 Found 110 hits with Last Name = 'tsujimoto' and Initial = 'g'
Found 110 hits with Last Name = 'tsujimoto' and Initial = 'g' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Alpha-1B adrenergic receptor
(C.H.O.) | BDBM29568

(CHEMBL2 | PRAZOSIN | PRAZOSIN HYDROCHLORIDE | [3H]...)Show SMILES COc1cc2nc(nc(N)c2cc1OC)N1CCN(CC1)C(=O)c1ccco1 Show InChI InChI=1S/C19H21N5O4/c1-26-15-10-12-13(11-16(15)27-2)21-19(22-17(12)20)24-7-5-23(6-8-24)18(25)14-4-3-9-28-14/h3-4,9-11H,5-8H2,1-2H3,(H2,20,21,22) | Reactome pathway
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Children's Medical Research Center
Curated by PDSP Ki Database
| |
Eur J Pharmacol 268: 399-407 (1994)
Article DOI: 10.1016/0922-4106(94)90065-5
BindingDB Entry DOI: 10.7270/Q26W98KS |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Homo sapiens (Human)) | BDBM29568

(CHEMBL2 | PRAZOSIN | PRAZOSIN HYDROCHLORIDE | [3H]...)Show SMILES COc1cc2nc(nc(N)c2cc1OC)N1CCN(CC1)C(=O)c1ccco1 Show InChI InChI=1S/C19H21N5O4/c1-26-15-10-12-13(11-16(15)27-2)21-19(22-17(12)20)24-7-5-23(6-8-24)18(25)14-4-3-9-28-14/h3-4,9-11H,5-8H2,1-2H3,(H2,20,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Children's Medical Research Center, Tokyo
Curated by PDSP Ki Database
| |
Biochem Biophys Res Commun 195: 902-9 (1993)
Article DOI: 10.1006/bbrc.1993.2130
BindingDB Entry DOI: 10.7270/Q2FQ9V30 |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Homo sapiens (Human)) | BDBM81444
(CAS_185453 | NSC_185453 | WB 4101 | WB-4101)Show InChI InChI=1S/C25H27NO5/c1-27-21-13-8-14-22(28-2)25(21)29-16-15-26-17-23-24(18-9-4-3-5-10-18)31-20-12-7-6-11-19(20)30-23/h3-14,23-24,26H,15-17H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Children's Medical Research Center, Tokyo
Curated by PDSP Ki Database
| |
Biochem Biophys Res Commun 195: 902-9 (1993)
Article DOI: 10.1006/bbrc.1993.2130
BindingDB Entry DOI: 10.7270/Q2FQ9V30 |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM35723
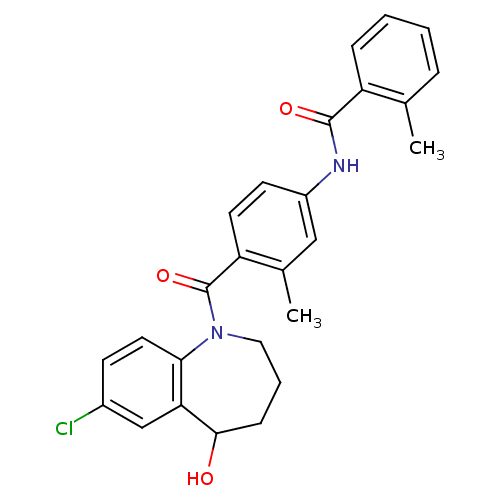
(CHEMBL344159 | N-[4-(7-Chloro-5-hydroxy-2,3,4,5-te...)Show SMILES Cc1ccccc1C(=O)Nc1ccc(C(=O)N2CCCC(O)c3cc(Cl)ccc23)c(C)c1 Show InChI InChI=1S/C26H25ClN2O3/c1-16-6-3-4-7-20(16)25(31)28-19-10-11-21(17(2)14-19)26(32)29-13-5-8-24(30)22-15-18(27)9-12-23(22)29/h3-4,6-7,9-12,14-15,24,30H,5,8,13H2,1-2H3,(H,28,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Second Tokushima Institute of New Drug Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 287: 860-7 (1998)
BindingDB Entry DOI: 10.7270/Q2S46QGC |
More data for this
Ligand-Target Pair | |
Vasopressin V1b receptor
(Homo sapiens (Human)) | BDBM35667
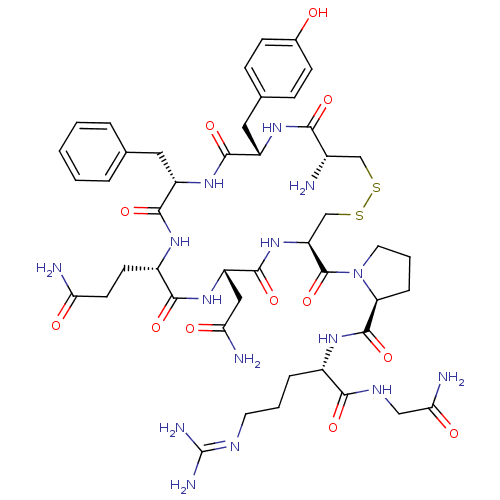
(AVP | CHEMBL373742 | US10131692, 44 (AVP) | [3H]Ar...)Show SMILES [#7]-[#6@H]-1-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccccc2)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6]-1=O)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6]-[#6](-[#7])=O Show InChI InChI=1S/C46H65N15O12S2/c47-27-22-74-75-23-33(45(73)61-17-5-9-34(61)44(72)56-28(8-4-16-53-46(51)52)39(67)54-21-37(50)65)60-43(71)32(20-36(49)64)59-40(68)29(14-15-35(48)63)55-41(69)31(18-24-6-2-1-3-7-24)58-42(70)30(57-38(27)66)19-25-10-12-26(62)13-11-25/h1-3,6-7,10-13,27-34,62H,4-5,8-9,14-23,47H2,(H2,48,63)(H2,49,64)(H2,50,65)(H,54,67)(H,55,69)(H,56,72)(H,57,66)(H,58,70)(H,59,68)(H,60,71)(H4,51,52,53)/t27-,28-,29-,30-,31-,32-,33-,34-/m0/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Second Tokushima Institute of New Drug Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 287: 860-7 (1998)
BindingDB Entry DOI: 10.7270/Q2S46QGC |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM35667

(AVP | CHEMBL373742 | US10131692, 44 (AVP) | [3H]Ar...)Show SMILES [#7]-[#6@H]-1-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccccc2)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6]-1=O)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6]-[#6](-[#7])=O Show InChI InChI=1S/C46H65N15O12S2/c47-27-22-74-75-23-33(45(73)61-17-5-9-34(61)44(72)56-28(8-4-16-53-46(51)52)39(67)54-21-37(50)65)60-43(71)32(20-36(49)64)59-40(68)29(14-15-35(48)63)55-41(69)31(18-24-6-2-1-3-7-24)58-42(70)30(57-38(27)66)19-25-10-12-26(62)13-11-25/h1-3,6-7,10-13,27-34,62H,4-5,8-9,14-23,47H2,(H2,48,63)(H2,49,64)(H2,50,65)(H,54,67)(H,55,69)(H,56,72)(H,57,66)(H,58,70)(H,59,68)(H,60,71)(H4,51,52,53)/t27-,28-,29-,30-,31-,32-,33-,34-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Second Tokushima Institute of New Drug Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 287: 860-7 (1998)
BindingDB Entry DOI: 10.7270/Q2S46QGC |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Rattus norvegicus (Rat)) | BDBM35667

(AVP | CHEMBL373742 | US10131692, 44 (AVP) | [3H]Ar...)Show SMILES [#7]-[#6@H]-1-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccccc2)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6]-1=O)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6]-[#6](-[#7])=O Show InChI InChI=1S/C46H65N15O12S2/c47-27-22-74-75-23-33(45(73)61-17-5-9-34(61)44(72)56-28(8-4-16-53-46(51)52)39(67)54-21-37(50)65)60-43(71)32(20-36(49)64)59-40(68)29(14-15-35(48)63)55-41(69)31(18-24-6-2-1-3-7-24)58-42(70)30(57-38(27)66)19-25-10-12-26(62)13-11-25/h1-3,6-7,10-13,27-34,62H,4-5,8-9,14-23,47H2,(H2,48,63)(H2,49,64)(H2,50,65)(H,54,67)(H,55,69)(H,56,72)(H,57,66)(H,58,70)(H,59,68)(H,60,71)(H4,51,52,53)/t27-,28-,29-,30-,31-,32-,33-,34-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Second Tokushima Institute of New Drug Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 287: 860-7 (1998)
BindingDB Entry DOI: 10.7270/Q2S46QGC |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Rattus norvegicus (Rat)) | BDBM35723

