 Found 188 hits with Last Name = 'tuffnell' and Initial = 'ar'
Found 188 hits with Last Name = 'tuffnell' and Initial = 'ar' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Adenosine receptor A2b
(Homo sapiens (Human)) | BDBM50166735
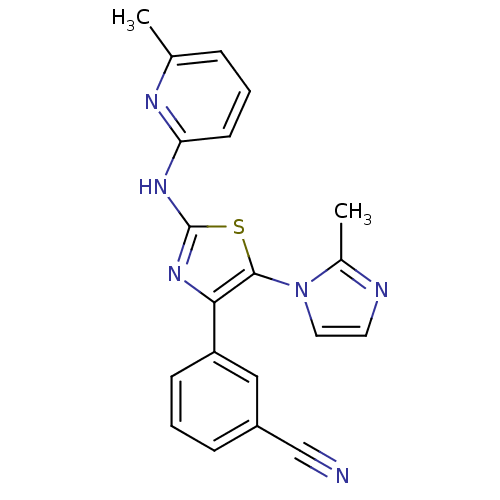
(3-[5-(2-Methyl-imidazol-1-yl)-2-(pyrazin-2-ylamino...)Show SMILES Cc1nccn1-c1sc(Nc2cccc(C)n2)nc1-c1cccc(c1)C#N Show InChI InChI=1S/C20H16N6S/c1-13-5-3-8-17(23-13)24-20-25-18(16-7-4-6-15(11-16)12-21)19(27-20)26-10-9-22-14(26)2/h3-11H,1-2H3,(H,23,24,25) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of luciferase production elicited by NECA by compound in CHO cells transfected with human adenosine A2b receptor and a luciferase expressi... |
Bioorg Med Chem Lett 15: 3081-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.021
BindingDB Entry DOI: 10.7270/Q2X63NQG |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2b
(Homo sapiens (Human)) | BDBM50166742
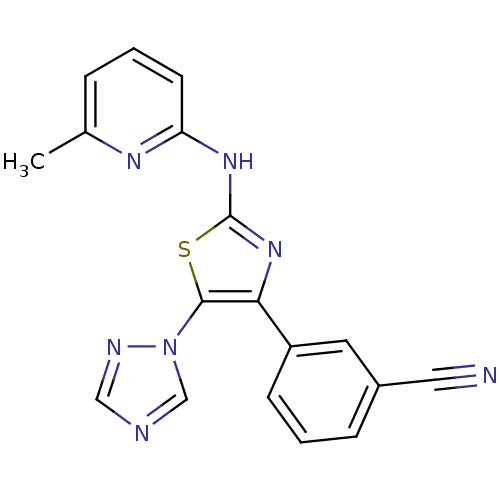
(3-[2-(3-Methyl-pyridin-2-ylamino)-5-[1,2,4]triazol...)Show SMILES Cc1cccc(Nc2nc(c(s2)-n2cncn2)-c2cccc(c2)C#N)n1 Show InChI InChI=1S/C18H13N7S/c1-12-4-2-7-15(22-12)23-18-24-16(14-6-3-5-13(8-14)9-19)17(26-18)25-11-20-10-21-25/h2-8,10-11H,1H3,(H,22,23,24) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of luciferase production elicited by NECA by compound in CHO cells transfected with human adenosine A2b receptor and a luciferase expressi... |
Bioorg Med Chem Lett 15: 3081-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.021
BindingDB Entry DOI: 10.7270/Q2X63NQG |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50166739
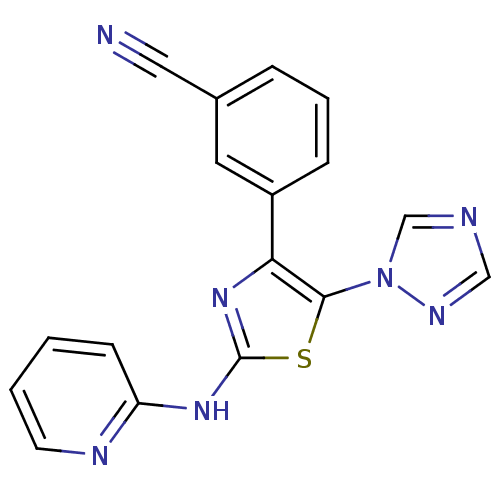
(3-[2-(Pyridin-2-ylamino)-5-[1,2,4]triazol-1-yl-thi...)Show InChI InChI=1S/C17H11N7S/c18-9-12-4-3-5-13(8-12)15-16(24-11-19-10-21-24)25-17(23-15)22-14-6-1-2-7-20-14/h1-8,10-11H,(H,20,22,23) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Displacement of [125I]-AB-MECA from human adenosine A3 receptors transfected in CHO cells |
Bioorg Med Chem Lett 15: 3081-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.021
BindingDB Entry DOI: 10.7270/Q2X63NQG |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50166742

(3-[2-(3-Methyl-pyridin-2-ylamino)-5-[1,2,4]triazol...)Show SMILES Cc1cccc(Nc2nc(c(s2)-n2cncn2)-c2cccc(c2)C#N)n1 Show InChI InChI=1S/C18H13N7S/c1-12-4-2-7-15(22-12)23-18-24-16(14-6-3-5-13(8-14)9-19)17(26-18)25-11-20-10-21-25/h2-8,10-11H,1H3,(H,22,23,24) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Displacement of [125I]-AB-MECA from human adenosine A3 receptors transfected in CHO cells |
Bioorg Med Chem Lett 15: 3081-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.021
BindingDB Entry DOI: 10.7270/Q2X63NQG |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2b
(Homo sapiens (Human)) | BDBM50166739

