

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM10404 ((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.54E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University | Assay Description The IC50 values were determined using the spectrophotometric Ellman's method. All of the tested compounds were dissolved in 0.01 M DMSO and then ... | Bioorg Chem 71: 244-256 (2017) Article DOI: 10.1016/j.bioorg.2017.02.010 BindingDB Entry DOI: 10.7270/Q2222SNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM10404 ((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University | Assay Description The IC50 values were determined using the spectrophotometric Ellman's method. All of the tested compounds were dissolved in 0.01 M DMSO and then ... | Bioorg Chem 71: 244-256 (2017) Article DOI: 10.1016/j.bioorg.2017.02.010 BindingDB Entry DOI: 10.7270/Q2222SNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM10404 ((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pardubice Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE using acetylthiocholine as substrate after 10 mins by Ellman's method | Bioorg Med Chem 21: 1735-48 (2013) Article DOI: 10.1016/j.bmc.2013.01.052 BindingDB Entry DOI: 10.7270/Q2V989F2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM10404 ((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 7.96E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pardubice Curated by ChEMBL | Assay Description Inhibition of equine BChE using after 10 mins by Ellman's method | Bioorg Med Chem 21: 1735-48 (2013) Article DOI: 10.1016/j.bmc.2013.01.052 BindingDB Entry DOI: 10.7270/Q2V989F2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM222329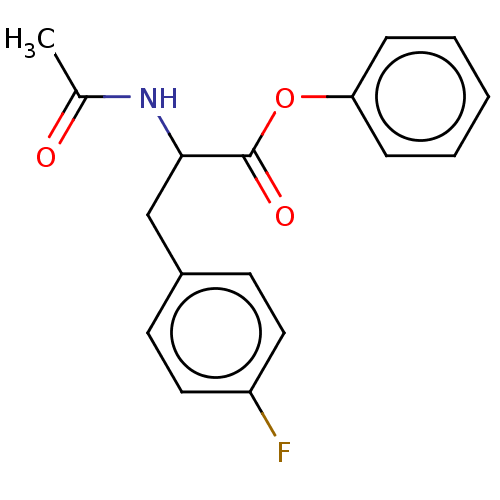 (Phenyl 2-acetamido-3-(4-fluorophenyl)propanoate (3...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University | Assay Description The IC50 values were determined using the spectrophotometric Ellman's method. All of the tested compounds were dissolved in 0.01 M DMSO and then ... | Bioorg Chem 71: 244-256 (2017) Article DOI: 10.1016/j.bioorg.2017.02.010 BindingDB Entry DOI: 10.7270/Q2222SNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50430780 (CHEMBL2334730) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.41E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pardubice Curated by ChEMBL | Assay Description Inhibition of equine BChE using after 10 mins by Ellman's method | Bioorg Med Chem 21: 1735-48 (2013) Article DOI: 10.1016/j.bmc.2013.01.052 BindingDB Entry DOI: 10.7270/Q2V989F2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50430779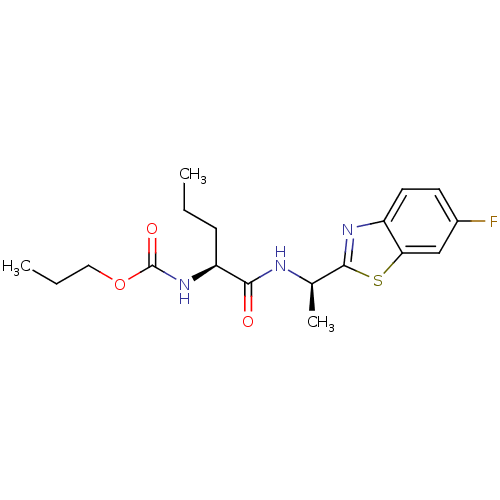 (CHEMBL2334731) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.93E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pardubice Curated by ChEMBL | Assay Description Inhibition of equine BChE using after 10 mins by Ellman's method | Bioorg Med Chem 21: 1735-48 (2013) Article DOI: 10.1016/j.bmc.2013.01.052 BindingDB Entry DOI: 10.7270/Q2V989F2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50430777 (CHEMBL2334733) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.96E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pardubice