 Found 1240 hits with Last Name = 'turner' and Initial = 'm'
Found 1240 hits with Last Name = 'turner' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM4690
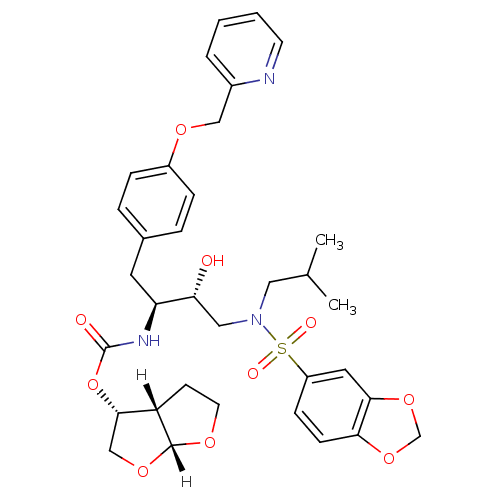
((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...)Show SMILES [H][C@@]12CCO[C@]1([H])OC[C@@H]2OC(=O)N[C@@H](Cc1ccc(OCc2ccccn2)cc1)[C@H](O)CN(CC(C)C)S(=O)(=O)c1ccc2OCOc2c1 |r| Show InChI InChI=1S/C34H41N3O10S/c1-22(2)17-37(48(40,41)26-10-11-30-31(16-26)46-21-45-30)18-29(38)28(36-34(39)47-32-20-44-33-27(32)12-14-42-33)15-23-6-8-25(9-7-23)43-19-24-5-3-4-13-35-24/h3-11,13,16,22,27-29,32-33,38H,12,14-15,17-21H2,1-2H3,(H,36,39)/t27-,28-,29+,32-,33+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.00000600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]GW0385 from HIV1 protease |
Bioorg Med Chem Lett 16: 1788-94 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.035
BindingDB Entry DOI: 10.7270/Q2WS8V1F |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM4685

((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...)Show SMILES [H][C@]12OCC[C@@]1([H])[C@H](CO2)OC(=O)N[C@@H](Cc1ccc(OCc2csc(C)n2)cc1)[C@H](O)CN(CC(C)C)S(=O)(=O)c1ccc2OCOc2c1 |r| Show InChI InChI=1S/C33H41N3O10S2/c1-20(2)14-36(48(39,40)25-8-9-29-30(13-25)45-19-44-29)15-28(37)27(35-33(38)46-31-17-43-32-26(31)10-11-41-32)12-22-4-6-24(7-5-22)42-16-23-18-47-21(3)34-23/h4-9,13,18,20,26-28,31-32,37H,10-12,14-17,19H2,1-3H3,(H,35,38)/t26-,27-,28+,31-,32+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.0000150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]GW0385 from HIV1 protease |
Bioorg Med Chem Lett 16: 1788-94 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.035
BindingDB Entry DOI: 10.7270/Q2WS8V1F |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50128419
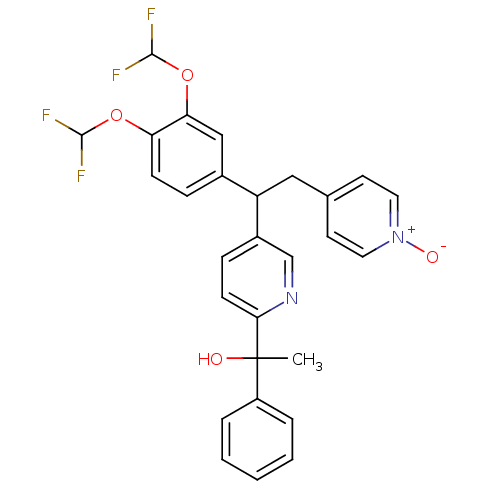
(1-{5-[1-(3,4-Bis-difluoromethoxy-phenyl)-2-(1-oxy-...)Show SMILES CC(O)(c1ccccc1)c1ccc(cn1)C(Cc1cc[n+]([O-])cc1)c1ccc(OC(F)F)c(OC(F)F)c1 Show InChI InChI=1S/C28H24F4N2O4/c1-28(35,21-5-3-2-4-6-21)25-10-8-20(17-33-25)22(15-18-11-13-34(36)14-12-18)19-7-9-23(37-26(29)30)24(16-19)38-27(31)32/h2-14,16-17,22,26-27,35H,15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
In vitro displacement of [35S]-MK- 499 from HEK 293 cells stably transfected with hERG voltage-gated potassium channel subunit Kv11.1 |
J Med Chem 46: 2413-26 (2003)
Article DOI: 10.1021/jm0204542
BindingDB Entry DOI: 10.7270/Q24X5756 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50409702
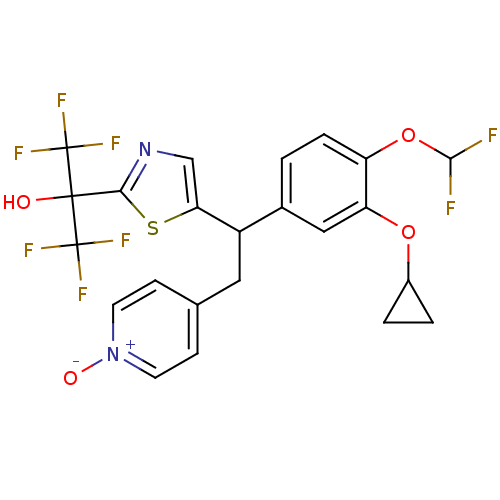
(CHEMBL2112296)Show SMILES OC(c1ncc(s1)C(Cc1cc[n+]([O-])cc1)c1ccc(OC(F)F)c(OC2CC2)c1)(C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C23H18F8N2O4S/c24-20(25)37-16-4-1-13(10-17(16)36-14-2-3-14)15(9-12-5-7-33(35)8-6-12)18-11-32-19(38-18)21(34,22(26,27)28)23(29,30)31/h1,4-8,10-11,14-15,20,34H,2-3,9H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
In vitro displacement of [35S]-MK- 499 from HEK 293 cells stably transfected with hERG voltage-gated potassium channel subunit Kv11.1 |
J Med Chem 46: 2413-26 (2003)
Article DOI: 10.1021/jm0204542
BindingDB Entry DOI: 10.7270/Q24X5756 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50409699
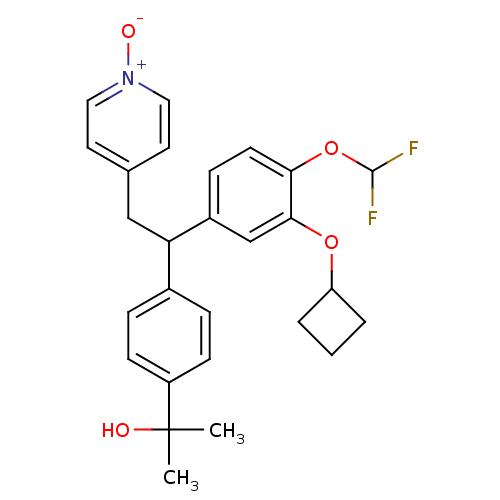
(CHEMBL2112294)Show SMILES CC(C)(O)c1ccc(cc1)C(Cc1cc[n+]([O-])cc1)c1ccc(OC(F)F)c(OC2CCC2)c1 Show InChI InChI=1S/C27H29F2NO4/c1-27(2,31)21-9-6-19(7-10-21)23(16-18-12-14-30(32)15-13-18)20-8-11-24(34-26(28)29)25(17-20)33-22-4-3-5-22/h6-15,17,22-23,26,31H,3-5,16H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.24E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
In vitro displacement of [35S]-MK- 499 from HEK 293 cells stably transfected with hERG voltage-gated potassium channel subunit Kv11.1 |
J Med Chem 46: 2413-26 (2003)
Article DOI: 10.1021/jm0204542
BindingDB Entry DOI: 10.7270/Q24X5756 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50128424

