 Found 62 hits with Last Name = 'tulasi' and Initial = 'vk'
Found 62 hits with Last Name = 'tulasi' and Initial = 'vk' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50367254

(ENALAPRILAT)Show SMILES C[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1CCC[C@H]1C(O)=O |r| Show InChI InChI=1S/C18H24N2O5/c1-12(16(21)20-11-5-8-15(20)18(24)25)19-14(17(22)23)10-9-13-6-3-2-4-7-13/h2-4,6-7,12,14-15,19H,5,8-11H2,1H3,(H,22,23)(H,24,25)/t12-,14-,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibitory activity against human renin inhibition (at pH 7.4) |
Bioorg Med Chem Lett 27: 2313-2318 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.036
BindingDB Entry DOI: 10.7270/Q2ZC858S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50242264

(CHEMBL4086264)Show SMILES CC[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1C[C@H](C[C@H]1C(O)=O)NC(=O)C[C@H](N)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C29H35F3N4O6/c1-2-23(35-24(28(39)40)9-8-16-6-4-3-5-7-16)27(38)36-15-19(13-25(36)29(41)42)34-26(37)12-18(33)10-17-11-21(31)22(32)14-20(17)30/h3-7,11,14,18-19,23-25,35H,2,8-10,12-13,15,33H2,1H3,(H,34,37)(H,39,40)(H,41,42)/t18-,19+,23+,24+,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibition of human plasma ACE |
Bioorg Med Chem Lett 27: 2313-2318 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.036
BindingDB Entry DOI: 10.7270/Q2ZC858S |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM50367254

(ENALAPRILAT)Show SMILES C[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1CCC[C@H]1C(O)=O |r| Show InChI InChI=1S/C18H24N2O5/c1-12(16(21)20-11-5-8-15(20)18(24)25)19-14(17(22)23)10-9-13-6-3-2-4-7-13/h2-4,6-7,12,14-15,19H,5,8-11H2,1H3,(H,22,23)(H,24,25)/t12-,14-,15-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibition of Wistar rat plasma ACE |
Bioorg Med Chem Lett 27: 2313-2318 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.036
BindingDB Entry DOI: 10.7270/Q2ZC858S |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Mus musculus) | BDBM50367254

(ENALAPRILAT)Show SMILES C[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1CCC[C@H]1C(O)=O |r| Show InChI InChI=1S/C18H24N2O5/c1-12(16(21)20-11-5-8-15(20)18(24)25)19-14(17(22)23)10-9-13-6-3-2-4-7-13/h2-4,6-7,12,14-15,19H,5,8-11H2,1H3,(H,22,23)(H,24,25)/t12-,14-,15-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibitory activity against human renin inhibition (at pH 7.4) |
Bioorg Med Chem Lett 27: 2313-2318 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.036
BindingDB Entry DOI: 10.7270/Q2ZC858S |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM11162
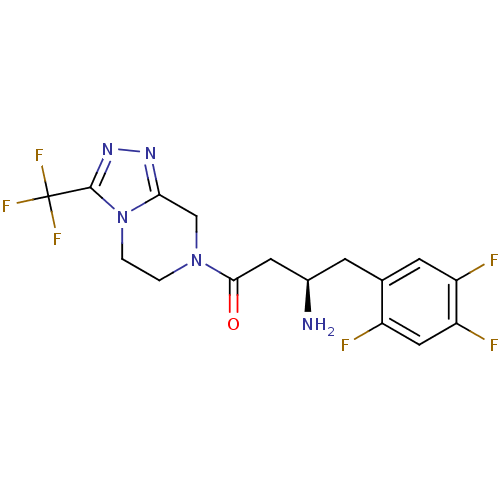
((1R)-3-oxo-3-[3-(trifluoroethyl)-5,6-dihydro[1,2,4...)Show SMILES N[C@@H](CC(=O)N1CCn2c(C1)nnc2C(F)(F)F)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C16H15F6N5O/c17-10-6-12(19)11(18)4-8(10)3-9(23)5-14(28)26-1-2-27-13(7-26)24-25-15(27)16(20,21)22/h4,6,9H,1-3,5,7,23H2/t9-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibition of human plasma DPP4 |
Bioorg Med Chem Lett 27: 2313-2318 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.036
BindingDB Entry DOI: 10.7270/Q2ZC858S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM50242264

(CHEMBL4086264)Show SMILES CC[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1C[C@H](C[C@H]1C(O)=O)NC(=O)C[C@H](N)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C29H35F3N4O6/c1-2-23(35-24(28(39)40)9-8-16-6-4-3-5-7-16)27(38)36-15-19(13-25(36)29(41)42)34-26(37)12-18(33)10-17-11-21(31)22(32)14-20(17)30/h3-7,11,14,18-19,23-25,35H,2,8-10,12-13,15,33H2,1H3,(H,34,37)(H,39,40)(H,41,42)/t18-,19+,23+,24+,25+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibitory activity against human renin inhibition (at pH 7.4) |
Bioorg Med Chem Lett 27: 2313-2318 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.036
BindingDB Entry DOI: 10.7270/Q2ZC858S |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Rattus norvegicus (rat)) | BDBM11162

