

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histone acetyltransferase p300 (Homo sapiens (Human)) | BDBM50081125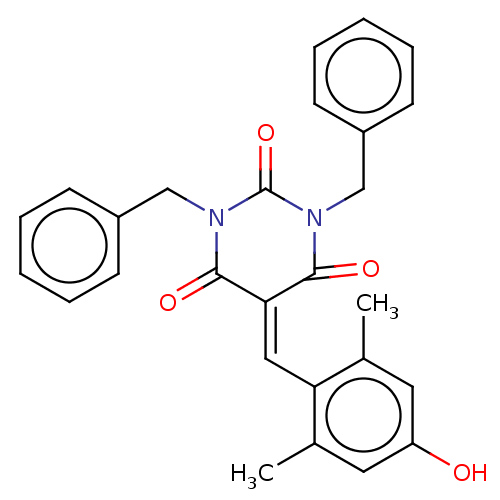 (CHEMBL3421961) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Salerno Curated by ChEMBL | Assay Description Noncompetitive inhibition of P300 (unknown origin) using acetyl CoA as substrate after 15 mins by double reciprocal plot analysis | J Med Chem 58: 2779-98 (2015) Article DOI: 10.1021/jm5019687 BindingDB Entry DOI: 10.7270/Q2736SMP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase p300 (Homo sapiens (Human)) | BDBM50081125 (CHEMBL3421961) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 9.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Salerno Curated by ChEMBL | Assay Description Noncompetitive inhibition of P300 (unknown origin) using biotinylated H3 as substrate after 15 mins by double reciprocal plot analysis | J Med Chem 58: 2779-98 (2015) Article DOI: 10.1021/jm5019687 BindingDB Entry DOI: 10.7270/Q2736SMP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| CREB-binding protein (Homo sapiens (Human)) | BDBM50081125 (CHEMBL3421961) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Salerno Curated by ChEMBL | Assay Description Inhibition of recombinant CBP (unknown origin) | J Med Chem 58: 2779-98 (2015) Article DOI: 10.1021/jm5019687 BindingDB Entry DOI: 10.7270/Q2736SMP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase p300 (Homo sapiens (Human)) | BDBM50081162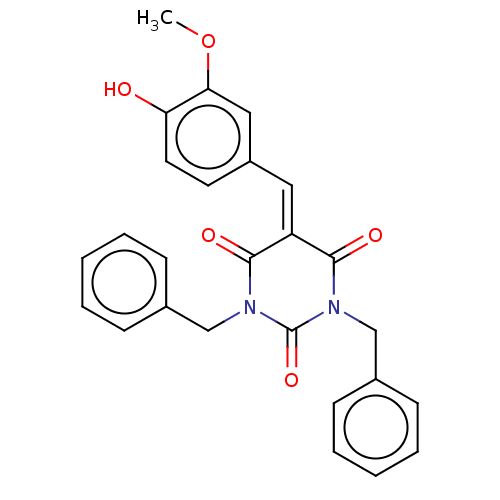 (CHEMBL3421943) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Salerno Curated by ChEMBL | Assay Description Inhibition of P300 (unknown origin) using histone H3/[acetyl-3H]-acetyl coenzyme A as substrate | J Med Chem 58: 2779-98 (2015) Article DOI: 10.1021/jm5019687 BindingDB Entry DOI: 10.7270/Q2736SMP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase p300 (Homo sapiens (Human)) | BDBM50081170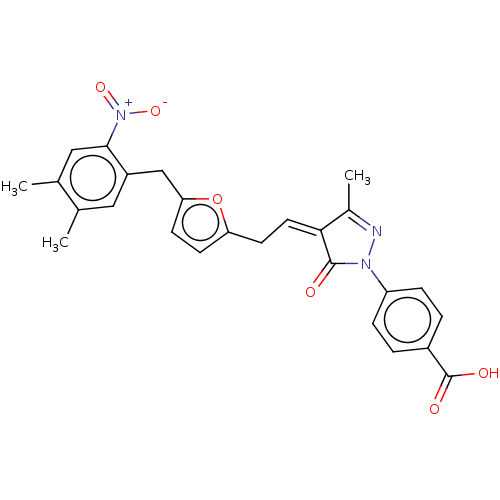 (CHEMBL3421960) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Salerno Curated by ChEMBL | Assay Description Inhibition of P300 (unknown origin) using histone H3/[acetyl-3H]-acetyl coenzyme A as substrate | J Med Chem 58: 2779-98 (2015) Article DOI: 10.1021/jm5019687 BindingDB Entry DOI: 10.7270/Q2736SMP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase p300 (Homo sapiens (Human)) | BDBM50081160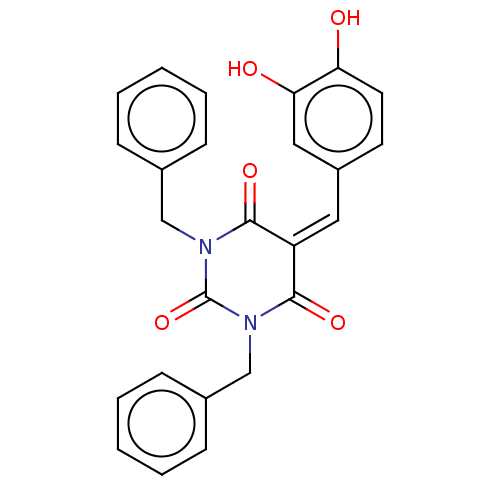 (CHEMBL3421941) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Salerno Curated by ChEMBL | Assay Description Inhibition of P300 (unknown origin) using histone H3/[acetyl-3H]-acetyl coenzyme A as substrate | J Med Chem 58: 2779-98 (2015) Article DOI: 10.1021/jm5019687 BindingDB Entry DOI: 10.7270/Q2736SMP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase p300 (Homo sapiens (Human)) | BDBM50081159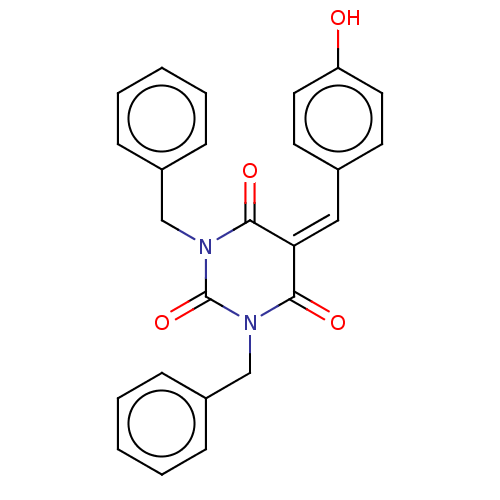 (CHEMBL3421940) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Salerno Curated by ChEMBL | Assay Description Inhibition of P300 (unknown origin) using histone H3/[acetyl-3H]-acetyl coenzyme A as substrate | J Med Chem 58: 2779-98 (2015) Article DOI: 10.1021/jm5019687 BindingDB Entry DOI: 10.7270/Q2736SMP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase p300 (Homo sapiens (Human)) | BDBM50081125 (CHEMBL3421961) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Salerno Curated by ChEMBL | Assay Description Inhibition of recombinant P300 (unknown origin) | J Med Chem 58: 2779-98 (2015) Article DOI: 10.1021/jm5019687 BindingDB Entry DOI: 10.7270/Q2736SMP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase p300 (Homo sapiens (Human)) | BDBM50081168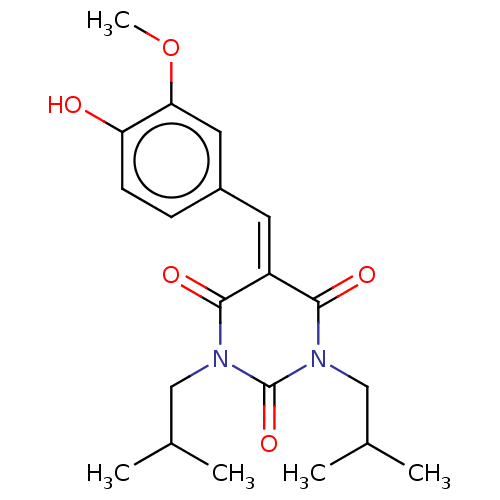 (CHEMBL3421950) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Salerno Curated by ChEMBL | Assay Description Inhibition of P300 (unknown origin) using histone H3/[acetyl-3H]-acetyl coenzyme A as substrate | J Med Chem 58: 2779-98 (2015) Article DOI: 10.1021/jm5019687 BindingDB Entry DOI: 10.7270/Q2736SMP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase p300 (Homo sapiens (Human)) | BDBM50081166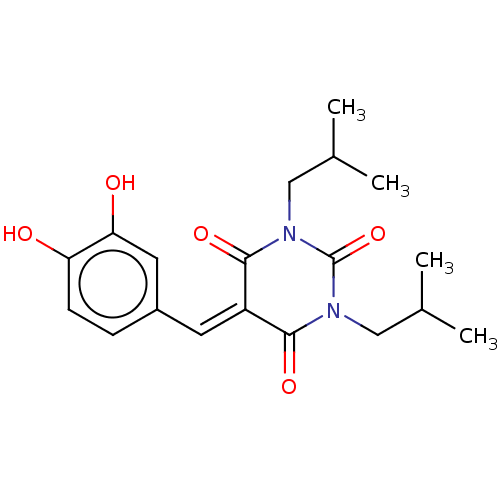 (CHEMBL3421948) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Salerno Curated by ChEMBL | Assay Description Inhibition of P300 (unknown origin) using histone H3/[acetyl-3H]-acetyl coenzyme A as substrate | J Med Chem 58: 2779-98 (2015) Article DOI: 10.1021/jm5019687 BindingDB Entry DOI: 10.7270/Q2736SMP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase p300 (Homo sapiens (Human)) | BDBM50081161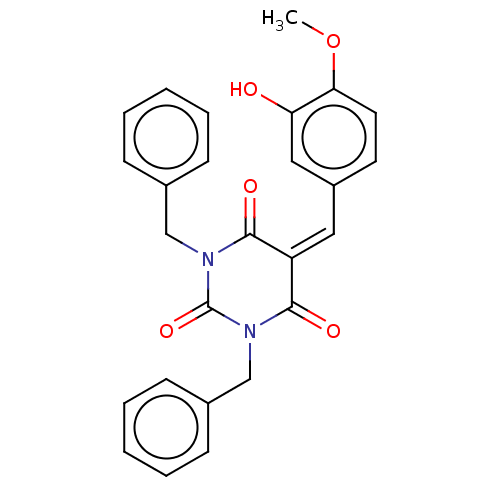 (CHEMBL3421942) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Salerno Curated by ChEMBL | Assay Description Inhibition of P300 (unknown origin) using histone H3/[acetyl-3H]-acetyl coenzyme A as substrate | J Med Chem 58: 2779-98 (2015) Article DOI: 10.1021/jm5019687 BindingDB Entry DOI: 10.7270/Q2736SMP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase p300 (Homo sapiens (Human)) | BDBM50067040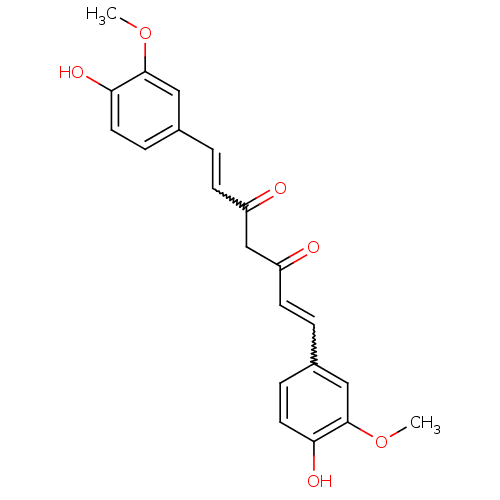 (((E,E)-1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-hept...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Salerno Curated by ChEMBL | Assay Description Inhibition of P300 (unknown origin) using histone H3/[acetyl-3H]-acetyl coenzyme A as substrate | J Med Chem 58: 2779-98 (2015) Article DOI: 10.1021/jm5019687 BindingDB Entry DOI: 10.7270/Q2736SMP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase p300 (Homo sapiens (Human)) | BDBM50081164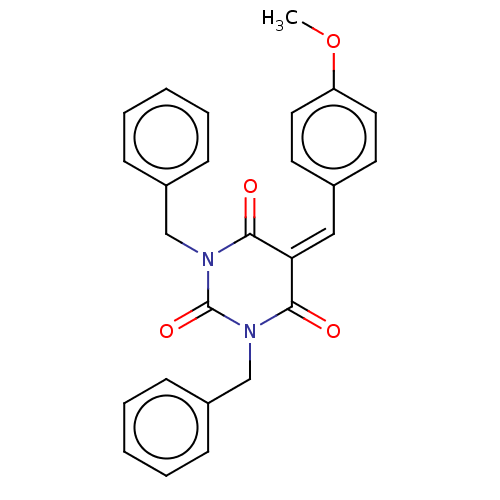 (CHEMBL3421945) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Salerno Curated by ChEMBL | Assay Description Inhibition of P300 (unknown origin) using histone H3/[acetyl-3H]-acetyl coenzyme A as substrate | J Med Chem 58: 2779-98 (2015) Article