 Found 145 hits with Last Name = 'turner' and Initial = 'mj'
Found 145 hits with Last Name = 'turner' and Initial = 'mj' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50128419
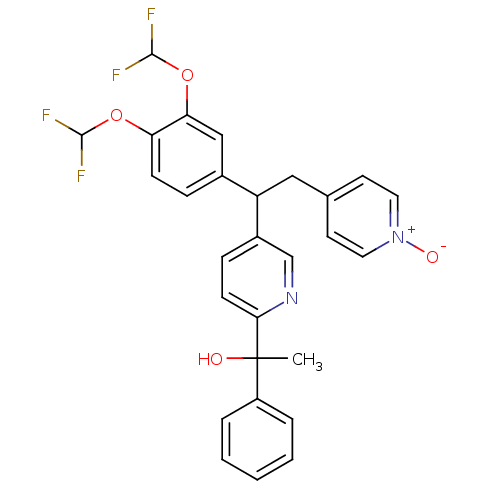
(1-{5-[1-(3,4-Bis-difluoromethoxy-phenyl)-2-(1-oxy-...)Show SMILES CC(O)(c1ccccc1)c1ccc(cn1)C(Cc1cc[n+]([O-])cc1)c1ccc(OC(F)F)c(OC(F)F)c1 Show InChI InChI=1S/C28H24F4N2O4/c1-28(35,21-5-3-2-4-6-21)25-10-8-20(17-33-25)22(15-18-11-13-34(36)14-12-18)19-7-9-23(37-26(29)30)24(16-19)38-27(31)32/h2-14,16-17,22,26-27,35H,15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
In vitro displacement of [35S]-MK- 499 from HEK 293 cells stably transfected with hERG voltage-gated potassium channel subunit Kv11.1 |
J Med Chem 46: 2413-26 (2003)
Article DOI: 10.1021/jm0204542
BindingDB Entry DOI: 10.7270/Q24X5756 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50409702
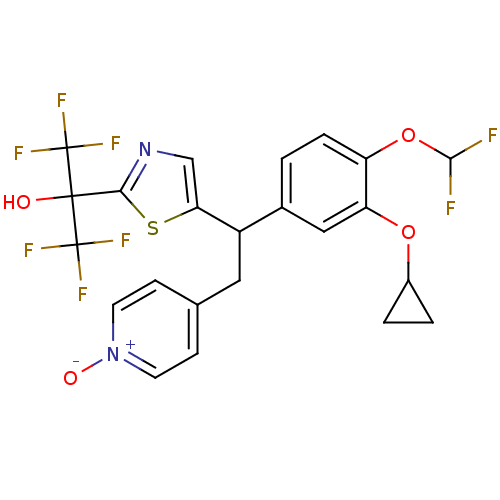
(CHEMBL2112296)Show SMILES OC(c1ncc(s1)C(Cc1cc[n+]([O-])cc1)c1ccc(OC(F)F)c(OC2CC2)c1)(C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C23H18F8N2O4S/c24-20(25)37-16-4-1-13(10-17(16)36-14-2-3-14)15(9-12-5-7-33(35)8-6-12)18-11-32-19(38-18)21(34,22(26,27)28)23(29,30)31/h1,4-8,10-11,14-15,20,34H,2-3,9H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
In vitro displacement of [35S]-MK- 499 from HEK 293 cells stably transfected with hERG voltage-gated potassium channel subunit Kv11.1 |
J Med Chem 46: 2413-26 (2003)
Article DOI: 10.1021/jm0204542
BindingDB Entry DOI: 10.7270/Q24X5756 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50409699
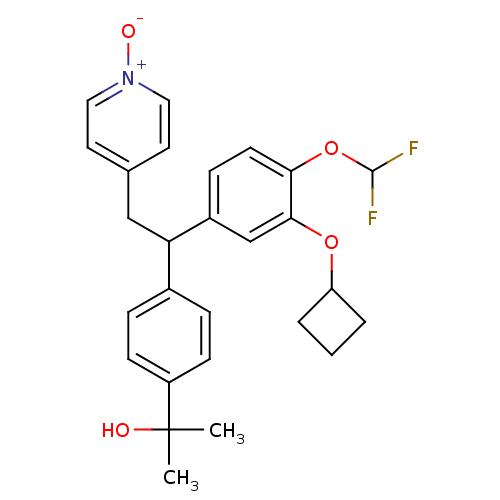
(CHEMBL2112294)Show SMILES CC(C)(O)c1ccc(cc1)C(Cc1cc[n+]([O-])cc1)c1ccc(OC(F)F)c(OC2CCC2)c1 Show InChI InChI=1S/C27H29F2NO4/c1-27(2,31)21-9-6-19(7-10-21)23(16-18-12-14-30(32)15-13-18)20-8-11-24(34-26(28)29)25(17-20)33-22-4-3-5-22/h6-15,17,22-23,26,31H,3-5,16H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.24E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
In vitro displacement of [35S]-MK- 499 from HEK 293 cells stably transfected with hERG voltage-gated potassium channel subunit Kv11.1 |
J Med Chem 46: 2413-26 (2003)
Article DOI: 10.1021/jm0204542
BindingDB Entry DOI: 10.7270/Q24X5756 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50128424

(2-{5-[1-(3,4-Bis-difluoromethoxy-phenyl)-2-(1-oxy-...)Show SMILES CC(C)(O)c1ccc(cn1)C(Cc1cc[n+]([O-])cc1)c1ccc(OC(F)F)c(OC(F)F)c1 Show InChI InChI=1S/C23H22F4N2O4/c1-23(2,30)20-6-4-16(13-28-20)17(11-14-7-9-29(31)10-8-14)15-3-5-18(32-21(24)25)19(12-15)33-22(26)27/h3-10,12-13,17,21-22,30H,11H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.27E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
In vitro displacement of [35S]-MK- 499 from HEK 293 cells stably transfected with hERG voltage-gated potassium channel subunit Kv11.1 |
J Med Chem 46: 2413-26 (2003)
Article DOI: 10.1021/jm0204542
BindingDB Entry DOI: 10.7270/Q24X5756 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50128424

(2-{5-[1-(3,4-Bis-difluoromethoxy-phenyl)-2-(1-oxy-...)Show SMILES CC(C)(O)c1ccc(cn1)C(Cc1cc[n+]([O-])cc1)c1ccc(OC(F)F)c(OC(F)F)c1 Show InChI InChI=1S/C23H22F4N2O4/c1-23(2,30)20-6-4-16(13-28-20)17(11-14-7-9-29(31)10-8-14)15-3-5-18(32-21(24)25)19(12-15)33-22(26)27/h3-10,12-13,17,21-22,30H,11H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.27E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
In vitro displacement of [35S]-MK- 499 from HEK 293 cells stably transfected with hERG voltage-gated potassium channel subunit Kv11.1 |
J Med Chem 46: 2413-26 (2003)
Article DOI: 10.1021/jm0204542
BindingDB Entry DOI: 10.7270/Q24X5756 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50128686

