 Found 751 hits with Last Name = 'tremblay' and Initial = 'n'
Found 751 hits with Last Name = 'tremblay' and Initial = 'n' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM85177
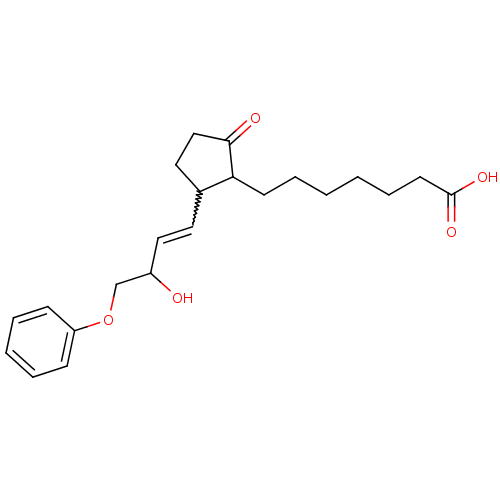
(CAS_80558-61-8 | M&B-28767 | NSC_119139)Show SMILES OC(COc1ccccc1)C=CC1CCC(=O)C1CCCCCCC(O)=O |w:11.12| Show InChI InChI=1S/C22H30O5/c23-18(16-27-19-8-4-3-5-9-19)14-12-17-13-15-21(24)20(17)10-6-1-2-7-11-22(25)26/h3-5,8-9,12,14,17-18,20,23H,1-2,6-7,10-11,13,15-16H2,(H,25,26) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
Biochim Biophys Acta 1483: 285-93 (2000)
Article DOI: 10.1016/s1388-1981(99)00164-x
BindingDB Entry DOI: 10.7270/Q2J964XQ |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM85603
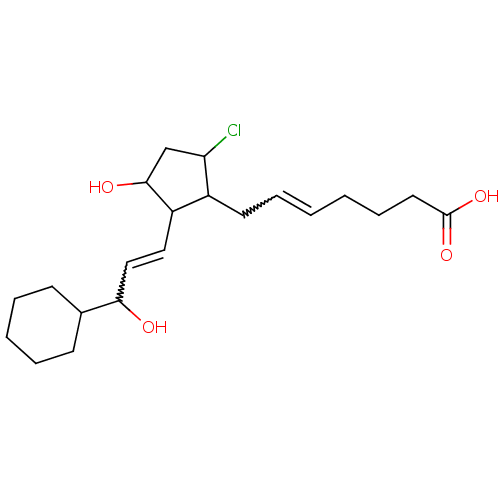
(CAS_5311503 | NSC_5311503 | ZK110841)Show SMILES OC(C=CC1C(O)CC(Cl)C1CC=CCCCC(O)=O)C1CCCCC1 |w:2.1,12.12| Show InChI InChI=1S/C21H33ClO4/c22-18-14-20(24)17(12-13-19(23)15-8-4-3-5-9-15)16(18)10-6-1-2-7-11-21(25)26/h1,6,12-13,15-20,23-24H,2-5,7-11,14H2,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
Biochim Biophys Acta 1483: 285-93 (2000)
Article DOI: 10.1016/s1388-1981(99)00164-x
BindingDB Entry DOI: 10.7270/Q2J964XQ |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM35847