(2-{5-[1-(3,4-Bis-difluoromethoxy-phenyl)-2-(1-oxy-...)Show SMILES CC(C)(O)c1ccc(cn1)C(Cc1cc[n+]([O-])cc1)c1ccc(OC(F)F)c(OC(F)F)c1 Show InChI InChI=1S/C23H22F4N2O4/c1-23(2,30)20-6-4-16(13-28-20)17(11-14-7-9-29(31)10-8-14)15-3-5-18(32-21(24)25)19(12-15)33-22(26)27/h3-10,12-13,17,21-22,30H,11H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.27E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
In vitro displacement of [35S]-MK- 499 from HEK 293 cells stably transfected with hERG voltage-gated potassium channel subunit Kv11.1 |
J Med Chem 46: 2413-26 (2003)
Article DOI: 10.1021/jm0204542
BindingDB Entry DOI: 10.7270/Q24X5756 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50128424

(2-{5-[1-(3,4-Bis-difluoromethoxy-phenyl)-2-(1-oxy-...)Show SMILES CC(C)(O)c1ccc(cn1)C(Cc1cc[n+]([O-])cc1)c1ccc(OC(F)F)c(OC(F)F)c1 Show InChI InChI=1S/C23H22F4N2O4/c1-23(2,30)20-6-4-16(13-28-20)17(11-14-7-9-29(31)10-8-14)15-3-5-18(32-21(24)25)19(12-15)33-22(26)27/h3-10,12-13,17,21-22,30H,11H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.27E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
In vitro displacement of [35S]-MK- 499 from HEK 293 cells stably transfected with hERG voltage-gated potassium channel subunit Kv11.1 |
J Med Chem 46: 2413-26 (2003)
Article DOI: 10.1021/jm0204542
BindingDB Entry DOI: 10.7270/Q24X5756 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50128686

(2-{5-[1-(3-Cyclobutoxy-4-difluoromethoxy-phenyl)-2...)Show SMILES CC(C)(O)c1ccc(cn1)C(Cc1cc[n+]([O-])cc1)c1ccc(OC(F)F)c(OC2CCC2)c1 Show InChI InChI=1S/C26H28F2N2O4/c1-26(2,31)24-9-7-19(16-29-24)21(14-17-10-12-30(32)13-11-17)18-6-8-22(34-25(27)28)23(15-18)33-20-4-3-5-20/h6-13,15-16,20-21,25,31H,3-5,14H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
In vitro displacement of [35S]-MK- 499 from HEK 293 cells stably transfected with hERG voltage-gated potassium channel subunit Kv11.1 |
J Med Chem 46: 2413-26 (2003)
Article DOI: 10.1021/jm0204542
BindingDB Entry DOI: 10.7270/Q24X5756 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50128689
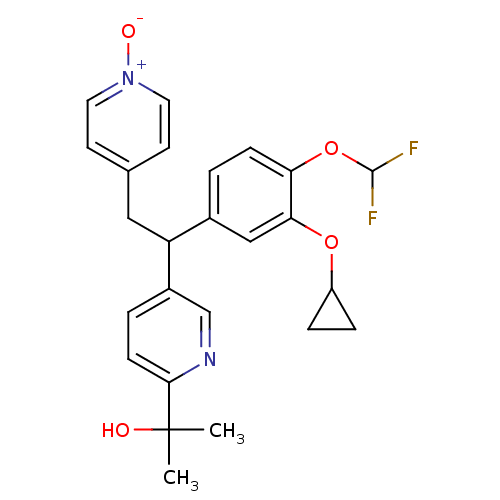
(2-{5-[1-(3-Cyclopropoxy-4-difluoromethoxy-phenyl)-...)Show SMILES CC(C)(O)c1ccc(cn1)C(Cc1cc[n+]([O-])cc1)c1ccc(OC(F)F)c(OC2CC2)c1 Show InChI InChI=1S/C25H26F2N2O4/c1-25(2,30)23-8-4-18(15-28-23)20(13-16-9-11-29(31)12-10-16)17-3-7-21(33-24(26)27)22(14-17)32-19-5-6-19/h3-4,7-12,14-15,19-20,24,30H,5-6,13H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.64E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
In vitro displacement of [35S]-MK- 499 from HEK 293 cells stably transfected with hERG voltage-gated potassium channel subunit Kv11.1 |
J Med Chem 46: 2413-26 (2003)
Article DOI: 10.1021/jm0204542
BindingDB Entry DOI: 10.7270/Q24X5756 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50128689

(2-{5-[1-(3-Cyclopropoxy-4-difluoromethoxy-phenyl)-...)Show SMILES CC(C)(O)c1ccc(cn1)C(Cc1cc[n+]([O-])cc1)c1ccc(OC(F)F)c(OC2CC2)c1 Show InChI InChI=1S/C25H26F2N2O4/c1-25(2,30)23-8-4-18(15-28-23)20(13-16-9-11-29(31)12-10-16)17-3-7-21(33-24(26)27)22(14-17)32-19-5-6-19/h3-4,7-12,14-15,19-20,24,30H,5-6,13H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.64E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
In vitro displacement of [35S]-MK- 499 from HEK 293 cells stably transfected with hERG voltage-gated potassium channel subunit Kv11.1 |
J Med Chem 46: 2413-26 (2003)
Article DOI: 10.1021/jm0204542
BindingDB Entry DOI: 10.7270/Q24X5756 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50409700
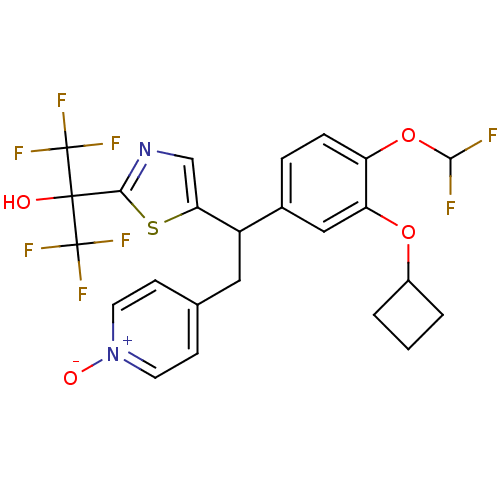
(CHEMBL2112295)Show SMILES OC(c1ncc(s1)C(Cc1cc[n+]([O-])cc1)c1ccc(OC(F)F)c(OC2CCC2)c1)(C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C24H20F8N2O4S/c25-21(26)38-17-5-4-14(11-18(17)37-15-2-1-3-15)16(10-13-6-8-34(36)9-7-13)19-12-33-20(39-19)22(35,23(27,28)29)24(30,31)32/h4-9,11-12,15-16,21,35H,1-3,10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.81E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
In vitro displacement of [35S]-MK- 499 from HEK 293 cells stably transfected with hERG voltage-gated potassium channel subunit Kv11.1 |
J Med Chem 46: 2413-26 (2003)
Article DOI: 10.1021/jm0204542
BindingDB Entry DOI: 10.7270/Q24X5756 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50409701