((1R)-3-oxo-3-[3-(trifluoroethyl)-5,6-dihydro[1,2,4...)Show SMILES N[C@@H](CC(=O)N1CCn2c(C1)nnc2C(F)(F)F)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C16H15F6N5O/c17-10-6-12(19)11(18)4-8(10)3-9(23)5-14(28)26-1-2-27-13(7-26)24-25-15(27)16(20,21)22/h4,6,9H,1-3,5,7,23H2/t9-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Laboratories Limited
Curated by ChEMBL
| Assay Description
Compound was tested in vitro for binding affinity against human carbonic anhydrase II; (ki*10e-9) |
Bioorg Med Chem Lett 27: 2313-2318 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.036
BindingDB Entry DOI: 10.7270/Q2ZC858S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dipeptidyl peptidase 4
(Mus musculus) | BDBM11162

((1R)-3-oxo-3-[3-(trifluoroethyl)-5,6-dihydro[1,2,4...)Show SMILES N[C@@H](CC(=O)N1CCn2c(C1)nnc2C(F)(F)F)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C16H15F6N5O/c17-10-6-12(19)11(18)4-8(10)3-9(23)5-14(28)26-1-2-27-13(7-26)24-25-15(27)16(20,21)22/h4,6,9H,1-3,5,7,23H2/t9-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibitory activity against human renin inhibition (at pH 7.4) |
Bioorg Med Chem Lett 27: 2313-2318 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.036
BindingDB Entry DOI: 10.7270/Q2ZC858S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50242265
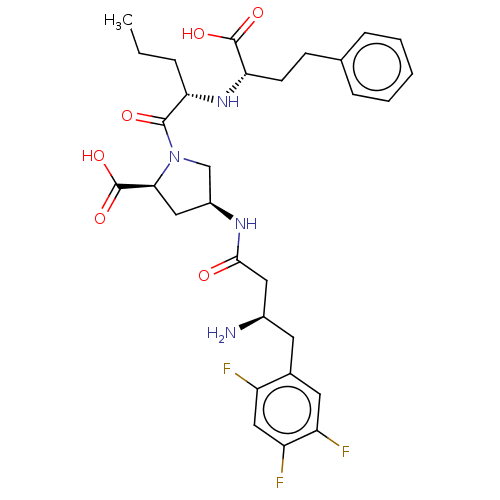
(CHEMBL4078112)Show SMILES CCC[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1C[C@H](C[C@H]1C(O)=O)NC(=O)C[C@H](N)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C30H37F3N4O6/c1-2-6-24(36-25(29(40)41)10-9-17-7-4-3-5-8-17)28(39)37-16-20(14-26(37)30(42)43)35-27(38)13-19(34)11-18-12-22(32)23(33)15-21(18)31/h3-5,7-8,12,15,19-20,24-26,36H,2,6,9-11,13-14,16,34H2,1H3,(H,35,38)(H,40,41)(H,42,43)/t19-,20+,24+,25+,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibitory activity against human renin inhibition (at pH 7.4) |
Bioorg Med Chem Lett 27: 2313-2318 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.036
BindingDB Entry DOI: 10.7270/Q2ZC858S |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50242264

(CHEMBL4086264)Show SMILES CC[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1C[C@H](C[C@H]1C(O)=O)NC(=O)C[C@H](N)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C29H35F3N4O6/c1-2-23(35-24(28(39)40)9-8-16-6-4-3-5-7-16)27(38)36-15-19(13-25(36)29(41)42)34-26(37)12-18(33)10-17-11-21(31)22(32)14-20(17)30/h3-7,11,14,18-19,23-25,35H,2,8-10,12-13,15,33H2,1H3,(H,34,37)(H,39,40)(H,41,42)/t18-,19+,23+,24+,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibitory activity against human renin inhibition (at pH 7.4) |
Bioorg Med Chem Lett 27: 2313-2318 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.036
BindingDB Entry DOI: 10.7270/Q2ZC858S |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Mus musculus) | BDBM50242266
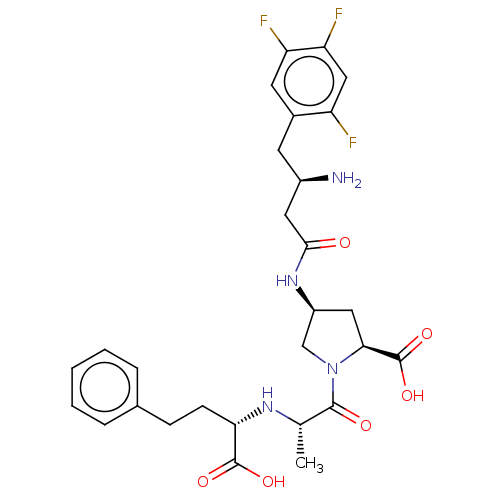
(CHEMBL4067185)Show SMILES C[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1C[C@H](C[C@H]1C(O)=O)NC(=O)C[C@H](N)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C28H33F3N4O6/c1-15(33-23(27(38)39)8-7-16-5-3-2-4-6-16)26(37)35-14-19(12-24(35)28(40)41)34-25(36)11-18(32)9-17-10-21(30)22(31)13-20(17)29/h2-6,10,13,15,18-19,23-24,33H,7-9,11-12,14,32H2,1H3,(H,34,36)(H,38,39)(H,40,41)/t15-,18+,19-,23-,24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibition of ob/ob mouse plasma ACE |
Bioorg Med Chem Lett 27: 2313-2318 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.036
BindingDB Entry DOI: 10.7270/Q2ZC858S |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50242265

