

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM518 ((3S,4aS,8aS)-N-tert-butyl-2-[(2R,3R)-2-hydroxy-3-[...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.0100 | -62.8 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
GlaxoSmithKline | Assay Description The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... | Bioorg Med Chem Lett 10: 1159-62 (2000) Article DOI: 10.1016/s0960-894x(00)00163-3 BindingDB Entry DOI: 10.7270/Q2X63K5G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM520 (1,3-thiazol-5-ylmethyl N-[(2S,3S,5S)-3-hydroxy-5-[...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.0200 | -61.1 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
GlaxoSmithKline | Assay Description The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... | Bioorg Med Chem Lett 10: 1159-62 (2000) Article DOI: 10.1016/s0960-894x(00)00163-3 BindingDB Entry DOI: 10.7270/Q2X63K5G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM519 ((2S)-N-[(2S,3R)-4-[(3S,4aS,8aS)-3-(tert-butylcarba...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0400 | -59.3 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
GlaxoSmithKline | Assay Description The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... | Bioorg Med Chem Lett 10: 1159-62 (2000) Article DOI: 10.1016/s0960-894x(00)00163-3 BindingDB Entry DOI: 10.7270/Q2X63K5G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM577 ((3S)-oxolan-3-yl N-[(2S,3R)-4-[(4-aminobenzene)(2-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.0400 | -59.3 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
GlaxoSmithKline | Assay Description The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... | Bioorg Med Chem Lett 10: 1159-62 (2000) Article DOI: 10.1016/s0960-894x(00)00163-3 BindingDB Entry DOI: 10.7270/Q2X63K5G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM9183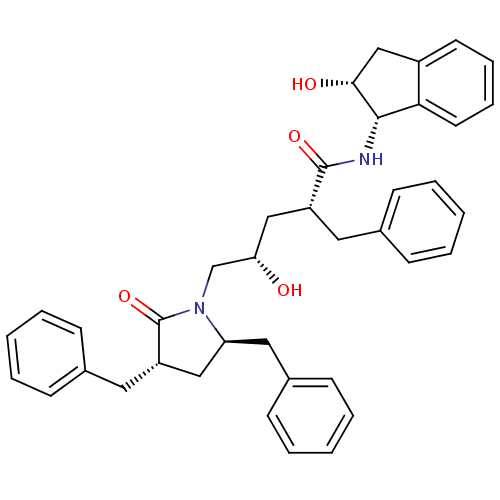 ((2R,4S)-2-benzyl-5-[(3S,5R)-3,5-dibenzyl-2-oxopyrr...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0500 | -58.8 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
