 Found 157 hits with Last Name = 'tsuji' and Initial = 'k'
Found 157 hits with Last Name = 'tsuji' and Initial = 'k' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM35723

(CHEMBL344159 | N-[4-(7-Chloro-5-hydroxy-2,3,4,5-te...)Show SMILES Cc1ccccc1C(=O)Nc1ccc(C(=O)N2CCCC(O)c3cc(Cl)ccc23)c(C)c1 Show InChI InChI=1S/C26H25ClN2O3/c1-16-6-3-4-7-20(16)25(31)28-19-10-11-21(17(2)14-19)26(32)29-13-5-8-24(30)22-15-18(27)9-12-23(22)29/h3-4,6-7,9-12,14-15,24,30H,5,8,13H2,1-2H3,(H,28,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Second Tokushima Institute of New Drug Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 287: 860-7 (1998)
BindingDB Entry DOI: 10.7270/Q2S46QGC |
More data for this
Ligand-Target Pair | |
Vasopressin V1b receptor
(Homo sapiens (Human)) | BDBM35667

(AVP | CHEMBL373742 | US10131692, 44 (AVP) | [3H]Ar...)Show SMILES [#7]-[#6@H]-1-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccccc2)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6]-1=O)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6]-[#6](-[#7])=O Show InChI InChI=1S/C46H65N15O12S2/c47-27-22-74-75-23-33(45(73)61-17-5-9-34(61)44(72)56-28(8-4-16-53-46(51)52)39(67)54-21-37(50)65)60-43(71)32(20-36(49)64)59-40(68)29(14-15-35(48)63)55-41(69)31(18-24-6-2-1-3-7-24)58-42(70)30(57-38(27)66)19-25-10-12-26(62)13-11-25/h1-3,6-7,10-13,27-34,62H,4-5,8-9,14-23,47H2,(H2,48,63)(H2,49,64)(H2,50,65)(H,54,67)(H,55,69)(H,56,72)(H,57,66)(H,58,70)(H,59,68)(H,60,71)(H4,51,52,53)/t27-,28-,29-,30-,31-,32-,33-,34-/m0/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Second Tokushima Institute of New Drug Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 287: 860-7 (1998)
BindingDB Entry DOI: 10.7270/Q2S46QGC |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM35667

(AVP | CHEMBL373742 | US10131692, 44 (AVP) | [3H]Ar...)Show SMILES [#7]-[#6@H]-1-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccccc2)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6]-1=O)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6]-[#6](-[#7])=O Show InChI InChI=1S/C46H65N15O12S2/c47-27-22-74-75-23-33(45(73)61-17-5-9-34(61)44(72)56-28(8-4-16-53-46(51)52)39(67)54-21-37(50)65)60-43(71)32(20-36(49)64)59-40(68)29(14-15-35(48)63)55-41(69)31(18-24-6-2-1-3-7-24)58-42(70)30(57-38(27)66)19-25-10-12-26(62)13-11-25/h1-3,6-7,10-13,27-34,62H,4-5,8-9,14-23,47H2,(H2,48,63)(H2,49,64)(H2,50,65)(H,54,67)(H,55,69)(H,56,72)(H,57,66)(H,58,70)(H,59,68)(H,60,71)(H4,51,52,53)/t27-,28-,29-,30-,31-,32-,33-,34-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Second Tokushima Institute of New Drug Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 287: 860-7 (1998)
BindingDB Entry DOI: 10.7270/Q2S46QGC |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Rattus norvegicus (Rat)) | BDBM35667

(AVP | CHEMBL373742 | US10131692, 44 (AVP) | [3H]Ar...)Show SMILES [#7]-[#6@H]-1-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccccc2)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6]-1=O)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6]-[#6](-[#7])=O Show InChI InChI=1S/C46H65N15O12S2/c47-27-22-74-75-23-33(45(73)61-17-5-9-34(61)44(72)56-28(8-4-16-53-46(51)52)39(67)54-21-37(50)65)60-43(71)32(20-36(49)64)59-40(68)29(14-15-35(48)63)55-41(69)31(18-24-6-2-1-3-7-24)58-42(70)30(57-38(27)66)19-25-10-12-26(62)13-11-25/h1-3,6-7,10-13,27-34,62H,4-5,8-9,14-23,47H2,(H2,48,63)(H2,49,64)(H2,50,65)(H,54,67)(H,55,69)(H,56,72)(H,57,66)(H,58,70)(H,59,68)(H,60,71)(H4,51,52,53)/t27-,28-,29-,30-,31-,32-,33-,34-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Second Tokushima Institute of New Drug Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 287: 860-7 (1998)
BindingDB Entry DOI: 10.7270/Q2S46QGC |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Rattus norvegicus (Rat)) | BDBM35723

(CHEMBL344159 | N-[4-(7-Chloro-5-hydroxy-2,3,4,5-te...)Show SMILES Cc1ccccc1C(=O)Nc1ccc(C(=O)N2CCCC(O)c3cc(Cl)ccc23)c(C)c1 Show InChI InChI=1S/C26H25ClN2O3/c1-16-6-3-4-7-20(16)25(31)28-19-10-11-21(17(2)14-19)26(32)29-13-5-8-24(30)22-15-18(27)9-12-23(22)29/h3-4,6-7,9-12,14-15,24,30H,5,8,13H2,1-2H3,(H,28,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Second Tokushima Institute of New Drug Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 287: 860-7 (1998)
BindingDB Entry DOI: 10.7270/Q2S46QGC |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM35667

(AVP | CHEMBL373742 | US10131692, 44 (AVP) | [3H]Ar...)Show SMILES [#7]-[#6@H]-1-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccccc2)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6]-1=O)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6]-[#6](-[#7])=O Show InChI InChI=1S/C46H65N15O12S2/c47-27-22-74-75-23-33(45(73)61-17-5-9-34(61)44(72)56-28(8-4-16-53-46(51)52)39(67)54-21-37(50)65)60-43(71)32(20-36(49)64)59-40(68)29(14-15-35(48)63)55-41(69)31(18-24-6-2-1-3-7-24)58-42(70)30(57-38(27)66)19-25-10-12-26(62)13-11-25/h1-3,6-7,10-13,27-34,62H,4-5,8-9,14-23,47H2,(H2,48,63)(H2,49,64)(H2,50,65)(H,54,67)(H,55,69)(H,56,72)(H,57,66)(H,58,70)(H,59,68)(H,60,71)(H4,51,52,53)/t27-,28-,29-,30-,31-,32-,33-,34-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Second Tokushima Institute of New Drug Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 287: 860-7 (1998)
BindingDB Entry DOI: 10.7270/Q2S46QGC |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Rattus norvegicus (Rat)) | BDBM35714

(CHEMBL420762 | Mozavaptan | N-[4-(5-Dimethylamino-...)Show SMILES CN(C)C1CCCN(C(=O)c2ccc(NC(=O)c3ccccc3C)cc2)c2ccccc12 Show InChI InChI=1S/C27H29N3O2/c1-19-9-4-5-10-22(19)26(31)28-21-16-14-20(15-17-21)27(32)30-18-8-13-24(29(2)3)23-11-6-7-12-25(23)30/h4-7,9-12,14-17,24H,8,13,18H2,1-3H3,(H,28,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 6.36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Second Tokushima Institute of New Drug Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 287: 860-7 (1998)
BindingDB Entry DOI: 10.7270/Q2S46QGC |
More data for this
Ligand-Target Pair | |
Adenosine deaminase
(Homo sapiens (Human)) | BDBM22920

(1-[(2R)-1-hydroxy-4-{6-[3-(1-methyl-1H-1,3-benzodi...)Show SMILES Cn1c(CCC(=O)Nc2ccc3ccn(CC[C@H](CO)n4cnc(c4)C(N)=O)c3c2)nc2ccccc12 |r| Show InChI InChI=1S/C27H29N7O3/c1-32-23-5-3-2-4-21(23)31-25(32)8-9-26(36)30-19-7-6-18-10-12-33(24(18)14-19)13-11-20(16-35)34-15-22(27(28)37)29-17-34/h2-7,10,12,14-15,17,20,35H,8-9,11,13,16H2,1H3,(H2,28,37)(H,30,36)/t20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 7.70 | -45.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Fujisawa Pharmaceutical Co., Ltd.
| Assay Description
The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a... |
J Med Chem 47: 2728-31 (2004)
Article DOI: 10.1021/jm0499559
BindingDB Entry DOI: 10.7270/Q22F7KQT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM35714