(CHEMBL344159 | N-[4-(7-Chloro-5-hydroxy-2,3,4,5-te...)Show SMILES Cc1ccccc1C(=O)Nc1ccc(C(=O)N2CCCC(O)c3cc(Cl)ccc23)c(C)c1 Show InChI InChI=1S/C26H25ClN2O3/c1-16-6-3-4-7-20(16)25(31)28-19-10-11-21(17(2)14-19)26(32)29-13-5-8-24(30)22-15-18(27)9-12-23(22)29/h3-4,6-7,9-12,14-15,24,30H,5,8,13H2,1-2H3,(H,28,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Second Tokushima Institute of New Drug Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 287: 860-7 (1998)
BindingDB Entry DOI: 10.7270/Q2S46QGC |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Homo sapiens (Human)) | BDBM31046
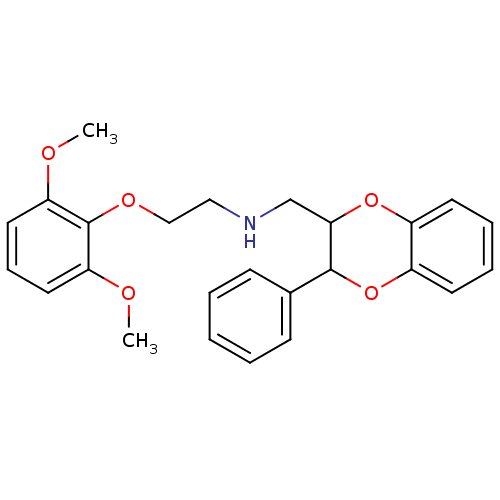
(3-[4,5-dihydro-1H-imidazol-2-ylmethyl-(4-methylphe...)Show InChI InChI=1S/C17H19N3O/c1-13-5-7-14(8-6-13)20(12-17-18-9-10-19-17)15-3-2-4-16(21)11-15/h2-8,11,21H,9-10,12H2,1H3,(H,18,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Similars
| DrugBank
Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Children's Medical Research Center, Tokyo
Curated by PDSP Ki Database
| |
Biochem Biophys Res Commun 195: 902-9 (1993)
Article DOI: 10.1006/bbrc.1993.2130
BindingDB Entry DOI: 10.7270/Q2FQ9V30 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM35667

(AVP | CHEMBL373742 | US10131692, 44 (AVP) | [3H]Ar...)Show SMILES [#7]-[#6@H]-1-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccccc2)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6]-1=O)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6]-[#6](-[#7])=O Show InChI InChI=1S/C46H65N15O12S2/c47-27-22-74-75-23-33(45(73)61-17-5-9-34(61)44(72)56-28(8-4-16-53-46(51)52)39(67)54-21-37(50)65)60-43(71)32(20-36(49)64)59-40(68)29(14-15-35(48)63)55-41(69)31(18-24-6-2-1-3-7-24)58-42(70)30(57-38(27)66)19-25-10-12-26(62)13-11-25/h1-3,6-7,10-13,27-34,62H,4-5,8-9,14-23,47H2,(H2,48,63)(H2,49,64)(H2,50,65)(H,54,67)(H,55,69)(H,56,72)(H,57,66)(H,58,70)(H,59,68)(H,60,71)(H4,51,52,53)/t27-,28-,29-,30-,31-,32-,33-,34-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Second Tokushima Institute of New Drug Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 287: 860-7 (1998)
BindingDB Entry DOI: 10.7270/Q2S46QGC |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Homo sapiens (Human)) | BDBM50033112
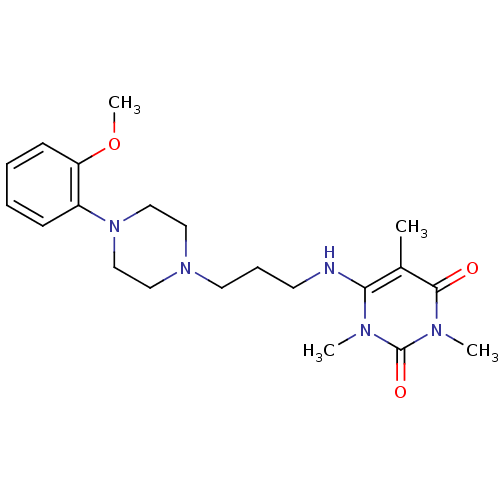
(6-{3-[4-(2-Methoxy-phenyl)-piperazin-1-yl]-propyla...)Show SMILES COc1ccccc1N1CCN(CCCNc2c(C)c(=O)n(C)c(=O)n2C)CC1 Show InChI InChI=1S/C21H31N5O3/c1-16-19(23(2)21(28)24(3)20(16)27)22-10-7-11-25-12-14-26(15-13-25)17-8-5-6-9-18(17)29-4/h5-6,8-9,22H,7,10-15H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Children's Medical Research Center, Tokyo
Curated by PDSP Ki Database
| |
Biochem Biophys Res Commun 195: 902-9 (1993)
Article DOI: 10.1006/bbrc.1993.2130
BindingDB Entry DOI: 10.7270/Q2FQ9V30 |
More data for this
Ligand-Target Pair | |
Alpha-1B adrenergic receptor
(C.H.O.) | BDBM81444

(CAS_185453 | NSC_185453 | WB 4101 | WB-4101)Show InChI InChI=1S/C25H27NO5/c1-27-21-13-8-14-22(28-2)25(21)29-16-15-26-17-23-24(18-9-4-3-5-10-18)31-20-12-7-6-11-19(20)30-23/h3-14,23-24,26H,15-17H2,1-2H3 | Reactome pathway
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Children's Medical Research Center
Curated by PDSP Ki Database
| |
Eur J Pharmacol 268: 399-407 (1994)
Article DOI: 10.1016/0922-4106(94)90065-5
BindingDB Entry DOI: 10.7270/Q26W98KS |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Rattus norvegicus (Rat)) | BDBM35714
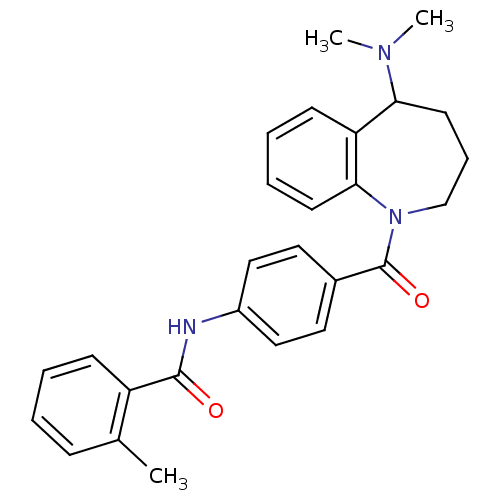
(CHEMBL420762 | Mozavaptan | N-[4-(5-Dimethylamino-...)Show SMILES CN(C)C1CCCN(C(=O)c2ccc(NC(=O)c3ccccc3C)cc2)c2ccccc12 Show InChI InChI=1S/C27H29N3O2/c1-19-9-4-5-10-22(19)26(31)28-21-16-14-20(15-17-21)27(32)30-18-8-13-24(29(2)3)23-11-6-7-12-25(23)30/h4-7,9-12,14-17,24H,8,13,18H2,1-3H3,(H,28,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 6.36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Second Tokushima Institute of New Drug Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 287: 860-7 (1998)
BindingDB Entry DOI: 10.7270/Q2S46QGC |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM35714

(CHEMBL420762 | Mozavaptan | N-[4-(5-Dimethylamino-...)Show SMILES CN(C)C1CCCN(C(=O)c2ccc(NC(=O)c3ccccc3C)cc2)c2ccccc12 Show InChI InChI=1S/C27H29N3O2/c1-19-9-4-5-10-22(19)26(31)28-21-16-14-20(15-17-21)27(32)30-18-8-13-24(29(2)3)23-11-6-7-12-25(23)30/h4-7,9-12,14-17,24H,8,13,18H2,1-3H3,(H,28,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 9.42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Second Tokushima Institute of New Drug Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 287: 860-7 (1998)
BindingDB Entry DOI: 10.7270/Q2S46QGC |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM35723

