 Found 820 hits with Last Name = 'traeger' and Initial = 's'
Found 820 hits with Last Name = 'traeger' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Androgen receptor
(Homo sapiens (Human)) | BDBM50204245

(CHEMBL3898284)Show SMILES [H][C@@]12C[C@@]([H])(C=C1)[C@]1(C)[C@@]2([H])CN(c2ccc(C#N)c(c2)C(F)(F)F)S1(=O)=O |r,c:5| Show InChI InChI=1S/C17H15F3N2O2S/c1-16-12-4-2-10(6-12)15(16)9-22(25(16,23)24)13-5-3-11(8-21)14(7-13)17(18,19)20/h2-5,7,10,12,15H,6,9H2,1H3/t10-,12+,15-,16+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from androgen receptor in human MDA-MB-453 cells |
Bioorg Med Chem Lett 26: 5707-5711 (2016)
Article DOI: 10.1016/j.bmcl.2016.10.059
BindingDB Entry DOI: 10.7270/Q2PK0J43 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50204241

(CHEMBL3917372)Show SMILES [H][C@]12CC[C@]([H])(C1)[C@]1([H])[C@@]2([H])CN(c2ccc(C#N)c(c2)C(F)(F)F)S1(=O)=O |r| Show InChI InChI=1S/C16H15F3N2O2S/c17-16(18,19)14-6-12(4-3-11(14)7-20)21-8-13-9-1-2-10(5-9)15(13)24(21,22)23/h3-4,6,9-10,13,15H,1-2,5,8H2/t9-,10+,13-,15+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from androgen receptor in human MDA-MB-453 cells |
Bioorg Med Chem Lett 26: 5707-5711 (2016)
Article DOI: 10.1016/j.bmcl.2016.10.059
BindingDB Entry DOI: 10.7270/Q2PK0J43 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50204243

(CHEMBL3926358)Show SMILES [H][C@]12CC[C@]([H])(C1)[C@]1(C)[C@@]2([H])CN(c2ccc(C#N)c(c2)C(F)(F)F)S1(=O)=O |r| Show InChI InChI=1S/C17H17F3N2O2S/c1-16-12-4-2-10(6-12)15(16)9-22(25(16,23)24)13-5-3-11(8-21)14(7-13)17(18,19)20/h3,5,7,10,12,15H,2,4,6,9H2,1H3/t10-,12+,15-,16+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from androgen receptor in human MDA-MB-453 cells |
Bioorg Med Chem Lett 26: 5707-5711 (2016)
Article DOI: 10.1016/j.bmcl.2016.10.059
BindingDB Entry DOI: 10.7270/Q2PK0J43 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50204246

(CHEMBL3893320)Show SMILES [H][C@]12CC[C@]([H])(C1)[C@]1(C(N)=O)[C@@]2([H])CN(c2ccc(C#N)c(c2)C(F)(F)F)S1(=O)=O |r| Show InChI InChI=1S/C17H16F3N3O3S/c18-17(19,20)13-6-12(4-2-10(13)7-21)23-8-14-9-1-3-11(5-9)16(14,15(22)24)27(23,25)26/h2,4,6,9,11,14H,1,3,5,8H2,(H2,22,24)/t9-,11+,14-,16+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from androgen receptor in human MDA-MB-453 cells |
Bioorg Med Chem Lett 26: 5707-5711 (2016)
Article DOI: 10.1016/j.bmcl.2016.10.059
BindingDB Entry DOI: 10.7270/Q2PK0J43 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50204247
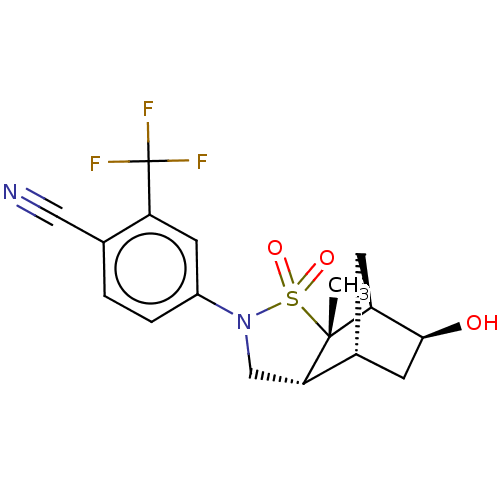
(CHEMBL3920247)Show SMILES [H][C@]12C[C@H](O)[C@]([H])(C1)[C@]1(C)[C@@]2([H])CN(c2ccc(C#N)c(c2)C(F)(F)F)S1(=O)=O |r| Show InChI InChI=1S/C17H17F3N2O3S/c1-16-13-4-10(5-15(13)23)14(16)8-22(26(16,24)25)11-3-2-9(7-21)12(6-11)17(18,19)20/h2-3,6,10,13-15,23H,4-5,8H2,1H3/t10-,13+,14+,15+,16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from androgen receptor in human MDA-MB-453 cells |
Bioorg Med Chem Lett 26: 5707-5711 (2016)
Article DOI: 10.1016/j.bmcl.2016.10.059
BindingDB Entry DOI: 10.7270/Q2PK0J43 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50204251
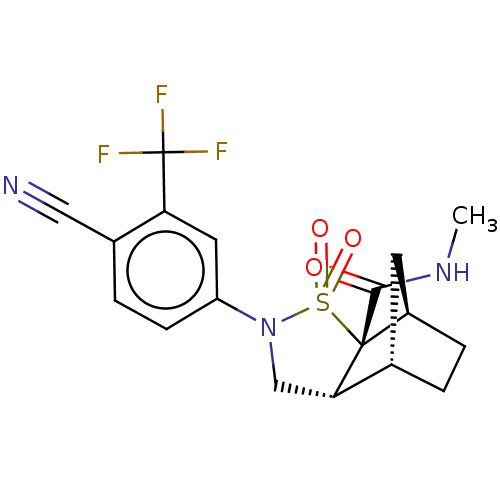
(CHEMBL3921315)Show SMILES [H][C@]12CC[C@]([H])(C1)[C@]1(C(=O)NC)[C@@]2([H])CN(c2ccc(C#N)c(c2)C(F)(F)F)S1(=O)=O |r| Show InChI InChI=1S/C18H18F3N3O3S/c1-23-16(25)17-12-4-2-10(6-12)15(17)9-24(28(17,26)27)13-5-3-11(8-22)14(7-13)18(19,20)21/h3,5,7,10,12,15H,2,4,6,9H2,1H3,(H,23,25)/t10-,12+,15-,17+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from androgen receptor in human MDA-MB-453 cells |
Bioorg Med Chem Lett 26: 5707-5711 (2016)
Article DOI: 10.1016/j.bmcl.2016.10.059
BindingDB Entry DOI: 10.7270/Q2PK0J43 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50204242

(CHEMBL3902310)Show SMILES [H][C@]12C[C@H](F)[C@]([H])(C1)[C@]1(C(=O)NC)[C@@]2([H])CN(c2ccc(C#N)c(c2)C(F)(F)F)S1(=O)=O |r| Show InChI InChI=1S/C18H17F4N3O3S/c1-24-16(26)17-13-4-10(5-15(13)19)14(17)8-25(29(17,27)28)11-3-2-9(7-23)12(6-11)18(20,21)22/h2-3,6,10,13-15H,4-5,8H2,1H3,(H,24,26)/t10-,13+,14+,15+,17-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from androgen receptor in human MDA-MB-453 cells |
Bioorg Med Chem Lett 26: 5707-5711 (2016)
Article DOI: 10.1016/j.bmcl.2016.10.059
BindingDB Entry DOI: 10.7270/Q2PK0J43 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50204250