DOI: 10.1021/jm5019687 BindingDB Entry DOI: 10.7270/Q2736SMP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase p300 (Homo sapiens (Human)) | BDBM50081165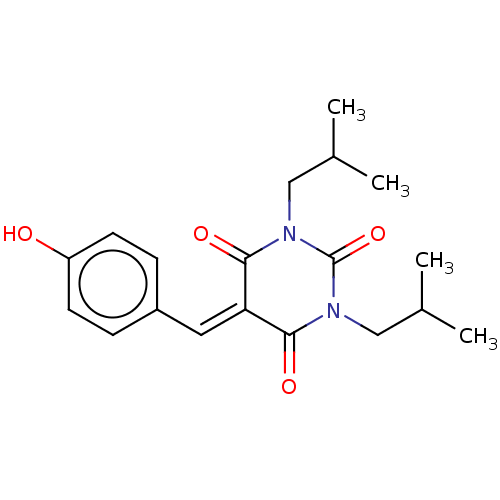 (CHEMBL3421947) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Salerno Curated by ChEMBL | Assay Description Inhibition of P300 (unknown origin) using histone H3/[acetyl-3H]-acetyl coenzyme A as substrate | J Med Chem 58: 2779-98 (2015) Article DOI: 10.1021/jm5019687 BindingDB Entry DOI: 10.7270/Q2736SMP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase p300 (Homo sapiens (Human)) | BDBM50081163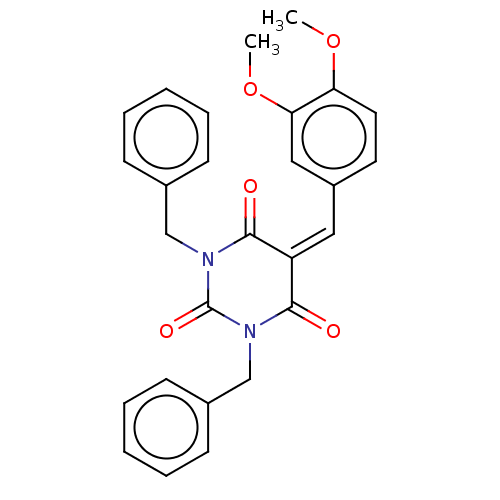 (CHEMBL3421944) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.14E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Salerno Curated by ChEMBL | Assay Description Inhibition of P300 (unknown origin) using histone H3/[acetyl-3H]-acetyl coenzyme A as substrate | J Med Chem 58: 2779-98 (2015) Article DOI: 10.1021/jm5019687 BindingDB Entry DOI: 10.7270/Q2736SMP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase p300 (Homo sapiens (Human)) | BDBM50081169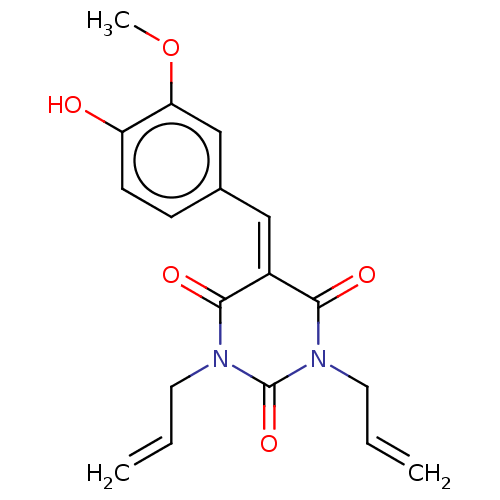 (CHEMBL3421957) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.64E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Salerno Curated by ChEMBL | Assay Description Inhibition of P300 (unknown origin) using histone H3/[acetyl-3H]-acetyl coenzyme A as substrate | J Med Chem 58: 2779-98 (2015) Article DOI: 10.1021/jm5019687 BindingDB Entry DOI: 10.7270/Q2736SMP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase p300 (Homo sapiens (Human)) | BDBM50081167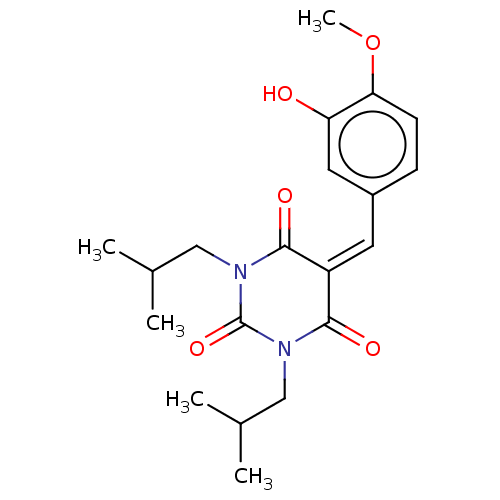 (CHEMBL3421949) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.14E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Salerno Curated by ChEMBL | Assay Description Inhibition of P300 (unknown origin) using histone H3/[acetyl-3H]-acetyl coenzyme A as substrate | J Med Chem 58: 2779-98 (2015) Article DOI: 10.1021/jm5019687 BindingDB Entry DOI: 10.7270/Q2736SMP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase KAT2B (Homo sapiens (Human)) | BDBM50292429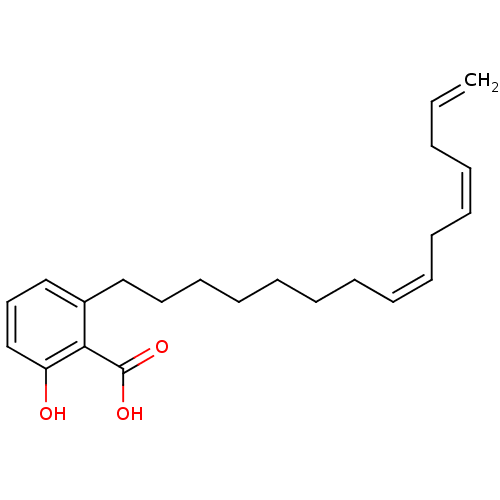 (2-Hydroxy-6-pentadecyl-benzoic acid | 2-Pentadecyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.39E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Salerno Curated by ChEMBL | Assay Description Inhibition of PCAF (unknown origin) using histone H3/[acetyl-3H]-acetyl coenzyme A as substrate | J Med Chem 58: 2779-98 (2015) Article DOI: 10.1021/jm5019687 BindingDB Entry DOI: 10.7270/Q2736SMP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin--tyrosine ligase (Homo sapiens (Human)) | BDBM50293292 (6alpha,15(S),23-trihydroxy-labd-8(22),13(14),17-tr...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a |
