

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50166893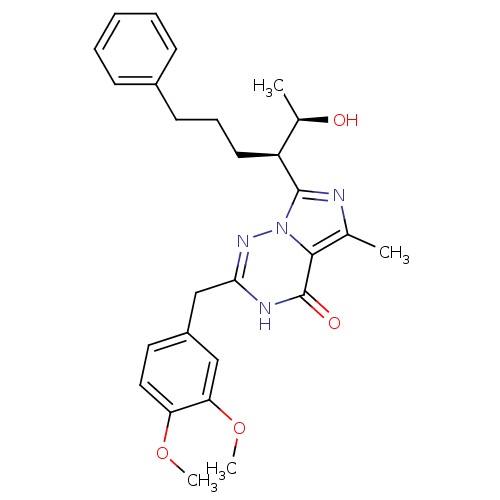 (2-(3,4-Dimethoxy-benzyl)-7-[(R)-1-((R)-1-hydroxy-e...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents | PDB Article PubMed | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human PDE2A1 using 3',5'-[3H]cGMP as substrate after 30 mins by scintillation proximity assay | J Med Chem 61: 3626-3640 (2018) Article DOI: 10.1021/acs.jmedchem.8b00116 BindingDB Entry DOI: 10.7270/Q2BV7K2X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50263751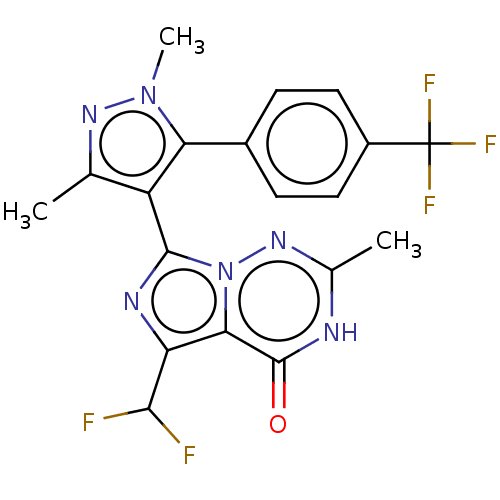 (CHEMBL4071033) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human PDE2A1 using 3',5'-[3H]cGMP as substrate after 30 mins by scintillation proximity assay | J Med Chem 61: 3626-3640 (2018) Article DOI: 10.1021/acs.jmedchem.8b00116 BindingDB Entry DOI: 10.7270/Q2BV7K2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50263733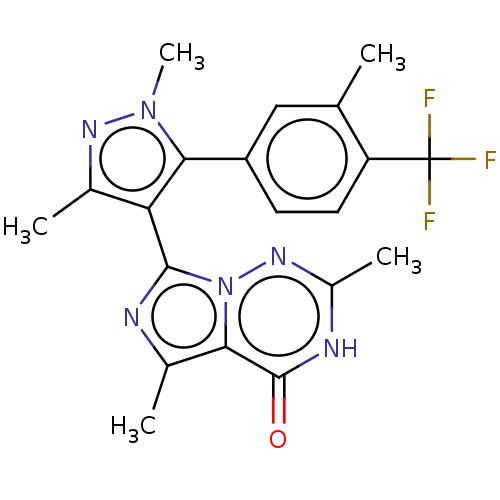 (CHEMBL4077126) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human PDE2A1 using 3',5'-[3H]cGMP as substrate after 30 mins by scintillation proximity assay | J Med Chem 61: 3626-3640 (2018) Article DOI: 10.1021/acs.jmedchem.8b00116 BindingDB Entry DOI: 10.7270/Q2BV7K2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50263737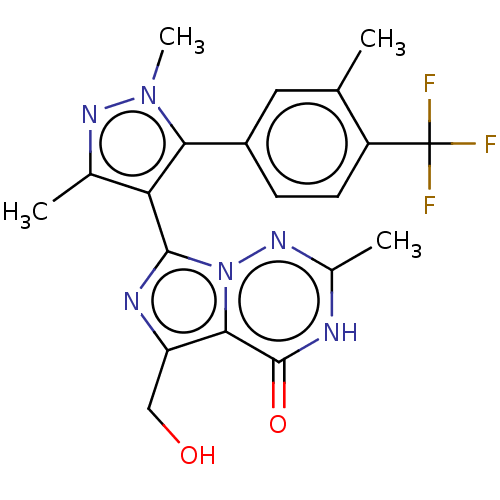 (CHEMBL4076642) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human PDE2A1 using 3',5'-[3H]cGMP as substrate after 30 mins by scintillation proximity assay | J Med Chem 61: 3626-3640 (2018) Article DOI: 10.1021/acs.jmedchem.8b00116 BindingDB Entry DOI: 10.7270/Q2BV7K2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM4566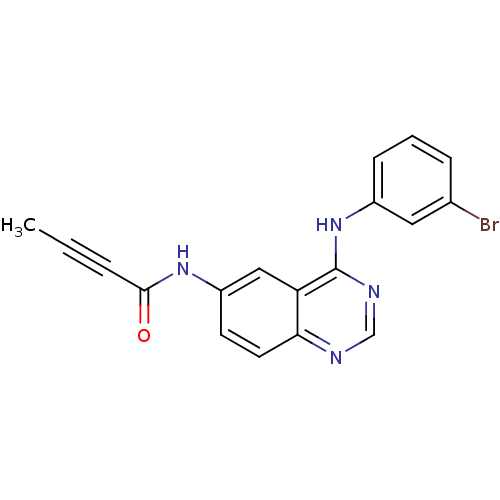 (4-anilinoquinazoline deriv. 1 | CHEMBL91867 | N-{4...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of erbB1 fusion protein expressed in baculovirus by ELISA | J Med Chem 49: 1475-85 (2006) Article DOI: 10.1021/jm050936o BindingDB Entry DOI: 10.7270/Q2ZS2X96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50263752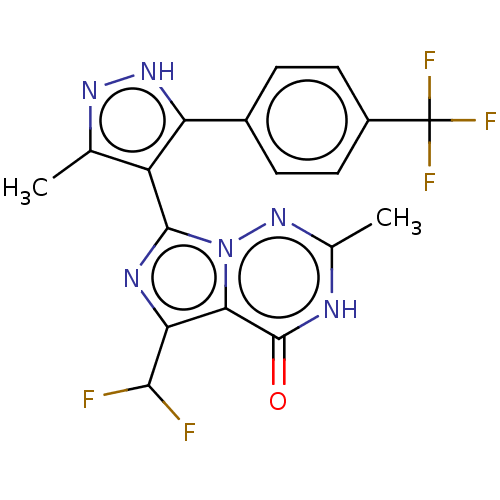 (CHEMBL4092829) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human PDE2A1 using 3',5'-[3H]cGMP as substrate after 30 mins by scintillation proximity assay | J Med Chem 61: 3626-3640 (2018) Article DOI: 10.1021/acs.jmedchem.8b00116 BindingDB Entry DOI: 