(CHEMBL3949176)Show SMILES [H][C@]12C[C@H](F)[C@]([H])(C1)[C@]1(C)[C@@]2([H])CN(c2ccc(C#N)c(c2)C(F)(F)F)S1(=O)=O |r| Show InChI InChI=1S/C17H16F4N2O2S/c1-16-13-4-10(5-15(13)18)14(16)8-23(26(16,24)25)11-3-2-9(7-22)12(6-11)17(19,20)21/h2-3,6,10,13-15H,4-5,8H2,1H3/t10-,13+,14+,15+,16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from androgen receptor in human MDA-MB-453 cells |
Bioorg Med Chem Lett 26: 5707-5711 (2016)
Article DOI: 10.1016/j.bmcl.2016.10.059
BindingDB Entry DOI: 10.7270/Q2PK0J43 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50204249

(CHEMBL3935242)Show SMILES [H][C@]12CC[C@]([H])(C1=O)[C@]1(C)[C@@]2([H])CN(c2ccc(C#N)c(c2)C(F)(F)F)S1(=O)=O |r,TLB:7:6:2.3:8.10| Show InChI InChI=1S/C17H15F3N2O3S/c1-16-12-5-4-11(15(12)23)14(16)8-22(26(16,24)25)10-3-2-9(7-21)13(6-10)17(18,19)20/h2-3,6,11-12,14H,4-5,8H2,1H3/t11-,12-,14+,16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from androgen receptor in human MDA-MB-453 cells |
Bioorg Med Chem Lett 26: 5707-5711 (2016)
Article DOI: 10.1016/j.bmcl.2016.10.059
BindingDB Entry DOI: 10.7270/Q2PK0J43 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50204248
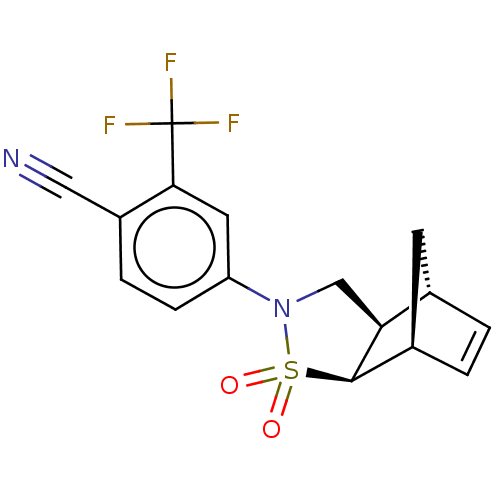
(CHEMBL3987109)Show SMILES [H][C@@]12C[C@@]([H])(C=C1)[C@]1([H])[C@@]2([H])CN(c2ccc(C#N)c(c2)C(F)(F)F)S1(=O)=O |r,c:5| Show InChI InChI=1S/C16H13F3N2O2S/c17-16(18,19)14-6-12(4-3-11(14)7-20)21-8-13-9-1-2-10(5-9)15(13)24(21,22)23/h1-4,6,9-10,13,15H,5,8H2/t9-,10+,13-,15+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from androgen receptor in human MDA-MB-453 cells |
Bioorg Med Chem Lett 26: 5707-5711 (2016)
Article DOI: 10.1016/j.bmcl.2016.10.059
BindingDB Entry DOI: 10.7270/Q2PK0J43 |
More data for this
Ligand-Target Pair | |
ATP-binding cassette sub-family C member 9/ATP-sensitive inward rectifier potassium channel 11
(Homo sapiens (Human)) | BDBM50062397
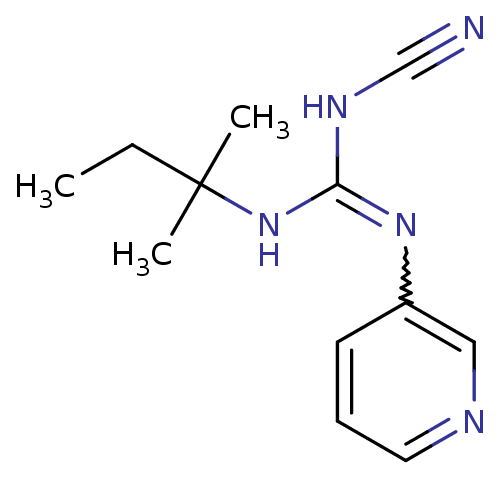
(3N-cyanoimino(tert-pentylamino)methyl-3-pyridinami...)Show InChI InChI=1S/C12H17N5/c1-4-12(2,3)17-11(15-9-13)16-10-6-5-7-14-8-10/h5-8H,4H2,1-3H3,(H2,15,16,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity was determined by displacement of [3H]-P1075 from its binding sites in canine cardiac membranes |
J Med Chem 41: 271-5 (1998)
Article DOI: 10.1021/jm970762d
BindingDB Entry DOI: 10.7270/Q2FN159P |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50204240

(CHEMBL3939272)Show SMILES [H][C@@]12C[C@@H](O)[C@@]([H])(C1)[C@]1([H])CN(c3ccc(C#N)c(c3)C(F)(F)F)S(=O)(=O)[C@]21C |r| Show InChI InChI=1S/C17H17F3N2O3S/c1-16-10-4-12(15(23)5-10)14(16)8-22(26(16,24)25)11-3-2-9(7-21)13(6-11)17(18,19)20/h2-3,6,10,12,14-15,23H,4-5,8H2,1H3/t10-,12-,14-,15+,16+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from androgen receptor in human MDA-MB-453 cells |
Bioorg Med Chem Lett 26: 5707-5711 (2016)
Article DOI: 10.1016/j.bmcl.2016.10.059
BindingDB Entry DOI: 10.7270/Q2PK0J43 |
More data for this
Ligand-Target Pair | |
ATP-binding cassette sub-family C member 9/ATP-sensitive inward rectifier potassium channel 11
(Homo sapiens (Human)) | BDBM50062402

(1N-cyanoimino(tert-pentylamino)methyl-3-azido-4-io...)Show SMILES CCC(C)(C)NC(Nc1ccc(I)c(c1)N=[N+]=[N-])=NC#N |w:18.19| Show InChI InChI=1S/C13H16IN7/c1-4-13(2,3)19-12(17-8-15)18-9-5-6-10(14)11(7-9)20-21-16/h5-7H,4H2,1-3H3,(H2,17,18,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity was determined by displacement of [3H]-P1075 from its binding sites in canine cardiac membranes |
J Med Chem 41: 271-5 (1998)
Article DOI: 10.1021/jm970762d
BindingDB Entry DOI: 10.7270/Q2FN159P |
More data for this
Ligand-Target Pair | |
ATP-binding cassette sub-family C member 9/ATP-sensitive inward rectifier potassium channel 11
(Homo sapiens (Human)) | BDBM50062394
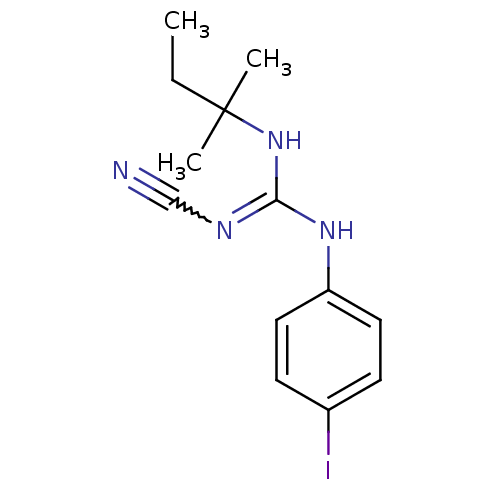
(1N-cyanoimino(tert-pentylamino)methyl-4-iodoanilin...)Show InChI InChI=1S/C13H17IN4/c1-4-13(2,3)18-12(16-9-15)17-11-7-5-10(14)6-8-11/h5-8H,4H2,1-3H3,(H2,16,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 74 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity was determined by displacement of [3H]-P1075 from its binding sites in canine cardiac membranes |
J Med Chem 41: 271-5 (1998)
Article DOI: 10.1021/jm970762d
BindingDB Entry DOI: 10.7270/Q2FN159P |
More data for this
Ligand-Target Pair | |
ATP-binding cassette sub-family C member 9/ATP-sensitive inward rectifier potassium channel 11
(Homo sapiens (Human)) | BDBM50062396
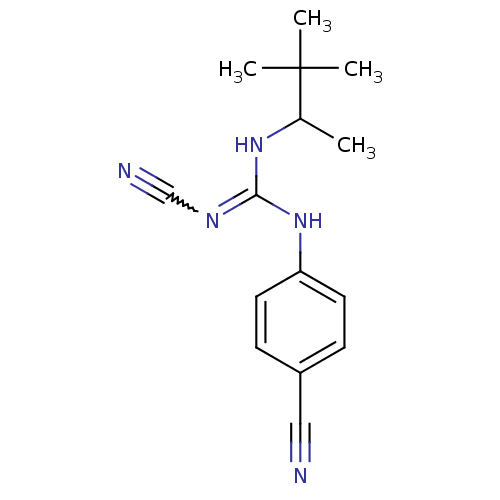
(4-cyanoimino(1,2,2-trimethylpropylamino)methylamin...)Show SMILES CC(NC(Nc1ccc(cc1)C#N)=NC#N)C(C)(C)C |w:13.14| Show InChI InChI=1S/C15H19N5/c1-11(15(2,3)4)19-14(18-10-17)20-13-7-5-12(9-16)6-8-13/h5-8,11H,1-4H3,(H2,18,19,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 99 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity was determined by displacement of [3H]-P1075 from its binding sites in canine cardiac membranes |
J Med Chem 41: 271-5 (1998)
Article DOI: 10.1021/jm970762d
BindingDB Entry DOI: 10.7270/Q2FN159P |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50204244