(2-{5-[1-(3-Cyclobutoxy-4-difluoromethoxy-phenyl)-2...)Show SMILES CC(C)(O)c1ccc(cn1)C(Cc1cc[n+]([O-])cc1)c1ccc(OC(F)F)c(OC2CCC2)c1 Show InChI InChI=1S/C26H28F2N2O4/c1-26(2,31)24-9-7-19(16-29-24)21(14-17-10-12-30(32)13-11-17)18-6-8-22(34-25(27)28)23(15-18)33-20-4-3-5-20/h6-13,15-16,20-21,25,31H,3-5,14H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
In vitro displacement of [35S]-MK- 499 from HEK 293 cells stably transfected with hERG voltage-gated potassium channel subunit Kv11.1 |
J Med Chem 46: 2413-26 (2003)
Article DOI: 10.1021/jm0204542
BindingDB Entry DOI: 10.7270/Q24X5756 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50128689
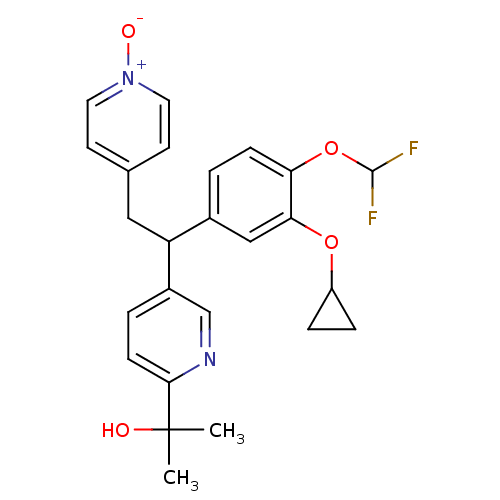
(2-{5-[1-(3-Cyclopropoxy-4-difluoromethoxy-phenyl)-...)Show SMILES CC(C)(O)c1ccc(cn1)C(Cc1cc[n+]([O-])cc1)c1ccc(OC(F)F)c(OC2CC2)c1 Show InChI InChI=1S/C25H26F2N2O4/c1-25(2,30)23-8-4-18(15-28-23)20(13-16-9-11-29(31)12-10-16)17-3-7-21(33-24(26)27)22(14-17)32-19-5-6-19/h3-4,7-12,14-15,19-20,24,30H,5-6,13H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.64E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
In vitro displacement of [35S]-MK- 499 from HEK 293 cells stably transfected with hERG voltage-gated potassium channel subunit Kv11.1 |
J Med Chem 46: 2413-26 (2003)
Article DOI: 10.1021/jm0204542
BindingDB Entry DOI: 10.7270/Q24X5756 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50128689

(2-{5-[1-(3-Cyclopropoxy-4-difluoromethoxy-phenyl)-...)Show SMILES CC(C)(O)c1ccc(cn1)C(Cc1cc[n+]([O-])cc1)c1ccc(OC(F)F)c(OC2CC2)c1 Show InChI InChI=1S/C25H26F2N2O4/c1-25(2,30)23-8-4-18(15-28-23)20(13-16-9-11-29(31)12-10-16)17-3-7-21(33-24(26)27)22(14-17)32-19-5-6-19/h3-4,7-12,14-15,19-20,24,30H,5-6,13H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.64E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
In vitro displacement of [35S]-MK- 499 from HEK 293 cells stably transfected with hERG voltage-gated potassium channel subunit Kv11.1 |
J Med Chem 46: 2413-26 (2003)
Article DOI: 10.1021/jm0204542
BindingDB Entry DOI: 10.7270/Q24X5756 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50409700
(CHEMBL2112295)Show SMILES OC(c1ncc(s1)C(Cc1cc[n+]([O-])cc1)c1ccc(OC(F)F)c(OC2CCC2)c1)(C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C24H20F8N2O4S/c25-21(26)38-17-5-4-14(11-18(17)37-15-2-1-3-15)16(10-13-6-8-34(36)9-7-13)19-12-33-20(39-19)22(35,23(27,28)29)24(30,31)32/h4-9,11-12,15-16,21,35H,1-3,10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.81E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
In vitro displacement of [35S]-MK- 499 from HEK 293 cells stably transfected with hERG voltage-gated potassium channel subunit Kv11.1 |
J Med Chem 46: 2413-26 (2003)
Article DOI: 10.1021/jm0204542
BindingDB Entry DOI: 10.7270/Q24X5756 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50409701

(CHEMBL383762)Show SMILES OC(c1ncc(s1)[C@H](Cc1ccc[n+]([O-])c1)c1ccc(OC(F)F)c(OC2CC2)c1)(C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C23H18F8N2O4S/c24-20(25)37-16-6-3-13(9-17(16)36-14-4-5-14)15(8-12-2-1-7-33(35)11-12)18-10-32-19(38-18)21(34,22(26,27)28)23(29,30)31/h1-3,6-7,9-11,14-15,20,34H,4-5,8H2/t15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
In vitro displacement of [35S]-MK- 499 from HEK 293 cells stably transfected with hERG voltage-gated potassium channel subunit Kv11.1 |
J Med Chem 46: 2413-26 (2003)
Article DOI: 10.1021/jm0204542
BindingDB Entry DOI: 10.7270/Q24X5756 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50128683
(2-{5-[1-(3-Cyclopropoxy-4-difluoromethoxy-phenyl)-...)Show SMILES CC(C)(O)c1ccc(cn1)C(Cc1ccc[n+]([O-])c1)c1ccc(OC(F)F)c(OC2CC2)c1 Show InChI InChI=1S/C25H26F2N2O4/c1-25(2,30)23-10-6-18(14-28-23)20(12-16-4-3-11-29(31)15-16)17-5-9-21(33-24(26)27)22(13-17)32-19-7-8-19/h3-6,9-11,13-15,19-20,24,30H,7-8,12H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.22E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
In vitro displacement of [35S]-MK- 499 from HEK 293 cells stably transfected with hERG voltage-gated potassium channel subunit Kv11.1 |
J Med Chem 46: 2413-26 (2003)
Article DOI: 10.1021/jm0204542
BindingDB Entry DOI: 10.7270/Q24X5756 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50128683

(2-{5-[1-(3-Cyclopropoxy-4-difluoromethoxy-phenyl)-...)Show SMILES CC(C)(O)c1ccc(cn1)C(Cc1ccc[n+]([O-])c1)c1ccc(OC(F)F)c(OC2CC2)c1 Show InChI InChI=1S/C25H26F2N2O4/c1-25(2,30)23-10-6-18(14-28-23)20(12-16-4-3-11-29(31)15-16)17-5-9-21(33-24(26)27)22(13-17)32-19-7-8-19/h3-6,9-11,13-15,19-20,24,30H,7-8,12H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.47E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
In vitro displacement of [35S]-MK- 499 from HEK 293 cells stably transfected with hERG voltage-gated potassium channel subunit Kv11.1 |
J Med Chem 46: 2413-26 (2003)
Article DOI: 10.1021/jm0204542
BindingDB Entry DOI: 10.7270/Q24X5756 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50128692
(5-{2-(3-Cyclopropoxy-4-difluoromethoxy-phenyl)-2-[...)Show SMILES CC(C)(O)c1ccc(cn1)C(Cc1ccc(=O)[nH]c1)c1ccc(OC(F)F)c(OC2CC2)c1 Show InChI InChI=1S/C25H26F2N2O4/c1-25(2,31)22-9-5-17(14-28-22)19(11-15-3-10-23(30)29-13-15)16-4-8-20(33-24(26)27)21(12-16)32-18-6-7-18/h3-5,8-10,12-14,18-19,24,31H,6-7,11H2,1-2H3,(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.59E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
In vitro displacement of [35S]-MK- 499 from HEK 293 cells stably transfected with hERG voltage-gated potassium channel subunit Kv11.1 |
J Med Chem 46: 2413-26 (2003)
Article DOI: 10.1021/jm0204542
BindingDB Entry DOI: 10.7270/Q24X5756 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50128685