((15S)-prostaglandin E2 | (5Z,11alpha,13E,15S)-11,1...)Show SMILES CCCCC[C@H](O)\C=C\[C@H]1[C@H](O)CC(=O)[C@@H]1C\C=C/CCCC(O)=O Show InChI InChI=1S/C20H32O5/c1-2-3-6-9-15(21)12-13-17-16(18(22)14-19(17)23)10-7-4-5-8-11-20(24)25/h4,7,12-13,15-17,19,21,23H,2-3,5-6,8-11,14H2,1H3,(H,24,25)/b7-4-,13-12+/t15-,16+,17+,19+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
Biochim Biophys Acta 1483: 285-93 (2000)
Article DOI: 10.1016/s1388-1981(99)00164-x
BindingDB Entry DOI: 10.7270/Q2J964XQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM85183
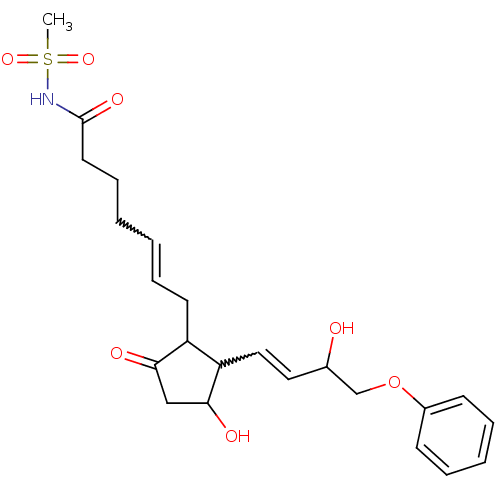
(CAS_60325-46-4 | NSC_43251 | SULPROSTONE)Show SMILES CS(=O)(=O)NC(=O)CCCC=CCC1C(C=CC(O)COc2ccccc2)C(O)CC1=O |w:10.9,15.14| Show InChI InChI=1S/C23H31NO7S/c1-32(29,30)24-23(28)12-8-3-2-7-11-19-20(22(27)15-21(19)26)14-13-17(25)16-31-18-9-5-4-6-10-18/h2,4-7,9-10,13-14,17,19-20,22,25,27H,3,8,11-12,15-16H2,1H3,(H,24,28) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
Biochim Biophys Acta 1483: 285-93 (2000)
Article DOI: 10.1016/s1388-1981(99)00164-x
BindingDB Entry DOI: 10.7270/Q2J964XQ |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM85175
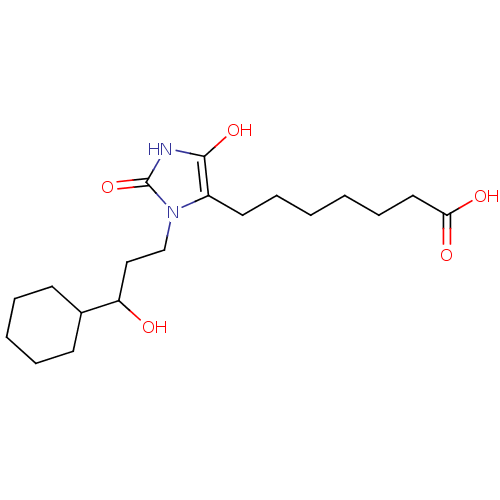
(BW245C | CAS_72814-32-5 | NSC_3080928)Show SMILES OC(CCn1c(CCCCCCC(O)=O)c(O)[nH]c1=O)C1CCCCC1 Show InChI InChI=1S/C19H32N2O5/c22-16(14-8-4-3-5-9-14)12-13-21-15(18(25)20-19(21)26)10-6-1-2-7-11-17(23)24/h14,16,22,25H,1-13H2,(H,20,26)(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
Biochim Biophys Acta 1483: 285-93 (2000)
Article DOI: 10.1016/s1388-1981(99)00164-x
BindingDB Entry DOI: 10.7270/Q2J964XQ |
More data for this
Ligand-Target Pair | |
Prostaglandin F2-alpha receptor
(Homo sapiens (Human)) | BDBM50085910
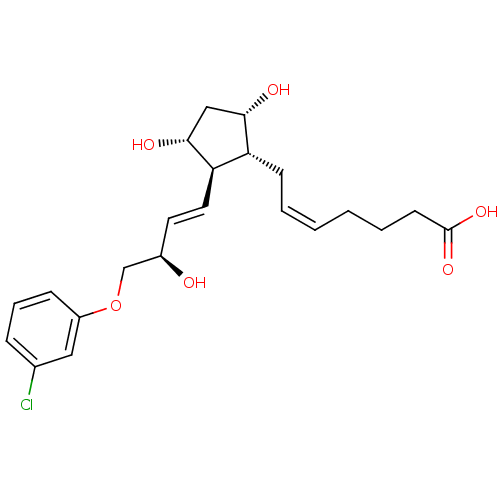
((Z)-7-{(1R,2R,3R,5S)-2-[(E)-(R)-4-(3-Chloro-phenox...)Show SMILES O[C@@H](COc1cccc(Cl)c1)\C=C\[C@H]1[C@H](O)C[C@H](O)[C@@H]1C\C=C/CCCC(O)=O Show InChI InChI=1S/C22H29ClO6/c23-15-6-5-7-17(12-15)29-14-16(24)10-11-19-18(20(25)13-21(19)26)8-3-1-2-4-9-22(27)28/h1,3,5-7,10-12,16,18-21,24-26H,2,4,8-9,13-14H2,(H,27,28)/b3-1-,11-10+/t16-,18-,19-,20+,21-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
Biochim Biophys Acta 1483: 285-93 (2000)
Article DOI: 10.1016/s1388-1981(99)00164-x
BindingDB Entry DOI: 10.7270/Q2J964XQ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50067735
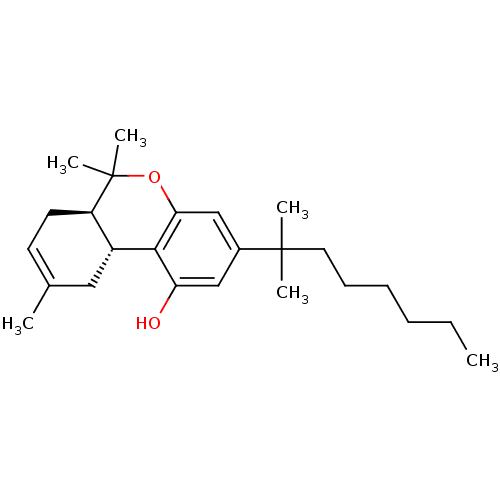
((6aR,10aR)-3-(1,1-Dimethyl-heptyl)-6,6,9-trimethyl...)Show SMILES CCCCCCC(C)(C)c1cc(O)c2[C@@H]3CC(C)=CC[C@H]3C(C)(C)Oc2c1 |c:17| Show InChI InChI=1S/C25H38O2/c1-7-8-9-10-13-24(3,4)18-15-21(26)23-19-14-17(2)11-12-20(19)25(5,6)27-22(23)16-18/h11,15-16,19-20,26H,7-10,12-14H2,1-6H3/t19-,20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against human Cannabinoid receptor 2 by using radioligand ([3H]-CP-55,940) assay. |
Bioorg Med Chem Lett 6: 189-194 (1996)
Article DOI: 10.1016/0960-894X(95)00573-C
BindingDB Entry DOI: 10.7270/Q2KS6RHX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50287934
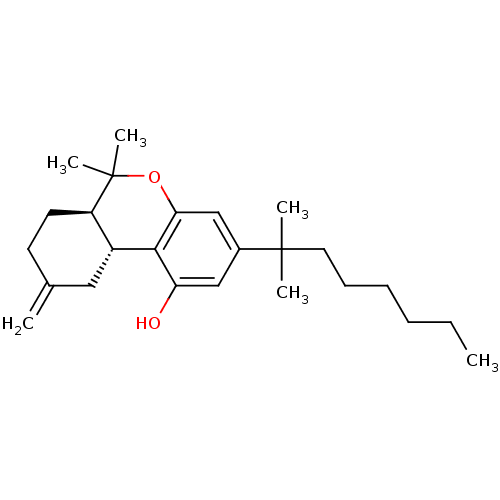
((6aR,10aR)-3-(1,1-Dimethyl-heptyl)-6,6-dimethyl-9-...)Show SMILES CCCCCCC(C)(C)c1cc(O)c2[C@@H]3CC(=C)CC[C@H]3C(C)(C)Oc2c1 Show InChI InChI=1S/C25H38O2/c1-7-8-9-10-13-24(3,4)18-15-21(26)23-19-14-17(2)11-12-20(19)25(5,6)27-22(23)16-18/h15-16,19-20,26H,2,7-14H2,1,3-6H3/t19-,20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against human Cannabinoid receptor 2 by using radioligand ([3H]-CP-55,940) assay. |
Bioorg Med Chem Lett 6: 189-194 (1996)
Article DOI: 10.1016/0960-894X(95)00573-C
BindingDB Entry DOI: 10.7270/Q2KS6RHX |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50193920
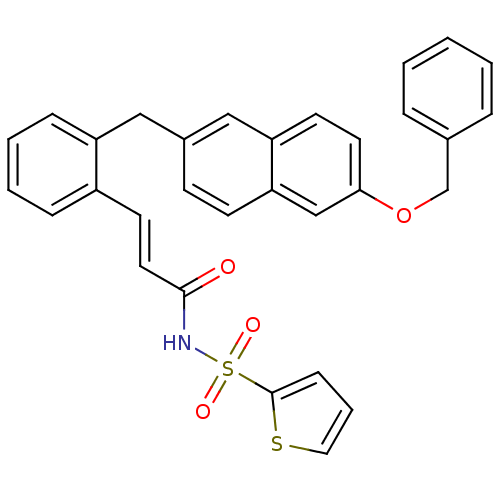
(3-(2-((6-(benzyloxy)naphthalen-2-yl)methyl)phenyl)...)Show SMILES O=C(NS(=O)(=O)c1cccs1)\C=C\c1ccccc1Cc1ccc2cc(OCc3ccccc3)ccc2c1 Show InChI InChI=1S/C31H25NO4S2/c33-30(32-38(34,35)31-11-6-18-37-31)17-15-25-9-4-5-10-26(25)19-24-12-13-28-21-29(16-14-27(28)20-24)36-22-23-7-2-1-3-8-23/h1-18,20-21H,19,22H2,(H,32,33)/b17-15+ | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity to EP3 receptor |
Bioorg Med Chem Lett 16: 5639-42 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.025
BindingDB Entry DOI: 10.7270/Q2NS0VQ6 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50193922
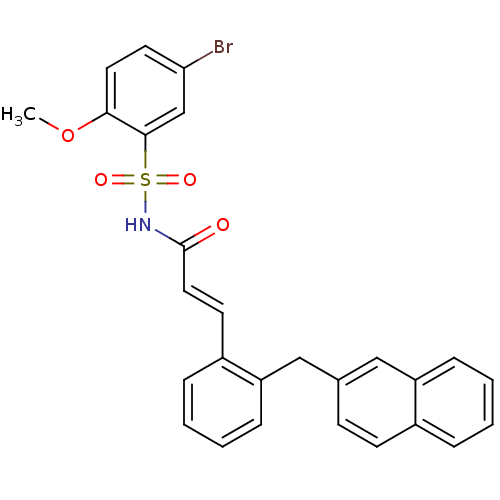
(CHEMBL218071 | N-(5-bromo-2-methoxyphenylsulfonyl)...)Show SMILES COc1ccc(Br)cc1S(=O)(=O)NC(=O)\C=C\c1ccccc1Cc1ccc2ccccc2c1 Show InChI InChI=1S/C27H22BrNO4S/c1-33-25-14-13-24(28)18-26(25)34(31,32)29-27(30)15-12-21-7-3-5-9-23(21)17-19-10-11-20-6-2-4-8-22(20)16-19/h2-16,18H,17H2,1H3,(H,29,30)/b15-12+ | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity to EP3 receptor |
Bioorg Med Chem Lett 16: 5639-42 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.025
BindingDB Entry DOI: 10.7270/Q2NS0VQ6 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50193935

(3-(2-((6-(benzyloxy)naphthalen-2-yl)methyl)phenyl)...)Show SMILES COc1ccc(Br)cc1S(=O)(=O)NC(=O)\C=C\c1ccccc1Cc1ccc2cc(OCc3ccccc3)ccc2c1 Show InChI InChI=1S/C34H28BrNO5S/c1-40-32-17-15-30(35)22-33(32)42(38,39)36-34(37)18-14-26-9-5-6-10-27(26)19-25-11-12-29-21-31(16-13-28(29)20-25)41-23-24-7-3-2-4-8-24/h2-18,20-22H,19,23H2,1H3,(H,36,37)/b18-14+ | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity to EP3 receptor in presence of HSA |
Bioorg Med Chem Lett 16: 5639-42 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.025
BindingDB Entry DOI: 10.7270/Q2NS0VQ6 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM35847