Curated by ChEMBL | Assay Description Inhibition of equine BChE using after 10 mins by Ellman's method | Bioorg Med Chem 21: 1735-48 (2013) Article DOI: 10.1016/j.bmc.2013.01.052 BindingDB Entry DOI: 10.7270/Q2V989F2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM10620 ((S)-3-(1-(dimethylamino)ethyl)phenyl ethyl(methyl)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pardubice Curated by ChEMBL | Assay Description Inhibition of equine BChE using after 10 mins by Ellman's method | Bioorg Med Chem 21: 1735-48 (2013) Article DOI: 10.1016/j.bmc.2013.01.052 BindingDB Entry DOI: 10.7270/Q2V989F2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50430776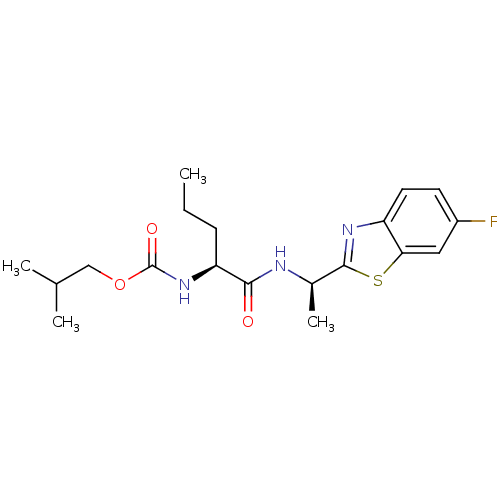 (CHEMBL2334734) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pardubice Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE using acetylthiocholine as substrate after 10 mins by Ellman's method | Bioorg Med Chem 21: 1735-48 (2013) Article DOI: 10.1016/j.bmc.2013.01.052 BindingDB Entry DOI: 10.7270/Q2V989F2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50430781 (CHEMBL2334729) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pardubice Curated by ChEMBL | Assay Description Inhibition of equine BChE using after 10 mins by Ellman's method | Bioorg Med Chem 21: 1735-48 (2013) Article DOI: 10.1016/j.bmc.2013.01.052 BindingDB Entry DOI: 10.7270/Q2V989F2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50430778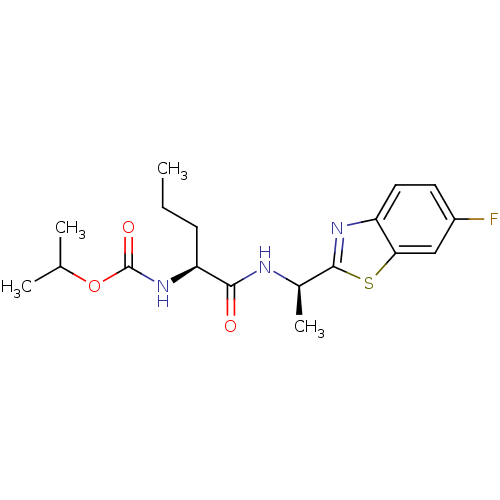 (CHEMBL2334732) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.13E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pardubice Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE using acetylthiocholine as substrate after 10 mins by Ellman's method | Bioorg Med Chem 21: 1735-48 (2013) Article DOI: 10.1016/j.bmc.2013.01.052 BindingDB Entry DOI: 10.7270/Q2V989F2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50430776 (CHEMBL2334734) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.61E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pardubice Curated by ChEMBL | Assay Description Inhibition of equine BChE using after 10 mins by Ellman's method | Bioorg Med Chem 21: 1735-48 (2013) Article DOI: 10.1016/j.bmc.2013.01.052 BindingDB Entry DOI: 10.7270/Q2V989F2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50430763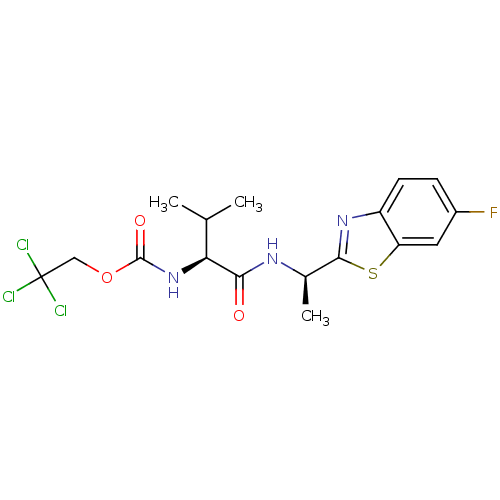 (CHEMBL2334725) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.66E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pardubice Curated by ChEMBL | Assay Description Inhibition of equine BChE using after 10 mins by Ellman's method | Bioorg Med Chem 21: 1735-48 (2013) Article DOI: 10.1016/j.bmc.2013.01.052 BindingDB Entry DOI: 10.7270/Q2V989F2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50430777 (CHEMBL2334733) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pardubice Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE using acetylthiocholine as substrate after 10 mins by Ellman's method | Bioorg Med Chem 21: 1735-48 (2013) Article DOI: 10.1016/j.bmc.2013.01.052 BindingDB Entry DOI: 