Universita di Salerno Curated by ChEMBL | Assay Description Binding affinity to TTL by surface plasmon resonance | J Med Chem 52: 3814-28 (2009) Article DOI: 10.1021/jm801637f BindingDB Entry DOI: 10.7270/Q2JD4WQD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin--tyrosine ligase (Homo sapiens (Human)) | BDBM50293293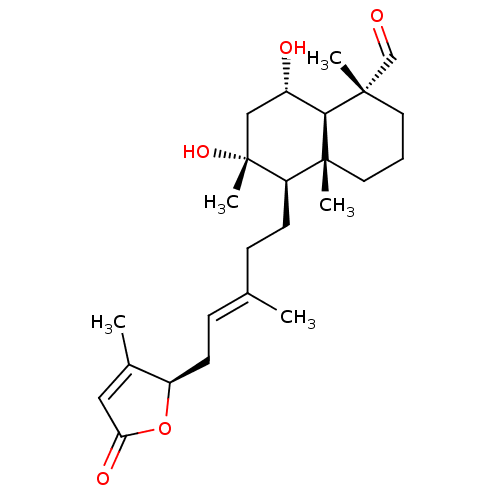 (6alpha,8alpha-dihydroxy-23-oxo-labd-13(14),17-dien...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 810 | n/a | n/a | n/a | n/a | n/a |
Universita di Salerno Curated by ChEMBL | Assay Description Binding affinity to TTL by surface plasmon resonance | J Med Chem 52: 3814-28 (2009) Article DOI: 10.1021/jm801637f BindingDB Entry DOI: 10.7270/Q2JD4WQD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin--tyrosine ligase (Homo sapiens (Human)) | BDBM50293294 (6alpha,8alpha,15(S)-trihydroxy-23-oxo-labd-13(14),...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a |
Universita di Salerno Curated by ChEMBL | Assay Description Binding affinity to TTL by surface plasmon resonance | J Med Chem 52: 3814-28 (2009) Article DOI: 10.1021/jm801637f BindingDB Entry DOI: 10.7270/Q2JD4WQD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin--tyrosine ligase (Homo sapiens (Human)) | BDBM50293295 (6alpha,8alpha,15(S)-trihydroxy-23-carbossimethylla...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 6.20E+3 | n/a | n/a | n/a | n/a | n/a |
Universita di Salerno Curated by ChEMBL | Assay Description Binding affinity to TTL by surface plasmon resonance | J Med Chem 52: 3814-28 (2009) Article DOI: 10.1021/jm801637f BindingDB Entry DOI: 10.7270/Q2JD4WQD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin--tyrosine ligase (Homo sapiens (Human)) | BDBM50293296 (6alpha,8alpha-dihydroxy-23-carbossi-labd-13(14),17...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a |
Universita di Salerno Curated by ChEMBL | Assay Description Binding affinity to TTL by surface plasmon resonance | J Med Chem 52: 3814-28 (2009) Article DOI: 10.1021/jm801637f BindingDB Entry DOI: 10.7270/Q2JD4WQD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin--tyrosine ligase (Homo sapiens (Human)) | BDBM50293297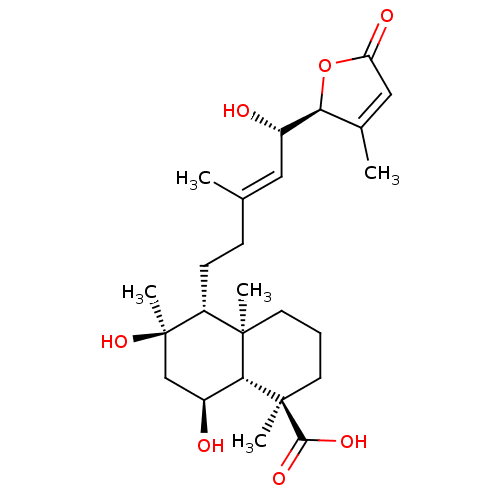 (6alpha,8alpha,15(S)-trihydroxy-23-carbossi-labd-13...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a |
Universita di Salerno Curated by ChEMBL | Assay Description Binding affinity to TTL by surface plasmon resonance | J Med Chem 52: 3814-28 (2009) Article DOI: 10.1021/jm801637f BindingDB Entry DOI: 10.7270/Q2JD4WQD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin--tyrosine ligase (Homo sapiens (Human)) | BDBM50293298 (6alpha,8alpha,23-trihydroxy-labd-13(14),17-dien-16...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a |
Universita di Salerno Curated by ChEMBL | Assay Description Binding affinity to TTL by surface plasmon resonance | J Med Chem 52: 3814-28 (2009) Article DOI: 10.1021/jm801637f BindingDB Entry DOI: 10.7270/Q2JD4WQD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin--tyrosine ligase (Homo sapiens (Human)) | BDBM50293299 (6alpha,8alpha,15(S),23-tetrahydroxy-labd-13(14),17...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | n/a |