((15S)-prostaglandin E2 | (5Z,11alpha,13E,15S)-11,1...)Show SMILES CCCCC[C@H](O)\C=C\[C@H]1[C@H](O)CC(=O)[C@@H]1C\C=C/CCCC(O)=O Show InChI InChI=1S/C20H32O5/c1-2-3-6-9-15(21)12-13-17-16(18(22)14-19(17)23)10-7-4-5-8-11-20(24)25/h4,7,12-13,15-17,19,21,23H,2-3,5-6,8-11,14H2,1H3,(H,24,25)/b7-4-,13-12+/t15-,16+,17+,19+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
Biochim Biophys Acta 1483: 285-93 (2000)
Article DOI: 10.1016/s1388-1981(99)00164-x
BindingDB Entry DOI: 10.7270/Q2J964XQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50067735

((6aR,10aR)-3-(1,1-Dimethyl-heptyl)-6,6,9-trimethyl...)Show SMILES CCCCCCC(C)(C)c1cc(O)c2[C@@H]3CC(C)=CC[C@H]3C(C)(C)Oc2c1 |c:17| Show InChI InChI=1S/C25H38O2/c1-7-8-9-10-13-24(3,4)18-15-21(26)23-19-14-17(2)11-12-20(19)25(5,6)27-22(23)16-18/h11,15-16,19-20,26H,7-10,12-14H2,1-6H3/t19-,20-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 0.830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against human cannabinoid receptor by using radioligand ([3H]-CP-55,940) assay. |
Bioorg Med Chem Lett 6: 189-194 (1996)
Article DOI: 10.1016/0960-894X(95)00573-C
BindingDB Entry DOI: 10.7270/Q2KS6RHX |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50193935

(3-(2-((6-(benzyloxy)naphthalen-2-yl)methyl)phenyl)...)Show SMILES COc1ccc(Br)cc1S(=O)(=O)NC(=O)\C=C\c1ccccc1Cc1ccc2cc(OCc3ccccc3)ccc2c1 Show InChI InChI=1S/C34H28BrNO5S/c1-40-32-17-15-30(35)22-33(32)42(38,39)36-34(37)18-14-26-9-5-6-10-27(26)19-25-11-12-29-21-31(16-13-28(29)20-25)41-23-24-7-3-2-4-8-24/h2-18,20-22H,19,23H2,1H3,(H,36,37)/b18-14+ | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity to EP3 receptor |
Bioorg Med Chem Lett 16: 5639-42 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.025
BindingDB Entry DOI: 10.7270/Q2NS0VQ6 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50193921
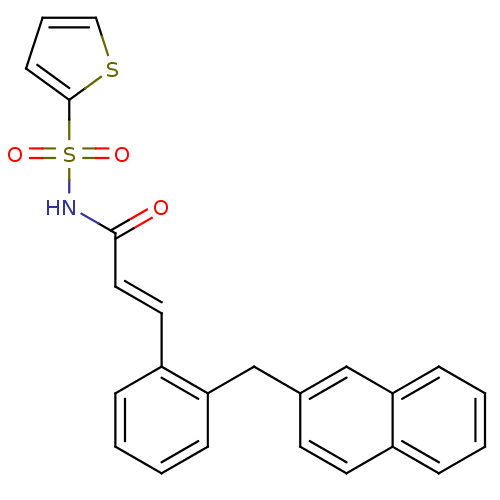
(3-(2-(naphthalen-2-ylmethyl)phenyl)-N-(thiophen-2-...)Show SMILES O=C(NS(=O)(=O)c1cccs1)\C=C\c1ccccc1Cc1ccc2ccccc2c1 Show InChI InChI=1S/C24H19NO3S2/c26-23(25-30(27,28)24-10-5-15-29-24)14-13-20-7-2-4-9-22(20)17-18-11-12-19-6-1-3-8-21(19)16-18/h1-16H,17H2,(H,25,26)/b14-13+ | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity to EP3 receptor |
Bioorg Med Chem Lett 16: 5639-42 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.025
BindingDB Entry DOI: 10.7270/Q2NS0VQ6 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50193938

(3-(2-(4-(benzyloxy)-3-methoxycinnamyl)phenyl)-N-(t...)Show SMILES COc1cc(\C=C\Cc2ccccc2\C=C\C(=O)NS(=O)(=O)c2cccs2)ccc1OCc1ccccc1 Show InChI InChI=1S/C30H27NO5S2/c1-35-28-21-23(16-18-27(28)36-22-24-9-3-2-4-10-24)11-7-14-25-12-5-6-13-26(25)17-19-29(32)31-38(33,34)30-15-8-20-37-30/h2-13,15-21H,14,22H2,1H3,(H,31,32)/b11-7+,19-17+ | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity to EP3 receptor |
Bioorg Med Chem Lett 16: 5639-42 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.025
BindingDB Entry DOI: 10.7270/Q2NS0VQ6 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50193918
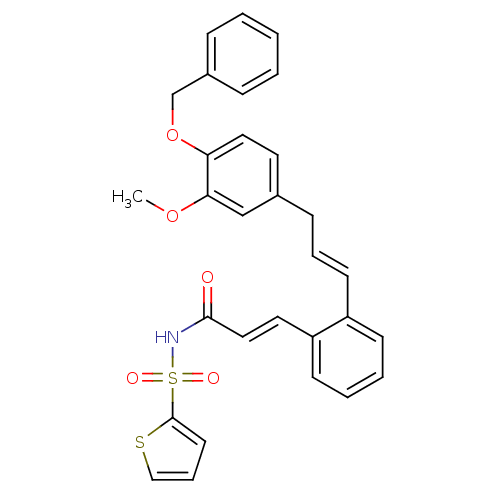
(3-(2-((E)-3-(4-(benzyloxy)-3-methoxyphenyl)prop-1-...)Show SMILES COc1cc(C\C=C\c2ccccc2\C=C\C(=O)NS(=O)(=O)c2cccs2)ccc1OCc1ccccc1 Show InChI InChI=1S/C30H27NO5S2/c1-35-28-21-23(16-18-27(28)36-22-24-9-3-2-4-10-24)11-7-14-25-12-5-6-13-26(25)17-19-29(32)31-38(33,34)30-15-8-20-37-30/h2-10,12-21H,11,22H2,1H3,(H,31,32)/b14-7+,19-17+ | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity to EP3 receptor |
Bioorg Med Chem Lett 16: 5639-42 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.025
BindingDB Entry DOI: 10.7270/Q2NS0VQ6 |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM82213
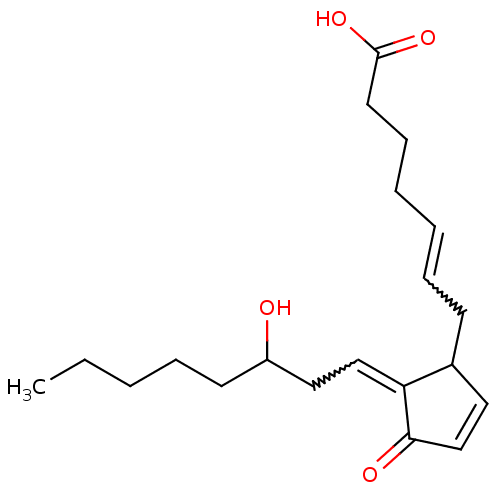
(CAS_41598-07-6 | NSC_114678 | PGD2)Show SMILES CCCCCC(O)CC=C1C(CC=CCCCC(O)=O)C=CC1=O |w:8.7,12.11,c:20| Show InChI InChI=1S/C20H30O4/c1-2-3-6-10-17(21)13-14-18-16(12-15-19(18)22)9-7-4-5-8-11-20(23)24/h4,7,12,14-17,21H,2-3,5-6,8-11,13H2,1H3,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
Biochim Biophys Acta 1483: 285-93 (2000)
Article DOI: 10.1016/s1388-1981(99)00164-x
BindingDB Entry DOI: 10.7270/Q2J964XQ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50287941
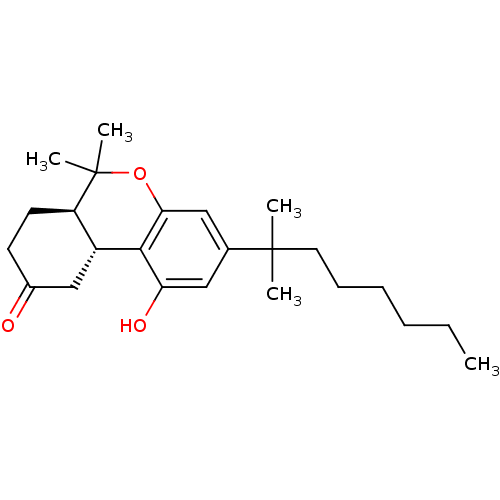
((6aR,10aR)-1-hydroxy-6,6-dimethyl-3-(2-methyloctan...)Show SMILES CCCCCCC(C)(C)c1cc(O)c2[C@@H]3CC(=O)CC[C@H]3C(C)(C)Oc2c1 Show InChI InChI=1S/C24H36O3/c1-6-7-8-9-12-23(2,3)16-13-20(26)22-18-15-17(25)10-11-19(18)24(4,5)27-21(22)14-16/h13-14,18-19,26H,6-12,15H2,1-5H3/t18-,19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against human Cannabinoid receptor 2 by using radioligand ([3H]-CP-55,940) assay. |
Bioorg Med Chem Lett 6: 189-194 (1996)
Article DOI: 10.1016/0960-894X(95)00573-C
BindingDB Entry DOI: 10.7270/Q2KS6RHX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50287934

