

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aromatase (Homo sapiens (Human)) | BDBM50047260 (4,4'-((1H-imidazol-1-yl)methylene)dibenzonitrile |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Moncton Curated by ChEMBL | Assay Description Inhibition of aromatase using 7-methoxy-4-trifluoromethyl coumarin as substrate after 30 mins by fluorescence-based colorimetric analysis | Eur J Med Chem 46: 4010-24 (2011) Article DOI: 10.1016/j.ejmech.2011.05.074 BindingDB Entry DOI: 10.7270/Q2C24WTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50008733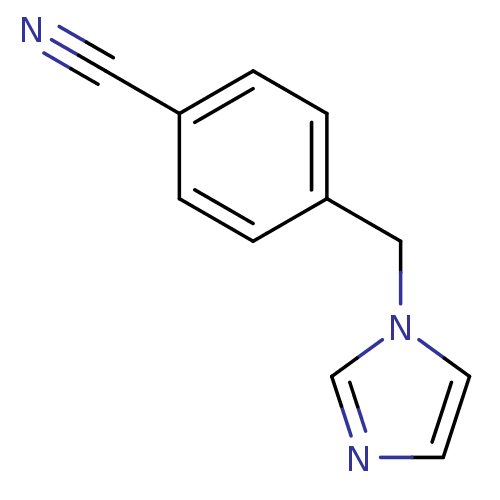 (1-(4-Cyanobenzyl)-1H-imidazole | 4-((1H-imidazol-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Moncton Curated by ChEMBL | Assay Description Inhibition of aromatase using 7-methoxy-4-trifluoromethyl coumarin as substrate after 30 mins by fluorescence-based colorimetric analysis | Eur J Med Chem 46: 4010-24 (2011) Article DOI: 10.1016/j.ejmech.2011.05.074 BindingDB Entry DOI: 10.7270/Q2C24WTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50351827 (CHEMBL224789) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Moncton Curated by ChEMBL | Assay Description Inhibition of aromatase using 7-methoxy-4-trifluoromethyl coumarin as substrate after 30 mins by fluorescence-based colorimetric analysis | Eur J Med Chem 46: 4010-24 (2011) Article DOI: 10.1016/j.ejmech.2011.05.074 BindingDB Entry DOI: 10.7270/Q2C24WTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM13061 (4,4 -(1H-1,2,4-triazol-1-ylmethanediyl)dibenzonitr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Moncton Curated by ChEMBL | Assay Description Inhibition of aromatase using 7-methoxy-4-trifluoromethyl coumarin as substrate after 30 mins by fluorescence-based colorimetric analysis | Eur J Med Chem 46: 4010-24 (2011) Article DOI: 10.1016/j.ejmech.2011.05.074 BindingDB Entry DOI: 10.7270/Q2C24WTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50014788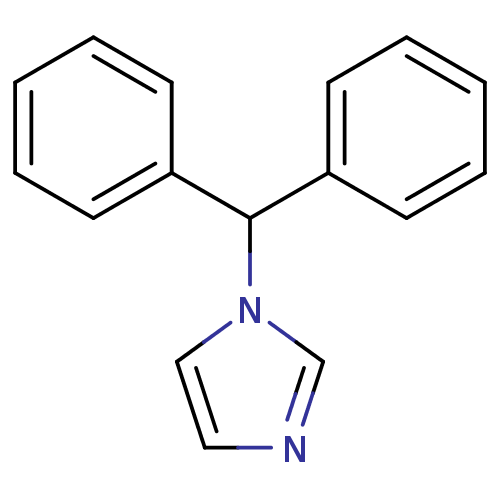 (1-Benzhydryl-1H-imidazole | CHEMBL336638) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Moncton Curated by ChEMBL | Assay Description Inhibition of aromatase using 7-methoxy-4-trifluoromethyl coumarin as substrate after 30 mins by fluorescence-based colorimetric analysis | Eur J Med Chem 46: 4010-24 (2011) Article DOI: 10.1016/j.ejmech.2011.05.074 BindingDB Entry DOI: 10.7270/Q2C24WTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50351855 (CHEMBL1824769) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Moncton Curated by ChEMBL | Assay Description Inhibition of aromatase using 7-methoxy-4-trifluoromethyl coumarin as substrate after 30 mins by fluorescence-based colorimetric analysis | Eur J Med Chem 46: 4010-24 (2011) Article DOI: 10.1016/j.ejmech.2011.05.074 BindingDB Entry DOI: 10.7270/Q2C24WTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, membrane associated (Homo sapiens (Human)) | BDBM23763 (3-({4-[(4-icosylpiperazin-1-yl)carbonyl]phenyl}met...