(2-{5-[1-(3-Cyclobutoxy-4-difluoromethoxy-phenyl)-2...)Show SMILES CC(C)(O)c1ncc(s1)C(Cc1cc[n+]([O-])cc1)c1ccc(OC(F)F)c(OC2CCC2)c1 Show InChI InChI=1S/C24H26F2N2O4S/c1-24(2,29)22-27-14-21(33-22)18(12-15-8-10-28(30)11-9-15)16-6-7-19(32-23(25)26)20(13-16)31-17-4-3-5-17/h6-11,13-14,17-18,23,29H,3-5,12H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.84E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
In vitro displacement of [35S]-MK- 499 from HEK 293 cells stably transfected with hERG voltage-gated potassium channel subunit Kv11.1 |
J Med Chem 46: 2413-26 (2003)
Article DOI: 10.1021/jm0204542
BindingDB Entry DOI: 10.7270/Q24X5756 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50174020
((S)-(+)-3-{2-[(3-Cyclopropyloxy-4-difluromethoxy)-...)Show SMILES OC(c1ncc(s1)[C@@H](Cc1ccc[n+]([O-])c1)c1ccc(OC(F)F)c(OC2CC2)c1)(C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C23H18F8N2O4S/c24-20(25)37-16-6-3-13(9-17(16)36-14-4-5-14)15(8-12-2-1-7-33(35)11-12)18-10-32-19(38-18)21(34,22(26,27)28)23(29,30)31/h1-3,6-7,9-11,14-15,20,34H,4-5,8H2/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.14E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
In vitro displacement of [35S]-MK- 499 from HEK 293 cells stably transfected with hERG voltage-gated potassium channel subunit Kv11.1 |
J Med Chem 46: 2413-26 (2003)
Article DOI: 10.1021/jm0204542
BindingDB Entry DOI: 10.7270/Q24X5756 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(Homo sapiens (Human)) | BDBM50128685

(2-{5-[1-(3-Cyclobutoxy-4-difluoromethoxy-phenyl)-2...)Show SMILES CC(C)(O)c1ncc(s1)C(Cc1cc[n+]([O-])cc1)c1ccc(OC(F)F)c(OC2CCC2)c1 Show InChI InChI=1S/C24H26F2N2O4S/c1-24(2,29)22-27-14-21(33-22)18(12-15-8-10-28(30)11-9-15)16-6-7-19(32-23(25)26)20(13-16)31-17-4-3-5-17/h6-11,13-14,17-18,23,29H,3-5,12H2,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity was determined against human Phosphodiesterase 4 isoform using a construct representing the common region of spliced var... |
J Med Chem 46: 2413-26 (2003)
Article DOI: 10.1021/jm0204542
BindingDB Entry DOI: 10.7270/Q24X5756 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(Homo sapiens (Human)) | BDBM50128692

(5-{2-(3-Cyclopropoxy-4-difluoromethoxy-phenyl)-2-[...)Show SMILES CC(C)(O)c1ccc(cn1)C(Cc1ccc(=O)[nH]c1)c1ccc(OC(F)F)c(OC2CC2)c1 Show InChI InChI=1S/C25H26F2N2O4/c1-25(2,31)22-9-5-17(14-28-22)19(11-15-3-10-23(30)29-13-15)16-4-8-20(33-24(26)27)21(12-16)32-18-6-7-18/h3-5,8-10,12-14,18-19,24,31H,6-7,11H2,1-2H3,(H,29,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity was determined against human Phosphodiesterase 4 isoform using a construct representing the common region of spliced var... |
J Med Chem 46: 2413-26 (2003)
Article DOI: 10.1021/jm0204542
BindingDB Entry DOI: 10.7270/Q24X5756 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(Homo sapiens (Human)) | BDBM50128686

(2-{5-[1-(3-Cyclobutoxy-4-difluoromethoxy-phenyl)-2...)Show SMILES CC(C)(O)c1ccc(cn1)C(Cc1cc[n+]([O-])cc1)c1ccc(OC(F)F)c(OC2CCC2)c1 Show InChI InChI=1S/C26H28F2N2O4/c1-26(2,31)24-9-7-19(16-29-24)21(14-17-10-12-30(32)13-11-17)18-6-8-22(34-25(27)28)23(15-18)33-20-4-3-5-20/h6-13,15-16,20-21,25,31H,3-5,14H2,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity was determined against human Phosphodiesterase 4 isoform using a construct representing the common region of spliced var... |
J Med Chem 46: 2413-26 (2003)
Article DOI: 10.1021/jm0204542
BindingDB Entry DOI: 10.7270/Q24X5756 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(Homo sapiens (Human)) | BDBM50128419

(1-{5-[1-(3,4-Bis-difluoromethoxy-phenyl)-2-(1-oxy-...)Show SMILES CC(O)(c1ccccc1)c1ccc(cn1)C(Cc1cc[n+]([O-])cc1)c1ccc(OC(F)F)c(OC(F)F)c1 Show InChI InChI=1S/C28H24F4N2O4/c1-28(35,21-5-3-2-4-6-21)25-10-8-20(17-33-25)22(15-18-11-13-34(36)14-12-18)19-7-9-23(37-26(29)30)24(16-19)38-27(31)32/h2-14,16-17,22,26-27,35H,15H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity was determined against human Phosphodiesterase 4 isoform using a construct representing the common region of spliced var... |
J Med Chem 46: 2413-26 (2003)
Article DOI: 10.1021/jm0204542
BindingDB Entry DOI: 10.7270/Q24X5756 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(Homo sapiens (Human)) | BDBM50128683

(2-{5-[1-(3-Cyclopropoxy-4-difluoromethoxy-phenyl)-...)Show SMILES CC(C)(O)c1ccc(cn1)C(Cc1ccc[n+]([O-])c1)c1ccc(OC(F)F)c(OC2CC2)c1 Show InChI InChI=1S/C25H26F2N2O4/c1-25(2,30)23-10-6-18(14-28-23)20(12-16-4-3-11-29(31)15-16)17-5-9-21(33-24(26)27)22(13-17)32-19-7-8-19/h3-6,9-11,13-15,19-20,24,30H,7-8,12H2,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity was determined against human Phosphodiesterase 4 isoform using a construct representing the common region of spliced var... |
J Med Chem 46: 2413-26 (2003)
Article DOI: 10.1021/jm0204542
BindingDB Entry DOI: 10.7270/Q24X5756 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(Homo sapiens (Human)) | BDBM50128683