(CHEMBL420762 | Mozavaptan | N-[4-(5-Dimethylamino-...)Show SMILES CN(C)C1CCCN(C(=O)c2ccc(NC(=O)c3ccccc3C)cc2)c2ccccc12 Show InChI InChI=1S/C27H29N3O2/c1-19-9-4-5-10-22(19)26(31)28-21-16-14-20(15-17-21)27(32)30-18-8-13-24(29(2)3)23-11-6-7-12-25(23)30/h4-7,9-12,14-17,24H,8,13,18H2,1-3H3,(H,28,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 9.42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Second Tokushima Institute of New Drug Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 287: 860-7 (1998)
BindingDB Entry DOI: 10.7270/Q2S46QGC |
More data for this
Ligand-Target Pair | |
Adenosine deaminase
(Homo sapiens (Human)) | BDBM22950

(1-[(3R,4S)-4-hydroxy-1-(naphthalen-2-yloxy)pentan-...)Show SMILES C[C@H](O)[C@@H](CCOc1ccc2ccccc2c1)n1cnc(c1)C(N)=O |r| Show InChI InChI=1S/C19H21N3O3/c1-13(23)18(22-11-17(19(20)24)21-12-22)8-9-25-16-7-6-14-4-2-3-5-15(14)10-16/h2-7,10-13,18,23H,8-9H2,1H3,(H2,20,24)/t13-,18+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
| MMDB
PDB
Article
PubMed
| 9.80 | -45.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Fujisawa Pharmaceutical Co., Ltd.
| Assay Description
The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a... |
J Med Chem 47: 2728-31 (2004)
Article DOI: 10.1021/jm0499559
BindingDB Entry DOI: 10.7270/Q22F7KQT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenosine deaminase
(Homo sapiens (Human)) | BDBM22948
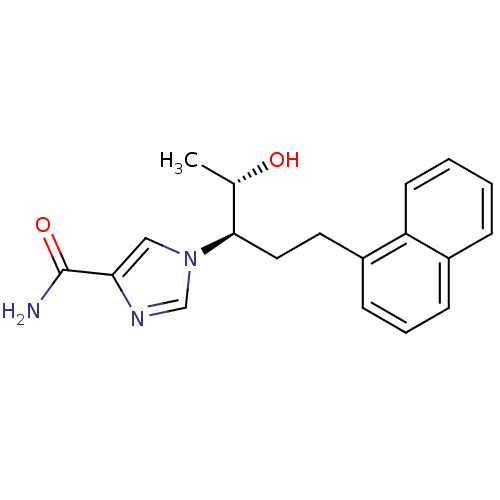
(1-[(1R,2S)-2-hydroxy-1-(2-naphthalen-1-ylethyl)pro...)Show SMILES C[C@H](O)[C@@H](CCc1cccc2ccccc12)n1cnc(c1)C(N)=O |r| Show InChI InChI=1S/C19H21N3O2/c1-13(23)18(22-11-17(19(20)24)21-12-22)10-9-15-7-4-6-14-5-2-3-8-16(14)15/h2-8,11-13,18,23H,9-10H2,1H3,(H2,20,24)/t13-,18+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 11 | -45.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Fujisawa Pharmaceutical Co., Ltd.
| Assay Description
The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a... |
J Med Chem 47: 2728-31 (2004)
Article DOI: 10.1021/jm0499559
BindingDB Entry DOI: 10.7270/Q22F7KQT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM35723

(CHEMBL344159 | N-[4-(7-Chloro-5-hydroxy-2,3,4,5-te...)Show SMILES Cc1ccccc1C(=O)Nc1ccc(C(=O)N2CCCC(O)c3cc(Cl)ccc23)c(C)c1 Show InChI InChI=1S/C26H25ClN2O3/c1-16-6-3-4-7-20(16)25(31)28-19-10-11-21(17(2)14-19)26(32)29-13-5-8-24(30)22-15-18(27)9-12-23(22)29/h3-4,6-7,9-12,14-15,24,30H,5,8,13H2,1-2H3,(H,28,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 12.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Second Tokushima Institute of New Drug Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 287: 860-7 (1998)
BindingDB Entry DOI: 10.7270/Q2S46QGC |
More data for this
Ligand-Target Pair | |
Adenosine deaminase
(Homo sapiens (Human)) | BDBM22949
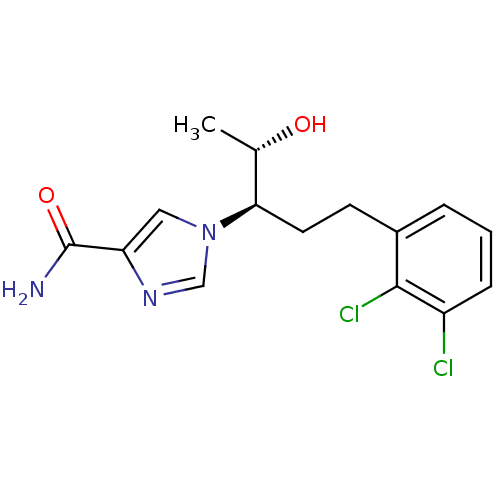
(1-[(3R,4S)-1-(2,3-dichlorophenyl)-4-hydroxypentan-...)Show SMILES C[C@H](O)[C@@H](CCc1cccc(Cl)c1Cl)n1cnc(c1)C(N)=O |r| Show InChI InChI=1S/C15H17Cl2N3O2/c1-9(21)13(20-7-12(15(18)22)19-8-20)6-5-10-3-2-4-11(16)14(10)17/h2-4,7-9,13,21H,5-6H2,1H3,(H2,18,22)/t9-,13+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| MMDB
PDB
Article
PubMed
| 13 | -44.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Fujisawa Pharmaceutical Co., Ltd.
| Assay Description
The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a... |
J Med Chem 47: 2728-31 (2004)
Article DOI: 10.1021/jm0499559
BindingDB Entry DOI: 10.7270/Q22F7KQT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM35714

(CHEMBL420762 | Mozavaptan | N-[4-(5-Dimethylamino-...)Show SMILES CN(C)C1CCCN(C(=O)c2ccc(NC(=O)c3ccccc3C)cc2)c2ccccc12 Show InChI InChI=1S/C27H29N3O2/c1-19-9-4-5-10-22(19)26(31)28-21-16-14-20(15-17-21)27(32)30-18-8-13-24(29(2)3)23-11-6-7-12-25(23)30/h4-7,9-12,14-17,24H,8,13,18H2,1-3H3,(H,28,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Second Tokushima Institute of New Drug Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 287: 860-7 (1998)
BindingDB Entry DOI: 10.7270/Q2S46QGC |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM35723

(CHEMBL344159 | N-[4-(7-Chloro-5-hydroxy-2,3,4,5-te...)Show SMILES Cc1ccccc1C(=O)Nc1ccc(C(=O)N2CCCC(O)c3cc(Cl)ccc23)c(C)c1 Show InChI InChI=1S/C26H25ClN2O3/c1-16-6-3-4-7-20(16)25(31)28-19-10-11-21(17(2)14-19)26(32)29-13-5-8-24(30)22-15-18(27)9-12-23(22)29/h3-4,6-7,9-12,14-15,24,30H,5,8,13H2,1-2H3,(H,28,31) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 325 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Second Tokushima Institute of New Drug Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 287: 860-7 (1998)
BindingDB Entry DOI: 10.7270/Q2S46QGC |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM35714