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Universite Paris 7-Denis Diderot | Assay Description PLA2 activity is by using the fluorescent phospholipid analogue, beta-pyC-10-PG as the substrate. The increase in fluorescence (ex @342 nm and em@388... | Bioorg Med Chem 16: 1242-53 (2008) Article DOI: 10.1016/j.bmc.2007.10.077 BindingDB Entry DOI: 10.7270/Q2J964PK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, membrane associated (Homo sapiens (Human)) | BDBM23764 (3-({4-[(4-docosylpiperazin-1-yl)carbonyl]phenyl}me...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Universite Paris 7-Denis Diderot | Assay Description PLA2 activity is by using the fluorescent phospholipid analogue, beta-pyC-10-PG as the substrate. The increase in fluorescence (ex @342 nm and em@388... | Bioorg Med Chem 16: 1242-53 (2008) Article DOI: 10.1016/j.bmc.2007.10.077 BindingDB Entry DOI: 10.7270/Q2J964PK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, membrane associated (Homo sapiens (Human)) | BDBM50206910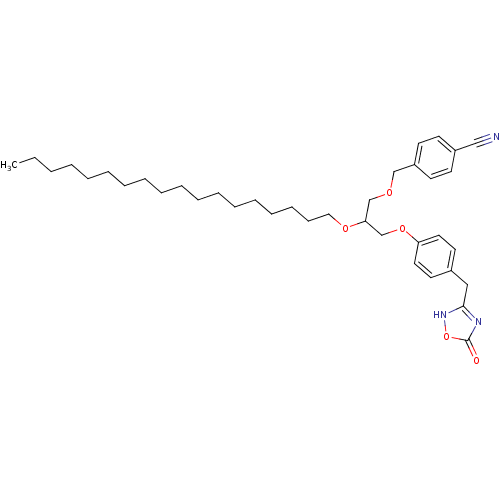 ((+/-)-1-O-(4-cyanomethylphenyl)-3-O-[4-(4,5-dihydr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris7-Denis Diderot Curated by ChEMBL | Assay Description Inhibition of human group IIA PLA2 by fluorimetric assay | J Med Chem 50: 1618-26 (2007) Article DOI: 10.1021/jm060082n BindingDB Entry DOI: 10.7270/Q2TQ6174 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM7887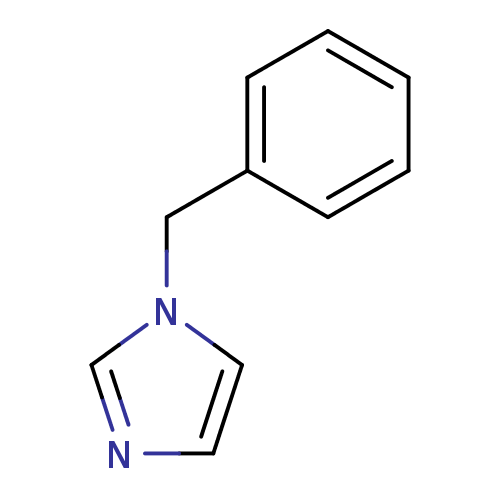 (1-Benzylimidazole (BI) | 1-benzyl-1H-imidazole | 1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Moncton Curated by ChEMBL | Assay Description Inhibition of aromatase using 7-methoxy-4-trifluoromethyl coumarin as substrate after 30 mins by fluorescence-based colorimetric analysis | Eur J Med Chem 46: 4010-24 (2011) Article DOI: 10.1016/j.ejmech.2011.05.074 BindingDB Entry DOI: 10.7270/Q2C24WTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50351859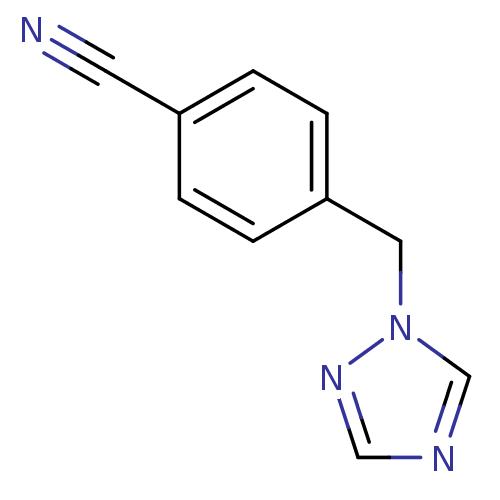 (CHEMBL1825020) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Moncton Curated by ChEMBL | Assay Description Inhibition of aromatase using 7-methoxy-4-trifluoromethyl coumarin as substrate after 30 mins by fluorescence-based colorimetric analysis | Eur J Med Chem 46: 4010-24 (2011) Article DOI: 10.1016/j.ejmech.2011.05.074 BindingDB Entry DOI: 10.7270/Q2C24WTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, membrane associated (Homo sapiens (Human)) | BDBM50055371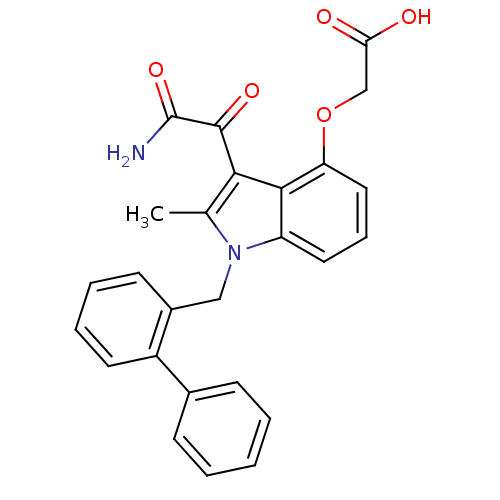 ((3-aminooxalyl-1-biphenyl-2-ylmethyl-2-methyl-1H-i...