(CHEMBL344159 | N-[4-(7-Chloro-5-hydroxy-2,3,4,5-te...)Show SMILES Cc1ccccc1C(=O)Nc1ccc(C(=O)N2CCCC(O)c3cc(Cl)ccc23)c(C)c1 Show InChI InChI=1S/C26H25ClN2O3/c1-16-6-3-4-7-20(16)25(31)28-19-10-11-21(17(2)14-19)26(32)29-13-5-8-24(30)22-15-18(27)9-12-23(22)29/h3-4,6-7,9-12,14-15,24,30H,5,8,13H2,1-2H3,(H,28,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 12.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Second Tokushima Institute of New Drug Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 287: 860-7 (1998)
BindingDB Entry DOI: 10.7270/Q2S46QGC |
More data for this
Ligand-Target Pair | |
Alpha-1B adrenergic receptor
(C.H.O.) | BDBM31046

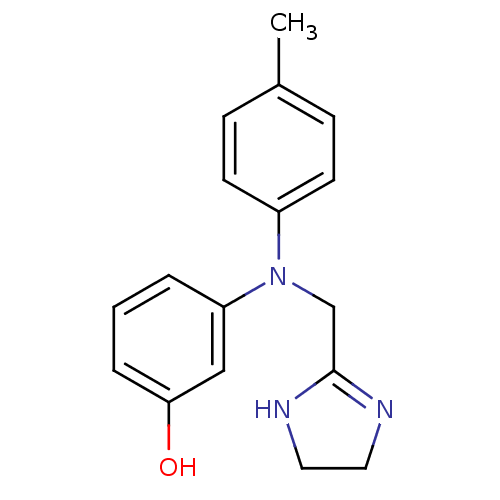
(3-[4,5-dihydro-1H-imidazol-2-ylmethyl-(4-methylphe...)Show InChI InChI=1S/C17H19N3O/c1-13-5-7-14(8-6-13)20(12-17-18-9-10-19-17)15-3-2-4-16(21)11-15/h2-8,11,21H,9-10,12H2,1H3,(H,18,19) | Reactome pathway
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Children's Medical Research Center
Curated by PDSP Ki Database
| |
Eur J Pharmacol 268: 399-407 (1994)
Article DOI: 10.1016/0922-4106(94)90065-5
BindingDB Entry DOI: 10.7270/Q26W98KS |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Homo sapiens (Human)) | BDBM30712
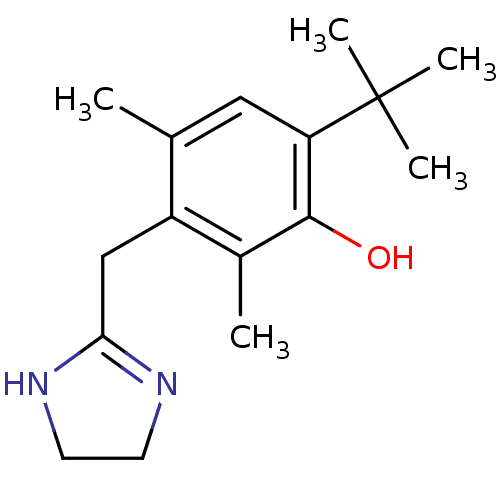
(6-tert-butyl-3-(2-imidazolin-2-ylmethyl)-2,4-dimet...)Show InChI InChI=1S/C16H24N2O/c1-10-8-13(16(3,4)5)15(19)11(2)12(10)9-14-17-6-7-18-14/h8,19H,6-7,9H2,1-5H3,(H,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
Article
PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Children's Medical Research Center, Tokyo
Curated by PDSP Ki Database
| |
Biochem Biophys Res Commun 195: 902-9 (1993)
Article DOI: 10.1006/bbrc.1993.2130
BindingDB Entry DOI: 10.7270/Q2FQ9V30 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM35714

(CHEMBL420762 | Mozavaptan | N-[4-(5-Dimethylamino-...)Show SMILES CN(C)C1CCCN(C(=O)c2ccc(NC(=O)c3ccccc3C)cc2)c2ccccc12 Show InChI InChI=1S/C27H29N3O2/c1-19-9-4-5-10-22(19)26(31)28-21-16-14-20(15-17-21)27(32)30-18-8-13-24(29(2)3)23-11-6-7-12-25(23)30/h4-7,9-12,14-17,24H,8,13,18H2,1-3H3,(H,28,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Second Tokushima Institute of New Drug Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 287: 860-7 (1998)
BindingDB Entry DOI: 10.7270/Q2S46QGC |
More data for this
Ligand-Target Pair | |
Alpha-1B adrenergic receptor
(C.H.O.) | BDBM50033112

(6-{3-[4-(2-Methoxy-phenyl)-piperazin-1-yl]-propyla...)Show SMILES COc1ccccc1N1CCN(CCCNc2c(C)c(=O)n(C)c(=O)n2C)CC1 Show InChI InChI=1S/C21H31N5O3/c1-16-19(23(2)21(28)24(3)20(16)27)22-10-7-11-25-12-14-26(15-13-25)17-8-5-6-9-18(17)29-4/h5-6,8-9,22H,7,10-15H2,1-4H3 | Reactome pathway
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Children's Medical Research Center
Curated by PDSP Ki Database
| |
Eur J Pharmacol 268: 399-407 (1994)
Article DOI: 10.1016/0922-4106(94)90065-5
BindingDB Entry DOI: 10.7270/Q26W98KS |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM35723

(CHEMBL344159 | N-[4-(7-Chloro-5-hydroxy-2,3,4,5-te...)Show SMILES Cc1ccccc1C(=O)Nc1ccc(C(=O)N2CCCC(O)c3cc(Cl)ccc23)c(C)c1 Show InChI InChI=1S/C26H25ClN2O3/c1-16-6-3-4-7-20(16)25(31)28-19-10-11-21(17(2)14-19)26(32)29-13-5-8-24(30)22-15-18(27)9-12-23(22)29/h3-4,6-7,9-12,14-15,24,30H,5,8,13H2,1-2H3,(H,28,31) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 325 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Second Tokushima Institute of New Drug Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 287: 860-7 (1998)
BindingDB Entry DOI: 10.7270/Q2S46QGC |
More data for this
Ligand-Target Pair | |
Alpha-1B adrenergic receptor
(C.H.O.) | BDBM30712

(6-tert-butyl-3-(2-imidazolin-2-ylmethyl)-2,4-dimet...)Show InChI InChI=1S/C16H24N2O/c1-10-8-13(16(3,4)5)15(19)11(2)12(10)9-14-17-6-7-18-14/h8,19H,6-7,9H2,1-5H3,(H,17,18) | Reactome pathway
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Children's Medical Research Center
Curated by PDSP Ki Database
| |
Eur J Pharmacol 268: 399-407 (1994)
Article DOI: 10.1016/0922-4106(94)90065-5
BindingDB Entry DOI: 10.7270/Q26W98KS |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM35714

(CHEMBL420762 | Mozavaptan | N-[4-(5-Dimethylamino-...)Show SMILES CN(C)C1CCCN(C(=O)c2ccc(NC(=O)c3ccccc3C)cc2)c2ccccc12 Show InChI InChI=1S/C27H29N3O2/c1-19-9-4-5-10-22(19)26(31)28-21-16-14-20(15-17-21)27(32)30-18-8-13-24(29(2)3)23-11-6-7-12-25(23)30/h4-7,9-12,14-17,24H,8,13,18H2,1-3H3,(H,28,31) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 524 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Second Tokushima Institute of New Drug Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 287: 860-7 (1998)
BindingDB Entry DOI: 10.7270/Q2S46QGC |
More data for this
Ligand-Target Pair | |
Alpha-1B adrenergic receptor
(C.H.O.) | BDBM50013515

((+)-yohimbine | (16alpha,17alpha)-17-hydroxyyohimb...)Show SMILES COC(=O)[C@H]1[C@@H](O)CC[C@H]2CN3CCc4c([nH]c5ccccc45)[C@@H]3C[C@H]12 |r| Show InChI InChI=1S/C21H26N2O3/c1-26-21(25)19-15-10-17-20-14(13-4-2-3-5-16(13)22-20)8-9-23(17)11-12(15)6-7-18(19)24/h2-5,12,15,17-19,22,24H,6-11H2,1H3/t12-,15-,17-,18-,19+/m0/s1 | Reactome pathway
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Children's Medical Research Center
Curated by PDSP Ki Database
| |
Eur J Pharmacol 268: 399-407 (1994)
Article DOI: 10.1016/0922-4106(94)90065-5
BindingDB Entry DOI: 10.7270/Q26W98KS |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Homo sapiens (Human)) | BDBM50013515