((6aR,10aR)-3-(1,1-Dimethyl-heptyl)-6,6-dimethyl-9-...)Show SMILES CCCCCCC(C)(C)c1cc(O)c2[C@@H]3CC(=C)CC[C@H]3C(C)(C)Oc2c1 Show InChI InChI=1S/C25H38O2/c1-7-8-9-10-13-24(3,4)18-15-21(26)23-19-14-17(2)11-12-20(19)25(5,6)27-22(23)16-18/h15-16,19-20,26H,2,7-14H2,1,3-6H3/t19-,20-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against human cannabinoid receptor by using radioligand ([3H]-CP-55,940) assay. |
Bioorg Med Chem Lett 6: 189-194 (1996)
Article DOI: 10.1016/0960-894X(95)00573-C
BindingDB Entry DOI: 10.7270/Q2KS6RHX |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM85603

(CAS_5311503 | NSC_5311503 | ZK110841)Show SMILES OC(C=CC1C(O)CC(Cl)C1CC=CCCCC(O)=O)C1CCCCC1 |w:2.1,12.12| Show InChI InChI=1S/C21H33ClO4/c22-18-14-20(24)17(12-13-19(23)15-8-4-3-5-9-15)16(18)10-6-1-2-7-11-21(25)26/h1,6,12-13,15-20,23-24H,2-5,7-11,14H2,(H,25,26) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
Biochim Biophys Acta 1483: 285-93 (2000)
Article DOI: 10.1016/s1388-1981(99)00164-x
BindingDB Entry DOI: 10.7270/Q2J964XQ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50287935
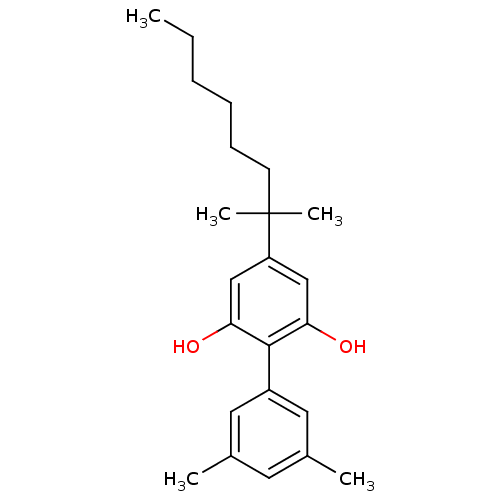
(4-(1,1-Dimethyl-heptyl)-3',5'-dimethyl-biphenyl-2,...)Show SMILES CCCCCCC(C)(C)c1cc(O)c(c(O)c1)-c1cc(C)cc(C)c1 Show InChI InChI=1S/C23H32O2/c1-6-7-8-9-10-23(4,5)19-14-20(24)22(21(25)15-19)18-12-16(2)11-17(3)13-18/h11-15,24-25H,6-10H2,1-5H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against human Cannabinoid receptor 2 by using radioligand ([3H]-CP-55,940) assay. |
Bioorg Med Chem Lett 6: 189-194 (1996)
Article DOI: 10.1016/0960-894X(95)00573-C
BindingDB Entry DOI: 10.7270/Q2KS6RHX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50287930
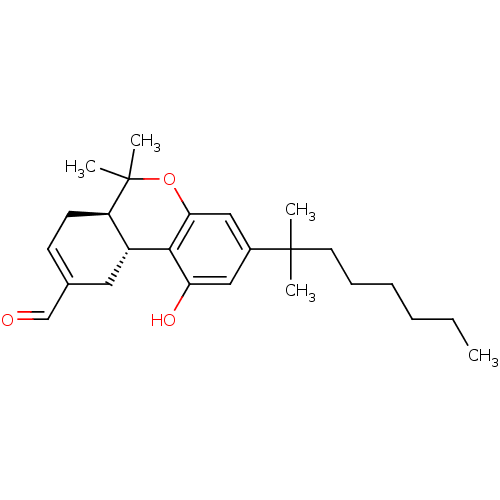
((6aR,10aR)-3-(1,1-Dimethyl-heptyl)-1-hydroxy-6,6-d...)Show SMILES CCCCCCC(C)(C)c1cc(O)c2[C@@H]3CC(C=O)=CC[C@H]3C(C)(C)Oc2c1 |c:18| Show InChI InChI=1S/C25H36O3/c1-6-7-8-9-12-24(2,3)18-14-21(27)23-19-13-17(16-26)10-11-20(19)25(4,5)28-22(23)15-18/h10,14-16,19-20,27H,6-9,11-13H2,1-5H3/t19-,20-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against human cannabinoid receptor by using radioligand ([3H]-CP-55,940) assay. |
Bioorg Med Chem Lett 6: 189-194 (1996)
Article DOI: 10.1016/0960-894X(95)00573-C
BindingDB Entry DOI: 10.7270/Q2KS6RHX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50287941

((6aR,10aR)-1-hydroxy-6,6-dimethyl-3-(2-methyloctan...)Show SMILES CCCCCCC(C)(C)c1cc(O)c2[C@@H]3CC(=O)CC[C@H]3C(C)(C)Oc2c1 Show InChI InChI=1S/C24H36O3/c1-6-7-8-9-12-23(2,3)16-13-20(26)22-18-15-17(25)10-11-19(18)24(4,5)27-21(22)14-16/h13-14,18-19,26H,6-12,15H2,1-5H3/t18-,19-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against human cannabinoid receptor by using radioligand ([3H]-CP-55,940) assay. |
Bioorg Med Chem Lett 6: 189-194 (1996)
Article DOI: 10.1016/0960-894X(95)00573-C
BindingDB Entry DOI: 10.7270/Q2KS6RHX |
More data for this
Ligand-Target Pair | |
Prostaglandin F2-alpha receptor
(Homo sapiens (Human)) | BDBM85173
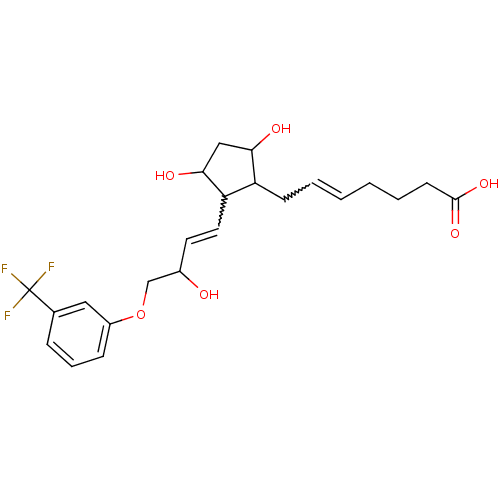
(CAS_40666-16-8 | FLUPROSTENOL | NSC_5311100)Show SMILES OC(COc1cccc(c1)C(F)(F)F)C=CC1C(O)CC(O)C1CC=CCCCC(O)=O |w:15.16,24.25| Show InChI InChI=1S/C23H29F3O6/c24-23(25,26)15-6-5-7-17(12-15)32-14-16(27)10-11-19-18(20(28)13-21(19)29)8-3-1-2-4-9-22(30)31/h1,3,5-7,10-12,16,18-21,27-29H,2,4,8-9,13-14H2,(H,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
Biochim Biophys Acta 1483: 285-93 (2000)
Article DOI: 10.1016/s1388-1981(99)00164-x
BindingDB Entry DOI: 10.7270/Q2J964XQ |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50193937