(CHEMBL3907304)Show SMILES [H][C@]12CC[C@]([H])(C1O)[C@]1(C)[C@@]2([H])CN(c2ccc(C#N)c(c2)C(F)(F)F)S1(=O)=O |r,TLB:7:6:2.3:8.10| Show InChI InChI=1S/C17H17F3N2O3S/c1-16-12-5-4-11(15(12)23)14(16)8-22(26(16,24)25)10-3-2-9(7-21)13(6-10)17(18,19)20/h2-3,6,11-12,14-15,23H,4-5,8H2,1H3/t11-,12-,14+,15?,16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 106 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from androgen receptor in human MDA-MB-453 cells |
Bioorg Med Chem Lett 26: 5707-5711 (2016)
Article DOI: 10.1016/j.bmcl.2016.10.059
BindingDB Entry DOI: 10.7270/Q2PK0J43 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50204244

(CHEMBL3907304)Show SMILES [H][C@]12CC[C@]([H])(C1O)[C@]1(C)[C@@]2([H])CN(c2ccc(C#N)c(c2)C(F)(F)F)S1(=O)=O |r,TLB:7:6:2.3:8.10| Show InChI InChI=1S/C17H17F3N2O3S/c1-16-12-5-4-11(15(12)23)14(16)8-22(26(16,24)25)10-3-2-9(7-21)13(6-10)17(18,19)20/h2-3,6,11-12,14-15,23H,4-5,8H2,1H3/t11-,12-,14+,15?,16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 106 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from androgen receptor in human MDA-MB-453 cells |
Bioorg Med Chem Lett 26: 5707-5711 (2016)
Article DOI: 10.1016/j.bmcl.2016.10.059
BindingDB Entry DOI: 10.7270/Q2PK0J43 |
More data for this
Ligand-Target Pair | |
ATP-binding cassette sub-family C member 9/ATP-sensitive inward rectifier potassium channel 11
(Homo sapiens (Human)) | BDBM50012957
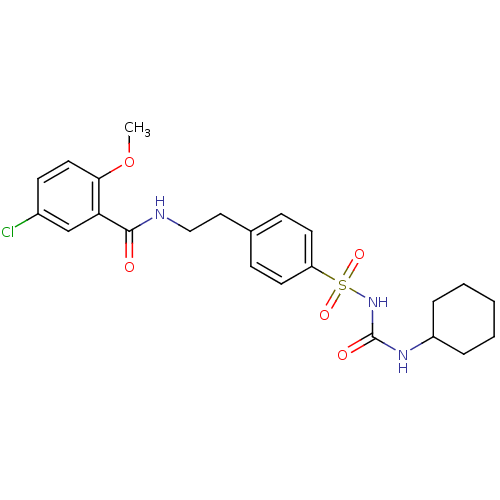
(1-((p-(2-(5-chloro-o-anisamido)ethyl)phenyl)sulfon...)Show SMILES COc1ccc(Cl)cc1C(=O)NCCc1ccc(cc1)S(=O)(=O)NC(=O)NC1CCCCC1 Show InChI InChI=1S/C23H28ClN3O5S/c1-32-21-12-9-17(24)15-20(21)22(28)25-14-13-16-7-10-19(11-8-16)33(30,31)27-23(29)26-18-5-3-2-4-6-18/h7-12,15,18H,2-6,13-14H2,1H3,(H,25,28)(H2,26,27,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity was determined by displacement of [3H]-P1075 from its binding sites in canine cardiac membranes |
J Med Chem 41: 271-5 (1998)
Article DOI: 10.1021/jm970762d
BindingDB Entry DOI: 10.7270/Q2FN159P |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
ATP-binding cassette sub-family C member 9/ATP-sensitive inward rectifier potassium channel 11
(Homo sapiens (Human)) | BDBM50062398

(4-cyanoimino(tert-pentylamino)methylaminobenzonitr...)Show InChI InChI=1S/C14H17N5/c1-4-14(2,3)19-13(17-10-16)18-12-7-5-11(9-15)6-8-12/h5-8H,4H2,1-3H3,(H2,17,18,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity was determined by displacement of [3H]-P1075 from its binding sites in canine cardiac membranes |
J Med Chem 41: 271-5 (1998)
Article DOI: 10.1021/jm970762d
BindingDB Entry DOI: 10.7270/Q2FN159P |
More data for this
Ligand-Target Pair | |
ATP-binding cassette sub-family C member 9/ATP-sensitive inward rectifier potassium channel 11
(Homo sapiens (Human)) | BDBM50240750

