

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Substance-P receptor (Homo sapiens (Human)) | BDBM50262566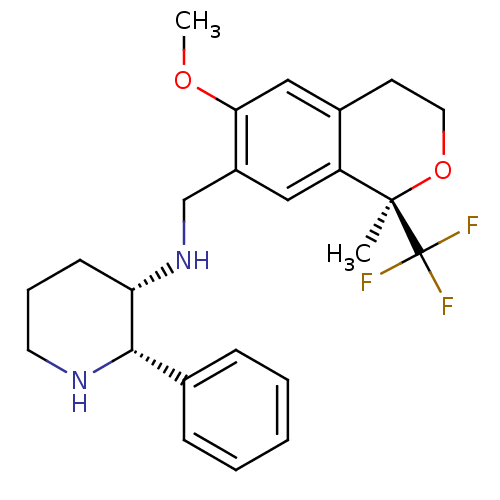 ((2S,3S)-3-[(1R)-6-Methoxy-1-methyl-1-trifluorometh...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from NK1 receptor in human IM9 cells | Bioorg Med Chem 16: 7193-205 (2008) Article DOI: 10.1016/j.bmc.2008.06.047 BindingDB Entry DOI: 10.7270/Q2RV0NHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50067935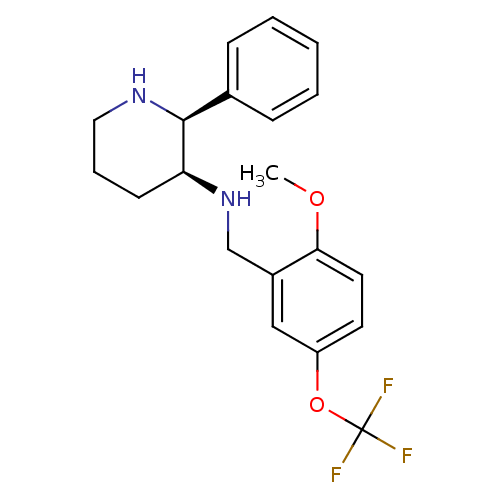 ((2-Methoxy-5-trifluoromethoxy-benzyl)-((2S,3S)-2-p...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Binding affinity to human CYP2D6 using bufuralol as substrate | Bioorg Med Chem 16: 7193-205 (2008) Article DOI: 10.1016/j.bmc.2008.06.047 BindingDB Entry DOI: 10.7270/Q2RV0NHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50262395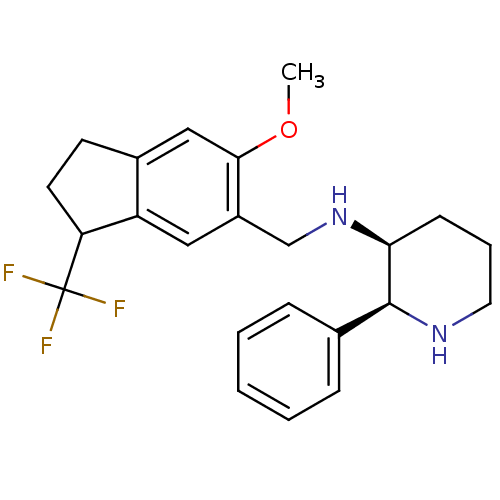 (CHEMBL478620 | R/S-(2S,3S)-N-((6-methoxy-3-(triflu...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Binding affinity to human CYP2D6 using bufuralol as substrate | Bioorg Med Chem 16: 7193-205 (2008) Article DOI: 10.1016/j.bmc.2008.06.047 BindingDB Entry DOI: 10.7270/Q2RV0NHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50262280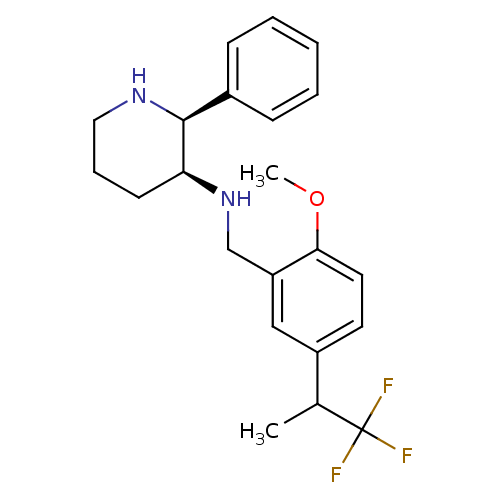 (CHEMBL513351 | R/S-(2S,3S)-N-(2-methoxy-5-(1,1,1-t...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Binding affinity to human CYP2D6 using bufuralol as substrate | Bioorg Med Chem 16: 7193-205 (2008) Article DOI: 10.1016/j.bmc.2008.06.047 BindingDB Entry DOI: 10.7270/Q2RV0NHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50262510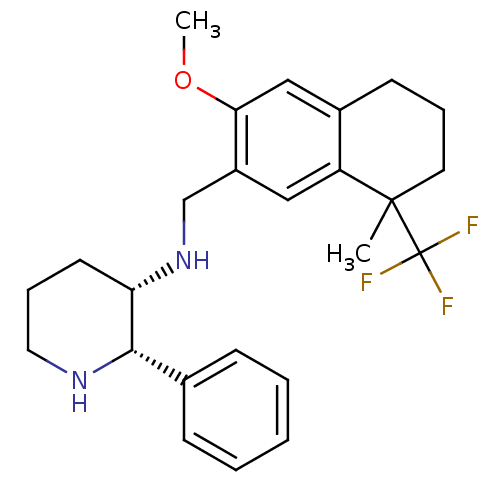 (CHEMBL477365 | R/S-(2S,3S)-3-[((6-Methoxy-1-methyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Binding affinity to human CYP2D6 using bufuralol as substrate | Bioorg Med Chem 16: 7193-205 (2008) Article DOI: 10.1016/j.bmc.2008.06.047 BindingDB Entry DOI: 10.7270/Q2RV0NHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50262567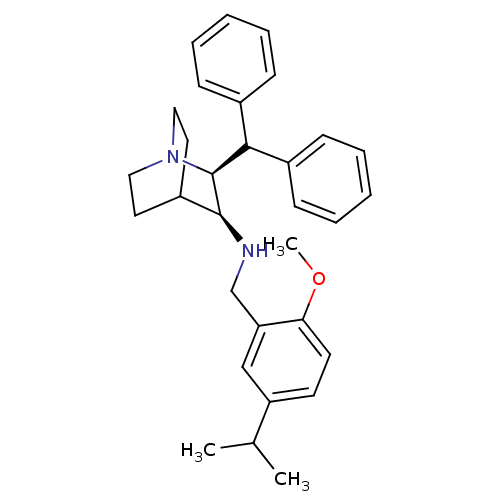 (((2S,3S)-2-Benzhydryl-1-aza-bicyclo[2.2.2]oct-3-yl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Binding affinity to human CYP2D6 using bufuralol as substrate | Bioorg Med Chem 16: 7193-205 (2008) Article DOI: 10.1016/j.bmc.2008.06.047 BindingDB Entry DOI: 10.7270/Q2RV0NHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50262279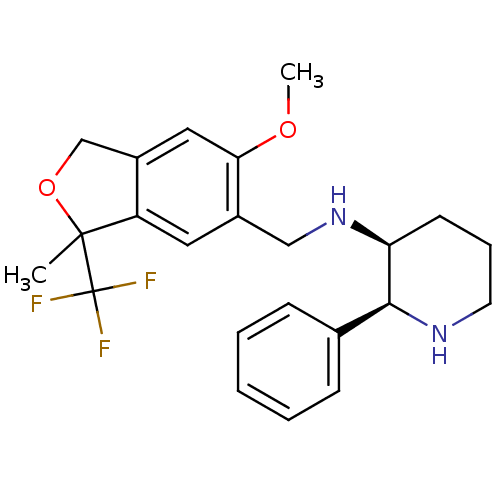 ((2S,3S)-N-((6-methoxy-3-methyl-3-(trifluoromethyl)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Binding affinity to human CYP2D6 using bufuralol as substrate | Bioorg Med Chem 16: 7193-205 (2008) Article DOI: 10.1016/j.bmc.2008.06.047 BindingDB Entry DOI: 10.7270/Q2RV0NHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50224198 ((2S,3S)-N-(2-methoxy-5-(1,1,1-trifluoro-2-methylpr...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Binding affinity to human CYP2D6 using bufuralol as substrate | Bioorg Med Chem 16: 7193-205 (2008) Article DOI: 10.1016/j.bmc.2008.06.047 BindingDB Entry DOI: 10.7270/Q2RV0NHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50262337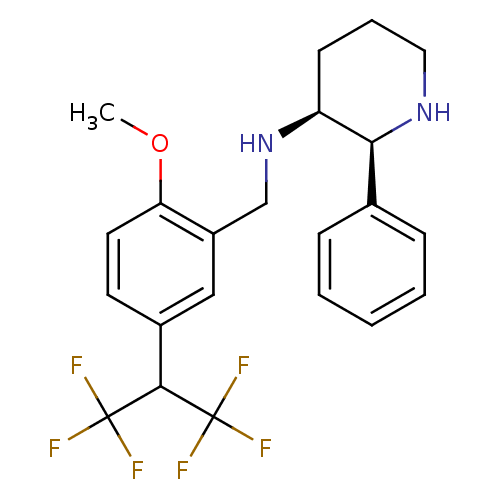 ((2S,3S)-N-(5-(1,1,1,3,3,3-hexafluoropropan-2-yl)-2...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Binding affinity to human CYP2D6 using bufuralol as substrate | Bioorg Med Chem 16: 7193-205 (2008) Article DOI: 10.1016/j.bmc.2008.06.047 BindingDB Entry DOI: 10.7270/Q2RV0NHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50262281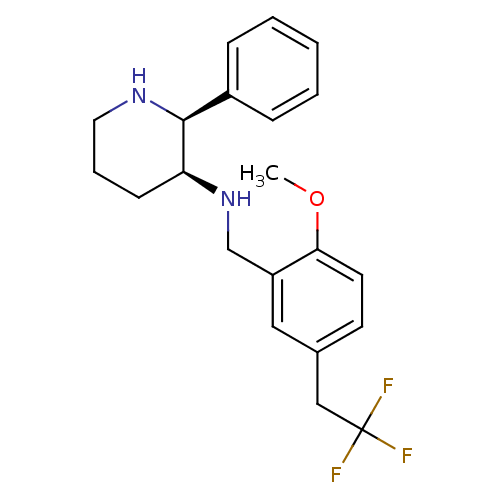 ((2S,3S)-3-[2-Methoxy-5-(2,2,2-trifluoroethyl)benzy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Binding affinity to human CYP2D6 using bufuralol as substrate | Bioorg Med Chem 16: 7193-205 (2008) Article DOI: 10.1016/j.bmc.2008.06.047 BindingDB Entry DOI: 10.7270/Q2RV0NHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50069868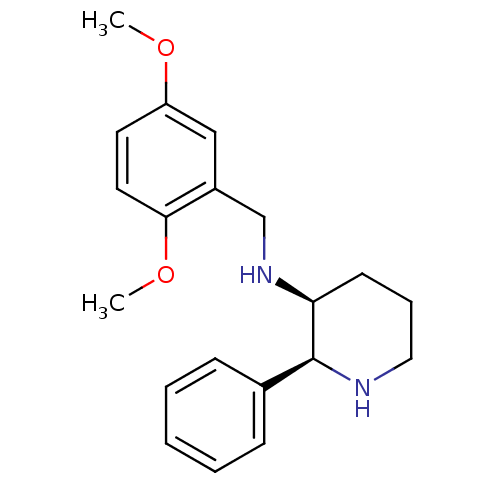 ((2,5-Dimethoxy-benzyl)-((2S,3S)-2-phenyl-piperidin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity for Tachykinin receptor 1 as displacement of [3H]-Substance P in human IM-9 cells | Bioorg Med Chem Lett 8: 281-4 (1999) BindingDB Entry DOI: 10.7270/Q2Z60PK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50069863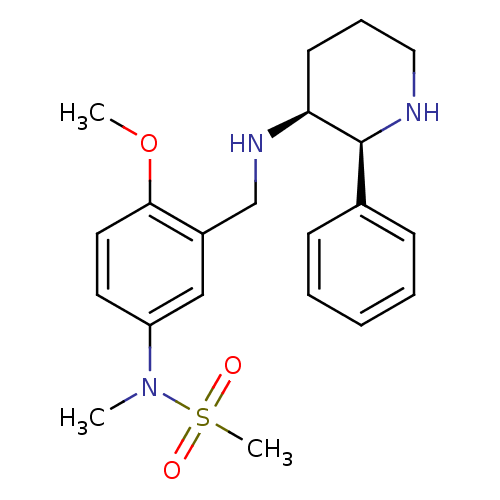 (CHEMBL329007 | N-{4-Methoxy-3-[((2S,3S)-2-phenyl-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity for Tachykinin receptor 1 as displacement of [3H]-Substance P in human IM-9 cells | Bioorg Med Chem Lett 8: 281-4 (1999) BindingDB Entry DOI: 10.7270/Q2Z60PK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50069870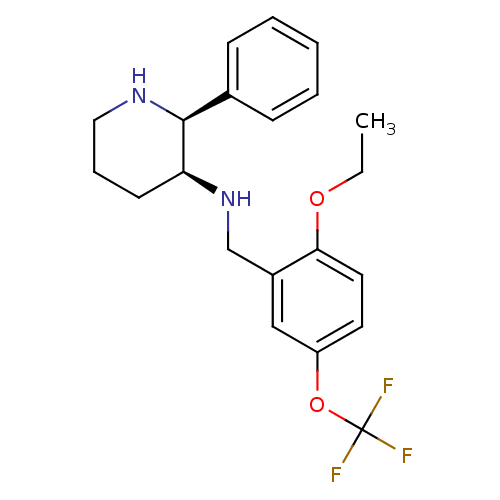 ((2-Ethoxy-5-trifluoromethoxy-benzyl)-((2S,3S)-2-ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity for Tachykinin receptor 1 as displacement of [3H]-Substance P in human IM-9 cells | Bioorg Med Chem