

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50005799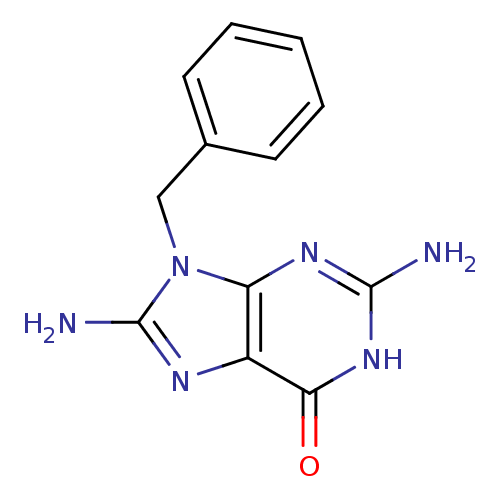 (2,8-Diamino-9-benzyl-1,9-dihydro-purin-6-one | CHE...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Defense Medical Center Curated by ChEMBL | Assay Description PNP activity in human RBC | J Med Chem 36: 1024-31 (1993) BindingDB Entry DOI: 10.7270/Q2CV4JCQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50368825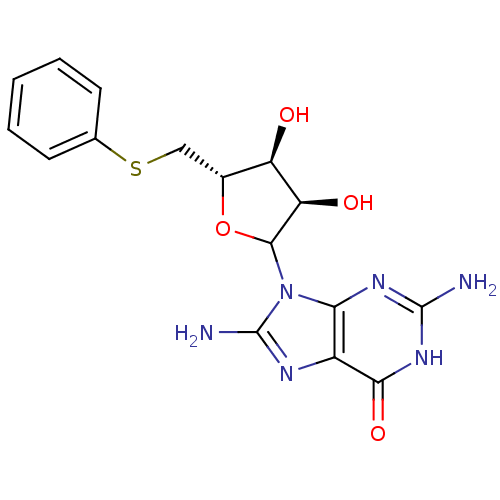 (CHEMBL603291) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Defense Medical Center Curated by ChEMBL | Assay Description PNP activity in human RBC | J Med Chem 36: 1024-31 (1993) BindingDB Entry DOI: 10.7270/Q2CV4JCQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50022243 (2,8-Diamino-1,9-dihydro-purin-6-one | 8-AMINOGUANI...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid UniChem Patents Similars | PubMed | 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Defense Medical Center Curated by ChEMBL | Assay Description PNP activity in human RBC | J Med Chem 36: 1024-31 (1993) BindingDB Entry DOI: 10.7270/Q2CV4JCQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50368823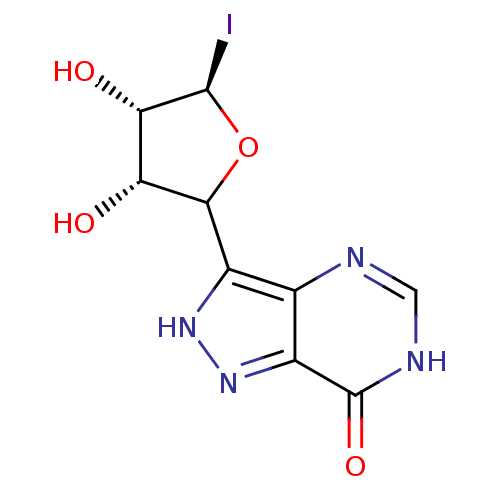 (CHEMBL606329) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Defense Medical Center Curated by ChEMBL | Assay Description PNP activity in human RBC | J Med Chem 36: 1024-31 (1993) BindingDB Entry DOI: 10.7270/Q2CV4JCQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50404028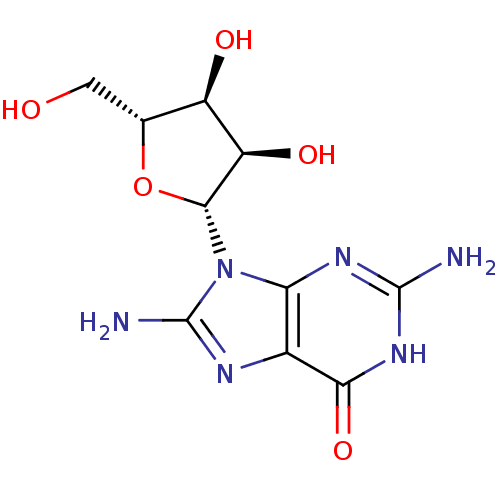 (CHEMBL2021376) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Defense Medical Center Curated by ChEMBL | Assay Description Compound was evaluated for the inhibitory activity against PNP activity in dialyzed extracts from human erythrocytes | J Med Chem 36: 1024-31 (1993) BindingDB Entry DOI: 10.7270/Q2CV4JCQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50046864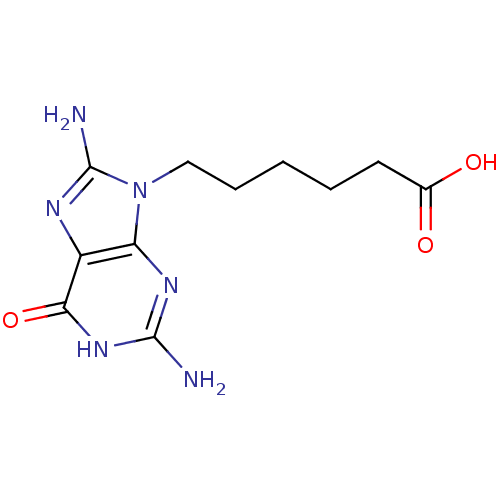 (6-(2,8-Diamino-6-oxo-1,6-dihydro-purin-9-yl)-hexan...