10.7270/Q2BV7K2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50263791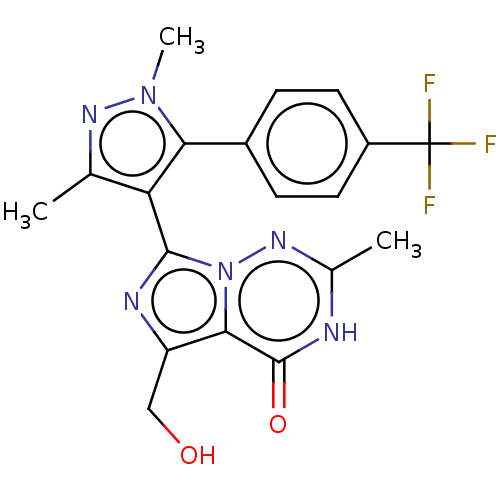 (CHEMBL4062397) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human PDE2A1 using 3',5'-[3H]cGMP as substrate after 30 mins by scintillation proximity assay | J Med Chem 61: 3626-3640 (2018) Article DOI: 10.1021/acs.jmedchem.8b00116 BindingDB Entry DOI: 10.7270/Q2BV7K2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50182693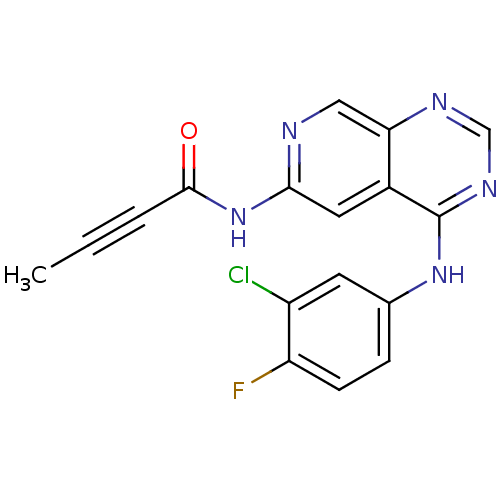 (CHEMBL203644 | N-[4-[(3-chloro-4-fluorophenyl)amin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of erbB1 fusion protein expressed in baculovirus by ELISA | J Med Chem 49: 1475-85 (2006) Article DOI: 10.1021/jm050936o BindingDB Entry DOI: 10.7270/Q2ZS2X96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50263791 (CHEMBL4062397) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human PDE2A1 using 3',5'-[3H]cGMP as substrate measured after 30 mins by Yttrium silicate scintillation proximity assay | ACS Med Chem Lett 9: 68-72 (2018) Article DOI: 10.1021/acsmedchemlett.7b00343 BindingDB Entry DOI: 10.7270/Q2MG7S23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50182697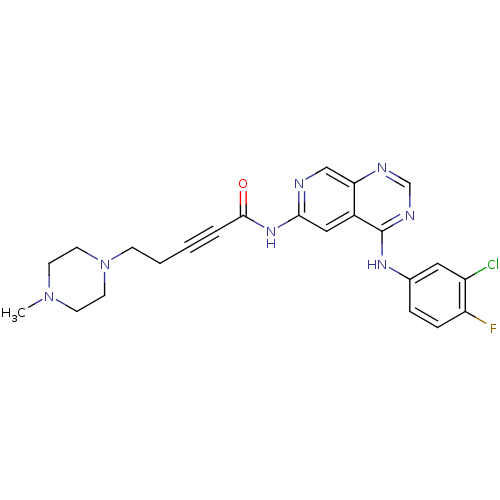 (CHEMBL203599 | N-[4-[(3-chloro-4-fluorophenyl)amin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of erbB1 fusion protein expressed in baculovirus by ELISA | J Med Chem 49: 1475-85 (2006) Article DOI: 10.1021/jm050936o BindingDB Entry DOI: 10.7270/Q2ZS2X96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor tyrosine-protein kinase erbB-4 (Homo sapiens (Human)) | BDBM50182693 (CHEMBL203644 | N-[4-[(3-chloro-4-fluorophenyl)amin...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of erbB4 fusion protein expressed in baculovirus by ELISA | J Med Chem 49: 1475-85 (2006) Article DOI: 10.1021/jm050936o BindingDB Entry DOI: 10.7270/Q2ZS2X96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor tyrosine-protein kinase erbB-2 (Homo sapiens (Human)) | BDBM50182688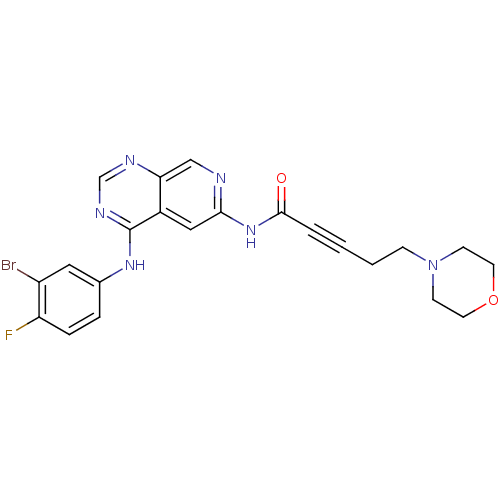 (CHEMBL204638 | N-[4-[(3-bromo-4-fluorophenyl)amino...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of HER stimulated human erbB autophosphorylation in MDA-MB-453 cells | J Med Chem 49: 1475-85 (2006) Article DOI: 10.1021/jm050936o BindingDB Entry DOI: 10.7270/Q2ZS2X96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor tyrosine-protein kinase erbB-2 (Homo sapiens (Human)) | BDBM50182684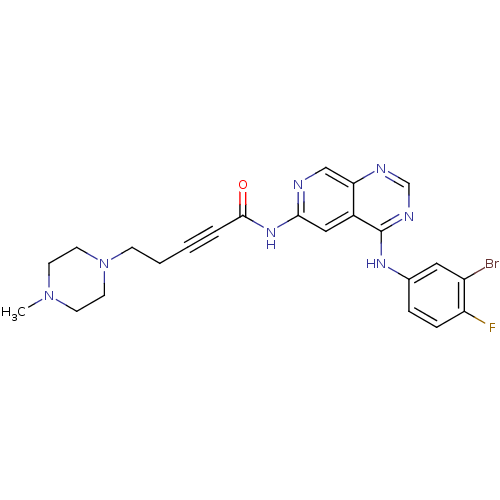 (CHEMBL437890 | N-[4-[(3-bromo-4-fluorophenyl)amino...