Lett 8: 281-4 (1999) BindingDB Entry DOI: 10.7270/Q2Z60PK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50069864 ((5-Chloro-2-methoxy-benzyl)-((2S,3S)-2-phenyl-pipe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity for Tachykinin receptor 1 as displacement of [3H]-Substance P in human IM-9 cells | Bioorg Med Chem Lett 8: 281-4 (1999) BindingDB Entry DOI: 10.7270/Q2Z60PK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50262279 ((2S,3S)-N-((6-methoxy-3-methyl-3-(trifluoromethyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from NK1 receptor in human IM9 cells | Bioorg Med Chem 16: 7193-205 (2008) Article DOI: 10.1016/j.bmc.2008.06.047 BindingDB Entry DOI: 10.7270/Q2RV0NHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50069865 ((2-Isopropoxy-5-trifluoromethoxy-benzyl)-((2S,3S)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity for Tachykinin receptor 1 as displacement of [3H]-Substance P in human IM-9 cells | Bioorg Med Chem Lett 8: 281-4 (1999) BindingDB Entry DOI: 10.7270/Q2Z60PK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50067935 ((2-Methoxy-5-trifluoromethoxy-benzyl)-((2S,3S)-2-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity for Tachykinin receptor 1 as displacement of [3H]-Substance P in human IM-9 cells | Bioorg Med Chem Lett 8: 281-4 (1999) BindingDB Entry DOI: 10.7270/Q2Z60PK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50224198 ((2S,3S)-N-(2-methoxy-5-(1,1,1-trifluoro-2-methylpr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from NK1 receptor in human IM9 cells | Bioorg Med Chem 16: 7193-205 (2008) Article DOI: 10.1016/j.bmc.2008.06.047 BindingDB Entry DOI: 10.7270/Q2RV0NHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine kinase (Homo sapiens (Human)) | BDBM50178014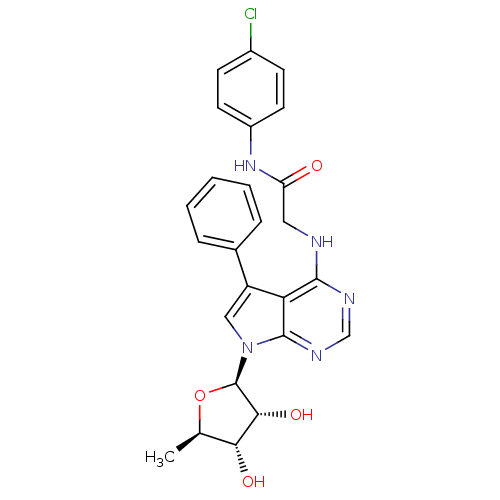 (CHEMBL370164 | N-(4-chlorophenyl)-2-(5-phenyl-7-(5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Metabasis Therapeutics, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against recombinant human adenosine kinase | J Med Chem 48: 7808-20 (2005) Article DOI: 10.1021/jm050394a BindingDB Entry DOI: 10.7270/Q22V2FPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine kinase (Homo sapiens (Human)) | BDBM50174602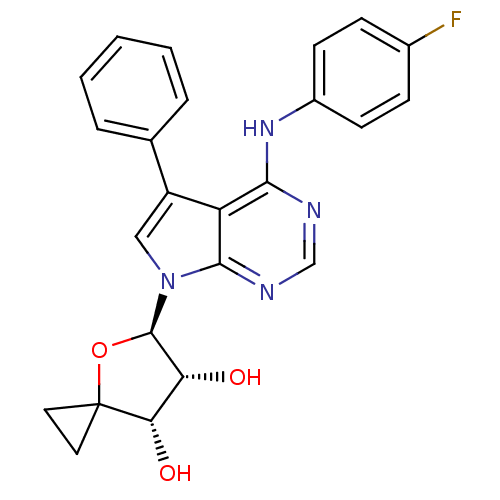 ((4R,5R,6S)-5-[4-(4-Fluoro-phenylamino)-5-phenyl-py...