10.7270/Q2V989F2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50430779 (CHEMBL2334731) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.85E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pardubice Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE using acetylthiocholine as substrate after 10 mins by Ellman's method | Bioorg Med Chem 21: 1735-48 (2013) Article DOI: 10.1016/j.bmc.2013.01.052 BindingDB Entry DOI: 10.7270/Q2V989F2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50430774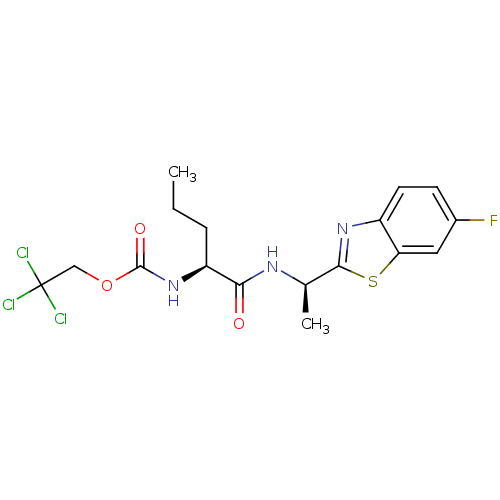 (CHEMBL2334736) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.86E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pardubice Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE using acetylthiocholine as substrate after 10 mins by Ellman's method | Bioorg Med Chem 21: 1735-48 (2013) Article DOI: 10.1016/j.bmc.2013.01.052 BindingDB Entry DOI: 10.7270/Q2V989F2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50430761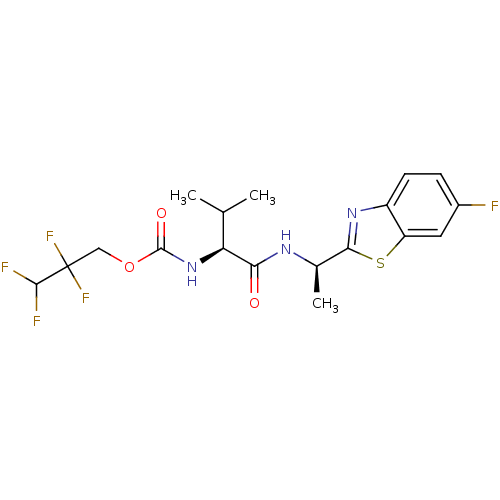 (CHEMBL2334727) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pardubice Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE using acetylthiocholine as substrate after 10 mins by Ellman's method | Bioorg Med Chem 21: 1735-48 (2013) Article DOI: 10.1016/j.bmc.2013.01.052 BindingDB Entry DOI: 10.7270/Q2V989F2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50430773 (CHEMBL2334737) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.93E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pardubice Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE using acetylthiocholine as substrate after 10 mins by Ellman's method | Bioorg Med Chem 21: 1735-48 (2013) Article DOI: 10.1016/j.bmc.2013.01.052 BindingDB Entry DOI: 10.7270/Q2V989F2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM222327 (Phenyl 2-acetamido-3-(2-fluorophenyl)propanoate (1...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University | Assay Description The IC50 values were determined using the spectrophotometric Ellman's method. All of the tested compounds were dissolved in 0.01 M DMSO and then ... | Bioorg Chem 71: 244-256 (2017) Article DOI: 10.1016/j.bioorg.2017.02.010 BindingDB Entry DOI: 10.7270/Q2222SNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50430763 (CHEMBL2334725) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.03E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pardubice Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE using acetylthiocholine as substrate after 10 mins by Ellman's method | Bioorg Med Chem 21: 1735-48 (2013) Article DOI: 10.1016/j.bmc.2013.01.052 BindingDB Entry DOI: 10.7270/Q2V989F2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50430772 (CHEMBL2334738) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.07E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pardubice Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE using acetylthiocholine as substrate after 10 mins by Ellman's method | Bioorg Med Chem 21: 1735-48 (2013) Article DOI: 10.1016/j.bmc.2013.01.052 BindingDB Entry DOI: 10.7270/Q2V989F2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50430770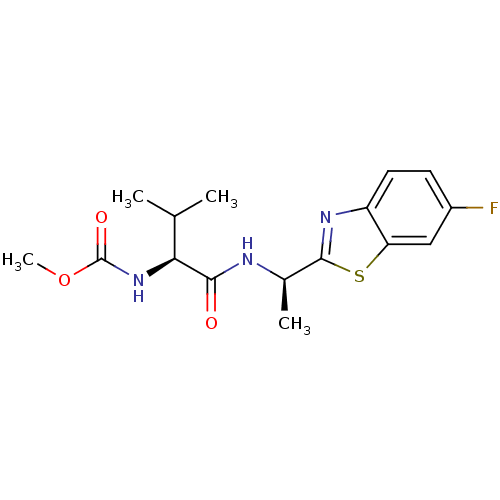 (CHEMBL2334740) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pardubice Curated by ChEMBL | Assay Description Inhibition of equine BChE using after 10 mins by Ellman's method | Bioorg Med Chem 21: 1735-48 (2013) Article DOI: 10.1016/j.bmc.2013.01.052 BindingDB Entry DOI: 10.7270/Q2V989F2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50430778 (CHEMBL2334732) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.22E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pardubice Curated by ChEMBL | Assay Description Inhibition of equine BChE using after 10 mins by Ellman's method | Bioorg Med Chem 21: 1735-48 (2013) Article DOI: 10.1016/j.bmc.2013.01.052 BindingDB Entry DOI: 10.7270/Q2V989F2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50430761 (CHEMBL2334727) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.38E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pardubice Curated by ChEMBL | Assay Description Inhibition of equine BChE using after 10 mins by Ellman's method | Bioorg Med Chem 21: 1735-48 (2013) Article DOI: 10.1016/j.bmc.2013.01.052 BindingDB Entry DOI: 10.7270/Q2V989F2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM222325 (4-(Trifluoromethyl)phenyl 2-acetamido-3-(3-fluorop...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University | Assay Description The IC50 values were determined using the spectrophotometric Ellman's method. All of the tested compounds were dissolved in 0.01 M DMSO and then ... | Bioorg Chem 71: 244-256 (2017) Article DOI: 10.1016/j.bioorg.2017.02.010 BindingDB Entry DOI: 10.7270/Q2222SNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50430780 (CHEMBL2334730) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.48E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pardubice Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE using acetylthiocholine as substrate after 10 mins by Ellman's method | Bioorg Med Chem 21: 1735-48 (2013) Article DOI: 10.1016/j.bmc.2013.01.052 BindingDB Entry DOI: 10.7270/Q2V989F2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50430775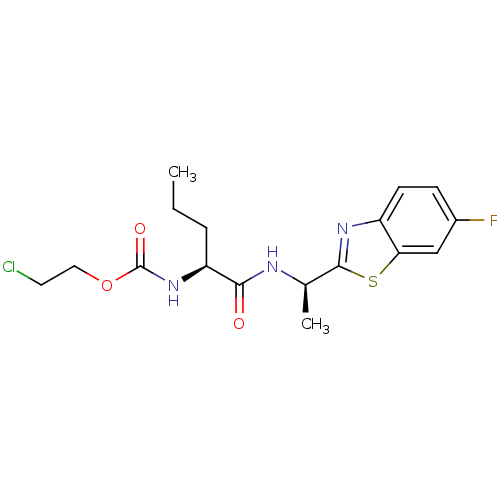 (CHEMBL2334735) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.54E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pardubice Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE using acetylthiocholine as substrate after 10 mins by Ellman's method | Bioorg Med Chem 21: 1735-48 (2013) Article DOI: 10.1016/j.bmc.2013.01.052 BindingDB Entry DOI: 10.7270/Q2V989F2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50430769 (CHEMBL2334741) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.56E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pardubice Curated by ChEMBL | Assay Description Inhibition of equine BChE using after 10 mins by Ellman's method | Bioorg Med Chem 21: 1735-48 (2013) Article DOI: 10.1016/j.bmc.2013.01.052 BindingDB Entry DOI: 10.7270/Q2V989F2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM222324 (4-(Trifluoromethyl)phenyl 2-acetamido-3-(2-fluorop...