GlaxoSmithKline | Assay Description The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... | Bioorg Med Chem Lett 10: 1159-62 (2000) Article DOI: 10.1016/s0960-894x(00)00163-3 BindingDB Entry DOI: 10.7270/Q2X63K5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM517 ((2S)-1-[(2S,4R)-4-benzyl-2-hydroxy-4-{[(1S,2R)-2-h...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0700 | -58.0 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
GlaxoSmithKline | Assay Description The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... | Bioorg Med Chem Lett 10: 1159-62 (2000) Article DOI: 10.1016/s0960-894x(00)00163-3 BindingDB Entry DOI: 10.7270/Q2X63K5G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM577 ((3S)-oxolan-3-yl N-[(2S,3R)-4-[(4-aminobenzene)(2-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc Curated by ChEMBL | Assay Description Binding affinity to HIV-1 protease was determined | Bioorg Med Chem Lett 8: 3631-6 (1999) BindingDB Entry DOI: 10.7270/Q2MC8Z5V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM9235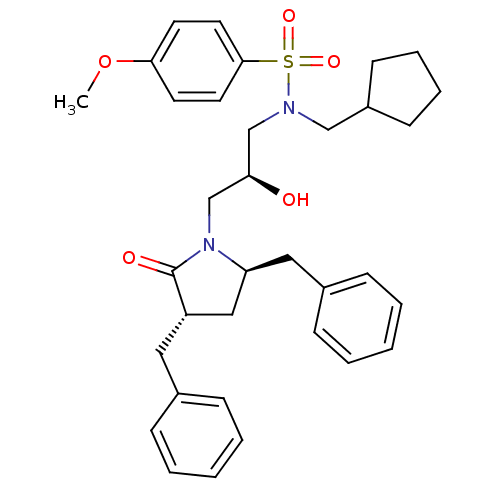 ((2S)-N-(cyclopentylmethyl)-3-[(3S,5R)-3,5-dibenzyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc Curated by ChEMBL | Assay Description Compound was assayed for inhibition against HIV protease activity | Bioorg Med Chem Lett 8: 3637-42 (1999) BindingDB Entry DOI: 10.7270/Q2GM86FH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM9225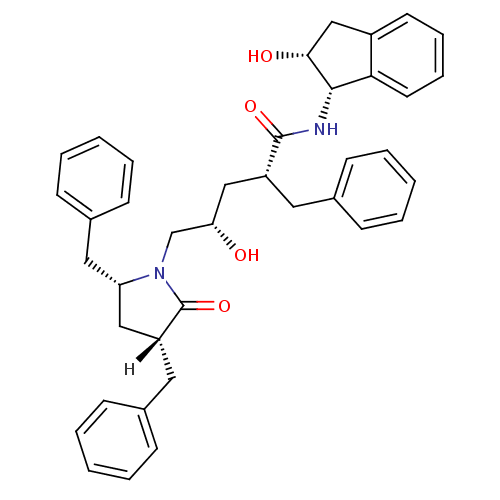 ((2R,4S)-2-benzyl-5-[(3R,5R)-3,5-dibenzyl-2-oxopyrr...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >0.5 | >-53.1 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
GlaxoSmithKline | Assay Description The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... | Bioorg Med Chem Lett 10: 1159-62 (2000) Article DOI: 10.1016/s0960-894x(00)00163-3 BindingDB Entry DOI: 10.7270/Q2X63K5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM577 ((3S)-oxolan-3-yl N-[(2S,3R)-4-[(4-aminobenzene)(2-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc Curated by ChEMBL | Assay Description Compound was assayed for inhibition against HIV protease activity | Bioorg Med Chem Lett 8: 3637-42 (1999) BindingDB Entry DOI: 