(3-[2-(Pyridin-2-ylamino)-5-[1,2,4]triazol-1-yl-thi...)Show InChI InChI=1S/C17H11N7S/c18-9-12-4-3-5-13(8-12)15-16(24-11-19-10-21-24)25-17(23-15)22-14-6-1-2-7-20-14/h1-8,10-11H,(H,20,22,23) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of luciferase production elicited by NECA by compound in CHO cells transfected with human adenosine A2b receptor and a luciferase expressi... |
Bioorg Med Chem Lett 15: 3081-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.021
BindingDB Entry DOI: 10.7270/Q2X63NQG |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2b
(Homo sapiens (Human)) | BDBM50166745
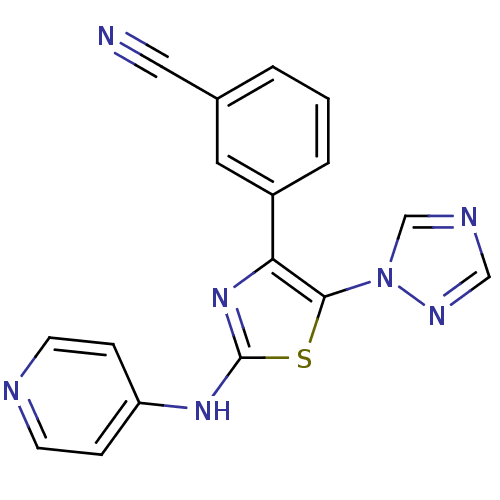
(3-[2-(Pyridin-4-ylamino)-5-[1,2,4]triazol-1-yl-thi...)Show InChI InChI=1S/C17H11N7S/c18-9-12-2-1-3-13(8-12)15-16(24-11-20-10-21-24)25-17(23-15)22-14-4-6-19-7-5-14/h1-8,10-11H,(H,19,22,23) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of luciferase production elicited by NECA by compound in CHO cells transfected with human adenosine A2b receptor and a luciferase expressi... |
Bioorg Med Chem Lett 15: 3081-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.021
BindingDB Entry DOI: 10.7270/Q2X63NQG |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2b
(Homo sapiens (Human)) | BDBM50166737
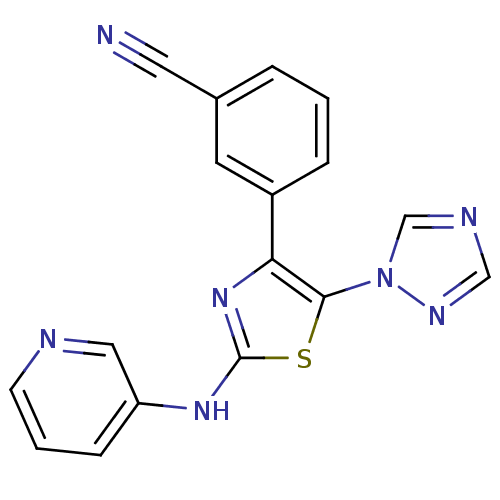
(3-[2-(Pyridin-3-ylamino)-5-[1,2,4]triazol-1-yl-thi...)Show InChI InChI=1S/C17H11N7S/c18-8-12-3-1-4-13(7-12)15-16(24-11-20-10-21-24)25-17(23-15)22-14-5-2-6-19-9-14/h1-7,9-11H,(H,22,23) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 86 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of luciferase production elicited by NECA by compound in CHO cells transfected with human adenosine A2b receptor and a luciferase expressi... |
Bioorg Med Chem Lett 15: 3081-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.021
BindingDB Entry DOI: 10.7270/Q2X63NQG |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2b
(Homo sapiens (Human)) | BDBM50166743

(3-[5-Imidazol-1-yl-2-(pyridin-3-ylamino)-thiazol-4...)Show InChI InChI=1S/C18H12N6S/c19-10-13-3-1-4-14(9-13)16-17(24-8-7-21-12-24)25-18(23-16)22-15-5-2-6-20-11-15/h1-9,11-12H,(H,22,23) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 92 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of luciferase production elicited by NECA by compound in CHO cells transfected with human adenosine A2b receptor and a luciferase expressi... |
Bioorg Med Chem Lett 15: 3081-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.021
BindingDB Entry DOI: 10.7270/Q2X63NQG |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50166743

(3-[5-Imidazol-1-yl-2-(pyridin-3-ylamino)-thiazol-4...)Show InChI InChI=1S/C18H12N6S/c19-10-13-3-1-4-14(9-13)16-17(24-8-7-21-12-24)25-18(23-16)22-15-5-2-6-20-11-15/h1-9,11-12H,(H,22,23) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 124 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Displacement of [125I]-AB-MECA from human adenosine A3 receptors transfected in CHO cells |
Bioorg Med Chem Lett 15: 3081-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.021
BindingDB Entry DOI: 10.7270/Q2X63NQG |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50166741
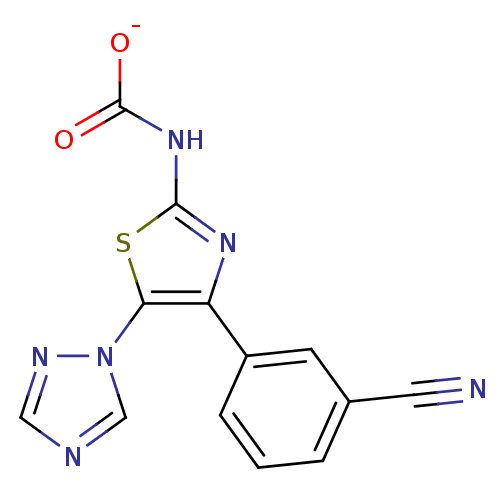
(4-(3-cyanophenyl)-5-(1H-1,2,4-triazol-1-yl)-1,3-th...)Show SMILES [O-]C(=O)Nc1nc(c(s1)-n1cncn1)-c1cccc(c1)C#N Show InChI InChI=1S/C13H8N6O2S/c14-5-8-2-1-3-9(4-8)10-11(19-7-15-6-16-19)22-12(17-10)18-13(20)21/h1-4,6-7H,(H,17,18)(H,20,21)/p-1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 134 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Displacement of [125I]-AB-MECA from human adenosine A3 receptors transfected in CHO cells |
Bioorg Med Chem Lett 15: 3081-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.021
BindingDB Entry DOI: 10.7270/Q2X63NQG |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50166735