Universita di Salerno Curated by ChEMBL | Assay Description Binding affinity to TTL by surface plasmon resonance | J Med Chem 52: 3814-28 (2009) Article DOI: 10.1021/jm801637f BindingDB Entry DOI: 10.7270/Q2JD4WQD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin--tyrosine ligase (Homo sapiens (Human)) | BDBM50293300 (8alpha-hydroxy,23alpha-O-ethyl-23,6alpha-epoxy-lab...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a |
Universita di Salerno Curated by ChEMBL | Assay Description Binding affinity to TTL by surface plasmon resonance | J Med Chem 52: 3814-28 (2009) Article DOI: 10.1021/jm801637f BindingDB Entry DOI: 10.7270/Q2JD4WQD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin--tyrosine ligase (Homo sapiens (Human)) | BDBM50293301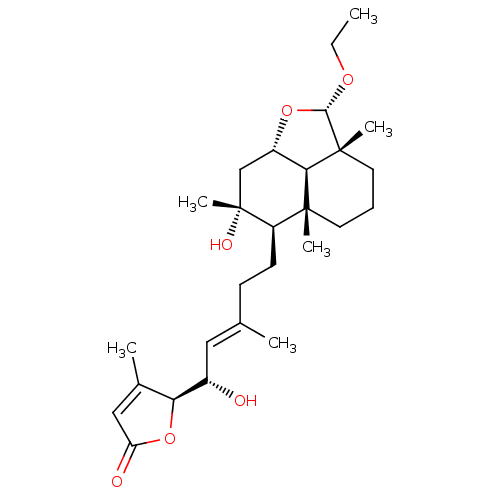 (8alpha,15(S)-dihydroxy,23alpha-O-ethyl-23,6alpha-e...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 960 | n/a | n/a | n/a | n/a | n/a |
Universita di Salerno Curated by ChEMBL | Assay Description Binding affinity to TTL by surface plasmon resonance | J Med Chem 52: 3814-28 (2009) Article DOI: 10.1021/jm801637f BindingDB Entry DOI: 10.7270/Q2JD4WQD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin--tyrosine ligase (Homo sapiens (Human)) | BDBM50293302 (8alpha,15(S),23alpha-trihydroxy-23,6alpha-epoxy-la...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 2.10E+5 | n/a | n/a | n/a | n/a | n/a |
Universita di Salerno Curated by ChEMBL | Assay Description Binding affinity to TTL by surface plasmon resonance | J Med Chem 52: 3814-28 (2009) Article DOI: 10.1021/jm801637f BindingDB Entry DOI: 10.7270/Q2JD4WQD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin--tyrosine ligase (Homo sapiens (Human)) | BDBM50293291 (6alpha,15(S)-dihydroxy-23-oxo-labd-8(22),13(14),17...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a |
Universita di Salerno Curated by ChEMBL | Assay Description Binding affinity to TTL by surface plasmon resonance | J Med Chem 52: 3814-28 (2009) Article DOI: 10.1021/jm801637f BindingDB Entry DOI: 10.7270/Q2JD4WQD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin--tyrosine ligase (Homo sapiens (Human)) | BDBM50293290 (6alpha,8alpha,23-trihydroxy-labd-13(14),15,17-trie...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a |
Universita di Salerno Curated by ChEMBL | Assay Description Binding affinity to TTL by surface plasmon resonance | J Med Chem 52: 3814-28 (2009) Article DOI: 10.1021/jm801637f BindingDB Entry DOI: 10.7270/Q2JD4WQD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin--tyrosine ligase (Homo sapiens (Human)) | BDBM50293289 (6alpha,8alpha-dihydroxy-23-carbossi-labd-13(14),15...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 93 | n/a | n/a | n/a | n/a | n/a |