(()-N-Cyano-N'-4-pyridinyl-N''-(1,2,2-trimethylprop...)Show InChI InChI=1S/C13H19N5/c1-10(13(2,3)4)17-12(16-9-14)18-11-5-7-15-8-6-11/h5-8,10H,1-4H3,(H2,15,16,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity was determined by displacement of [3H]-P1075 from its binding sites in canine cardiac membranes |
J Med Chem 41: 271-5 (1998)
Article DOI: 10.1021/jm970762d
BindingDB Entry DOI: 10.7270/Q2FN159P |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50089354
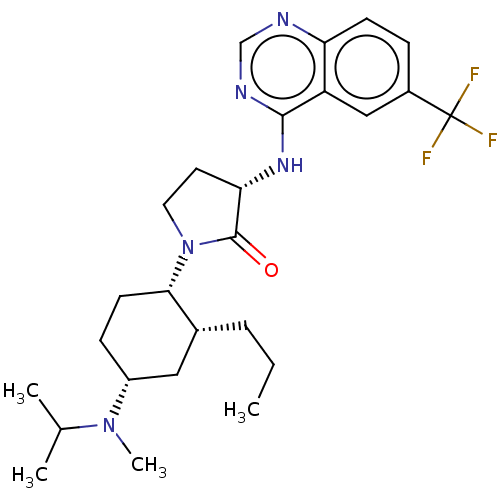
(CHEMBL3577945)Show SMILES CCC[C@@H]1C[C@@H](CC[C@@H]1N1CC[C@H](Nc2ncnc3ccc(cc23)C(F)(F)F)C1=O)N(C)C(C)C |r| Show InChI InChI=1S/C26H36F3N5O/c1-5-6-17-13-19(33(4)16(2)3)8-10-23(17)34-12-11-22(25(34)35)32-24-20-14-18(26(27,28)29)7-9-21(20)30-15-31-24/h7,9,14-17,19,22-23H,5-6,8,10-13H2,1-4H3,(H,30,31,32)/t17-,19-,22+,23+/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 341 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of muscarinic acetylcholine receptor M1 (unknown origin) |
ACS Med Chem Lett 6: 439-44 (2015)
Article DOI: 10.1021/ml500505q
BindingDB Entry DOI: 10.7270/Q2668FWM |
More data for this
Ligand-Target Pair | |
ATP-binding cassette sub-family C member 9/ATP-sensitive inward rectifier potassium channel 11
(Homo sapiens (Human)) | BDBM50062393
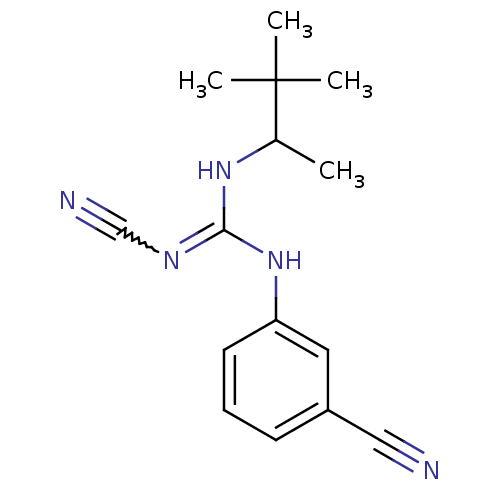
(3-cyanoimino(1,2,2-trimethylpropylamino)methylamin...)Show SMILES CC(NC(Nc1cccc(c1)C#N)=NC#N)C(C)(C)C |w:13.14| Show InChI InChI=1S/C15H19N5/c1-11(15(2,3)4)19-14(18-10-17)20-13-7-5-6-12(8-13)9-16/h5-8,11H,1-4H3,(H2,18,19,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity was determined by displacement of [3H]-P1075 from its binding sites in canine cardiac membranes |
J Med Chem 41: 271-5 (1998)
Article DOI: 10.1021/jm970762d
BindingDB Entry DOI: 10.7270/Q2FN159P |
More data for this
Ligand-Target Pair | |
ATP-binding cassette sub-family C member 9/ATP-sensitive inward rectifier potassium channel 11
(Homo sapiens (Human)) | BDBM50062399

(4-[tert-butylamino(cyanoimino)methylamino]benzonit...)Show InChI InChI=1S/C13H15N5/c1-13(2,3)18-12(16-9-15)17-11-6-4-10(8-14)5-7-11/h4-7H,1-3H3,(H2,16,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity was determined by displacement of [3H]-P1075 from its binding sites in canine cardiac membranes |
J Med Chem 41: 271-5 (1998)
Article DOI: 10.1021/jm970762d
BindingDB Entry DOI: 10.7270/Q2FN159P |
More data for this
Ligand-Target Pair | |
ATP-binding cassette sub-family C member 9/ATP-sensitive inward rectifier potassium channel 11
(Homo sapiens (Human)) | BDBM50062400

(1N-cyanoimino(1,2,2-trimethylpropylamino)methylani...)Show InChI InChI=1S/C14H20N4/c1-11(14(2,3)4)17-13(16-10-15)18-12-8-6-5-7-9-12/h5-9,11H,1-4H3,(H2,16,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity was determined by displacement of [3H]-P1075 from its binding sites in canine cardiac membranes |
J Med Chem 41: 271-5 (1998)
Article DOI: 10.1021/jm970762d
BindingDB Entry DOI: 10.7270/Q2FN159P |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM50089354

(CHEMBL3577945)Show SMILES CCC[C@@H]1C[C@@H](CC[C@@H]1N1CC[C@H](Nc2ncnc3ccc(cc23)C(F)(F)F)C1=O)N(C)C(C)C |r| Show InChI InChI=1S/C26H36F3N5O/c1-5-6-17-13-19(33(4)16(2)3)8-10-23(17)34-12-11-22(25(34)35)32-24-20-14-18(26(27,28)29)7-9-21(20)30-15-31-24/h7,9,14-17,19,22-23H,5-6,8,10-13H2,1-4H3,(H,30,31,32)/t17-,19-,22+,23+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 865 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of muscarinic acetylcholine receptor M4 (unknown origin) |
ACS Med Chem Lett 6: 439-44 (2015)
Article DOI: 10.1021/ml500505q
BindingDB Entry DOI: 10.7270/Q2668FWM |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50089354

(CHEMBL3577945)Show SMILES CCC[C@@H]1C[C@@H](CC[C@@H]1N1CC[C@H](Nc2ncnc3ccc(cc23)C(F)(F)F)C1=O)N(C)C(C)C |r| Show InChI InChI=1S/C26H36F3N5O/c1-5-6-17-13-19(33(4)16(2)3)8-10-23(17)34-12-11-22(25(34)35)32-24-20-14-18(26(27,28)29)7-9-21(20)30-15-31-24/h7,9,14-17,19,22-23H,5-6,8,10-13H2,1-4H3,(H,30,31,32)/t17-,19-,22+,23+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 4.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of muscarinic acetylcholine receptor M2 (unknown origin) |
ACS Med Chem Lett 6: 439-44 (2015)
Article DOI: 10.1021/ml500505q
BindingDB Entry DOI: 10.7270/Q2668FWM |
More data for this
Ligand-Target Pair | |
ATP-binding cassette sub-family C member 9/ATP-sensitive inward rectifier potassium channel 11
(Homo sapiens (Human)) | BDBM50062392
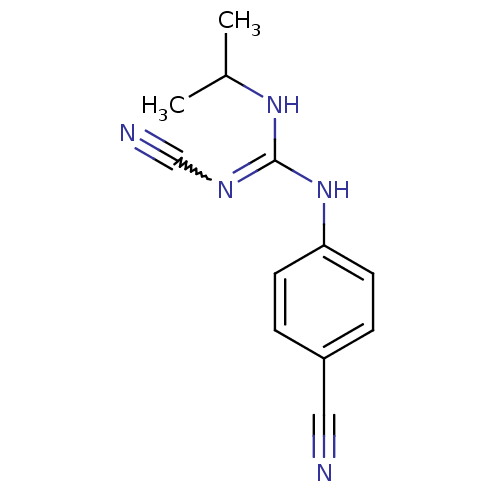
(4-isopropylamino(cyanoimino)methylaminobenzonitril...)Show InChI InChI=1S/C12H13N5/c1-9(2)16-12(15-8-14)17-11-5-3-10(7-13)4-6-11/h3-6,9H,1-2H3,(H2,15,16,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity was determined by displacement of [3H]-P1075 from its binding sites in canine cardiac membranes |
J Med Chem 41: 271-5 (1998)
Article DOI: 10.1021/jm970762d
BindingDB Entry DOI: 10.7270/Q2FN159P |
More data for this
Ligand-Target Pair | |
ATP-binding cassette sub-family C member 9/ATP-sensitive inward rectifier potassium channel 11
(Homo sapiens (Human)) | BDBM50062401