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.74E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University | Assay Description The IC50 values were determined using the spectrophotometric Ellman's method. All of the tested compounds were dissolved in 0.01 M DMSO and then ... | Bioorg Chem 71: 244-256 (2017) Article DOI: 10.1016/j.bioorg.2017.02.010 BindingDB Entry DOI: 10.7270/Q2222SNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM10620 ((S)-3-(1-(dimethylamino)ethyl)phenyl ethyl(methyl)...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.84E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University | Assay Description The IC50 values were determined using the spectrophotometric Ellman's method. All of the tested compounds were dissolved in 0.01 M DMSO and then ... | Bioorg Chem 71: 244-256 (2017) Article DOI: 10.1016/j.bioorg.2017.02.010 BindingDB Entry DOI: 10.7270/Q2222SNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50430765 (CHEMBL2334745) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.11E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pardubice Curated by ChEMBL | Assay Description Inhibition of equine BChE using after 10 mins by Ellman's method | Bioorg Med Chem 21: 1735-48 (2013) Article DOI: 10.1016/j.bmc.2013.01.052 BindingDB Entry DOI: 10.7270/Q2V989F2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50430767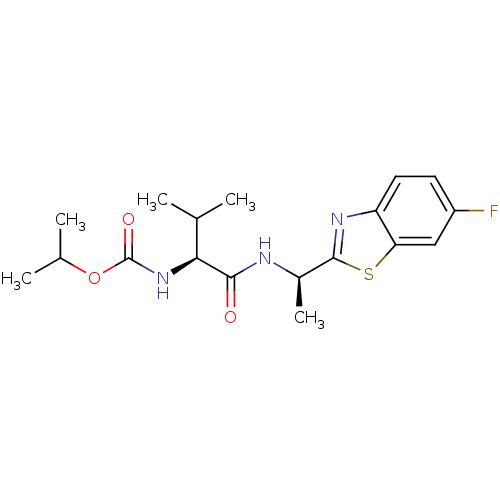 (CHEMBL2334743) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.11E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pardubice Curated by ChEMBL | Assay Description Inhibition of equine BChE using after 10 mins by Ellman's method | Bioorg Med Chem 21: 1735-48 (2013) Article DOI: 10.1016/j.bmc.2013.01.052 BindingDB Entry DOI: 10.7270/Q2V989F2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50430772 (CHEMBL2334738) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pardubice Curated by ChEMBL | Assay Description Inhibition of equine BChE using after 10 mins by Ellman's method | Bioorg Med Chem 21: 1735-48 (2013) Article DOI: 10.1016/j.bmc.2013.01.052 BindingDB Entry DOI: 10.7270/Q2V989F2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50430771 (CHEMBL2334739) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pardubice Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE using acetylthiocholine as substrate after 10 mins by Ellman's method | Bioorg Med Chem 21: 1735-48 (2013) Article DOI: 10.1016/j.bmc.2013.01.052 BindingDB Entry DOI: 10.7270/Q2V989F2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50430782 (CHEMBL2334747) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.72E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pardubice Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE using acetylthiocholine as substrate after 10 mins by Ellman's method | Bioorg Med Chem 21: 1735-48 (2013) Article DOI: 10.1016/j.bmc.2013.01.052 BindingDB Entry DOI: 10.7270/Q2V989F2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50430764 (CHEMBL2334746) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.75E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pardubice Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE using acetylthiocholine as substrate after 