((+)-yohimbine | (16alpha,17alpha)-17-hydroxyyohimb...)Show SMILES COC(=O)[C@H]1[C@@H](O)CC[C@H]2CN3CCc4c([nH]c5ccccc45)[C@@H]3C[C@H]12 |r| Show InChI InChI=1S/C21H26N2O3/c1-26-21(25)19-15-10-17-20-14(13-4-2-3-5-16(13)22-20)8-9-23(17)11-12(15)6-7-18(19)24/h2-5,12,15,17-19,22,24H,6-11H2,1H3/t12-,15-,17-,18-,19+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Children's Medical Research Center, Tokyo
Curated by PDSP Ki Database
| |
Biochem Biophys Res Commun 195: 902-9 (1993)
Article DOI: 10.1006/bbrc.1993.2130
BindingDB Entry DOI: 10.7270/Q2FQ9V30 |
More data for this
Ligand-Target Pair | |
Alpha-1B adrenergic receptor
(C.H.O.) | BDBM35234

(DL-[7-3H]norepinephrine | NOREPINEPHRINE | Noradre...)Show InChI InChI=1S/C8H11NO3/c9-4-8(12)5-1-2-6(10)7(11)3-5/h1-3,8,10-12H,4,9H2 | Reactome pathway
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 920 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Children's Medical Research Center
Curated by PDSP Ki Database
| |
Eur J Pharmacol 268: 399-407 (1994)
Article DOI: 10.1016/0922-4106(94)90065-5
BindingDB Entry DOI: 10.7270/Q26W98KS |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Homo sapiens (Human)) | BDBM84342

(4-[1-hydroxy-2-(methylamino)ethyl]benzene-1,2-diol...)Show InChI InChI=1S/C9H13NO3/c1-10-5-9(13)6-2-3-7(11)8(12)4-6/h2-4,9-13H,5H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Children's Medical Research Center, Tokyo
Curated by PDSP Ki Database
| |
Biochem Biophys Res Commun 195: 902-9 (1993)
Article DOI: 10.1006/bbrc.1993.2130
BindingDB Entry DOI: 10.7270/Q2FQ9V30 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Alpha-1A adrenergic receptor
(Homo sapiens (Human)) | BDBM84342

(4-[1-hydroxy-2-(methylamino)ethyl]benzene-1,2-diol...)Show InChI InChI=1S/C9H13NO3/c1-10-5-9(13)6-2-3-7(11)8(12)4-6/h2-4,9-13H,5H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Children's Medical Research Center, Tokyo
Curated by PDSP Ki Database
| |
Biochem Biophys Res Commun 195: 902-9 (1993)
Article DOI: 10.1006/bbrc.1993.2130
BindingDB Entry DOI: 10.7270/Q2FQ9V30 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Alpha-1A adrenergic receptor
(Homo sapiens (Human)) | BDBM35234

(DL-[7-3H]norepinephrine | NOREPINEPHRINE | Noradre...)Show InChI InChI=1S/C8H11NO3/c9-4-8(12)5-1-2-6(10)7(11)3-5/h1-3,8,10-12H,4,9H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Children's Medical Research Center, Tokyo
Curated by PDSP Ki Database
| |
Biochem Biophys Res Commun 195: 902-9 (1993)
Article DOI: 10.1006/bbrc.1993.2130
BindingDB Entry DOI: 10.7270/Q2FQ9V30 |
More data for this
Ligand-Target Pair | |
Alpha-1B adrenergic receptor
(C.H.O.) | BDBM84342

(4-[1-hydroxy-2-(methylamino)ethyl]benzene-1,2-diol...)Show InChI InChI=1S/C9H13NO3/c1-10-5-9(13)6-2-3-7(11)8(12)4-6/h2-4,9-13H,5H2,1H3 | Reactome pathway
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 9.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Children's Medical Research Center
Curated by PDSP Ki Database
| |
Eur J Pharmacol 268: 399-407 (1994)
Article DOI: 10.1016/0922-4106(94)90065-5
BindingDB Entry DOI: 10.7270/Q26W98KS |
More data for this
Ligand-Target Pair | |
Alpha-1B adrenergic receptor
(C.H.O.) | BDBM50026777
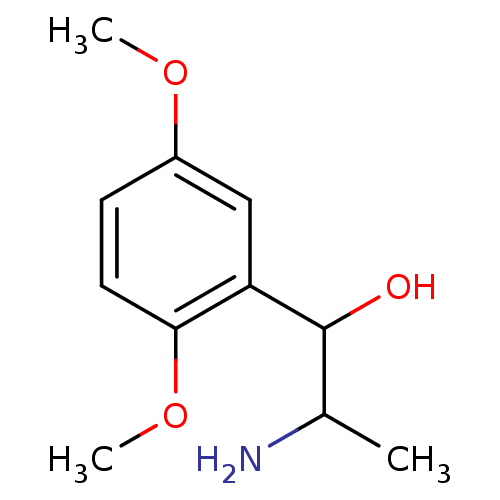
(2-Amino-1-(2,5-dimethoxy-phenyl)-propan-1-ol | 2-A...)Show InChI InChI=1S/C11H17NO3/c1-7(12)11(13)9-6-8(14-2)4-5-10(9)15-3/h4-7,11,13H,12H2,1-3H3 | Reactome pathway
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Children's Medical Research Center
Curated by PDSP Ki Database
| |
Eur J Pharmacol 268: 399-407 (1994)
Article DOI: 10.1016/0922-4106(94)90065-5
BindingDB Entry DOI: 10.7270/Q26W98KS |
More data for this
Ligand-Target Pair | |
Alpha-1B adrenergic receptor
(C.H.O.) | BDBM36024
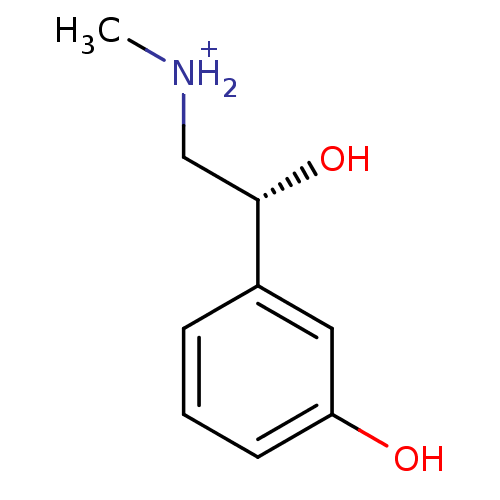
((R)-(-)-phenylephrine | PHENYLEPHRINE | Phenylephr...)Show InChI InChI=1S/C9H13NO2/c1-10-6-9(12)7-3-2-4-8(11)5-7/h2-5,9-12H,6H2,1H3/p+1/t9-/m0/s1 | Reactome pathway
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Children's Medical Research Center
Curated by PDSP Ki Database
| |
Eur J Pharmacol 268: 399-407 (1994)
Article DOI: 10.1016/0922-4106(94)90065-5
BindingDB Entry DOI: 10.7270/Q26W98KS |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Homo sapiens (Human)) | BDBM25392
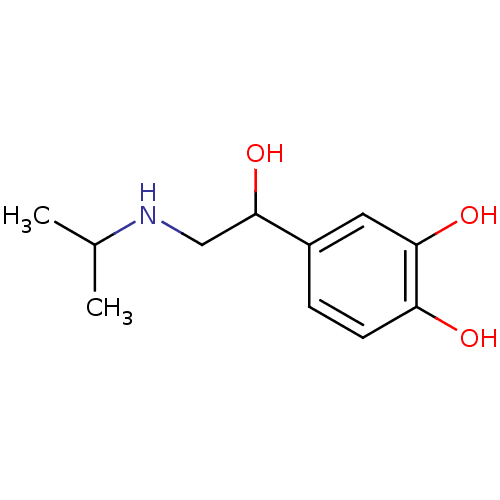
(4-[1-hydroxy-2-(isopropylamino)ethyl]pyrocatechol;...)Show InChI InChI=1S/C11H17NO3/c1-7(2)12-6-11(15)8-3-4-9(13)10(14)5-8/h3-5,7,11-15H,6H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Children's Medical Research Center, Tokyo
Curated by PDSP Ki Database
| |
Biochem Biophys Res Commun 195: 902-9 (1993)
Article DOI: 10.1006/bbrc.1993.2130
BindingDB Entry DOI: 10.7270/Q2FQ9V30 |
More data for this
Ligand-Target Pair | |
Alpha-1B adrenergic receptor
(C.H.O.) | BDBM25392