(CHEMBL383762)Show SMILES OC(c1ncc(s1)[C@H](Cc1ccc[n+]([O-])c1)c1ccc(OC(F)F)c(OC2CC2)c1)(C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C23H18F8N2O4S/c24-20(25)37-16-6-3-13(9-17(16)36-14-4-5-14)15(8-12-2-1-7-33(35)11-12)18-10-32-19(38-18)21(34,22(26,27)28)23(29,30)31/h1-3,6-7,9-11,14-15,20,34H,4-5,8H2/t15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
In vitro displacement of [35S]-MK- 499 from HEK 293 cells stably transfected with hERG voltage-gated potassium channel subunit Kv11.1 |
J Med Chem 46: 2413-26 (2003)
Article DOI: 10.1021/jm0204542
BindingDB Entry DOI: 10.7270/Q24X5756 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50128683
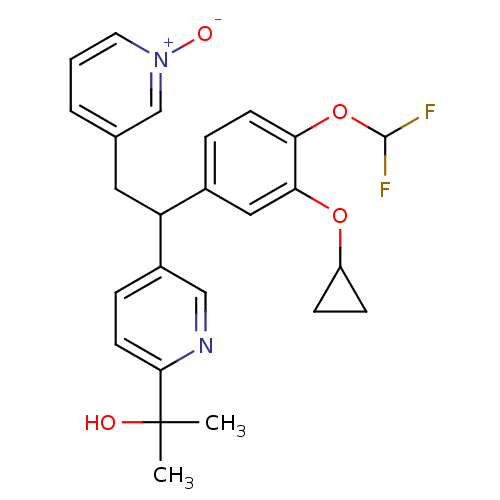
(2-{5-[1-(3-Cyclopropoxy-4-difluoromethoxy-phenyl)-...)Show SMILES CC(C)(O)c1ccc(cn1)C(Cc1ccc[n+]([O-])c1)c1ccc(OC(F)F)c(OC2CC2)c1 Show InChI InChI=1S/C25H26F2N2O4/c1-25(2,30)23-10-6-18(14-28-23)20(12-16-4-3-11-29(31)15-16)17-5-9-21(33-24(26)27)22(13-17)32-19-7-8-19/h3-6,9-11,13-15,19-20,24,30H,7-8,12H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.22E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
In vitro displacement of [35S]-MK- 499 from HEK 293 cells stably transfected with hERG voltage-gated potassium channel subunit Kv11.1 |
J Med Chem 46: 2413-26 (2003)
Article DOI: 10.1021/jm0204542
BindingDB Entry DOI: 10.7270/Q24X5756 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50128683

(2-{5-[1-(3-Cyclopropoxy-4-difluoromethoxy-phenyl)-...)Show SMILES CC(C)(O)c1ccc(cn1)C(Cc1ccc[n+]([O-])c1)c1ccc(OC(F)F)c(OC2CC2)c1 Show InChI InChI=1S/C25H26F2N2O4/c1-25(2,30)23-10-6-18(14-28-23)20(12-16-4-3-11-29(31)15-16)17-5-9-21(33-24(26)27)22(13-17)32-19-7-8-19/h3-6,9-11,13-15,19-20,24,30H,7-8,12H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.47E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
In vitro displacement of [35S]-MK- 499 from HEK 293 cells stably transfected with hERG voltage-gated potassium channel subunit Kv11.1 |
J Med Chem 46: 2413-26 (2003)
Article DOI: 10.1021/jm0204542
BindingDB Entry DOI: 10.7270/Q24X5756 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50128692
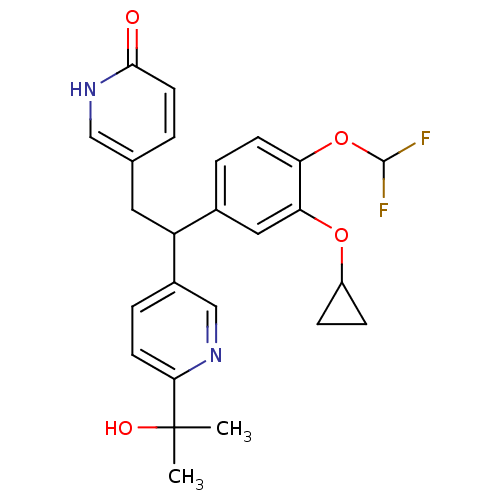
(5-{2-(3-Cyclopropoxy-4-difluoromethoxy-phenyl)-2-[...)Show SMILES CC(C)(O)c1ccc(cn1)C(Cc1ccc(=O)[nH]c1)c1ccc(OC(F)F)c(OC2CC2)c1 Show InChI InChI=1S/C25H26F2N2O4/c1-25(2,31)22-9-5-17(14-28-22)19(11-15-3-10-23(30)29-13-15)16-4-8-20(33-24(26)27)21(12-16)32-18-6-7-18/h3-5,8-10,12-14,18-19,24,31H,6-7,11H2,1-2H3,(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.59E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
In vitro displacement of [35S]-MK- 499 from HEK 293 cells stably transfected with hERG voltage-gated potassium channel subunit Kv11.1 |
J Med Chem 46: 2413-26 (2003)
Article DOI: 10.1021/jm0204542
BindingDB Entry DOI: 10.7270/Q24X5756 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50128685

(2-{5-[1-(3-Cyclobutoxy-4-difluoromethoxy-phenyl)-2...)Show SMILES CC(C)(O)c1ncc(s1)C(Cc1cc[n+]([O-])cc1)c1ccc(OC(F)F)c(OC2CCC2)c1 Show InChI InChI=1S/C24H26F2N2O4S/c1-24(2,29)22-27-14-21(33-22)18(12-15-8-10-28(30)11-9-15)16-6-7-19(32-23(25)26)20(13-16)31-17-4-3-5-17/h6-11,13-14,17-18,23,29H,3-5,12H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.84E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
In vitro displacement of [35S]-MK- 499 from HEK 293 cells stably transfected with hERG voltage-gated potassium channel subunit Kv11.1 |
J Med Chem 46: 2413-26 (2003)
Article DOI: 10.1021/jm0204542
BindingDB Entry DOI: 10.7270/Q24X5756 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50174020
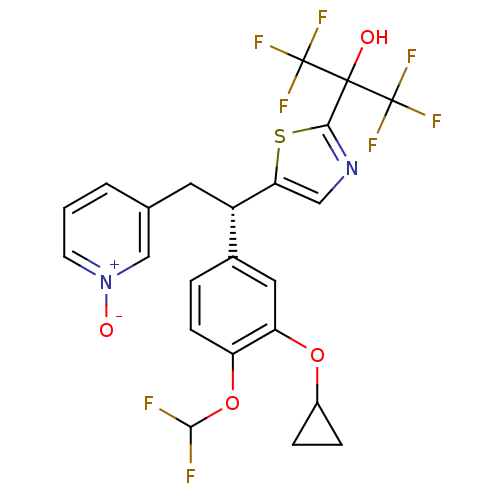
((S)-(+)-3-{2-[(3-Cyclopropyloxy-4-difluromethoxy)-...)Show SMILES OC(c1ncc(s1)[C@@H](Cc1ccc[n+]([O-])c1)c1ccc(OC(F)F)c(OC2CC2)c1)(C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C23H18F8N2O4S/c24-20(25)37-16-6-3-13(9-17(16)36-14-4-5-14)15(8-12-2-1-7-33(35)11-12)18-10-32-19(38-18)21(34,22(26,27)28)23(29,30)31/h1-3,6-7,9-11,14-15,20,34H,4-5,8H2/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.14E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
In vitro displacement of [35S]-MK- 499 from HEK 293 cells stably transfected with hERG voltage-gated potassium channel subunit Kv11.1 |
J Med Chem 46: 2413-26 (2003)
Article DOI: 10.1021/jm0204542
BindingDB Entry DOI: 10.7270/Q24X5756 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50174030
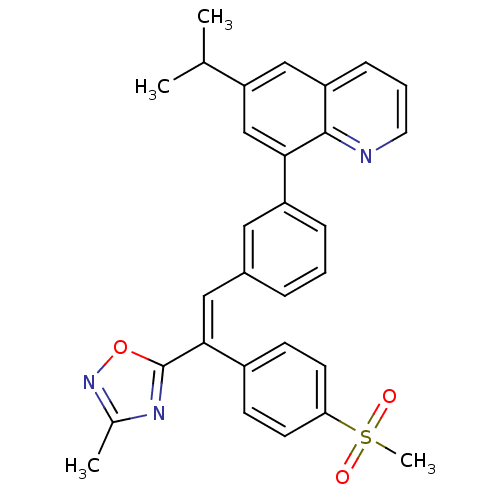
((E)-6-isopropyl-8-(3-(2-(3-methyl-1,2,4-oxadiazol-...)Show SMILES CC(C)c1cc(-c2cccc(\C=C(\c3nc(C)no3)c3ccc(cc3)S(C)(=O)=O)c2)c2ncccc2c1 Show InChI InChI=1S/C30H27N3O3S/c1-19(2)25-17-24-9-6-14-31-29(24)27(18-25)23-8-5-7-21(15-23)16-28(30-32-20(3)33-36-30)22-10-12-26(13-11-22)37(4,34)35/h5-19H,1-4H3/b28-16+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against PDE4B |
Bioorg Med Chem Lett 15: 5241-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.036
BindingDB Entry DOI: 10.7270/Q2B56KHG |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(Homo sapiens (Human)) | BDBM50174030

