Description
Inhibition of Equine serum BChE using butyrylthiocoline iodide as a substrate after 20 mins by Ellman's assay |
Eur J Med Chem 46: 2170-84 (2011)
Article DOI: 10.1016/j.ejmech.2011.02.071
BindingDB Entry DOI: 10.7270/Q2BK1CPG |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Genova
Curated by ChEMBL
| Assay Description
Inhibition of human plasma AChE |
Eur J Med Chem 46: 2170-84 (2011)
Article DOI: 10.1016/j.ejmech.2011.02.071
BindingDB Entry DOI: 10.7270/Q2BK1CPG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Genova
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocytes AChE |
Eur J Med Chem 46: 2170-84 (2011)
Article DOI: 10.1016/j.ejmech.2011.02.071
BindingDB Entry DOI: 10.7270/Q2BK1CPG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Bos taurus (bovine)) | BDBM50346417

(6-chloro-2-methoxy-N-(3-(((1R,9aR)-octahydro-1H-qu...)Show SMILES COc1ccc2nc3cc(Cl)ccc3c(NCCCSC[C@@H]3CCCN4CCCC[C@H]34)c2c1 |r| Show InChI InChI=1S/C27H34ClN3OS/c1-32-21-9-11-24-23(17-21)27(22-10-8-20(28)16-25(22)30-24)29-12-5-15-33-18-19-6-4-14-31-13-3-2-7-26(19)31/h8-11,16-17,19,26H,2-7,12-15,18H2,1H3,(H,29,30)/t19-,26+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Genova
Curated by ChEMBL
| Assay Description
Inhibition of bovine erythrocyte AChE using acetylthiocholine iodide as a substrate after 20 mins by Ellman's assay |
Eur J Med Chem 46: 2170-84 (2011)
Article DOI: 10.1016/j.ejmech.2011.02.071
BindingDB Entry DOI: 10.7270/Q2BK1CPG |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50346422
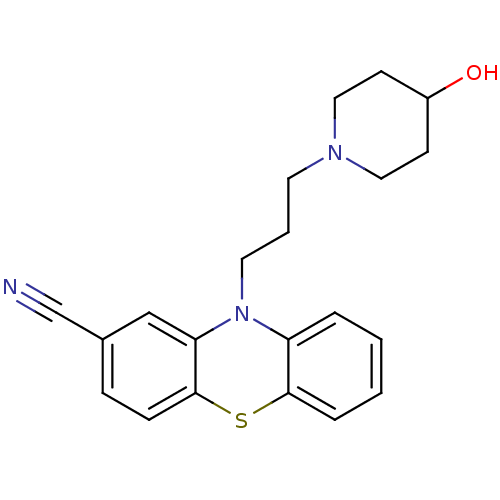
(10-(3-(4-hydroxypiperidin-1-yl)propyl)-10H-phenoth...)Show InChI InChI=1S/C21H23N3OS/c22-15-16-6-7-21-19(14-16)24(18-4-1-2-5-20(18)26-21)11-3-10-23-12-8-17(25)9-13-23/h1-2,4-7,14,17,25H,3,8-13H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Genova
Curated by ChEMBL
| Assay Description
Inhibition of Equine serum BChE using butyrylthiocoline iodide as a substrate after 20 mins by Ellman's assay |
Eur J Med Chem 46: 2170-84 (2011)
Article DOI: 10.1016/j.ejmech.2011.02.071
BindingDB Entry DOI: 10.7270/Q2BK1CPG |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM8958
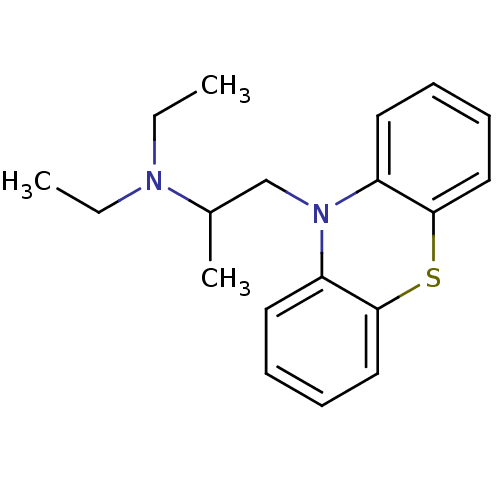
(10-(2-diethylaminopropyl)phenothiazine | CHEMBL120...)Show InChI InChI=1S/C19H24N2S/c1-4-20(5-2)15(3)14-21-16-10-6-8-12-18(16)22-19-13-9-7-11-17(19)21/h6-13,15H,4-5,14H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Genova
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocytes AChE |
Eur J Med Chem 46: 2170-84 (2011)
Article DOI: 10.1016/j.ejmech.2011.02.071
BindingDB Entry DOI: 10.7270/Q2BK1CPG |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM8958

