 Found 2433 hits with Last Name = 'tora' and Initial = 'g'
Found 2433 hits with Last Name = 'tora' and Initial = 'g' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50430060
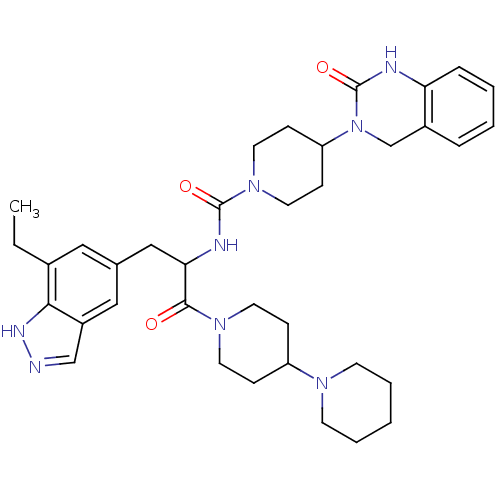
(CHEMBL2336421)Show SMILES CCc1cc(CC(NC(=O)N2CCC(CC2)N2Cc3ccccc3NC2=O)C(=O)N2CCC(CC2)N2CCCCC2)cc2cn[nH]c12 Show InChI InChI=1S/C36H48N8O3/c1-2-26-20-25(21-28-23-37-40-33(26)28)22-32(34(45)42-16-10-29(11-17-42)41-14-6-3-7-15-41)39-35(46)43-18-12-30(13-19-43)44-24-27-8-4-5-9-31(27)38-36(44)47/h4-5,8-9,20-21,23,29-30,32H,2-3,6-7,10-19,22,24H2,1H3,(H,37,40)(H,38,47)(H,39,46) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research& Development
Curated by ChEMBL
| Assay Description
Binding affinity to CGRP receptor (unknown origin) |
Bioorg Med Chem Lett 23: 1870-3 (2013)
Article DOI: 10.1016/j.bmcl.2013.01.011
BindingDB Entry DOI: 10.7270/Q2WD41XV |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50273292
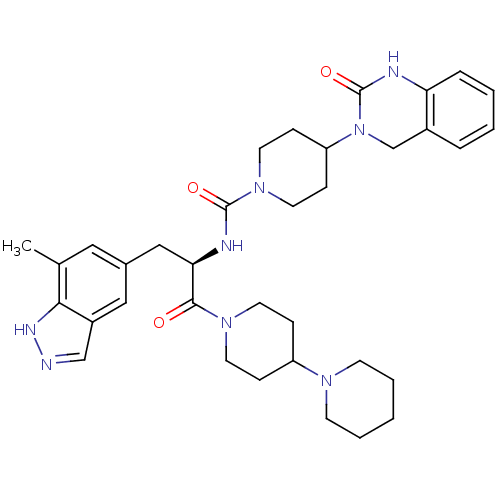
((R)-N-(1-(1,4'-bipiperidin-1'-yl)-3-(7-methyl-1H-i...)Show SMILES Cc1cc(C[C@@H](NC(=O)N2CCC(CC2)N2Cc3ccccc3NC2=O)C(=O)N2CCC(CC2)N2CCCCC2)cc2cn[nH]c12 |r| Show InChI InChI=1S/C35H46N8O3/c1-24-19-25(20-27-22-36-39-32(24)27)21-31(33(44)41-15-9-28(10-16-41)40-13-5-2-6-14-40)38-34(45)42-17-11-29(12-18-42)43-23-26-7-3-4-8-30(26)37-35(43)46/h3-4,7-8,19-20,22,28-29,31H,2,5-6,9-18,21,23H2,1H3,(H,36,39)(H,37,46)(H,38,45)/t31-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.00730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research& Development
Curated by ChEMBL
| Assay Description
Binding affinity to CGRP receptor (unknown origin) |
Bioorg Med Chem Lett 23: 1870-3 (2013)
Article DOI: 10.1016/j.bmcl.2013.01.011
BindingDB Entry DOI: 10.7270/Q2WD41XV |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50430061
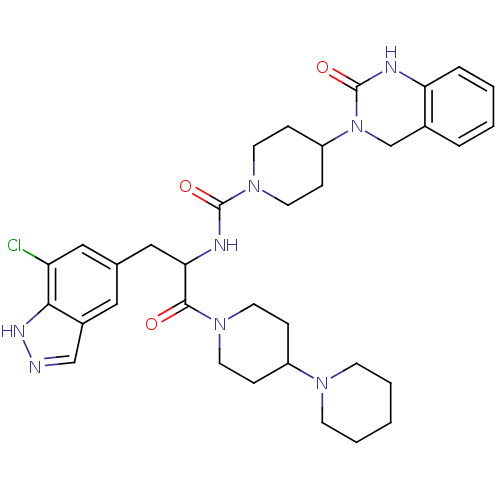
(CHEMBL2336422)Show SMILES Clc1cc(CC(NC(=O)N2CCC(CC2)N2Cc3ccccc3NC2=O)C(=O)N2CCC(CC2)N2CCCCC2)cc2cn[nH]c12 Show InChI InChI=1S/C34H43ClN8O3/c35-28-19-23(18-25-21-36-39-31(25)28)20-30(32(44)41-14-8-26(9-15-41)40-12-4-1-5-13-40)38-33(45)42-16-10-27(11-17-42)43-22-24-6-2-3-7-29(24)37-34(43)46/h2-3,6-7,18-19,21,26-27,30H,1,4-5,8-17,20,22H2,(H,36,39)(H,37,46)(H,38,45) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research& Development
Curated by ChEMBL
| Assay Description
Binding affinity to CGRP receptor (unknown origin) |
Bioorg Med Chem Lett 23: 1870-3 (2013)
Article DOI: 10.1016/j.bmcl.2013.01.011
BindingDB Entry DOI: 10.7270/Q2WD41XV |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50273292