10.7270/Q2GM86FH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxytocin receptor (RAT) | BDBM50406692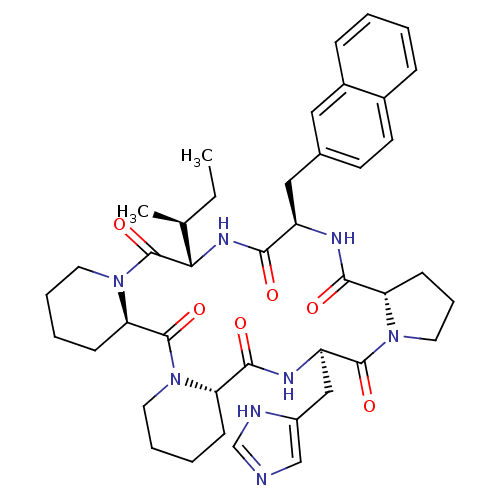 (CHEMBL2112249) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for OT receptor affinity by displacement of [3H]OT from binding sites in uterine tissue taken from pregnant rats | J Med Chem 35: 3905-18 (1992) BindingDB Entry DOI: 10.7270/Q2K64JP2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM81891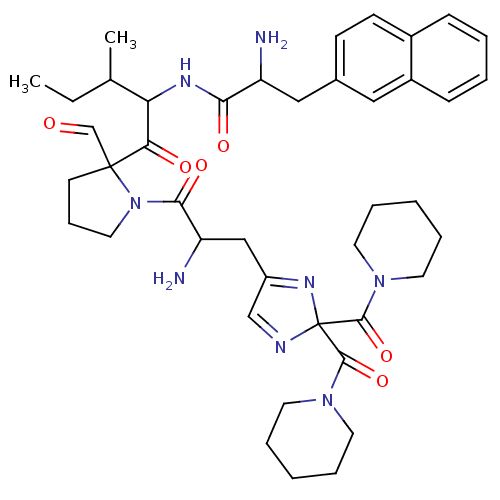 (CAS_188397 | L-366,948 | NSC_188397) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by PDSP Ki Database | J Pharmacol Exp Ther 256: 304-8 (1991) BindingDB Entry DOI: 10.7270/Q2028Q1G | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM9229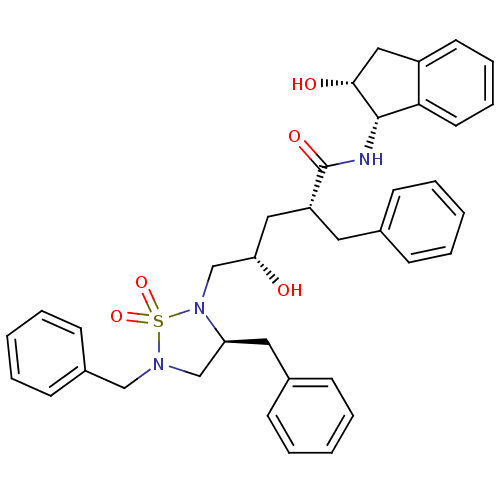 ((2R,4S)-2-benzyl-5-[(3S)-3,5-dibenzyl-1,1-dioxo-1,...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.800 | -51.9 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
GlaxoSmithKline | Assay Description The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... | Bioorg Med Chem Lett 10: 1159-62 (2000) Article DOI: 10.1016/s0960-894x(00)00163-3 BindingDB Entry DOI: 10.7270/Q2X63K5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM81891 (CAS_188397 | L-366,948 | NSC_188397) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by PDSP Ki Database | J Pharmacol Exp Ther 256: 304-8 (1991) BindingDB Entry DOI: 10.7270/Q2028Q1G | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM9230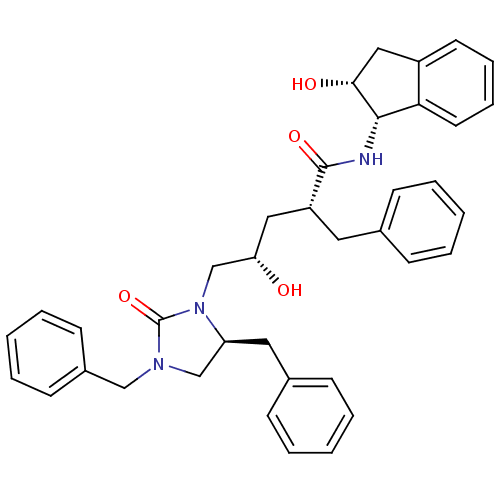 ((2R,4S)-2-benzyl-5-[(5S)-3,5-dibenzyl-2-oxoimidazo...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.20 | -50.9 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
GlaxoSmithKline | Assay Description The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... | Bioorg Med Chem Lett 10: 1159-62 (2000) Article DOI: 10.1016/s0960-894x(00)00163-3 BindingDB Entry DOI: 10.7270/Q2X63K5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM81894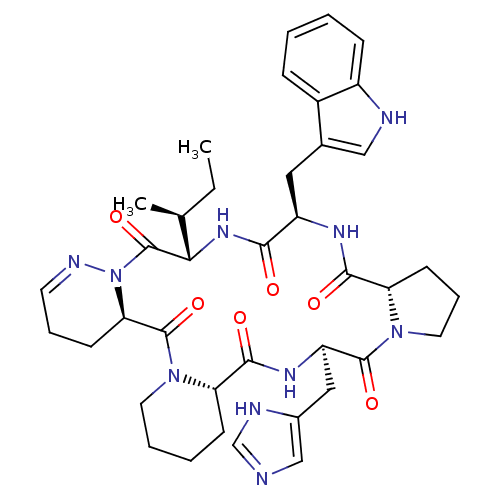 (Cyclo[L-Pro-D-Trp-L-Ile-1,6-didehydro-D-Pyz-L-Pip-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by PDSP Ki Database | J Pharmacol Exp Ther 256: 304-8 (1991) BindingDB Entry DOI: 10.7270/Q2028Q1G | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM81894 (Cyclo[L-Pro-D-Trp-L-Ile-1,6-didehydro-D-Pyz-L-Pip-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Ability to inhibit AVP stimulation of adenylate cyclase activity in the rat kidney medulla (AVP-V2) receptor | J Med Chem 35: 3905-18 (1992) BindingDB Entry DOI: 10.7270/Q2K64JP2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM81894 (Cyclo[L-Pro-D-Trp-L-Ile-1,6-didehydro-D-Pyz-L-Pip-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for OT receptor affinity by displacement of [3H]OT from binding sites in uterine tissue taken from nonlabor pregnant women | J Med Chem 35: 3905-18 (1992) BindingDB Entry DOI: 10.7270/Q2K64JP2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM81894 (Cyclo[L-Pro-D-Trp-L-Ile-1,6-didehydro-D-Pyz-L-Pip-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for OT receptor affinity by displacement of [3H]OT from binding sites in uterine tissue taken from near-term pregnant rhesus m... | J Med Chem 35: 3905-18 (1992) BindingDB Entry DOI: 10.7270/Q2K64JP2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM81894 (Cyclo[L-Pro-D-Trp-L-Ile-1,6-didehydro-D-Pyz-L-Pip-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by PDSP Ki Database | J Pharmacol Exp Ther 256: 304-8 (1991) BindingDB Entry DOI: 10.7270/Q2028Q1G | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Rhesus) | BDBM81894 (Cyclo[L-Pro-D-Trp-L-Ile-1,6-didehydro-D-Pyz-L-Pip-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by PDSP Ki Database | J Pharmacol Exp Ther 256: 304-8 (1991) BindingDB Entry DOI: 10.7270/Q2028Q1G | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM81894 (Cyclo[L-Pro-D-Trp-L-Ile-1,6-didehydro-D-Pyz-L-Pip-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by PDSP Ki Database | J Pharmacol Exp Ther 256: 304-8 (1991) BindingDB Entry DOI: 10.7270/Q2028Q1G | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50001311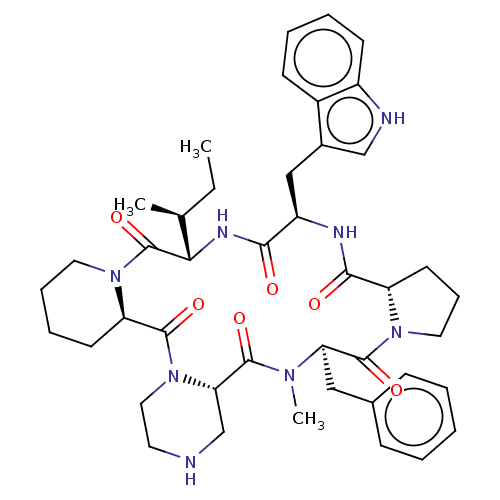 (24-benzyl-16-(1H-3-indolylmethyl)-25-methyl-13-[1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for