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris7-Denis Diderot Curated by ChEMBL | Assay Description Inhibition of human group IIA PLA2 in [3H]oleate-labeled Escherichia coli membrane by radiometric assay | J Med Chem 50: 1618-26 (2007) Article DOI: 10.1021/jm060082n BindingDB Entry DOI: 10.7270/Q2TQ6174 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50583291 (CHEMBL5085074) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of 5-LO in thapsigargin stimulated human PMNL cells assessed as reduction in 5-LO product level preincubated for 5 mins followed by thapsi... | Citation and Details Article DOI: 10.1021/acs.jnatprod.1c00982 BindingDB Entry DOI: 10.7270/Q2W099TK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50583293 (CHEMBL5088008) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of 5-LO in thapsigargin stimulated human PMNL cells assessed as reduction in 5-LO product level preincubated for 5 mins followed by thapsi... | Citation and Details Article DOI: 10.1021/acs.jnatprod.1c00982 BindingDB Entry DOI: 10.7270/Q2W099TK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50583295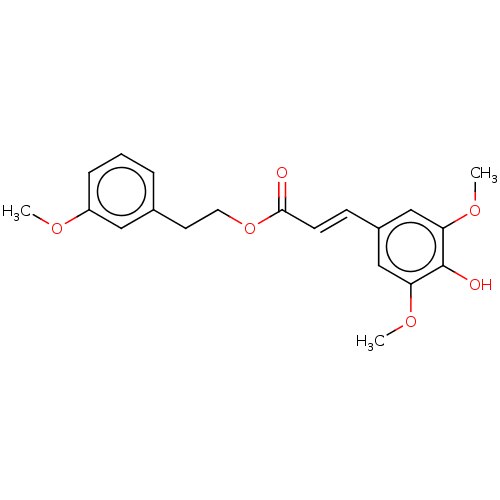 (CHEMBL5093372) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of 5-LO in thapsigargin stimulated human PMNL cells assessed as reduction in 5-LO product level preincubated for 5 mins followed by thapsi... | Citation and Details Article DOI: 10.1021/acs.jnatprod.1c00982 BindingDB Entry DOI: 10.7270/Q2W099TK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50583289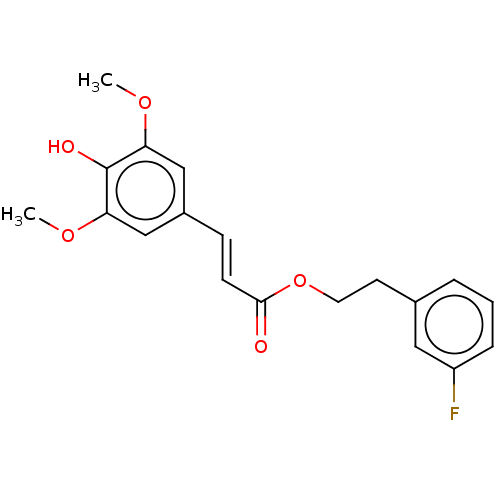 (CHEMBL5080803) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of 5-LO in thapsigargin stimulated human PMNL cells assessed as reduction in 5-LO product level preincubated for 5 mins followed by thapsi... | Citation and Details Article DOI: 10.1021/acs.jnatprod.1c00982 BindingDB Entry DOI: 10.7270/Q2W099TK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50583290 (CHEMBL5088166) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of 5-LO in thapsigargin stimulated human PMNL cells assessed as reduction in 5-LO product level preincubated for 5 mins followed by thapsi... | Citation and Details Article DOI: 10.1021/acs.jnatprod.1c00982 BindingDB Entry DOI: 10.7270/Q2W099TK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50583294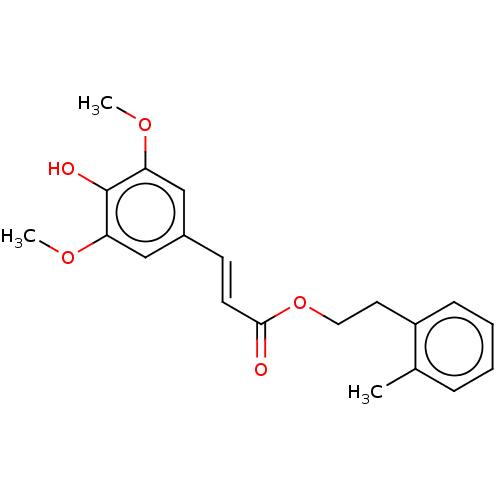 (CHEMBL5072274) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of 5-LO in thapsigargin stimulated human PMNL cells assessed as reduction in 5-LO product level preincubated for 5 mins followed by thapsi... | Citation and Details Article DOI: 10.1021/acs.jnatprod.1c00982 BindingDB Entry DOI: 10.7270/Q2W099TK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50583296 (CHEMBL5085633) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of 5-LO in thapsigargin stimulated human PMNL cells assessed as reduction in 5-LO product level preincubated for 5 mins followed by thapsi... | Citation and Details Article DOI: 10.1021/acs.jnatprod.1c00982 BindingDB Entry DOI: 