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of erbB2 fusion protein expressed in baculovirus by ELISA | J Med Chem 49: 1475-85 (2006) Article DOI: 10.1021/jm050936o BindingDB Entry DOI: 10.7270/Q2ZS2X96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM5446 (CHEMBL553 | ERLOTINIB HYDROCHLORIDE | Erlotinib | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of erbB1 fusion protein expressed in baculovirus by ELISA | J Med Chem 49: 1475-85 (2006) Article DOI: 10.1021/jm050936o BindingDB Entry DOI: 10.7270/Q2ZS2X96 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50182689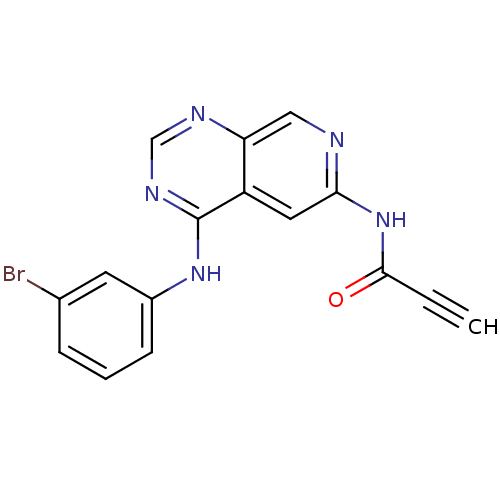 (CHEMBL378144 | N-[4-[(3-bromophenyl)amino]pyrido[3...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of erbB1 fusion protein expressed in baculovirus by ELISA | J Med Chem 49: 1475-85 (2006) Article DOI: 10.1021/jm050936o BindingDB Entry DOI: 10.7270/Q2ZS2X96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50286695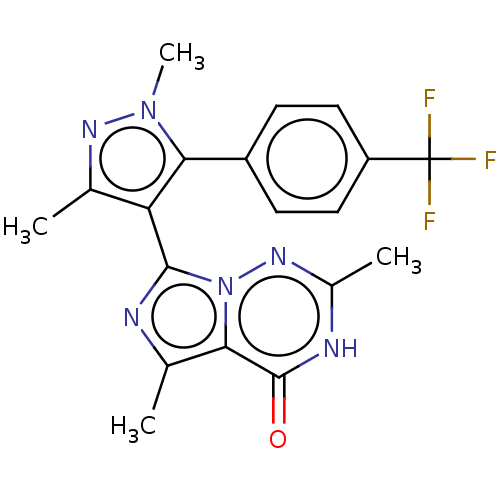 (CHEMBL4160171) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human PDE2A1 using 3',5'-[3H]cGMP as substrate measured after 30 mins by Yttrium silicate scintillation proximity assay | ACS Med Chem Lett 9: 68-72 (2018) Article DOI: 10.1021/acsmedchemlett.7b00343 BindingDB Entry DOI: 10.7270/Q2MG7S23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor tyrosine-protein kinase erbB-2 (Homo sapiens (Human)) | BDBM50182688 (CHEMBL204638 | N-[4-[(3-bromo-4-fluorophenyl)amino...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of erbB2 fusion protein expressed in baculovirus by ELISA | J Med Chem 49: 1475-85 (2006) Article DOI: 10.1021/jm050936o BindingDB Entry DOI: 10.7270/Q2ZS2X96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor tyrosine-protein kinase erbB-2 (Homo sapiens (Human)) | BDBM50182697 (CHEMBL203599 | N-[4-[(3-chloro-4-fluorophenyl)amin...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of erbB2 fusion protein expressed in baculovirus by ELISA | J Med Chem 49: 1475-85 (2006) Article DOI: 10.1021/jm050936o BindingDB Entry DOI: 10.7270/Q2ZS2X96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor tyrosine-protein kinase erbB-4 (Homo sapiens (Human)) | BDBM50182684 (CHEMBL437890 | N-[4-[(3-bromo-4-fluorophenyl)amino...