(10-(2-diethylaminopropyl)phenothiazine | CHEMBL120...)Show InChI InChI=1S/C19H24N2S/c1-4-20(5-2)15(3)14-21-16-10-6-8-12-18(16)22-19-13-9-7-11-17(19)21/h6-13,15H,4-5,14H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Genova
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocytes BChE |
Eur J Med Chem 46: 2170-84 (2011)
Article DOI: 10.1016/j.ejmech.2011.02.071
BindingDB Entry DOI: 10.7270/Q2BK1CPG |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM8958

(10-(2-diethylaminopropyl)phenothiazine | CHEMBL120...)Show InChI InChI=1S/C19H24N2S/c1-4-20(5-2)15(3)14-21-16-10-6-8-12-18(16)22-19-13-9-7-11-17(19)21/h6-13,15H,4-5,14H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Genova
Curated by ChEMBL
| Assay Description
Inhibition of human plasma BChE |
Eur J Med Chem 46: 2170-84 (2011)
Article DOI: 10.1016/j.ejmech.2011.02.071
BindingDB Entry DOI: 10.7270/Q2BK1CPG |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50346416
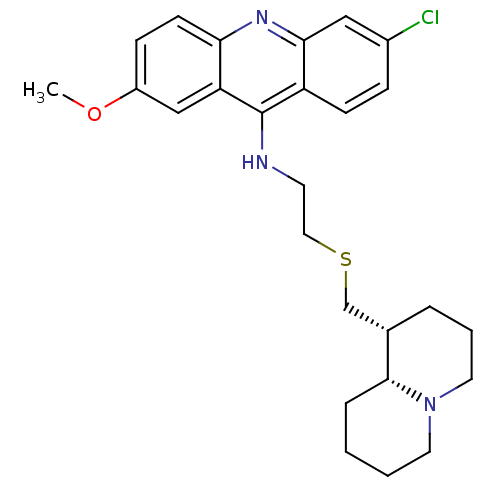
(6-chloro-2-methoxy-N-(2-(((1R,9aR)-octahydro-1H-qu...)Show SMILES COc1ccc2nc3cc(Cl)ccc3c(NCCSC[C@@H]3CCCN4CCCC[C@H]34)c2c1 |r| Show InChI InChI=1S/C26H32ClN3OS/c1-31-20-8-10-23-22(16-20)26(21-9-7-19(27)15-24(21)29-23)28-11-14-32-17-18-5-4-13-30-12-3-2-6-25(18)30/h7-10,15-16,18,25H,2-6,11-14,17H2,1H3,(H,28,29)/t18-,25+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Genova
Curated by ChEMBL
| Assay Description
Inhibition of Equine serum BChE using butyrylthiocoline iodide as a substrate after 20 mins by Ellman's assay |
Eur J Med Chem 46: 2170-84 (2011)
Article DOI: 10.1016/j.ejmech.2011.02.071
BindingDB Entry DOI: 10.7270/Q2BK1CPG |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50346436

(5-Methyl-1-(9H-thioxanthen-9-ylmethyl)-(1S,9aR)-oc...)Show SMILES C[N+]12CCCC[C@@H]1[C@H](CC1c3ccccc3Sc3ccccc13)CCC2 |r| Show InChI InChI=1S/C24H30NS/c1-25-15-7-6-12-22(25)18(9-8-16-25)17-21-19-10-2-4-13-23(19)26-24-14-5-3-11-20(21)24/h2-5,10-11,13-14,18,21-22H,6-9,12,15-17H2,1H3/q+1/t18-,22+,25?/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Genova
Curated by ChEMBL
| Assay Description
Inhibition of Equine serum BChE using butyrylthiocoline iodide as a substrate after 20 mins by Ellman's assay |
Eur J Med Chem 46: 2170-84 (2011)
Article DOI: 10.1016/j.ejmech.2011.02.071
BindingDB Entry DOI: 10.7270/Q2BK1CPG |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50346408