(CHEMBL420762 | Mozavaptan | N-[4-(5-Dimethylamino-...)Show SMILES CN(C)C1CCCN(C(=O)c2ccc(NC(=O)c3ccccc3C)cc2)c2ccccc12 Show InChI InChI=1S/C27H29N3O2/c1-19-9-4-5-10-22(19)26(31)28-21-16-14-20(15-17-21)27(32)30-18-8-13-24(29(2)3)23-11-6-7-12-25(23)30/h4-7,9-12,14-17,24H,8,13,18H2,1-3H3,(H,28,31) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 524 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Second Tokushima Institute of New Drug Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 287: 860-7 (1998)
BindingDB Entry DOI: 10.7270/Q2S46QGC |
More data for this
Ligand-Target Pair | |
Chymotrypsin-like elastase family member 1/2A
(Sus scrofa (Pig)) | BDBM50226399

(CHEMBL3143932)Show SMILES COC(=O)CCC(=O)N[C@@H](C)C(=O)N[C@@H](C)C(=O)N1CCC[C@H]1C(=O)CN(C(C)C)C(=O)Sc1nnnn1-c1ccccc1 |r| Show InChI InChI=1S/C28H38N8O7S/c1-17(2)35(28(42)44-27-31-32-33-36(27)20-10-7-6-8-11-20)16-22(37)21-12-9-15-34(21)26(41)19(4)30-25(40)18(3)29-23(38)13-14-24(39)43-5/h6-8,10-11,17-19,21H,9,12-16H2,1-5H3,(H,29,38)(H,30,40)/t18-,19-,21-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound is evaluated for the inhibition of porcine pancreatic (PP) elastase |
J Med Chem 29: 1468-76 (1986)
BindingDB Entry DOI: 10.7270/Q2NG4ST4 |
More data for this
Ligand-Target Pair | |
Vasopressin V1b receptor
(Homo sapiens (Human)) | BDBM35714

(CHEMBL420762 | Mozavaptan | N-[4-(5-Dimethylamino-...)Show SMILES CN(C)C1CCCN(C(=O)c2ccc(NC(=O)c3ccccc3C)cc2)c2ccccc12 Show InChI InChI=1S/C27H29N3O2/c1-19-9-4-5-10-22(19)26(31)28-21-16-14-20(15-17-21)27(32)30-18-8-13-24(29(2)3)23-11-6-7-12-25(23)30/h4-7,9-12,14-17,24H,8,13,18H2,1-3H3,(H,28,31) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Second Tokushima Institute of New Drug Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 287: 860-7 (1998)
BindingDB Entry DOI: 10.7270/Q2S46QGC |
More data for this
Ligand-Target Pair | |
Vasopressin V1b receptor
(Homo sapiens (Human)) | BDBM35723

(CHEMBL344159 | N-[4-(7-Chloro-5-hydroxy-2,3,4,5-te...)Show SMILES Cc1ccccc1C(=O)Nc1ccc(C(=O)N2CCCC(O)c3cc(Cl)ccc23)c(C)c1 Show InChI InChI=1S/C26H25ClN2O3/c1-16-6-3-4-7-20(16)25(31)28-19-10-11-21(17(2)14-19)26(32)29-13-5-8-24(30)22-15-18(27)9-12-23(22)29/h3-4,6-7,9-12,14-15,24,30H,5,8,13H2,1-2H3,(H,28,31) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Second Tokushima Institute of New Drug Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 287: 860-7 (1998)
BindingDB Entry DOI: 10.7270/Q2S46QGC |
More data for this
Ligand-Target Pair | |
Chymotrypsin-like elastase family member 1/2A
(Sus scrofa (Pig)) | BDBM50226400
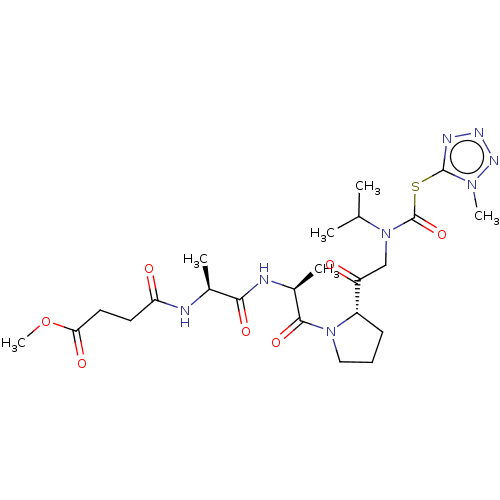
(CHEMBL3143923)Show SMILES COC(=O)CCC(=O)N[C@@H](C)C(=O)N[C@@H](C)C(=O)N1CCC[C@H]1C(=O)CN(C(C)C)C(=O)Sc1nnnn1C |r| Show InChI InChI=1S/C23H36N8O7S/c1-13(2)31(23(37)39-22-26-27-28-29(22)5)12-17(32)16-8-7-11-30(16)21(36)15(4)25-20(35)14(3)24-18(33)9-10-19(34)38-6/h13-16H,7-12H2,1-6H3,(H,24,33)(H,25,35)/t14-,15-,16-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.95E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound is evaluated for the inhibition of porcine pancreatic (PP) elastase |
J Med Chem 29: 1468-76 (1986)
BindingDB Entry DOI: 10.7270/Q2NG4ST4 |
More data for this
Ligand-Target Pair | |
Chymotrypsin-like elastase family member 1/2A
(Sus scrofa (Pig)) | BDBM50226396

(CHEMBL3143922)Show SMILES COC(=O)CCC(=O)N[C@@H](C)C(=O)N[C@@H](C)C(=O)N1CCC[C@H]1C(=O)CN(C(C)C)C(=O)Oc1ccccc1 |r| Show InChI InChI=1S/C27H38N4O8/c1-17(2)31(27(37)39-20-10-7-6-8-11-20)16-22(32)21-12-9-15-30(21)26(36)19(4)29-25(35)18(3)28-23(33)13-14-24(34)38-5/h6-8,10-11,17-19,21H,9,12-16H2,1-5H3,(H,28,33)(H,29,35)/t18-,19-,21-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.15E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound is evaluated for the inhibition of porcine pancreatic (PP) elastase |
J Med Chem 29: 1468-76 (1986)
BindingDB Entry DOI: 10.7270/Q2NG4ST4 |
More data for this
Ligand-Target Pair | |
Chymotrypsin-like elastase family member 1/2A
(Sus scrofa (Pig)) | BDBM50226401
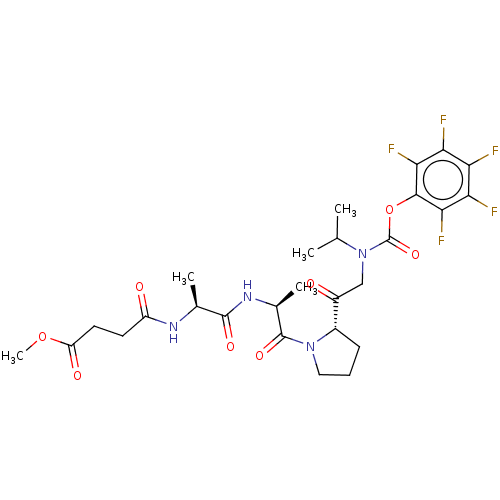
(CHEMBL3143927)Show SMILES COC(=O)CCC(=O)N[C@@H](C)C(=O)N[C@@H](C)C(=O)N1CCC[C@H]1C(=O)CN(C(C)C)C(=O)Oc1c(F)c(F)c(F)c(F)c1F |r| Show InChI InChI=1S/C27H33F5N4O8/c1-12(2)36(27(42)44-24-22(31)20(29)19(28)21(30)23(24)32)11-16(37)15-7-6-10-35(15)26(41)14(4)34-25(40)13(3)33-17(38)8-9-18(39)43-5/h12-15H,6-11H2,1-5H3,(H,33,38)(H,34,40)/t13-,14-,15-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound is evaluated for the inhibition of porcine pancreatic (PP) elastase |
J Med Chem 29: 1468-76 (1986)
BindingDB Entry DOI: 10.7270/Q2NG4ST4 |
More data for this
Ligand-Target Pair | |
Chymotrypsin-like elastase family member 1/2A
(Sus scrofa (Pig)) | BDBM50226397