(CHEMBL4078112)Show SMILES CCC[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1C[C@H](C[C@H]1C(O)=O)NC(=O)C[C@H](N)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C30H37F3N4O6/c1-2-6-24(36-25(29(40)41)10-9-17-7-4-3-5-8-17)28(39)37-16-20(14-26(37)30(42)43)35-27(38)13-19(34)11-18-12-22(32)23(33)15-21(18)31/h3-5,7-8,12,15,19-20,24-26,36H,2,6,9-11,13-14,16,34H2,1H3,(H,35,38)(H,40,41)(H,42,43)/t19-,20+,24+,25+,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibition of human plasma DPP4 |
Bioorg Med Chem Lett 27: 2313-2318 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.036
BindingDB Entry DOI: 10.7270/Q2ZC858S |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Mus musculus) | BDBM50242264

(CHEMBL4086264)Show SMILES CC[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1C[C@H](C[C@H]1C(O)=O)NC(=O)C[C@H](N)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C29H35F3N4O6/c1-2-23(35-24(28(39)40)9-8-16-6-4-3-5-7-16)27(38)36-15-19(13-25(36)29(41)42)34-26(37)12-18(33)10-17-11-21(31)22(32)14-20(17)30/h3-7,11,14,18-19,23-25,35H,2,8-10,12-13,15,33H2,1H3,(H,34,37)(H,39,40)(H,41,42)/t18-,19+,23+,24+,25+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibition of ob/ob mouse plasma ACE |
Bioorg Med Chem Lett 27: 2313-2318 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.036
BindingDB Entry DOI: 10.7270/Q2ZC858S |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50242274
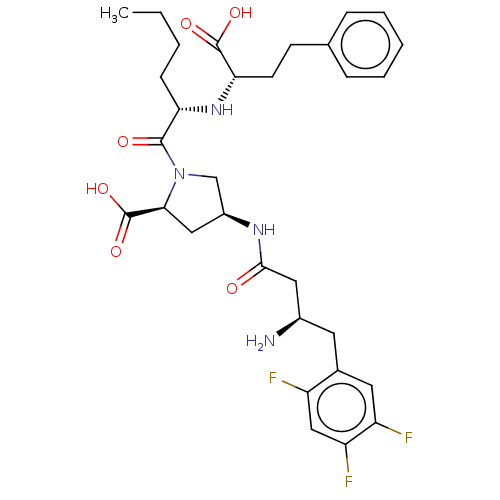
(CHEMBL4090635)Show SMILES CCCC[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1C[C@H](C[C@H]1C(O)=O)NC(=O)C[C@H](N)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C31H39F3N4O6/c1-2-3-9-25(37-26(30(41)42)11-10-18-7-5-4-6-8-18)29(40)38-17-21(15-27(38)31(43)44)36-28(39)14-20(35)12-19-13-23(33)24(34)16-22(19)32/h4-8,13,16,20-21,25-27,37H,2-3,9-12,14-15,17,35H2,1H3,(H,36,39)(H,41,42)(H,43,44)/t20-,21+,25+,26+,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 102 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibition of human plasma DPP4 |
Bioorg Med Chem Lett 27: 2313-2318 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.036
BindingDB Entry DOI: 10.7270/Q2ZC858S |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Rattus norvegicus (rat)) | BDBM50242266

(CHEMBL4067185)Show SMILES C[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1C[C@H](C[C@H]1C(O)=O)NC(=O)C[C@H](N)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C28H33F3N4O6/c1-15(33-23(27(38)39)8-7-16-5-3-2-4-6-16)26(37)35-14-19(12-24(35)28(40)41)34-25(36)11-18(32)9-17-10-21(30)22(31)13-20(17)29/h2-6,10,13,15,18-19,23-24,33H,7-9,11-12,14,32H2,1H3,(H,34,36)(H,38,39)(H,40,41)/t15-,18+,19-,23-,24-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 155 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibition of Wistar rat plasma DPP4 |
Bioorg Med Chem Lett 27: 2313-2318 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.036
BindingDB Entry DOI: 10.7270/Q2ZC858S |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50242266

(CHEMBL4067185)Show SMILES C[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1C[C@H](C[C@H]1C(O)=O)NC(=O)C[C@H](N)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C28H33F3N4O6/c1-15(33-23(27(38)39)8-7-16-5-3-2-4-6-16)26(37)35-14-19(12-24(35)28(40)41)34-25(36)11-18(32)9-17-10-21(30)22(31)13-20(17)29/h2-6,10,13,15,18-19,23-24,33H,7-9,11-12,14,32H2,1H3,(H,34,36)(H,38,39)(H,40,41)/t15-,18+,19-,23-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 158 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibition of human plasma ACE |
Bioorg Med Chem Lett 27: 2313-2318 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.036
BindingDB Entry DOI: 10.7270/Q2ZC858S |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Mus musculus) | BDBM50242266

(CHEMBL4067185)Show SMILES C[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1C[C@H](C[C@H]1C(O)=O)NC(=O)C[C@H](N)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C28H33F3N4O6/c1-15(33-23(27(38)39)8-7-16-5-3-2-4-6-16)26(37)35-14-19(12-24(35)28(40)41)34-25(36)11-18(32)9-17-10-21(30)22(31)13-20(17)29/h2-6,10,13,15,18-19,23-24,33H,7-9,11-12,14,32H2,1H3,(H,34,36)(H,38,39)(H,40,41)/t15-,18+,19-,23-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 217 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibition of ob/ob mouse plasma DPP4 |
Bioorg Med Chem Lett 27: 2313-2318 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.036
BindingDB Entry DOI: 10.7270/Q2ZC858S |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50242266