(4-methylamino(cyanoimino)methylaminobenzonitrile |...)Show InChI InChI=1S/C10H9N5/c1-13-10(14-7-12)15-9-4-2-8(6-11)3-5-9/h2-5H,1H3,(H2,13,14,15) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity was determined by displacement of [3H]-P1075 from its binding sites in canine cardiac membranes |
J Med Chem 41: 271-5 (1998)
Article DOI: 10.1021/jm970762d
BindingDB Entry DOI: 10.7270/Q2FN159P |
More data for this
Ligand-Target Pair | |
ATP-binding cassette sub-family C member 9/ATP-sensitive inward rectifier potassium channel 11
(Homo sapiens (Human)) | BDBM50062395
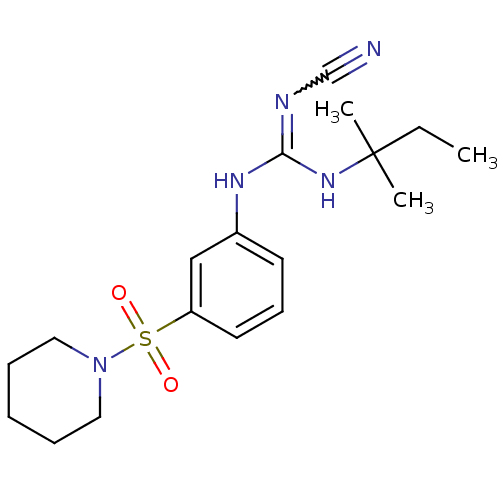
(1N-cyanoimino(tert-pentylamino)methyl-3-hexahydro-...)Show SMILES CCC(C)(C)NC(Nc1cccc(c1)S(=O)(=O)N1CCCCC1)=NC#N |w:23.25| Show InChI InChI=1S/C18H27N5O2S/c1-4-18(2,3)22-17(20-14-19)21-15-9-8-10-16(13-15)26(24,25)23-11-6-5-7-12-23/h8-10,13H,4-7,11-12H2,1-3H3,(H2,20,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity was determined by displacement of [3H]-P1075 from its binding sites in canine cardiac membranes |
J Med Chem 41: 271-5 (1998)
Article DOI: 10.1021/jm970762d
BindingDB Entry DOI: 10.7270/Q2FN159P |
More data for this
Ligand-Target Pair | |
ATP-binding cassette sub-family C member 9/ATP-sensitive inward rectifier potassium channel 11
(Homo sapiens (Human)) | BDBM50062391
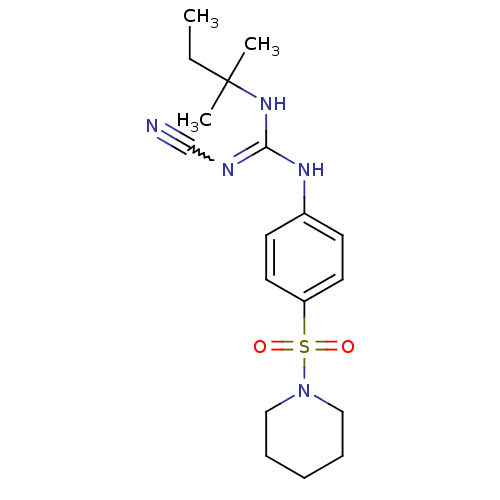
(1N-cyanoimino(tert-pentylamino)methyl-4-hexahydro-...)Show SMILES CCC(C)(C)NC(Nc1ccc(cc1)S(=O)(=O)N1CCCCC1)=NC#N |w:23.25| Show InChI InChI=1S/C18H27N5O2S/c1-4-18(2,3)22-17(20-14-19)21-15-8-10-16(11-9-15)26(24,25)23-12-6-5-7-13-23/h8-11H,4-7,12-13H2,1-3H3,(H2,20,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity was determined by displacement of [3H]-P1075 from its binding sites in canine cardiac membranes |
J Med Chem 41: 271-5 (1998)
Article DOI: 10.1021/jm970762d
BindingDB Entry DOI: 10.7270/Q2FN159P |
More data for this
Ligand-Target Pair | |
ATP-binding cassette sub-family C member 9/ATP-sensitive inward rectifier potassium channel 11
(Homo sapiens (Human)) | BDBM50044253

((3R,4R)-3-Hydroxy-2,2-dimethyl-4-(2-oxo-pyrrolidin...)Show SMILES CC1(C)Oc2ccc(cc2[C@@H]([C@H]1O)N1CCCC1=O)C#N |r| Show InChI InChI=1S/C16H18N2O3/c1-16(2)15(20)14(18-7-3-4-13(18)19)11-8-10(9-17)5-6-12(11)21-16/h5-6,8,14-15,20H,3-4,7H2,1-2H3/t14-,15+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity was determined by displacement of [3H]-P1075 from its binding sites in canine cardiac membranes |
J Med Chem 41: 271-5 (1998)
Article DOI: 10.1021/jm970762d
BindingDB Entry DOI: 10.7270/Q2FN159P |
More data for this
Ligand-Target Pair | |
ATP-binding cassette sub-family C member 9/ATP-sensitive inward rectifier potassium channel 11
(Homo sapiens (Human)) | BDBM50044253

((3R,4R)-3-Hydroxy-2,2-dimethyl-4-(2-oxo-pyrrolidin...)Show SMILES CC1(C)Oc2ccc(cc2[C@@H]([C@H]1O)N1CCCC1=O)C#N |r| Show InChI InChI=1S/C16H18N2O3/c1-16(2)15(20)14(18-7-3-4-13(18)19)11-8-10(9-17)5-6-12(11)21-16/h5-6,8,14-15,20H,3-4,7H2,1-2H3/t14-,15+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity was determined by displacement of [3H]-P1075 from its binding sites in canine cardiac membranes |
J Med Chem 41: 271-5 (1998)
Article DOI: 10.1021/jm970762d
BindingDB Entry DOI: 10.7270/Q2FN159P |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50231346
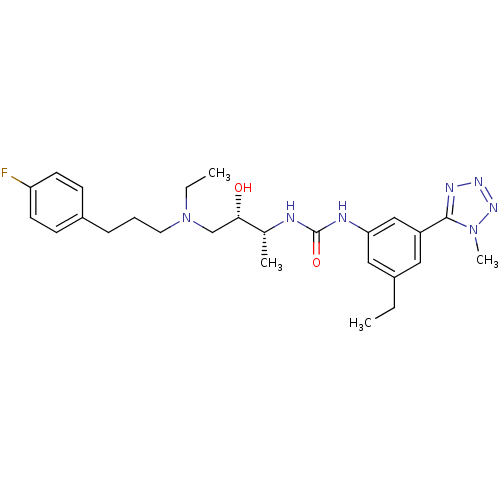
(1-((2R,3S)-4-(ethyl(3-(4-fluorophenyl)propyl)amino...)Show SMILES CCN(CCCc1ccc(F)cc1)C[C@H](O)[C@@H](C)NC(=O)Nc1cc(CC)cc(c1)-c1nnnn1C Show InChI InChI=1S/C26H36FN7O2/c1-5-19-14-21(25-30-31-32-33(25)4)16-23(15-19)29-26(36)28-18(3)24(35)17-34(6-2)13-7-8-20-9-11-22(27)12-10-20/h9-12,14-16,18,24,35H,5-8,13,17H2,1-4H3,(H2,28,29,36)/t18-,24+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR3 receptor assessed as inhibition of chemotaxis in eosinophil |
Bioorg Med Chem Lett 18: 586-95 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.087
BindingDB Entry DOI: 10.7270/Q2FF3S4K |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50231377
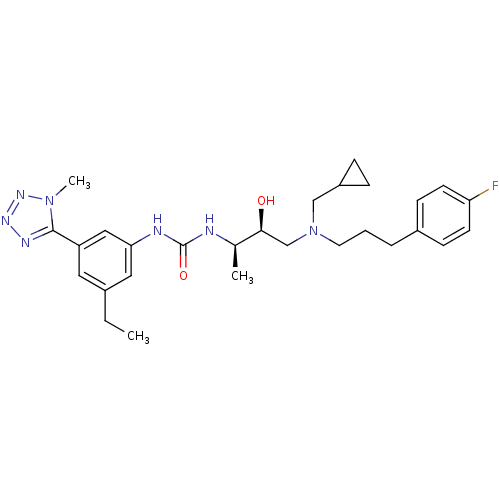
(1-((2R,3S)-4-((cyclopropylmethyl)(3-(4-fluoropheny...)Show SMILES CCc1cc(NC(=O)N[C@H](C)[C@@H](O)CN(CCCc2ccc(F)cc2)CC2CC2)cc(c1)-c1nnnn1C Show InChI InChI=1S/C28H38FN7O2/c1-4-20-14-23(27-32-33-34-35(27)3)16-25(15-20)31-28(38)30-19(2)26(37)18-36(17-22-7-8-22)13-5-6-21-9-11-24(29)12-10-21/h9-12,14-16,19,22,26,37H,4-8,13,17-18H2,1-3H3,(H2,30,31,38)/t19-,26+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR3 receptor assessed as inhibition of chemotaxis in eosinophil |
Bioorg Med Chem Lett 18: 586-95 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.087
BindingDB Entry DOI: 10.7270/Q2FF3S4K |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50163634