(2-{5-[1-(3-Cyclopropoxy-4-difluoromethoxy-phenyl)-...)Show SMILES CC(C)(O)c1ccc(cn1)C(Cc1ccc[n+]([O-])c1)c1ccc(OC(F)F)c(OC2CC2)c1 Show InChI InChI=1S/C25H26F2N2O4/c1-25(2,30)23-10-6-18(14-28-23)20(12-16-4-3-11-29(31)15-16)17-5-9-21(33-24(26)27)22(13-17)32-19-7-8-19/h3-6,9-11,13-15,19-20,24,30H,7-8,12H2,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity was determined against human Phosphodiesterase 4 isoform using a construct representing the common region of spliced var... |
J Med Chem 46: 2413-26 (2003)
Article DOI: 10.1021/jm0204542
BindingDB Entry DOI: 10.7270/Q24X5756 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(Homo sapiens (Human)) | BDBM50064858
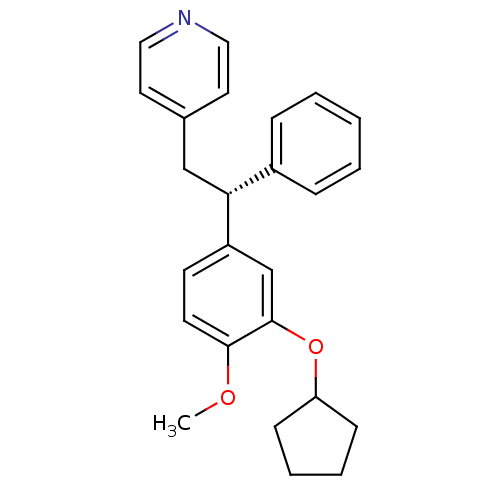
((R)-4-(2-(3-(cyclopentyloxy)-4-methoxyphenyl)-2-ph...)Show SMILES COc1ccc(cc1OC1CCCC1)[C@H](Cc1ccncc1)c1ccccc1 |r| Show InChI InChI=1S/C25H27NO2/c1-27-24-12-11-21(18-25(24)28-22-9-5-6-10-22)23(20-7-3-2-4-8-20)17-19-13-15-26-16-14-19/h2-4,7-8,11-16,18,22-23H,5-6,9-10,17H2,1H3/t23-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity was determined against human Phosphodiesterase 4 isoform using a construct representing the common region of spliced var... |
J Med Chem 46: 2413-26 (2003)
Article DOI: 10.1021/jm0204542
BindingDB Entry DOI: 10.7270/Q24X5756 |
More data for this
Ligand-Target Pair | |
Beta-secretase 2
(Homo sapiens (Human)) | BDBM50314607

(2-amino-4-(3'-methoxybiphenyl-3-yl)-1-methyl-4-(py...)Show SMILES COc1cccc(c1)-c1cccc(c1)C1(N=C(N)N(C)C1=O)c1ccncc1 |t:17| Show InChI InChI=1S/C22H20N4O2/c1-26-20(27)22(25-21(26)23,17-9-11-24-12-10-17)18-7-3-5-15(13-18)16-6-4-8-19(14-16)28-2/h3-14H,1-2H3,(H2,23,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of BACE2 by FRET assay |
Bioorg Med Chem Lett 20: 2326-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.136
BindingDB Entry DOI: 10.7270/Q28C9WDF |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50314606
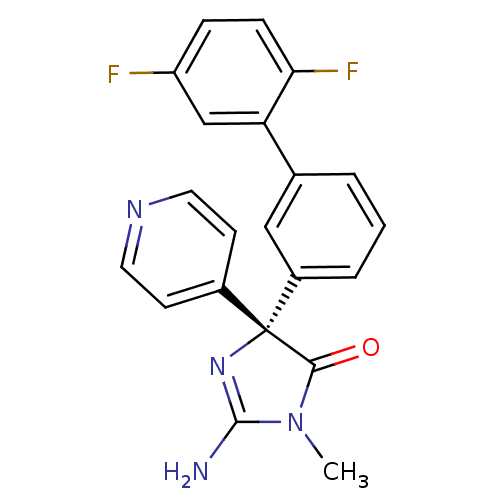
((5S)-2-amino-5-(2',5'-difluorobiphenyl-3-yl)-3-met...)Show SMILES CN1C(N)=N[C@](C1=O)(c1ccncc1)c1cccc(c1)-c1cc(F)ccc1F |r,c:3| Show InChI InChI=1S/C21H16F2N4O/c1-27-19(28)21(26-20(27)24,14-7-9-25-10-8-14)15-4-2-3-13(11-15)17-12-16(22)5-6-18(17)23/h2-12H,1H3,(H2,24,26)/t21-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 by FRET assay |
Bioorg Med Chem Lett 20: 2326-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.136
BindingDB Entry DOI: 10.7270/Q28C9WDF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50314603
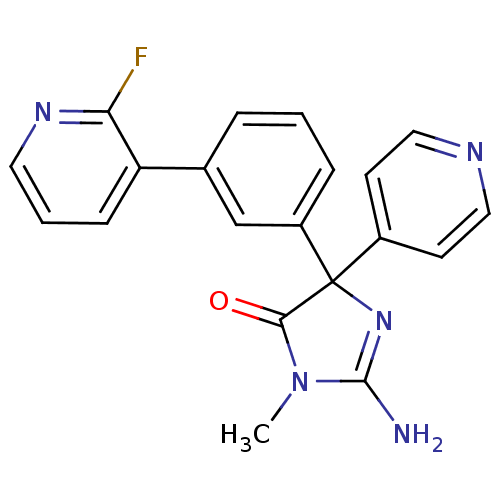
(2-amino-4-(3-(2-fluoropyridin-3-yl)phenyl)-1-methy...)Show SMILES CN1C(N)=NC(C1=O)(c1ccncc1)c1cccc(c1)-c1cccnc1F |c:3| Show InChI InChI=1S/C20H16FN5O/c1-26-18(27)20(25-19(26)22,14-7-10-23-11-8-14)15-5-2-4-13(12-15)16-6-3-9-24-17(16)21/h2-12H,1H3,(H2,22,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 by FRET assay |
Bioorg Med Chem Lett 20: 2326-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.136
BindingDB Entry DOI: 10.7270/Q28C9WDF |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50314607

(2-amino-4-(3'-methoxybiphenyl-3-yl)-1-methyl-4-(py...)Show SMILES COc1cccc(c1)-c1cccc(c1)C1(N=C(N)N(C)C1=O)c1ccncc1 |t:17| Show InChI InChI=1S/C22H20N4O2/c1-26-20(27)22(25-21(26)23,17-9-11-24-12-10-17)18-7-3-5-15(13-18)16-6-4-8-19(14-16)28-2/h3-14H,1-2H3,(H2,23,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 by FRET assay |
Bioorg Med Chem Lett 20: 2326-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.136
BindingDB Entry DOI: 10.7270/Q28C9WDF |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(Homo sapiens (Human)) | BDBM50128424