(CHEMBL4067185)Show SMILES C[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1C[C@H](C[C@H]1C(O)=O)NC(=O)C[C@H](N)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C28H33F3N4O6/c1-15(33-23(27(38)39)8-7-16-5-3-2-4-6-16)26(37)35-14-19(12-24(35)28(40)41)34-25(36)11-18(32)9-17-10-21(30)22(31)13-20(17)29/h2-6,10,13,15,18-19,23-24,33H,7-9,11-12,14,32H2,1H3,(H,34,36)(H,38,39)(H,40,41)/t15-,18+,19-,23-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 255 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibitory activity against human renin inhibition (at pH 7.4) |
Bioorg Med Chem Lett 27: 2313-2318 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.036
BindingDB Entry DOI: 10.7270/Q2ZC858S |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50242274

(CHEMBL4090635)Show SMILES CCCC[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1C[C@H](C[C@H]1C(O)=O)NC(=O)C[C@H](N)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C31H39F3N4O6/c1-2-3-9-25(37-26(30(41)42)11-10-18-7-5-4-6-8-18)29(40)38-17-21(15-27(38)31(43)44)36-28(39)14-20(35)12-19-13-23(33)24(34)16-22(19)32/h4-8,13,16,20-21,25-27,37H,2-3,9-12,14-15,17,35H2,1H3,(H,36,39)(H,41,42)(H,43,44)/t20-,21+,25+,26+,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 279 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibition of human plasma ACE |
Bioorg Med Chem Lett 27: 2313-2318 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.036
BindingDB Entry DOI: 10.7270/Q2ZC858S |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Rattus norvegicus (rat)) | BDBM50242264

(CHEMBL4086264)Show SMILES CC[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1C[C@H](C[C@H]1C(O)=O)NC(=O)C[C@H](N)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C29H35F3N4O6/c1-2-23(35-24(28(39)40)9-8-16-6-4-3-5-7-16)27(38)36-15-19(13-25(36)29(41)42)34-26(37)12-18(33)10-17-11-21(31)22(32)14-20(17)30/h3-7,11,14,18-19,23-25,35H,2,8-10,12-13,15,33H2,1H3,(H,34,37)(H,39,40)(H,41,42)/t18-,19+,23+,24+,25+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 319 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibition of Wistar rat plasma DPP4 |
Bioorg Med Chem Lett 27: 2313-2318 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.036
BindingDB Entry DOI: 10.7270/Q2ZC858S |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM50242266

(CHEMBL4067185)Show SMILES C[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1C[C@H](C[C@H]1C(O)=O)NC(=O)C[C@H](N)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C28H33F3N4O6/c1-15(33-23(27(38)39)8-7-16-5-3-2-4-6-16)26(37)35-14-19(12-24(35)28(40)41)34-25(36)11-18(32)9-17-10-21(30)22(31)13-20(17)29/h2-6,10,13,15,18-19,23-24,33H,7-9,11-12,14,32H2,1H3,(H,34,36)(H,38,39)(H,40,41)/t15-,18+,19-,23-,24-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 334 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibitory activity against human renin inhibition (at pH 7.4) |
Bioorg Med Chem Lett 27: 2313-2318 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.036
BindingDB Entry DOI: 10.7270/Q2ZC858S |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Mus musculus) | BDBM50242264

(CHEMBL4086264)Show SMILES CC[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1C[C@H](C[C@H]1C(O)=O)NC(=O)C[C@H](N)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C29H35F3N4O6/c1-2-23(35-24(28(39)40)9-8-16-6-4-3-5-7-16)27(38)36-15-19(13-25(36)29(41)42)34-26(37)12-18(33)10-17-11-21(31)22(32)14-20(17)30/h3-7,11,14,18-19,23-25,35H,2,8-10,12-13,15,33H2,1H3,(H,34,37)(H,39,40)(H,41,42)/t18-,19+,23+,24+,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 488 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibition of ob/ob mouse plasma DPP4 |
Bioorg Med Chem Lett 27: 2313-2318 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.036
BindingDB Entry DOI: 10.7270/Q2ZC858S |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM50242265

(CHEMBL4078112)Show SMILES CCC[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1C[C@H](C[C@H]1C(O)=O)NC(=O)C[C@H](N)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C30H37F3N4O6/c1-2-6-24(36-25(29(40)41)10-9-17-7-4-3-5-8-17)28(39)37-16-20(14-26(37)30(42)43)35-27(38)13-19(34)11-18-12-22(32)23(33)15-21(18)31/h3-5,7-8,12,15,19-20,24-26,36H,2,6,9-11,13-14,16,34H2,1H3,(H,35,38)(H,40,41)(H,42,43)/t19-,20+,24+,25+,26+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 893 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibition of Wistar rat plasma ACE |
Bioorg Med Chem Lett 27: 2313-2318 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.036
BindingDB Entry DOI: 10.7270/Q2ZC858S |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Rattus norvegicus (rat)) | BDBM50242265

(CHEMBL4078112)Show SMILES CCC[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1C[C@H](C[C@H]1C(O)=O)NC(=O)C[C@H](N)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C30H37F3N4O6/c1-2-6-24(36-25(29(40)41)10-9-17-7-4-3-5-8-17)28(39)37-16-20(14-26(37)30(42)43)35-27(38)13-19(34)11-18-12-22(32)23(33)15-21(18)31/h3-5,7-8,12,15,19-20,24-26,36H,2,6,9-11,13-14,16,34H2,1H3,(H,35,38)(H,40,41)(H,42,43)/t19-,20+,24+,25+,26+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibition of Wistar rat plasma DPP4 |
Bioorg Med Chem Lett 27: 2313-2318 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.036
BindingDB Entry DOI: 10.7270/Q2ZC858S |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Mus musculus) | BDBM50242265