((E)-6-isopropyl-8-(3-(2-(3-methyl-1,2,4-oxadiazol-...)Show SMILES CC(C)c1cc(-c2cccc(\C=C(\c3nc(C)no3)c3ccc(cc3)S(C)(=O)=O)c2)c2ncccc2c1 Show InChI InChI=1S/C30H27N3O3S/c1-19(2)25-17-24-9-6-14-31-29(24)27(18-25)23-8-5-7-21(15-23)16-28(30-32-20(3)33-36-30)22-10-12-26(13-11-22)37(4,34)35/h5-19H,1-4H3/b28-16+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against PDE4A |
Bioorg Med Chem Lett 15: 5241-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.036
BindingDB Entry DOI: 10.7270/Q2B56KHG |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-6/beta-2
(Homo sapiens (Human)) | BDBM50481208

(CHEMBL594330)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](CS)NC(=O)[C@@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](CC(N)=O)NC(=O)[C@H](CO)NC(=O)[C@H](CS)NC(=O)[C@H](CS)NC(=O)CN)C(C)C)C(=O)N[C@@H](C)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CS)C(O)=O |r| Show InChI InChI=1S/C65H104N22O21S4/c1-28(2)11-34(52(94)73-31(7)51(93)75-36(13-32-18-69-26-71-32)55(97)81-40(20-88)57(99)79-38(15-47(67)90)56(98)77-35(12-29(3)4)53(95)85-45(25-112)65(107)108)76-54(96)37(14-33-19-70-27-72-33)78-60(102)44(24-111)84-63(105)50(30(5)6)86-62(104)46-9-8-10-87(46)64(106)39(16-48(68)91)80-58(100)41(21-89)82-61(103)43(23-110)83-59(101)42(22-109)74-49(92)17-66/h18-19,26-31,34-46,50,88-89,109-112H,8-17,20-25,66H2,1-7H3,(H2,67,90)(H2,68,91)(H,69,71)(H,70,72)(H,73,94)(H,74,92)(H,75,93)(H,76,96)(H,77,98)(H,78,102)(H,79,99)(H,80,100)(H,81,97)(H,82,103)(H,83,101)(H,84,105)(H,85,95)(H,86,104)(H,107,108)/t31-,34-,35-,36-,37-,38-,39-,40-,41-,42-,43-,44-,45-,46-,50-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Boise State University
Curated by ChEMBL
| Assay Description
Inhibition of alpha6beta2 nAChR |
Bioorg Med Chem 17: 5894-9 (2009)
Article DOI: 10.1016/j.bmc.2009.07.005
BindingDB Entry DOI: 10.7270/Q2474DN8 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50174025
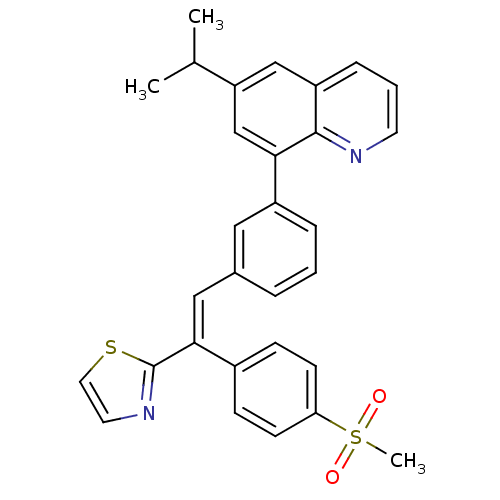
(6-isopropyl-8-(3-(2-(4-(methylsulfonyl)phenyl)-2-(...)Show SMILES CC(C)c1cc(-c2cccc(\C=C(\c3nccs3)c3ccc(cc3)S(C)(=O)=O)c2)c2ncccc2c1 Show InChI InChI=1S/C30H26N2O2S2/c1-20(2)25-18-24-8-5-13-31-29(24)27(19-25)23-7-4-6-21(16-23)17-28(30-32-14-15-35-30)22-9-11-26(12-10-22)36(3,33)34/h4-20H,1-3H3/b28-17+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against PDE4D |
Bioorg Med Chem Lett 15: 5241-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.036
BindingDB Entry DOI: 10.7270/Q2B56KHG |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(Homo sapiens (Human)) | BDBM50174028
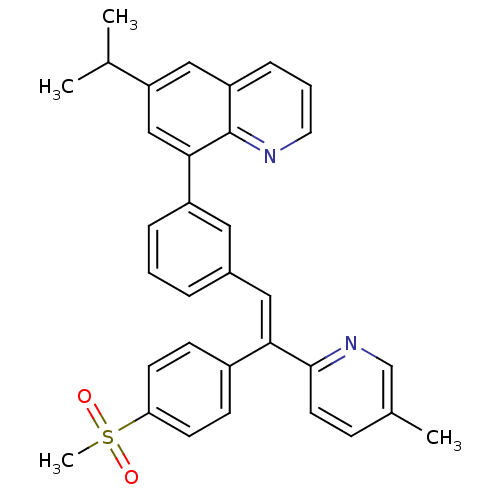
((E)-6-isopropyl-8-(3-(2-(5-methylpyridin-2-yl)-2-(...)Show SMILES CC(C)c1cc(-c2cccc(\C=C(/c3ccc(cc3)S(C)(=O)=O)c3ccc(C)cn3)c2)c2ncccc2c1 Show InChI InChI=1S/C33H30N2O2S/c1-22(2)28-19-27-9-6-16-34-33(27)31(20-28)26-8-5-7-24(17-26)18-30(32-15-10-23(3)21-35-32)25-11-13-29(14-12-25)38(4,36)37/h5-22H,1-4H3/b30-18+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against PDE4A |
Bioorg Med Chem Lett 15: 5241-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.036
BindingDB Entry DOI: 10.7270/Q2B56KHG |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM19386
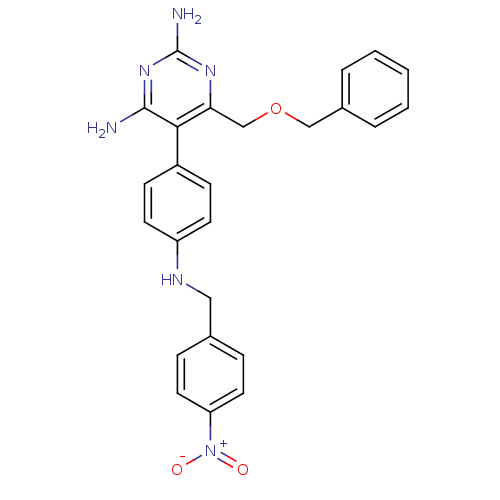
(2,4-diaminopyrimidine derivative, 8b | 6-[(benzylo...)Show SMILES Nc1nc(N)c(c(COCc2ccccc2)n1)-c1ccc(NCc2ccc(cc2)[N+]([O-])=O)cc1 Show InChI InChI=1S/C25H24N6O3/c26-24-23(22(29-25(27)30-24)16-34-15-18-4-2-1-3-5-18)19-8-10-20(11-9-19)28-14-17-6-12-21(13-7-17)31(32)33/h1-13,28H,14-16H2,(H4,26,27,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | 5.80 | n/a | n/a | 7.4 | 22 |
Abbott Laboratories
| Assay Description
Specific binding was determined by incubation of membranes from GHS-R1a transfected CHO-K cells with 125I-His9-ghrelin in the presence of increasing ... |
J Med Chem 49: 2568-78 (2006)
Article DOI: 10.1021/jm0510934
BindingDB Entry DOI: 10.7270/Q27W69G1 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50174030