(CHEMBL3143925)Show SMILES COC(=O)CCC(=O)N[C@@H](C)C(=O)N[C@@H](C)C(=O)N1CCC[C@H]1C(=O)CN(C(C)C)C(=O)Oc1ccc(cc1)[N+]([O-])=O |r| Show InChI InChI=1S/C27H37N5O10/c1-16(2)31(27(38)42-20-10-8-19(9-11-20)32(39)40)15-22(33)21-7-6-14-30(21)26(37)18(4)29-25(36)17(3)28-23(34)12-13-24(35)41-5/h8-11,16-18,21H,6-7,12-15H2,1-5H3,(H,28,34)(H,29,36)/t17-,18-,21-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound is evaluated for the inhibition of porcine pancreatic (PP) elastase |
J Med Chem 29: 1468-76 (1986)
BindingDB Entry DOI: 10.7270/Q2NG4ST4 |
More data for this
Ligand-Target Pair | |
Chymotrypsin-like elastase family member 1/2A
(Sus scrofa (Pig)) | BDBM50226398
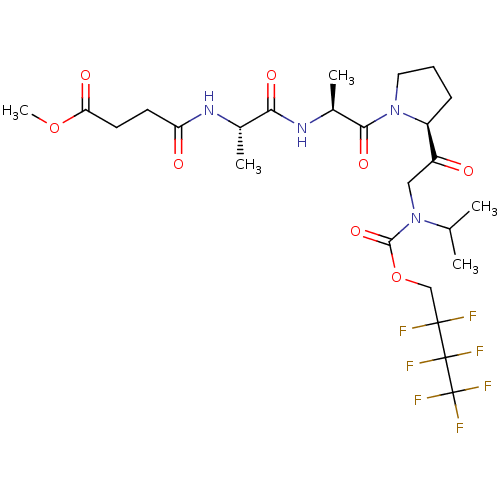
(CHEMBL3143926)Show SMILES COC(=O)CCC(=O)N[C@@H](C)C(=O)N[C@@H](C)C(=O)N1CCC[C@H]1C(=O)CN(C(C)C)C(=O)OCC(F)(F)C(F)(F)C(F)(F)F |r| Show InChI InChI=1S/C25H35F7N4O8/c1-13(2)36(22(42)44-12-23(26,27)24(28,29)25(30,31)32)11-17(37)16-7-6-10-35(16)21(41)15(4)34-20(40)14(3)33-18(38)8-9-19(39)43-5/h13-16H,6-12H2,1-5H3,(H,33,38)(H,34,40)/t14-,15-,16-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 8.21E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound is evaluated for the inhibition of porcine pancreatic (PP) elastase |
J Med Chem 29: 1468-76 (1986)
BindingDB Entry DOI: 10.7270/Q2NG4ST4 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50403016

(CHEMBL2207845)Show SMILES CC(C)C[C@H](NC(=O)[C@@H]1CCCN1C(C)=O)C(=O)N[C@@H](Cc1cncn1CCCCCCCCc1ccccc1)C(=O)N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)OP(O)(O)=O)C(N)=O |r| Show InChI InChI=1S/C40H63N8O11P/c1-26(2)21-31(44-40(55)34-18-14-20-48(34)28(4)50)37(52)43-32(38(53)45-33(24-49)39(54)46-35(36(41)51)27(3)59-60(56,57)58)22-30-23-42-25-47(30)19-13-8-6-5-7-10-15-29-16-11-9-12-17-29/h9,11-12,16-17,23,25-27,31-35,49H,5-8,10,13-15,18-22,24H2,1-4H3,(H2,41,51)(H,43,52)(H,44,55)(H,45,53)(H,46,54)(H2,56,57,58)/t27-,31+,32+,33+,34+,35+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute-Frederick
Curated by ChEMBL
| Assay Description
Inhibition of 5CF-GPMQSpTPLNG-NH2 binding to Plk1 C-terminal polo-box domain (unknown origin) preincubated for 30 mins followed by probe addition mea... |
Bioorg Med Chem Lett 28: 3202-3205 (2018)
Article DOI: 10.1016/j.bmcl.2018.08.018
BindingDB Entry DOI: 10.7270/Q25Q4ZST |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50467110
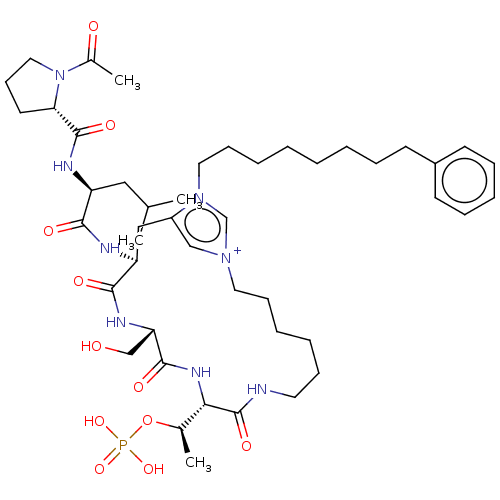
(CHEMBL4278200)Show SMILES CC(C)C[C@H](NC(=O)[C@@H]1CCCN1C(C)=O)C(=O)N[C@H]1Cc2c[n+](CCCCCCNC(=O)[C@@H](NC(=O)[C@H](CO)NC1=O)[C@@H](C)OP(O)(O)=O)cn2CCCCCCCCc1ccccc1 |r| Show InChI InChI=1S/C46H73N8O11P/c1-32(2)27-37(49-45(60)40-22-18-26-54(40)34(4)56)42(57)48-38-28-36-29-52(31-53(36)25-17-9-6-5-7-12-19-35-20-13-11-14-21-35)24-16-10-8-15-23-47-46(61)41(33(3)65-66(62,63)64)51-44(59)39(30-55)50-43(38)58/h11,13-14,20-21,29,31-33,37-41,55H,5-10,12,15-19,22-28,30H2,1-4H3,(H6-,47,48,49,50,51,57,58,59,60,61,62,63,64)/p+1/t33-,37+,38+,39+,40+,41+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute-Frederick
Curated by ChEMBL
| Assay Description
Inhibition of 5CF-GPMQSpTPLNG-NH2 binding to Plk1 C-terminal polo-box domain (unknown origin) preincubated for 30 mins followed by probe addition mea... |
Bioorg Med Chem Lett 28: 3202-3205 (2018)
Article DOI: 10.1016/j.bmcl.2018.08.018
BindingDB Entry DOI: 10.7270/Q25Q4ZST |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50467111
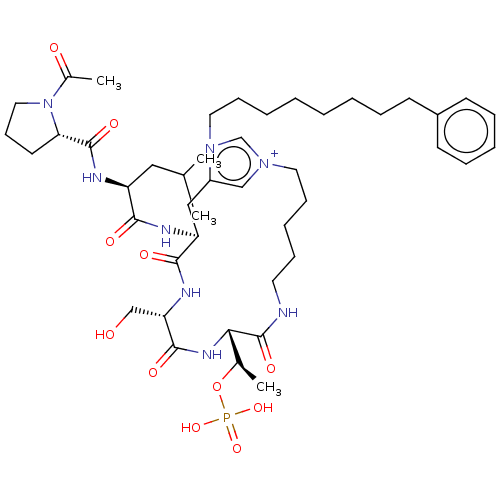
(CHEMBL4281659)Show SMILES CC(C)C[C@H](NC(=O)[C@@H]1CCCN1C(C)=O)C(=O)N[C@H]1Cc2c[n+](CCCCCNC(=O)[C@@H](NC(=O)[C@H](CO)NC1=O)[C@@H](C)OP(O)(O)=O)cn2CCCCCCCCc1ccccc1 |r| Show InChI InChI=1S/C45H71N8O11P/c1-31(2)26-36(48-44(59)39-21-17-25-53(39)33(4)55)41(56)47-37-27-35-28-51(30-52(35)24-16-8-6-5-7-11-18-34-19-12-9-13-20-34)23-15-10-14-22-46-45(60)40(32(3)64-65(61,62)63)50-43(58)38(29-54)49-42(37)57/h9,12-13,19-20,28,30-32,36-40,54H,5-8,10-11,14-18,21-27,29H2,1-4H3,(H6-,46,47,48,49,50,56,57,58,59,60,61,62,63)/p+1/t32-,36+,37+,38+,39+,40+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute-Frederick
Curated by ChEMBL
| Assay Description
Inhibition of 5CF-GPMQSpTPLNG-NH2 binding to Plk1 C-terminal polo-box domain (unknown origin) preincubated for 30 mins followed by probe addition mea... |
Bioorg Med Chem Lett 28: 3202-3205 (2018)
Article DOI: 10.1016/j.bmcl.2018.08.018
BindingDB Entry DOI: 10.7270/Q25Q4ZST |
More data for this
Ligand-Target Pair | |
Adenosine deaminase
(Homo sapiens (Human)) | BDBM22947
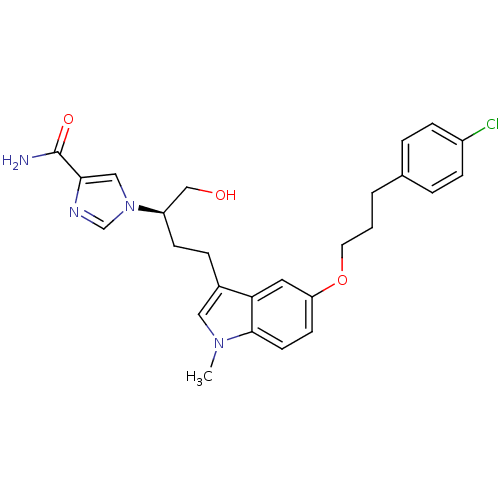
(1-[(2R)-4-{5-[3-(4-chlorophenyl)propoxy]-1-methyl-...)Show SMILES Cn1cc(CC[C@H](CO)n2cnc(c2)C(N)=O)c2cc(OCCCc3ccc(Cl)cc3)ccc12 |r| Show InChI InChI=1S/C26H29ClN4O3/c1-30-14-19(6-9-21(16-32)31-15-24(26(28)33)29-17-31)23-13-22(10-11-25(23)30)34-12-2-3-18-4-7-20(27)8-5-18/h4-5,7-8,10-11,13-15,17,21,32H,2-3,6,9,12,16H2,1H3,(H2,28,33)/t21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory concentration against adenosine deaminase |
J Med Chem 48: 4750-3 (2005)
Article DOI: 10.1021/jm050413g
BindingDB Entry DOI: 10.7270/Q2JD4WBG |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50467112