10.7270/Q2W099TK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50583302 (CHEMBL5071392) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of 5-LO in thapsigargin stimulated human PMNL cells assessed as reduction in 5-LO product level preincubated for 5 mins followed by thapsi... | Citation and Details Article DOI: 10.1021/acs.jnatprod.1c00982 BindingDB Entry DOI: 10.7270/Q2W099TK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50583308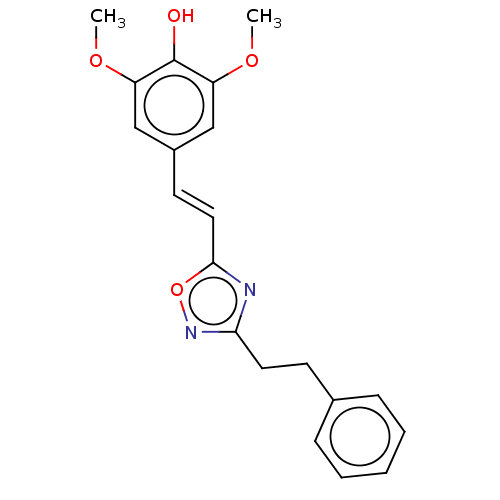 (CHEMBL5089203) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of 5-LO in thapsigargin stimulated human PMNL cells assessed as reduction in 5-LO product level preincubated for 5 mins followed by thapsi... | Citation and Details Article DOI: 10.1021/acs.jnatprod.1c00982 BindingDB Entry DOI: 10.7270/Q2W099TK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2 group V (Homo sapiens (Human)) | BDBM50055371 ((3-aminooxalyl-1-biphenyl-2-ylmethyl-2-methyl-1H-i...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris7-Denis Diderot Curated by ChEMBL | Assay Description Inhibition of human group V PLA2 in [3H]oleate-labeled Escherichia coli membrane by radiometric assay | J Med Chem 50: 1618-26 (2007) Article DOI: 10.1021/jm060082n BindingDB Entry DOI: 10.7270/Q2TQ6174 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, membrane associated (Homo sapiens (Human)) | BDBM50206911 ((+/+)-1-O-decyl-3-O-[4-(4,5-dihydro-5-oxo-1,2,4-4H...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris7-Denis Diderot Curated by ChEMBL | Assay Description Inhibition of human group IIA PLA2 by fluorimetric assay | J Med Chem 50: 1618-26 (2007) Article DOI: 10.1021/jm060082n BindingDB Entry DOI: 10.7270/Q2TQ6174 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, membrane associated (Homo sapiens (Human)) | BDBM50206913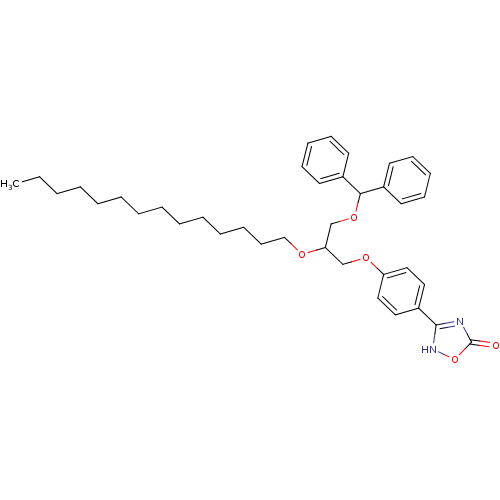 ((+/-)-3-O-[4-(4,5-dihydro-5-oxo-1,2,4-4H-oxadiazol...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris7-Denis Diderot Curated by ChEMBL | Assay Description Inhibition of human group IIA PLA2 by fluorimetric assay | J Med Chem 50: 1618-26 (2007) Article DOI: 10.1021/jm060082n BindingDB Entry DOI: 10.7270/Q2TQ6174 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, membrane associated (Homo sapiens (Human)) | BDBM50206912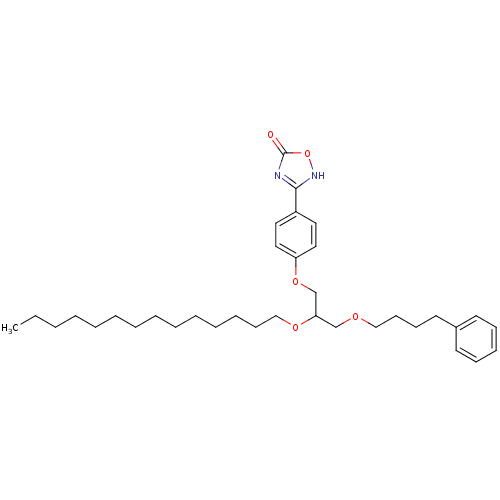 ((+/-)-3-O-[4-(4,5-dihydro-5-oxo-1,2,4-4H-oxadiazol...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris7-Denis Diderot Curated by ChEMBL | Assay Description Inhibition of human group IIA PLA2 by fluorimetric assay | J Med Chem 50: 1618-26 (2007) Article DOI: 10.1021/jm060082n BindingDB Entry DOI: 10.7270/Q2TQ6174 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50583288 (CHEMBL4794186) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of 5-LO in thapsigargin stimulated human PMNL cells assessed as reduction in 5-LO product level