10 mins by Ellman's method | Bioorg Med Chem 21: 1735-48 (2013) Article DOI: 10.1016/j.bmc.2013.01.052 BindingDB Entry DOI: 10.7270/Q2V989F2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50430781 (CHEMBL2334729) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.88E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pardubice Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE using acetylthiocholine as substrate after 10 mins by Ellman's method | Bioorg Med Chem 21: 1735-48 (2013) Article DOI: 10.1016/j.bmc.2013.01.052 BindingDB Entry DOI: 10.7270/Q2V989F2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50430765 (CHEMBL2334745) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.93E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pardubice Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE using acetylthiocholine as substrate after 10 mins by Ellman's method | Bioorg Med Chem 21: 1735-48 (2013) Article DOI: 10.1016/j.bmc.2013.01.052 BindingDB Entry DOI: 10.7270/Q2V989F2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50430762 (CHEMBL2334726) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.94E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pardubice Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE using acetylthiocholine as substrate after 10 mins by Ellman's method | Bioorg Med Chem 21: 1735-48 (2013) Article DOI: 10.1016/j.bmc.2013.01.052 BindingDB Entry DOI: 10.7270/Q2V989F2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM222316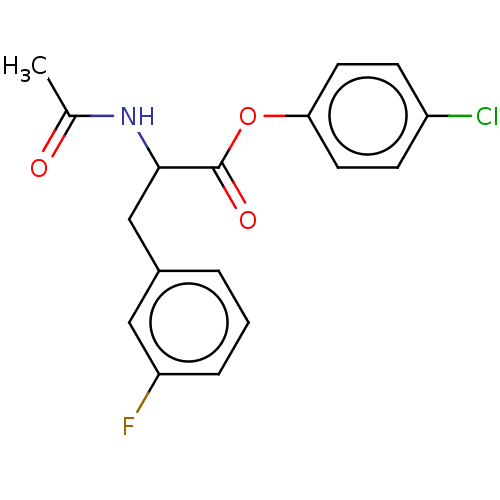 (4-Chlorophenyl 2-acetamido-3-(3-fluorophenyl)propa...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.27E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University | Assay Description The IC50 values were determined using the spectrophotometric Ellman's method. All of the tested compounds were dissolved in 0.01 M DMSO and then ... | Bioorg Chem 71: 244-256 (2017) Article DOI: 10.1016/j.bioorg.2017.02.010 BindingDB Entry DOI: 10.7270/Q2222SNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM222328 (Phenyl 2-acetamido-3-(3-fluorophenyl)propanoate (2...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.37E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University | Assay Description The IC50 values were determined using the spectrophotometric Ellman's method. All of the tested compounds were dissolved in 0.01 M DMSO and then ... | Bioorg Chem 71: 244-256 (2017) Article DOI: 10.1016/j.bioorg.2017.02.010 BindingDB Entry DOI: 10.7270/Q2222SNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50430775 (CHEMBL2334735) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.41E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pardubice Curated by ChEMBL | Assay Description Inhibition of equine BChE using after 10 mins by Ellman's method | Bioorg Med Chem 21: 1735-48 (2013) Article DOI: 10.1016/j.bmc.2013.01.052 BindingDB Entry DOI: 10.7270/Q2V989F2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50430764 (CHEMBL2334746) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.42E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pardubice Curated by ChEMBL | Assay Description Inhibition of equine BChE using after 10 mins by Ellman's method | Bioorg Med Chem 21: 1735-48 (2013) Article DOI: 10.1016/j.bmc.2013.01.052 BindingDB Entry DOI: 10.7270/Q2V989F2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM10620 ((S)-3-(1-(dimethylamino)ethyl)phenyl ethyl(methyl)...