Universita di Salerno Curated by ChEMBL | Assay Description Binding affinity to TTL by surface plasmon resonance | J Med Chem 52: 3814-28 (2009) Article DOI: 10.1021/jm801637f BindingDB Entry DOI: 10.7270/Q2JD4WQD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin--tyrosine ligase (Homo sapiens (Human)) | BDBM50293288 (6alpha,8alpha-dihydroxy-23-oxo-13(14),15,17-trien-...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a |
Universita di Salerno Curated by ChEMBL | Assay Description Binding affinity to TTL by surface plasmon resonance | J Med Chem 52: 3814-28 (2009) Article DOI: 10.1021/jm801637f BindingDB Entry DOI: 10.7270/Q2JD4WQD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin--tyrosine ligase (Homo sapiens (Human)) | BDBM50293287 (8alpha-hydroxylabd-13(14),15,17-trien-6alpha,23-16...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a |
Universita di Salerno Curated by ChEMBL | Assay Description Binding affinity to TTL by surface plasmon resonance | J Med Chem 52: 3814-28 (2009) Article DOI: 10.1021/jm801637f BindingDB Entry DOI: 10.7270/Q2JD4WQD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin--tyrosine ligase (Homo sapiens (Human)) | BDBM50293286 (8alpha-23-dihydroxy-23,6alpha-epoxy-labd-13(14),15...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a |
Universita di Salerno Curated by ChEMBL | Assay Description Binding affinity to TTL by surface plasmon resonance | J Med Chem 52: 3814-28 (2009) Article DOI: 10.1021/jm801637f BindingDB Entry DOI: 10.7270/Q2JD4WQD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin--tyrosine ligase (Homo sapiens (Human)) | BDBM50293285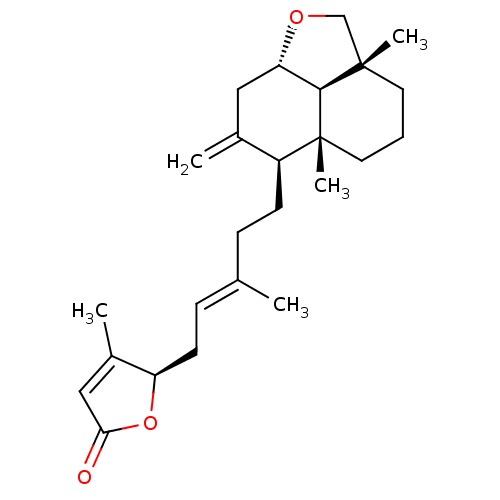 (23,6alpha-epoxy-labd-8,13(14),17-trien-16(R),19-ol...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 8.70E+3 | n/a | n/a | n/a | n/a | n/a |
Universita di Salerno Curated by ChEMBL | Assay Description Binding affinity to TTL by surface plasmon resonance | J Med Chem 52: 3814-28 (2009) Article DOI: 10.1021/jm801637f BindingDB Entry DOI: 10.7270/Q2JD4WQD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase p300 (Homo sapiens (Human)) | BDBM50081172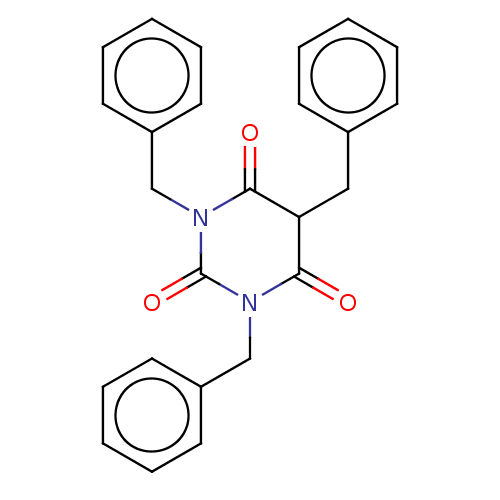 (CHEMBL3421930) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Salerno Curated by ChEMBL | Assay Description Binding affinity to human recombinant P300 catalytic domain by SPR analysis | J Med Chem 58: 2779-98 (2015) Article DOI: 10.1021/jm5019687 BindingDB Entry DOI: 10.7270/Q2736SMP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase p300 (Homo sapiens (Human)) | BDBM50081171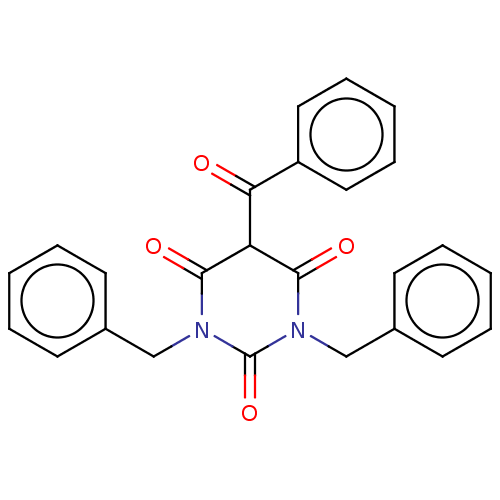 (CHEMBL3421905) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Salerno Curated by ChEMBL | Assay Description Binding affinity to human recombinant P300 catalytic domain by SPR analysis | J Med Chem 58: 2779-98 (2015) Article DOI: 10.1021/jm5019687 BindingDB Entry DOI: 10.7270/Q2736SMP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Farnesyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50024797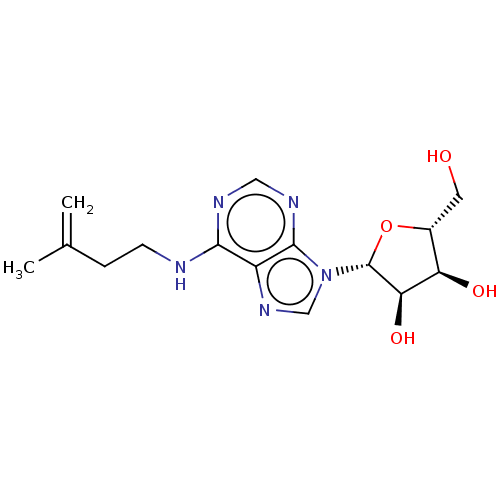 (CHEBI:60283 | CHEMBL1163500 | N6-Isopentenyladenos...