(2-{5-[1-(3,4-Bis-difluoromethoxy-phenyl)-2-(1-oxy-...)Show SMILES CC(C)(O)c1ccc(cn1)C(Cc1cc[n+]([O-])cc1)c1ccc(OC(F)F)c(OC(F)F)c1 Show InChI InChI=1S/C23H22F4N2O4/c1-23(2,30)20-6-4-16(13-28-20)17(11-14-7-9-29(31)10-8-14)15-3-5-18(32-21(24)25)19(12-15)33-22(26)27/h3-10,12-13,17,21-22,30H,11H2,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity was determined against human Phosphodiesterase 4 isoform using a construct representing the common region of spliced var... |
J Med Chem 46: 2413-26 (2003)
Article DOI: 10.1021/jm0204542
BindingDB Entry DOI: 10.7270/Q24X5756 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(Homo sapiens (Human)) | BDBM50128424

(2-{5-[1-(3,4-Bis-difluoromethoxy-phenyl)-2-(1-oxy-...)Show SMILES CC(C)(O)c1ccc(cn1)C(Cc1cc[n+]([O-])cc1)c1ccc(OC(F)F)c(OC(F)F)c1 Show InChI InChI=1S/C23H22F4N2O4/c1-23(2,30)20-6-4-16(13-28-20)17(11-14-7-9-29(31)10-8-14)15-3-5-18(32-21(24)25)19(12-15)33-22(26)27/h3-10,12-13,17,21-22,30H,11H2,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity was determined against human Phosphodiesterase 4 isoform using a construct representing the common region of spliced var... |
J Med Chem 46: 2413-26 (2003)
Article DOI: 10.1021/jm0204542
BindingDB Entry DOI: 10.7270/Q24X5756 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(Homo sapiens (Human)) | BDBM50128690
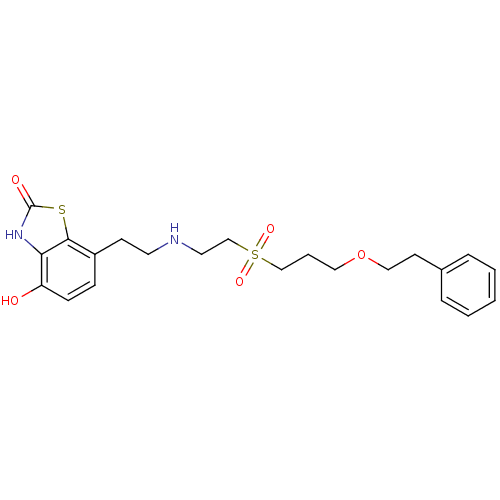
(4-Hydroxy-7-{2-[2-(3-phenethyloxy-propane-1-sulfon...)Show SMILES Oc1ccc(CCNCCS(=O)(=O)CCCOCCc2ccccc2)c2sc(=O)[nH]c12 Show InChI InChI=1S/C22H28N2O5S2/c25-19-8-7-18(21-20(19)24-22(26)30-21)9-11-23-12-16-31(27,28)15-4-13-29-14-10-17-5-2-1-3-6-17/h1-3,5-8,23,25H,4,9-16H2,(H,24,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity was determined against human Phosphodiesterase 4 isoform using a construct representing the common region of spliced var... |
J Med Chem 46: 2413-26 (2003)
Article DOI: 10.1021/jm0204542
BindingDB Entry DOI: 10.7270/Q24X5756 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50314611
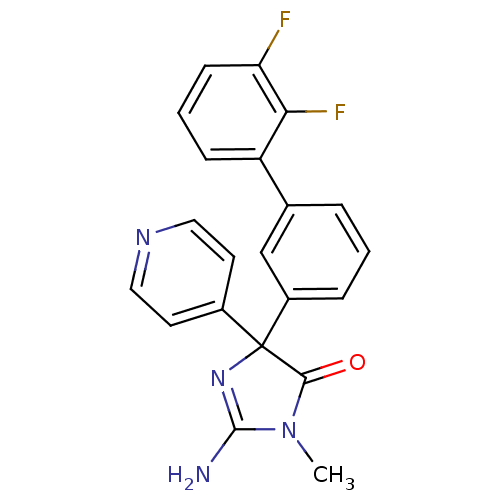
(2-amino-4-(2',3'-difluorobiphenyl-3-yl)-1-methyl-4...)Show SMILES CN1C(N)=NC(C1=O)(c1ccncc1)c1cccc(c1)-c1cccc(F)c1F |c:3| Show InChI InChI=1S/C21H16F2N4O/c1-27-19(28)21(26-20(27)24,14-8-10-25-11-9-14)15-5-2-4-13(12-15)16-6-3-7-17(22)18(16)23/h2-12H,1H3,(H2,24,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 by FRET assay |
Bioorg Med Chem Lett 20: 2326-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.136
BindingDB Entry DOI: 10.7270/Q28C9WDF |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50314609
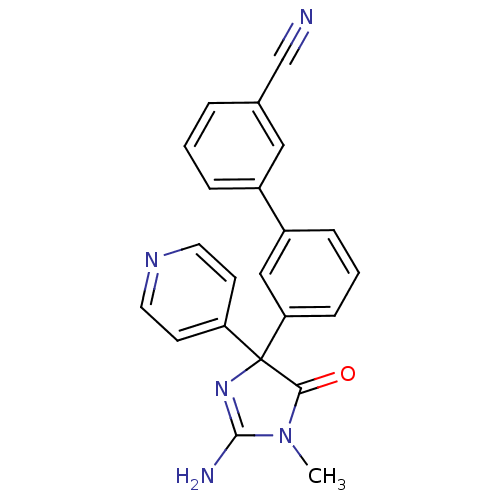
(3'-(2-amino-1-methyl-5-oxo-4-(pyridin-4-yl)-4,5-di...)Show SMILES CN1C(N)=NC(C1=O)(c1ccncc1)c1cccc(c1)-c1cccc(c1)C#N |c:3| Show InChI InChI=1S/C22H17N5O/c1-27-20(28)22(26-21(27)24,18-8-10-25-11-9-18)19-7-3-6-17(13-19)16-5-2-4-15(12-16)14-23/h2-13H,1H3,(H2,24,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 by FRET assay |
Bioorg Med Chem Lett 20: 2326-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.136
BindingDB Entry DOI: 10.7270/Q28C9WDF |
More data for this
Ligand-Target Pair | |
Beta-secretase 2
(Homo sapiens (Human)) | BDBM50314609