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of erbB4 fusion protein expressed in baculovirus by ELISA | J Med Chem 49: 1475-85 (2006) Article DOI: 10.1021/jm050936o BindingDB Entry DOI: 10.7270/Q2ZS2X96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor tyrosine-protein kinase erbB-2 (Homo sapiens (Human)) | BDBM50182697 (CHEMBL203599 | N-[4-[(3-chloro-4-fluorophenyl)amin...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of ligand stimulated erbB2 autophosphorylation in T24 NIH cells | J Med Chem 49: 1475-85 (2006) Article DOI: 10.1021/jm050936o BindingDB Entry DOI: 10.7270/Q2ZS2X96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor tyrosine-protein kinase erbB-2 (Homo sapiens (Human)) | BDBM50182682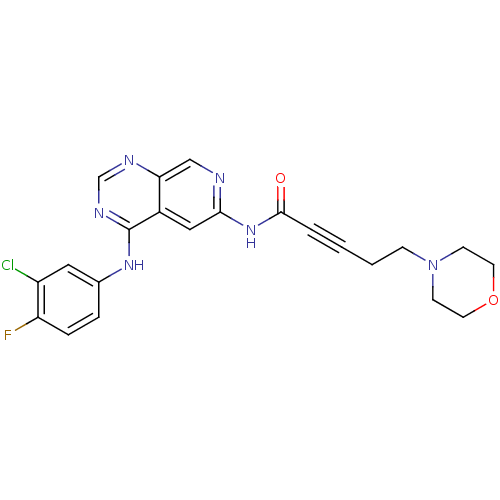 (CHEMBL203661 | N-[4-[(3-chloro-4-fluorophenyl)amin...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of erbB2 fusion protein expressed in baculovirus by ELISA | J Med Chem 49: 1475-85 (2006) Article DOI: 10.1021/jm050936o BindingDB Entry DOI: 10.7270/Q2ZS2X96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50182678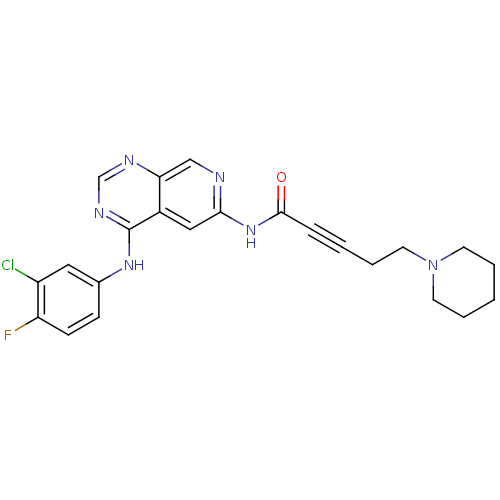 (CHEMBL205059 | N-[4-[(3-chloro-4-fluorophenyl)amin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of erbB1 fusion protein expressed in baculovirus by ELISA | J Med Chem 49: 1475-85 (2006) Article DOI: 10.1021/jm050936o BindingDB Entry DOI: 10.7270/Q2ZS2X96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50263793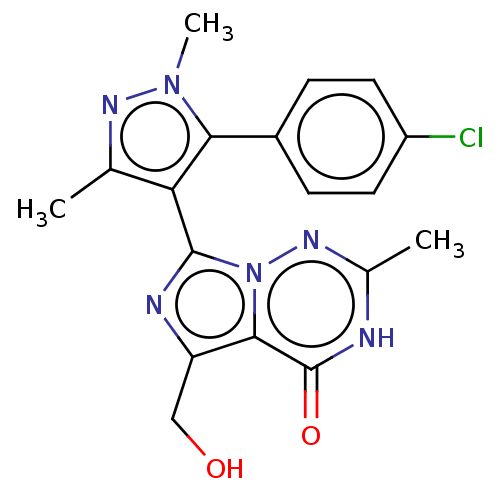 (CHEMBL4073753) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.920 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human PDE2A1 using 3',5'-[3H]cGMP as substrate after 30 mins by scintillation proximity assay | J Med Chem 61: 3626-3640 (2018) Article DOI: 10.1021/acs.jmedchem.8b00116 BindingDB Entry DOI: 10.7270/Q2BV7K2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50263792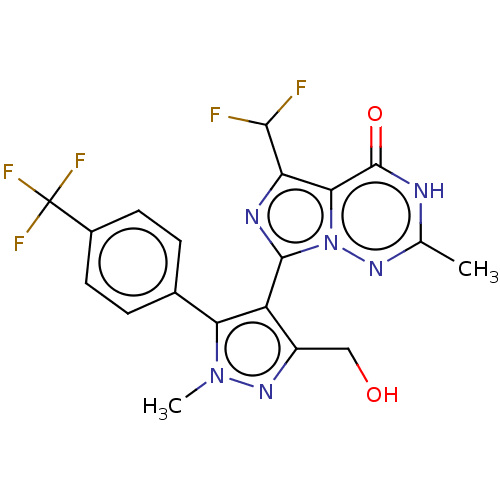 (CHEMBL4091870) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human PDE2A1 using 3',5'-[3H]cGMP as substrate after 30 mins by scintillation proximity assay | J Med Chem 61: 3626-3640 (2018) Article DOI: 10.1021/acs.jmedchem.8b00116 BindingDB Entry DOI: 10.7270/Q2BV7K2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor tyrosine-protein kinase erbB-4 (Homo sapiens (Human)) | BDBM50182682 (CHEMBL203661 | N-[4-[(3-chloro-4-fluorophenyl)amin...