(3-(2-((E)-3-(4-methoxyphenyl)prop-1-enyl)phenyl)-N...)Show SMILES COc1ccc(C\C=C\c2ccccc2\C=C\C(=O)NS(=O)(=O)c2cccs2)cc1 Show InChI InChI=1S/C23H21NO4S2/c1-28-21-14-11-18(12-15-21)6-4-9-19-7-2-3-8-20(19)13-16-22(25)24-30(26,27)23-10-5-17-29-23/h2-5,7-17H,6H2,1H3,(H,24,25)/b9-4+,16-13+ | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity to EP3 receptor |
Bioorg Med Chem Lett 16: 5639-42 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.025
BindingDB Entry DOI: 10.7270/Q2NS0VQ6 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50193923
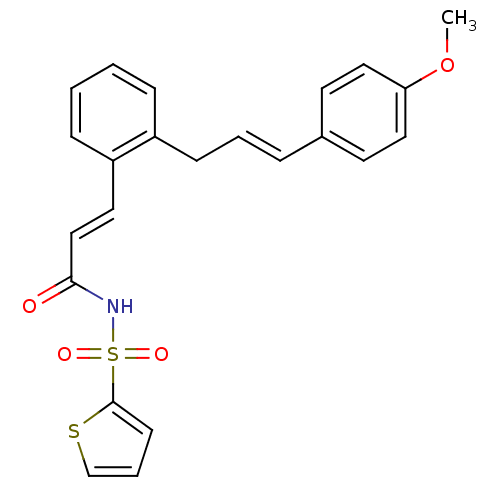
(3-(2-(4-methoxycinnamyl)phenyl)-N-(thiophen-2-ylsu...)Show SMILES COc1ccc(\C=C\Cc2ccccc2\C=C\C(=O)NS(=O)(=O)c2cccs2)cc1 Show InChI InChI=1S/C23H21NO4S2/c1-28-21-14-11-18(12-15-21)6-4-9-19-7-2-3-8-20(19)13-16-22(25)24-30(26,27)23-10-5-17-29-23/h2-8,10-17H,9H2,1H3,(H,24,25)/b6-4+,16-13+ | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity to EP3 receptor |
Bioorg Med Chem Lett 16: 5639-42 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.025
BindingDB Entry DOI: 10.7270/Q2NS0VQ6 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50193920

(3-(2-((6-(benzyloxy)naphthalen-2-yl)methyl)phenyl)...)Show SMILES O=C(NS(=O)(=O)c1cccs1)\C=C\c1ccccc1Cc1ccc2cc(OCc3ccccc3)ccc2c1 Show InChI InChI=1S/C31H25NO4S2/c33-30(32-38(34,35)31-11-6-18-37-31)17-15-25-9-4-5-10-26(25)19-24-12-13-28-21-29(16-14-27(28)20-24)36-22-23-7-2-1-3-8-23/h1-18,20-21H,19,22H2,(H,32,33)/b17-15+ | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity to EP3 receptor in presence of HSA |
Bioorg Med Chem Lett 16: 5639-42 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.025
BindingDB Entry DOI: 10.7270/Q2NS0VQ6 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50287930

((6aR,10aR)-3-(1,1-Dimethyl-heptyl)-1-hydroxy-6,6-d...)Show SMILES CCCCCCC(C)(C)c1cc(O)c2[C@@H]3CC(C=O)=CC[C@H]3C(C)(C)Oc2c1 |c:18| Show InChI InChI=1S/C25H36O3/c1-6-7-8-9-12-24(2,3)18-14-21(27)23-19-13-17(16-26)10-11-20(19)25(4,5)28-22(23)15-18/h10,14-16,19-20,27H,6-9,11-13H2,1-5H3/t19-,20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against human Cannabinoid receptor 2 by using radioligand ([3H]-CP-55,940) assay. |
Bioorg Med Chem Lett 6: 189-194 (1996)
Article DOI: 10.1016/0960-894X(95)00573-C
BindingDB Entry DOI: 10.7270/Q2KS6RHX |
More data for this
Ligand-Target Pair | |
Prostaglandin F2-alpha receptor
(Homo sapiens (Human)) | BDBM50240648
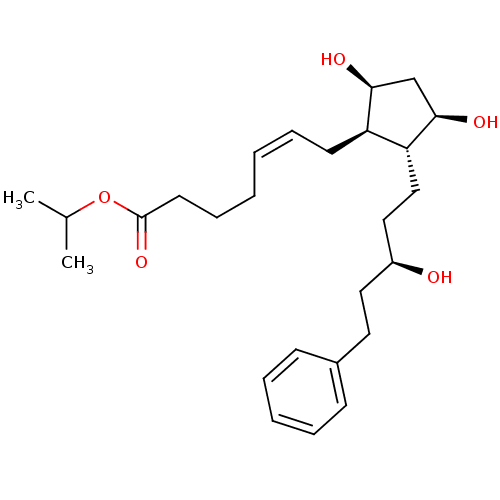
(LATANOPROST (FREE ACID) | PhXA 41 | Xalatan | isop...)Show SMILES CC(C)OC(=O)CCC\C=C/C[C@H]1[C@@H](O)C[C@@H](O)[C@@H]1CC[C@@H](O)CCc1ccccc1 |r| Show InChI InChI=1S/C26H40O5/c1-19(2)31-26(30)13-9-4-3-8-12-22-23(25(29)18-24(22)28)17-16-21(27)15-14-20-10-6-5-7-11-20/h3,5-8,10-11,19,21-25,27-29H,4,9,12-18H2,1-2H3/b8-3-/t21-,22+,23+,24-,25+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
Biochim Biophys Acta 1483: 285-93 (2000)
Article DOI: 10.1016/s1388-1981(99)00164-x
BindingDB Entry DOI: 10.7270/Q2J964XQ |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50193926