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Defense Medical Center Curated by ChEMBL | Assay Description Compound was evaluated for the inhibitory activity against PNP activity in dialyzed extracts from human erythrocytes | J Med Chem 36: 1024-31 (1993) BindingDB Entry DOI: 10.7270/Q2CV4JCQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50046869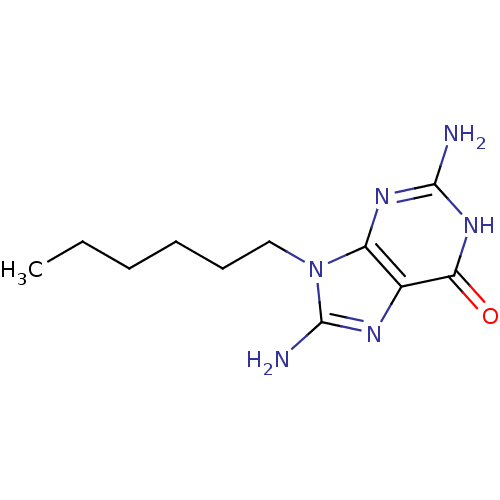 (2,8-Diamino-9-hexyl-1,9-dihydro-purin-6-one | CHEM...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.24E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Defense Medical Center Curated by ChEMBL | Assay Description Compound was evaluated for the inhibitory activity against PNP activity in dialyzed extracts from human erythrocytes | J Med Chem 36: 1024-31 (1993) BindingDB Entry DOI: 10.7270/Q2CV4JCQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50207253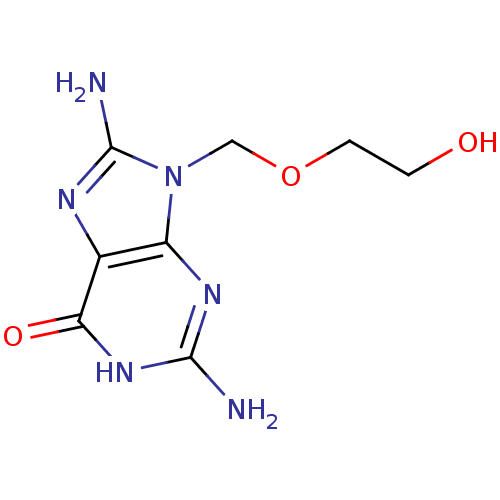 (2,8-Diamino-9-(2-hydroxy-ethoxymethyl)-1,9-dihydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.34E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Defense Medical Center Curated by ChEMBL | Assay Description PNP activity in human RBC | J Med Chem 36: 1024-31 (1993) BindingDB Entry DOI: 10.7270/Q2CV4JCQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50046867 (2-Amino-9-(2-iodo-ethoxymethyl)-1,9-dihydro-purin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Defense Medical Center Curated by ChEMBL | Assay Description PNP activity in human RBC | J Med Chem 36: 1024-31 (1993) BindingDB Entry DOI: 10.7270/Q2CV4JCQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50207401 (2-Amino-8-bromo-9-(2-hydroxy-ethoxymethyl)-1,9-dih...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.87E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Defense Medical Center Curated by ChEMBL | Assay Description PNP activity in human RBC | J Med Chem 36: 1024-31 (1993) BindingDB Entry DOI: 10.7270/Q2CV4JCQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50046868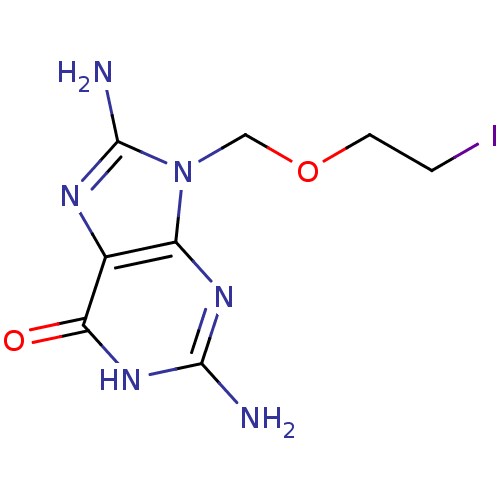 (2,8-Diamino-9-(2-iodo-ethoxymethyl)-1,9-dihydro-pu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Defense Medical Center Curated by ChEMBL | Assay Description PNP activity in human RBC | J Med Chem 36: 1024-31 (1993) BindingDB Entry DOI: 10.7270/Q2CV4JCQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50368828 (CHEMBL603290) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Defense