OT receptor affinity by displacement of [3H]OT from binding sites in uterine tissue taken from pregnant rats | J Med Chem 35: 3905-18 (1992) BindingDB Entry DOI: 10.7270/Q2K64JP2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM81893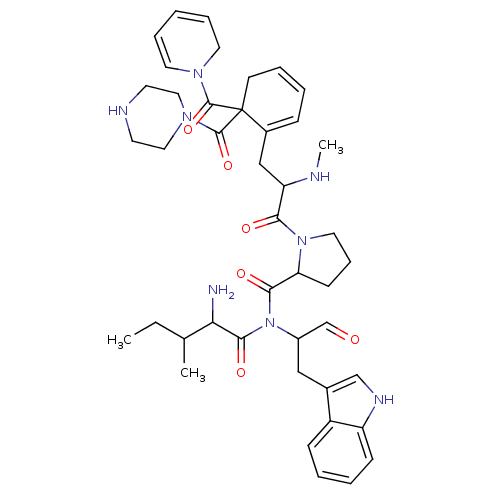 (CAS_3083084 | L-366,811 | NSC_3083084) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by PDSP Ki Database | J Pharmacol Exp Ther 256: 304-8 (1991) BindingDB Entry DOI: 10.7270/Q2028Q1G | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM81889 (Cyclo[L-Pro-D-Phe-L-Ile-1,6-didehydro-D-Pyz-5-[(di...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by PDSP Ki Database | J Pharmacol Exp Ther 256: 304-8 (1991) BindingDB Entry DOI: 10.7270/Q2028Q1G | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM81893 (CAS_3083084 | L-366,811 | NSC_3083084) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by PDSP Ki Database | J Pharmacol Exp Ther 256: 304-8 (1991) BindingDB Entry DOI: 10.7270/Q2028Q1G | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50013636 (22-benzyl-13-(1H-3-indolylmethyl)-23-methyl-10-[1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [3H]- oxytocin binding to rat uterine Oxytocin receptor | J Med Chem 33: 1843-5 (1990) BindingDB Entry DOI: 10.7270/Q2JS9PDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM81889 (Cyclo[L-Pro-D-Phe-L-Ile-1,6-didehydro-D-Pyz-5-[(di...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by PDSP Ki Database | J Pharmacol Exp Ther 256: 304-8 (1991) BindingDB Entry DOI: 10.7270/Q2028Q1G | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50001311 (24-benzyl-16-(1H-3-indolylmethyl)-25-methyl-13-[1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for OT receptor affinity by displacement of [3H]OT from binding sites in uterine tissue taken from nonlabor pregnant women | J Med Chem 35: 3905-18 (1992) BindingDB Entry DOI: 10.7270/Q2K64JP2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM81893 (CAS_3083084 | L-366,811 | NSC_3083084) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by PDSP Ki Database | J Pharmacol Exp Ther 256: 304-8 (1991) BindingDB