preincubated for 5 mins followed by thapsi... | Citation and Details Article DOI: 10.1021/acs.jnatprod.1c00982 BindingDB Entry DOI: 10.7270/Q2W099TK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50583307 (CHEMBL5072348) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of 5-LO in thapsigargin stimulated human PMNL cells assessed as reduction in 5-LO product level preincubated for 5 mins followed by thapsi... | Citation and Details Article DOI: 10.1021/acs.jnatprod.1c00982 BindingDB Entry DOI: 10.7270/Q2W099TK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50029225 ((E)-3-(2,5-Dihydroxy-phenyl)-acrylic acid phenethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Moncton Curated by ChEMBL | Assay Description Inhibition of 5-LO in human PMNL cells assessed as reduction in leukotriene formation preincubated for 5 mins followed by thapsigargin stimulation me... | Eur J Med Chem 179: 347-357 (2019) Article DOI: 10.1016/j.ejmech.2019.06.060 BindingDB Entry DOI: 10.7270/Q2125X31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50029225 ((E)-3-(2,5-Dihydroxy-phenyl)-acrylic acid phenethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Moncton Curated by ChEMBL | Assay Description Inhibition of 5-LO in human PMNL cells assessed as reduction in leukotriene formation preincubated for 5 mins followed by thapsigargin stimulation me... | Eur J Med Chem 179: 347-357 (2019) Article DOI: 10.1016/j.ejmech.2019.06.060 BindingDB Entry DOI: 10.7270/Q2125X31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, membrane associated (Homo sapiens (Human)) | BDBM50206905 ((S)-3-(4-(2-(tetradecyloxy)-3-(trityloxy)propoxy)p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris7-Denis Diderot Curated by ChEMBL | Assay Description Inhibition of human group IIA PLA2 by fluorimetric assay | J Med Chem 50: 1618-26 (2007) Article DOI: 10.1021/jm060082n BindingDB Entry DOI: 10.7270/Q2TQ6174 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50583298 (CHEMBL4759754) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of 5-LO in thapsigargin stimulated human PMNL cells assessed as reduction in 5-LO product level preincubated for 5 mins followed by thapsi... | Citation and Details Article DOI: 10.1021/acs.jnatprod.1c00982 BindingDB Entry DOI: 10.7270/Q2W099TK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50583292 (CHEMBL5073724) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of 5-LO in thapsigargin stimulated human PMNL cells assessed as reduction in 5-LO product level preincubated for 5 mins followed by thapsi... | Citation and Details Article DOI: 10.1021/acs.jnatprod.1c00982 BindingDB Entry DOI: 10.7270/Q2W099TK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50583303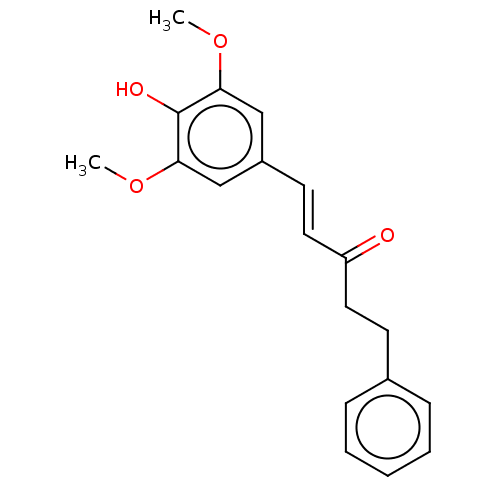 (CHEMBL5092055) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of 5-LO in thapsigargin stimulated