((E)-6-isopropyl-8-(3-(2-(3-methyl-1,2,4-oxadiazol-...)Show SMILES CC(C)c1cc(-c2cccc(\C=C(\c3nc(C)no3)c3ccc(cc3)S(C)(=O)=O)c2)c2ncccc2c1 Show InChI InChI=1S/C30H27N3O3S/c1-19(2)25-17-24-9-6-14-31-29(24)27(18-25)23-8-5-7-21(15-23)16-28(30-32-20(3)33-36-30)22-10-12-26(13-11-22)37(4,34)35/h5-19H,1-4H3/b28-16+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against PDE4D |
Bioorg Med Chem Lett 15: 5241-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.036
BindingDB Entry DOI: 10.7270/Q2B56KHG |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50174028

((E)-6-isopropyl-8-(3-(2-(5-methylpyridin-2-yl)-2-(...)Show SMILES CC(C)c1cc(-c2cccc(\C=C(/c3ccc(cc3)S(C)(=O)=O)c3ccc(C)cn3)c2)c2ncccc2c1 Show InChI InChI=1S/C33H30N2O2S/c1-22(2)28-19-27-9-6-16-34-33(27)31(20-28)26-8-5-7-24(17-26)18-30(32-15-10-23(3)21-35-32)25-11-13-29(14-12-25)38(4,36)37/h5-22H,1-4H3/b30-18+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against PDE4B |
Bioorg Med Chem Lett 15: 5241-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.036
BindingDB Entry DOI: 10.7270/Q2B56KHG |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50174028

((E)-6-isopropyl-8-(3-(2-(5-methylpyridin-2-yl)-2-(...)Show SMILES CC(C)c1cc(-c2cccc(\C=C(/c3ccc(cc3)S(C)(=O)=O)c3ccc(C)cn3)c2)c2ncccc2c1 Show InChI InChI=1S/C33H30N2O2S/c1-22(2)28-19-27-9-6-16-34-33(27)31(20-28)26-8-5-7-24(17-26)18-30(32-15-10-23(3)21-35-32)25-11-13-29(14-12-25)38(4,36)37/h5-22H,1-4H3/b30-18+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against PDE4D |
Bioorg Med Chem Lett 15: 5241-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.036
BindingDB Entry DOI: 10.7270/Q2B56KHG |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-6/beta-2
(Homo sapiens (Human)) | BDBM50481207
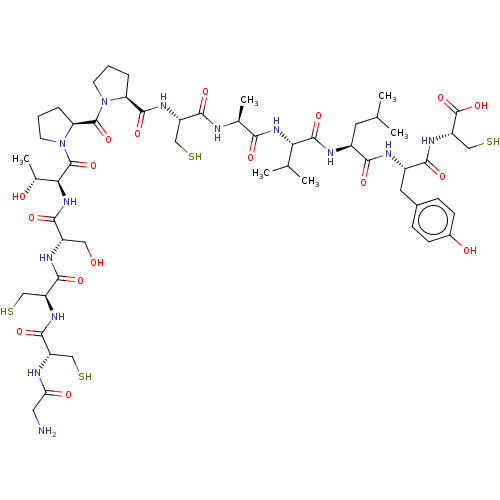
(CHEMBL595286)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](CS)NC(=O)[C@@H]1CCCN1C(=O)[C@@H]1CCCN1C(=O)[C@@H](NC(=O)[C@H](CO)NC(=O)[C@H](CS)NC(=O)[C@H](CS)NC(=O)CN)[C@@H](C)O)C(C)C)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CS)C(O)=O |r| Show InChI InChI=1S/C54H85N13O17S4/c1-25(2)17-31(44(73)58-32(18-29-11-13-30(70)14-12-29)45(74)63-37(24-88)54(83)84)59-51(80)41(26(3)4)64-43(72)27(5)56-47(76)35(22-86)62-50(79)38-9-7-15-66(38)52(81)39-10-8-16-67(39)53(82)42(28(6)69)65-46(75)33(20-68)60-49(78)36(23-87)61-48(77)34(21-85)57-40(71)19-55/h11-14,25-28,31-39,41-42,68-70,85-88H,7-10,15-24,55H2,1-6H3,(H,56,76)(H,57,71)(H,58,73)(H,59,80)(H,60,78)(H,61,77)(H,62,79)(H,63,74)(H,64,72)(H,65,75)(H,83,84)/t27-,28+,31-,32-,33-,34-,35-,36-,37-,38-,39-,41-,42-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
Boise State University
Curated by ChEMBL
| Assay Description
Inhibition of alpha6beta2 nAChR |
Bioorg Med Chem 17: 5894-9 (2009)
Article DOI: 10.1016/j.bmc.2009.07.005
BindingDB Entry DOI: 10.7270/Q2474DN8 |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM19387
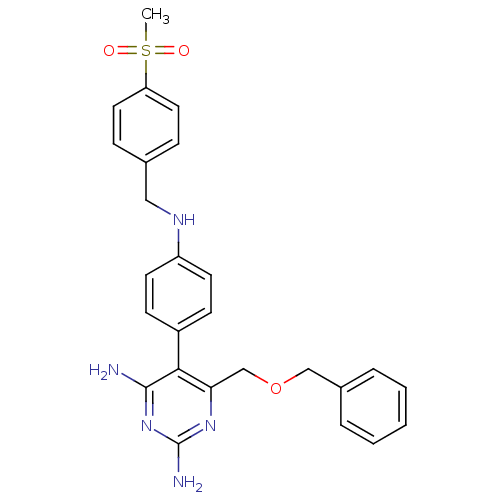
(2,4-diaminopyrimidine derivative, 8c | 6-[(benzylo...)Show SMILES CS(=O)(=O)c1ccc(CNc2ccc(cc2)-c2c(N)nc(N)nc2COCc2ccccc2)cc1 Show InChI InChI=1S/C26H27N5O3S/c1-35(32,33)22-13-7-18(8-14-22)15-29-21-11-9-20(10-12-21)24-23(30-26(28)31-25(24)27)17-34-16-19-5-3-2-4-6-19/h2-14,29H,15-17H2,1H3,(H4,27,28,30,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | 7.20 | n/a | n/a | 7.4 | 22 |
Abbott Laboratories
| Assay Description
Specific binding was determined by incubation of membranes from GHS-R1a transfected CHO-K cells with 125I-His9-ghrelin in the presence of increasing ... |
J Med Chem 49: 2568-78 (2006)
Article DOI: 10.1021/jm0510934
BindingDB Entry DOI: 10.7270/Q27W69G1 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50174025