(3-(2-(2-(2,6-dichlorobenzyloxy)-5-methylcinnamyl)p...)Show SMILES Cc1ccc(OCc2c(Cl)cccc2Cl)c(\C=C\Cc2ccccc2\C=C\C(O)=O)c1 Show InChI InChI=1S/C26H22Cl2O3/c1-18-12-14-25(31-17-22-23(27)10-5-11-24(22)28)21(16-18)9-4-8-19-6-2-3-7-20(19)13-15-26(29)30/h2-7,9-16H,8,17H2,1H3,(H,29,30)/b9-4+,15-13+ | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity to EP3 receptor |
Bioorg Med Chem Lett 16: 5639-42 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.025
BindingDB Entry DOI: 10.7270/Q2NS0VQ6 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50159778
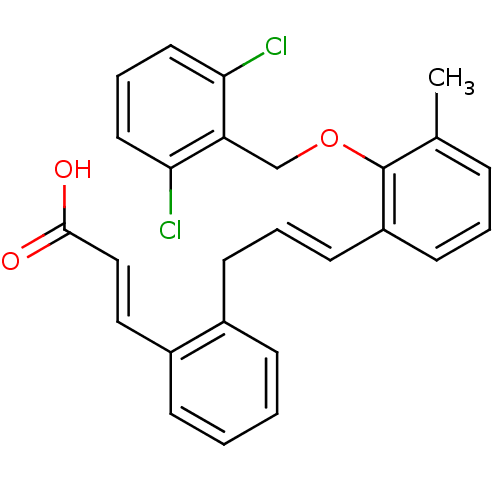
((E)-3-(2-{(E)-3-[2-(2,6-Dichloro-benzyloxy)-3-meth...)Show SMILES Cc1cccc(\C=C\Cc2ccccc2\C=C\C(O)=O)c1OCc1c(Cl)cccc1Cl Show InChI InChI=1S/C26H22Cl2O3/c1-18-7-4-11-21(26(18)31-17-22-23(27)13-6-14-24(22)28)12-5-10-19-8-2-3-9-20(19)15-16-25(29)30/h2-9,11-16H,10,17H2,1H3,(H,29,30)/b12-5+,16-15+ | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity for human prostanoid EP3 receptor |
Bioorg Med Chem Lett 15: 527-30 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.051
BindingDB Entry DOI: 10.7270/Q26H4J51 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50193924
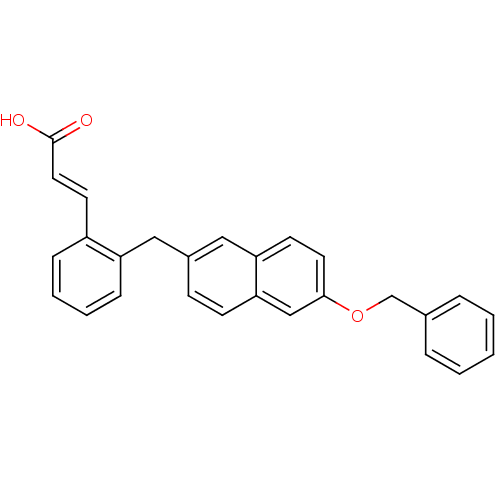
(3-(2-((6-(benzyloxy)naphthalen-2-yl)methyl)phenyl)...)Show SMILES OC(=O)\C=C\c1ccccc1Cc1ccc2cc(OCc3ccccc3)ccc2c1 Show InChI InChI=1S/C27H22O3/c28-27(29)15-13-22-8-4-5-9-23(22)16-21-10-11-25-18-26(14-12-24(25)17-21)30-19-20-6-2-1-3-7-20/h1-15,17-18H,16,19H2,(H,28,29)/b15-13+ | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity to EP3 receptor |
Bioorg Med Chem Lett 16: 5639-42 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.025
BindingDB Entry DOI: 10.7270/Q2NS0VQ6 |
More data for this
Ligand-Target Pair | |
Prostaglandin F2-alpha receptor
(Homo sapiens (Human)) | BDBM50020300
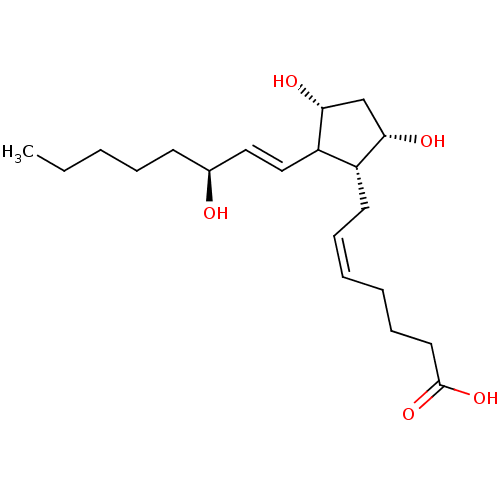
((S-isomer)7-[3,5-Dihydroxy-2-(3-hydroxy-oct-1-enyl...)Show SMILES CCCCC[C@H](O)\C=C\C1[C@H](O)C[C@H](O)[C@@H]1C\C=C/CCCC(O)=O Show InChI InChI=1S/C20H34O5/c1-2-3-6-9-15(21)12-13-17-16(18(22)14-19(17)23)10-7-4-5-8-11-20(24)25/h4,7,12-13,15-19,21-23H,2-3,5-6,8-11,14H2,1H3,(H,24,25)/b7-4-,13-12+/t15-,16+,17?,18-,19+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
Biochim Biophys Acta 1483: 285-93 (2000)
Article DOI: 10.1016/s1388-1981(99)00164-x
BindingDB Entry DOI: 10.7270/Q2J964XQ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50213163
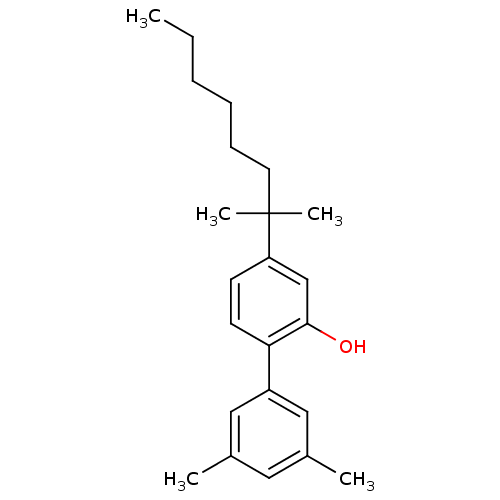
(4-(1,1-dimethyl-heptyl)-3',5'-dimethyl-biphenyl-2-...)Show InChI InChI=1S/C23H32O/c1-6-7-8-9-12-23(4,5)20-10-11-21(22(24)16-20)19-14-17(2)13-18(3)15-19/h10-11,13-16,24H,6-9,12H2,1-5H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against human Cannabinoid receptor 2 by using radioligand ([3H]-CP-55,940) assay. |
Bioorg Med Chem Lett 6: 189-194 (1996)
Article DOI: 10.1016/0960-894X(95)00573-C
BindingDB Entry DOI: 10.7270/Q2KS6RHX |
More data for this
Ligand-Target Pair | |
Solute carrier organic anion transporter family member 2A1
(Homo sapiens (Human)) | BDBM50008781
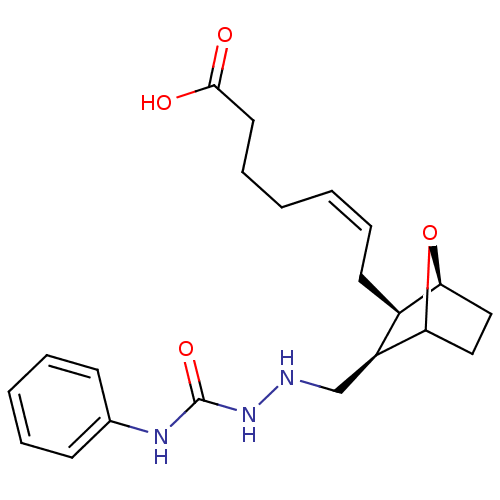
(7-(3-(2-ethyl-N-phenylhydrazinecarboxamide)-7-oxa-...)Show SMILES OC(=O)CCC\C=C/C[C@H]1[C@@H]2CCC(O2)[C@H]1CNNC(=O)Nc1ccccc1 Show InChI InChI=1S/C21H29N3O4/c25-20(26)11-7-2-1-6-10-16-17(19-13-12-18(16)28-19)14-22-24-21(27)23-15-8-4-3-5-9-15/h1,3-6,8-9,16-19,22H,2,7,10-14H2,(H,25,26)(H2,23,24,27)/b6-1-/t16-,17+,18+,19?/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
Biochim Biophys Acta 1483: 285-93 (2000)
Article DOI: 10.1016/s1388-1981(99)00164-x
BindingDB Entry DOI: 10.7270/Q2J964XQ |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50085910

((Z)-7-{(1R,2R,3R,5S)-2-[(E)-(R)-4-(3-Chloro-phenox...)Show SMILES O[C@@H](COc1cccc(Cl)c1)\C=C\[C@H]1[C@H](O)C[C@H](O)[C@@H]1C\C=C/CCCC(O)=O Show InChI InChI=1S/C22H29ClO6/c23-15-6-5-7-17(12-15)29-14-16(24)10-11-19-18(20(25)13-21(19)26)8-3-1-2-4-9-22(27)28/h1,3,5-7,10-12,16,18-21,24-26H,2,4,8-9,13-14H2,(H,27,28)/b3-1-,11-10+/t16-,18-,19-,20+,21-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
Biochim Biophys Acta 1483: 285-93 (2000)
Article DOI: 10.1016/s1388-1981(99)00164-x
BindingDB Entry DOI: 10.7270/Q2J964XQ |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM85605
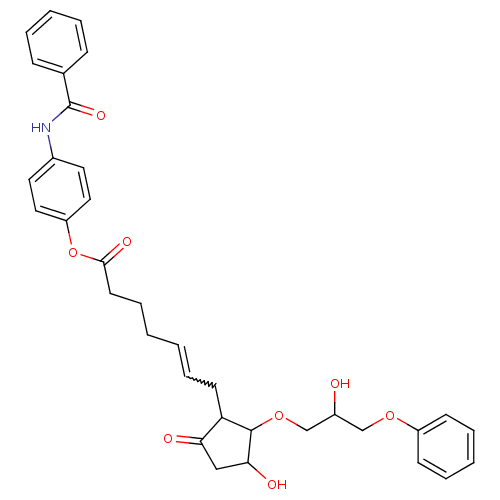
(GR 63799)Show SMILES OC(COC1C(O)CC(=O)C1CC=CCCCC(=O)Oc1ccc(NC(=O)c2ccccc2)cc1)COc1ccccc1 |w:12.12| Show InChI InChI=1S/C34H37NO8/c36-26(22-41-27-13-7-4-8-14-27)23-42-33-29(30(37)21-31(33)38)15-9-1-2-10-16-32(39)43-28-19-17-25(18-20-28)35-34(40)24-11-5-3-6-12-24/h1,3-9,11-14,17-20,26,29,31,33,36,38H,2,10,15-16,21-23H2,(H,35,40) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.77 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
Biochim Biophys Acta 1483: 285-93 (2000)
Article DOI: 10.1016/s1388-1981(99)00164-x
BindingDB Entry DOI: 10.7270/Q2J964XQ |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM35847