Medical Center Curated by ChEMBL | Assay Description PNP activity in human RBC | J Med Chem 36: 1024-31 (1993) BindingDB Entry DOI: 10.7270/Q2CV4JCQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50368824 (CHEMBL606113) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Defense Medical Center Curated by ChEMBL | Assay Description PNP activity in human RBC | J Med Chem 36: 1024-31 (1993) BindingDB Entry DOI: 10.7270/Q2CV4JCQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50368827 (CHEMBL606330) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Defense Medical Center Curated by ChEMBL | Assay Description PNP activity in human RBC | J Med Chem 36: 1024-31 (1993) BindingDB Entry DOI: 10.7270/Q2CV4JCQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50367285 (CHEMBL603183) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Defense Medical Center Curated by ChEMBL | Assay Description PNP activity in human RBC | J Med Chem 36: 1024-31 (1993) BindingDB Entry DOI: 10.7270/Q2CV4JCQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50039559 (CHEMBL267803 | [5-(2-Amino-6-oxo-1,6-dihydro-purin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Defense Medical Center Curated by ChEMBL | Assay Description PNP activity in human RBC | J Med Chem 36: 1024-31 (1993) BindingDB Entry DOI: 10.7270/Q2CV4JCQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50021776 (2-Amino-9-(2-hydroxy-ethoxymethyl)-5,9-dihydro-pur...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 1.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Defense Medical Center Curated by ChEMBL | Assay Description PNP activity in human RBC | J Med Chem 36: 1024-31 (1993) BindingDB Entry DOI: 10.7270/Q2CV4JCQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50368826 (CHEMBL603298) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Defense Medical Center Curated by ChEMBL | Assay Description PNP activity in human RBC | J Med Chem 36: 1024-31 (1993) BindingDB Entry DOI: 10.7270/Q2CV4JCQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50002692 ((AZT) 1-(4-Azido-5-hydroxymethyl-tetrahydro-furan-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Antiviral activity against Human Immunodeficiency Virus by Reverse