(CHEMBL4078112)Show SMILES CCC[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1C[C@H](C[C@H]1C(O)=O)NC(=O)C[C@H](N)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C30H37F3N4O6/c1-2-6-24(36-25(29(40)41)10-9-17-7-4-3-5-8-17)28(39)37-16-20(14-26(37)30(42)43)35-27(38)13-19(34)11-18-12-22(32)23(33)15-21(18)31/h3-5,7-8,12,15,19-20,24-26,36H,2,6,9-11,13-14,16,34H2,1H3,(H,35,38)(H,40,41)(H,42,43)/t19-,20+,24+,25+,26+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibition of ob/ob mouse plasma ACE |
Bioorg Med Chem Lett 27: 2313-2318 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.036
BindingDB Entry DOI: 10.7270/Q2ZC858S |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Mus musculus) | BDBM50242274

(CHEMBL4090635)Show SMILES CCCC[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1C[C@H](C[C@H]1C(O)=O)NC(=O)C[C@H](N)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C31H39F3N4O6/c1-2-3-9-25(37-26(30(41)42)11-10-18-7-5-4-6-8-18)29(40)38-17-21(15-27(38)31(43)44)36-28(39)14-20(35)12-19-13-23(33)24(34)16-22(19)32/h4-8,13,16,20-21,25-27,37H,2-3,9-12,14-15,17,35H2,1H3,(H,36,39)(H,41,42)(H,43,44)/t20-,21+,25+,26+,27+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibition of ob/ob mouse plasma ACE |
Bioorg Med Chem Lett 27: 2313-2318 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.036
BindingDB Entry DOI: 10.7270/Q2ZC858S |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Mus musculus) | BDBM50242265

(CHEMBL4078112)Show SMILES CCC[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1C[C@H](C[C@H]1C(O)=O)NC(=O)C[C@H](N)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C30H37F3N4O6/c1-2-6-24(36-25(29(40)41)10-9-17-7-4-3-5-8-17)28(39)37-16-20(14-26(37)30(42)43)35-27(38)13-19(34)11-18-12-22(32)23(33)15-21(18)31/h3-5,7-8,12,15,19-20,24-26,36H,2,6,9-11,13-14,16,34H2,1H3,(H,35,38)(H,40,41)(H,42,43)/t19-,20+,24+,25+,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.47E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibition of ob/ob mouse plasma DPP4 |
Bioorg Med Chem Lett 27: 2313-2318 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.036
BindingDB Entry DOI: 10.7270/Q2ZC858S |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM50242274

(CHEMBL4090635)Show SMILES CCCC[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1C[C@H](C[C@H]1C(O)=O)NC(=O)C[C@H](N)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C31H39F3N4O6/c1-2-3-9-25(37-26(30(41)42)11-10-18-7-5-4-6-8-18)29(40)38-17-21(15-27(38)31(43)44)36-28(39)14-20(35)12-19-13-23(33)24(34)16-22(19)32/h4-8,13,16,20-21,25-27,37H,2-3,9-12,14-15,17,35H2,1H3,(H,36,39)(H,41,42)(H,43,44)/t20-,21+,25+,26+,27+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibition of Wistar rat plasma ACE |
Bioorg Med Chem Lett 27: 2313-2318 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.036
BindingDB Entry DOI: 10.7270/Q2ZC858S |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Rattus norvegicus (rat)) | BDBM50242274

(CHEMBL4090635)Show SMILES CCCC[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1C[C@H](C[C@H]1C(O)=O)NC(=O)C[C@H](N)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C31H39F3N4O6/c1-2-3-9-25(37-26(30(41)42)11-10-18-7-5-4-6-8-18)29(40)38-17-21(15-27(38)31(43)44)36-28(39)14-20(35)12-19-13-23(33)24(34)16-22(19)32/h4-8,13,16,20-21,25-27,37H,2-3,9-12,14-15,17,35H2,1H3,(H,36,39)(H,41,42)(H,43,44)/t20-,21+,25+,26+,27+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibition of Wistar rat plasma DPP4 |
Bioorg Med Chem Lett 27: 2313-2318 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.036
BindingDB Entry DOI: 10.7270/Q2ZC858S |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Mus musculus) | BDBM50242274

(CHEMBL4090635)Show SMILES CCCC[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1C[C@H](C[C@H]1C(O)=O)NC(=O)C[C@H](N)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C31H39F3N4O6/c1-2-3-9-25(37-26(30(41)42)11-10-18-7-5-4-6-8-18)29(40)38-17-21(15-27(38)31(43)44)36-28(39)14-20(35)12-19-13-23(33)24(34)16-22(19)32/h4-8,13,16,20-21,25-27,37H,2-3,9-12,14-15,17,35H2,1H3,(H,36,39)(H,41,42)(H,43,44)/t20-,21+,25+,26+,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibition of ob/ob mouse plasma DPP4 |
Bioorg Med Chem Lett 27: 2313-2318 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.036
BindingDB Entry DOI: 10.7270/Q2ZC858S |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM11162

((1R)-3-oxo-3-[3-(trifluoroethyl)-5,6-dihydro[1,2,4...)Show SMILES N[C@@H](CC(=O)N1CCn2c(C1)nnc2C(F)(F)F)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C16H15F6N5O/c17-10-6-12(19)11(18)4-8(10)3-9(23)5-14(28)26-1-2-27-13(7-26)24-25-15(27)16(20,21)22/h4,6,9H,1-3,5,7,23H2/t9-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibition of human plasma ACE |
Bioorg Med Chem Lett 27: 2313-2318 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.036
BindingDB Entry DOI: 10.7270/Q2ZC858S |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 8
(Homo sapiens (Human)) | BDBM50242266