((R)-N-(1-(1,4'-bipiperidin-1'-yl)-3-(7-methyl-1H-i...)Show SMILES Cc1cc(C[C@@H](NC(=O)N2CCC(CC2)N2Cc3ccccc3NC2=O)C(=O)N2CCC(CC2)N2CCCCC2)cc2cn[nH]c12 |r| Show InChI InChI=1S/C35H46N8O3/c1-24-19-25(20-27-22-36-39-32(24)27)21-31(33(44)41-15-9-28(10-16-41)40-13-5-2-6-14-40)38-34(45)42-17-11-29(12-18-42)43-23-26-7-3-4-8-30(26)37-35(43)46/h3-4,7-8,19-20,22,28-29,31H,2,5-6,9-18,21,23H2,1H3,(H,36,39)(H,37,46)(H,38,45)/t31-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research& Development
Curated by ChEMBL
| Assay Description
Binding affinity to CGRP receptor (unknown origin) |
Bioorg Med Chem Lett 23: 1870-3 (2013)
Article DOI: 10.1016/j.bmcl.2013.01.011
BindingDB Entry DOI: 10.7270/Q2WD41XV |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50268484
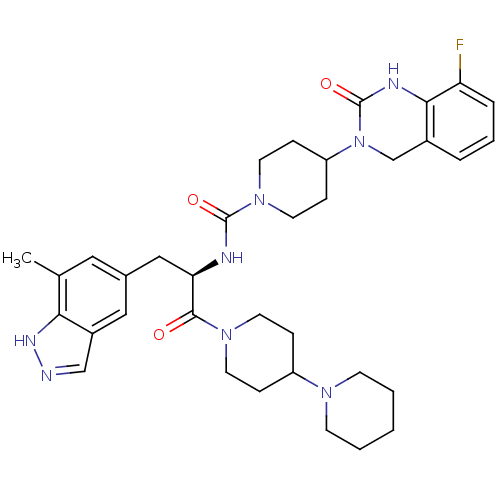
((R)-4-(8-Fluoro-2-oxo-1,2-dihydroquinazolin-3(4H)-...)Show SMILES Cc1cc(C[C@@H](NC(=O)N2CCC(CC2)N2Cc3cccc(F)c3NC2=O)C(=O)N2CCC(CC2)N2CCCCC2)cc2cn[nH]c12 |r| Show InChI InChI=1S/C35H45FN8O3/c1-23-18-24(19-26-21-37-40-31(23)26)20-30(33(45)42-14-8-27(9-15-42)41-12-3-2-4-13-41)38-34(46)43-16-10-28(11-17-43)44-22-25-6-5-7-29(36)32(25)39-35(44)47/h5-7,18-19,21,27-28,30H,2-4,8-17,20,22H2,1H3,(H,37,40)(H,38,46)(H,39,47)/t30-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research& Development
Curated by ChEMBL
| Assay Description
Binding affinity to CGRP receptor (unknown origin) |
Bioorg Med Chem Lett 23: 1870-3 (2013)
Article DOI: 10.1016/j.bmcl.2013.01.011
BindingDB Entry DOI: 10.7270/Q2WD41XV |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50430062
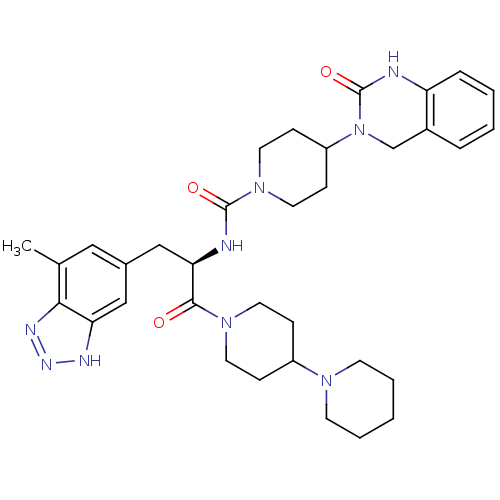
(CHEMBL2336411)Show SMILES Cc1cc(C[C@@H](NC(=O)N2CCC(CC2)N2Cc3ccccc3NC2=O)C(=O)N2CCC(CC2)N2CCCCC2)cc2[nH]nnc12 |r| Show InChI InChI=1S/C34H45N9O3/c1-23-19-24(20-29-31(23)38-39-37-29)21-30(32(44)41-15-9-26(10-16-41)40-13-5-2-6-14-40)36-33(45)42-17-11-27(12-18-42)43-22-25-7-3-4-8-28(25)35-34(43)46/h3-4,7-8,19-20,26-27,30H,2,5-6,9-18,21-22H2,1H3,(H,35,46)(H,36,45)(H,37,38,39)/t30-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research& Development
Curated by ChEMBL
| Assay Description
Binding affinity to CGRP receptor (unknown origin) |
Bioorg Med Chem Lett 23: 1870-3 (2013)
Article DOI: 10.1016/j.bmcl.2013.01.011
BindingDB Entry DOI: 10.7270/Q2WD41XV |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50430066
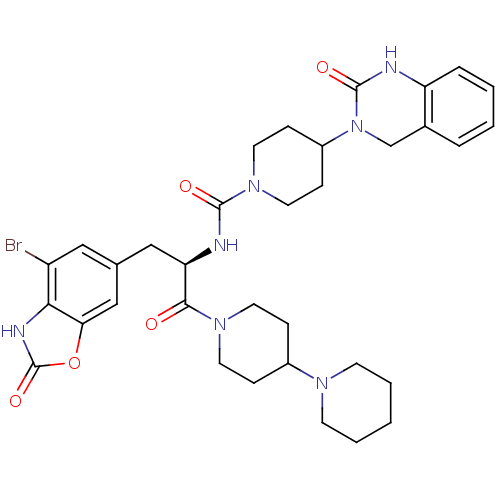
(CHEMBL2336416)Show SMILES Brc1cc(C[C@@H](NC(=O)N2CCC(CC2)N2Cc3ccccc3NC2=O)C(=O)N2CCC(CC2)N2CCCCC2)cc2oc(=O)[nH]c12 |r| Show InChI InChI=1S/C34H42BrN7O5/c35-26-18-22(20-29-30(26)38-34(46)47-29)19-28(31(43)40-14-8-24(9-15-40)39-12-4-1-5-13-39)37-32(44)41-16-10-25(11-17-41)42-21-23-6-2-3-7-27(23)36-33(42)45/h2-3,6-7,18,20,24-25,28H,1,4-5,8-17,19,21H2,(H,36,45)(H,37,44)(H,38,46)/t28-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research& Development
Curated by ChEMBL
| Assay Description
Binding affinity to CGRP receptor (unknown origin) |
Bioorg Med Chem Lett 23: 1870-3 (2013)
Article DOI: 10.1016/j.bmcl.2013.01.011
BindingDB Entry DOI: 10.7270/Q2WD41XV |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50430058
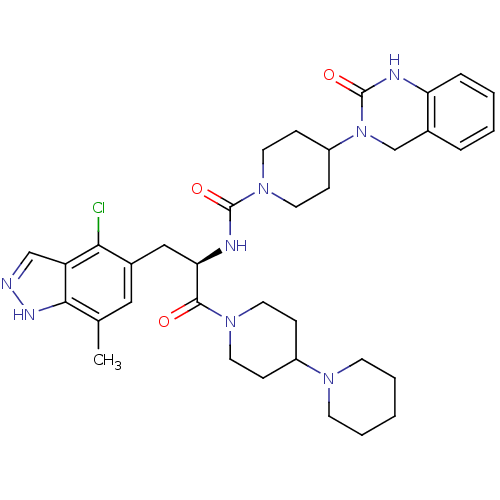
(CHEMBL2336418)Show SMILES Cc1cc(C[C@@H](NC(=O)N2CCC(CC2)N2Cc3ccccc3NC2=O)C(=O)N2CCC(CC2)N2CCCCC2)c(Cl)c2cn[nH]c12 |r| Show InChI InChI=1S/C35H45ClN8O3/c1-23-19-25(31(36)28-21-37-40-32(23)28)20-30(33(45)42-15-9-26(10-16-42)41-13-5-2-6-14-41)39-34(46)43-17-11-27(12-18-43)44-22-24-7-3-4-8-29(24)38-35(44)47/h3-4,7-8,19,21,26-27,30H,2,5-6,9-18,20,22H2,1H3,(H,37,40)(H,38,47)(H,39,46)/t30-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research& Development
Curated by ChEMBL
| Assay Description
Binding affinity to CGRP receptor (unknown origin) |
Bioorg Med Chem Lett 23: 1870-3 (2013)
Article DOI: 10.1016/j.bmcl.2013.01.011
BindingDB Entry DOI: 10.7270/Q2WD41XV |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50430057
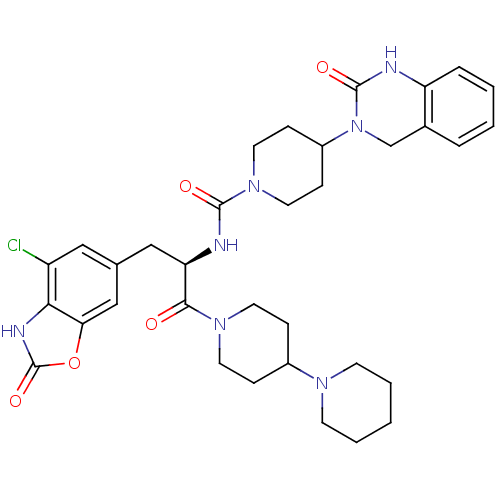
(CHEMBL2336417)Show SMILES Clc1cc(C[C@@H](NC(=O)N2CCC(CC2)N2Cc3ccccc3NC2=O)C(=O)N2CCC(CC2)N2CCCCC2)cc2oc(=O)[nH]c12 |r| Show InChI InChI=1S/C34H42ClN7O5/c35-26-18-22(20-29-30(26)38-34(46)47-29)19-28(31(43)40-14-8-24(9-15-40)39-12-4-1-5-13-39)37-32(44)41-16-10-25(11-17-41)42-21-23-6-2-3-7-27(23)36-33(42)45/h2-3,6-7,18,20,24-25,28H,1,4-5,8-17,19,21H2,(H,36,45)(H,37,44)(H,38,46)/t28-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research& Development
Curated by ChEMBL
| Assay Description
Binding affinity to CGRP receptor (unknown origin) |
Bioorg Med Chem Lett 23: 1870-3 (2013)
Article DOI: 10.1016/j.bmcl.2013.01.011
BindingDB Entry DOI: 10.7270/Q2WD41XV |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50430059