(2-(((1R,9aR)-octahydro-1H-quinolizin-1-yl)methylth...)Show SMILES O=C(CSC[C@@H]1CCCN2CCCC[C@H]12)N1c2ccccc2Sc2ccccc12 |r| Show InChI InChI=1S/C24H28N2OS2/c27-24(17-28-16-18-8-7-15-25-14-6-5-9-19(18)25)26-20-10-1-3-12-22(20)29-23-13-4-2-11-21(23)26/h1-4,10-13,18-19H,5-9,14-17H2/t18-,19+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Genova
Curated by ChEMBL
| Assay Description
Inhibition of Equine serum BChE using butyrylthiocoline iodide as a substrate after 20 mins by Ellman's assay |
Eur J Med Chem 46: 2170-84 (2011)
Article DOI: 10.1016/j.ejmech.2011.02.071
BindingDB Entry DOI: 10.7270/Q2BK1CPG |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50346410

(2-[(1R,9aR)-(Octahydro-1H-quinolizin-1-yl)methylth...)Show SMILES CC(SC[C@@H]1CCCN2CCCC[C@H]12)C(=O)N1c2ccccc2Sc2ccccc12 |r| Show InChI InChI=1S/C25H30N2OS2/c1-18(29-17-19-9-8-16-26-15-7-6-10-20(19)26)25(28)27-21-11-2-4-13-23(21)30-24-14-5-3-12-22(24)27/h2-5,11-14,18-20H,6-10,15-17H2,1H3/t18?,19-,20+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Genova
Curated by ChEMBL
| Assay Description
Inhibition of Equine serum BChE using butyrylthiocoline iodide as a substrate after 20 mins by Ellman's assay |
Eur J Med Chem 46: 2170-84 (2011)
Article DOI: 10.1016/j.ejmech.2011.02.071
BindingDB Entry DOI: 10.7270/Q2BK1CPG |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50346425
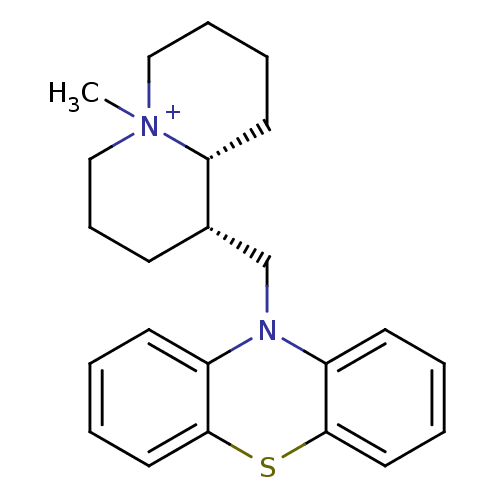
((1S,9aR)-1-((10H-phenothiazin-10-yl)methyl)-5-meth...)Show SMILES C[N+]12CCCC[C@@H]1[C@H](CN1c3ccccc3Sc3ccccc13)CCC2 |r| Show InChI InChI=1S/C23H29N2S/c1-25-15-7-6-12-21(25)18(9-8-16-25)17-24-19-10-2-4-13-22(19)26-23-14-5-3-11-20(23)24/h2-5,10-11,13-14,18,21H,6-9,12,15-17H2,1H3/q+1/t18-,21+,25?/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Genova
Curated by ChEMBL
| Assay Description
Inhibition of Equine serum BChE using butyrylthiocoline iodide as a substrate after 20 mins by Ellman's assay |
Eur J Med Chem 46: 2170-84 (2011)
Article DOI: 10.1016/j.ejmech.2011.02.071
BindingDB Entry DOI: 10.7270/Q2BK1CPG |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50346442

((1S,9aR)-1-((9H-fluoren-9-yl)methyl)octahydro-1H-q...)Show SMILES C(C1c2ccccc2-c2ccccc12)[C@@H]1CCCN2CCCC[C@H]12 |r| Show InChI InChI=1S/C23H27N/c1-3-11-20-18(9-1)19-10-2-4-12-21(19)22(20)16-17-8-7-15-24-14-6-5-13-23(17)24/h1-4,9-12,17,22-23H,5-8,13-16H2/t17-,23+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 630 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Genova
Curated by ChEMBL
| Assay Description
Inhibition of Equine serum BChE using butyrylthiocoline iodide as a substrate after 20 mins by Ellman's assay |
Eur J Med Chem 46: 2170-84 (2011)
Article DOI: 10.1016/j.ejmech.2011.02.071
BindingDB Entry DOI: 10.7270/Q2BK1CPG |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Bos taurus (bovine)) | BDBM50346421