(6-isopropyl-8-(3-(2-(4-(methylsulfonyl)phenyl)-2-(...)Show SMILES CC(C)c1cc(-c2cccc(\C=C(\c3nccs3)c3ccc(cc3)S(C)(=O)=O)c2)c2ncccc2c1 Show InChI InChI=1S/C30H26N2O2S2/c1-20(2)25-18-24-8-5-13-31-29(24)27(19-25)23-7-4-6-21(16-23)17-28(30-32-14-15-35-30)22-9-11-26(12-10-22)36(3,33)34/h4-20H,1-3H3/b28-17+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against PDE4B |
Bioorg Med Chem Lett 15: 5241-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.036
BindingDB Entry DOI: 10.7270/Q2B56KHG |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50174029
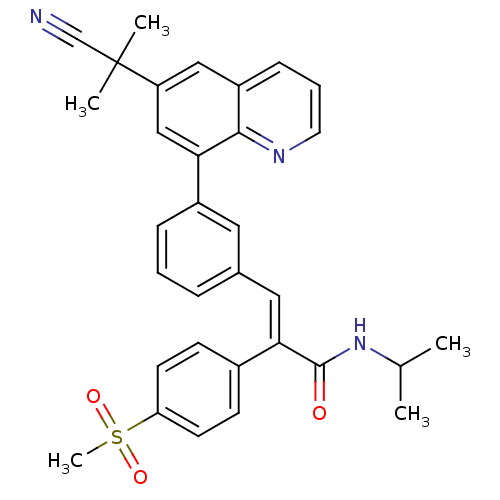
((E)-3-(3-(6-(2-cyanopropan-2-yl)quinolin-8-yl)phen...)Show SMILES CC(C)NC(=O)C(=C\c1cccc(c1)-c1cc(cc2cccnc12)C(C)(C)C#N)\c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C32H31N3O3S/c1-21(2)35-31(36)29(23-11-13-27(14-12-23)39(5,37)38)17-22-8-6-9-24(16-22)28-19-26(32(3,4)20-33)18-25-10-7-15-34-30(25)28/h6-19,21H,1-5H3,(H,35,36)/b29-17+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against PDE4B |
Bioorg Med Chem Lett 15: 5241-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.036
BindingDB Entry DOI: 10.7270/Q2B56KHG |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50174023
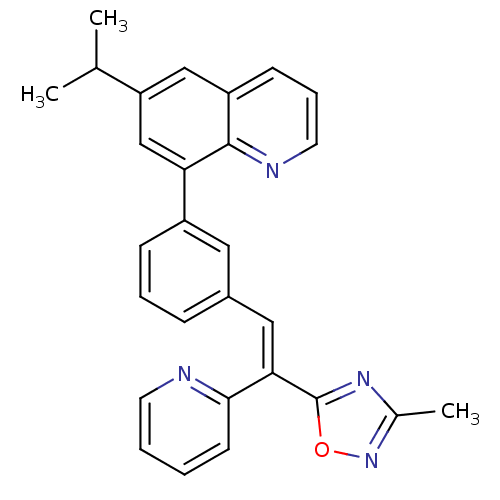
((E)-6-isopropyl-8-(3-(2-(3-methyl-1,2,4-oxadiazol-...)Show SMILES CC(C)c1cc(-c2cccc(\C=C(\c3nc(C)no3)c3ccccn3)c2)c2ncccc2c1 Show InChI InChI=1S/C28H24N4O/c1-18(2)23-16-22-10-7-13-30-27(22)24(17-23)21-9-6-8-20(14-21)15-25(26-11-4-5-12-29-26)28-31-19(3)32-33-28/h4-18H,1-3H3/b25-15+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against PDE4B |
Bioorg Med Chem Lett 15: 5241-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.036
BindingDB Entry DOI: 10.7270/Q2B56KHG |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(Homo sapiens (Human)) | BDBM50174013
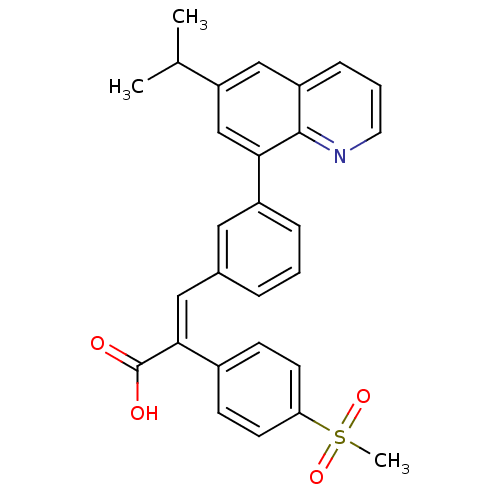
((E)-3-(3-(6-isopropylquinolin-8-yl)phenyl)-2-(4-(m...)Show SMILES CC(C)c1cc(-c2cccc(\C=C(\C(O)=O)c3ccc(cc3)S(C)(=O)=O)c2)c2ncccc2c1 Show InChI InChI=1S/C28H25NO4S/c1-18(2)23-16-22-8-5-13-29-27(22)25(17-23)21-7-4-6-19(14-21)15-26(28(30)31)20-9-11-24(12-10-20)34(3,32)33/h4-18H,1-3H3,(H,30,31)/b26-15+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against PDE4A |
Bioorg Med Chem Lett 15: 5241-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.036
BindingDB Entry DOI: 10.7270/Q2B56KHG |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(Homo sapiens (Human)) | BDBM50174018
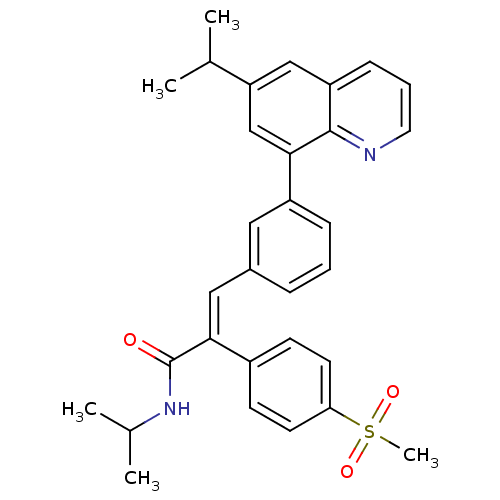
((E)-N-isopropyl-3-(3-(6-isopropylquinolin-8-yl)phe...)Show SMILES CC(C)NC(=O)C(=C\c1cccc(c1)-c1cc(cc2cccnc12)C(C)C)\c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C31H32N2O3S/c1-20(2)26-18-25-10-7-15-32-30(25)28(19-26)24-9-6-8-22(16-24)17-29(31(34)33-21(3)4)23-11-13-27(14-12-23)37(5,35)36/h6-21H,1-5H3,(H,33,34)/b29-17+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against PDE4A |
Bioorg Med Chem Lett 15: 5241-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.036
BindingDB Entry DOI: 10.7270/Q2B56KHG |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50174013

((E)-3-(3-(6-isopropylquinolin-8-yl)phenyl)-2-(4-(m...)Show SMILES CC(C)c1cc(-c2cccc(\C=C(\C(O)=O)c3ccc(cc3)S(C)(=O)=O)c2)c2ncccc2c1 Show InChI InChI=1S/C28H25NO4S/c1-18(2)23-16-22-8-5-13-29-27(22)25(17-23)21-7-4-6-19(14-21)15-26(28(30)31)20-9-11-24(12-10-20)34(3,32)33/h4-18H,1-3H3,(H,30,31)/b26-15+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against PDE4B |
Bioorg Med Chem Lett 15: 5241-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.036
BindingDB Entry DOI: 10.7270/Q2B56KHG |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50174018

((E)-N-isopropyl-3-(3-(6-isopropylquinolin-8-yl)phe...)Show SMILES CC(C)NC(=O)C(=C\c1cccc(c1)-c1cc(cc2cccnc12)C(C)C)\c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C31H32N2O3S/c1-20(2)26-18-25-10-7-15-32-30(25)28(19-26)24-9-6-8-22(16-24)17-29(31(34)33-21(3)4)23-11-13-27(14-12-23)37(5,35)36/h6-21H,1-5H3,(H,33,34)/b29-17+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against PDE4B |
Bioorg Med Chem Lett 15: 5241-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.036
BindingDB Entry DOI: 10.7270/Q2B56KHG |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50174031
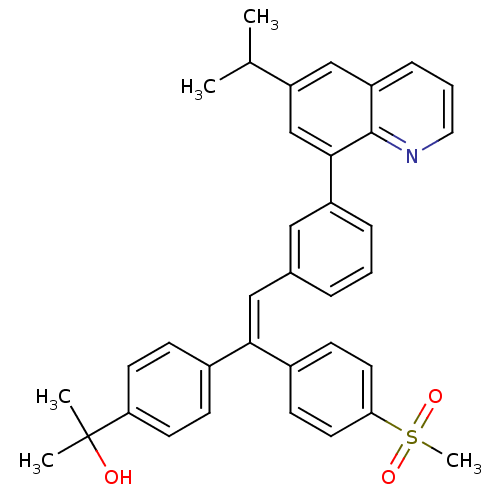
((Z)-2-(4-(2-(3-(6-isopropylquinolin-8-yl)phenyl)-1...)Show SMILES CC(C)c1cc(-c2cccc(\C=C(\c3ccc(cc3)C(C)(C)O)c3ccc(cc3)S(C)(=O)=O)c2)c2ncccc2c1 Show InChI InChI=1S/C36H35NO3S/c1-24(2)30-22-29-10-7-19-37-35(29)34(23-30)28-9-6-8-25(20-28)21-33(26-11-15-31(16-12-26)36(3,4)38)27-13-17-32(18-14-27)41(5,39)40/h6-24,38H,1-5H3/b33-21- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against PDE4B |
Bioorg Med Chem Lett 15: 5241-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.036
BindingDB Entry DOI: 10.7270/Q2B56KHG |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-6/beta-2
(Homo sapiens (Human)) | BDBM50481206