Entry DOI: 10.7270/Q2028Q1G | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Rhesus) | BDBM81893 (CAS_3083084 | L-366,811 | NSC_3083084) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by PDSP Ki Database | J Pharmacol Exp Ther 256: 304-8 (1991) BindingDB Entry DOI: 10.7270/Q2028Q1G | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50001311 (24-benzyl-16-(1H-3-indolylmethyl)-25-methyl-13-[1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for OT receptor affinity by displacement of [3H]OT from binding sites in uterine tissue taken from near-term pregnant rhesus m... | J Med Chem 35: 3905-18 (1992) BindingDB Entry DOI: 10.7270/Q2K64JP2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50406692 (CHEMBL2112249) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for OT receptor affinity by displacement of [3H]OT from binding sites in uterine tissue taken from nonlabor pregnant women | J Med Chem 35: 3905-18 (1992) BindingDB Entry DOI: 10.7270/Q2K64JP2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50406692 (CHEMBL2112249) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for OT receptor affinity by displacement of [3H]OT from binding sites in uterine tissue taken from pregnant rats | J Med Chem 35: 3905-18 (1992) BindingDB Entry DOI: 10.7270/Q2K64JP2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM81891 (CAS_188397 | L-366,948 | NSC_188397) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by PDSP Ki Database | J Pharmacol Exp Ther 256: 304-8 (1991) BindingDB Entry DOI: 10.7270/Q2028Q1G | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50013628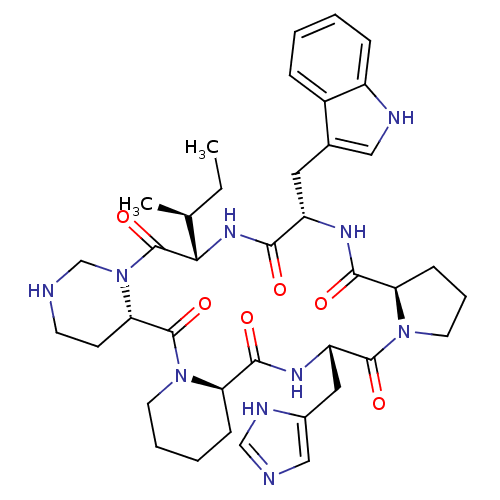 (24-(1H-5-imidazolylmethyl)-16-(1H-3-indolylmethyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [3H]- oxytocin binding to rat uterine Oxytocin receptor | J Med Chem 33: 1843-5 (1990) BindingDB Entry DOI: 10.7270/Q2JS9PDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50013630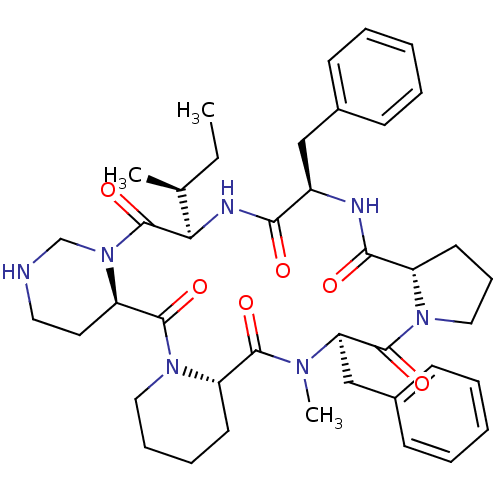 (16,24-dibenzyl-25-methyl-13-[1-methyl-(1S)-propyl]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [3H]- oxytocin binding to rat uterine Oxytocin receptor | J Med