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of erbB4 fusion protein expressed in baculovirus by ELISA | J Med Chem 49: 1475-85 (2006) Article DOI: 10.1021/jm050936o BindingDB Entry DOI: 10.7270/Q2ZS2X96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor tyrosine-protein kinase erbB-2 (Homo sapiens (Human)) | BDBM50182697 (CHEMBL203599 | N-[4-[(3-chloro-4-fluorophenyl)amin...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of HER stimulated human erbB autophosphorylation in MDA-MB-453 cells | J Med Chem 49: 1475-85 (2006) Article DOI: 10.1021/jm050936o BindingDB Entry DOI: 10.7270/Q2ZS2X96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor tyrosine-protein kinase erbB-4 (Homo sapiens (Human)) | BDBM50182688 (CHEMBL204638 | N-[4-[(3-bromo-4-fluorophenyl)amino...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of erbB4 fusion protein expressed in baculovirus by ELISA | J Med Chem 49: 1475-85 (2006) Article DOI: 10.1021/jm050936o BindingDB Entry DOI: 10.7270/Q2ZS2X96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor tyrosine-protein kinase erbB-2 (Homo sapiens (Human)) | BDBM50182693 (CHEMBL203644 | N-[4-[(3-chloro-4-fluorophenyl)amin...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of erbB2 fusion protein expressed in baculovirus by ELISA | J Med Chem 49: 1475-85 (2006) Article DOI: 10.1021/jm050936o BindingDB Entry DOI: 10.7270/Q2ZS2X96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50263739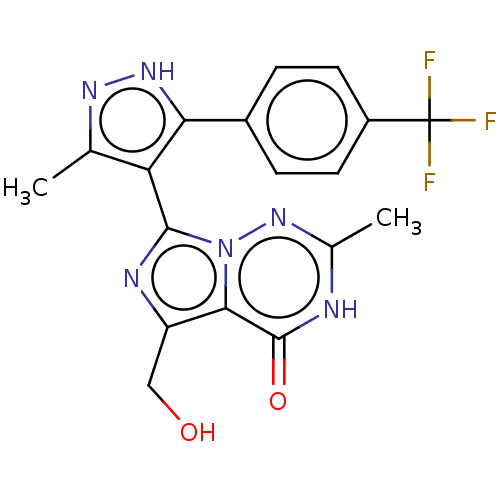 (CHEMBL4059875) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human PDE2A1 using 3',5'-[3H]cGMP as substrate after 30 mins by scintillation proximity assay | J Med Chem 61: 3626-3640 (2018) Article DOI: 10.1021/acs.jmedchem.8b00116 BindingDB Entry DOI: 10.7270/Q2BV7K2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50182684 (CHEMBL437890 | N-[4-[(3-bromo-4-fluorophenyl)amino...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of erbB1 fusion protein expressed in baculovirus by ELISA | J Med Chem 49: 1475-85 (2006) Article DOI: 10.1021/jm050936o BindingDB Entry DOI: 10.7270/Q2ZS2X96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor tyrosine-protein kinase erbB-4 (Homo sapiens (Human)) | BDBM50182697 (CHEMBL203599 | N-[4-[(3-chloro-4-fluorophenyl)amin...