(3-[5-(2-Methyl-imidazol-1-yl)-2-(pyrazin-2-ylamino...)Show SMILES Cc1nccn1-c1sc(Nc2cccc(C)n2)nc1-c1cccc(c1)C#N Show InChI InChI=1S/C20H16N6S/c1-13-5-3-8-17(23-13)24-20-25-18(16-7-4-6-15(11-16)12-21)19(27-20)26-10-9-22-14(26)2/h3-11H,1-2H3,(H,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 175 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]ZM-241,385 from human adenosine A2a receptors transfected in HEK 293 cells |
Bioorg Med Chem Lett 15: 3081-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.021
BindingDB Entry DOI: 10.7270/Q2X63NQG |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50166735

(3-[5-(2-Methyl-imidazol-1-yl)-2-(pyrazin-2-ylamino...)Show SMILES Cc1nccn1-c1sc(Nc2cccc(C)n2)nc1-c1cccc(c1)C#N Show InChI InChI=1S/C20H16N6S/c1-13-5-3-8-17(23-13)24-20-25-18(16-7-4-6-15(11-16)12-21)19(27-20)26-10-9-22-14(26)2/h3-11H,1-2H3,(H,23,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 224 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPCPX from human adenosine A1 receptors transfected in CHO cells |
Bioorg Med Chem Lett 15: 3081-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.021
BindingDB Entry DOI: 10.7270/Q2X63NQG |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50166745

(3-[2-(Pyridin-4-ylamino)-5-[1,2,4]triazol-1-yl-thi...)Show InChI InChI=1S/C17H11N7S/c18-9-12-2-1-3-13(8-12)15-16(24-11-20-10-21-24)25-17(23-15)22-14-4-6-19-7-5-14/h1-8,10-11H,(H,19,22,23) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 304 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Displacement of [125I]-AB-MECA from human adenosine A3 receptors transfected in CHO cells |
Bioorg Med Chem Lett 15: 3081-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.021
BindingDB Entry DOI: 10.7270/Q2X63NQG |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2b
(Homo sapiens (Human)) | BDBM50166741

(4-(3-cyanophenyl)-5-(1H-1,2,4-triazol-1-yl)-1,3-th...)Show SMILES [O-]C(=O)Nc1nc(c(s1)-n1cncn1)-c1cccc(c1)C#N Show InChI InChI=1S/C13H8N6O2S/c14-5-8-2-1-3-9(4-8)10-11(19-7-15-6-16-19)22-12(17-10)18-13(20)21/h1-4,6-7H,(H,17,18)(H,20,21)/p-1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 365 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of luciferase production elicited by NECA by compound in CHO cells transfected with human adenosine A2b receptor and a luciferase expressi... |
Bioorg Med Chem Lett 15: 3081-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.021
BindingDB Entry DOI: 10.7270/Q2X63NQG |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50166735

(3-[5-(2-Methyl-imidazol-1-yl)-2-(pyrazin-2-ylamino...)Show SMILES Cc1nccn1-c1sc(Nc2cccc(C)n2)nc1-c1cccc(c1)C#N Show InChI InChI=1S/C20H16N6S/c1-13-5-3-8-17(23-13)24-20-25-18(16-7-4-6-15(11-16)12-21)19(27-20)26-10-9-22-14(26)2/h3-11H,1-2H3,(H,23,24,25) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 419 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Displacement of [125I]-AB-MECA from human adenosine A3 receptors transfected in CHO cells |
Bioorg Med Chem Lett 15: 3081-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.021
BindingDB Entry DOI: 10.7270/Q2X63NQG |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50166743

(3-[5-Imidazol-1-yl-2-(pyridin-3-ylamino)-thiazol-4...)Show InChI InChI=1S/C18H12N6S/c19-10-13-3-1-4-14(9-13)16-17(24-8-7-21-12-24)25-18(23-16)22-15-5-2-6-20-11-15/h1-9,11-12H,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPCPX from human adenosine A1 receptors transfected in CHO cells |
Bioorg Med Chem Lett 15: 3081-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.021
BindingDB Entry DOI: 10.7270/Q2X63NQG |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50166737

(3-[2-(Pyridin-3-ylamino)-5-[1,2,4]triazol-1-yl-thi...)Show InChI InChI=1S/C17H11N7S/c18-8-12-3-1-4-13(7-12)15-16(24-11-20-10-21-24)25-17(23-15)22-14-5-2-6-19-9-14/h1-7,9-11H,(H,22,23) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 698 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Displacement of [125I]-AB-MECA from human adenosine A3 receptors transfected in CHO cells |
Bioorg Med Chem Lett 15: 3081-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.021
BindingDB Entry DOI: 10.7270/Q2X63NQG |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50166737