transcriptase assay in CEM-SS cells | J Med Chem 41: 1252-62 (1998) Article DOI: 10.1021/jm970559i BindingDB Entry DOI: 10.7270/Q2P26X8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50002692 ((AZT) 1-(4-Azido-5-hydroxymethyl-tetrahydro-furan-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description antiviral activity in Human immunodeficiency virus-1 as reverse transcriptase activity in culture supernatant | J Med Chem 38: 4106-14 (1995) BindingDB Entry DOI: 10.7270/Q25B01HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50031364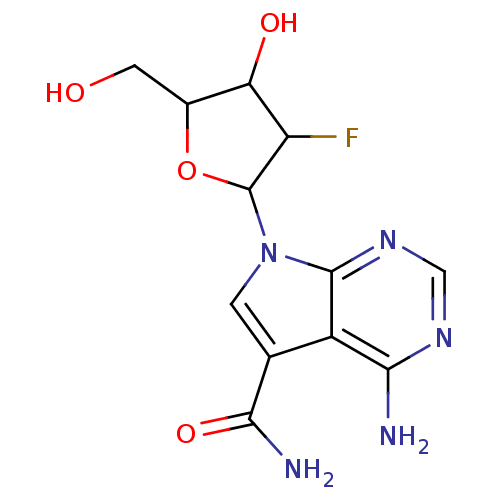 (4-Amino-7-(3-fluoro-4-hydroxy-5-hydroxymethyl-tetr...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description antiviral activity in Human immunodeficiency virus-1 as reverse transcriptase activity in culture supernatant | J Med Chem 38: 4106-14 (1995) BindingDB Entry DOI: 10.7270/Q25B01HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50369541 (CHEMBL608901) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Antiviral activity was evaluated by RT (reverse transcriptase) activity in cells acutely infected with HIV-1 | J Med Chem 43: 2457-63 (2000) BindingDB Entry DOI: 10.7270/Q22N52Z2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50422306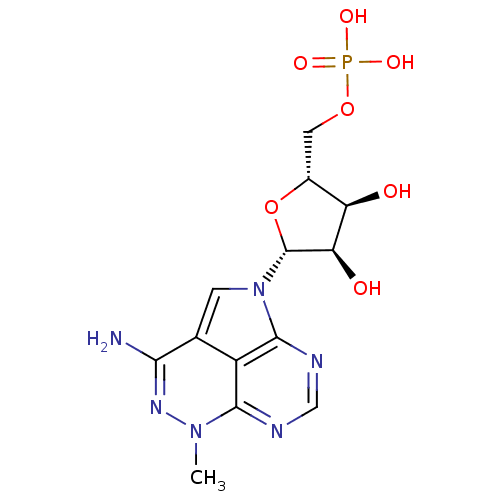 (Phosphate Salt Of Tricyclic Nucleoside | TRICIRIBI...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Antiviral activity was tested in cells acutely infected with HIV-1 RT (reverse transcriptase) | J Med Chem 43: 2438-48 (2000) BindingDB Entry DOI: 10.7270/Q26D5TNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50089379 ((2R,3R,4S,5R)-2-(3-Amino-5-methyl-5H-1,4,5,6,8-pen...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibitory concentration against human immunodeficiency virus type 1 reverse transcriptase | J Med Chem 48: 3840-51 (2005) Article DOI: 10.1021/jm0402014 BindingDB Entry DOI: 10.7270/Q2D50MH7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50369528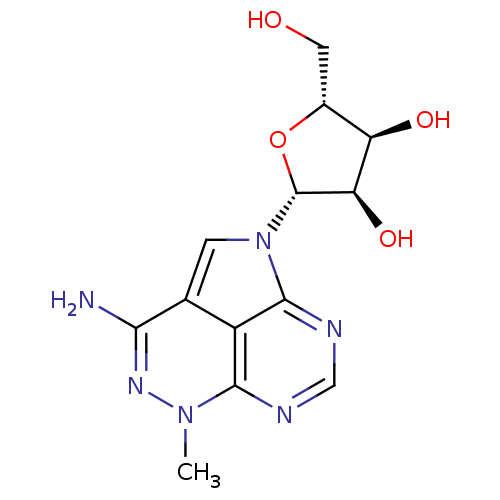 (NSC-154020 | TRICIRIBINE) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Antiviral activity was tested in cells acutely infected with HIV-1 RT (reverse transcriptase) | J Med Chem 43: 