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Metabasis Therapeutics Inc. Curated by ChEMBL | Assay Description Inhibitory concentration against human recombinant adenosine kinase using [14C]-AMP as radioligand | J Med Chem 48: 6430-41 (2005) Article DOI: 10.1021/jm0503650 BindingDB Entry DOI: 10.7270/Q2DV1JFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50262510 (CHEMBL477365 | R/S-(2S,3S)-3-[((6-Methoxy-1-methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from NK1 receptor in human IM9 cells | Bioorg Med Chem 16: 7193-205 (2008) Article DOI: 10.1016/j.bmc.2008.06.047 BindingDB Entry DOI: 10.7270/Q2RV0NHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50262510 (CHEMBL477365 | R/S-(2S,3S)-3-[((6-Methoxy-1-methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from NK1 receptor in human IM9 cells | Bioorg Med Chem 16: 7193-205 (2008) Article DOI: 10.1016/j.bmc.2008.06.047 BindingDB Entry DOI: 10.7270/Q2RV0NHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50261732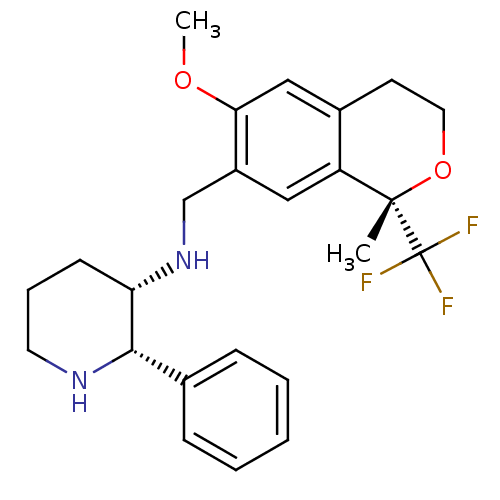 ((2S,3S)-N-(((S)-6-methoxy-1-methyl-1-(trifluoromet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from NK1 receptor in human IM9 cells | Bioorg Med Chem 16: 7193-205 (2008) Article DOI: 10.1016/j.bmc.2008.06.047 BindingDB Entry DOI: 10.7270/Q2RV0NHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50262280 (CHEMBL513351 | R/S-(2S,3S)-N-(2-methoxy-5-(1,1,1-t...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from NK1 receptor in human IM9 cells | Bioorg Med Chem 16: 7193-205 (2008) Article DOI: 10.1016/j.bmc.2008.06.047 BindingDB Entry DOI: 10.7270/Q2RV0NHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50067935 ((2-Methoxy-5-trifluoromethoxy-benzyl)-((2S,3S)-2-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from NK1 receptor in human IM9 cells | Bioorg Med Chem 16: 7193-205 (2008) Article DOI: 10.1016/j.bmc.2008.06.047 BindingDB Entry DOI: 10.7270/Q2RV0NHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50069862 ((2-Difluoromethoxy-benzyl)-((2S,3S)-2-phenyl-piper...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity for Tachykinin receptor 1 as displacement of [3H]-Substance P in human IM-9 cells | Bioorg Med Chem Lett 8: 281-4 (1999) BindingDB Entry DOI: 10.7270/Q2Z60PK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50069867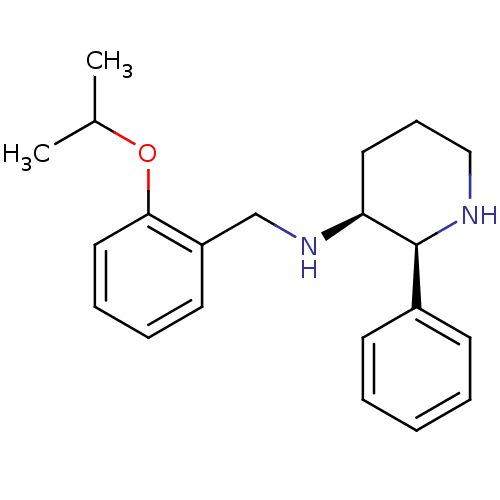 ((2-Isopropoxy-benzyl)-((2S,3S)-2-phenyl-piperidin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity for Tachykinin receptor 1 as displacement of [3H]-Substance P in human IM-9 cells | Bioorg Med Chem Lett 8: 281-4 (1999) BindingDB Entry DOI: 10.7270/Q2Z60PK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine kinase (Homo sapiens (Human)) | BDBM50134740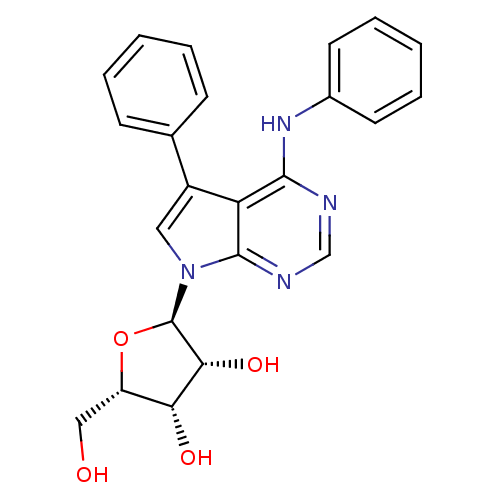 ((1R,4S,5R)-2-Hydroxymethyl-5-(5-phenyl-4-phenylami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