(3-[2-(Pyridin-3-ylamino)-5-[1,2,4]triazol-1-yl-thi...)Show InChI InChI=1S/C17H11N7S/c18-8-12-3-1-4-13(7-12)15-16(24-11-20-10-21-24)25-17(23-15)22-14-5-2-6-19-9-14/h1-7,9-11H,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.53E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPCPX from human adenosine A1 receptors transfected in CHO cells |
Bioorg Med Chem Lett 15: 3081-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.021
BindingDB Entry DOI: 10.7270/Q2X63NQG |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50166739

(3-[2-(Pyridin-2-ylamino)-5-[1,2,4]triazol-1-yl-thi...)Show InChI InChI=1S/C17H11N7S/c18-9-12-4-3-5-13(8-12)15-16(24-11-19-10-21-24)25-17(23-15)22-14-6-1-2-7-20-14/h1-8,10-11H,(H,20,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.76E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPCPX from human adenosine A1 receptors transfected in CHO cells |
Bioorg Med Chem Lett 15: 3081-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.021
BindingDB Entry DOI: 10.7270/Q2X63NQG |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50166739

(3-[2-(Pyridin-2-ylamino)-5-[1,2,4]triazol-1-yl-thi...)Show InChI InChI=1S/C17H11N7S/c18-9-12-4-3-5-13(8-12)15-16(24-11-19-10-21-24)25-17(23-15)22-14-6-1-2-7-20-14/h1-8,10-11H,(H,20,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]ZM-241,385 from human adenosine A2a receptors transfected in HEK 293 cells |
Bioorg Med Chem Lett 15: 3081-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.021
BindingDB Entry DOI: 10.7270/Q2X63NQG |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50166737

(3-[2-(Pyridin-3-ylamino)-5-[1,2,4]triazol-1-yl-thi...)Show InChI InChI=1S/C17H11N7S/c18-8-12-3-1-4-13(7-12)15-16(24-11-20-10-21-24)25-17(23-15)22-14-5-2-6-19-9-14/h1-7,9-11H,(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.57E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]ZM-241,385 from human adenosine A2a receptors transfected in HEK 293 cells |
Bioorg Med Chem Lett 15: 3081-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.021
BindingDB Entry DOI: 10.7270/Q2X63NQG |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50166743

(3-[5-Imidazol-1-yl-2-(pyridin-3-ylamino)-thiazol-4...)Show InChI InChI=1S/C18H12N6S/c19-10-13-3-1-4-14(9-13)16-17(24-8-7-21-12-24)25-18(23-16)22-15-5-2-6-20-11-15/h1-9,11-12H,(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]ZM-241,385 from human adenosine A2a receptors transfected in HEK 293 cells |
Bioorg Med Chem Lett 15: 3081-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.021
BindingDB Entry DOI: 10.7270/Q2X63NQG |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50166741

(4-(3-cyanophenyl)-5-(1H-1,2,4-triazol-1-yl)-1,3-th...)Show SMILES [O-]C(=O)Nc1nc(c(s1)-n1cncn1)-c1cccc(c1)C#N Show InChI InChI=1S/C13H8N6O2S/c14-5-8-2-1-3-9(4-8)10-11(19-7-15-6-16-19)22-12(17-10)18-13(20)21/h1-4,6-7H,(H,17,18)(H,20,21)/p-1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.84E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPCPX from human adenosine A1 receptors transfected in CHO cells |
Bioorg Med Chem Lett 15: 3081-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.021
BindingDB Entry DOI: 10.7270/Q2X63NQG |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50166745

(3-[2-(Pyridin-4-ylamino)-5-[1,2,4]triazol-1-yl-thi...)Show InChI InChI=1S/C17H11N7S/c18-9-12-2-1-3-13(8-12)15-16(24-11-20-10-21-24)25-17(23-15)22-14-4-6-19-7-5-14/h1-8,10-11H,(H,19,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.78E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPCPX from human adenosine A1 receptors transfected in CHO cells |
Bioorg Med Chem Lett 15: 3081-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.021
BindingDB Entry DOI: 10.7270/Q2X63NQG |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50166745

(3-[2-(Pyridin-4-ylamino)-5-[1,2,4]triazol-1-yl-thi...)Show InChI InChI=1S/C17H11N7S/c18-9-12-2-1-3-13(8-12)15-16(24-11-20-10-21-24)25-17(23-15)22-14-4-6-19-7-5-14/h1-8,10-11H,(H,19,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]ZM-241,385 from human adenosine A2a receptors transfected in HEK 293 cells |
Bioorg Med Chem Lett 15: 3081-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.021
BindingDB Entry DOI: 10.7270/Q2X63NQG |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50166741

(4-(3-cyanophenyl)-5-(1H-1,2,4-triazol-1-yl)-1,3-th...)Show SMILES [O-]C(=O)Nc1nc(c(s1)-n1cncn1)-c1cccc(c1)C#N Show InChI InChI=1S/C13H8N6O2S/c14-5-8-2-1-3-9(4-8)10-11(19-7-15-6-16-19)22-12(17-10)18-13(20)21/h1-4,6-7H,(H,17,18)(H,20,21)/p-1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.61E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]ZM-241,385 from human adenosine A2a receptors transfected in HEK 293 cells |
Bioorg Med Chem Lett 15: 3081-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.021
BindingDB Entry DOI: 10.7270/Q2X63NQG |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50166742