(CHEMBL4287557)Show SMILES C[C@@H](OP(O)(O)=O)[C@@H]1NC(=O)[C@H](CO)NC(=O)[C@H](Cc2c[n+](CCCCCNC1=O)cn2CCCCCCCCc1ccccc1)NC(C)=O |r| Show InChI InChI=1S/C34H53N6O9P/c1-25(49-50(46,47)48)31-34(45)35-18-12-8-13-19-39-22-28(21-29(36-26(2)42)32(43)37-30(23-41)33(44)38-31)40(24-39)20-14-6-4-3-5-9-15-27-16-10-7-11-17-27/h7,10-11,16-17,22,24-25,29-31,41H,3-6,8-9,12-15,18-21,23H2,1-2H3,(H5-,35,36,37,38,42,43,44,45,46,47,48)/p+1/t25-,29+,30+,31+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute-Frederick
Curated by ChEMBL
| Assay Description
Inhibition of 5CF-GPMQSpTPLNG-NH2 binding to Plk1 C-terminal polo-box domain (unknown origin) preincubated for 30 mins followed by probe addition mea... |
Bioorg Med Chem Lett 28: 3202-3205 (2018)
Article DOI: 10.1016/j.bmcl.2018.08.018
BindingDB Entry DOI: 10.7270/Q25Q4ZST |
More data for this
Ligand-Target Pair | |
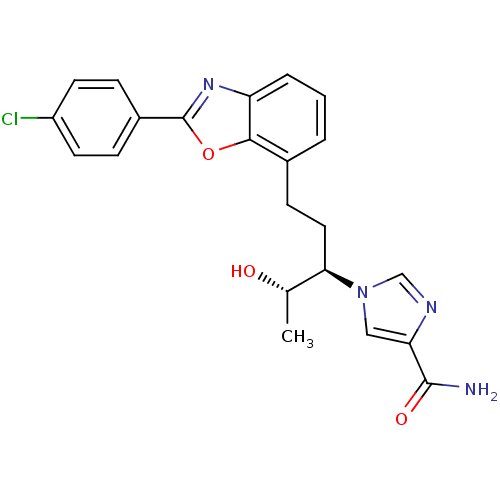
Adenosine deaminase
(Homo sapiens (Human)) | BDBM50170633
(1-((1R,2S)-1-{2-[2-(4-Chloro-phenyl)-benzooxazol-7...)Show SMILES C[C@H](O)[C@@H](CCc1cccc2nc(oc12)-c1ccc(Cl)cc1)n1cnc(c1)C(N)=O Show InChI InChI=1S/C22H21ClN4O3/c1-13(28)19(27-11-18(21(24)29)25-12-27)10-7-14-3-2-4-17-20(14)30-22(26-17)15-5-8-16(23)9-6-15/h2-6,8-9,11-13,19,28H,7,10H2,1H3,(H2,24,29)/t13-,19+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory concentration against adenosine deaminase |
J Med Chem 48: 4750-3 (2005)
Article DOI: 10.1021/jm050413g
BindingDB Entry DOI: 10.7270/Q2JD4WBG |
More data for this
Ligand-Target Pair | |
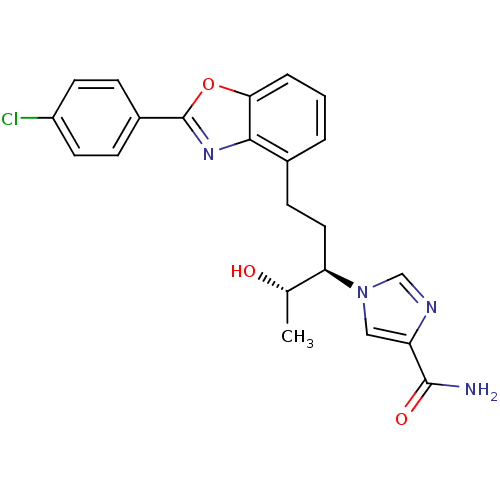
Adenosine deaminase
(Homo sapiens (Human)) | BDBM50170634
(1-((1R,2S)-1-{2-[2-(4-Chloro-phenyl)-benzooxazol-4...)Show SMILES C[C@H](O)[C@@H](CCc1cccc2oc(nc12)-c1ccc(Cl)cc1)n1cnc(c1)C(N)=O Show InChI InChI=1S/C22H21ClN4O3/c1-13(28)18(27-11-17(21(24)29)25-12-27)10-7-14-3-2-4-19-20(14)26-22(30-19)15-5-8-16(23)9-6-15/h2-6,8-9,11-13,18,28H,7,10H2,1H3,(H2,24,29)/t13-,18+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory concentration against adenosine deaminase |
J Med Chem 48: 4750-3 (2005)
Article DOI: 10.1021/jm050413g
BindingDB Entry DOI: 10.7270/Q2JD4WBG |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50467109