(1-((1R,2S)-2-(((S)-3-(4-fluorobenzyl)piperidin-1-y...)Show SMILES Cn1nnnc1-c1cccc(NC(=O)N[C@@H]2CCCC[C@H]2CN2CCC[C@@H](Cc3ccc(F)cc3)C2)c1 |r| Show InChI InChI=1S/C28H36FN7O/c1-35-27(32-33-34-35)22-8-4-9-25(17-22)30-28(37)31-26-10-3-2-7-23(26)19-36-15-5-6-21(18-36)16-20-11-13-24(29)14-12-20/h4,8-9,11-14,17,21,23,26H,2-3,5-7,10,15-16,18-19H2,1H3,(H2,30,31,37)/t21-,23-,26+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR3 receptor assessed as inhibition of chemotaxis in eosinophil at 30 nM |
Bioorg Med Chem Lett 18: 586-95 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.087
BindingDB Entry DOI: 10.7270/Q2FF3S4K |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50163634

(1-((1R,2S)-2-(((S)-3-(4-fluorobenzyl)piperidin-1-y...)Show SMILES Cn1nnnc1-c1cccc(NC(=O)N[C@@H]2CCCC[C@H]2CN2CCC[C@@H](Cc3ccc(F)cc3)C2)c1 |r| Show InChI InChI=1S/C28H36FN7O/c1-35-27(32-33-34-35)22-8-4-9-25(17-22)30-28(37)31-26-10-3-2-7-23(26)19-36-15-5-6-21(18-36)16-20-11-13-24(29)14-12-20/h4,8-9,11-14,17,21,23,26H,2-3,5-7,10,15-16,18-19H2,1H3,(H2,30,31,37)/t21-,23-,26+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR3 receptor assessed as inhibition of chemotaxis in eosinophil |
Bioorg Med Chem Lett 18: 586-95 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.087
BindingDB Entry DOI: 10.7270/Q2FF3S4K |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50231350
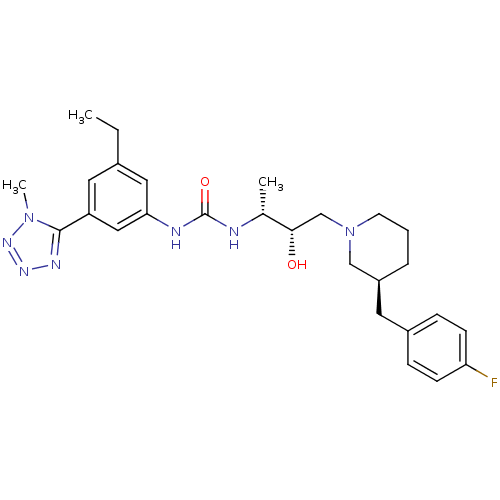
(1-((2R,3S)-4-(ethyl(3-(4-fluorophenyl)propyl)amino...)Show SMILES CCc1cc(NC(=O)N[C@H](C)[C@@H](O)CN2CCC[C@@H](Cc3ccc(F)cc3)C2)cc(c1)-c1nnnn1C Show InChI InChI=1S/C27H36FN7O2/c1-4-19-13-22(26-31-32-33-34(26)3)15-24(14-19)30-27(37)29-18(2)25(36)17-35-11-5-6-21(16-35)12-20-7-9-23(28)10-8-20/h7-10,13-15,18,21,25,36H,4-6,11-12,16-17H2,1-3H3,(H2,29,30,37)/t18-,21+,25+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR3 receptor assessed as inhibition of chemotaxis in eosinophil |
Bioorg Med Chem Lett 18: 586-95 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.087
BindingDB Entry DOI: 10.7270/Q2FF3S4K |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50231358
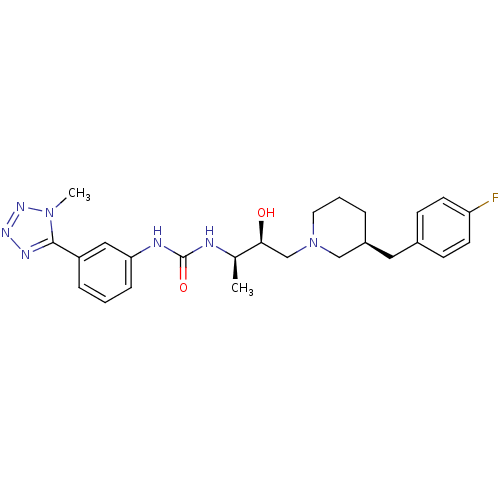
(1-((2R,3S)-4-((S)-3-(4-fluorobenzyl)piperidin-1-yl...)Show SMILES C[C@@H](NC(=O)Nc1cccc(c1)-c1nnnn1C)[C@@H](O)CN1CCC[C@@H](Cc2ccc(F)cc2)C1 |r| Show InChI InChI=1S/C25H32FN7O2/c1-17(27-25(35)28-22-7-3-6-20(14-22)24-29-30-31-32(24)2)23(34)16-33-12-4-5-19(15-33)13-18-8-10-21(26)11-9-18/h3,6-11,14,17,19,23,34H,4-5,12-13,15-16H2,1-2H3,(H2,27,28,35)/t17-,19+,23+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0380 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR3 receptor assessed as inhibition of chemotaxis in eosinophil |
Bioorg Med Chem Lett 18: 586-95 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.087
BindingDB Entry DOI: 10.7270/Q2FF3S4K |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50231354
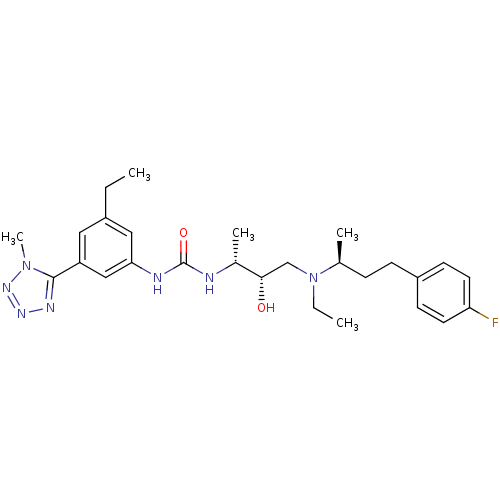
(1-((2R,3S)-4-(ethyl((S)-4-(4-fluorophenyl)butan-2-...)Show SMILES CCN(C[C@H](O)[C@@H](C)NC(=O)Nc1cc(CC)cc(c1)-c1nnnn1C)[C@@H](C)CCc1ccc(F)cc1 Show InChI InChI=1S/C27H38FN7O2/c1-6-20-14-22(26-31-32-33-34(26)5)16-24(15-20)30-27(37)29-19(4)25(36)17-35(7-2)18(3)8-9-21-10-12-23(28)13-11-21/h10-16,18-19,25,36H,6-9,17H2,1-5H3,(H2,29,30,37)/t18-,19+,25-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Displacement of [125I] eotaxin from human CCR3 receptor in CHO cells |
Bioorg Med Chem Lett 18: 586-95 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.087
BindingDB Entry DOI: 10.7270/Q2FF3S4K |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50231371
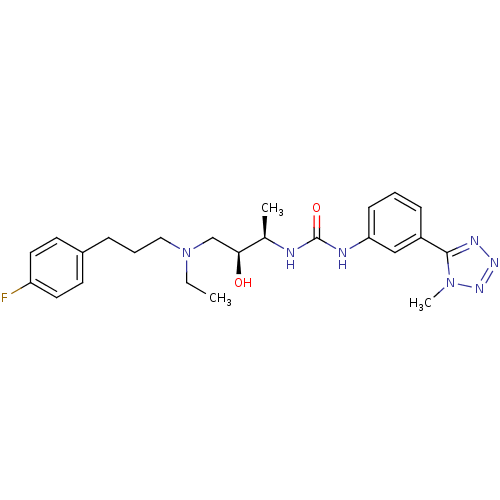
(1-((2R,3S)-4-(ethyl(3-(4-fluorophenyl)propyl)amino...)Show SMILES CCN(CCCc1ccc(F)cc1)C[C@H](O)[C@@H](C)NC(=O)Nc1cccc(c1)-c1nnnn1C Show InChI InChI=1S/C24H32FN7O2/c1-4-32(14-6-7-18-10-12-20(25)13-11-18)16-22(33)17(2)26-24(34)27-21-9-5-8-19(15-21)23-28-29-30-31(23)3/h5,8-13,15,17,22,33H,4,6-7,14,16H2,1-3H3,(H2,26,27,34)/t17-,22+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Displacement of [125I] eotaxin from human CCR3 receptor in CHO cells |
Bioorg Med Chem Lett 18: 586-95 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.087
BindingDB Entry DOI: 10.7270/Q2FF3S4K |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50231360
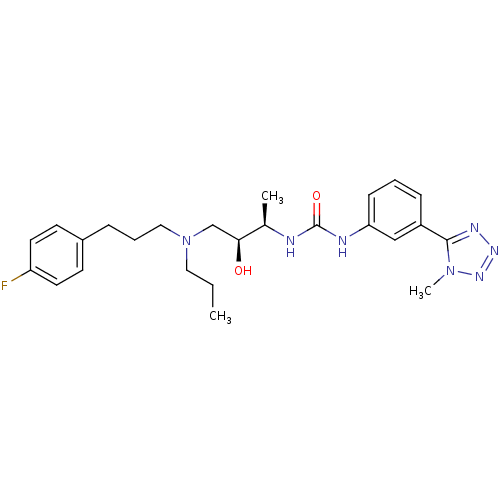
(1-((2R,3S)-4-((3-(4-fluorophenyl)propyl)(propyl)am...)Show SMILES CCCN(CCCc1ccc(F)cc1)C[C@H](O)[C@@H](C)NC(=O)Nc1cccc(c1)-c1nnnn1C Show InChI InChI=1S/C25H34FN7O2/c1-4-14-33(15-6-7-19-10-12-21(26)13-11-19)17-23(34)18(2)27-25(35)28-22-9-5-8-20(16-22)24-29-30-31-32(24)3/h5,8-13,16,18,23,34H,4,6-7,14-15,17H2,1-3H3,(H2,27,28,35)/t18-,23+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Displacement of [125I] eotaxin from human CCR3 receptor in CHO cells |
Bioorg Med Chem Lett 18: 586-95 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.087
BindingDB Entry DOI: 10.7270/Q2FF3S4K |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50089354