(3-[2-(3-Methyl-pyridin-2-ylamino)-5-[1,2,4]triazol...)Show SMILES Cc1cccc(Nc2nc(c(s2)-n2cncn2)-c2cccc(c2)C#N)n1 Show InChI InChI=1S/C18H13N7S/c1-12-4-2-7-15(22-12)23-18-24-16(14-6-3-5-13(8-14)9-19)17(26-18)25-11-20-10-21-25/h2-8,10-11H,1H3,(H,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]ZM-241,385 from human adenosine A2a receptors transfected in HEK 293 cells |
Bioorg Med Chem Lett 15: 3081-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.021
BindingDB Entry DOI: 10.7270/Q2X63NQG |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50166742

(3-[2-(3-Methyl-pyridin-2-ylamino)-5-[1,2,4]triazol...)Show SMILES Cc1cccc(Nc2nc(c(s2)-n2cncn2)-c2cccc(c2)C#N)n1 Show InChI InChI=1S/C18H13N7S/c1-12-4-2-7-15(22-12)23-18-24-16(14-6-3-5-13(8-14)9-19)17(26-18)25-11-20-10-21-25/h2-8,10-11H,1H3,(H,22,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPCPX from human adenosine A1 receptors transfected in CHO cells |
Bioorg Med Chem Lett 15: 3081-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.021
BindingDB Entry DOI: 10.7270/Q2X63NQG |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50108504
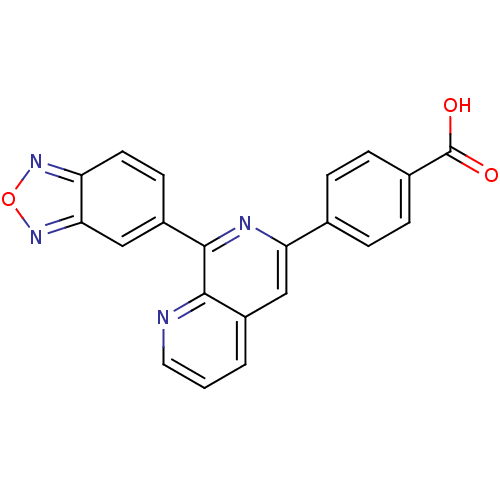
(4-(8-(benzo[c][1,2,5]oxadiazol-5-yl)-1,7-naphthyri...)Show SMILES OC(=O)c1ccc(cc1)-c1cc2cccnc2c(n1)-c1ccc2nonc2c1 Show InChI InChI=1S/C21H12N4O3/c26-21(27)13-5-3-12(4-6-13)17-10-14-2-1-9-22-19(14)20(23-17)15-7-8-16-18(11-15)25-28-24-16/h1-11H,(H,26,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4D |
J Med Chem 58: 6747-52 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00902
BindingDB Entry DOI: 10.7270/Q2319XPF |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50108504

(4-(8-(benzo[c][1,2,5]oxadiazol-5-yl)-1,7-naphthyri...)Show SMILES OC(=O)c1ccc(cc1)-c1cc2cccnc2c(n1)-c1ccc2nonc2c1 Show InChI InChI=1S/C21H12N4O3/c26-21(27)13-5-3-12(4-6-13)17-10-14-2-1-9-22-19(14)20(23-17)15-7-8-16-18(11-15)25-28-24-16/h1-11H,(H,26,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4D |
J Med Chem 55: 7472-9 (2012)
Article DOI: 10.1021/jm300459a
BindingDB Entry DOI: 10.7270/Q2RN390V |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50063917

((2S,3R)-N(4)-[(2S)-3,3-dimethyl-1-(methylamino)-1-...)Show SMILES CNC(=O)[C@@H](NC(=O)[C@H](CC(C)C)[C@H](O)C(=O)NO)C(C)(C)C |r| Show InChI InChI=1S/C15H29N3O5/c1-8(2)7-9(10(19)13(21)18-23)12(20)17-11(14(22)16-6)15(3,4)5/h8-11,19,23H,7H2,1-6H3,(H,16,22)(H,17,20)(H,18,21)/t9-,10+,11-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of Coll-3 MMP-13 |
Bioorg Med Chem Lett 9: 2887-92 (1999)
BindingDB Entry DOI: 10.7270/Q25H7FGN |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50081851

((2R,3R)-3-(cyclopentylmethyl)-2-((1-(dimethylamino...)Show SMILES CN(C)c1cccc2c(cccc12)S(=O)(=O)N(C)C[C@@H]([C@@H](CC1CCCC1)C(=O)N1CCCCC1)C(=O)NO Show InChI InChI=1S/C29H42N4O5S/c1-31(2)26-15-9-14-23-22(26)13-10-16-27(23)39(37,38)32(3)20-25(28(34)30-36)24(19-21-11-5-6-12-21)29(35)33-17-7-4-8-18-33/h9-10,13-16,21,24-25,36H,4-8,11-12,17-20H2,1-3H3,(H,30,34)/t24-,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of HNC MMP-8 |
Bioorg Med Chem Lett 9: 2887-92 (1999)
BindingDB Entry DOI: 10.7270/Q25H7FGN |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50063917