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of erbB4 fusion protein expressed in baculovirus by ELISA | J Med Chem 49: 1475-85 (2006) Article DOI: 10.1021/jm050936o BindingDB Entry DOI: 10.7270/Q2ZS2X96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50182682 (CHEMBL203661 | N-[4-[(3-chloro-4-fluorophenyl)amin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of erbB1 fusion protein expressed in baculovirus by ELISA | J Med Chem 49: 1475-85 (2006) Article DOI: 10.1021/jm050936o BindingDB Entry DOI: 10.7270/Q2ZS2X96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50182690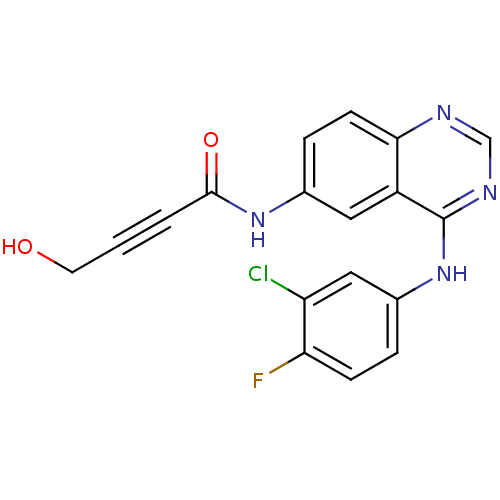 (CHEMBL202411 | N-[4-[(3-Chloro-4-fluorophenyl)amin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of erbB1 fusion protein expressed in baculovirus by ELISA | J Med Chem 49: 1475-85 (2006) Article DOI: 10.1021/jm050936o BindingDB Entry DOI: 10.7270/Q2ZS2X96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50182688 (CHEMBL204638 | N-[4-[(3-bromo-4-fluorophenyl)amino...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of erbB1 fusion protein expressed in baculovirus by ELISA | J Med Chem 49: 1475-85 (2006) Article DOI: 10.1021/jm050936o BindingDB Entry DOI: 10.7270/Q2ZS2X96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50263785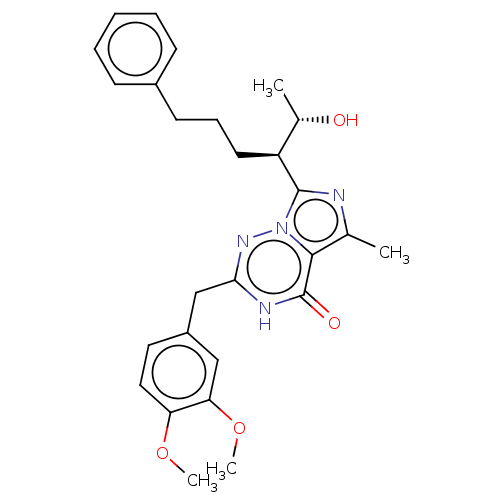 (CHEMBL4069753) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human PDE2A1 using 3',5'-[3H]cGMP as substrate after 30 mins by scintillation proximity assay | J Med Chem 61: 3626-3640 (2018) Article DOI: 10.1021/acs.jmedchem.8b00116 BindingDB Entry DOI: 10.7270/Q2BV7K2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50182695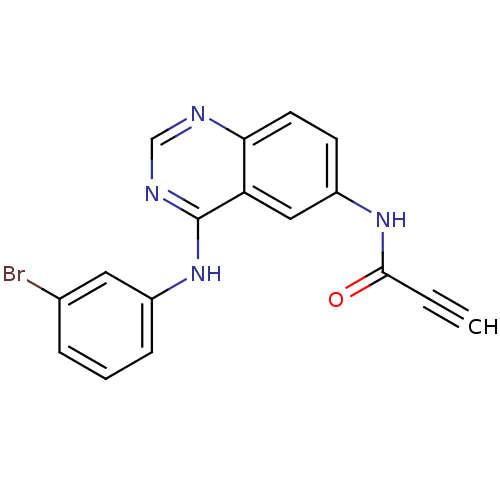 (CHEMBL202360 | N-[4-[(3-bromophenyl)amino]-6-quina...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of erbB1 fusion protein expressed in baculovirus by ELISA | J Med Chem 49: 1475-85 (2006) Article DOI: 10.1021/jm050936o BindingDB Entry DOI: 10.7270/Q2ZS2X96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor tyrosine-protein kinase erbB-4 (Homo sapiens (Human)) | BDBM50182695 (CHEMBL202360 | N-[4-[(3-bromophenyl)amino]-6-quina...