2438-48 (2000) BindingDB Entry DOI: 10.7270/Q26D5TNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50369528 (NSC-154020 | TRICIRIBINE) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Antiviral activity was evaluated by RT (reverse transcriptase) activity in cells acutely infected with HIV-1 | J Med Chem 43: 2457-63 (2000) BindingDB Entry DOI: 10.7270/Q22N52Z2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50422306 (Phosphate Salt Of Tricyclic Nucleoside | TRICIRIBI...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Antiviral activity was evaluated by RT (reverse transcriptase) activity in cells acutely infected with HIV-1 | J Med Chem 43: 2457-63 (2000) BindingDB Entry DOI: 10.7270/Q22N52Z2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50031365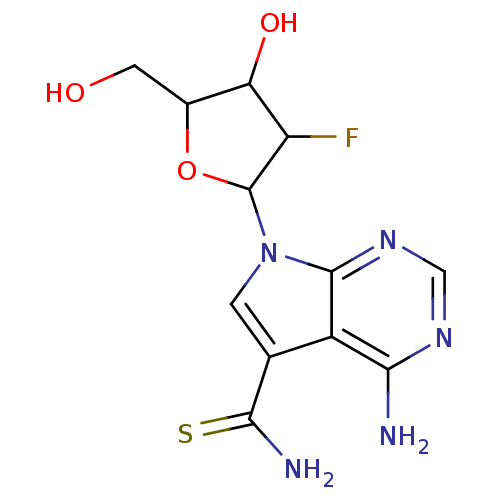 (4-Amino-7-(3-fluoro-4-hydroxy-5-hydroxymethyl-tetr...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description antiviral activity in Human immunodeficiency virus-1 as reverse transcriptase activity in culture supernatant | J Med Chem 38: 4106-14 (1995) BindingDB Entry DOI: 10.7270/Q25B01HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50369542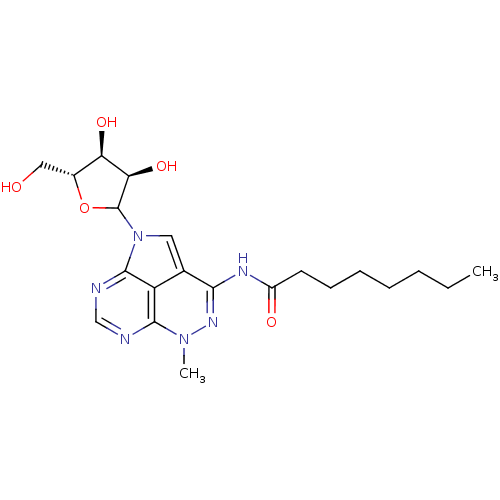 (CHEMBL611850) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Antiviral activity was evaluated by Reverse transcriptase activity in cells acutely infected with HIV-1 | J Med Chem 43: 2457-63 (2000) BindingDB Entry DOI: 10.7270/Q22N52Z2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50369534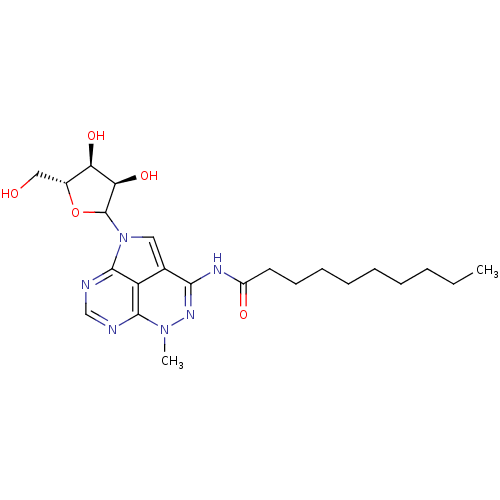 (CHEMBL607723) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Antiviral activity was evaluated by Reverse transcriptase activity in cells acutely infected with HIV-1 | J Med Chem 43: 2457-63 (2000) BindingDB Entry DOI: 10.7270/Q22N52Z2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GMP synthase [glutamine-hydrolyzing] (Mus musculus) | BDBM50042853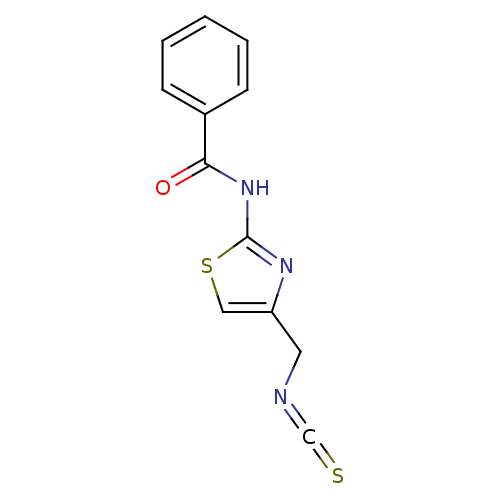 (CHEMBL127366 | N-(4-Isothiocyanatomethyl-thiazol-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description In vitro inhibitory activity against partially purified GMP synthetase in L1210 cells | J Med Chem 36: 3849-52 (1994) BindingDB Entry DOI: 