(4-[1-hydroxy-2-(isopropylamino)ethyl]pyrocatechol;...)Show InChI InChI=1S/C11H17NO3/c1-7(2)12-6-11(15)8-3-4-9(13)10(14)5-8/h3-5,7,11-15H,6H2,1-2H3 | Reactome pathway
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Children's Medical Research Center
Curated by PDSP Ki Database
| |
Eur J Pharmacol 268: 399-407 (1994)
Article DOI: 10.1016/0922-4106(94)90065-5
BindingDB Entry DOI: 10.7270/Q26W98KS |
More data for this
Ligand-Target Pair | |
Vasopressin V1b receptor
(Homo sapiens (Human)) | BDBM35714

(CHEMBL420762 | Mozavaptan | N-[4-(5-Dimethylamino-...)Show SMILES CN(C)C1CCCN(C(=O)c2ccc(NC(=O)c3ccccc3C)cc2)c2ccccc12 Show InChI InChI=1S/C27H29N3O2/c1-19-9-4-5-10-22(19)26(31)28-21-16-14-20(15-17-21)27(32)30-18-8-13-24(29(2)3)23-11-6-7-12-25(23)30/h4-7,9-12,14-17,24H,8,13,18H2,1-3H3,(H,28,31) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Second Tokushima Institute of New Drug Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 287: 860-7 (1998)
BindingDB Entry DOI: 10.7270/Q2S46QGC |
More data for this
Ligand-Target Pair | |
Vasopressin V1b receptor
(Homo sapiens (Human)) | BDBM35723

(CHEMBL344159 | N-[4-(7-Chloro-5-hydroxy-2,3,4,5-te...)Show SMILES Cc1ccccc1C(=O)Nc1ccc(C(=O)N2CCCC(O)c3cc(Cl)ccc23)c(C)c1 Show InChI InChI=1S/C26H25ClN2O3/c1-16-6-3-4-7-20(16)25(31)28-19-10-11-21(17(2)14-19)26(32)29-13-5-8-24(30)22-15-18(27)9-12-23(22)29/h3-4,6-7,9-12,14-15,24,30H,5,8,13H2,1-2H3,(H,28,31) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Second Tokushima Institute of New Drug Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 287: 860-7 (1998)
BindingDB Entry DOI: 10.7270/Q2S46QGC |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Homo sapiens (Human)) | BDBM50026777

(2-Amino-1-(2,5-dimethoxy-phenyl)-propan-1-ol | 2-A...)Show InChI InChI=1S/C11H17NO3/c1-7(12)11(13)9-6-8(14-2)4-5-10(9)15-3/h4-7,11,13H,12H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Children's Medical Research Center, Tokyo
Curated by PDSP Ki Database
| |
Biochem Biophys Res Commun 195: 902-9 (1993)
Article DOI: 10.1006/bbrc.1993.2130
BindingDB Entry DOI: 10.7270/Q2FQ9V30 |
More data for this
Ligand-Target Pair | |
KiSS-1 receptor
(Homo sapiens (Human)) | BDBM26339
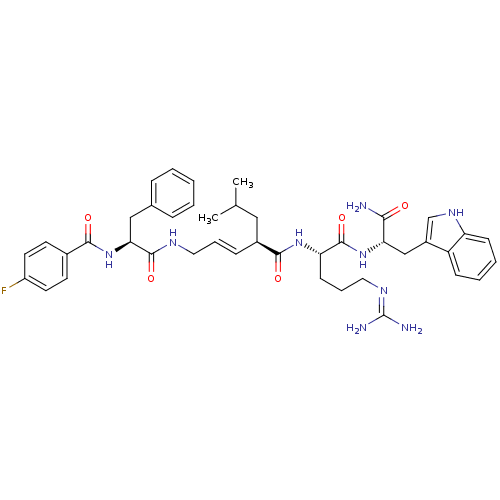
((2R,3E)-N-[(1S)-4-carbamimidamido-1-{[(1S)-1-carba...)Show SMILES CC(C)C[C@H](\C=C\CNC(=O)[C@H](Cc1ccccc1)NC(=O)c1ccc(F)cc1)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(N)=O |r,wU:32.33,wD:11.19,4.4,43.44,(10.76,-1.42,;10.76,.12,;12.1,.89,;9.43,.89,;9.43,2.43,;8.09,3.2,;6.76,2.43,;5.43,3.2,;4.09,2.43,;2.76,3.2,;2.76,4.74,;1.43,2.43,;1.43,.89,;.66,-.45,;-.87,-.66,;-1.45,-2.09,;-.5,-3.3,;1.02,-3.09,;1.6,-1.66,;.09,3.2,;-1.24,2.43,;-1.24,.89,;-2.57,3.2,;-2.57,4.74,;-3.91,5.51,;-5.24,4.74,;-6.58,5.51,;-5.24,3.2,;-3.91,2.43,;10.76,3.2,;10.76,4.74,;12.1,2.43,;13.43,3.2,;13.43,4.74,;12.1,5.51,;12.1,7.05,;10.76,7.82,;10.76,9.36,;12.1,10.13,;9.43,10.13,;14.76,2.43,;14.76,.89,;16.1,3.2,;17.43,2.43,;17.43,.89,;18.76,.12,;18.15,-1.3,;19.3,-2.32,;20.63,-1.53,;22.1,-2,;23.23,-.96,;22.9,.55,;21.43,1.01,;20.3,-.03,;18.76,3.2,;20.1,2.43,;18.76,4.74,)| Show InChI InChI=1S/C42H52FN9O5/c1-26(2)22-29(12-8-20-47-40(56)36(23-27-10-4-3-5-11-27)52-38(54)28-16-18-31(43)19-17-28)39(55)50-34(15-9-21-48-42(45)46)41(57)51-35(37(44)53)24-30-25-49-33-14-7-6-13-32(30)33/h3-8,10-14,16-19,25-26,29,34-36,49H,9,15,20-24H2,1-2H3,(H2,44,53)(H,47,56)(H,50,55)(H,51,57)(H,52,54)(H4,45,46,48)/b12-8+/t29-,34-,35-,36-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [125I]kisspeptin-15 from GPR54 |
ACS Med Chem Lett 2: 53-57 (2011)
Article DOI: 10.1021/ml1002053
BindingDB Entry DOI: 10.7270/Q2K07586 |
More data for this
Ligand-Target Pair | |
KiSS-1 receptor
(Homo sapiens (Human)) | BDBM26349