(CHEMBL3577945)Show SMILES CCC[C@@H]1C[C@@H](CC[C@@H]1N1CC[C@H](Nc2ncnc3ccc(cc23)C(F)(F)F)C1=O)N(C)C(C)C |r| Show InChI InChI=1S/C26H36F3N5O/c1-5-6-17-13-19(33(4)16(2)3)8-10-23(17)34-12-11-22(25(34)35)32-24-20-14-18(26(27,28)29)7-9-21(20)30-15-31-24/h7,9,14-17,19,22-23H,5-6,8,10-13H2,1-4H3,(H,30,31,32)/t17-,19-,22+,23+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Antagonist activity against CCR2 in human PBMC assessed as inhibition of MCP1-induced chemotaxis at 37 degC |
ACS Med Chem Lett 6: 439-44 (2015)
Article DOI: 10.1021/ml500505q
BindingDB Entry DOI: 10.7270/Q2668FWM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50194718
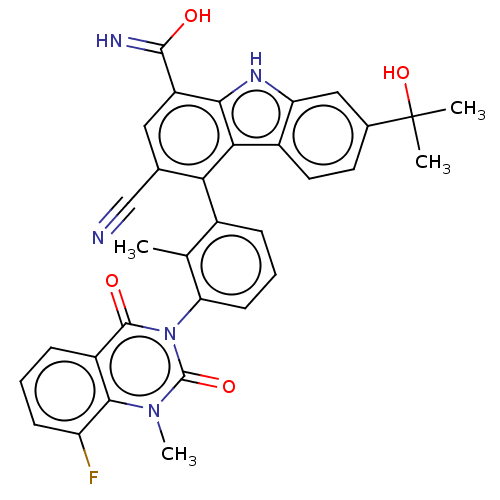
(CHEMBL3899411)Show SMILES Cc1c(cccc1-n1c(=O)n(C)c2c(F)cccc2c1=O)-c1c(cc(C(O)=N)c2[nH]c3cc(ccc3c12)C(C)(C)O)C#N |(-2.03,-1.34,;-1.6,.13,;-.1,.5,;.33,1.98,;-.74,3.09,;-2.23,2.73,;-2.66,1.25,;-4.16,.88,;-4.59,-.6,;-3.52,-1.71,;-6.08,-.97,;-6.51,-2.44,;-7.15,.15,;-8.65,-.22,;-9.08,-1.7,;-9.71,.89,;-9.28,2.37,;-7.78,2.74,;-6.72,1.63,;-5.22,1.99,;-4.79,3.47,;.96,-.61,;.53,-2.09,;1.6,-3.2,;3.09,-2.84,;4.16,-3.95,;5.66,-3.58,;3.73,-5.43,;3.52,-1.36,;5.06,-1.52,;6.34,-.67,;7.62,.19,;7.51,1.73,;6.13,2.41,;4.85,1.55,;4.95,.01,;2.46,-.24,;8.79,2.58,;7.94,3.86,;9.65,1.3,;10.07,3.44,;-.96,-2.46,;-2.46,-2.82,)| Show InChI InChI=1S/C33H26FN5O4/c1-16-19(7-6-10-25(16)39-31(41)21-8-5-9-23(34)29(21)38(4)32(39)42)26-17(15-35)13-22(30(36)40)28-27(26)20-12-11-18(33(2,3)43)14-24(20)37-28/h5-14,37,43H,1-4H3,(H2,36,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of full length recombinant human His-tagged BTK expressed in baculovirus using fluoresceinated peptide incubated for 60 mins by fluorescen... |
J Med Chem 59: 9173-9200 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01088
BindingDB Entry DOI: 10.7270/Q23T9K5H |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50231379
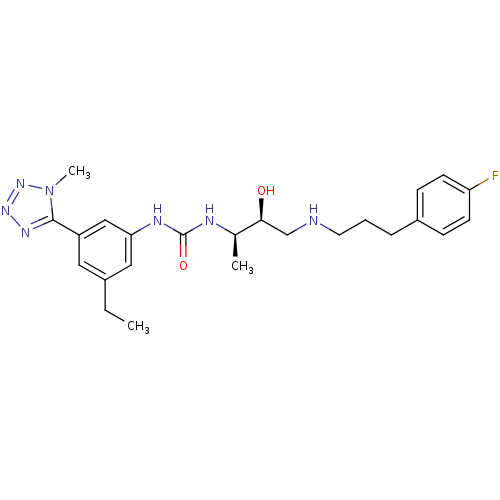
(1-(3-ethyl-5-(1-methyl-1H-tetrazol-5-yl)phenyl)-3-...)Show SMILES CCc1cc(NC(=O)N[C@H](C)[C@@H](O)CNCCCc2ccc(F)cc2)cc(c1)-c1nnnn1C Show InChI InChI=1S/C24H32FN7O2/c1-4-17-12-19(23-29-30-31-32(23)3)14-21(13-17)28-24(34)27-16(2)22(33)15-26-11-5-6-18-7-9-20(25)10-8-18/h7-10,12-14,16,22,26,33H,4-6,11,15H2,1-3H3,(H2,27,28,34)/t16-,22+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Displacement of [125I] eotaxin from human CCR3 receptor in CHO cells |
Bioorg Med Chem Lett 18: 586-95 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.087
BindingDB Entry DOI: 10.7270/Q2FF3S4K |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50231358