(CHEMBL2336419)Show SMILES CCc1cc(CC(NC(=O)N2CCC(CC2)N2Cc3ccccc3NC2=O)C(=O)N2CCC(CC2)N2CCCCC2)cc2c(C)n[nH]c12 Show InChI InChI=1S/C37H50N8O3/c1-3-27-21-26(22-31-25(2)40-41-34(27)31)23-33(35(46)43-17-11-29(12-18-43)42-15-7-4-8-16-42)39-36(47)44-19-13-30(14-20-44)45-24-28-9-5-6-10-32(28)38-37(45)48/h5-6,9-10,21-22,29-30,33H,3-4,7-8,11-20,23-24H2,1-2H3,(H,38,48)(H,39,47)(H,40,41) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research& Development
Curated by ChEMBL
| Assay Description
Binding affinity to CGRP receptor (unknown origin) |
Bioorg Med Chem Lett 23: 1870-3 (2013)
Article DOI: 10.1016/j.bmcl.2013.01.011
BindingDB Entry DOI: 10.7270/Q2WD41XV |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50430065
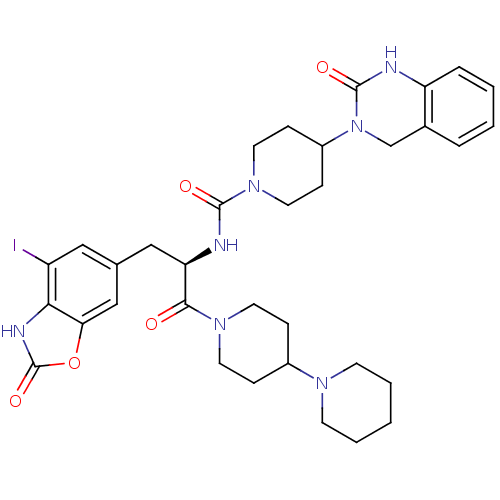
(CHEMBL2336415)Show SMILES Ic1cc(C[C@@H](NC(=O)N2CCC(CC2)N2Cc3ccccc3NC2=O)C(=O)N2CCC(CC2)N2CCCCC2)cc2oc(=O)[nH]c12 |r| Show InChI InChI=1S/C34H42IN7O5/c35-26-18-22(20-29-30(26)38-34(46)47-29)19-28(31(43)40-14-8-24(9-15-40)39-12-4-1-5-13-39)37-32(44)41-16-10-25(11-17-41)42-21-23-6-2-3-7-27(23)36-33(42)45/h2-3,6-7,18,20,24-25,28H,1,4-5,8-17,19,21H2,(H,36,45)(H,37,44)(H,38,46)/t28-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research& Development
Curated by ChEMBL
| Assay Description
Binding affinity to CGRP receptor (unknown origin) |
Bioorg Med Chem Lett 23: 1870-3 (2013)
Article DOI: 10.1016/j.bmcl.2013.01.011
BindingDB Entry DOI: 10.7270/Q2WD41XV |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50430063
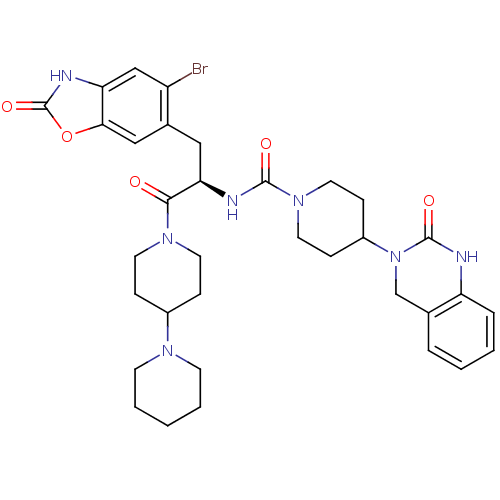
(CHEMBL2336412)Show SMILES Brc1cc2[nH]c(=O)oc2cc1C[C@@H](NC(=O)N1CCC(CC1)N1Cc2ccccc2NC1=O)C(=O)N1CCC(CC1)N1CCCCC1 |r| Show InChI InChI=1S/C34H42BrN7O5/c35-26-20-28-30(47-34(46)38-28)19-23(26)18-29(31(43)40-14-8-24(9-15-40)39-12-4-1-5-13-39)37-32(44)41-16-10-25(11-17-41)42-21-22-6-2-3-7-27(22)36-33(42)45/h2-3,6-7,19-20,24-25,29H,1,4-5,8-18,21H2,(H,36,45)(H,37,44)(H,38,46)/t29-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research& Development
Curated by ChEMBL
| Assay Description
Binding affinity to CGRP receptor (unknown origin) |
Bioorg Med Chem Lett 23: 1870-3 (2013)
Article DOI: 10.1016/j.bmcl.2013.01.011
BindingDB Entry DOI: 10.7270/Q2WD41XV |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50430067
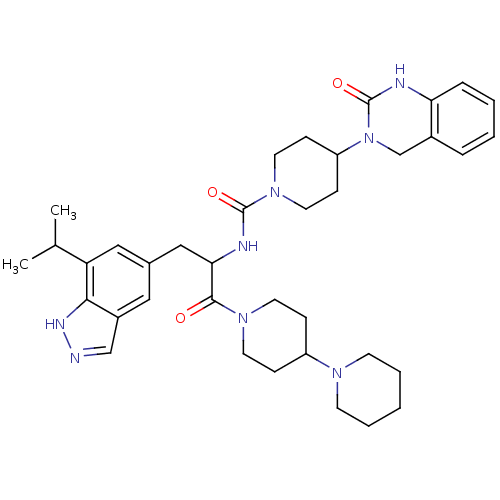
(CHEMBL2336420)Show SMILES CC(C)c1cc(CC(NC(=O)N2CCC(CC2)N2Cc3ccccc3NC2=O)C(=O)N2CCC(CC2)N2CCCCC2)cc2cn[nH]c12 Show InChI InChI=1S/C37H50N8O3/c1-25(2)31-21-26(20-28-23-38-41-34(28)31)22-33(35(46)43-16-10-29(11-17-43)42-14-6-3-7-15-42)40-36(47)44-18-12-30(13-19-44)45-24-27-8-4-5-9-32(27)39-37(45)48/h4-5,8-9,20-21,23,25,29-30,33H,3,6-7,10-19,22,24H2,1-2H3,(H,38,41)(H,39,48)(H,40,47) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research& Development
Curated by ChEMBL
| Assay Description
Binding affinity to CGRP receptor (unknown origin) |
Bioorg Med Chem Lett 23: 1870-3 (2013)
Article DOI: 10.1016/j.bmcl.2013.01.011
BindingDB Entry DOI: 10.7270/Q2WD41XV |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50273291
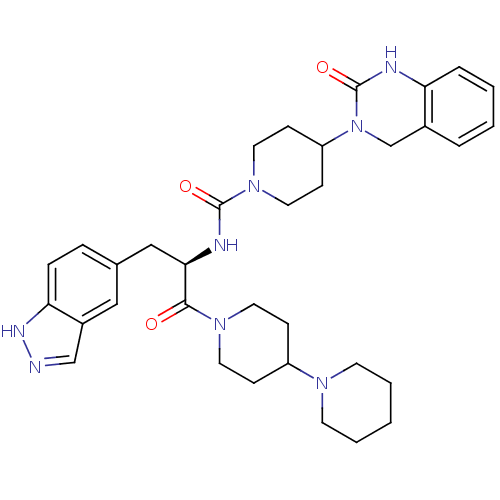
((R)-N-(1-(1,4'-bipiperidin-1'-yl)-3-(1H-indazol-5-...)Show SMILES O=C(N[C@H](Cc1ccc2[nH]ncc2c1)C(=O)N1CCC(CC1)N1CCCCC1)N1CCC(CC1)N1Cc2ccccc2NC1=O |r| Show InChI InChI=1S/C34H44N8O3/c43-32(40-16-10-27(11-17-40)39-14-4-1-5-15-39)31(21-24-8-9-30-26(20-24)22-35-38-30)37-33(44)41-18-12-28(13-19-41)42-23-25-6-2-3-7-29(25)36-34(42)45/h2-3,6-9,20,22,27-28,31H,1,4-5,10-19,21,23H2,(H,35,38)(H,36,45)(H,37,44)/t31-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research& Development
Curated by ChEMBL
| Assay Description
Binding affinity to CGRP receptor (unknown origin) |
Bioorg Med Chem Lett 23: 1870-3 (2013)
Article DOI: 10.1016/j.bmcl.2013.01.011
BindingDB Entry DOI: 10.7270/Q2WD41XV |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50430064
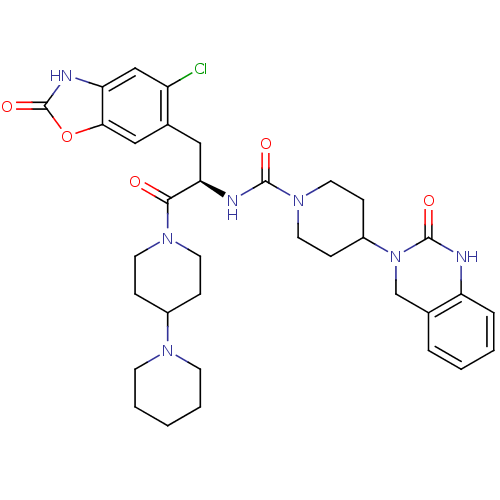
(CHEMBL2336413)Show SMILES Clc1cc2[nH]c(=O)oc2cc1C[C@@H](NC(=O)N1CCC(CC1)N1Cc2ccccc2NC1=O)C(=O)N1CCC(CC1)N1CCCCC1 |r| Show InChI InChI=1S/C34H42ClN7O5/c35-26-20-28-30(47-34(46)38-28)19-23(26)18-29(31(43)40-14-8-24(9-15-40)39-12-4-1-5-13-39)37-32(44)41-16-10-25(11-17-41)42-21-22-6-2-3-7-27(22)36-33(42)45/h2-3,6-7,19-20,24-25,29H,1,4-5,8-18,21H2,(H,36,45)(H,37,44)(H,38,46)/t29-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research& Development
Curated by ChEMBL
| Assay Description
Binding affinity to CGRP receptor (unknown origin) |
Bioorg Med Chem Lett 23: 1870-3 (2013)
Article DOI: 10.1016/j.bmcl.2013.01.011
BindingDB Entry DOI: 10.7270/Q2WD41XV |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50430068