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of erbB4 fusion protein expressed in baculovirus by ELISA | J Med Chem 49: 1475-85 (2006) Article DOI: 10.1021/jm050936o BindingDB Entry DOI: 10.7270/Q2ZS2X96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM4779 (CHEMBL31965 | CHEMBL545315 | CI-1033 | Canertinib ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of EGF stimulated human erbB1 autophosphorylation in NIH3T3 cells | J Med Chem 49: 1475-85 (2006) Article DOI: 10.1021/jm050936o BindingDB Entry DOI: 10.7270/Q2ZS2X96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50182687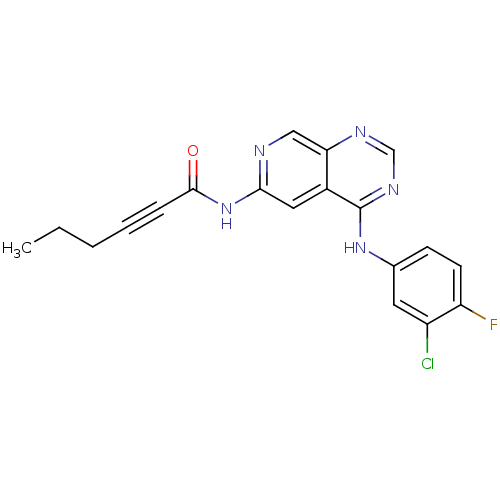 (CHEMBL203645 | N-[4-[(3-chloro-4-fluorophenyl)amin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of erbB1 fusion protein expressed in baculovirus by ELISA | J Med Chem 49: 1475-85 (2006) Article DOI: 10.1021/jm050936o BindingDB Entry DOI: 10.7270/Q2ZS2X96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50182698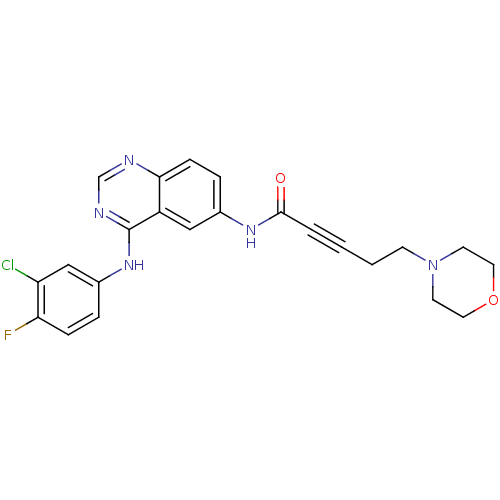 (CHEMBL204085 | N-[4-[(3-chloro-4-fluorophenyl)amin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of erbB1 fusion protein expressed in baculovirus by ELISA | J Med Chem 49: 1475-85 (2006) Article DOI: 10.1021/jm050936o BindingDB Entry DOI: 10.7270/Q2ZS2X96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50182682 (CHEMBL203661 | N-[4-[(3-chloro-4-fluorophenyl)amin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of EGF stimulated human erbB1 autophosphorylation in NIH3T3 cells | J Med Chem 49: 1475-85 (2006) Article DOI: 10.1021/jm050936o BindingDB Entry DOI: 10.7270/Q2ZS2X96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50182686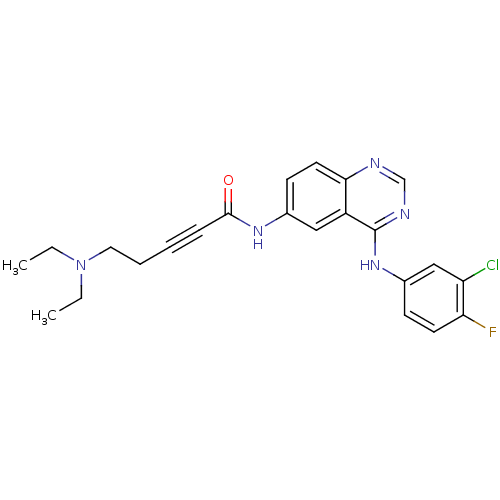 (CHEMBL382073 | N-[4-[(3-chloro-4-fluorophenyl)amin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of erbB1 fusion protein expressed in baculovirus by ELISA | J Med Chem 49: 1475-85 (2006) Article DOI: 10.1021/jm050936o BindingDB Entry DOI: 10.7270/Q2ZS2X96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor tyrosine-protein kinase erbB-4 (Homo sapiens (Human)) | BDBM50182678 (CHEMBL205059 | N-[4-[(3-chloro-4-fluorophenyl)amin...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of erbB4 fusion protein expressed in baculovirus by ELISA | J Med Chem 49: 1475-85 (2006) Article DOI: 10.1021/jm050936o BindingDB Entry DOI: 10.7270/Q2ZS2X96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50182697 (CHEMBL203599 | N-[4-[(3-chloro-4-fluorophenyl)amin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of EGF stimulated human erbB1 autophosphorylation in NIH3T3 cells | J Med Chem 49: 1475-85 (2006) Article DOI: 10.1021/jm050936o BindingDB Entry DOI: 10.7270/Q2ZS2X96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor tyrosine-protein kinase erbB-2 (Homo sapiens (Human)) | BDBM50182695 (CHEMBL202360 | N-[4-[(3-bromophenyl)amino]-6-quina...