((2S)-2-[(2S)-2-[(2S)-2-[(2S)-2-amino-3-(4-hydroxyp...)Show SMILES CC(C)C[C@H](NC(=O)CNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CO)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |wU:83.86,72.75,12.20,29.34,37.50,51.58,59.62,wD:4.4,23.26,(23.93,-21.81,;22.46,-22.29,;22.15,-23.79,;21.33,-21.26,;21.65,-19.76,;20.51,-18.74,;19.04,-19.2,;18.73,-20.72,;17.9,-18.17,;16.44,-18.65,;15.29,-17.62,;15.61,-16.12,;13.83,-18.1,;12.69,-17.06,;13.01,-15.57,;11.86,-14.54,;12.18,-13.03,;13.65,-12.55,;14.79,-13.59,;14.47,-15.08,;13.51,-19.6,;12.05,-20.08,;10.9,-19.05,;11.73,-21.57,;12.87,-22.61,;14.34,-22.12,;10.27,-22.05,;9.94,-23.55,;11.09,-24.58,;8.49,-24.03,;7.34,-23,;7.67,-21.49,;6.52,-20.46,;9.12,-21.02,;8.16,-25.53,;6.7,-26.01,;5.56,-24.98,;6.38,-27.51,;4.92,-27.99,;4.6,-29.49,;5.63,-30.63,;4.86,-31.97,;3.35,-31.65,;2.11,-32.56,;.71,-31.92,;.55,-30.4,;1.79,-29.49,;3.2,-30.12,;7.53,-28.54,;8.98,-28.07,;9.31,-26.56,;10.13,-29.1,;9.81,-30.6,;8.35,-31.08,;8.02,-32.58,;7.2,-30.05,;11.59,-28.62,;12.74,-29.65,;12.42,-31.16,;14.21,-29.18,;14.52,-27.67,;15.34,-30.2,;16.8,-29.73,;17.12,-28.22,;18.58,-27.75,;19.72,-28.77,;21.19,-28.3,;19.41,-30.28,;17.94,-30.75,;23.11,-19.28,;23.42,-17.77,;24.24,-20.31,;25.71,-19.83,;26.03,-18.32,;27.49,-17.85,;27.81,-16.34,;29.28,-15.87,;29.59,-14.36,;28.45,-13.34,;31.06,-13.9,;26.85,-20.86,;26.53,-22.37,;28.32,-20.39,;29.46,-21.42,;30.93,-20.94,;32.07,-21.97,;33.53,-21.49,;34.68,-22.52,;34.35,-24.03,;32.89,-24.5,;31.75,-23.47,;29.14,-22.92,;27.68,-23.4,;30.28,-23.95,)| Show InChI InChI=1S/C63H83N17O14/c1-34(2)24-45(58(90)74-43(18-11-23-70-63(68)69)57(89)75-44(54(67)86)26-35-12-5-3-6-13-35)73-53(85)32-72-56(88)46(27-36-14-7-4-8-15-36)77-62(94)50(33-81)80-61(93)49(30-52(66)84)79-59(91)47(28-38-31-71-42-17-10-9-16-40(38)42)78-60(92)48(29-51(65)83)76-55(87)41(64)25-37-19-21-39(82)22-20-37/h3-10,12-17,19-22,31,34,41,43-50,71,81-82H,11,18,23-30,32-33,64H2,1-2H3,(H2,65,83)(H2,66,84)(H2,67,86)(H,72,88)(H,73,85)(H,74,90)(H,75,89)(H,76,87)(H,77,94)(H,78,92)(H,79,91)(H,80,93)(H4,68,69,70)/t41-,43-,44-,45-,46-,47-,48-,49-,50-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [125I]kisspeptin-15 from GPR54 |
ACS Med Chem Lett 2: 53-57 (2011)
Article DOI: 10.1021/ml1002053
BindingDB Entry DOI: 10.7270/Q2K07586 |
More data for this
Ligand-Target Pair | |
KiSS-1 receptor
(Homo sapiens (Human)) | BDBM26338
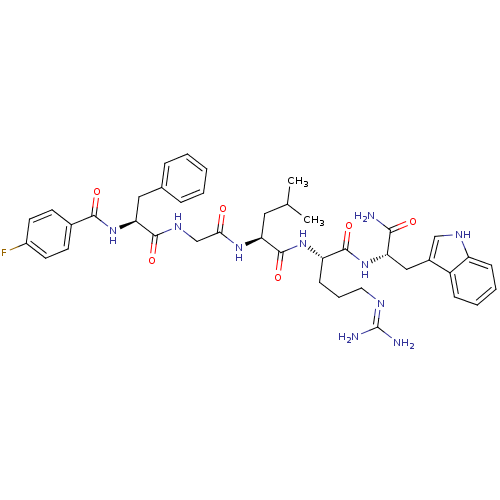
((2S)-N-[(1S)-4-carbamimidamido-1-{[(1S)-1-carbamoy...)Show SMILES CC(C)C[C@H](NC(=O)CNC(=O)[C@H](Cc1ccccc1)NC(=O)c1ccc(F)cc1)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(N)=O |r,wU:33.34,wD:12.20,4.4,44.45,(10.76,-1.42,;10.76,.12,;12.1,.89,;9.43,.89,;9.43,2.43,;8.09,3.2,;6.76,2.43,;6.76,.89,;5.43,3.2,;4.09,2.43,;2.76,3.2,;2.76,4.74,;1.43,2.43,;1.43,.89,;.66,-.45,;-.87,-.66,;-1.45,-2.09,;-.5,-3.3,;1.02,-3.09,;1.6,-1.66,;.09,3.2,;-1.24,2.43,;-1.24,.89,;-2.57,3.2,;-2.57,4.74,;-3.91,5.51,;-5.24,4.74,;-6.58,5.51,;-5.24,3.2,;-3.91,2.43,;10.76,3.2,;10.76,4.74,;12.1,2.43,;13.43,3.2,;13.43,4.74,;12.1,5.51,;12.1,7.05,;10.76,7.82,;10.76,9.36,;12.1,10.13,;9.43,10.13,;14.76,2.43,;14.76,.89,;16.1,3.2,;17.43,2.43,;17.43,.89,;18.76,.12,;18.15,-1.3,;19.3,-2.32,;20.63,-1.53,;22.1,-2,;23.23,-.96,;22.9,.55,;21.43,1.01,;20.3,-.03,;18.76,3.2,;20.1,2.43,;18.76,4.74,)| Show InChI InChI=1S/C41H51FN10O6/c1-24(2)19-33(49-35(53)23-48-38(56)34(20-25-9-4-3-5-10-25)52-37(55)26-14-16-28(42)17-15-26)40(58)50-31(13-8-18-46-41(44)45)39(57)51-32(36(43)54)21-27-22-47-30-12-7-6-11-29(27)30/h3-7,9-12,14-17,22,24,31-34,47H,8,13,18-21,23H2,1-2H3,(H2,43,54)(H,48,56)(H,49,53)(H,50,58)(H,51,57)(H,52,55)(H4,44,45,46)/t31-,32-,33-,34-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [125I]kisspeptin-15 from GPR54 |
ACS Med Chem Lett 2: 53-57 (2011)
Article DOI: 10.1021/ml1002053
BindingDB Entry DOI: 10.7270/Q2K07586 |
More data for this
Ligand-Target Pair | |
Neuropeptide FF receptor 2
(Homo sapiens (Human)) | BDBM50336911
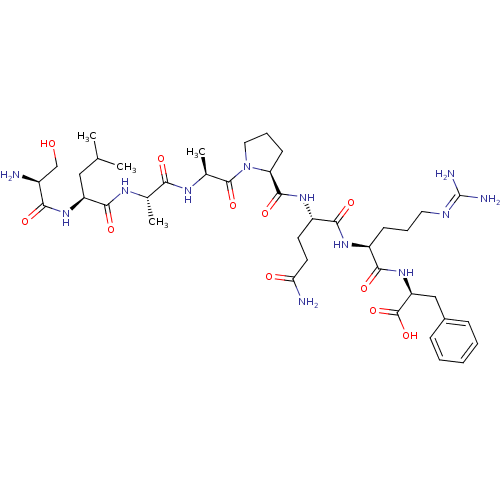
((S)-2-((S)-2-((S)-5-amino-2-((S)-1-((S)-2-((S)-2-(...)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](-[#8])=O |r| Show InChI InChI=1S/C40H64N12O11/c1-21(2)18-28(50-33(56)25(41)20-53)36(59)46-22(3)32(55)47-23(4)38(61)52-17-9-13-30(52)37(60)49-27(14-15-31(42)54)35(58)48-26(12-8-16-45-40(43)44)34(57)51-29(39(62)63)19-24-10-6-5-7-11-24/h5-7,10-11,21-23,25-30,53H,8-9,12-20,41H2,1-4H3,(H2,42,54)(H,46,59)(H,47,55)(H,48,58)(H,49,60)(H,50,56)(H,51,57)(H,62,63)(H4,43,44,45)/t22-,23-,25-,26-,27-,28-,29-,30-/m0/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of (D-[125I]Tyr1, MePhe3)-NPFF from NPFFR2 after 2 hrs |
ACS Med Chem Lett 2: 53-57 (2011)
Article DOI: 10.1021/ml1002053
BindingDB Entry DOI: 10.7270/Q2K07586 |
More data for this
Ligand-Target Pair | |
Neuropeptide FF receptor 1
(Homo sapiens (Human)) | BDBM50336911