human PMNL cells assessed as reduction in 5-LO product level preincubated for 5 mins followed by thapsi... | Citation and Details Article DOI: 10.1021/acs.jnatprod.1c00982 BindingDB Entry DOI: 10.7270/Q2W099TK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50583304 (CHEMBL5077729) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of 5-LO in thapsigargin stimulated human PMNL cells assessed as reduction in 5-LO product level preincubated for 5 mins followed by thapsi... | Citation and Details Article DOI: 10.1021/acs.jnatprod.1c00982 BindingDB Entry DOI: 10.7270/Q2W099TK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, membrane associated (Homo sapiens (Human)) | BDBM23771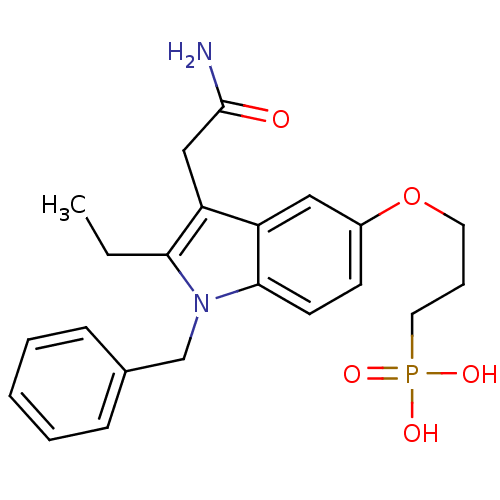 ((3-{[1-benzyl-3-(carbamoylmethyl)-2-ethyl-1H-indol...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 470 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Universite Paris 7-Denis Diderot | Assay Description PLA2 activity is by using the fluorescent phospholipid analogue, beta-pyC-10-PG as the substrate. The increase in fluorescence (ex @342 nm and em@388... | Bioorg Med Chem 16: 1242-53 (2008) Article DOI: 10.1016/j.bmc.2007.10.077 BindingDB Entry DOI: 10.7270/Q2J964PK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Phospholipase A2, membrane associated (Homo sapiens (Human)) | BDBM23771 ((3-{[1-benzyl-3-(carbamoylmethyl)-2-ethyl-1H-indol...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris7-Denis Diderot Curated by ChEMBL | Assay Description Inhibition of human group IIA PLA2 by fluorimetric assay | J Med Chem 50: 1618-26 (2007) Article DOI: 10.1021/jm060082n BindingDB Entry DOI: 10.7270/Q2TQ6174 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50530377 (CHEMBL4579523) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 530 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Moncton Curated by ChEMBL | Assay Description Inhibition of 5-LO in human PMNL cells assessed as reduction in leukotriene formation preincubated for 5 mins followed by thapsigargin stimulation me... | Eur J Med Chem 179: 347-357 (2019) Article DOI: 10.1016/j.ejmech.2019.06.060 BindingDB Entry DOI: 10.7270/Q2125X31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50530377 (CHEMBL4579523) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 530 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Moncton Curated by ChEMBL | Assay Description Inhibition of 5-LO in human PMNL cells assessed as reduction in leukotriene formation preincubated for 5 mins followed by thapsigargin stimulation me... | Eur J Med Chem 179: 347-357 (2019) Article DOI: 10.1016/j.ejmech.2019.06.060 BindingDB Entry DOI: 10.7270/Q2125X31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2 group V (Homo sapiens (Human)) | BDBM50206911 ((+/+)-1-O-decyl-3-O-[4-(4,5-dihydro-5-oxo-1,2,4-4H...