((2S,3R)-N(4)-[(2S)-3,3-dimethyl-1-(methylamino)-1-...)Show SMILES CNC(=O)[C@@H](NC(=O)[C@H](CC(C)C)[C@H](O)C(=O)NO)C(C)(C)C |r| Show InChI InChI=1S/C15H29N3O5/c1-8(2)7-9(10(19)13(21)18-23)12(20)17-11(14(22)16-6)15(3,4)5/h8-11,19,23H,7H2,1-6H3,(H,16,22)(H,17,20)(H,18,21)/t9-,10+,11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of HNC MMP-8 |
Bioorg Med Chem Lett 9: 2887-92 (1999)
BindingDB Entry DOI: 10.7270/Q25H7FGN |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50081851

((2R,3R)-3-(cyclopentylmethyl)-2-((1-(dimethylamino...)Show SMILES CN(C)c1cccc2c(cccc12)S(=O)(=O)N(C)C[C@@H]([C@@H](CC1CCCC1)C(=O)N1CCCCC1)C(=O)NO Show InChI InChI=1S/C29H42N4O5S/c1-31(2)26-15-9-14-23-22(26)13-10-16-27(23)39(37,38)32(3)20-25(28(34)30-36)24(19-21-11-5-6-12-21)29(35)33-17-7-4-8-18-33/h9-10,13-16,21,24-25,36H,4-8,11-12,17-20H2,1-3H3,(H,30,34)/t24-,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of HFC MMP-1 |
Bioorg Med Chem Lett 9: 2887-92 (1999)
BindingDB Entry DOI: 10.7270/Q25H7FGN |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50081866

((2R,3R)-3-(cyclopentylmethyl)-N-hydroxy-2-((4-meth...)Show SMILES COc1ccc(cc1)S(=O)(=O)N(C)C[C@@H]([C@@H](CC1CCCC1)C(=O)N1CCOCC1)C(=O)NO Show InChI InChI=1S/C23H35N3O7S/c1-25(34(30,31)19-9-7-18(32-2)8-10-19)16-21(22(27)24-29)20(15-17-5-3-4-6-17)23(28)26-11-13-33-14-12-26/h7-10,17,20-21,29H,3-6,11-16H2,1-2H3,(H,24,27)/t20-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of HNC MMP-8 |
Bioorg Med Chem Lett 9: 2887-92 (1999)
BindingDB Entry DOI: 10.7270/Q25H7FGN |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50113973
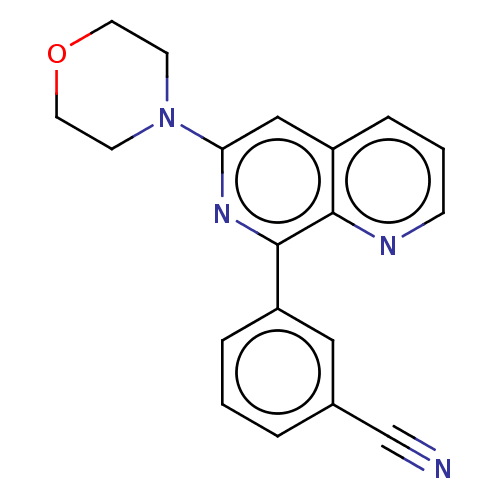
(CHEMBL3605513)Show InChI InChI=1S/C19H16N4O/c20-13-14-3-1-4-15(11-14)19-18-16(5-2-6-21-18)12-17(22-19)23-7-9-24-10-8-23/h1-6,11-12H,7-10H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4D |
J Med Chem 58: 6747-52 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00902
BindingDB Entry DOI: 10.7270/Q2319XPF |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50081869
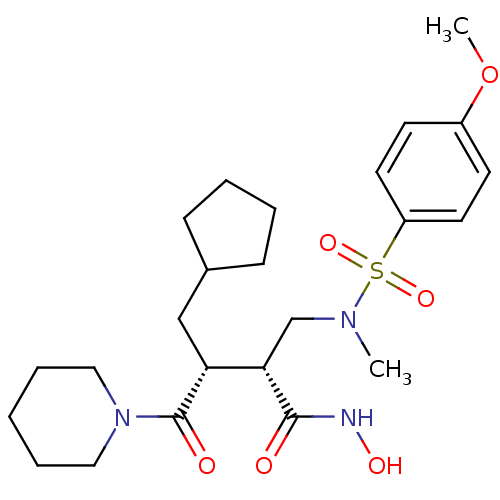
((2R,3R)-3-(cyclopentylmethyl)-N-hydroxy-2-((4-meth...)Show SMILES COc1ccc(cc1)S(=O)(=O)N(C)C[C@@H]([C@@H](CC1CCCC1)C(=O)N1CCCCC1)C(=O)NO Show InChI InChI=1S/C24H37N3O6S/c1-26(34(31,32)20-12-10-19(33-2)11-13-20)17-22(23(28)25-30)21(16-18-8-4-5-9-18)24(29)27-14-6-3-7-15-27/h10-13,18,21-22,30H,3-9,14-17H2,1-2H3,(H,25,28)/t21-,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of HNC MMP-8 |
Bioorg Med Chem Lett 9: 2887-92 (1999)
BindingDB Entry DOI: 10.7270/Q25H7FGN |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50081852

((2R,3R)-3-(cyclopentylmethyl)-N-hydroxy-2-((N-meth...)Show SMILES CN(C[C@@H]([C@@H](CC1CCCC1)C(=O)N1CCCCC1)C(=O)NO)S(=O)(=O)c1cccc2ccccc12 Show InChI InChI=1S/C27H37N3O5S/c1-29(36(34,35)25-15-9-13-21-12-5-6-14-22(21)25)19-24(26(31)28-33)23(18-20-10-3-4-11-20)27(32)30-16-7-2-8-17-30/h5-6,9,12-15,20,23-24,33H,2-4,7-8,10-11,16-19H2,1H3,(H,28,31)/t23-,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of HNC MMP-8 |
Bioorg Med Chem Lett 9: 2887-92 (1999)
BindingDB Entry DOI: 10.7270/Q25H7FGN |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50081859