((S)-2-((S)-2-((S)-5-amino-2-((S)-1-((S)-2-((S)-2-(...)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](-[#8])=O |r| Show InChI InChI=1S/C40H64N12O11/c1-21(2)18-28(50-33(56)25(41)20-53)36(59)46-22(3)32(55)47-23(4)38(61)52-17-9-13-30(52)37(60)49-27(14-15-31(42)54)35(58)48-26(12-8-16-45-40(43)44)34(57)51-29(39(62)63)19-24-10-6-5-7-11-24/h5-7,10-11,21-23,25-30,53H,8-9,12-20,41H2,1-4H3,(H2,42,54)(H,46,59)(H,47,55)(H,48,58)(H,49,60)(H,50,56)(H,51,57)(H,62,63)(H4,43,44,45)/t22-,23-,25-,26-,27-,28-,29-,30-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of (D-[125I]Tyr1, MePhe3)-NPFF from NPFFR1 after 2 hrs |
ACS Med Chem Lett 2: 53-57 (2011)
Article DOI: 10.1021/ml1002053
BindingDB Entry DOI: 10.7270/Q2K07586 |
More data for this
Ligand-Target Pair | |
Neuropeptide FF receptor 1
(Homo sapiens (Human)) | BDBM26349

((2S)-2-[(2S)-2-[(2S)-2-[(2S)-2-amino-3-(4-hydroxyp...)Show SMILES CC(C)C[C@H](NC(=O)CNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CO)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |wU:83.86,72.75,12.20,29.34,37.50,51.58,59.62,wD:4.4,23.26,(23.93,-21.81,;22.46,-22.29,;22.15,-23.79,;21.33,-21.26,;21.65,-19.76,;20.51,-18.74,;19.04,-19.2,;18.73,-20.72,;17.9,-18.17,;16.44,-18.65,;15.29,-17.62,;15.61,-16.12,;13.83,-18.1,;12.69,-17.06,;13.01,-15.57,;11.86,-14.54,;12.18,-13.03,;13.65,-12.55,;14.79,-13.59,;14.47,-15.08,;13.51,-19.6,;12.05,-20.08,;10.9,-19.05,;11.73,-21.57,;12.87,-22.61,;14.34,-22.12,;10.27,-22.05,;9.94,-23.55,;11.09,-24.58,;8.49,-24.03,;7.34,-23,;7.67,-21.49,;6.52,-20.46,;9.12,-21.02,;8.16,-25.53,;6.7,-26.01,;5.56,-24.98,;6.38,-27.51,;4.92,-27.99,;4.6,-29.49,;5.63,-30.63,;4.86,-31.97,;3.35,-31.65,;2.11,-32.56,;.71,-31.92,;.55,-30.4,;1.79,-29.49,;3.2,-30.12,;7.53,-28.54,;8.98,-28.07,;9.31,-26.56,;10.13,-29.1,;9.81,-30.6,;8.35,-31.08,;8.02,-32.58,;7.2,-30.05,;11.59,-28.62,;12.74,-29.65,;12.42,-31.16,;14.21,-29.18,;14.52,-27.67,;15.34,-30.2,;16.8,-29.73,;17.12,-28.22,;18.58,-27.75,;19.72,-28.77,;21.19,-28.3,;19.41,-30.28,;17.94,-30.75,;23.11,-19.28,;23.42,-17.77,;24.24,-20.31,;25.71,-19.83,;26.03,-18.32,;27.49,-17.85,;27.81,-16.34,;29.28,-15.87,;29.59,-14.36,;28.45,-13.34,;31.06,-13.9,;26.85,-20.86,;26.53,-22.37,;28.32,-20.39,;29.46,-21.42,;30.93,-20.94,;32.07,-21.97,;33.53,-21.49,;34.68,-22.52,;34.35,-24.03,;32.89,-24.5,;31.75,-23.47,;29.14,-22.92,;27.68,-23.4,;30.28,-23.95,)| Show InChI InChI=1S/C63H83N17O14/c1-34(2)24-45(58(90)74-43(18-11-23-70-63(68)69)57(89)75-44(54(67)86)26-35-12-5-3-6-13-35)73-53(85)32-72-56(88)46(27-36-14-7-4-8-15-36)77-62(94)50(33-81)80-61(93)49(30-52(66)84)79-59(91)47(28-38-31-71-42-17-10-9-16-40(38)42)78-60(92)48(29-51(65)83)76-55(87)41(64)25-37-19-21-39(82)22-20-37/h3-10,12-17,19-22,31,34,41,43-50,71,81-82H,11,18,23-30,32-33,64H2,1-2H3,(H2,65,83)(H2,66,84)(H2,67,86)(H,72,88)(H,73,85)(H,74,90)(H,75,89)(H,76,87)(H,77,94)(H,78,92)(H,79,91)(H,80,93)(H4,68,69,70)/t41-,43-,44-,45-,46-,47-,48-,49-,50-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of (D-[125I]Tyr1, MePhe3)-NPFF from NPFFR1 after 2 hrs |
ACS Med Chem Lett 2: 53-57 (2011)
Article DOI: 10.1021/ml1002053
BindingDB Entry DOI: 10.7270/Q2K07586 |
More data for this
Ligand-Target Pair | |
Casein kinase II subunit alpha
(Homo sapiens (Human)) | BDBM50420563

(CHEMBL2087023)Show SMILES COc1ccc(cc1)C(=O)Nc1ncc(s1)-c1ccc(cc1)C(O)=O Show InChI InChI=1S/C18H14N2O4S/c1-24-14-8-6-12(7-9-14)16(21)20-18-19-10-15(25-18)11-2-4-13(5-3-11)17(22)23/h2-10H,1H3,(H,22,23)(H,19,20,21) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Competitive inhibition of GST fused human CK2alpha expressed in Escherichia coli BL21 after 10 mins in presence of ATP |
J Med Chem 55: 2899-903 (2012)
Article DOI: 10.1021/jm2015167
BindingDB Entry DOI: 10.7270/Q2445NRV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Casein kinase II subunit alpha
(Homo sapiens (Human)) | BDBM4078

(6,7,13,14-tetrahydroxy-2,9-dioxatetracyclo[6.6.2.0...)Show InChI InChI=1S/C14H6O8/c15-5-1-3-7-8-4(14(20)22-11(7)9(5)17)2-6(16)10(18)12(8)21-13(3)19/h1-2,15-18H | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka Prefecture University
Curated by ChEMBL
| Assay Description
Inhibition of GST-fused human recombinant CK2alpha expressed in Escherichia coli HMS174 (DE3) |
Bioorg Med Chem Lett 19: 2920-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.076
BindingDB Entry DOI: 10.7270/Q25B02CC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Casein kinase II subunit alpha'
(Homo sapiens (Human)) | BDBM50420563

(CHEMBL2087023)Show SMILES COc1ccc(cc1)C(=O)Nc1ncc(s1)-c1ccc(cc1)C(O)=O Show InChI InChI=1S/C18H14N2O4S/c1-24-14-8-6-12(7-9-14)16(21)20-18-19-10-15(25-18)11-2-4-13(5-3-11)17(22)23/h2-10H,1H3,(H,22,23)(H,19,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Competitive inhibition of GST fused human CK2alpha' expressed in Escherichia coli BL21 after 10 mins in presence of ATP |
J Med Chem 55: 2899-903 (2012)
Article DOI: 10.1021/jm2015167
BindingDB Entry DOI: 10.7270/Q2445NRV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Casein kinase II subunit alpha'
(Homo sapiens (Human)) | BDBM50420565

(CHEMBL2087025)Show SMILES COc1ccc(cc1)C(=O)Nc1cc(n[nH]1)-c1ccc(cc1)C(O)=O Show InChI InChI=1S/C18H15N3O4/c1-25-14-8-6-12(7-9-14)17(22)19-16-10-15(20-21-16)11-2-4-13(5-3-11)18(23)24/h2-10H,1H3,(H,23,24)(H2,19,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Competitive inhibition of GST fused human CK2alpha' expressed in Escherichia coli BL21 after 10 mins in presence of ATP |
J Med Chem 55: 2899-903 (2012)
Article DOI: 10.1021/jm2015167
BindingDB Entry DOI: 10.7270/Q2445NRV |
More data for this
Ligand-Target Pair | |
Neuropeptide FF receptor 2
(Homo sapiens (Human)) | BDBM26349