((15S)-prostaglandin E2 | (5Z,11alpha,13E,15S)-11,1...)Show SMILES CCCCC[C@H](O)\C=C\[C@H]1[C@H](O)CC(=O)[C@@H]1C\C=C/CCCC(O)=O Show InChI InChI=1S/C20H32O5/c1-2-3-6-9-15(21)12-13-17-16(18(22)14-19(17)23)10-7-4-5-8-11-20(24)25/h4,7,12-13,15-17,19,21,23H,2-3,5-6,8-11,14H2,1H3,(H,24,25)/b7-4-,13-12+/t15-,16+,17+,19+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
Biochim Biophys Acta 1483: 285-93 (2000)
Article DOI: 10.1016/s1388-1981(99)00164-x
BindingDB Entry DOI: 10.7270/Q2J964XQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50193930
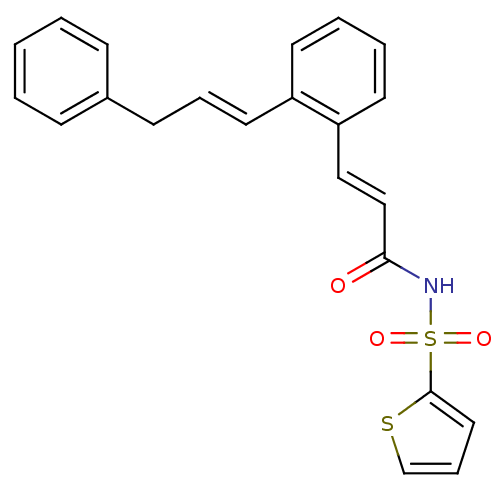
(3-(2-((E)-3-phenylprop-1-enyl)phenyl)-N-(thiophen-...)Show SMILES O=C(NS(=O)(=O)c1cccs1)\C=C\c1ccccc1\C=C\Cc1ccccc1 Show InChI InChI=1S/C22H19NO3S2/c24-21(23-28(25,26)22-14-7-17-27-22)16-15-20-12-5-4-11-19(20)13-6-10-18-8-2-1-3-9-18/h1-9,11-17H,10H2,(H,23,24)/b13-6+,16-15+ | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity to EP3 receptor |
Bioorg Med Chem Lett 16: 5639-42 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.025
BindingDB Entry DOI: 10.7270/Q2NS0VQ6 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50193925
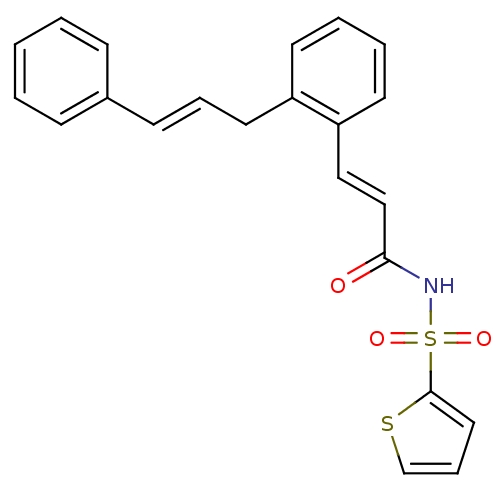
(3-(2-cinnamylphenyl)-N-(thiophen-2-ylsulfonyl)acry...)Show SMILES O=C(NS(=O)(=O)c1cccs1)\C=C\c1ccccc1C\C=C\c1ccccc1 Show InChI InChI=1S/C22H19NO3S2/c24-21(23-28(25,26)22-14-7-17-27-22)16-15-20-12-5-4-11-19(20)13-6-10-18-8-2-1-3-9-18/h1-12,14-17H,13H2,(H,23,24)/b10-6+,16-15+ | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity to EP3 receptor |
Bioorg Med Chem Lett 16: 5639-42 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.025
BindingDB Entry DOI: 10.7270/Q2NS0VQ6 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM85603

(CAS_5311503 | NSC_5311503 | ZK110841)Show SMILES OC(C=CC1C(O)CC(Cl)C1CC=CCCCC(O)=O)C1CCCCC1 |w:2.1,12.12| Show InChI InChI=1S/C21H33ClO4/c22-18-14-20(24)17(12-13-19(23)15-8-4-3-5-9-15)16(18)10-6-1-2-7-11-21(25)26/h1,6,12-13,15-20,23-24H,2-5,7-11,14H2,(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
Biochim Biophys Acta 1483: 285-93 (2000)
Article DOI: 10.1016/s1388-1981(99)00164-x
BindingDB Entry DOI: 10.7270/Q2J964XQ |
More data for this
Ligand-Target Pair | |
Prostaglandin F2-alpha receptor
(Homo sapiens (Human)) | BDBM82213

(CAS_41598-07-6 | NSC_114678 | PGD2)Show SMILES CCCCCC(O)CC=C1C(CC=CCCCC(O)=O)C=CC1=O |w:8.7,12.11,c:20| Show InChI InChI=1S/C20H30O4/c1-2-3-6-10-17(21)13-14-18-16(12-15-19(18)22)9-7-4-5-8-11-20(23)24/h4,7,12,14-17,21H,2-3,5-6,8-11,13H2,1H3,(H,23,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 6.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
Biochim Biophys Acta 1483: 285-93 (2000)
Article DOI: 10.1016/s1388-1981(99)00164-x
BindingDB Entry DOI: 10.7270/Q2J964XQ |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50193922