(CHEMBL2336414)Show SMILES O=C(N[C@H](Cc1cc(C#N)c2[nH]c(=O)oc2c1)C(=O)N1CCC(CC1)N1CCCCC1)N1CCC(CC1)N1Cc2ccccc2NC1=O |r| Show InChI InChI=1S/C35H42N8O5/c36-21-25-18-23(20-30-31(25)39-35(47)48-30)19-29(32(44)41-14-8-26(9-15-41)40-12-4-1-5-13-40)38-33(45)42-16-10-27(11-17-42)43-22-24-6-2-3-7-28(24)37-34(43)46/h2-3,6-7,18,20,26-27,29H,1,4-5,8-17,19,22H2,(H,37,46)(H,38,45)(H,39,47)/t29-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research& Development
Curated by ChEMBL
| Assay Description
Binding affinity to CGRP receptor (unknown origin) |
Bioorg Med Chem Lett 23: 1870-3 (2013)
Article DOI: 10.1016/j.bmcl.2013.01.011
BindingDB Entry DOI: 10.7270/Q2WD41XV |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50430069
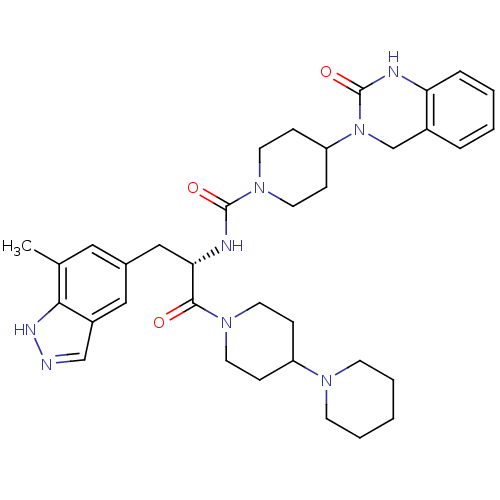
(CHEMBL2336423)Show SMILES Cc1cc(C[C@H](NC(=O)N2CCC(CC2)N2Cc3ccccc3NC2=O)C(=O)N2CCC(CC2)N2CCCCC2)cc2cn[nH]c12 |r| Show InChI InChI=1S/C35H46N8O3/c1-24-19-25(20-27-22-36-39-32(24)27)21-31(33(44)41-15-9-28(10-16-41)40-13-5-2-6-14-40)38-34(45)42-17-11-29(12-18-42)43-23-26-7-3-4-8-30(26)37-35(43)46/h3-4,7-8,19-20,22,28-29,31H,2,5-6,9-18,21,23H2,1H3,(H,36,39)(H,37,46)(H,38,45)/t31-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research& Development
Curated by ChEMBL
| Assay Description
Binding affinity to CGRP receptor (unknown origin) |
Bioorg Med Chem Lett 23: 1870-3 (2013)
Article DOI: 10.1016/j.bmcl.2013.01.011
BindingDB Entry DOI: 10.7270/Q2WD41XV |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50187039

(1-(3,4-dichlorobenzyl)-5-hydroxy-1H-indole-2-carbo...)Show InChI InChI=1S/C16H11Cl2NO3/c17-12-3-1-9(5-13(12)18)8-19-14-4-2-11(20)6-10(14)7-15(19)16(21)22/h1-7,20H,8H2,(H,21,22) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]MCP1 from human CCR2 expressed in CHO cells |
Bioorg Med Chem Lett 16: 3735-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.045
BindingDB Entry DOI: 10.7270/Q2N01645 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50091438

((E)-3-(3,4-Dichloro-phenyl)-N-{5-[4-(5-hydroxy-1H-...)Show SMILES Oc1ccc2[nH]cc(C3CCN(CCCCCNC(=O)\C=C\c4ccc(Cl)c(Cl)c4)CC3)c2c1 Show InChI InChI=1S/C27H31Cl2N3O2/c28-24-7-4-19(16-25(24)29)5-9-27(34)30-12-2-1-3-13-32-14-10-20(11-15-32)23-18-31-26-8-6-21(33)17-22(23)26/h4-9,16-18,20,31,33H,1-3,10-15H2,(H,30,34)/b9-5+ | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]MCP1 from human CCR2 expressed in CHO cells |
Bioorg Med Chem Lett 16: 3735-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.045
BindingDB Entry DOI: 10.7270/Q2N01645 |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50268484

((R)-4-(8-Fluoro-2-oxo-1,2-dihydroquinazolin-3(4H)-...)Show SMILES Cc1cc(C[C@@H](NC(=O)N2CCC(CC2)N2Cc3cccc(F)c3NC2=O)C(=O)N2CCC(CC2)N2CCCCC2)cc2cn[nH]c12 |r| Show InChI InChI=1S/C35H45FN8O3/c1-23-18-24(19-26-21-37-40-31(23)26)20-30(33(45)42-14-8-27(9-15-42)41-12-3-2-4-13-41)38-34(46)43-16-10-28(11-17-43)44-22-25-6-5-7-29(36)32(25)39-35(44)47/h5-7,18-19,21,27-28,30H,2-4,8-17,20,22H2,1H3,(H,37,40)(H,38,46)(H,39,47)/t30-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0260 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Antagonist activity at human CGRP receptor expressed in human SK-N-MC cells assessed as inhibition of CGRP-stimulated cAMP production |
Bioorg Med Chem Lett 23: 5684-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.08.031
BindingDB Entry DOI: 10.7270/Q2BZ67H8 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Rattus norvegicus (rat)) | BDBM50108639
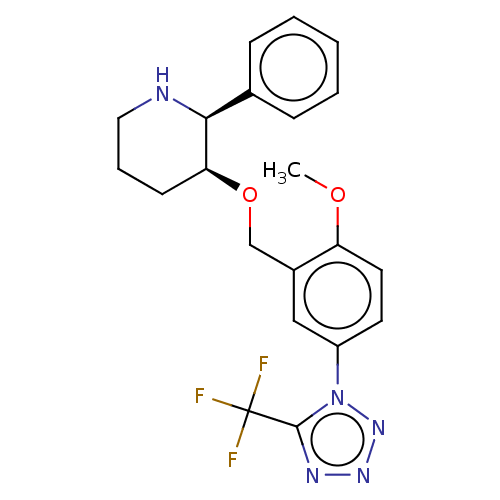
(CHEMBL3596448)Show SMILES COc1ccc(cc1CO[C@H]1CCCN[C@H]1c1ccccc1)-n1nnnc1C(F)(F)F |r| Show InChI InChI=1S/C21H22F3N5O2/c1-30-17-10-9-16(29-20(21(22,23)24)26-27-28-29)12-15(17)13-31-18-8-5-11-25-19(18)14-6-3-2-4-7-14/h2-4,6-7,9-10,12,18-19,25H,5,8,11,13H2,1H3/t18-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-substance P from gerbil NK1 receptor expressed in HEK293 cell membranes incubated for 30 mins by liquid scintillation counting... |
Bioorg Med Chem Lett 25: 3039-43 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.098
BindingDB Entry DOI: 10.7270/Q24M9690 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Rattus norvegicus (rat)) | BDBM50108631
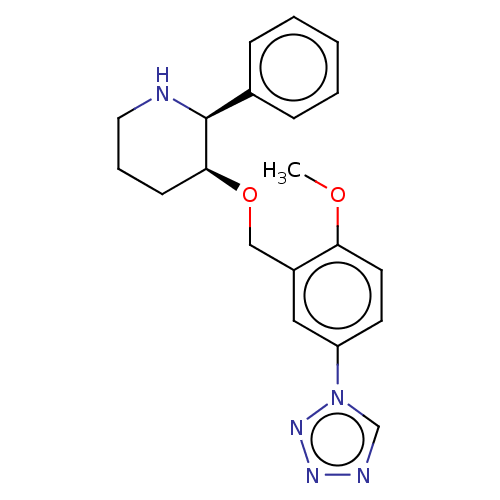
(CHEMBL3596446)Show SMILES COc1ccc(cc1CO[C@H]1CCCN[C@H]1c1ccccc1)-n1cnnn1 |r| Show InChI InChI=1S/C20H23N5O2/c1-26-18-10-9-17(25-14-22-23-24-25)12-16(18)13-27-19-8-5-11-21-20(19)15-6-3-2-4-7-15/h2-4,6-7,9-10,12,14,19-21H,5,8,11,13H2,1H3/t19-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-substance P from gerbil NK1 receptor expressed in HEK293 cell membranes incubated for 30 mins by liquid scintillation counting... |
Bioorg Med Chem Lett 25: 3039-43 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.098
BindingDB Entry DOI: 10.7270/Q24M9690 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Rattus norvegicus (rat)) | BDBM50108638
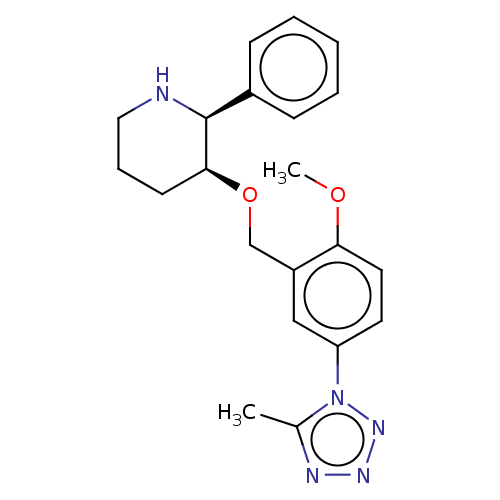
(CHEMBL3596447)Show SMILES COc1ccc(cc1CO[C@H]1CCCN[C@H]1c1ccccc1)-n1nnnc1C |r| Show InChI InChI=1S/C21H25N5O2/c1-15-23-24-25-26(15)18-10-11-19(27-2)17(13-18)14-28-20-9-6-12-22-21(20)16-7-4-3-5-8-16/h3-5,7-8,10-11,13,20-22H,6,9,12,14H2,1-2H3/t20-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-substance P from gerbil NK1 receptor expressed in HEK293 cell membranes incubated for 30 mins by liquid scintillation counting... |
Bioorg Med Chem Lett 25: 3039-43 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.098
BindingDB Entry DOI: 10.7270/Q24M9690 |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50441045
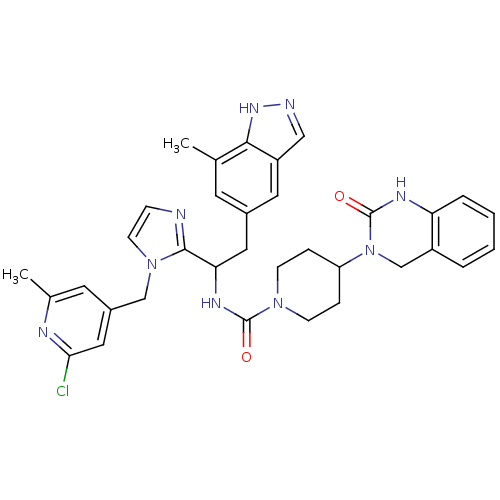
(CHEMBL2430167)Show SMILES Cc1cc(Cn2ccnc2C(Cc2cc(C)c3[nH]ncc3c2)NC(=O)N2CCC(CC2)N2Cc3ccccc3NC2=O)cc(Cl)n1 Show InChI InChI=1S/C34H36ClN9O2/c1-21-13-23(15-26-18-37-41-31(21)26)16-29(32-36-9-12-43(32)19-24-14-22(2)38-30(35)17-24)40-33(45)42-10-7-27(8-11-42)44-20-25-5-3-4-6-28(25)39-34(44)46/h3-6,9,12-15,17-18,27,29H,7-8,10-11,16,19-20H2,1-2H3,(H,37,41)(H,39,46)(H,40,45) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0780 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Antagonist activity at human CGRP receptor expressed in human SK-N-MC cells assessed as inhibition of CGRP-stimulated cAMP production |
Bioorg Med Chem Lett 23: 5684-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.08.031
BindingDB Entry DOI: 10.7270/Q2BZ67H8 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Rattus norvegicus (rat)) | BDBM50220136
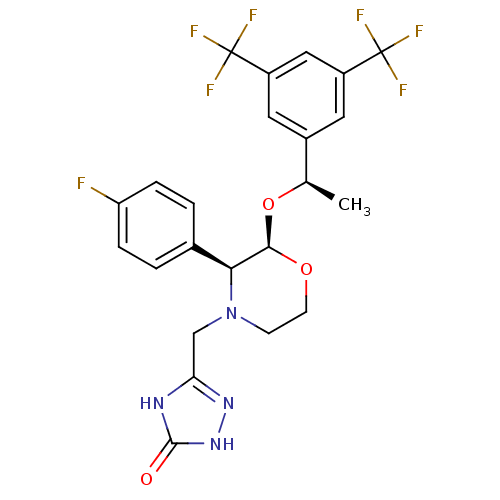
(3-[2-{1-[3,5-di(trifluoromethyl)phenyl]ethoxy}-3-(...)Show SMILES C[C@@H](O[C@H]1OCCN(Cc2n[nH]c(=O)[nH]2)[C@H]1c1ccc(F)cc1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C23H21F7N4O3/c1-12(14-8-15(22(25,26)27)10-16(9-14)23(28,29)30)37-20-19(13-2-4-17(24)5-3-13)34(6-7-36-20)11-18-31-21(35)33-32-18/h2-5,8-10,12,19-20H,6-7,11H2,1H3,(H2,31,32,33,35)/t12-,19+,20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-substance P from gerbil NK1 receptor expressed in HEK293 cell membranes incubated for 30 mins by liquid scintillation counting... |
Bioorg Med Chem Lett 25: 3039-43 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.098
BindingDB Entry DOI: 10.7270/Q24M9690 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50257994
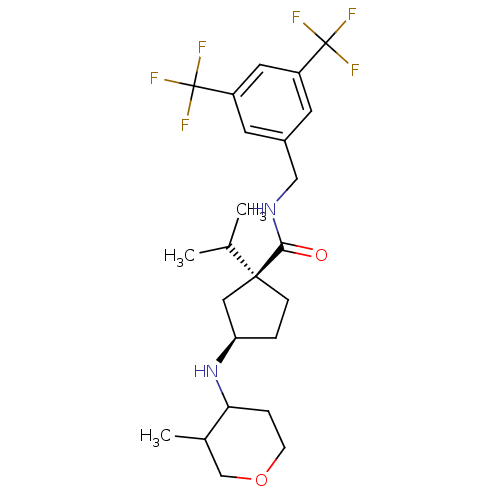
((1S,3R)-N-(3,5-bis(-trifluoromethyl)benzyl)-1-iso ...)Show SMILES CC(C)[C@@]1(CC[C@H](C1)NC1CCOCC1C)C(=O)NCc1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C24H32F6N2O2/c1-14(2)22(6-4-19(11-22)32-20-5-7-34-13-15(20)3)21(33)31-12-16-8-17(23(25,26)27)10-18(9-16)24(28,29)30/h8-10,14-15,19-20,32H,4-7,11-13H2,1-3H3,(H,31,33)/t15?,19-,20?,22+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR2 receptor expressed in CHO cells by chemotaxis assay |
Bioorg Med Chem Lett 19: 1830-4 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.050
BindingDB Entry DOI: 10.7270/Q2GH9HVZ |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50257994