(1-((2R,3S)-4-((S)-3-(4-fluorobenzyl)piperidin-1-yl...)Show SMILES C[C@@H](NC(=O)Nc1cccc(c1)-c1nnnn1C)[C@@H](O)CN1CCC[C@@H](Cc2ccc(F)cc2)C1 |r| Show InChI InChI=1S/C25H32FN7O2/c1-17(27-25(35)28-22-7-3-6-20(14-22)24-29-30-31-32(24)2)23(34)16-33-12-4-5-19(15-33)13-18-8-10-21(26)11-9-18/h3,6-11,14,17,19,23,34H,4-5,12-13,15-16H2,1-2H3,(H2,27,28,35)/t17-,19+,23+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Displacement of [125I] eotaxin from human CCR3 receptor in CHO cells |
Bioorg Med Chem Lett 18: 586-95 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.087
BindingDB Entry DOI: 10.7270/Q2FF3S4K |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM13320

(1-Methyl-1H-imidazole-4-sulfonic Acid [6-Cyano-1-(...)Show SMILES CC(=C)CN(C1CN(Cc2cncn2C)c2ccc(cc2C1)C#N)S(=O)(=O)c1cn(C)cn1 Show InChI InChI=1S/C23H27N7O2S/c1-17(2)11-30(33(31,32)23-14-27(3)16-26-23)20-8-19-7-18(9-24)5-6-22(19)29(12-20)13-21-10-25-15-28(21)4/h5-7,10,14-16,20H,1,8,11-13H2,2-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human farnesyltransferase |
Bioorg Med Chem Lett 15: 1895-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.004
BindingDB Entry DOI: 10.7270/Q2RB75CJ |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50231376

(1-(3-ethyl-5-(1-methyl-1H-tetrazol-5-yl)phenyl)-3-...)Show SMILES CCc1cc(NC(=O)N[C@H](C)[C@@H](O)CN(C)CCCc2ccc(F)cc2)cc(c1)-c1nnnn1C Show InChI InChI=1S/C25H34FN7O2/c1-5-18-13-20(24-29-30-31-33(24)4)15-22(14-18)28-25(35)27-17(2)23(34)16-32(3)12-6-7-19-8-10-21(26)11-9-19/h8-11,13-15,17,23,34H,5-7,12,16H2,1-4H3,(H2,27,28,35)/t17-,23+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Displacement of [125I] eotaxin from human CCR3 receptor in CHO cells |
Bioorg Med Chem Lett 18: 586-95 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.087
BindingDB Entry DOI: 10.7270/Q2FF3S4K |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50231346

(1-((2R,3S)-4-(ethyl(3-(4-fluorophenyl)propyl)amino...)Show SMILES CCN(CCCc1ccc(F)cc1)C[C@H](O)[C@@H](C)NC(=O)Nc1cc(CC)cc(c1)-c1nnnn1C Show InChI InChI=1S/C26H36FN7O2/c1-5-19-14-21(25-30-31-32-33(25)4)16-23(15-19)29-26(36)28-18(3)24(35)17-34(6-2)13-7-8-20-9-11-22(27)12-10-20/h9-12,14-16,18,24,35H,5-8,13,17H2,1-4H3,(H2,28,29,36)/t18-,24+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Displacement of [125I] eotaxin from human CCR3 receptor in CHO cells |
Bioorg Med Chem Lett 18: 586-95 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.087
BindingDB Entry DOI: 10.7270/Q2FF3S4K |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50089366
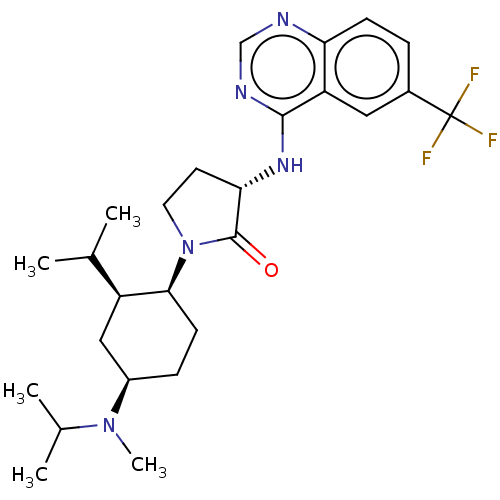
(CHEMBL3577948)Show SMILES CC(C)[C@@H]1C[C@@H](CC[C@@H]1N1CC[C@H](Nc2ncnc3ccc(cc23)C(F)(F)F)C1=O)N(C)C(C)C |r| Show InChI InChI=1S/C26H36F3N5O/c1-15(2)19-13-18(33(5)16(3)4)7-9-23(19)34-11-10-22(25(34)35)32-24-20-12-17(26(27,28)29)6-8-21(20)30-14-31-24/h6,8,12,14-16,18-19,22-23H,7,9-11,13H2,1-5H3,(H,30,31,32)/t18-,19+,22+,23+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of [125I]hMCP1 binding to CCR2 in human PBMC incubated for 30 mins at room temperature |
ACS Med Chem Lett 6: 439-44 (2015)
Article DOI: 10.1021/ml500505q
BindingDB Entry DOI: 10.7270/Q2668FWM |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50231364

(1-((2R,3S)-4-((cyclopropylmethyl)(3-(4-fluoropheny...)Show SMILES C[C@@H](NC(=O)Nc1cccc(c1)-c1nnnn1C)[C@@H](O)CN(CCCc1ccc(F)cc1)CC1CC1 Show InChI InChI=1S/C26H34FN7O2/c1-18(28-26(36)29-23-7-3-6-21(15-23)25-30-31-32-33(25)2)24(35)17-34(16-20-8-9-20)14-4-5-19-10-12-22(27)13-11-19/h3,6-7,10-13,15,18,20,24,35H,4-5,8-9,14,16-17H2,1-2H3,(H2,28,29,36)/t18-,24+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Displacement of [125I] eotaxin from human CCR3 receptor in CHO cells |
Bioorg Med Chem Lett 18: 586-95 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.087
BindingDB Entry DOI: 10.7270/Q2FF3S4K |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data