Metabasis Therapeutics Inc. Curated by ChEMBL | Assay Description Inhibitory concentration against human recombinant adenosine kinase using [14C]-AMP as radioligand | J Med Chem 48: 6430-41 (2005) Article DOI: 10.1021/jm0503650 BindingDB Entry DOI: 10.7270/Q2DV1JFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50030232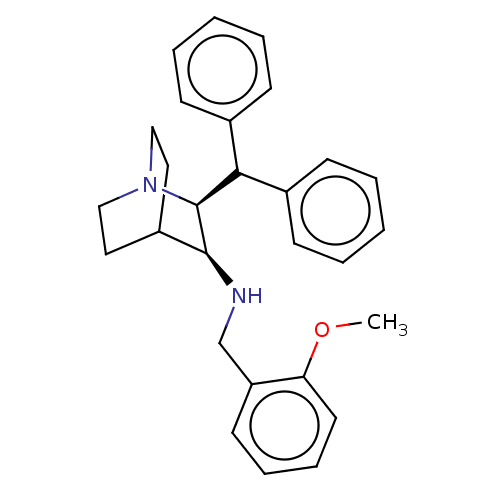 ((S)-((S)-2-Benzhydryl-1-aza-bicyclo[2.2.2]oct-3-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer, Inc. Curated by ChEMBL | Assay Description Tested in vitro for the binding affinity towards NK1 receptor in human IM-9 cells using [125I]-labeled bolton-hunter substance P as ligand | J Med Chem 37: 2831-40 (1994) BindingDB Entry DOI: 10.7270/Q2CZ365J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50000041 ((+) (2-Methoxy-benzyl)-(2-phenyl-piperidin-3-yl)-a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity for Tachykinin receptor 1 as displacement of [3H]-Substance P in human IM-9 cells | Bioorg Med Chem Lett 8: 281-4 (1999) BindingDB Entry DOI: 10.7270/Q2Z60PK8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50262453 (CHEMBL479049 | R/S-(2S,3S)-N-((3-methoxy-8-(triflu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from NK1 receptor in human IM9 cells | Bioorg Med Chem 16: 7193-205 (2008) Article DOI: 10.1016/j.bmc.2008.06.047 BindingDB Entry DOI: 10.7270/Q2RV0NHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine kinase (Homo sapiens (Human)) | BDBM50090887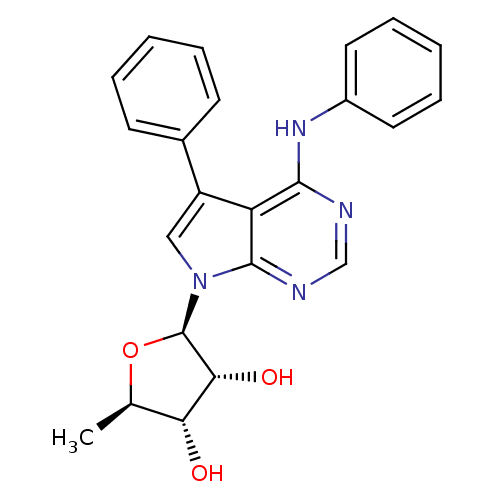 ((1R,4S,5R)-2-Methyl-5-(5-phenyl-4-phenylamino-pyrr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Metabasis Therapeutics, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against recombinant human adenosine kinase | J Med Chem 48: 7808-20 (2005) Article DOI: 10.1021/jm050394a BindingDB Entry DOI: 10.7270/Q22V2FPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine kinase (Homo sapiens (Human)) | BDBM50178015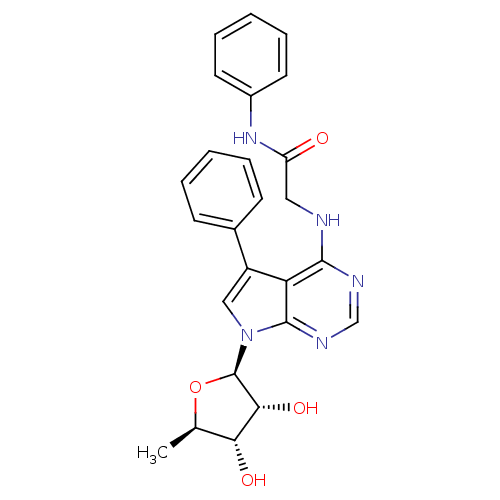 (2-(5-phenyl-7-(5-deoxy-beta-D-ribofuranosyl)-pyrro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Metabasis