Chem 33: 1843-5 (1990) BindingDB Entry DOI: 10.7270/Q2JS9PDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM81890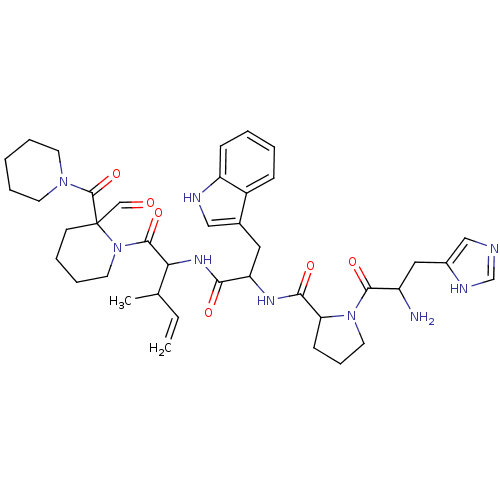 (CAS_196819 | L-366,682 | NSC_196819) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by PDSP Ki Database | J Pharmacol Exp Ther 256: 304-8 (1991) BindingDB Entry DOI: 10.7270/Q2028Q1G | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM81892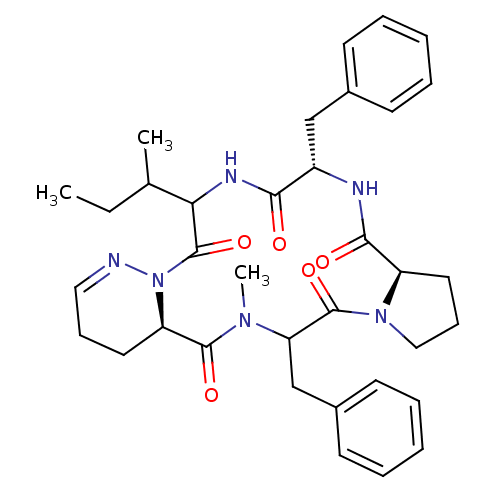 (Cyclo[L-Pro-D-Phe-L-Ile-1,6-didehydro-D-Pyz-N-meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by PDSP Ki Database | J Pharmacol Exp Ther 256: 304-8 (1991) BindingDB Entry DOI: 10.7270/Q2028Q1G | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50001307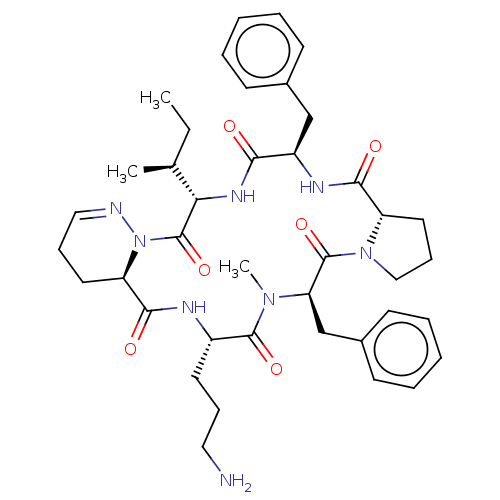 (18-(3-Amino-propyl)-6,15-dibenzyl-3-sec-butyl-16-m...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [3H]arginine vasopressin binding to AVP-V2 site in rat kidney medulla. | J Med Chem 35: 3905-18 (1992) BindingDB Entry DOI: 10.7270/Q2K64JP2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50368435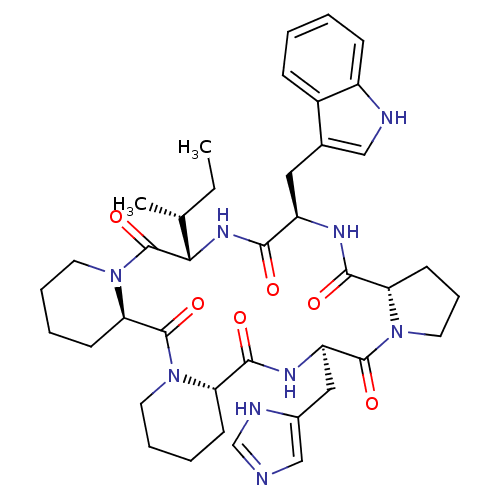 (CHEMBL1790937) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for OT receptor affinity by displacement of [3H]OT from binding sites in uterine tissue taken from pregnant rats | J Med Chem 35: 3905-18 (1992) BindingDB Entry DOI: 10.7270/Q2K64JP2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM81889 (Cyclo[L-Pro-D-Phe-L-Ile-1,6-didehydro-D-Pyz-5-[(di...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by PDSP Ki Database | J Pharmacol Exp Ther 256: 304-8 (1991) BindingDB Entry DOI: 10.7270/Q2028Q1G | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Rhesus) | BDBM81891 (CAS_188397 | L-366,948 | NSC_188397) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by PDSP Ki Database | J Pharmacol Exp Ther 256: 304-8 (1991) BindingDB Entry DOI: 10.7270/Q2028Q1G | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50073365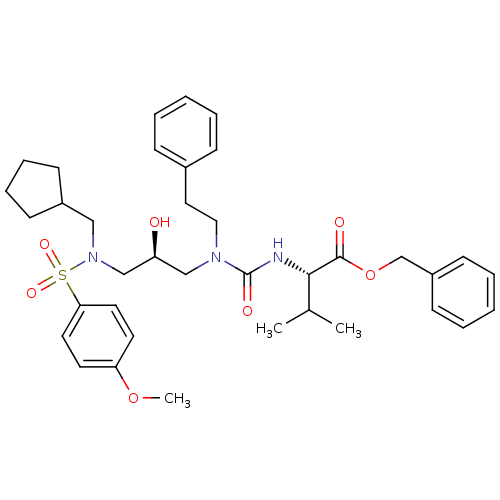 ((S)-2-(3-{(R)-3-[Cyclopentylmethyl-(4-methoxy-phen...