(3'-(2-amino-1-methyl-5-oxo-4-(pyridin-4-yl)-4,5-di...)Show SMILES CN1C(N)=NC(C1=O)(c1ccncc1)c1cccc(c1)-c1cccc(c1)C#N |c:3| Show InChI InChI=1S/C22H17N5O/c1-27-20(28)22(26-21(27)24,18-8-10-25-11-9-18)19-7-3-6-17(13-19)16-5-2-4-15(12-16)14-23/h2-13H,1H3,(H2,24,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of BACE2 by FRET assay |
Bioorg Med Chem Lett 20: 2326-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.136
BindingDB Entry DOI: 10.7270/Q28C9WDF |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50314604

((+/-)-2-amino-4-(2',5'-difluorobiphenyl-3-yl)-1-me...)Show SMILES CN1C(N)=NC(C1=O)(c1ccncc1)c1cccc(c1)-c1cc(F)ccc1F |c:3| Show InChI InChI=1S/C21H16F2N4O/c1-27-19(28)21(26-20(27)24,14-7-9-25-10-8-14)15-4-2-3-13(11-15)17-12-16(22)5-6-18(17)23/h2-12H,1H3,(H2,24,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 by FRET assay |
Bioorg Med Chem Lett 20: 2326-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.136
BindingDB Entry DOI: 10.7270/Q28C9WDF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50314610
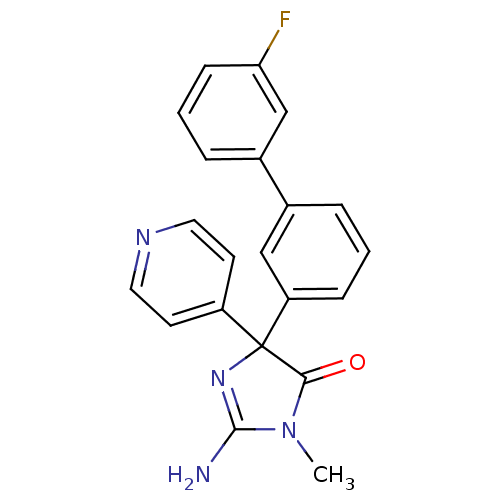
(2-amino-4-(3'-fluorobiphenyl-3-yl)-1-methyl-4-(pyr...)Show SMILES CN1C(N)=NC(C1=O)(c1ccncc1)c1cccc(c1)-c1cccc(F)c1 |c:3| Show InChI InChI=1S/C21H17FN4O/c1-26-19(27)21(25-20(26)23,16-8-10-24-11-9-16)17-6-2-4-14(12-17)15-5-3-7-18(22)13-15/h2-13H,1H3,(H2,23,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 by FRET assay |
Bioorg Med Chem Lett 20: 2326-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.136
BindingDB Entry DOI: 10.7270/Q28C9WDF |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(Homo sapiens (Human)) | BDBM50128689

(2-{5-[1-(3-Cyclopropoxy-4-difluoromethoxy-phenyl)-...)Show SMILES CC(C)(O)c1ccc(cn1)C(Cc1cc[n+]([O-])cc1)c1ccc(OC(F)F)c(OC2CC2)c1 Show InChI InChI=1S/C25H26F2N2O4/c1-25(2,30)23-8-4-18(15-28-23)20(13-16-9-11-29(31)12-10-16)17-3-7-21(33-24(26)27)22(14-17)32-19-5-6-19/h3-4,7-12,14-15,19-20,24,30H,5-6,13H2,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity was determined against human Phosphodiesterase 4 isoform using a construct representing the common region of spliced var... |
J Med Chem 46: 2413-26 (2003)
Article DOI: 10.1021/jm0204542
BindingDB Entry DOI: 10.7270/Q24X5756 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(Homo sapiens (Human)) | BDBM50128689

(2-{5-[1-(3-Cyclopropoxy-4-difluoromethoxy-phenyl)-...)Show SMILES CC(C)(O)c1ccc(cn1)C(Cc1cc[n+]([O-])cc1)c1ccc(OC(F)F)c(OC2CC2)c1 Show InChI InChI=1S/C25H26F2N2O4/c1-25(2,30)23-8-4-18(15-28-23)20(13-16-9-11-29(31)12-10-16)17-3-7-21(33-24(26)27)22(14-17)32-19-5-6-19/h3-4,7-12,14-15,19-20,24,30H,5-6,13H2,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity was determined against human Phosphodiesterase 4 isoform using a construct representing the common region of spliced var... |
J Med Chem 46: 2413-26 (2003)
Article DOI: 10.1021/jm0204542
BindingDB Entry DOI: 10.7270/Q24X5756 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50310155
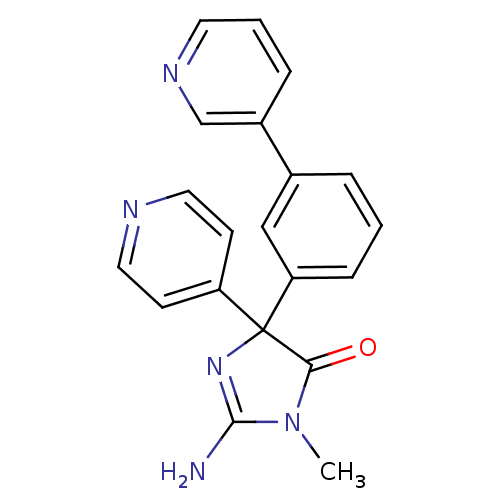
(2-amino-1-methyl-4-(3-(pyridin-3-yl)phenyl)-4-(pyr...)Show SMILES CN1C(N)=NC(C1=O)(c1ccncc1)c1cccc(c1)-c1cccnc1 |c:3| Show InChI InChI=1S/C20H17N5O/c1-25-18(26)20(24-19(25)21,16-7-10-22-11-8-16)17-6-2-4-14(12-17)15-5-3-9-23-13-15/h2-13H,1H3,(H2,21,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 by FRET assay |
Bioorg Med Chem Lett 20: 2326-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.136
BindingDB Entry DOI: 10.7270/Q28C9WDF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50314600
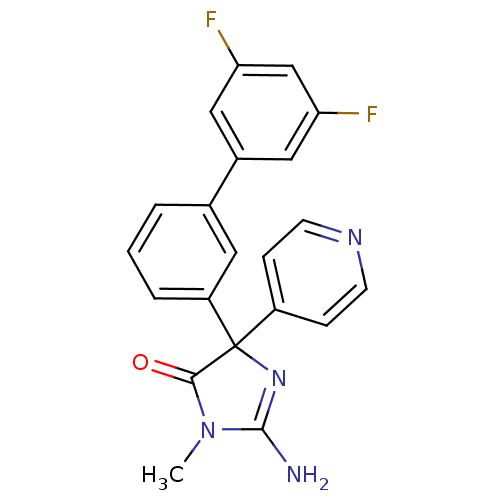
(2-amino-4-(3',5'-difluorobiphenyl-3-yl)-1-methyl-4...)Show SMILES CN1C(N)=NC(C1=O)(c1ccncc1)c1cccc(c1)-c1cc(F)cc(F)c1 |c:3| Show InChI InChI=1S/C21H16F2N4O/c1-27-19(28)21(26-20(27)24,15-5-7-25-8-6-15)16-4-2-3-13(9-16)14-10-17(22)12-18(23)11-14/h2-12H,1H3,(H2,24,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 by FRET assay |
Bioorg Med Chem Lett 20: 2326-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.136
BindingDB Entry DOI: 10.7270/Q28C9WDF |
More data for this
Ligand-Target Pair | |
Beta-secretase 2
(Homo sapiens (Human)) | BDBM50314600