10.7270/Q2W9588K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50369533 (CHEMBL611245) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Antiviral activity was evaluated by RT (reverse transcriptase) activity in cells acutely infected with HIV-1 | J Med Chem 43: 2457-63 (2000) BindingDB Entry DOI: 10.7270/Q22N52Z2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50369536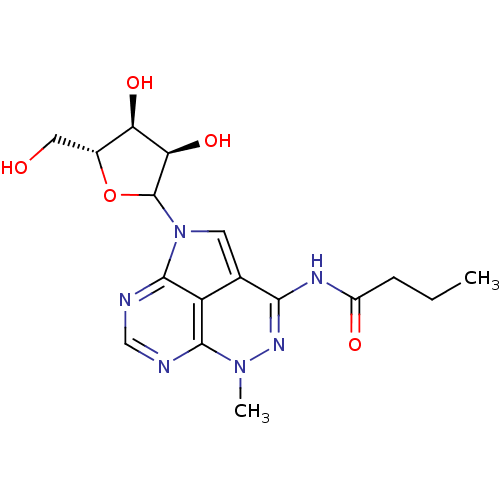 (CHEMBL611242) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Antiviral activity was evaluated by RT (reverse transcriptase) activity in cells acutely infected with HIV-1 | J Med Chem 43: 2457-63 (2000) BindingDB Entry DOI: 10.7270/Q22N52Z2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50369535 (CHEMBL607724) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Antiviral activity was evaluated by Reverse transcriptase activity in cells acutely infected with HIV-1 | J Med Chem 43: 2457-63 (2000) BindingDB Entry DOI: 10.7270/Q22N52Z2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50369540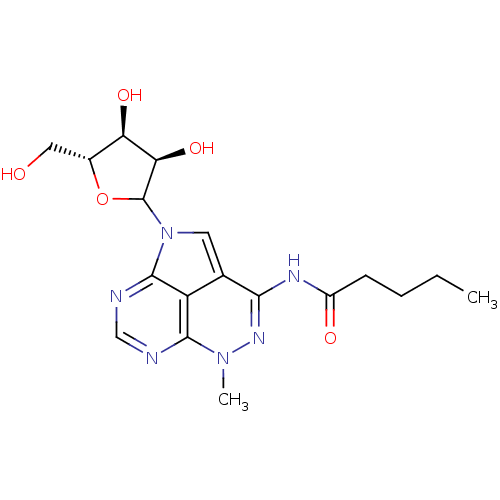 (CHEMBL608301) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Antiviral activity was evaluated by RT (reverse transcriptase) activity in cells acutely infected with HIV-1 | J Med Chem 43: 2457-63 (2000) BindingDB Entry DOI: 10.7270/Q22N52Z2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50369537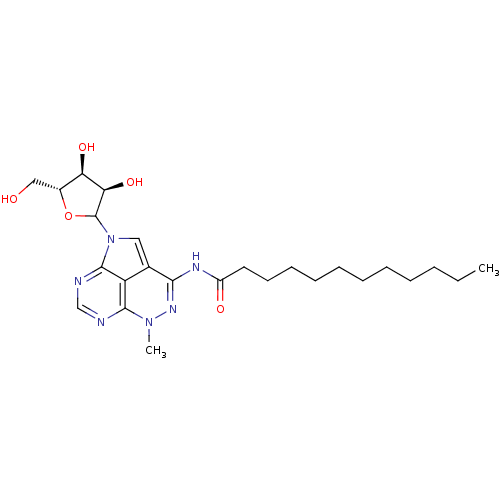 (CHEMBL611243) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Antiviral activity was evaluated by Reverse transcriptase activity in cells acutely infected with HIV-1 | J Med Chem 43: 2457-63 (2000) BindingDB Entry DOI: 10.7270/Q22N52Z2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50168027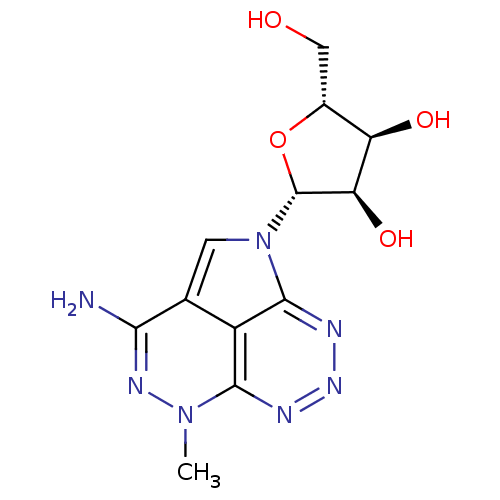 ((2R,3R,4S,5R)-2-(3-Amino-5-methyl-5H-1,4,5,6,7,8-h...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibitory concentration against human immunodeficiency virus type 1 reverse transcriptase | J Med Chem 48: 3840-51 (2005) Article DOI: 10.1021/jm0402014 BindingDB Entry DOI: 10.7270/Q2D50MH7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50369539 (CHEMBL610949) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Antiviral activity was evaluated by RT (reverse transcriptase) activity in cells acutely infected with HIV-1 | J Med Chem 43: 2457-63 (2000) BindingDB Entry DOI: 10.7270/Q22N52Z2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50369532 (CHEMBL610657) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Antiviral activity was evaluated by RT (reverse transcriptase) activity in cells acutely infected with HIV-1 | J Med Chem 43: 2457-63 (2000) BindingDB Entry DOI: 10.7270/Q22N52Z2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50369538 (CHEMBL609191) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Antiviral activity was evaluated by RT (reverse transcriptase) activity in cells acutely infected with HIV-1 | J Med Chem 43: 2457-63 (2000) BindingDB Entry DOI: 10.7270/Q22N52Z2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50063941 (5,6-Dichloro-1-(4-chloro-benzyl)-1H-benzoimidazol-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Antiviral activity against Human Immunodeficiency Virus by Reverse transcriptase (RT) assay in CEM-SS cells | J Med Chem 41: 1252-62 (1998) Article DOI: 10.1021/jm970559i BindingDB Entry DOI: 10.7270/Q2P26X8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50407560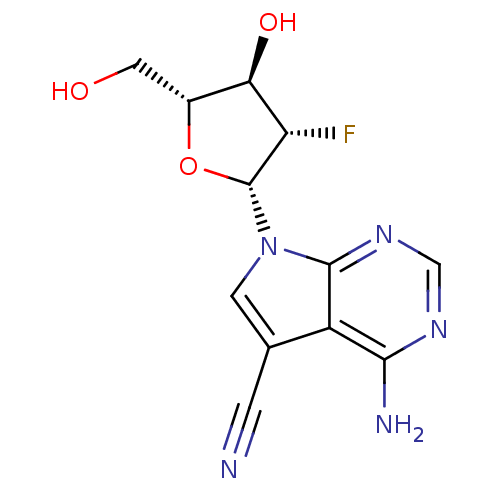 (CHEMBL473686) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description antiviral activity in Human immunodeficiency virus-1 as reverse transcriptase activity in culture supernatant | J Med Chem 38: 4106-14 (1995) BindingDB Entry DOI: 10.7270/Q25B01HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50407562 (CHEMBL2112687) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description antiviral activity in Human immunodeficiency virus-1 as reverse transcriptase activity in culture supernatant | J Med Chem 38: 4106-14 (1995) BindingDB Entry DOI: 10.7270/Q25B01HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50063977 (5,6-Dichloro-1-(4-trifluoromethyl-benzyl)-1H-benzo...