(CHEMBL4292295)Show SMILES CC(C)C[C@H](NC(=O)[C@@H]1CCCN1C(C)=O)C(=O)N[C@H]1Cc2c[n+](CCCCNC(=O)[C@@H](NC(=O)[C@H](CO)NC1=O)[C@@H](C)OP(O)(O)=O)cn2CCCCCCCCc1ccccc1 |r| Show InChI InChI=1S/C44H69N8O11P/c1-30(2)25-35(47-43(58)38-20-16-24-52(38)32(4)54)40(55)46-36-26-34-27-50(29-51(34)23-14-8-6-5-7-10-17-33-18-11-9-12-19-33)22-15-13-21-45-44(59)39(31(3)63-64(60,61)62)49-42(57)37(28-53)48-41(36)56/h9,11-12,18-19,27,29-31,35-39,53H,5-8,10,13-17,20-26,28H2,1-4H3,(H6-,45,46,47,48,49,55,56,57,58,59,60,61,62)/p+1/t31-,35+,36+,37+,38+,39+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute-Frederick
Curated by ChEMBL
| Assay Description
Inhibition of 5CF-GPMQSpTPLNG-NH2 binding to Plk1 C-terminal polo-box domain (unknown origin) preincubated for 30 mins followed by probe addition mea... |
Bioorg Med Chem Lett 28: 3202-3205 (2018)
Article DOI: 10.1016/j.bmcl.2018.08.018
BindingDB Entry DOI: 10.7270/Q25Q4ZST |
More data for this
Ligand-Target Pair | |
Adenosine deaminase
(Homo sapiens (Human)) | BDBM22949

(1-[(3R,4S)-1-(2,3-dichlorophenyl)-4-hydroxypentan-...)Show SMILES C[C@H](O)[C@@H](CCc1cccc(Cl)c1Cl)n1cnc(c1)C(N)=O |r| Show InChI InChI=1S/C15H17Cl2N3O2/c1-9(21)13(20-7-12(15(18)22)19-8-20)6-5-10-3-2-4-11(16)14(10)17/h2-4,7-9,13,21H,5-6H2,1H3,(H2,18,22)/t9-,13+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| MMDB
PDB
Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory concentration against adenosine deaminase |
J Med Chem 48: 4750-3 (2005)
Article DOI: 10.1021/jm050413g
BindingDB Entry DOI: 10.7270/Q2JD4WBG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenosine deaminase
(Homo sapiens (Human)) | BDBM22948

(1-[(1R,2S)-2-hydroxy-1-(2-naphthalen-1-ylethyl)pro...)Show SMILES C[C@H](O)[C@@H](CCc1cccc2ccccc12)n1cnc(c1)C(N)=O |r| Show InChI InChI=1S/C19H21N3O2/c1-13(23)18(22-11-17(19(20)24)21-12-22)10-9-15-7-4-6-14-5-2-3-8-16(14)15/h2-8,11-13,18,23H,9-10H2,1H3,(H2,20,24)/t13-,18+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory concentration against adenosine deaminase |
J Med Chem 48: 4750-3 (2005)
Article DOI: 10.1021/jm050413g
BindingDB Entry DOI: 10.7270/Q2JD4WBG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Envelope glycoprotein gp160
(Human immunodeficiency virus 1) | BDBM50568672

(CHEMBL4863234)Show SMILES COCCOCCOCCOCCOCCNC(=O)CCC(=O)OC(CCCNC(N)=N)C(=O)NCCN1CCC(CC11CCCCC1)NC(=O)C(=O)Nc1ccc(Cl)cc1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HIV1 KP-5mvcR gp120 interaction with CD4 in human TZM-bl cells assessed as decrease in viral entry by MTT assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01153
BindingDB Entry DOI: 10.7270/Q2BC4399 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50467110

(CHEMBL4278200)Show SMILES CC(C)C[C@H](NC(=O)[C@@H]1CCCN1C(C)=O)C(=O)N[C@H]1Cc2c[n+](CCCCCCNC(=O)[C@@H](NC(=O)[C@H](CO)NC1=O)[C@@H](C)OP(O)(O)=O)cn2CCCCCCCCc1ccccc1 |r| Show InChI InChI=1S/C46H73N8O11P/c1-32(2)27-37(49-45(60)40-22-18-26-54(40)34(4)56)42(57)48-38-28-36-29-52(31-53(36)25-17-9-6-5-7-12-19-35-20-13-11-14-21-35)24-16-10-8-15-23-47-46(61)41(33(3)65-66(62,63)64)51-44(59)39(30-55)50-43(38)58/h11,13-14,20-21,29,31-33,37-41,55H,5-10,12,15-19,22-28,30H2,1-4H3,(H6-,47,48,49,50,51,57,58,59,60,61,62,63,64)/p+1/t33-,37+,38+,39+,40+,41+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute-Frederick
Curated by ChEMBL
| Assay Description
Inhibition of full-length myc-tagged Plk1 C-terminal polo-box domain (unknown origin) assessed as inhibition of Plk1-Biotin-Ahx-PMQS(pT)PLN-NH2 pepti... |
Bioorg Med Chem Lett 28: 3202-3205 (2018)
Article DOI: 10.1016/j.bmcl.2018.08.018
BindingDB Entry DOI: 10.7270/Q25Q4ZST |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50467111

(CHEMBL4281659)Show SMILES CC(C)C[C@H](NC(=O)[C@@H]1CCCN1C(C)=O)C(=O)N[C@H]1Cc2c[n+](CCCCCNC(=O)[C@@H](NC(=O)[C@H](CO)NC1=O)[C@@H](C)OP(O)(O)=O)cn2CCCCCCCCc1ccccc1 |r| Show InChI InChI=1S/C45H71N8O11P/c1-31(2)26-36(48-44(59)39-21-17-25-53(39)33(4)55)41(56)47-37-27-35-28-51(30-52(35)24-16-8-6-5-7-11-18-34-19-12-9-13-20-34)23-15-10-14-22-46-45(60)40(32(3)64-65(61,62)63)50-43(58)38(29-54)49-42(37)57/h9,12-13,19-20,28,30-32,36-40,54H,5-8,10-11,14-18,21-27,29H2,1-4H3,(H6-,46,47,48,49,50,56,57,58,59,60,61,62,63)/p+1/t32-,36+,37+,38+,39+,40+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute-Frederick
Curated by ChEMBL
| Assay Description
Inhibition of full-length myc-tagged Plk1 C-terminal polo-box domain (unknown origin) assessed as inhibition of Plk1-Biotin-Ahx-PMQS(pT)PLN-NH2 pepti... |
Bioorg Med Chem Lett 28: 3202-3205 (2018)
Article DOI: 10.1016/j.bmcl.2018.08.018
BindingDB Entry DOI: 10.7270/Q25Q4ZST |
More data for this
Ligand-Target Pair | |
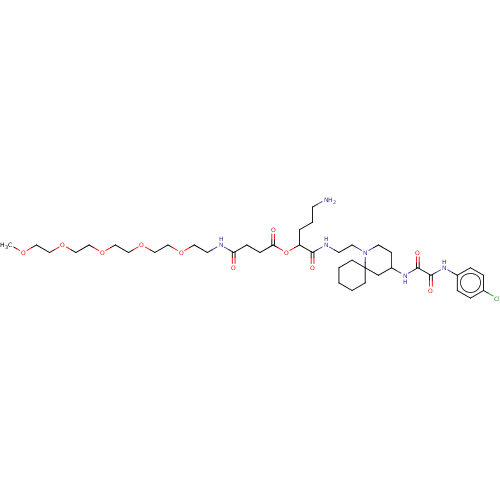
Envelope glycoprotein gp160
(Human immunodeficiency virus 1) | BDBM50568670
(CHEMBL4849790)Show SMILES COCCOCCOCCOCCOCCNC(=O)CCC(=O)OC(CCCN)C(=O)NCCN1CCC(CC11CCCCC1)NC(=O)C(=O)Nc1ccc(Cl)cc1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HIV1 KP-5mvcR gp120 interaction with CD4 in human TZM-bl cells assessed as decrease in viral entry by MTT assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01153
BindingDB Entry DOI: 10.7270/Q2BC4399 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK3
(Homo sapiens (Human)) | BDBM50467110