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc Curated by ChEMBL | Assay Description Binding affinity to HIV-1 protease was determined | Bioorg Med Chem Lett 8: 3631-6 (1999) BindingDB Entry DOI: 10.7270/Q2MC8Z5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM81895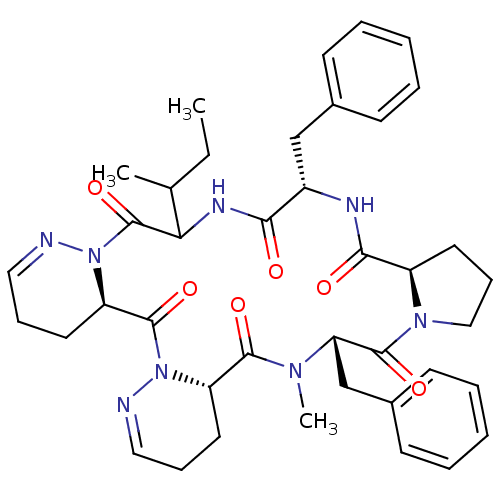 (Cyclo[L-Pro-D-Phe-L-Ile-1,6-didehydro-D-Pyz-1,6-di...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by PDSP Ki Database | J Pharmacol Exp Ther 256: 304-8 (1991) BindingDB Entry DOI: 10.7270/Q2028Q1G | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50001309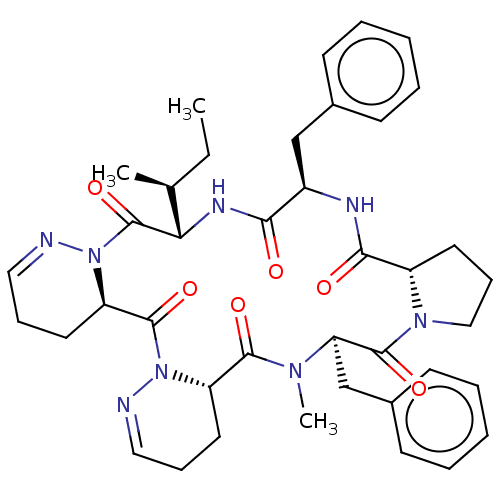 ((cyclo-[L-propyl-D-phenylalanyl-L-isoleucyl-D-dehy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [3H]- oxytocin binding to rat uterine Oxytocin receptor | J Med Chem 33: 1843-5 (1990) BindingDB Entry DOI: 10.7270/Q2JS9PDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50001309 ((cyclo-[L-propyl-D-phenylalanyl-L-isoleucyl-D-dehy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards rat uterine receptor was determined using [3H]oxytocin as radioligand | J Med Chem 35: 3905-18 (1992) BindingDB Entry DOI: 10.7270/Q2K64JP2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50013634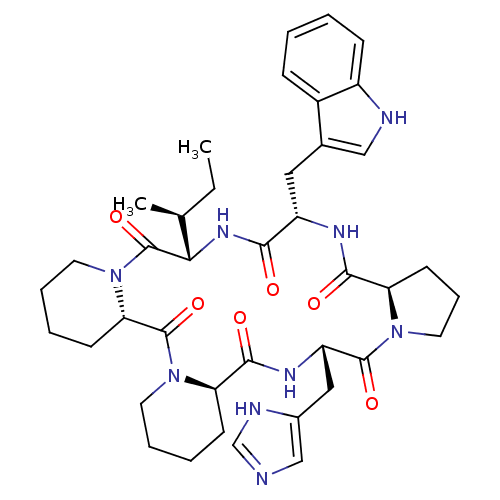 (24-(1H-5-imidazolylmethyl)-16-(1H-3-indolylmethyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [3H]- oxytocin binding to rat uterine Oxytocin receptor | J Med Chem 33: 1843-5 (1990) BindingDB Entry DOI: 10.7270/Q2JS9PDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Rhesus) | BDBM81889 (Cyclo[L-Pro-D-Phe-L-Ile-1,6-didehydro-D-Pyz-5-[(di...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 8.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by PDSP Ki Database | J Pharmacol Exp Ther 256: 304-8 (1991) BindingDB Entry DOI: 10.7270/Q2028Q1G | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50013638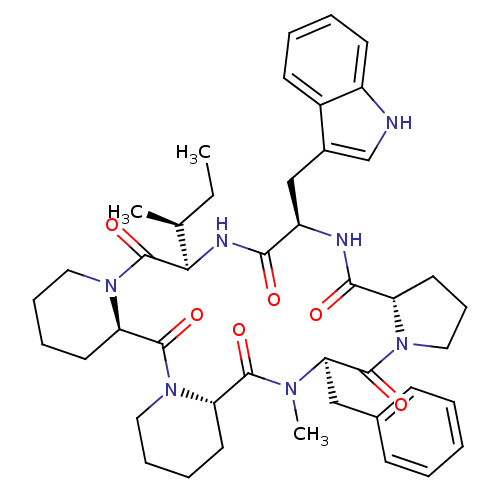 (24-benzyl-16-(1H-3-indolylmethyl)-25-methyl-13-[1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [3H]- oxytocin binding to rat uterine Oxytocin receptor | J Med Chem 33: 1843-5 (1990) BindingDB Entry DOI: 10.7270/Q2JS9PDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 326 total ) | Next | Last >> |