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris7-Denis Diderot Curated by ChEMBL | Assay Description Inhibition of human group V PLA2 in [3H]oleate-labeled Escherichia coli membrane by radiometric assay | J Med Chem 50: 1618-26 (2007) Article DOI: 10.1021/jm060082n BindingDB Entry DOI: 10.7270/Q2TQ6174 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50583306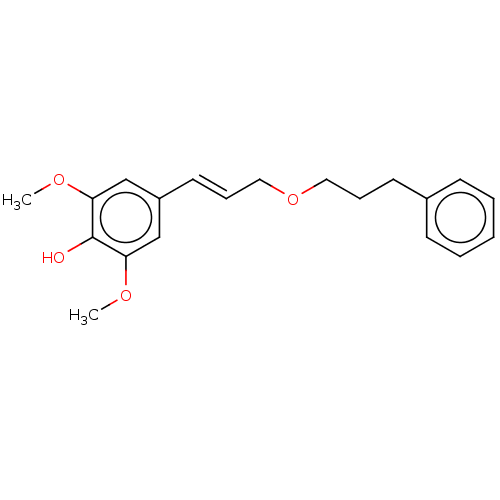 (CHEMBL5084797) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of 5-LO in thapsigargin stimulated human PMNL cells assessed as reduction in 5-LO product level preincubated for 5 mins followed by thapsi... | Citation and Details Article DOI: 10.1021/acs.jnatprod.1c00982 BindingDB Entry DOI: 10.7270/Q2W099TK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50583301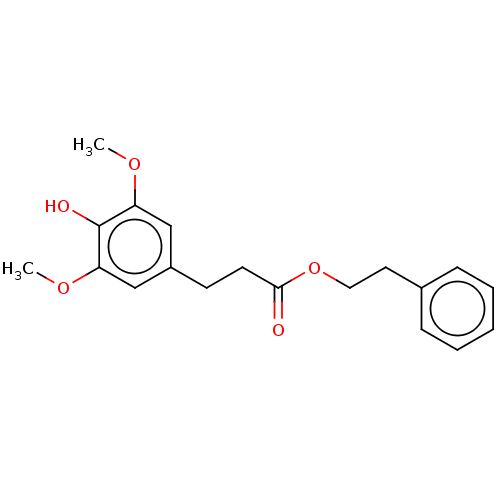 (CHEMBL5078319) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 590 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of 5-LO in thapsigargin stimulated human PMNL cells assessed as reduction in 5-LO product level preincubated for 5 mins followed by thapsi... | Citation and Details Article DOI: 10.1021/acs.jnatprod.1c00982 BindingDB Entry DOI: 10.7270/Q2W099TK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2 group V (Homo sapiens (Human)) | BDBM50206920 ((+/-)-3-O-[4-(4,5-dihydro-5-oxo-1,2,4-4H-oxadiazol...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 660 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris7-Denis Diderot Curated by ChEMBL | Assay Description Inhibition of human group V PLA2 in [3H]oleate-labeled Escherichia coli membrane by radiometric assay | J Med Chem 50: 1618-26 (2007) Article DOI: 10.1021/jm060082n BindingDB Entry DOI: 10.7270/Q2TQ6174 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50277011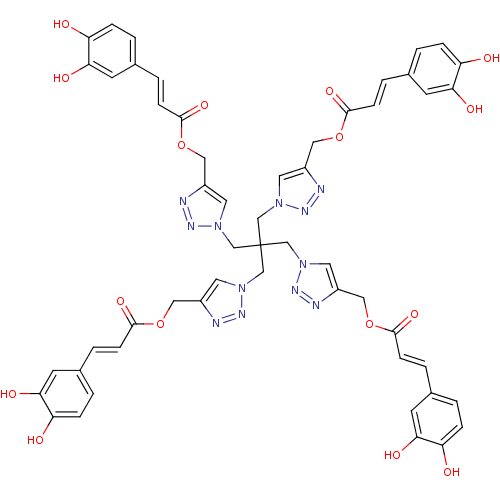 ((2E,2'E)-(1,1'-(2,2-bis((4-(((E)-3-(3,4-dihydroxyp...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 660 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Moncton Curated by ChEMBL | Assay Description Inhibition of human 5LOX expressed in HEK293 cells | Bioorg Med Chem Lett 19: 1118-21 (2009) Article DOI: 10.1016/j.bmcl.2008.12.108 BindingDB Entry DOI: 10.7270/Q21R6QCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50276981 ((E)-2-(4-(cinnamoyloxymethyl)-1H-1,2,3-triazol-1-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 680 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Moncton Curated by ChEMBL | Assay Description Inhibition of human 5LOX expressed in HEK293 cells | Bioorg Med Chem Lett 19: 1118-21 (2009) Article DOI: 10.1016/j.bmcl.2008.12.108 BindingDB Entry DOI: 10.7270/Q21R6QCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Patatin-like phospholipase domain-containing protein 2 (Homo sapiens (Human)) | BDBM50185419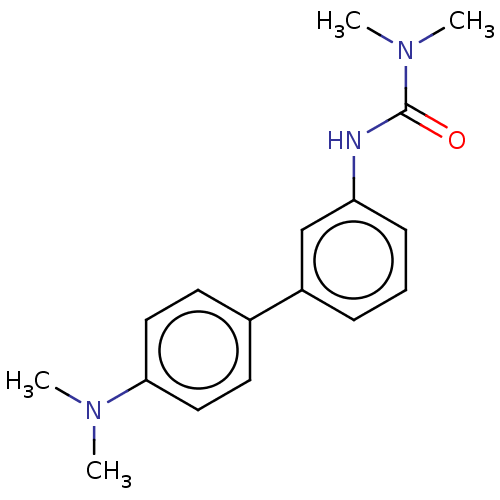 (CHEMBL3823931) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Moncton