(CHEMBL4067185)Show SMILES C[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1C[C@H](C[C@H]1C(O)=O)NC(=O)C[C@H](N)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C28H33F3N4O6/c1-15(33-23(27(38)39)8-7-16-5-3-2-4-6-16)26(37)35-14-19(12-24(35)28(40)41)34-25(36)11-18(32)9-17-10-21(30)22(31)13-20(17)29/h2-6,10,13,15,18-19,23-24,33H,7-9,11-12,14,32H2,1H3,(H,34,36)(H,38,39)(H,40,41)/t15-,18+,19-,23-,24-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibition of DPP8 (unknown origin) |
Bioorg Med Chem Lett 27: 2313-2318 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.036
BindingDB Entry DOI: 10.7270/Q2ZC858S |
More data for this
Ligand-Target Pair | |
Prolyl endopeptidase
(Homo sapiens (Human)) | BDBM50242266

(CHEMBL4067185)Show SMILES C[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1C[C@H](C[C@H]1C(O)=O)NC(=O)C[C@H](N)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C28H33F3N4O6/c1-15(33-23(27(38)39)8-7-16-5-3-2-4-6-16)26(37)35-14-19(12-24(35)28(40)41)34-25(36)11-18(32)9-17-10-21(30)22(31)13-20(17)29/h2-6,10,13,15,18-19,23-24,33H,7-9,11-12,14,32H2,1H3,(H,34,36)(H,38,39)(H,40,41)/t15-,18+,19-,23-,24-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibition of PPCE (unknown origin) |
Bioorg Med Chem Lett 27: 2313-2318 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.036
BindingDB Entry DOI: 10.7270/Q2ZC858S |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 9
(Homo sapiens (Human)) | BDBM50242266

(CHEMBL4067185)Show SMILES C[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1C[C@H](C[C@H]1C(O)=O)NC(=O)C[C@H](N)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C28H33F3N4O6/c1-15(33-23(27(38)39)8-7-16-5-3-2-4-6-16)26(37)35-14-19(12-24(35)28(40)41)34-25(36)11-18(32)9-17-10-21(30)22(31)13-20(17)29/h2-6,10,13,15,18-19,23-24,33H,7-9,11-12,14,32H2,1H3,(H,34,36)(H,38,39)(H,40,41)/t15-,18+,19-,23-,24-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Laboratories Limited
Curated by ChEMBL
| Assay Description
Compound was tested in vitro for binding affinity against human carbonic anhydrase II; (ki*10e-9) |
Bioorg Med Chem Lett 27: 2313-2318 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.036
BindingDB Entry DOI: 10.7270/Q2ZC858S |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 8
(Homo sapiens (Human)) | BDBM50242265

(CHEMBL4078112)Show SMILES CCC[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1C[C@H](C[C@H]1C(O)=O)NC(=O)C[C@H](N)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C30H37F3N4O6/c1-2-6-24(36-25(29(40)41)10-9-17-7-4-3-5-8-17)28(39)37-16-20(14-26(37)30(42)43)35-27(38)13-19(34)11-18-12-22(32)23(33)15-21(18)31/h3-5,7-8,12,15,19-20,24-26,36H,2,6,9-11,13-14,16,34H2,1H3,(H,35,38)(H,40,41)(H,42,43)/t19-,20+,24+,25+,26+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibition of DPP8 (unknown origin) |
Bioorg Med Chem Lett 27: 2313-2318 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.036
BindingDB Entry DOI: 10.7270/Q2ZC858S |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 8
(Homo sapiens (Human)) | BDBM50242264

(CHEMBL4086264)Show SMILES CC[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1C[C@H](C[C@H]1C(O)=O)NC(=O)C[C@H](N)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C29H35F3N4O6/c1-2-23(35-24(28(39)40)9-8-16-6-4-3-5-7-16)27(38)36-15-19(13-25(36)29(41)42)34-26(37)12-18(33)10-17-11-21(31)22(32)14-20(17)30/h3-7,11,14,18-19,23-25,35H,2,8-10,12-13,15,33H2,1H3,(H,34,37)(H,39,40)(H,41,42)/t18-,19+,23+,24+,25+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibitory activity against human renin inhibition (at pH 7.4) |
Bioorg Med Chem Lett 27: 2313-2318 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.036
BindingDB Entry DOI: 10.7270/Q2ZC858S |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 9
(Homo sapiens (Human)) | BDBM11162

((1R)-3-oxo-3-[3-(trifluoroethyl)-5,6-dihydro[1,2,4...)Show SMILES N[C@@H](CC(=O)N1CCn2c(C1)nnc2C(F)(F)F)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C16H15F6N5O/c17-10-6-12(19)11(18)4-8(10)3-9(23)5-14(28)26-1-2-27-13(7-26)24-25-15(27)16(20,21)22/h4,6,9H,1-3,5,7,23H2/t9-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibition of DPP9 (unknown origin) |
Bioorg Med Chem Lett 27: 2313-2318 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.036
BindingDB Entry DOI: 10.7270/Q2ZC858S |
More data for this
Ligand-Target Pair | |
Aminopeptidase N
(Homo sapiens (Human)) | BDBM50242265