((1S,3R)-N-(3,5-bis(-trifluoromethyl)benzyl)-1-iso ...)Show SMILES CC(C)[C@@]1(CC[C@H](C1)NC1CCOCC1C)C(=O)NCc1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C24H32F6N2O2/c1-14(2)22(6-4-19(11-22)32-20-5-7-34-13-15(20)3)21(33)31-12-16-8-17(23(25,26)27)10-18(9-16)24(28,29)30/h8-10,14-15,19-20,32H,4-7,11-13H2,1-3H3,(H,31,33)/t15?,19-,20?,22+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR2 receptor expressed in CHO cells by chemotaxis assay |
Bioorg Med Chem Lett 19: 1830-4 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.050
BindingDB Entry DOI: 10.7270/Q2GH9HVZ |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50257994

((1S,3R)-N-(3,5-bis(-trifluoromethyl)benzyl)-1-iso ...)Show SMILES CC(C)[C@@]1(CC[C@H](C1)NC1CCOCC1C)C(=O)NCc1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C24H32F6N2O2/c1-14(2)22(6-4-19(11-22)32-20-5-7-34-13-15(20)3)21(33)31-12-16-8-17(23(25,26)27)10-18(9-16)24(28,29)30/h8-10,14-15,19-20,32H,4-7,11-13H2,1-3H3,(H,31,33)/t15?,19-,20?,22+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR2 receptor expressed in CHO cells by chemotaxis assay |
Bioorg Med Chem Lett 19: 1830-4 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.050
BindingDB Entry DOI: 10.7270/Q2GH9HVZ |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50441046

(CHEMBL2430169)Show SMILES Cc1cc(Cn2ccnc2C(Cc2cc(C)c3[nH]ncc3c2)NC(=O)N2CCC(CC2)N2Cc3ccccc3NC2=O)ccn1 Show InChI InChI=1S/C34H37N9O2/c1-22-15-25(17-27-19-37-40-31(22)27)18-30(32-36-11-14-42(32)20-24-7-10-35-23(2)16-24)39-33(44)41-12-8-28(9-13-41)43-21-26-5-3-4-6-29(26)38-34(43)45/h3-7,10-11,14-17,19,28,30H,8-9,12-13,18,20-21H2,1-2H3,(H,37,40)(H,38,45)(H,39,44) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Antagonist activity at human CGRP receptor expressed in human SK-N-MC cells assessed as inhibition of CGRP-stimulated cAMP production |
Bioorg Med Chem Lett 23: 5684-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.08.031
BindingDB Entry DOI: 10.7270/Q2BZ67H8 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50212136
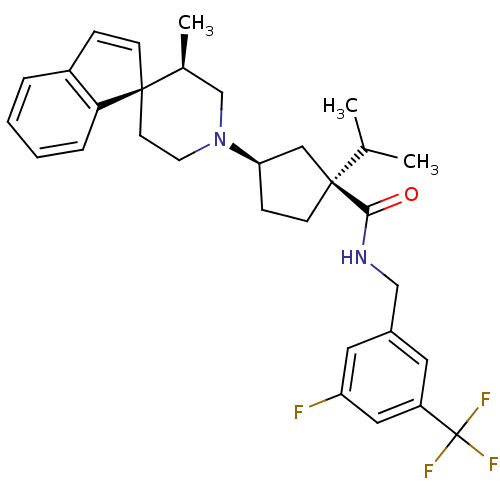
((1S,3R)-N-{[3-fluoro-5-(trifluoromethyl)phenyl]met...)Show SMILES CC(C)[C@@]1(CC[C@H](C1)N1CC[C@]2(C=Cc3ccccc23)[C@@H](C)C1)C(=O)NCc1cc(F)cc(c1)C(F)(F)F |c:13| Show InChI InChI=1S/C31H36F4N2O/c1-20(2)30(28(38)36-18-22-14-24(31(33,34)35)16-25(32)15-22)11-9-26(17-30)37-13-12-29(21(3)19-37)10-8-23-6-4-5-7-27(23)29/h4-8,10,14-16,20-21,26H,9,11-13,17-19H2,1-3H3,(H,36,38)/t21-,26+,29+,30-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of chemotaxis in human MCP1 monocytes |
Bioorg Med Chem Lett 17: 3636-41 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.053
BindingDB Entry DOI: 10.7270/Q23J3CN8 |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50441032