(2-amino-4-(3',5'-difluorobiphenyl-3-yl)-1-methyl-4...)Show SMILES CN1C(N)=NC(C1=O)(c1ccncc1)c1cccc(c1)-c1cc(F)cc(F)c1 |c:3| Show InChI InChI=1S/C21H16F2N4O/c1-27-19(28)21(26-20(27)24,15-5-7-25-8-6-15)16-4-2-3-13(9-16)14-10-17(22)12-18(23)11-14/h2-12H,1H3,(H2,24,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of BACE2 by FRET assay |
Bioorg Med Chem Lett 20: 2326-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.136
BindingDB Entry DOI: 10.7270/Q28C9WDF |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50314612

(2-amino-1-methyl-4-(3-(pyrazin-2-yl)phenyl)-4-(pyr...)Show SMILES CN1C(N)=NC(C1=O)(c1ccncc1)c1cccc(c1)-c1cnccn1 |c:3| Show InChI InChI=1S/C19H16N6O/c1-25-17(26)19(24-18(25)20,14-5-7-21-8-6-14)15-4-2-3-13(11-15)16-12-22-9-10-23-16/h2-12H,1H3,(H2,20,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 by FRET assay |
Bioorg Med Chem Lett 20: 2326-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.136
BindingDB Entry DOI: 10.7270/Q28C9WDF |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50372257
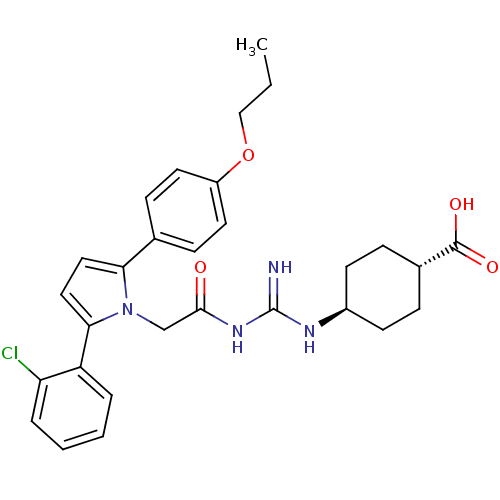
(CHEMBL271914)Show SMILES CCCOc1ccc(cc1)-c1ccc(-c2ccccc2Cl)n1CC(=O)NC(=N)N[C@H]1CC[C@@H](CC1)C(O)=O |wU:29.31,wD:32.38,(-10.39,1.86,;-10.37,.32,;-9.03,-.43,;-9.01,-1.97,;-7.56,-2.49,;-6.41,-1.46,;-4.95,-1.93,;-4.64,-3.44,;-5.77,-4.47,;-7.23,-4,;-3.17,-3.92,;-2.69,-5.38,;-1.15,-5.38,;-.68,-3.92,;.79,-3.44,;1.1,-1.94,;2.56,-1.46,;3.71,-2.49,;3.39,-4,;1.93,-4.47,;1.61,-5.98,;-1.92,-3.01,;-1.93,-1.47,;-.59,-.7,;.74,-1.47,;-.59,.84,;.74,1.61,;.74,3.15,;2.08,.84,;3.41,1.61,;3.4,3.14,;4.74,3.91,;6.08,3.13,;6.07,1.59,;4.74,.83,;7.41,3.9,;8.75,3.12,;7.42,5.44,)| Show InChI InChI=1S/C29H33ClN4O4/c1-2-17-38-22-13-9-19(10-14-22)25-15-16-26(23-5-3-4-6-24(23)30)34(25)18-27(35)33-29(31)32-21-11-7-20(8-12-21)28(36)37/h3-6,9-10,13-16,20-21H,2,7-8,11-12,17-18H2,1H3,(H,36,37)(H3,31,32,33,35)/t20-,21- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 |
Bioorg Med Chem Lett 18: 767-71 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.043
BindingDB Entry DOI: 10.7270/Q27S7PK8 |
More data for this
Ligand-Target Pair | |
Beta-secretase 2
(Homo sapiens (Human)) | BDBM50314610

(2-amino-4-(3'-fluorobiphenyl-3-yl)-1-methyl-4-(pyr...)Show SMILES CN1C(N)=NC(C1=O)(c1ccncc1)c1cccc(c1)-c1cccc(F)c1 |c:3| Show InChI InChI=1S/C21H17FN4O/c1-26-19(27)21(25-20(26)23,16-8-10-24-11-9-16)17-6-2-4-14(12-17)15-5-3-7-18(22)13-15/h2-13H,1H3,(H2,23,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of BACE2 by FRET assay |
Bioorg Med Chem Lett 20: 2326-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.136
BindingDB Entry DOI: 10.7270/Q28C9WDF |
More data for this
Ligand-Target Pair | |
Beta-secretase 2
(Homo sapiens (Human)) | BDBM50314606

((5S)-2-amino-5-(2',5'-difluorobiphenyl-3-yl)-3-met...)Show SMILES CN1C(N)=N[C@](C1=O)(c1ccncc1)c1cccc(c1)-c1cc(F)ccc1F |r,c:3| Show InChI InChI=1S/C21H16F2N4O/c1-27-19(28)21(26-20(27)24,14-7-9-25-10-8-14)15-4-2-3-13(11-15)17-12-16(22)5-6-18(17)23/h2-12H,1H3,(H2,24,26)/t21-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of BACE2 by FRET assay |
Bioorg Med Chem Lett 20: 2326-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.136
BindingDB Entry DOI: 10.7270/Q28C9WDF |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM16755
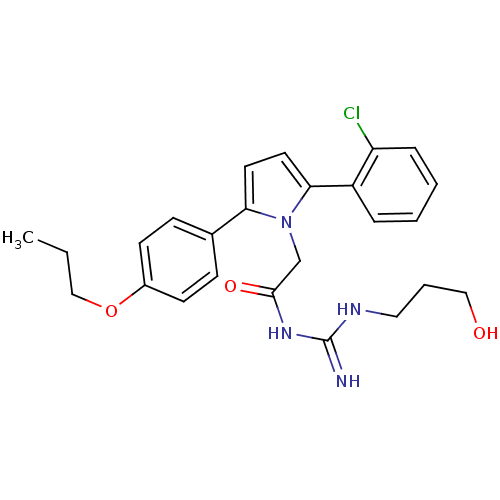
(Acylguanidine, 10d | N-[(1Z)-amino[(3-hydroxypropy...)Show SMILES CCCOc1ccc(cc1)-c1ccc(-c2ccccc2Cl)n1CC(=O)NC(=N)NCCCO Show InChI InChI=1S/C25H29ClN4O3/c1-2-16-33-19-10-8-18(9-11-19)22-12-13-23(20-6-3-4-7-21(20)26)30(22)17-24(32)29-25(27)28-14-5-15-31/h3-4,6-13,31H,2,5,14-17H2,1H3,(H3,27,28,29,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | 4.5 | 22 |
Wyeth Research
| Assay Description
A homogenous, continuous fluorescence resonance energy transfer (FRET) assay was used to assess compound inhibition of enzyme based on the cleavage o... |
J Med Chem 49: 6158-61 (2006)
Article DOI: 10.1021/jm0607451
BindingDB Entry DOI: 10.7270/Q2M043NG |
More data for this
Ligand-Target Pair | |
Beta-secretase 2
(Homo sapiens (Human)) | BDBM50314604