((2R,3R)-2-((4-chloro-N-methylphenylsulfonamido)met...)Show SMILES CN(C[C@@H]([C@@H](CC1CCCC1)C(=O)N1CCCCC1)C(=O)NO)S(=O)(=O)c1ccc(Cl)cc1 Show InChI InChI=1S/C23H34ClN3O5S/c1-26(33(31,32)19-11-9-18(24)10-12-19)16-21(22(28)25-30)20(15-17-7-3-4-8-17)23(29)27-13-5-2-6-14-27/h9-12,17,20-21,30H,2-8,13-16H2,1H3,(H,25,28)/t20-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of HNC MMP-8 |
Bioorg Med Chem Lett 9: 2887-92 (1999)
BindingDB Entry DOI: 10.7270/Q25H7FGN |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50063917

((2S,3R)-N(4)-[(2S)-3,3-dimethyl-1-(methylamino)-1-...)Show SMILES CNC(=O)[C@@H](NC(=O)[C@H](CC(C)C)[C@H](O)C(=O)NO)C(C)(C)C |r| Show InChI InChI=1S/C15H29N3O5/c1-8(2)7-9(10(19)13(21)18-23)12(20)17-11(14(22)16-6)15(3,4)5/h8-11,19,23H,7H2,1-6H3,(H,16,22)(H,17,20)(H,18,21)/t9-,10+,11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of HFC MMP-1 |
Bioorg Med Chem Lett 9: 2887-92 (1999)
BindingDB Entry DOI: 10.7270/Q25H7FGN |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50081871

((2R,3R)-3-(cyclopentylmethyl)-N-hydroxy-2-((N-meth...)Show SMILES CC(C)S(=O)(=O)N(C)C[C@@H]([C@@H](CC1CCCC1)C(=O)N1CCCCC1)C(=O)NO Show InChI InChI=1S/C20H37N3O5S/c1-15(2)29(27,28)22(3)14-18(19(24)21-26)17(13-16-9-5-6-10-16)20(25)23-11-7-4-8-12-23/h15-18,26H,4-14H2,1-3H3,(H,21,24)/t17-,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of HFC MMP-1 |
Bioorg Med Chem Lett 9: 2887-92 (1999)
BindingDB Entry DOI: 10.7270/Q25H7FGN |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50081860

((2R,3R)-3-(cyclopentylmethyl)-N-hydroxy-2-((N-meth...)Show SMILES CN(C[C@@H]([C@@H](CC1CCCC1)C(=O)N1CCOCC1)C(=O)NO)S(C)(=O)=O Show InChI InChI=1S/C17H31N3O6S/c1-19(27(2,24)25)12-15(16(21)18-23)14(11-13-5-3-4-6-13)17(22)20-7-9-26-10-8-20/h13-15,23H,3-12H2,1-2H3,(H,18,21)/t14-,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of HFC MMP-1 |
Bioorg Med Chem Lett 9: 2887-92 (1999)
BindingDB Entry DOI: 10.7270/Q25H7FGN |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50081864

((2R,3R)-3-(cyclopentylmethyl)-N-hydroxy-2-((N-meth...)Show SMILES CN(C[C@@H]([C@@H](CC1CCCC1)C(=O)N1CCCCC1)C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C18H33N3O5S/c1-20(27(2,25)26)13-16(17(22)19-24)15(12-14-8-4-5-9-14)18(23)21-10-6-3-7-11-21/h14-16,24H,3-13H2,1-2H3,(H,19,22)/t15-,16+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of HFC MMP-1 |
Bioorg Med Chem Lett 9: 2887-92 (1999)
BindingDB Entry DOI: 10.7270/Q25H7FGN |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50063917

((2S,3R)-N(4)-[(2S)-3,3-dimethyl-1-(methylamino)-1-...)Show SMILES CNC(=O)[C@@H](NC(=O)[C@H](CC(C)C)[C@H](O)C(=O)NO)C(C)(C)C |r| Show InChI InChI=1S/C15H29N3O5/c1-8(2)7-9(10(19)13(21)18-23)12(20)17-11(14(22)16-6)15(3,4)5/h8-11,19,23H,7H2,1-6H3,(H,16,22)(H,17,20)(H,18,21)/t9-,10+,11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of Gel-A MMP-2 |
Bioorg Med Chem Lett 9: 2887-92 (1999)
BindingDB Entry DOI: 10.7270/Q25H7FGN |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50081865

((2R,3R)-2-((1-(dimethylamino)-N-methylnaphthalene-...)Show SMILES CC(C)C[C@H]([C@H](CN(C)S(=O)(=O)c1cccc2c(cccc12)N(C)C)C(=O)NO)C(=O)N1CCCCC1 Show InChI InChI=1S/C27H40N4O5S/c1-19(2)17-22(27(33)31-15-7-6-8-16-31)23(26(32)28-34)18-30(5)37(35,36)25-14-10-11-20-21(25)12-9-13-24(20)29(3)4/h9-14,19,22-23,34H,6-8,15-18H2,1-5H3,(H,28,32)/t22-,23+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of HFC MMP-1 |
Bioorg Med Chem Lett 9: 2887-92 (1999)
BindingDB Entry DOI: 10.7270/Q25H7FGN |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50081872