(CHEMBL2430177)Show SMILES Cc1cc(CC(NC(=O)N2CCC(CC2)N2Cc3ccccc3NC2=O)c2nccn2Cc2ccc(cc2)C(C)(C)C)cc2cn[nH]c12 Show InChI InChI=1S/C38H44N8O2/c1-25-19-27(20-29-22-40-43-34(25)29)21-33(35-39-15-18-45(35)23-26-9-11-30(12-10-26)38(2,3)4)42-36(47)44-16-13-31(14-17-44)46-24-28-7-5-6-8-32(28)41-37(46)48/h5-12,15,18-20,22,31,33H,13-14,16-17,21,23-24H2,1-4H3,(H,40,43)(H,41,48)(H,42,47) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Antagonist activity at human CGRP receptor expressed in human SK-N-MC cells assessed as inhibition of CGRP-stimulated cAMP production |
Bioorg Med Chem Lett 23: 5684-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.08.031
BindingDB Entry DOI: 10.7270/Q2BZ67H8 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50106365
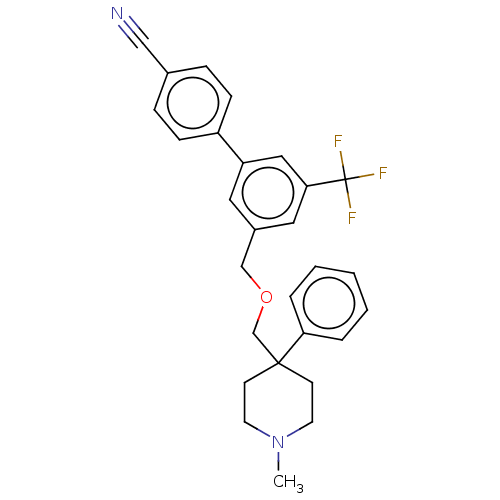
(CHEMBL3596492)Show SMILES CN1CCC(COCc2cc(cc(c2)C(F)(F)F)-c2ccc(cc2)C#N)(CC1)c1ccccc1 Show InChI InChI=1S/C28H27F3N2O/c1-33-13-11-27(12-14-33,25-5-3-2-4-6-25)20-34-19-22-15-24(17-26(16-22)28(29,30)31)23-9-7-21(18-32)8-10-23/h2-10,15-17H,11-14,19-20H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Binding affinity to human NK1 receptor |
Bioorg Med Chem Lett 25: 3039-43 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.098
BindingDB Entry DOI: 10.7270/Q24M9690 |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50441044

(CHEMBL2430166 | US9695176, 4)Show SMILES Cc1cc(CC(OC(=O)N2CCC(CC2)c2cc3ccccc3[nH]c2=O)c2ncc3CN(CCn23)C2CCCCC2)cc2cn[nH]c12 Show InChI InChI=1S/C37H43N7O3/c1-24-17-25(18-28-21-39-41-34(24)28)19-33(35-38-22-30-23-43(15-16-44(30)35)29-8-3-2-4-9-29)47-37(46)42-13-11-26(12-14-42)31-20-27-7-5-6-10-32(27)40-36(31)45/h5-7,10,17-18,20-22,26,29,33H,2-4,8-9,11-16,19,23H2,1H3,(H,39,41)(H,40,45) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Antagonist activity at human CGRP receptor expressed in human SK-N-MC cells assessed as inhibition of CGRP-stimulated cAMP production |
Bioorg Med Chem Lett 23: 5684-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.08.031
BindingDB Entry DOI: 10.7270/Q2BZ67H8 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50212127
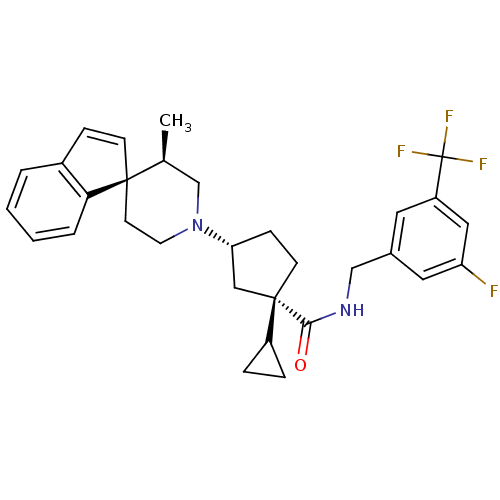
((1S,3R)-1-cyclopropyl-N-{[3-fluoro-5-(trifluoromet...)Show SMILES C[C@H]1CN(CC[C@]11C=Cc2ccccc12)[C@@H]1CC[C@](C1)(C1CC1)C(=O)NCc1cc(F)cc(c1)C(F)(F)F |c:8| Show InChI InChI=1S/C31H34F4N2O/c1-20-19-37(13-12-29(20)10-8-22-4-2-3-5-27(22)29)26-9-11-30(17-26,23-6-7-23)28(38)36-18-21-14-24(31(33,34)35)16-25(32)15-21/h2-5,8,10,14-16,20,23,26H,6-7,9,11-13,17-19H2,1H3,(H,36,38)/t20-,26+,29+,30-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of chemotaxis in human MCP1 monocytes |
Bioorg Med Chem Lett 17: 3636-41 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.053
BindingDB Entry DOI: 10.7270/Q23J3CN8 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Rattus norvegicus (rat)) | BDBM50106366
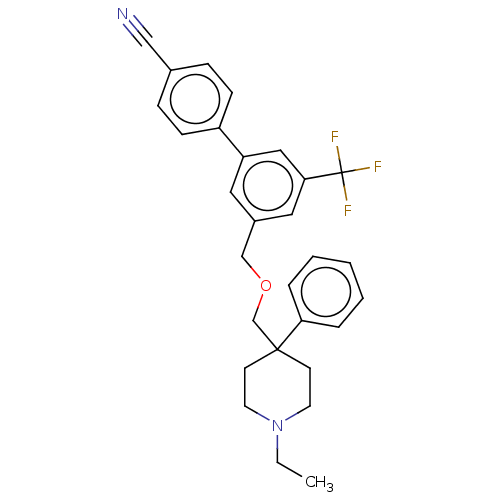
(CHEMBL3596493)Show SMILES CCN1CCC(COCc2cc(cc(c2)C(F)(F)F)-c2ccc(cc2)C#N)(CC1)c1ccccc1 Show InChI InChI=1S/C29H29F3N2O/c1-2-34-14-12-28(13-15-34,26-6-4-3-5-7-26)21-35-20-23-16-25(18-27(17-23)29(30,31)32)24-10-8-22(19-33)9-11-24/h3-11,16-18H,2,12-15,20-21H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-substance P from gerbil NK1 receptor expressed in HEK293 cell membranes incubated for 30 mins by liquid scintillation counting... |
Bioorg Med Chem Lett 25: 3039-43 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.098
BindingDB Entry DOI: 10.7270/Q24M9690 |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50441043

(CHEMBL2430165 | US9695176, 1)Show SMILES Cc1cc(CC(OC(=O)N2CCC(CC2)c2cc3ccccc3[nH]c2=O)c2ncc3CN(Cc4ccccc4)CCn23)cc2cn[nH]c12 Show InChI InChI=1S/C38H39N7O3/c1-25-17-27(18-30-21-40-42-35(25)30)19-34(36-39-22-31-24-43(15-16-45(31)36)23-26-7-3-2-4-8-26)48-38(47)44-13-11-28(12-14-44)32-20-29-9-5-6-10-33(29)41-37(32)46/h2-10,17-18,20-22,28,34H,11-16,19,23-24H2,1H3,(H,40,42)(H,41,46) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Antagonist activity at human CGRP receptor expressed in human SK-N-MC cells assessed as inhibition of CGRP-stimulated cAMP production |
Bioorg Med Chem Lett 23: 5684-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.08.031
BindingDB Entry DOI: 10.7270/Q2BZ67H8 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Rattus norvegicus (rat)) | BDBM50106365