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 2.02E+6 | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Salerno Curated by ChEMBL | Assay Description Binding affinity to FPPS (unknown origin) expressed in Escherichia coli BL21(DEscherichia ) assessed as binding constant of proton H2/H8 of purine ri... | J Med Chem 57: 7798-803 (2014) Article DOI: 10.1021/jm500869x BindingDB Entry DOI: 10.7270/Q25140SB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Farnesyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50024797 (CHEBI:60283 | CHEMBL1163500 | N6-Isopentenyladenos...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 2.21E+6 | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Salerno Curated by ChEMBL | Assay Description Binding affinity to FPPS (unknown origin) expressed in Escherichia coli BL21(DEscherichia ) assessed as binding constant of proton H12 of isopentenyl... | J Med Chem 57: 7798-803 (2014) Article DOI: 10.1021/jm500869x BindingDB Entry DOI: 10.7270/Q25140SB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Farnesyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50024797 (CHEBI:60283 | CHEMBL1163500 | N6-Isopentenyladenos...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 5.50E+5 | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Salerno Curated by ChEMBL | Assay Description Binding affinity to FPPS (unknown origin) expressed in Escherichia coli BL21(DEscherichia ) assessed as binding constant of proton H11 of isopentenyl... | J Med Chem 57: 7798-803 (2014) Article DOI: 10.1021/jm500869x BindingDB Entry DOI: 10.7270/Q25140SB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Farnesyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50024797 (CHEBI:60283 | CHEMBL1163500 | N6-Isopentenyladenos...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 1.21E+6 | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Salerno Curated by ChEMBL | Assay Description Binding affinity to FPPS (unknown origin) expressed in Escherichia coli BL21(DEscherichia ) assessed as binding constant of proton H14-H15 of isopent... | J Med Chem 57: 7798-803 (2014) Article DOI: 10.1021/jm500869x BindingDB Entry DOI: 10.7270/Q25140SB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase p300 (Homo sapiens (Human)) | BDBM84877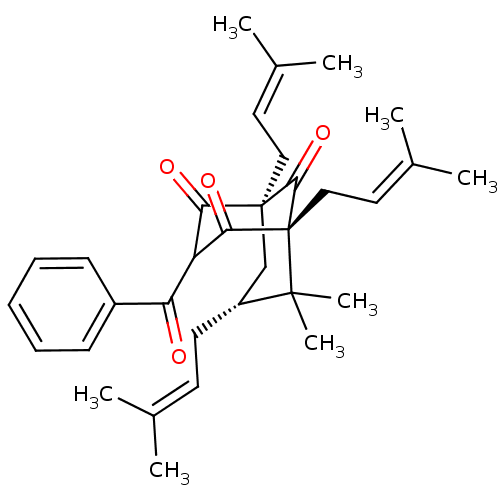 (Clusianone, 10) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 810 | n/a | n/a | n/a | n/a | 37 |