(CHEMBL4078112)Show SMILES CCC[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1C[C@H](C[C@H]1C(O)=O)NC(=O)C[C@H](N)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C30H37F3N4O6/c1-2-6-24(36-25(29(40)41)10-9-17-7-4-3-5-8-17)28(39)37-16-20(14-26(37)30(42)43)35-27(38)13-19(34)11-18-12-22(32)23(33)15-21(18)31/h3-5,7-8,12,15,19-20,24-26,36H,2,6,9-11,13-14,16,34H2,1H3,(H,35,38)(H,40,41)(H,42,43)/t19-,20+,24+,25+,26+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibition of APN (unknown origin) |
Bioorg Med Chem Lett 27: 2313-2318 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.036
BindingDB Entry DOI: 10.7270/Q2ZC858S |
More data for this
Ligand-Target Pair | |
Aminopeptidase N
(Homo sapiens (Human)) | BDBM11162

((1R)-3-oxo-3-[3-(trifluoroethyl)-5,6-dihydro[1,2,4...)Show SMILES N[C@@H](CC(=O)N1CCn2c(C1)nnc2C(F)(F)F)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C16H15F6N5O/c17-10-6-12(19)11(18)4-8(10)3-9(23)5-14(28)26-1-2-27-13(7-26)24-25-15(27)16(20,21)22/h4,6,9H,1-3,5,7,23H2/t9-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibition of APN (unknown origin) |
Bioorg Med Chem Lett 27: 2313-2318 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.036
BindingDB Entry DOI: 10.7270/Q2ZC858S |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50242266

(CHEMBL4067185)Show SMILES C[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1C[C@H](C[C@H]1C(O)=O)NC(=O)C[C@H](N)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C28H33F3N4O6/c1-15(33-23(27(38)39)8-7-16-5-3-2-4-6-16)26(37)35-14-19(12-24(35)28(40)41)34-25(36)11-18(32)9-17-10-21(30)22(31)13-20(17)29/h2-6,10,13,15,18-19,23-24,33H,7-9,11-12,14,32H2,1H3,(H,34,36)(H,38,39)(H,40,41)/t15-,18+,19-,23-,24-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibition of NEP (unknown origin) |
Bioorg Med Chem Lett 27: 2313-2318 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.036
BindingDB Entry DOI: 10.7270/Q2ZC858S |
More data for this
Ligand-Target Pair | |
Prolyl endopeptidase
(Homo sapiens (Human)) | BDBM50242265

(CHEMBL4078112)Show SMILES CCC[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1C[C@H](C[C@H]1C(O)=O)NC(=O)C[C@H](N)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C30H37F3N4O6/c1-2-6-24(36-25(29(40)41)10-9-17-7-4-3-5-8-17)28(39)37-16-20(14-26(37)30(42)43)35-27(38)13-19(34)11-18-12-22(32)23(33)15-21(18)31/h3-5,7-8,12,15,19-20,24-26,36H,2,6,9-11,13-14,16,34H2,1H3,(H,35,38)(H,40,41)(H,42,43)/t19-,20+,24+,25+,26+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Laboratories Limited
Curated by ChEMBL
| Assay Description
Compound was tested in vitro for binding affinity against human carbonic anhydrase II; (ki*10e-9) |
Bioorg Med Chem Lett 27: 2313-2318 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.036
BindingDB Entry DOI: 10.7270/Q2ZC858S |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 2
(Homo sapiens (Human)) | BDBM50242274

(CHEMBL4090635)Show SMILES CCCC[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1C[C@H](C[C@H]1C(O)=O)NC(=O)C[C@H](N)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C31H39F3N4O6/c1-2-3-9-25(37-26(30(41)42)11-10-18-7-5-4-6-8-18)29(40)38-17-21(15-27(38)31(43)44)36-28(39)14-20(35)12-19-13-23(33)24(34)16-22(19)32/h4-8,13,16,20-21,25-27,37H,2-3,9-12,14-15,17,35H2,1H3,(H,36,39)(H,41,42)(H,43,44)/t20-,21+,25+,26+,27+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Laboratories Limited
Curated by ChEMBL
| Assay Description
Compound was tested in vitro for binding affinity against human carbonic anhydrase II; (ki*10e-9) |
Bioorg Med Chem Lett 27: 2313-2318 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.036
BindingDB Entry DOI: 10.7270/Q2ZC858S |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 2
(Homo sapiens (Human)) | BDBM50242266

(CHEMBL4067185)Show SMILES C[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1C[C@H](C[C@H]1C(O)=O)NC(=O)C[C@H](N)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C28H33F3N4O6/c1-15(33-23(27(38)39)8-7-16-5-3-2-4-6-16)26(37)35-14-19(12-24(35)28(40)41)34-25(36)11-18(32)9-17-10-21(30)22(31)13-20(17)29/h2-6,10,13,15,18-19,23-24,33H,7-9,11-12,14,32H2,1H3,(H,34,36)(H,38,39)(H,40,41)/t15-,18+,19-,23-,24-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Laboratories Limited
Curated by ChEMBL
| Assay Description
Compound was tested in vitro for binding affinity against human carbonic anhydrase II; (ki*10e-9) |
Bioorg Med Chem Lett 27: 2313-2318 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.036
BindingDB Entry DOI: 10.7270/Q2ZC858S |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 2
(Homo sapiens (Human)) | BDBM11162