(CHEMBL4278200)Show SMILES CC(C)C[C@H](NC(=O)[C@@H]1CCCN1C(C)=O)C(=O)N[C@H]1Cc2c[n+](CCCCCCNC(=O)[C@@H](NC(=O)[C@H](CO)NC1=O)[C@@H](C)OP(O)(O)=O)cn2CCCCCCCCc1ccccc1 |r| Show InChI InChI=1S/C46H73N8O11P/c1-32(2)27-37(49-45(60)40-22-18-26-54(40)34(4)56)42(57)48-38-28-36-29-52(31-53(36)25-17-9-6-5-7-12-19-35-20-13-11-14-21-35)24-16-10-8-15-23-47-46(61)41(33(3)65-66(62,63)64)51-44(59)39(30-55)50-43(38)58/h11,13-14,20-21,29,31-33,37-41,55H,5-10,12,15-19,22-28,30H2,1-4H3,(H6-,47,48,49,50,51,57,58,59,60,61,62,63,64)/p+1/t33-,37+,38+,39+,40+,41+/m1/s1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute-Frederick
Curated by ChEMBL
| Assay Description
Inhibition of 5CF-PLATSpTPKNG-NH2 binding to Plk3 C-terminal polo-box domain (unknown origin) preincubated for 30 mins followed by probe addition mea... |
Bioorg Med Chem Lett 28: 3202-3205 (2018)
Article DOI: 10.1016/j.bmcl.2018.08.018
BindingDB Entry DOI: 10.7270/Q25Q4ZST |
More data for this
Ligand-Target Pair | |
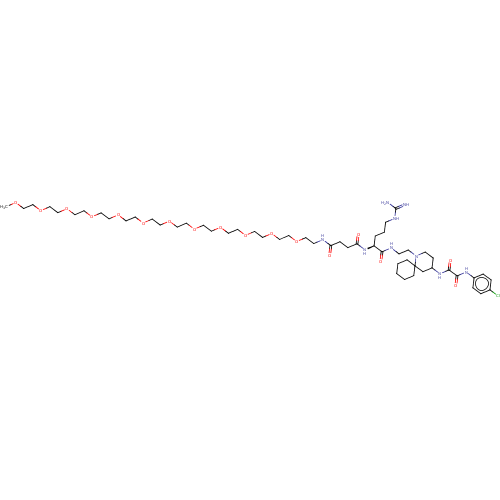
Envelope glycoprotein gp160
(Human immunodeficiency virus 1) | BDBM50568674
(CHEMBL4873341)Show SMILES COCCOCCOCCOCCOCCOCCOCCOCCOCCOCCOCCOCCNC(=O)CCC(=O)N[C@@H](CCCNC(N)=N)C(=O)NCCN1CCC(CC11CCCCC1)NC(=O)C(=O)Nc1ccc(Cl)cc1 |r| | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HIV1 KP-5mvcR gp120 interaction with CD4 in human TZM-bl cells assessed as decrease in viral entry by MTT assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01153
BindingDB Entry DOI: 10.7270/Q2BC4399 |
More data for this
Ligand-Target Pair | |
Envelope glycoprotein gp160
(Human immunodeficiency virus 1) | BDBM50568675

(CHEMBL4863569)Show SMILES COCCOCCOCCOCCOCCOCCOCCOCCOCCOCCOCCOCCOCCOCCOCCOCCOCCOCCOCCOCCOCCOCCOCCOCCNC(=O)CCC(=O)N[C@@H](CCCNC(N)=N)C(=O)NCCN1CCC(CC11CCCCC1)NC(=O)C(=O)Nc1ccc(Cl)cc1 |r| | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HIV1 KP-5mvcR gp120 interaction with CD4 in human TZM-bl cells assessed as decrease in viral entry by MTT assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01153
BindingDB Entry DOI: 10.7270/Q2BC4399 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK2
(Homo sapiens (Human)) | BDBM50467110

(CHEMBL4278200)Show SMILES CC(C)C[C@H](NC(=O)[C@@H]1CCCN1C(C)=O)C(=O)N[C@H]1Cc2c[n+](CCCCCCNC(=O)[C@@H](NC(=O)[C@H](CO)NC1=O)[C@@H](C)OP(O)(O)=O)cn2CCCCCCCCc1ccccc1 |r| Show InChI InChI=1S/C46H73N8O11P/c1-32(2)27-37(49-45(60)40-22-18-26-54(40)34(4)56)42(57)48-38-28-36-29-52(31-53(36)25-17-9-6-5-7-12-19-35-20-13-11-14-21-35)24-16-10-8-15-23-47-46(61)41(33(3)65-66(62,63)64)51-44(59)39(30-55)50-43(38)58/h11,13-14,20-21,29,31-33,37-41,55H,5-10,12,15-19,22-28,30H2,1-4H3,(H6-,47,48,49,50,51,57,58,59,60,61,62,63,64)/p+1/t33-,37+,38+,39+,40+,41+/m1/s1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute-Frederick
Curated by ChEMBL
| Assay Description
Inhibition of 5CF-GPMQTSpTPKNG-NH2 binding to Plk2 C-terminal polo-box domain (unknown origin) preincubated for 30 mins followed by probe addition me... |
Bioorg Med Chem Lett 28: 3202-3205 (2018)
Article DOI: 10.1016/j.bmcl.2018.08.018
BindingDB Entry DOI: 10.7270/Q25Q4ZST |
More data for this
Ligand-Target Pair | |
Envelope glycoprotein gp160
(Human immunodeficiency virus 1) | BDBM50568661

(CHEMBL4863990)Show SMILES NC(=N)NCCCCC(=O)NCCN1CCC(CC11CCCCC1)NC(=O)C(=O)Nc1ccc(Cl)cc1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HIV1 KP-5mvcR gp120 interaction with CD4 in human TZM-bl cells assessed as decrease in viral entry by MTT assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01153
BindingDB Entry DOI: 10.7270/Q2BC4399 |
More data for this
Ligand-Target Pair | |
Envelope glycoprotein gp160
(Human immunodeficiency virus 1) | BDBM50568673
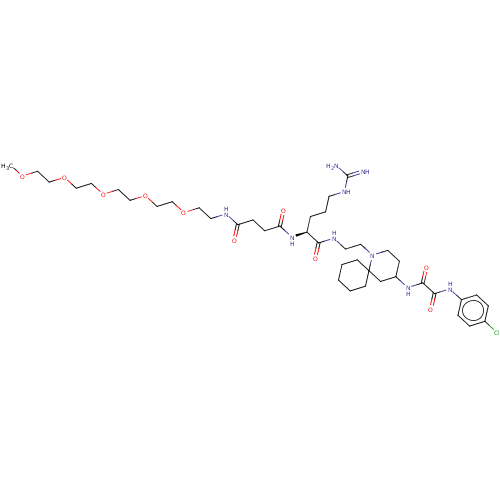
(CHEMBL4876176)Show SMILES COCCOCCOCCOCCOCCNC(=O)CCC(=O)N[C@@H](CCCNC(N)=N)C(=O)NCCN1CCC(CC11CCCCC1)NC(=O)C(=O)Nc1ccc(Cl)cc1 |r| | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HIV1 KP-5mvcR gp120 interaction with CD4 in human TZM-bl cells assessed as decrease in viral entry by MTT assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01153
BindingDB Entry DOI: 10.7270/Q2BC4399 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK3
(Homo sapiens (Human)) | BDBM50467111

(CHEMBL4281659)Show SMILES CC(C)C[C@H](NC(=O)[C@@H]1CCCN1C(C)=O)C(=O)N[C@H]1Cc2c[n+](CCCCCNC(=O)[C@@H](NC(=O)[C@H](CO)NC1=O)[C@@H](C)OP(O)(O)=O)cn2CCCCCCCCc1ccccc1 |r| Show InChI InChI=1S/C45H71N8O11P/c1-31(2)26-36(48-44(59)39-21-17-25-53(39)33(4)55)41(56)47-37-27-35-28-51(30-52(35)24-16-8-6-5-7-11-18-34-19-12-9-13-20-34)23-15-10-14-22-46-45(60)40(32(3)64-65(61,62)63)50-43(58)38(29-54)49-42(37)57/h9,12-13,19-20,28,30-32,36-40,54H,5-8,10-11,14-18,21-27,29H2,1-4H3,(H6-,46,47,48,49,50,56,57,58,59,60,61,62,63)/p+1/t32-,36+,37+,38+,39+,40+/m1/s1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute-Frederick
Curated by ChEMBL
| Assay Description
Inhibition of 5CF-PLATSpTPKNG-NH2 binding to Plk3 C-terminal polo-box domain (unknown origin) preincubated for 30 mins followed by probe addition mea... |
Bioorg Med Chem Lett 28: 3202-3205 (2018)
Article DOI: 10.1016/j.bmcl.2018.08.018
BindingDB Entry DOI: 10.7270/Q25Q4ZST |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK2
(Homo sapiens (Human)) | BDBM50467111