(CHEMBL3596492)Show SMILES CN1CCC(COCc2cc(cc(c2)C(F)(F)F)-c2ccc(cc2)C#N)(CC1)c1ccccc1 Show InChI InChI=1S/C28H27F3N2O/c1-33-13-11-27(12-14-33,25-5-3-2-4-6-25)20-34-19-22-15-24(17-26(16-22)28(29,30)31)23-9-7-21(18-32)8-10-23/h2-10,15-17H,11-14,19-20H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-substance P from gerbil NK1 receptor expressed in HEK293 cell membranes incubated for 30 mins by liquid scintillation counting... |
Bioorg Med Chem Lett 25: 3039-43 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.098
BindingDB Entry DOI: 10.7270/Q24M9690 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50257995
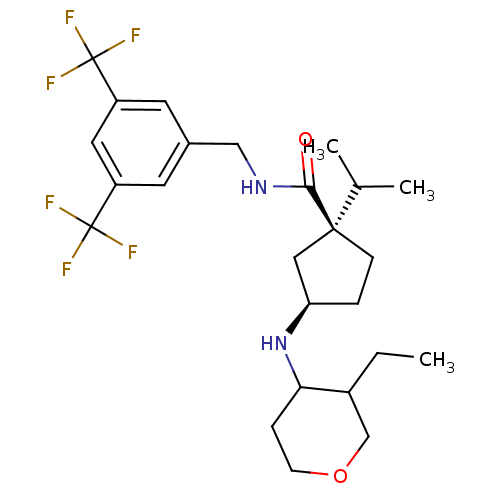
((1S,3R)-N-(3,5-bis(trifluoromethyl)benzyl)-3-(3-et...)Show SMILES CCC1COCCC1N[C@@H]1CC[C@](C1)(C(C)C)C(=O)NCc1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C25H34F6N2O2/c1-4-17-14-35-8-6-21(17)33-20-5-7-23(12-20,15(2)3)22(34)32-13-16-9-18(24(26,27)28)11-19(10-16)25(29,30)31/h9-11,15,17,20-21,33H,4-8,12-14H2,1-3H3,(H,32,34)/t17?,20-,21?,23+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR2 receptor expressed in CHO cells by chemotaxis assay |
Bioorg Med Chem Lett 19: 1830-4 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.050
BindingDB Entry DOI: 10.7270/Q2GH9HVZ |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Rattus norvegicus (rat)) | BDBM50106358
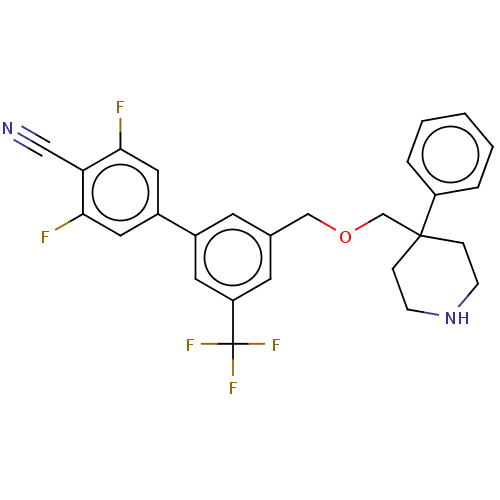
(CHEMBL3596485)Show SMILES Fc1cc(cc(F)c1C#N)-c1cc(COCC2(CCNCC2)c2ccccc2)cc(c1)C(F)(F)F Show InChI InChI=1S/C27H23F5N2O/c28-24-13-20(14-25(29)23(24)15-33)19-10-18(11-22(12-19)27(30,31)32)16-35-17-26(6-8-34-9-7-26)21-4-2-1-3-5-21/h1-5,10-14,34H,6-9,16-17H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-substance P from gerbil NK1 receptor expressed in HEK293 cell membranes incubated for 30 mins by liquid scintillation counting... |
Bioorg Med Chem Lett 25: 3039-43 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.098
BindingDB Entry DOI: 10.7270/Q24M9690 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50212128
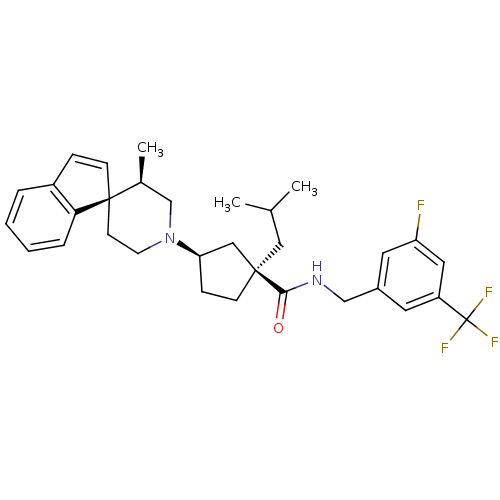
((1R,3R)-N-{[3-fluoro-5-(trifluoromethyl)phenyl]met...)Show SMILES CC(C)C[C@@]1(CC[C@H](C1)N1CC[C@]2(C=Cc3ccccc23)[C@@H](C)C1)C(=O)NCc1cc(F)cc(c1)C(F)(F)F |c:14| Show InChI InChI=1S/C32H38F4N2O/c1-21(2)17-30(29(39)37-19-23-14-25(32(34,35)36)16-26(33)15-23)10-9-27(18-30)38-13-12-31(22(3)20-38)11-8-24-6-4-5-7-28(24)31/h4-8,11,14-16,21-22,27H,9-10,12-13,17-20H2,1-3H3,(H,37,39)/t22-,27+,30+,31+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of chemotaxis in human MCP1 monocytes |
Bioorg Med Chem Lett 17: 3636-41 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.053
BindingDB Entry DOI: 10.7270/Q23J3CN8 |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50441052

(CHEMBL2430174)Show SMILES Cc1cnc(C(Cc2cc(C)c3[nH]ncc3c2)NC(=O)N2CCC(CC2)N2Cc3ccccc3NC2=O)n1Cc1cccc(F)c1 Show InChI InChI=1S/C35H37FN8O2/c1-22-14-25(15-27-19-38-41-32(22)27)17-31(33-37-18-23(2)43(33)20-24-6-5-8-28(36)16-24)40-34(45)42-12-10-29(11-13-42)44-21-26-7-3-4-9-30(26)39-35(44)46/h3-9,14-16,18-19,29,31H,10-13,17,20-21H2,1-2H3,(H,38,41)(H,39,46)(H,40,45) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Antagonist activity at human CGRP receptor expressed in human SK-N-MC cells assessed as inhibition of CGRP-stimulated cAMP production |
Bioorg Med Chem Lett 23: 5684-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.08.031
BindingDB Entry DOI: 10.7270/Q2BZ67H8 |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50441054
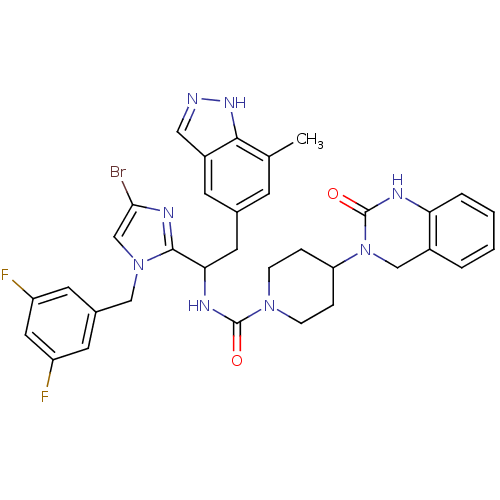
(CHEMBL2430176)Show SMILES Cc1cc(CC(NC(=O)N2CCC(CC2)N2Cc3ccccc3NC2=O)c2nc(Br)cn2Cc2cc(F)cc(F)c2)cc2cn[nH]c12 Show InChI InChI=1S/C34H33BrF2N8O2/c1-20-10-21(11-24-16-38-42-31(20)24)14-29(32-41-30(35)19-44(32)17-22-12-25(36)15-26(37)13-22)40-33(46)43-8-6-27(7-9-43)45-18-23-4-2-3-5-28(23)39-34(45)47/h2-5,10-13,15-16,19,27,29H,6-9,14,17-18H2,1H3,(H,38,42)(H,39,47)(H,40,46) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Antagonist activity at human CGRP receptor expressed in human SK-N-MC cells assessed as inhibition of CGRP-stimulated cAMP production |
Bioorg Med Chem Lett 23: 5684-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.08.031
BindingDB Entry DOI: 10.7270/Q2BZ67H8 |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50441033