((2R,3R)-3-(cyclopentylmethyl)-2-{[(dimethylsulfamo...)Show SMILES CN(C)S(=O)(=O)N(C)C[C@@H]([C@@H](CC1CCCC1)C(=O)N1CCCCC1)C(=O)NO Show InChI InChI=1S/C19H36N4O5S/c1-21(2)29(27,28)22(3)14-17(18(24)20-26)16(13-15-9-5-6-10-15)19(25)23-11-7-4-8-12-23/h15-17,26H,4-14H2,1-3H3,(H,20,24)/t16-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of HFC MMP-1 |
Bioorg Med Chem Lett 9: 2887-92 (1999)
BindingDB Entry DOI: 10.7270/Q25H7FGN |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50396765
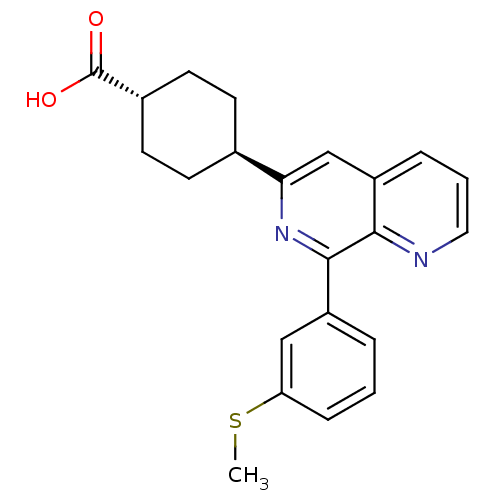
(CHEMBL2172376)Show SMILES CSc1cccc(c1)-c1nc(cc2cccnc12)[C@H]1CC[C@@H](CC1)C(O)=O |r,wU:21.27,wD:18.20,(8.68,-13.64,;8.67,-12.1,;7.34,-11.34,;6,-12.12,;4.66,-11.35,;4.66,-9.81,;6,-9.04,;7.33,-9.8,;6,-7.51,;7.33,-6.73,;7.32,-5.18,;5.99,-4.42,;4.66,-5.2,;3.32,-4.43,;2,-5.2,;1.99,-6.75,;3.33,-7.52,;4.66,-6.74,;8.66,-4.4,;9.99,-5.17,;11.32,-4.4,;11.32,-2.86,;9.98,-2.09,;8.64,-2.87,;12.65,-2.08,;13.98,-2.85,;12.65,-.54,)| Show InChI InChI=1S/C22H22N2O2S/c1-27-18-6-2-4-16(12-18)21-20-17(5-3-11-23-20)13-19(24-21)14-7-9-15(10-8-14)22(25)26/h2-6,11-15H,7-10H2,1H3,(H,25,26)/t14-,15- | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4D-mediated [3H]CAMP hydrolysis after 30 to 60 mins by scintillation proximity assay |
J Med Chem 55: 7472-9 (2012)
Article DOI: 10.1021/jm300459a
BindingDB Entry DOI: 10.7270/Q2RN390V |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50081852

((2R,3R)-3-(cyclopentylmethyl)-N-hydroxy-2-((N-meth...)Show SMILES CN(C[C@@H]([C@@H](CC1CCCC1)C(=O)N1CCCCC1)C(=O)NO)S(=O)(=O)c1cccc2ccccc12 Show InChI InChI=1S/C27H37N3O5S/c1-29(36(34,35)25-15-9-13-21-12-5-6-14-22(21)25)19-24(26(31)28-33)23(18-20-10-3-4-11-20)27(32)30-16-7-2-8-17-30/h5-6,9,12-15,20,23-24,33H,2-4,7-8,10-11,16-19H2,1H3,(H,28,31)/t23-,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of HFC MMP-1 |
Bioorg Med Chem Lett 9: 2887-92 (1999)
BindingDB Entry DOI: 10.7270/Q25H7FGN |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50081867

((2R,3R)-3-(cyclopentylmethyl)-N-hydroxy-2-((N-meth...)Show SMILES CCS(=O)(=O)N(C)C[C@@H]([C@@H](CC1CCCC1)C(=O)N1CCCCC1)C(=O)NO Show InChI InChI=1S/C19H35N3O5S/c1-3-28(26,27)21(2)14-17(18(23)20-25)16(13-15-9-5-6-10-15)19(24)22-11-7-4-8-12-22/h15-17,25H,3-14H2,1-2H3,(H,20,23)/t16-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of HFC MMP-1 |
Bioorg Med Chem Lett 9: 2887-92 (1999)
BindingDB Entry DOI: 10.7270/Q25H7FGN |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50081851

((2R,3R)-3-(cyclopentylmethyl)-2-((1-(dimethylamino...)Show SMILES CN(C)c1cccc2c(cccc12)S(=O)(=O)N(C)C[C@@H]([C@@H](CC1CCCC1)C(=O)N1CCCCC1)C(=O)NO Show InChI InChI=1S/C29H42N4O5S/c1-31(2)26-15-9-14-23-22(26)13-10-16-27(23)39(37,38)32(3)20-25(28(34)30-36)24(19-21-11-5-6-12-21)29(35)33-17-7-4-8-18-33/h9-10,13-16,21,24-25,36H,4-8,11-12,17-20H2,1-3H3,(H,30,34)/t24-,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of Strom-1 MMP-3 |
Bioorg Med Chem Lett 9: 2887-92 (1999)
BindingDB Entry DOI: 10.7270/Q25H7FGN |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data