Therapeutics, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against recombinant human adenosine kinase | J Med Chem 48: 7808-20 (2005) Article DOI: 10.1021/jm050394a BindingDB Entry DOI: 10.7270/Q22V2FPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine kinase (Homo sapiens (Human)) | BDBM50178003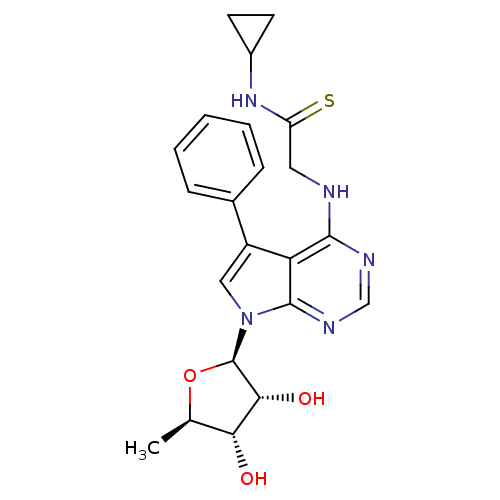 (CHEMBL370011 | N-cyclopropyl-2-(5-phenyl-7-(5-deox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Metabasis Therapeutics, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against recombinant human adenosine kinase | J Med Chem 48: 7808-20 (2005) Article DOI: 10.1021/jm050394a BindingDB Entry DOI: 10.7270/Q22V2FPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine kinase (Homo sapiens (Human)) | BDBM50090887 ((1R,4S,5R)-2-Methyl-5-(5-phenyl-4-phenylamino-pyrr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Metabasis Therapeutics Inc. Curated by ChEMBL | Assay Description Inhibitory concentration against human recombinant adenosine kinase using [14C]-AMP as radioligand | J Med Chem 48: 6430-41 (2005) Article DOI: 10.1021/jm0503650 BindingDB Entry DOI: 10.7270/Q2DV1JFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50069860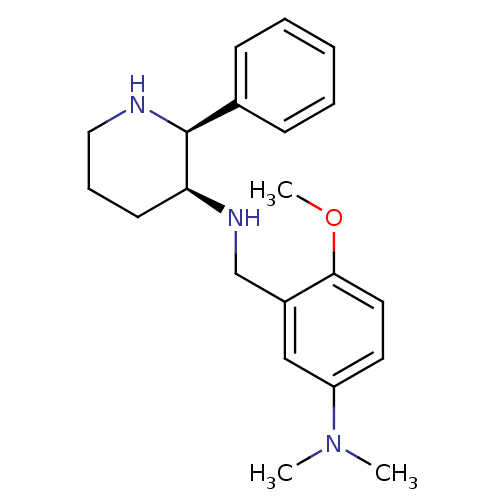 ((5-Dimethylamino-2-methoxy-benzyl)-((2S,3S)-2-phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity for Tachykinin receptor 1 as displacement of [3H]-Substance P in human IM-9 cells | Bioorg Med Chem Lett 8: 281-4 (1999) BindingDB Entry DOI: 10.7270/Q2Z60PK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine kinase (Homo sapiens (Human)) | BDBM50178007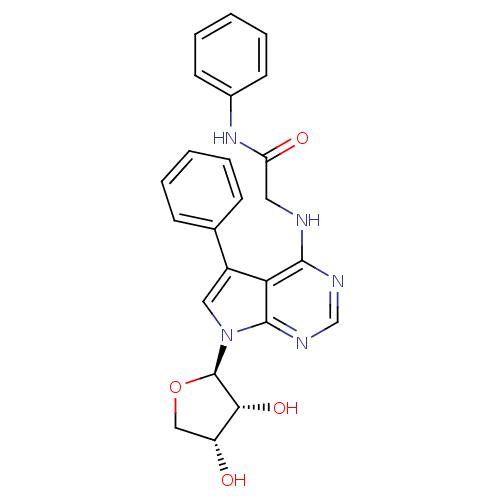 (2-(5-phenyl-7-(beta-D-erythrofuranosyl)-pyrrolo[2,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Metabasis Therapeutics, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against recombinant human adenosine kinase | J Med Chem 48: 7808-20 (2005) Article DOI: 10.1021/jm050394a BindingDB Entry DOI: 10.7270/Q22V2FPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50001450 ((SP)Arg-Pro-Lys-Pro-Gln-Gln-Phe-Phe-Gly-Leu-Met-NH...