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of erbB2 fusion protein expressed in baculovirus by ELISA | J Med Chem 49: 1475-85 (2006) Article DOI: 10.1021/jm050936o BindingDB Entry DOI: 10.7270/Q2ZS2X96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor tyrosine-protein kinase erbB-2 (Homo sapiens (Human)) | BDBM50182678 (CHEMBL205059 | N-[4-[(3-chloro-4-fluorophenyl)amin...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of HER stimulated human erbB autophosphorylation in MDA-MB-453 cells | J Med Chem 49: 1475-85 (2006) Article DOI: 10.1021/jm050936o BindingDB Entry DOI: 10.7270/Q2ZS2X96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor tyrosine-protein kinase erbB-2 (Homo sapiens (Human)) | BDBM50182678 (CHEMBL205059 | N-[4-[(3-chloro-4-fluorophenyl)amin...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of erbB2 fusion protein expressed in baculovirus by ELISA | J Med Chem 49: 1475-85 (2006) Article DOI: 10.1021/jm050936o BindingDB Entry DOI: 10.7270/Q2ZS2X96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor tyrosine-protein kinase erbB-2 (Homo sapiens (Human)) | BDBM50182690 (CHEMBL202411 | N-[4-[(3-Chloro-4-fluorophenyl)amin...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of erbB2 fusion protein expressed in baculovirus by ELISA | J Med Chem 49: 1475-85 (2006) Article DOI: 10.1021/jm050936o BindingDB Entry DOI: 10.7270/Q2ZS2X96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor tyrosine-protein kinase erbB-2 (Homo sapiens (Human)) | BDBM50182698 (CHEMBL204085 | N-[4-[(3-chloro-4-fluorophenyl)amin...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of erbB2 fusion protein expressed in baculovirus by ELISA | J Med Chem 49: 1475-85 (2006) Article DOI: 10.1021/jm050936o BindingDB Entry DOI: 10.7270/Q2ZS2X96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50263783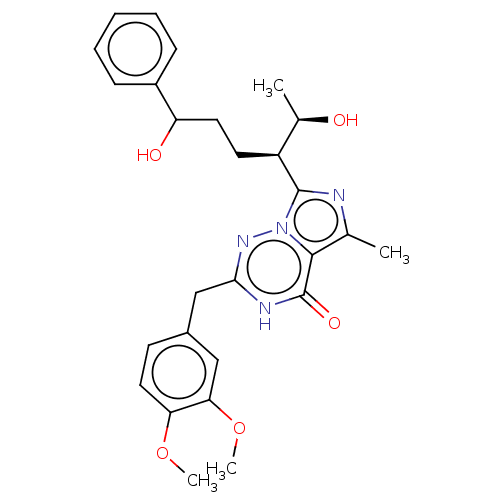 (CHEMBL4088306) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human PDE2A1 using 3',5'-[3H]cGMP as substrate after 30 mins by scintillation proximity assay | J Med Chem 61: 3626-3640 (2018) Article DOI: 10.1021/acs.jmedchem.8b00116 BindingDB Entry DOI: 10.7270/Q2BV7K2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 278 total ) | Next | Last >> |