(CHEMBL4281659)Show SMILES CC(C)C[C@H](NC(=O)[C@@H]1CCCN1C(C)=O)C(=O)N[C@H]1Cc2c[n+](CCCCCNC(=O)[C@@H](NC(=O)[C@H](CO)NC1=O)[C@@H](C)OP(O)(O)=O)cn2CCCCCCCCc1ccccc1 |r| Show InChI InChI=1S/C45H71N8O11P/c1-31(2)26-36(48-44(59)39-21-17-25-53(39)33(4)55)41(56)47-37-27-35-28-51(30-52(35)24-16-8-6-5-7-11-18-34-19-12-9-13-20-34)23-15-10-14-22-46-45(60)40(32(3)64-65(61,62)63)50-43(58)38(29-54)49-42(37)57/h9,12-13,19-20,28,30-32,36-40,54H,5-8,10-11,14-18,21-27,29H2,1-4H3,(H6-,46,47,48,49,50,56,57,58,59,60,61,62,63)/p+1/t32-,36+,37+,38+,39+,40+/m1/s1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute-Frederick
Curated by ChEMBL
| Assay Description
Inhibition of 5CF-GPMQTSpTPKNG-NH2 binding to Plk2 C-terminal polo-box domain (unknown origin) preincubated for 30 mins followed by probe addition me... |
Bioorg Med Chem Lett 28: 3202-3205 (2018)
Article DOI: 10.1016/j.bmcl.2018.08.018
BindingDB Entry DOI: 10.7270/Q25Q4ZST |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50403016

(CHEMBL2207845)Show SMILES CC(C)C[C@H](NC(=O)[C@@H]1CCCN1C(C)=O)C(=O)N[C@@H](Cc1cncn1CCCCCCCCc1ccccc1)C(=O)N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)OP(O)(O)=O)C(N)=O |r| Show InChI InChI=1S/C40H63N8O11P/c1-26(2)21-31(44-40(55)34-18-14-20-48(34)28(4)50)37(52)43-32(38(53)45-33(24-49)39(54)46-35(36(41)51)27(3)59-60(56,57)58)22-30-23-42-25-47(30)19-13-8-6-5-7-10-15-29-16-11-9-12-17-29/h9,11-12,16-17,23,25-27,31-35,49H,5-8,10,13-15,18-22,24H2,1-4H3,(H2,41,51)(H,43,52)(H,44,55)(H,45,53)(H,46,54)(H2,56,57,58)/t27-,31+,32+,33+,34+,35+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute-Frederick
Curated by ChEMBL
| Assay Description
Inhibition of full-length myc-tagged Plk1 C-terminal polo-box domain (unknown origin) assessed as inhibition of Plk1-Biotin-Ahx-PMQS(pT)PLN-NH2 pepti... |
Bioorg Med Chem Lett 28: 3202-3205 (2018)
Article DOI: 10.1016/j.bmcl.2018.08.018
BindingDB Entry DOI: 10.7270/Q25Q4ZST |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50467109

(CHEMBL4292295)Show SMILES CC(C)C[C@H](NC(=O)[C@@H]1CCCN1C(C)=O)C(=O)N[C@H]1Cc2c[n+](CCCCNC(=O)[C@@H](NC(=O)[C@H](CO)NC1=O)[C@@H](C)OP(O)(O)=O)cn2CCCCCCCCc1ccccc1 |r| Show InChI InChI=1S/C44H69N8O11P/c1-30(2)25-35(47-43(58)38-20-16-24-52(38)32(4)54)40(55)46-36-26-34-27-50(29-51(34)23-14-8-6-5-7-10-17-33-18-11-9-12-19-33)22-15-13-21-45-44(59)39(31(3)63-64(60,61)62)49-42(57)37(28-53)48-41(36)56/h9,11-12,18-19,27,29-31,35-39,53H,5-8,10,13-17,20-26,28H2,1-4H3,(H6-,45,46,47,48,49,55,56,57,58,59,60,61,62)/p+1/t31-,35+,36+,37+,38+,39+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute-Frederick
Curated by ChEMBL
| Assay Description
Inhibition of full-length myc-tagged Plk1 C-terminal polo-box domain (unknown origin) assessed as inhibition of Plk1-Biotin-Ahx-PMQS(pT)PLN-NH2 pepti... |
Bioorg Med Chem Lett 28: 3202-3205 (2018)
Article DOI: 10.1016/j.bmcl.2018.08.018
BindingDB Entry DOI: 10.7270/Q25Q4ZST |
More data for this
Ligand-Target Pair | |
Envelope glycoprotein gp160
(Human immunodeficiency virus 1) | BDBM50333241
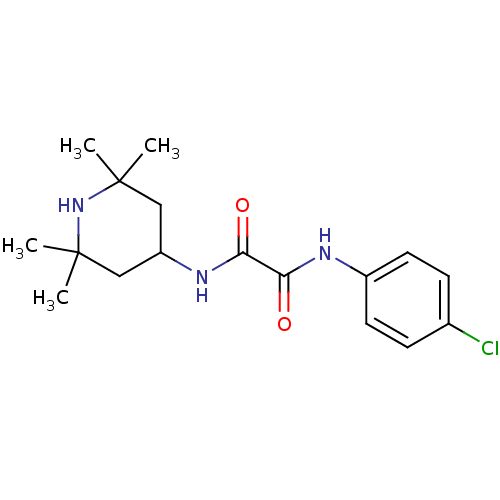
(CHEMBL254781 | N-(4-chlorophenyl)-N'-(2,2,6,6-tetr...)Show SMILES CC1(C)CC(CC(C)(C)N1)NC(=O)C(=O)Nc1ccc(Cl)cc1 Show InChI InChI=1S/C17H24ClN3O2/c1-16(2)9-13(10-17(3,4)21-16)20-15(23)14(22)19-12-7-5-11(18)6-8-12/h5-8,13,21H,9-10H2,1-4H3,(H,19,22)(H,20,23) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HIV1 KP-5mvcR gp120 interaction with CD4 in human TZM-bl cells assessed as decrease in viral entry by MTT assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01153
BindingDB Entry DOI: 10.7270/Q2BC4399 |
More data for this
Ligand-Target Pair | |
Envelope glycoprotein gp160
(Human immunodeficiency virus 1) | BDBM50568663
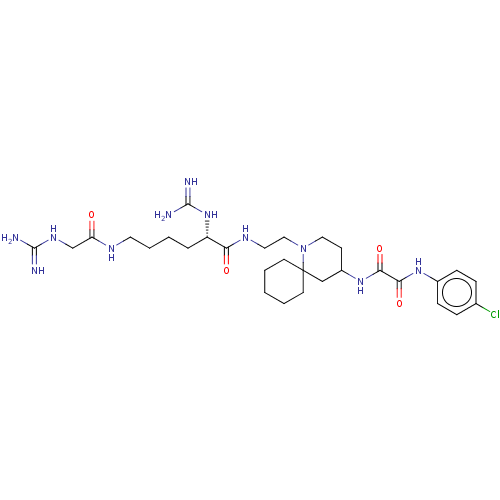
(CHEMBL4859187)Show SMILES NC(=N)NCC(=O)NCCCC[C@H](NC(N)=N)C(=O)NCCN1CCC(CC11CCCCC1)NC(=O)C(=O)Nc1ccc(Cl)cc1 |r| | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HIV1 KP-5mvcR gp120 interaction with CD4 in human TZM-bl cells assessed as decrease in viral entry by MTT assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01153
BindingDB Entry DOI: 10.7270/Q2BC4399 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data