((1R)-3-oxo-3-[3-(trifluoroethyl)-5,6-dihydro[1,2,4...)Show SMILES N[C@@H](CC(=O)N1CCn2c(C1)nnc2C(F)(F)F)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C16H15F6N5O/c17-10-6-12(19)11(18)4-8(10)3-9(23)5-14(28)26-1-2-27-13(7-26)24-25-15(27)16(20,21)22/h4,6,9H,1-3,5,7,23H2/t9-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibition of DPP2 (unknown origin) |
Bioorg Med Chem Lett 27: 2313-2318 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.036
BindingDB Entry DOI: 10.7270/Q2ZC858S |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 9
(Homo sapiens (Human)) | BDBM50242274

(CHEMBL4090635)Show SMILES CCCC[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1C[C@H](C[C@H]1C(O)=O)NC(=O)C[C@H](N)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C31H39F3N4O6/c1-2-3-9-25(37-26(30(41)42)11-10-18-7-5-4-6-8-18)29(40)38-17-21(15-27(38)31(43)44)36-28(39)14-20(35)12-19-13-23(33)24(34)16-22(19)32/h4-8,13,16,20-21,25-27,37H,2-3,9-12,14-15,17,35H2,1H3,(H,36,39)(H,41,42)(H,43,44)/t20-,21+,25+,26+,27+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Laboratories Limited
Curated by ChEMBL
| Assay Description
Compound was tested in vitro for binding affinity against human carbonic anhydrase II; (ki*10e-9) |
Bioorg Med Chem Lett 27: 2313-2318 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.036
BindingDB Entry DOI: 10.7270/Q2ZC858S |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 9
(Homo sapiens (Human)) | BDBM50242264

(CHEMBL4086264)Show SMILES CC[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1C[C@H](C[C@H]1C(O)=O)NC(=O)C[C@H](N)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C29H35F3N4O6/c1-2-23(35-24(28(39)40)9-8-16-6-4-3-5-7-16)27(38)36-15-19(13-25(36)29(41)42)34-26(37)12-18(33)10-17-11-21(31)22(32)14-20(17)30/h3-7,11,14,18-19,23-25,35H,2,8-10,12-13,15,33H2,1H3,(H,34,37)(H,39,40)(H,41,42)/t18-,19+,23+,24+,25+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Laboratories Limited
Curated by ChEMBL
| Assay Description
Compound was tested in vitro for binding affinity against human carbonic anhydrase II; (ki*10e-9) |
Bioorg Med Chem Lett 27: 2313-2318 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.036
BindingDB Entry DOI: 10.7270/Q2ZC858S |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 8
(Homo sapiens (Human)) | BDBM50242274

(CHEMBL4090635)Show SMILES CCCC[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1C[C@H](C[C@H]1C(O)=O)NC(=O)C[C@H](N)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C31H39F3N4O6/c1-2-3-9-25(37-26(30(41)42)11-10-18-7-5-4-6-8-18)29(40)38-17-21(15-27(38)31(43)44)36-28(39)14-20(35)12-19-13-23(33)24(34)16-22(19)32/h4-8,13,16,20-21,25-27,37H,2-3,9-12,14-15,17,35H2,1H3,(H,36,39)(H,41,42)(H,43,44)/t20-,21+,25+,26+,27+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Laboratories Limited
Curated by ChEMBL
| Assay Description
Binding affinity to HIV protease |
Bioorg Med Chem Lett 27: 2313-2318 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.036
BindingDB Entry DOI: 10.7270/Q2ZC858S |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 8
(Homo sapiens (Human)) | BDBM11162

((1R)-3-oxo-3-[3-(trifluoroethyl)-5,6-dihydro[1,2,4...)Show SMILES N[C@@H](CC(=O)N1CCn2c(C1)nnc2C(F)(F)F)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C16H15F6N5O/c17-10-6-12(19)11(18)4-8(10)3-9(23)5-14(28)26-1-2-27-13(7-26)24-25-15(27)16(20,21)22/h4,6,9H,1-3,5,7,23H2/t9-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibitory activity against human renin inhibition (at pH 7.4) |
Bioorg Med Chem Lett 27: 2313-2318 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.036
BindingDB Entry DOI: 10.7270/Q2ZC858S |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50367254

(ENALAPRILAT)Show SMILES C[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1CCC[C@H]1C(O)=O |r| Show InChI InChI=1S/C18H24N2O5/c1-12(16(21)20-11-5-8-15(20)18(24)25)19-14(17(22)23)10-9-13-6-3-2-4-7-13/h2-4,6-7,12,14-15,19H,5,8-11H2,1H3,(H,22,23)(H,24,25)/t12-,14-,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibition of human plasma DPP4 |
Bioorg Med Chem Lett 27: 2313-2318 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.036
BindingDB Entry DOI: 10.7270/Q2ZC858S |
More data for this
Ligand-Target Pair | |
Aminopeptidase N
(Homo sapiens (Human)) | BDBM50242274

(CHEMBL4090635)Show SMILES CCCC[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1C[C@H](C[C@H]1C(O)=O)NC(=O)C[C@H](N)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C31H39F3N4O6/c1-2-3-9-25(37-26(30(41)42)11-10-18-7-5-4-6-8-18)29(40)38-17-21(15-27(38)31(43)44)36-28(39)14-20(35)12-19-13-23(33)24(34)16-22(19)32/h4-8,13,16,20-21,25-27,37H,2-3,9-12,14-15,17,35H2,1H3,(H,36,39)(H,41,42)(H,43,44)/t20-,21+,25+,26+,27+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibition of APN (unknown origin) |
Bioorg Med Chem Lett 27: 2313-2318 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.036
BindingDB Entry DOI: 10.7270/Q2ZC858S |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data