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity for Tachykinin receptor 1 as displacement of [3H]-Substance P in human IM-9 cells | Bioorg Med Chem Lett 8: 281-4 (1999) BindingDB Entry DOI: 10.7270/Q2Z60PK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50262336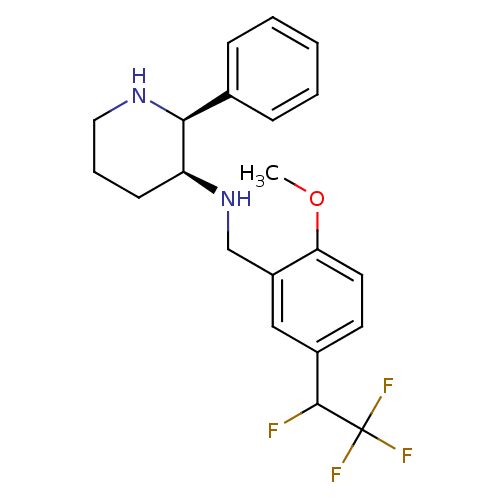 (CHEMBL468163 | R/S-(2S,3S)-N-(2-methoxy-5-(1,2,2,2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from NK1 receptor in human IM9 cells | Bioorg Med Chem 16: 7193-205 (2008) Article DOI: 10.1016/j.bmc.2008.06.047 BindingDB Entry DOI: 10.7270/Q2RV0NHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine kinase (Homo sapiens (Human)) | BDBM50090883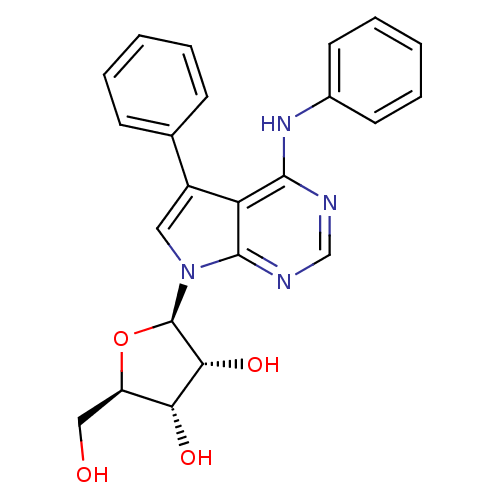 ((1R,4S,5R)-2-Hydroxymethyl-5-(5-phenyl-4-phenylami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Metabasis Therapeutics Inc. Curated by ChEMBL | Assay Description Inhibitory concentration against human recombinant adenosine kinase using [14C]-AMP as radioligand | J Med Chem 48: 6430-41 (2005) Article DOI: 10.1021/jm0503650 BindingDB Entry DOI: 10.7270/Q2DV1JFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50069869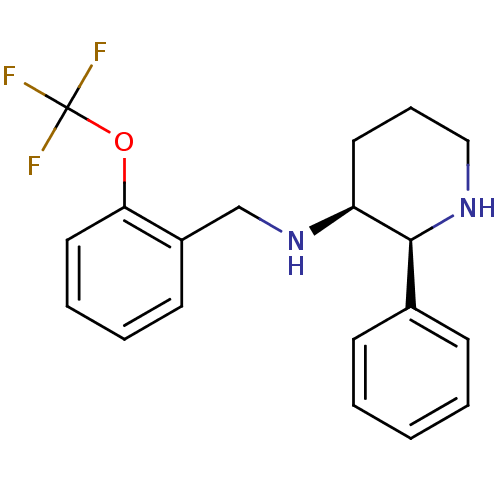 (((2S,3S)-2-Phenyl-piperidin-3-yl)-(2-trifluorometh...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity for Tachykinin receptor 1 as displacement of [3H]-Substance P in human IM-9 cells | Bioorg Med Chem Lett 8: 281-4 (1999) BindingDB Entry DOI: 10.7270/Q2Z60PK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine kinase (Homo sapiens (Human)) | BDBM50178023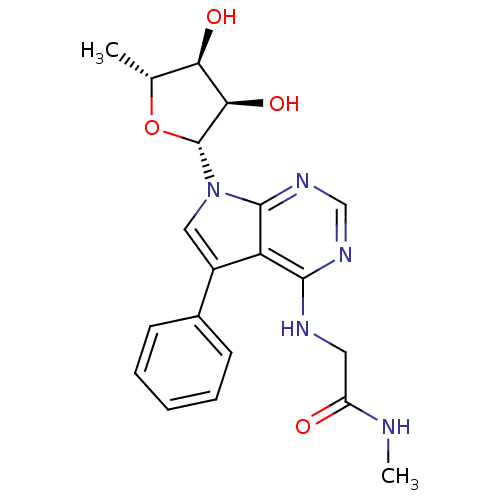 (2-(5-phenyl-7-(5-deoxy-beta-D-ribofuranosyl)-pyrro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Metabasis Therapeutics, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against recombinant human adenosine kinase | J Med Chem 48: 7808-20 (2005) Article DOI: 10.1021/jm050394a BindingDB Entry DOI: 10.7270/Q22V2FPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine kinase (Homo sapiens (Human)) | BDBM50134753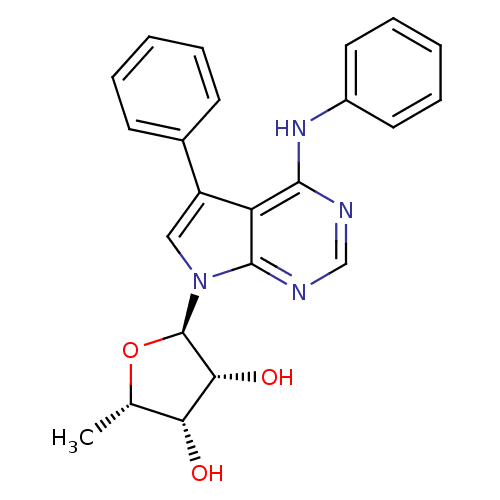 ((1R,4S,5R)-2-Methyl-5-(5-phenyl-4-phenylamino-pyrr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Metabasis Therapeutics Inc. Curated by ChEMBL | Assay Description Inhibitory concentration against human recombinant adenosine kinase using [14C]-AMP as radioligand | J Med Chem 48: 6430-41 (2005) Article DOI: 10.1021/jm0503650 BindingDB Entry DOI: 10.7270/Q2DV1JFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50262395 (CHEMBL478620 | R/S-(2S,3S)-N-((6-methoxy-3-(triflu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from NK1 receptor in human IM9 cells | Bioorg Med Chem 16: 7193-205 (2008) Article DOI: 10.1016/j.bmc.2008.06.047 BindingDB Entry DOI: 