Universitá degli Studi di Salerno | Assay Description The p300 activity assays were performed by a colorimetric kit (JM-K322-100, MBL) using active recombinant p300/HAT as positive control and acetyl-CoA... | Chembiochem 11: 818-27 (2010) Article DOI: 10.1002/cbic.200900721 BindingDB Entry DOI: 10.7270/Q2S1810J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase p300 (Homo sapiens (Human)) | BDBM84875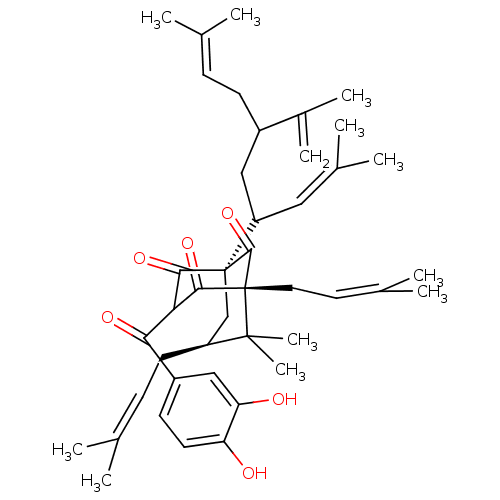 (Guttiferone E, 4) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 390 | n/a | n/a | n/a | n/a | 37 |
Universitá degli Studi di Salerno | Assay Description The p300 activity assays were performed by a colorimetric kit (JM-K322-100, MBL) using active recombinant p300/HAT as positive control and acetyl-CoA... | Chembiochem 11: 818-27 (2010) Article DOI: 10.1002/cbic.200900721 BindingDB Entry DOI: 10.7270/Q2S1810J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase p300 (Homo sapiens (Human)) | BDBM84874 (Guttiferone A, 3) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 100 | n/a | n/a | n/a | n/a | 37 |
Universitá degli Studi di Salerno | Assay Description The p300 activity assays were performed by a colorimetric kit (JM-K322-100, MBL) using active recombinant p300/HAT as positive control and acetyl-CoA... | Chembiochem 11: 818-27 (2010) Article DOI: 10.1002/cbic.200900721 BindingDB Entry DOI: 10.7270/Q2S1810J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 1 [11-371] (Homo sapiens (Human)) | BDBM50206427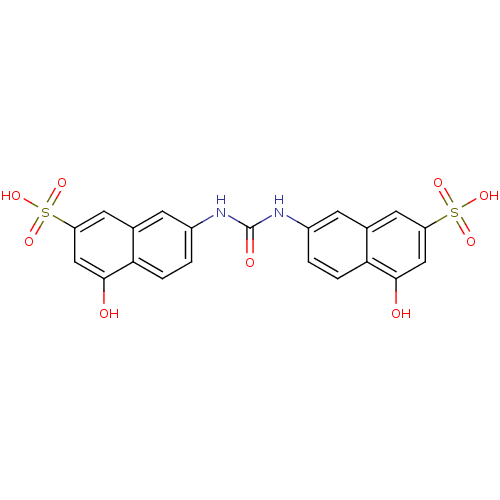 (7,7'-carbonylbis(azanediyl)bis(4-hydroxynaphthalen...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 810 | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Salerno Curated by ChEMBL | Assay Description Binding affinity to human recombinant GST-PRMT1 expressed Escherichia coli BL21 by SPR assay | J Med Chem 55: 9875-90 (2012) Article DOI: 10.1021/jm301097p BindingDB Entry DOI: 10.7270/Q2445NM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase p300 (Homo sapiens (Human)) | BDBM50241990 (CHEMBL502489 | Camboginol | Garcinol | Garcinol, 1) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 400 | n/a | n/a | n/a | n/a | 37 |
Universitá degli Studi di Salerno | Assay Description The p300 activity assays were performed by a colorimetric kit (JM-K322-100, MBL) using active recombinant p300/HAT as positive control and acetyl-CoA... | Chembiochem 11: 818-27 (2010) Article DOI: 10.1002/cbic.200900721 BindingDB Entry DOI: 10.7270/Q2S1810J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase p300 (Homo sapiens (Human)) | BDBM84873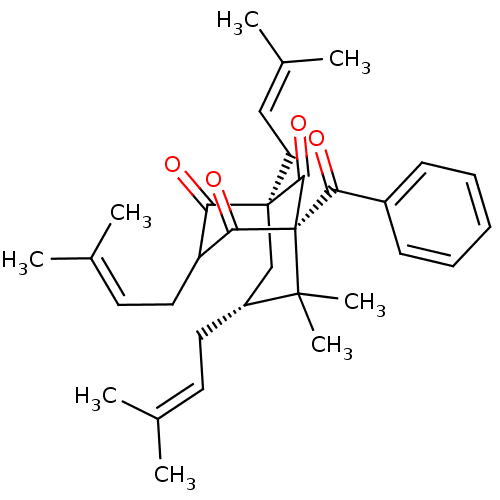 (Nemorosone, 2) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 250 | n/a | n/a | n/a | n/a | 37 |
Universitá degli Studi di Salerno | Assay Description The p300 activity assays were performed by a colorimetric kit (JM-K322-100, MBL) using active recombinant p300/HAT as positive control and acetyl-CoA... | Chembiochem 11: 818-27 (2010) Article DOI: 10.1002/cbic.200900721 BindingDB Entry DOI: 10.7270/Q2S1810J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||