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Antiviral activity against Human Immunodeficiency Virus by Reverse transcriptase assay in CEM-SS cells | J Med Chem 41: 1252-62 (1998) Article DOI: 10.1021/jm970559i BindingDB Entry DOI: 10.7270/Q2P26X8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50408925 (CHEMBL2112098) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Antiviral activity was tested in cells acutely infected with HIV-1 RT (reverse transcriptase) | J Med Chem 43: 2438-48 (2000) BindingDB Entry DOI: 10.7270/Q26D5TNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50407563 (CHEMBL2111874) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description antiviral activity in Human immunodeficiency virus-1 as reverse transcriptase activity in culture supernatant | J Med Chem 38: 4106-14 (1995) BindingDB Entry DOI: 10.7270/Q25B01HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50063948 (2-Bromo-5,6-dichloro-1-(3-nitro-benzyl)-1H-benzoim...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Antiviral activity against Human Immunodeficiency Virus by Reverse transcriptase (RT) assay in CEM-SS cells. | J Med Chem 41: 1252-62 (1998) Article DOI: 10.1021/jm970559i BindingDB Entry DOI: 10.7270/Q2P26X8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50063945 (2-Bromo-5,6-dichloro-1-(3-methyl-benzyl)-1H-benzoi...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Antiviral activity against Human Immunodeficiency Virus by Reverse transcriptase assay in CEM-SS cells | J Med Chem 41: 1252-62 (1998) Article DOI: 10.1021/jm970559i BindingDB Entry DOI: 10.7270/Q2P26X8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Solute carrier family 15 member 1 (Homo sapiens (Human)) | BDBM50370614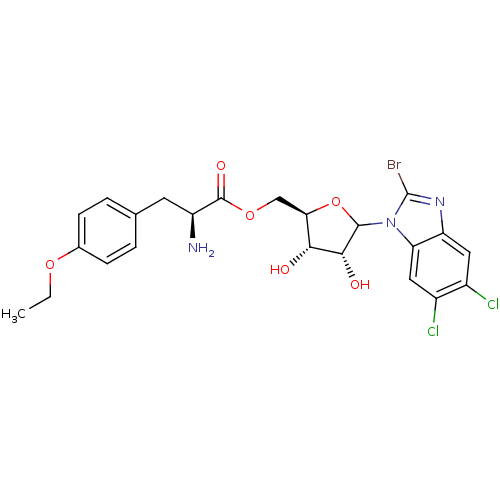 (CHEMBL608788) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibitory concentration against [3H]glycylsarcosine uptake in HeLa cells expressing human Intestinal peptide transporter PepT1 | J Med Chem 48: 1274-7 (2005) Article DOI: 10.1021/jm049450i BindingDB Entry DOI: 10.7270/Q2348M46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50063968 (1-(3,5-Bis-trifluoromethyl-benzyl)-2-bromo-5,6-dic...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Antiviral activity against Human Immunodeficiency Virus by Reverse transcriptase assay in CEM-SS cells | J Med Chem 41: 1252-62 (1998) Article DOI: 10.1021/jm970559i BindingDB Entry DOI: 10.7270/Q2P26X8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 75 total ) | Next | Last >> |