Curated by ChEMBL | Assay Description Inhibition of ATGL (unknown origin) overexpressed in Escherichia coli XL-1 cells using [9,10-3H(N)]triolein as substrate incubated for 60 mins by liq... | Eur J Med Chem 118: 290-8 (2016) Article DOI: 10.1016/j.ejmech.2016.04.021 BindingDB Entry DOI: 10.7270/Q20V8FQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50583297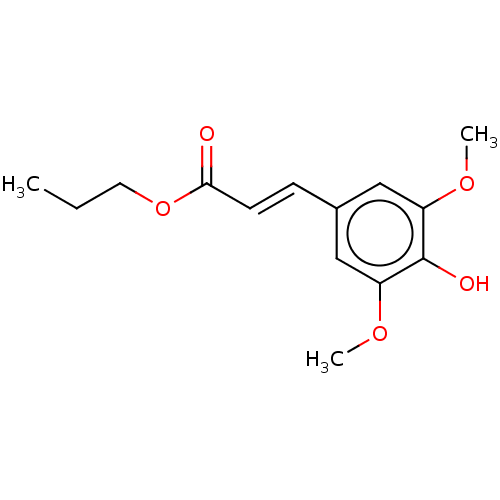 (CHEMBL5084822) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 710 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of 5-LO in thapsigargin stimulated human PMNL cells assessed as reduction in 5-LO product level preincubated for 5 mins followed by thapsi... | Citation and Details Article DOI: 10.1021/acs.jnatprod.1c00982 BindingDB Entry DOI: 10.7270/Q2W099TK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50277009 ((E)-(1-(2-((E)-3-(3,4-dihydroxyphenyl)acryloyloxy)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 740 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Moncton Curated by ChEMBL | Assay Description Inhibition of human 5LOX expressed in HEK293 cells | Bioorg Med Chem Lett 19: 1118-21 (2009) Article DOI: 10.1016/j.bmcl.2008.12.108 BindingDB Entry DOI: 10.7270/Q21R6QCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50277010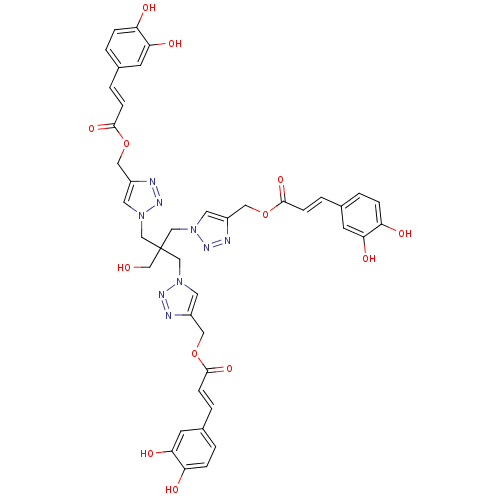 ((2E,2'E)-(1,1'-(2-((4-(((E)-3-(3,4-dihydroxyphenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 790 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Moncton Curated by ChEMBL | Assay Description Inhibition of human 5LOX expressed in HEK293 cells | Bioorg Med Chem Lett 19: 1118-21 (2009) Article DOI: 10.1016/j.bmcl.2008.12.108 BindingDB Entry DOI: 10.7270/Q21R6QCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, membrane associated (Homo sapiens (Human)) | BDBM23762 (3-({4-[(4-octadecylpiperazin-1-yl)carbonyl]phenyl}...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Universite Paris 7-Denis Diderot | Assay Description PLA2 activity is by using the fluorescent phospholipid analogue, beta-pyC-10-PG as the substrate. The increase in fluorescence (ex @342 nm and em@388... | Bioorg Med Chem 16: 1242-53 (2008) Article DOI: 10.1016/j.bmc.2007.10.077 BindingDB Entry DOI: 10.7270/Q2J964PK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50530376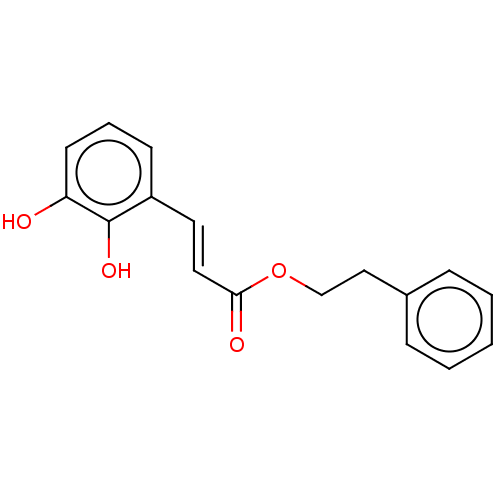 (CHEMBL4582170) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 890 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Moncton Curated by ChEMBL | Assay Description Inhibition of 5-LO in human PMNL cells assessed as reduction in leukotriene formation preincubated for 5 mins followed by thapsigargin stimulation me... | Eur J Med Chem 179: 347-357 (2019) Article DOI: 10.1016/j.ejmech.2019.06.060 BindingDB Entry DOI: 10.7270/Q2125X31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 226 total ) | Next | Last >> |