((2S)-2-[(2S)-2-[(2S)-2-[(2S)-2-amino-3-(4-hydroxyp...)Show SMILES CC(C)C[C@H](NC(=O)CNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CO)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |wU:83.86,72.75,12.20,29.34,37.50,51.58,59.62,wD:4.4,23.26,(23.93,-21.81,;22.46,-22.29,;22.15,-23.79,;21.33,-21.26,;21.65,-19.76,;20.51,-18.74,;19.04,-19.2,;18.73,-20.72,;17.9,-18.17,;16.44,-18.65,;15.29,-17.62,;15.61,-16.12,;13.83,-18.1,;12.69,-17.06,;13.01,-15.57,;11.86,-14.54,;12.18,-13.03,;13.65,-12.55,;14.79,-13.59,;14.47,-15.08,;13.51,-19.6,;12.05,-20.08,;10.9,-19.05,;11.73,-21.57,;12.87,-22.61,;14.34,-22.12,;10.27,-22.05,;9.94,-23.55,;11.09,-24.58,;8.49,-24.03,;7.34,-23,;7.67,-21.49,;6.52,-20.46,;9.12,-21.02,;8.16,-25.53,;6.7,-26.01,;5.56,-24.98,;6.38,-27.51,;4.92,-27.99,;4.6,-29.49,;5.63,-30.63,;4.86,-31.97,;3.35,-31.65,;2.11,-32.56,;.71,-31.92,;.55,-30.4,;1.79,-29.49,;3.2,-30.12,;7.53,-28.54,;8.98,-28.07,;9.31,-26.56,;10.13,-29.1,;9.81,-30.6,;8.35,-31.08,;8.02,-32.58,;7.2,-30.05,;11.59,-28.62,;12.74,-29.65,;12.42,-31.16,;14.21,-29.18,;14.52,-27.67,;15.34,-30.2,;16.8,-29.73,;17.12,-28.22,;18.58,-27.75,;19.72,-28.77,;21.19,-28.3,;19.41,-30.28,;17.94,-30.75,;23.11,-19.28,;23.42,-17.77,;24.24,-20.31,;25.71,-19.83,;26.03,-18.32,;27.49,-17.85,;27.81,-16.34,;29.28,-15.87,;29.59,-14.36,;28.45,-13.34,;31.06,-13.9,;26.85,-20.86,;26.53,-22.37,;28.32,-20.39,;29.46,-21.42,;30.93,-20.94,;32.07,-21.97,;33.53,-21.49,;34.68,-22.52,;34.35,-24.03,;32.89,-24.5,;31.75,-23.47,;29.14,-22.92,;27.68,-23.4,;30.28,-23.95,)| Show InChI InChI=1S/C63H83N17O14/c1-34(2)24-45(58(90)74-43(18-11-23-70-63(68)69)57(89)75-44(54(67)86)26-35-12-5-3-6-13-35)73-53(85)32-72-56(88)46(27-36-14-7-4-8-15-36)77-62(94)50(33-81)80-61(93)49(30-52(66)84)79-59(91)47(28-38-31-71-42-17-10-9-16-40(38)42)78-60(92)48(29-51(65)83)76-55(87)41(64)25-37-19-21-39(82)22-20-37/h3-10,12-17,19-22,31,34,41,43-50,71,81-82H,11,18,23-30,32-33,64H2,1-2H3,(H2,65,83)(H2,66,84)(H2,67,86)(H,72,88)(H,73,85)(H,74,90)(H,75,89)(H,76,87)(H,77,94)(H,78,92)(H,79,91)(H,80,93)(H4,68,69,70)/t41-,43-,44-,45-,46-,47-,48-,49-,50-/m0/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of (D-[125I]Tyr1, MePhe3)-NPFF from NPFFR2 after 2 hrs |
ACS Med Chem Lett 2: 53-57 (2011)
Article DOI: 10.1021/ml1002053
BindingDB Entry DOI: 10.7270/Q2K07586 |
More data for this
Ligand-Target Pair | |
Casein kinase II subunit alpha
(Homo sapiens (Human)) | BDBM50420562
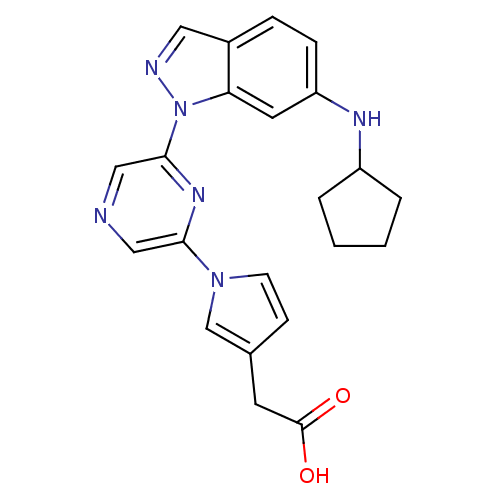
(CHEMBL2048144)Show SMILES OC(=O)Cc1ccn(c1)-c1cncc(n1)-n1ncc2ccc(NC3CCCC3)cc12 Show InChI InChI=1S/C22H22N6O2/c29-22(30)9-15-7-8-27(14-15)20-12-23-13-21(26-20)28-19-10-18(6-5-16(19)11-24-28)25-17-3-1-2-4-17/h5-8,10-14,17,25H,1-4,9H2,(H,29,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Competitive inhibition of GST fused human CK2alpha expressed in Escherichia coli BL21 after 10 mins in presence of ATP |
J Med Chem 55: 2899-903 (2012)
Article DOI: 10.1021/jm2015167
BindingDB Entry DOI: 10.7270/Q2445NRV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Casein kinase II subunit alpha
(Homo sapiens (Human)) | BDBM50420565

(CHEMBL2087025)Show SMILES COc1ccc(cc1)C(=O)Nc1cc(n[nH]1)-c1ccc(cc1)C(O)=O Show InChI InChI=1S/C18H15N3O4/c1-25-14-8-6-12(7-9-14)17(22)19-16-10-15(20-21-16)11-2-4-13(5-3-11)18(23)24/h2-10H,1H3,(H,23,24)(H2,19,20,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Competitive inhibition of GST fused human CK2alpha expressed in Escherichia coli BL21 after 10 mins in presence of ATP |
J Med Chem 55: 2899-903 (2012)
Article DOI: 10.1021/jm2015167
BindingDB Entry DOI: 10.7270/Q2445NRV |
More data for this
Ligand-Target Pair | |
Neuropeptide FF receptor 1
(Homo sapiens (Human)) | BDBM26338

((2S)-N-[(1S)-4-carbamimidamido-1-{[(1S)-1-carbamoy...)Show SMILES CC(C)C[C@H](NC(=O)CNC(=O)[C@H](Cc1ccccc1)NC(=O)c1ccc(F)cc1)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(N)=O |r,wU:33.34,wD:12.20,4.4,44.45,(10.76,-1.42,;10.76,.12,;12.1,.89,;9.43,.89,;9.43,2.43,;8.09,3.2,;6.76,2.43,;6.76,.89,;5.43,3.2,;4.09,2.43,;2.76,3.2,;2.76,4.74,;1.43,2.43,;1.43,.89,;.66,-.45,;-.87,-.66,;-1.45,-2.09,;-.5,-3.3,;1.02,-3.09,;1.6,-1.66,;.09,3.2,;-1.24,2.43,;-1.24,.89,;-2.57,3.2,;-2.57,4.74,;-3.91,5.51,;-5.24,4.74,;-6.58,5.51,;-5.24,3.2,;-3.91,2.43,;10.76,3.2,;10.76,4.74,;12.1,2.43,;13.43,3.2,;13.43,4.74,;12.1,5.51,;12.1,7.05,;10.76,7.82,;10.76,9.36,;12.1,10.13,;9.43,10.13,;14.76,2.43,;14.76,.89,;16.1,3.2,;17.43,2.43,;17.43,.89,;18.76,.12,;18.15,-1.3,;19.3,-2.32,;20.63,-1.53,;22.1,-2,;23.23,-.96,;22.9,.55,;21.43,1.01,;20.3,-.03,;18.76,3.2,;20.1,2.43,;18.76,4.74,)| Show InChI InChI=1S/C41H51FN10O6/c1-24(2)19-33(49-35(53)23-48-38(56)34(20-25-9-4-3-5-10-25)52-37(55)26-14-16-28(42)17-15-26)40(58)50-31(13-8-18-46-41(44)45)39(57)51-32(36(43)54)21-27-22-47-30-12-7-6-11-29(27)30/h3-7,9-12,14-17,22,24,31-34,47H,8,13,18-21,23H2,1-2H3,(H2,43,54)(H,48,56)(H,49,53)(H,50,58)(H,51,57)(H,52,55)(H4,44,45,46)/t31-,32-,33-,34-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of (D-[125I]Tyr1, MePhe3)-NPFF from NPFFR1 after 2 hrs |
ACS Med Chem Lett 2: 53-57 (2011)
Article DOI: 10.1021/ml1002053
BindingDB Entry DOI: 10.7270/Q2K07586 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data