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.61E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University | Assay Description The IC50 values were determined using the spectrophotometric Ellman's method. All of the tested compounds were dissolved in 0.01 M DMSO and then ... | Bioorg Chem 71: 244-256 (2017) Article DOI: 10.1016/j.bioorg.2017.02.010 BindingDB Entry DOI: 10.7270/Q2222SNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM222318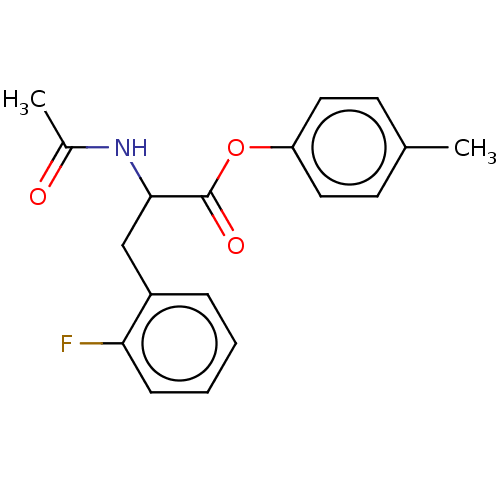 (4-Methylphenyl 2-acetamido-3-(2-fluorophenyl)propa...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.79E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University | Assay Description The IC50 values were determined using the spectrophotometric Ellman's method. All of the tested compounds were dissolved in 0.01 M DMSO and then ... | Bioorg Chem 71: 244-256 (2017) Article DOI: 10.1016/j.bioorg.2017.02.010 BindingDB Entry DOI: 10.7270/Q2222SNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50430773 (CHEMBL2334737) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pardubice Curated by ChEMBL | Assay Description Inhibition of equine BChE using after 10 mins by Ellman's method | Bioorg Med Chem 21: 1735-48 (2013) Article DOI: 10.1016/j.bmc.2013.01.052 BindingDB Entry DOI: 10.7270/Q2V989F2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM222317 (4-Chlorophenyl 2-acetamido-3-(4-fluorophenyl)propa...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.91E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University | Assay Description The IC50 values were determined using the spectrophotometric Ellman's method. All of the tested compounds were dissolved in 0.01 M DMSO and then ... | Bioorg Chem 71: 244-256 (2017) Article DOI: 10.1016/j.bioorg.2017.02.010 BindingDB Entry DOI: 10.7270/Q2222SNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50430771 (CHEMBL2334739) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.98E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pardubice Curated by ChEMBL | Assay Description Inhibition of equine BChE using after 10 mins by Ellman's method | Bioorg Med Chem 21: 1735-48 (2013) Article DOI: 10.1016/j.bmc.2013.01.052 BindingDB Entry DOI: 10.7270/Q2V989F2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM222324 (4-(Trifluoromethyl)phenyl 2-acetamido-3-(2-fluorop...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.98E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University | Assay Description The IC50 values were determined using the spectrophotometric Ellman's method. All of the tested compounds were dissolved in 0.01 M DMSO and then ... | Bioorg Chem 71: 244-256 (2017) Article DOI: 10.1016/j.bioorg.2017.02.010 BindingDB Entry DOI: 10.7270/Q2222SNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 119 total ) | Next | Last >> |