10.7270/Q2RV0NHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50262395 (CHEMBL478620 | R/S-(2S,3S)-N-((6-methoxy-3-(triflu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from NK1 receptor in human IM9 cells | Bioorg Med Chem 16: 7193-205 (2008) Article DOI: 10.1016/j.bmc.2008.06.047 BindingDB Entry DOI: 10.7270/Q2RV0NHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine kinase (Homo sapiens (Human)) | BDBM50178018 (2-(3-phenyl-1-(beta-D-ribofuranosyl)-pyrazolo[3,4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Metabasis Therapeutics, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against recombinant human adenosine kinase | J Med Chem 48: 7808-20 (2005) Article DOI: 10.1021/jm050394a BindingDB Entry DOI: 10.7270/Q22V2FPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50262281 ((2S,3S)-3-[2-Methoxy-5-(2,2,2-trifluoroethyl)benzy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from NK1 receptor in human IM9 cells | Bioorg Med Chem 16: 7193-205 (2008) Article DOI: 10.1016/j.bmc.2008.06.047 BindingDB Entry DOI: 10.7270/Q2RV0NHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50262394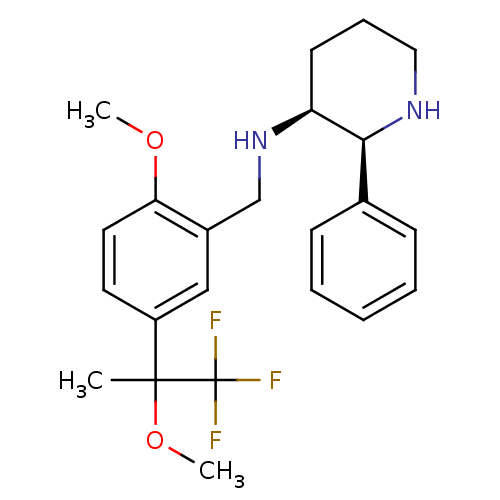 (CHEMBL515935 | R/S-(2S,3S)-N-(2-methoxy-5-(1,1,1-t...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from NK1 receptor in human IM9 cells | Bioorg Med Chem 16: 7193-205 (2008) Article DOI: 10.1016/j.bmc.2008.06.047 BindingDB Entry DOI: 10.7270/Q2RV0NHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50030236 ((S)-((S)-8-Benzhydryl-7-aza-tricyclo[4.3.1.0*3,7*]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.920 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer, Inc. Curated by ChEMBL | Assay Description Tested in vitro for the binding affinity towards NK1 receptor in human IM-9 cells using [125I]-labeled bolton-hunter substance P as ligand | J Med Chem 37: 2831-40 (1994) BindingDB Entry DOI: 10.7270/Q2CZ365J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine kinase (Homo sapiens (Human)) | BDBM50178027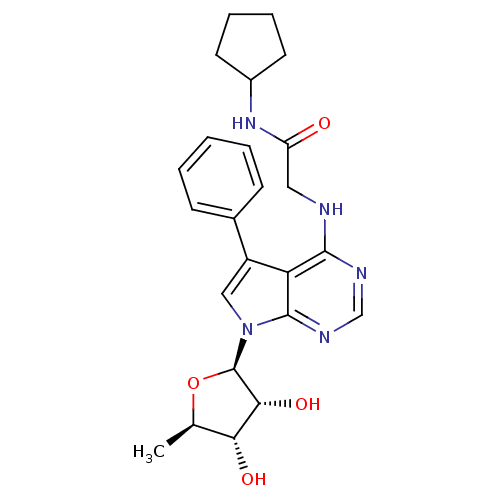 (CHEMBL438449 | N-cyclopentyl-2-(5-phenyl-7-(5-deox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Metabasis Therapeutics, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against recombinant human adenosine kinase | J Med Chem 48: 7808-20 (2005) Article DOI: 10.1021/jm050394a BindingDB Entry DOI: 10.7270/Q22V2FPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 127 total ) | Next | Last >> |