(CHEMBL595311)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](CS)NC(=O)[C@@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](CC(N)=O)NC(=O)[C@H](CO)NC(=O)[C@H](CS)NC(=O)[C@H](CS)NC(=O)CN)C(C)C)C(=O)N[C@@H](CS)C(O)=O |r| Show InChI InChI=1S/C67H106N22O23S4/c1-29(2)12-35(54(98)76-34(9-10-51(95)96)53(97)79-38(15-33-20-72-28-74-33)57(101)83-41(21-90)59(103)81-39(16-48(69)92)58(102)78-36(13-30(3)4)55(99)87-46(26-116)67(111)112)77-56(100)37(14-32-19-71-27-73-32)80-62(106)45(25-115)86-65(109)52(31(5)6)88-64(108)47-8-7-11-89(47)66(110)40(17-49(70)93)82-60(104)42(22-91)84-63(107)44(24-114)85-61(105)43(23-113)75-50(94)18-68/h19-20,27-31,34-47,52,90-91,113-116H,7-18,21-26,68H2,1-6H3,(H2,69,92)(H2,70,93)(H,71,73)(H,72,74)(H,75,94)(H,76,98)(H,77,100)(H,78,102)(H,79,97)(H,80,106)(H,81,103)(H,82,104)(H,83,101)(H,84,107)(H,85,105)(H,86,109)(H,87,99)(H,88,108)(H,95,96)(H,111,112)/t34-,35-,36-,37-,38-,39-,40-,41-,42-,43-,44-,45-,46-,47-,52-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a |
Boise State University
Curated by ChEMBL
| Assay Description
Inhibition of alpha6beta2 nAChR |
Bioorg Med Chem 17: 5894-9 (2009)
Article DOI: 10.1016/j.bmc.2009.07.005
BindingDB Entry DOI: 10.7270/Q2474DN8 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(Homo sapiens (Human)) | BDBM50174031

((Z)-2-(4-(2-(3-(6-isopropylquinolin-8-yl)phenyl)-1...)Show SMILES CC(C)c1cc(-c2cccc(\C=C(\c3ccc(cc3)C(C)(C)O)c3ccc(cc3)S(C)(=O)=O)c2)c2ncccc2c1 Show InChI InChI=1S/C36H35NO3S/c1-24(2)30-22-29-10-7-19-37-35(29)34(23-30)28-9-6-8-25(20-28)21-33(26-11-15-31(16-12-26)36(3,4)38)27-13-17-32(18-14-27)41(5,39)40/h6-24,38H,1-5H3/b33-21- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against PDE4A |
Bioorg Med Chem Lett 15: 5241-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.036
BindingDB Entry DOI: 10.7270/Q2B56KHG |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50174020

((S)-(+)-3-{2-[(3-Cyclopropyloxy-4-difluromethoxy)-...)Show SMILES OC(c1ncc(s1)[C@@H](Cc1ccc[n+]([O-])c1)c1ccc(OC(F)F)c(OC2CC2)c1)(C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C23H18F8N2O4S/c24-20(25)37-16-6-3-13(9-17(16)36-14-4-5-14)15(8-12-2-1-7-33(35)11-12)18-10-32-19(38-18)21(34,22(26,27)28)23(29,30)31/h1-3,6-7,9-11,14-15,20,34H,4-5,8H2/t15-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against PDE4B |
Bioorg Med Chem Lett 15: 5241-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.036
BindingDB Entry DOI: 10.7270/Q2B56KHG |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50174020

((S)-(+)-3-{2-[(3-Cyclopropyloxy-4-difluromethoxy)-...)Show SMILES OC(c1ncc(s1)[C@@H](Cc1ccc[n+]([O-])c1)c1ccc(OC(F)F)c(OC2CC2)c1)(C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C23H18F8N2O4S/c24-20(25)37-16-6-3-13(9-17(16)36-14-4-5-14)15(8-12-2-1-7-33(35)11-12)18-10-32-19(38-18)21(34,22(26,27)28)23(29,30)31/h1-3,6-7,9-11,14-15,20,34H,4-5,8H2/t15-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against PDE4D |
Bioorg Med Chem Lett 15: 5241-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.036
BindingDB Entry DOI: 10.7270/Q2B56KHG |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(Homo sapiens (Human)) | BDBM50174020

((S)-(+)-3-{2-[(3-Cyclopropyloxy-4-difluromethoxy)-...)Show SMILES OC(c1ncc(s1)[C@@H](Cc1ccc[n+]([O-])c1)c1ccc(OC(F)F)c(OC2CC2)c1)(C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C23H18F8N2O4S/c24-20(25)37-16-6-3-13(9-17(16)36-14-4-5-14)15(8-12-2-1-7-33(35)11-12)18-10-32-19(38-18)21(34,22(26,27)28)23(29,30)31/h1-3,6-7,9-11,14-15,20,34H,4-5,8H2/t15-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against PDE4A |
Bioorg Med Chem Lett 15: 5241-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.036
BindingDB Entry DOI: 10.7270/Q2B56KHG |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50174021
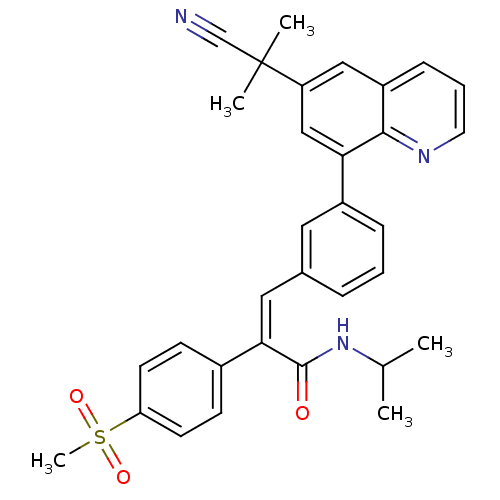
((Z)-3-(3-(6-(2-cyanopropan-2-yl)quinolin-8-yl)phen...)Show SMILES CC(C)NC(=O)C(=C/c1cccc(c1)-c1cc(cc2cccnc12)C(C)(C)C#N)\c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C32H31N3O3S/c1-21(2)35-31(36)29(23-11-13-27(14-12-23)39(5,37)38)17-22-8-6-9-24(16-22)28-19-26(32(3,4)20-33)18-25-10-7-15-34-30(25)28/h6-19,21H,1-5H3,(H,35,36)/b29-17- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against PDE4B |
Bioorg Med Chem Lett 15: 5241-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.036
BindingDB Entry DOI: 10.7270/Q2B56KHG |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4C
(Homo sapiens (Human)) | BDBM50174028