(CHEMBL2430178)Show SMILES Cc1cc(CC(NC(=O)N2CCC(CC2)N2Cc3ccccc3NC2=O)c2nccn2Cc2cc(F)cc(F)c2)cc2cn[nH]c12 Show InChI InChI=1S/C34H34F2N8O2/c1-21-12-22(13-25-18-38-41-31(21)25)16-30(32-37-8-11-43(32)19-23-14-26(35)17-27(36)15-23)40-33(45)42-9-6-28(7-10-42)44-20-24-4-2-3-5-29(24)39-34(44)46/h2-5,8,11-15,17-18,28,30H,6-7,9-10,16,19-20H2,1H3,(H,38,41)(H,39,46)(H,40,45) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Antagonist activity at human CGRP receptor expressed in human SK-N-MC cells assessed as inhibition of CGRP-stimulated cAMP production |
Bioorg Med Chem Lett 23: 5684-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.08.031
BindingDB Entry DOI: 10.7270/Q2BZ67H8 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Rattus norvegicus (rat)) | BDBM50106367
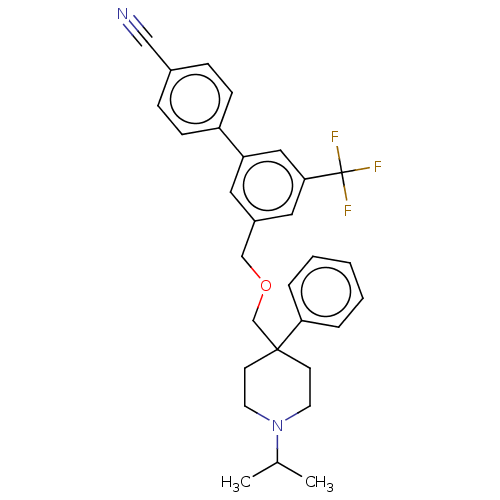
(CHEMBL3596494)Show SMILES CC(C)N1CCC(COCc2cc(cc(c2)C(F)(F)F)-c2ccc(cc2)C#N)(CC1)c1ccccc1 Show InChI InChI=1S/C30H31F3N2O/c1-22(2)35-14-12-29(13-15-35,27-6-4-3-5-7-27)21-36-20-24-16-26(18-28(17-24)30(31,32)33)25-10-8-23(19-34)9-11-25/h3-11,16-18,22H,12-15,20-21H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-substance P from gerbil NK1 receptor expressed in HEK293 cell membranes incubated for 30 mins by liquid scintillation counting... |
Bioorg Med Chem Lett 25: 3039-43 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.098
BindingDB Entry DOI: 10.7270/Q24M9690 |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50441048
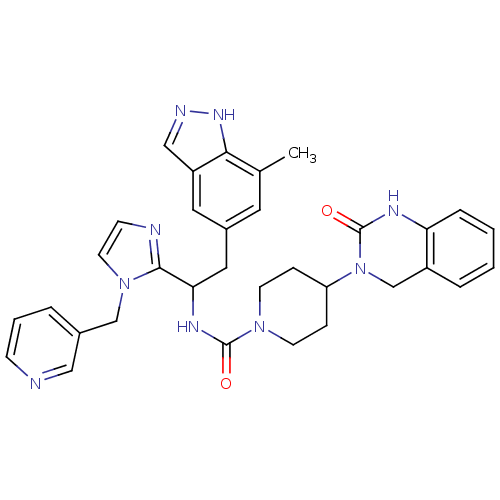
(CHEMBL2430170)Show SMILES Cc1cc(CC(NC(=O)N2CCC(CC2)N2Cc3ccccc3NC2=O)c2nccn2Cc2cccnc2)cc2cn[nH]c12 Show InChI InChI=1S/C33H35N9O2/c1-22-15-24(16-26-19-36-39-30(22)26)17-29(31-35-11-14-41(31)20-23-5-4-10-34-18-23)38-32(43)40-12-8-27(9-13-40)42-21-25-6-2-3-7-28(25)37-33(42)44/h2-7,10-11,14-16,18-19,27,29H,8-9,12-13,17,20-21H2,1H3,(H,36,39)(H,37,44)(H,38,43) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Antagonist activity at human CGRP receptor expressed in human SK-N-MC cells assessed as inhibition of CGRP-stimulated cAMP production |
Bioorg Med Chem Lett 23: 5684-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.08.031
BindingDB Entry DOI: 10.7270/Q2BZ67H8 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Rattus norvegicus (rat)) | BDBM50106356
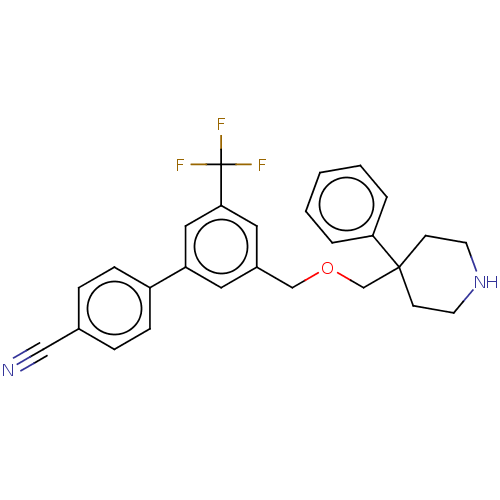
(CHEMBL3596483)Show SMILES FC(F)(F)c1cc(COCC2(CCNCC2)c2ccccc2)cc(c1)-c1ccc(cc1)C#N Show InChI InChI=1S/C27H25F3N2O/c28-27(29,30)25-15-21(14-23(16-25)22-8-6-20(17-31)7-9-22)18-33-19-26(10-12-32-13-11-26)24-4-2-1-3-5-24/h1-9,14-16,32H,10-13,18-19H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-substance P from gerbil NK1 receptor expressed in HEK293 cell membranes incubated for 30 mins by liquid scintillation counting... |
Bioorg Med Chem Lett 25: 3039-43 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.098
BindingDB Entry DOI: 10.7270/Q24M9690 |
More data for this
Ligand-Target Pair | |
Endothelial lipase
(Homo sapiens (Human)) | BDBM151415
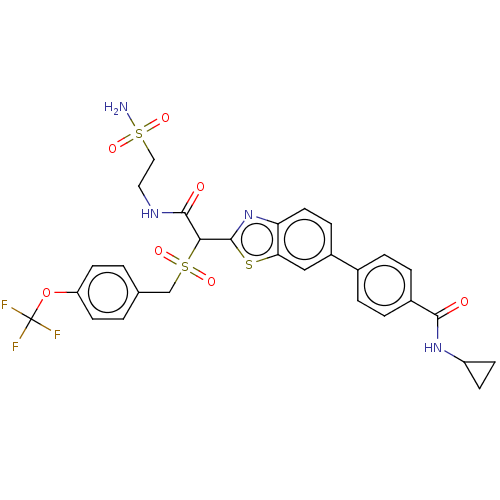
(US8987314, B226)Show SMILES NS(=O)(=O)CCNC(=O)C(c1nc2ccc(cc2s1)-c1ccc(cc1)C(=O)NC1CC1)S(=O)(=O)Cc1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C29H27F3N4O7S3/c30-29(31,32)43-22-10-1-17(2-11-22)16-45(39,40)25(27(38)34-13-14-46(33,41)42)28-36-23-12-7-20(15-24(23)44-28)18-3-5-19(6-4-18)26(37)35-21-8-9-21/h1-7,10-12,15,21,25H,8-9,13-14,16H2,(H,34,38)(H,35,37)(H2,33,41,42) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Bristol-Myers Squibb Company
US Patent
| Assay Description
Endothelial lipase (EL) and hepatic lipase (HL) activities were measured using a fluorescent substrate, A10070, (Invitrogen, CA) doped into an artifi... |
US Patent US8987314 (2015)
BindingDB Entry DOI: 10.7270/Q2W094N8 |
More data for this
Ligand-Target Pair | |
Endothelial lipase
(Homo sapiens (Human)) | BDBM151416
(US8987314, B227)Show SMILES NC(=O)c1ccc(cc1)-c1ccc2nc(sc2c1)C(C(=O)NCCS(N)(=O)=O)S(=O)(=O)Cc1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C26H23F3N4O7S3/c27-26(28,29)40-19-8-1-15(2-9-19)14-42(36,37)22(24(35)32-11-12-43(31,38)39)25-33-20-10-7-18(13-21(20)41-25)16-3-5-17(6-4-16)23(30)34/h1-10,13,22H,11-12,14H2,(H2,30,34)(H,32,35)(H2,31,38,39) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Bristol-Myers Squibb Company
US Patent
| Assay Description
Endothelial lipase (EL) and hepatic lipase (HL) activities were measured using a fluorescent substrate, A10070, (Invitrogen, CA) doped into an artifi... |
US Patent US8987314 (2015)
BindingDB Entry DOI: 10.7270/Q2W094N8 |
More data for this
Ligand-Target Pair | |
Endothelial lipase
(Homo sapiens (Human)) | BDBM151267
(US8987314, B63)Show SMILES COCc1ccc(cc1)-c1ccc2nc(sc2c1)C(C(=O)NCCS(N)(=O)=O)S(C)(=O)=O Show InChI InChI=1S/C20H23N3O6S3/c1-29-12-13-3-5-14(6-4-13)15-7-8-16-17(11-15)30-20(23-16)18(31(2,25)26)19(24)22-9-10-32(21,27)28/h3-8,11,18H,9-10,12H2,1-2H3,(H,22,24)(H2,21,27,28) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Bristol-Myers Squibb Company
US Patent
| Assay Description
Endothelial lipase (EL) and hepatic lipase (HL) activities were measured using a fluorescent substrate, A10070, (Invitrogen, CA) doped into an artifi... |
US Patent US8987314 (2015)
BindingDB Entry DOI: 10.7270/Q2W094N8 |
More data for this
Ligand-Target Pair | |
Endothelial lipase
(Homo sapiens (Human)) | BDBM151268

(US8987314, B64)Show SMILES COCCOC(=O)Nc1ccc(cc1)-c1ccc2nc(sc2c1)C(C(=O)NCCS(N)(=O)=O)S(C)(=O)=O Show InChI InChI=1S/C22H26N4O8S3/c1-33-10-11-34-22(28)25-16-6-3-14(4-7-16)15-5-8-17-18(13-15)35-21(26-17)19(36(2,29)30)20(27)24-9-12-37(23,31)32/h3-8,13,19H,9-12H2,1-2H3,(H,24,27)(H,25,28)(H2,23,31,32) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Bristol-Myers Squibb Company
US Patent
| Assay Description
Endothelial lipase (EL) and hepatic lipase (HL) activities were measured using a fluorescent substrate, A10070, (Invitrogen, CA) doped into an artifi... |
US Patent US8987314 (2015)
BindingDB Entry DOI: 10.7270/Q2W094N8 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data