(CHEMBL218071 | N-(5-bromo-2-methoxyphenylsulfonyl)...)Show SMILES COc1ccc(Br)cc1S(=O)(=O)NC(=O)\C=C\c1ccccc1Cc1ccc2ccccc2c1 Show InChI InChI=1S/C27H22BrNO4S/c1-33-25-14-13-24(28)18-26(25)34(31,32)29-27(30)15-12-21-7-3-5-9-23(21)17-19-10-11-20-6-2-4-8-22(20)16-19/h2-16,18H,17H2,1H3,(H,29,30)/b15-12+ | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity to EP3 receptor in presence of HSA |
Bioorg Med Chem Lett 16: 5639-42 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.025
BindingDB Entry DOI: 10.7270/Q2NS0VQ6 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50159762
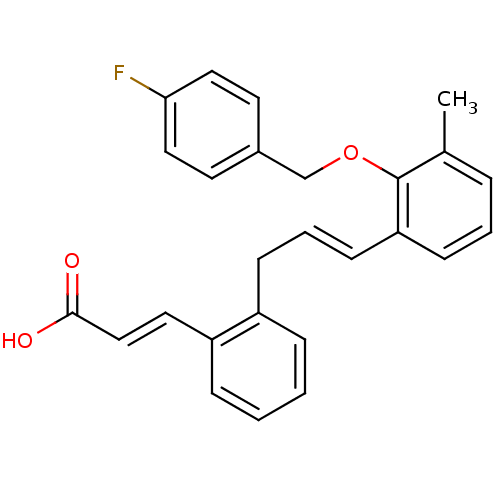
((E)-3-(2-{(E)-3-[2-(4-Fluoro-benzyloxy)-3-methyl-p...)Show SMILES Cc1cccc(\C=C\Cc2ccccc2\C=C\C(O)=O)c1OCc1ccc(F)cc1 Show InChI InChI=1S/C26H23FO3/c1-19-6-4-10-23(26(19)30-18-20-12-15-24(27)16-13-20)11-5-9-21-7-2-3-8-22(21)14-17-25(28)29/h2-8,10-17H,9,18H2,1H3,(H,28,29)/b11-5+,17-14+ | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity for human prostanoid EP3 receptor |
Bioorg Med Chem Lett 15: 527-30 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.051
BindingDB Entry DOI: 10.7270/Q26H4J51 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50134524
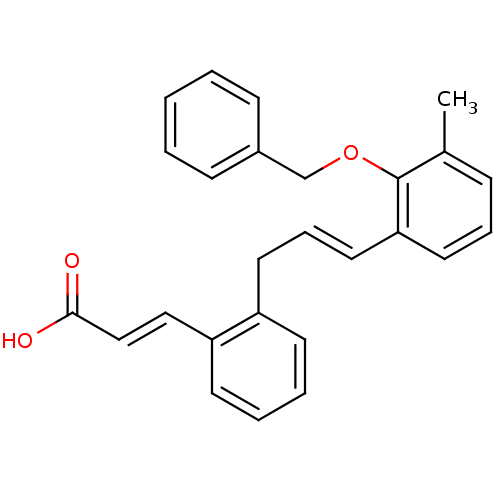
((E)-3-{2-[(E)-3-(2-Benzyloxy-3-methyl-phenyl)-ally...)Show SMILES Cc1cccc(\C=C\Cc2ccccc2\C=C\C(O)=O)c1OCc1ccccc1 Show InChI InChI=1S/C26H24O3/c1-20-9-7-15-24(26(20)29-19-21-10-3-2-4-11-21)16-8-14-22-12-5-6-13-23(22)17-18-25(27)28/h2-13,15-18H,14,19H2,1H3,(H,27,28)/b16-8+,18-17+ | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity for human prostanoid EP3 receptor |
Bioorg Med Chem Lett 15: 527-30 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.051
BindingDB Entry DOI: 10.7270/Q26H4J51 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM85606
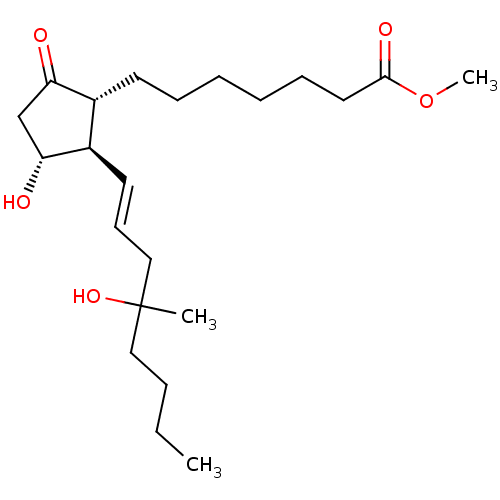
(CAS_59122-46-2 | MISOPROSTOL (Free Acid))Show SMILES CCCCC(C)(O)C\C=C\[C@H]1[C@H](O)CC(=O)[C@@H]1CCCCCCC(=O)OC |r| Show InChI InChI=1S/C22H38O5/c1-4-5-14-22(2,26)15-10-12-18-17(19(23)16-20(18)24)11-8-6-7-9-13-21(25)27-3/h10,12,17-18,20,24,26H,4-9,11,13-16H2,1-3H3/b12-10+/t17-,18-,20-,22?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
Biochim Biophys Acta 1483: 285-93 (2000)
Article DOI: 10.1016/s1388-1981(99)00164-x
BindingDB Entry DOI: 10.7270/Q2J964XQ |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50193931
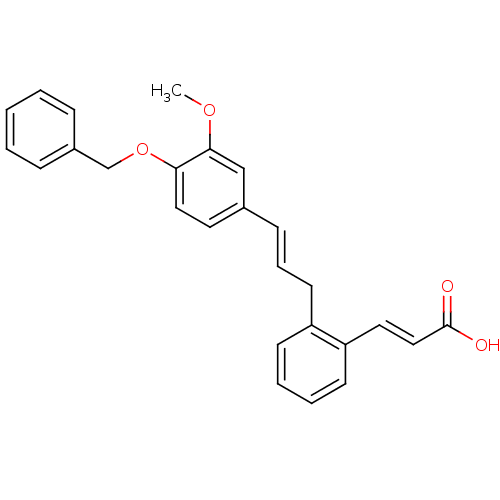
(3-(2-(4-(benzyloxy)-3-methoxycinnamyl)phenyl)acryl...)Show SMILES COc1cc(\C=C\Cc2ccccc2\C=C\C(O)=O)ccc1OCc1ccccc1 Show InChI InChI=1S/C26H24O4/c1-29-25-18-20(14-16-24(25)30-19-21-8-3-2-4-9-21)10-7-13-22-11-5-6-12-23(22)15-17-26(27)28/h2-12,14-18H,13,19H2,1H3,(H,27,28)/b10-7+,17-15+ | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity to EP3 receptor |
Bioorg Med Chem Lett 16: 5639-42 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.025
BindingDB Entry DOI: 10.7270/Q2NS0VQ6 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50159774
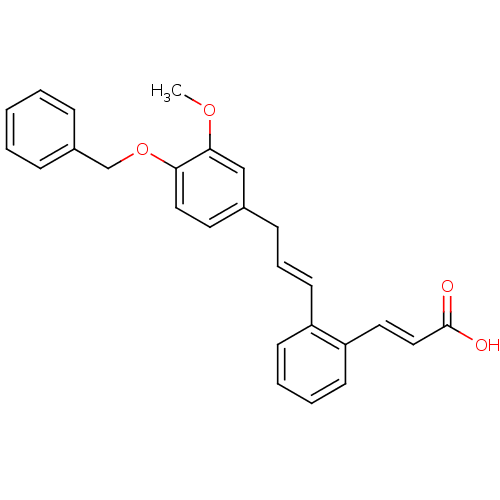
((E)-3-{2-[(E)-3-(4-Benzyloxy-3-methoxy-phenyl)-pro...)Show SMILES COc1cc(C\C=C\c2ccccc2\C=C\C(O)=O)ccc1OCc1ccccc1 Show InChI InChI=1S/C26H24O4/c1-29-25-18-20(14-16-24(25)30-19-21-8-3-2-4-9-21)10-7-13-22-11-5-6-12-23(22)15-17-26(27)28/h2-9,11-18H,10,19H2,1H3,(H,27,28)/b13-7+,17-15+ | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity to EP3 receptor |
Bioorg Med Chem Lett 16: 5639-42 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.025
BindingDB Entry DOI: 10.7270/Q2NS0VQ6 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM50159763
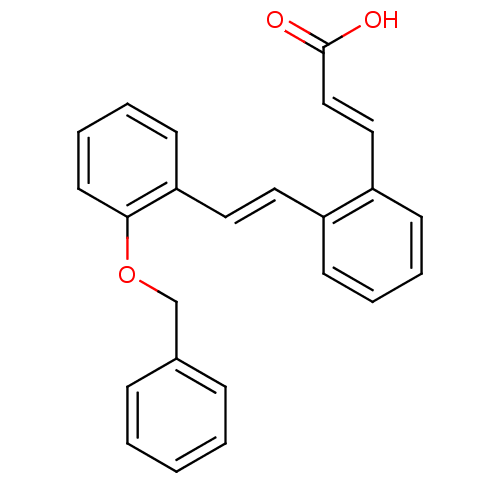
((E)-3-{2-[(E)-2-(2-Benzyloxy-phenyl)-vinyl]-phenyl...)Show InChI InChI=1S/C24H20O3/c25-24(26)17-16-21-11-5-4-10-20(21)14-15-22-12-6-7-13-23(22)27-18-19-8-2-1-3-9-19/h1-17H,18H2,(H,25,26)/b15-14+,17-16+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity for human prostanoid EP2 receptor |
Bioorg Med Chem Lett 15: 527-30 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.051
BindingDB Entry DOI: 10.7270/Q26H4J51 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data