((E)-6-isopropyl-8-(3-(2-(5-methylpyridin-2-yl)-2-(...)Show SMILES CC(C)c1cc(-c2cccc(\C=C(/c3ccc(cc3)S(C)(=O)=O)c3ccc(C)cn3)c2)c2ncccc2c1 Show InChI InChI=1S/C33H30N2O2S/c1-22(2)28-19-27-9-6-16-34-33(27)31(20-28)26-8-5-7-24(17-26)18-30(32-15-10-23(3)21-35-32)25-11-13-29(14-12-25)38(4,36)37/h5-22H,1-4H3/b30-18+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against PDE4C |
Bioorg Med Chem Lett 15: 5241-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.036
BindingDB Entry DOI: 10.7270/Q2B56KHG |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(Homo sapiens (Human)) | BDBM50174025

(6-isopropyl-8-(3-(2-(4-(methylsulfonyl)phenyl)-2-(...)Show SMILES CC(C)c1cc(-c2cccc(\C=C(\c3nccs3)c3ccc(cc3)S(C)(=O)=O)c2)c2ncccc2c1 Show InChI InChI=1S/C30H26N2O2S2/c1-20(2)25-18-24-8-5-13-31-29(24)27(19-25)23-7-4-6-21(16-23)17-28(30-32-14-15-35-30)22-9-11-26(12-10-22)36(3,33)34/h4-20H,1-3H3/b28-17+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against PDE4A |
Bioorg Med Chem Lett 15: 5241-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.036
BindingDB Entry DOI: 10.7270/Q2B56KHG |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50174013

((E)-3-(3-(6-isopropylquinolin-8-yl)phenyl)-2-(4-(m...)Show SMILES CC(C)c1cc(-c2cccc(\C=C(\C(O)=O)c3ccc(cc3)S(C)(=O)=O)c2)c2ncccc2c1 Show InChI InChI=1S/C28H25NO4S/c1-18(2)23-16-22-8-5-13-29-27(22)25(17-23)21-7-4-6-19(14-21)15-26(28(30)31)20-9-11-24(12-10-20)34(3,32)33/h4-18H,1-3H3,(H,30,31)/b26-15+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against PDE4D |
Bioorg Med Chem Lett 15: 5241-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.036
BindingDB Entry DOI: 10.7270/Q2B56KHG |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(Homo sapiens (Human)) | BDBM50174023

((E)-6-isopropyl-8-(3-(2-(3-methyl-1,2,4-oxadiazol-...)Show SMILES CC(C)c1cc(-c2cccc(\C=C(\c3nc(C)no3)c3ccccn3)c2)c2ncccc2c1 Show InChI InChI=1S/C28H24N4O/c1-18(2)23-16-22-10-7-13-30-27(22)24(17-23)21-9-6-8-20(14-21)15-25(26-11-4-5-12-29-26)28-31-19(3)32-33-28/h4-18H,1-3H3/b25-15+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against PDE4A |
Bioorg Med Chem Lett 15: 5241-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.036
BindingDB Entry DOI: 10.7270/Q2B56KHG |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4C
(Homo sapiens (Human)) | BDBM50174031

((Z)-2-(4-(2-(3-(6-isopropylquinolin-8-yl)phenyl)-1...)Show SMILES CC(C)c1cc(-c2cccc(\C=C(\c3ccc(cc3)C(C)(C)O)c3ccc(cc3)S(C)(=O)=O)c2)c2ncccc2c1 Show InChI InChI=1S/C36H35NO3S/c1-24(2)30-22-29-10-7-19-37-35(29)34(23-30)28-9-6-8-25(20-28)21-33(26-11-15-31(16-12-26)36(3,4)38)27-13-17-32(18-14-27)41(5,39)40/h6-24,38H,1-5H3/b33-21- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against PDE4C |
Bioorg Med Chem Lett 15: 5241-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.036
BindingDB Entry DOI: 10.7270/Q2B56KHG |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(Homo sapiens (Human)) | BDBM50128685

(2-{5-[1-(3-Cyclobutoxy-4-difluoromethoxy-phenyl)-2...)Show SMILES CC(C)(O)c1ncc(s1)C(Cc1cc[n+]([O-])cc1)c1ccc(OC(F)F)c(OC2CCC2)c1 Show InChI InChI=1S/C24H26F2N2O4S/c1-24(2,29)22-27-14-21(33-22)18(12-15-8-10-28(30)11-9-15)16-6-7-19(32-23(25)26)20(13-16)31-17-4-3-5-17/h6-11,13-14,17-18,23,29H,3-5,12H2,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity was determined against human Phosphodiesterase 4 isoform using a construct representing the common region of spliced var... |
J Med Chem 46: 2413-26 (2003)
Article DOI: 10.1021/jm0204542
BindingDB Entry DOI: 10.7270/Q24X5756 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50174022
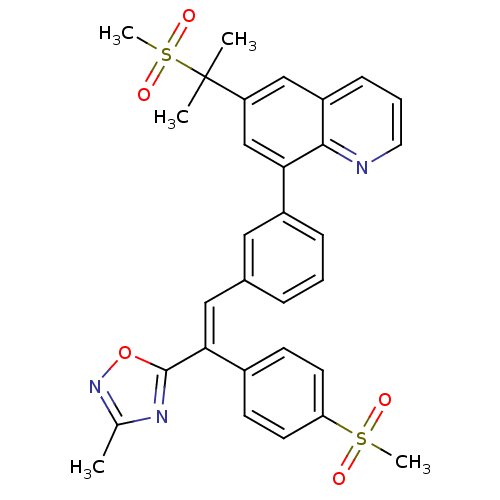
((E)-8-(3-(2-(3-methyl-1,2,4-oxadiazol-5-yl)-2-(4-(...)Show SMILES Cc1noc(n1)C(=C\c1cccc(c1)-c1cc(cc2cccnc12)C(C)(C)S(C)(=O)=O)\c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C31H29N3O5S2/c1-20-33-30(39-34-20)28(22-11-13-26(14-12-22)40(4,35)36)17-21-8-6-9-23(16-21)27-19-25(31(2,3)41(5,37)38)18-24-10-7-15-32-29(24)27/h6-19H,1-5H3/b28-17+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against PDE4B |
Bioorg Med Chem Lett 15: 5241-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.036
BindingDB Entry DOI: 10.7270/Q2B56KHG |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-3/beta-2
(Homo sapiens (Human)) | BDBM50481206

(CHEMBL595311)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](CS)NC(=O)[C@@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](CC(N)=O)NC(=O)[C@H](CO)NC(=O)[C@H](CS)NC(=O)[C@H](CS)NC(=O)CN)C(C)C)C(=O)N[C@@H](CS)C(O)=O |r| Show InChI InChI=1S/C67H106N22O23S4/c1-29(2)12-35(54(98)76-34(9-10-51(95)96)53(97)79-38(15-33-20-72-28-74-33)57(101)83-41(21-90)59(103)81-39(16-48(69)92)58(102)78-36(13-30(3)4)55(99)87-46(26-116)67(111)112)77-56(100)37(14-32-19-71-27-73-32)80-62(106)45(25-115)86-65(109)52(31(5)6)88-64(108)47-8-7-11-89(47)66(110)40(17-49(70)93)82-60(104)42(22-91)84-63(107)44(24-114)85-61(105)43(23-113)75-50(94)18-68/h19-20,27-31,34-47,52,90-91,113-116H,7-18,21-26,68H2,1-6H3,(H2,69,92)(H2,70,93)(H,71,73)(H,72,74)(H,75,94)(H,76,98)(H,77,100)(H,78,102)(H,79,97)(H,80,106)(H,81,103)(H,82,104)(H,83,101)(H,84,107)(H,85,105)(H,86,109)(H,87,99)(H,88,108)(H,95,96)(H,111,112)/t34-,35-,36-,37-,38-,39-,40-,41-,42-,43-,44-,45-,46-,47-,52-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boise State University
Curated by ChEMBL
| Assay Description
Inhibition of alpha3beta2 nAChR |
Bioorg Med Chem 17: 5894-9 (2009)
Article DOI: 10.1016/j.bmc.2009.07.005
BindingDB Entry DOI: 10.7270/Q2474DN8 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data