(6-(5-(((1R,9aR)-octahydro-1H-quinolizin-1-yl)methy...)Show SMILES O=c1ccc2cc(OCCCCCSC[C@@H]3CCCN4CCCC[C@H]34)ccc2o1 |r| Show InChI InChI=1S/C24H33NO3S/c26-24-12-9-19-17-21(10-11-23(19)28-24)27-15-4-1-5-16-29-18-20-7-6-14-25-13-3-2-8-22(20)25/h9-12,17,20,22H,1-8,13-16,18H2/t20-,22+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 680 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Genova
Curated by ChEMBL
| Assay Description
Inhibition of bovine erythrocyte AChE using acetylthiocholine iodide as a substrate after 20 mins by Ellman's assay |
Eur J Med Chem 46: 2170-84 (2011)
Article DOI: 10.1016/j.ejmech.2011.02.071
BindingDB Entry DOI: 10.7270/Q2BK1CPG |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50346417

(6-chloro-2-methoxy-N-(3-(((1R,9aR)-octahydro-1H-qu...)Show SMILES COc1ccc2nc3cc(Cl)ccc3c(NCCCSC[C@@H]3CCCN4CCCC[C@H]34)c2c1 |r| Show InChI InChI=1S/C27H34ClN3OS/c1-32-21-9-11-24-23(17-21)27(22-10-8-20(28)16-25(22)30-24)29-12-5-15-33-18-19-6-4-14-31-13-3-2-7-26(19)31/h8-11,16-17,19,26H,2-7,12-15,18H2,1H3,(H,29,30)/t19-,26+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 690 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Genova
Curated by ChEMBL
| Assay Description
Inhibition of Equine serum BChE using butyrylthiocoline iodide as a substrate after 20 mins by Ellman's assay |
Eur J Med Chem 46: 2170-84 (2011)
Article DOI: 10.1016/j.ejmech.2011.02.071
BindingDB Entry DOI: 10.7270/Q2BK1CPG |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM8958

(10-(2-diethylaminopropyl)phenothiazine | CHEMBL120...)Show InChI InChI=1S/C19H24N2S/c1-4-20(5-2)15(3)14-21-16-10-6-8-12-18(16)22-19-13-9-7-11-17(19)21/h6-13,15H,4-5,14H2,1-3H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 720 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Genova
Curated by ChEMBL
| Assay Description
Inhibition of Equine serum BChE using butyrylthiocoline iodide as a substrate after 20 mins by Ellman's assay |
Eur J Med Chem 46: 2170-84 (2011)
Article DOI: 10.1016/j.ejmech.2011.02.071
BindingDB Entry DOI: 10.7270/Q2BK1CPG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cholinesterase
(Equus caballus (Horse)) | BDBM50346412

(4-(((1R,9aR)-octahydro-1H-quinolizin-1-yl)methylth...)Show SMILES O=C(CCCSC[C@@H]1CCCN2CCCC[C@H]12)N1c2ccccc2Sc2ccccc12 |r| Show InChI InChI=1S/C26H32N2OS2/c29-26(15-8-18-30-19-20-9-7-17-27-16-6-5-10-21(20)27)28-22-11-1-3-13-24(22)31-25-14-4-2-12-23(25)28/h1-4,11-14,20-21H,5-10,15-19H2/t20-,21+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 740 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Genova
Curated by ChEMBL
| Assay Description
Inhibition of Equine serum BChE using butyrylthiocoline iodide as a substrate after 20 mins by Ellman's assay |
Eur J Med Chem 46: 2170-84 (2011)
Article DOI: 10.1016/j.ejmech.2011.02.071
BindingDB Entry DOI: 10.7270/Q2BK1CPG |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50346411

(3-(((1R,9aR)-octahydro-1H-quinolizin-1-yl)methylth...)Show SMILES O=C(CCSC[C@@H]1CCCN2CCCC[C@H]12)N1c2ccccc2Sc2ccccc12 |r| Show InChI InChI=1S/C25H30N2OS2/c28-25(14-17-29-18-19-8-7-16-26-15-6-5-9-20(19)26)27-21-10-1-3-12-23(21)30-24-13-4-2-11-22(24)27/h1-4,10-13,19-20H,5-9,14-18H2/t19-,20+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 750 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Genova
Curated by ChEMBL
| Assay Description
Inhibition of Equine serum BChE using butyrylthiocoline iodide as a substrate after 20 mins by Ellman's assay |
Eur J Med Chem 46: 2170-84 (2011)
Article DOI: 10.1016/j.ejmech.2011.02.071
BindingDB Entry DOI: 10.7270/Q2BK1CPG |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data