((+/-)-2-amino-4-(2',5'-difluorobiphenyl-3-yl)-1-me...)Show SMILES CN1C(N)=NC(C1=O)(c1ccncc1)c1cccc(c1)-c1cc(F)ccc1F |c:3| Show InChI InChI=1S/C21H16F2N4O/c1-27-19(28)21(26-20(27)24,14-7-9-25-10-8-14)15-4-2-3-13(11-15)17-12-16(22)5-6-18(17)23/h2-12H,1H3,(H2,24,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of BACE2 by FRET assay |
Bioorg Med Chem Lett 20: 2326-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.136
BindingDB Entry DOI: 10.7270/Q28C9WDF |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM16755

(Acylguanidine, 10d | N-[(1Z)-amino[(3-hydroxypropy...)Show SMILES CCCOc1ccc(cc1)-c1ccc(-c2ccccc2Cl)n1CC(=O)NC(=N)NCCCO Show InChI InChI=1S/C25H29ClN4O3/c1-2-16-33-19-10-8-18(9-11-19)22-12-13-23(20-6-3-4-7-21(20)26)30(22)17-24(32)29-25(27)28-14-5-15-31/h3-4,6-13,31H,2,5,14-17H2,1H3,(H3,27,28,29,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 121 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 |
Bioorg Med Chem Lett 18: 767-71 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.043
BindingDB Entry DOI: 10.7270/Q27S7PK8 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50372264
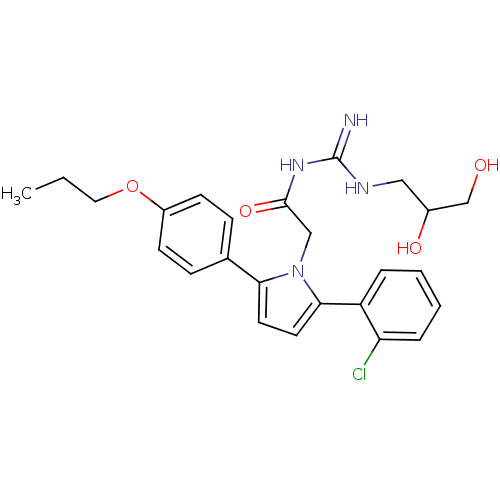
(CHEMBL430034)Show SMILES CCCOc1ccc(cc1)-c1ccc(-c2ccccc2Cl)n1CC(=O)NC(=N)NCC(O)CO Show InChI InChI=1S/C25H29ClN4O4/c1-2-13-34-19-9-7-17(8-10-19)22-11-12-23(20-5-3-4-6-21(20)26)30(22)15-24(33)29-25(27)28-14-18(32)16-31/h3-12,18,31-32H,2,13-16H2,1H3,(H3,27,28,29,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 124 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 |
Bioorg Med Chem Lett 18: 767-71 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.043
BindingDB Entry DOI: 10.7270/Q27S7PK8 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50372267
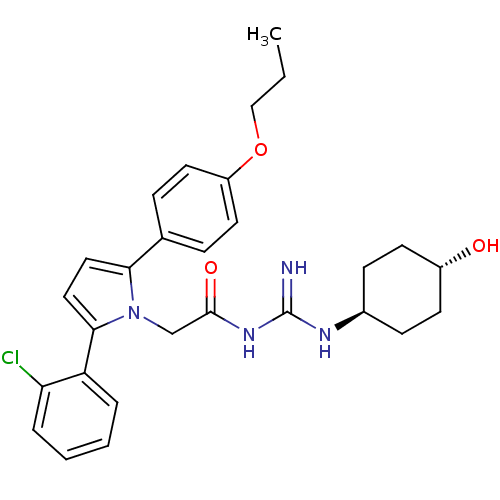
(CHEMBL428075)Show SMILES CCCOc1ccc(cc1)-c1ccc(-c2ccccc2Cl)n1CC(=O)NC(=N)N[C@H]1CC[C@H](O)CC1 |wU:29.31,wD:32.35,(11.88,2.57,;11.9,1.03,;13.24,.28,;13.26,-1.26,;14.71,-1.78,;15.86,-.75,;17.32,-1.22,;17.63,-2.73,;16.5,-3.76,;15.04,-3.29,;19.1,-3.21,;19.58,-4.67,;21.12,-4.67,;21.59,-3.21,;23.06,-2.73,;23.37,-1.23,;24.83,-.75,;25.98,-1.78,;25.66,-3.29,;24.2,-3.76,;23.88,-5.27,;20.35,-2.3,;20.35,-.76,;21.68,.01,;23.01,-.76,;21.68,1.55,;23.01,2.32,;23.01,3.86,;24.35,1.55,;25.68,2.32,;25.67,3.85,;27.02,4.62,;28.35,3.85,;29.69,4.61,;28.34,2.3,;27.01,1.54,)| Show InChI InChI=1S/C28H33ClN4O3/c1-2-17-36-22-13-7-19(8-14-22)25-15-16-26(23-5-3-4-6-24(23)29)33(25)18-27(35)32-28(30)31-20-9-11-21(34)12-10-20/h3-8,13-16,20-21,34H,2,9-12,17-18H2,1H3,(H3,30,31,32,35)/t20-,21- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 125 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 |
Bioorg Med Chem Lett 18: 767-71 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.043
BindingDB Entry DOI: 10.7270/Q27S7PK8 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50310149
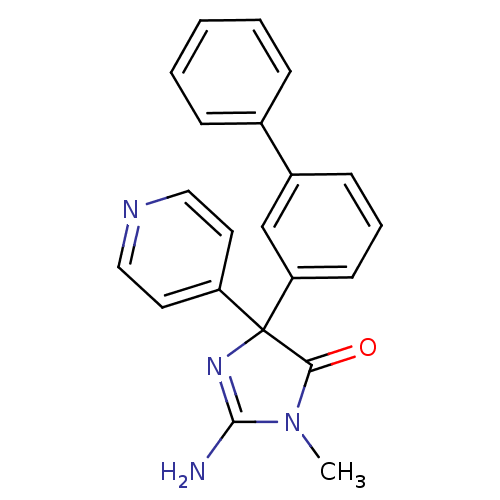
(2-amino-4-(biphenyl-3-yl)-1-methyl-4-(pyridin-4-yl...)Show SMILES CN1C(N)=NC(C1=O)(c1ccncc1)c1cccc(c1)-c1ccccc1 |c:3| Show InChI InChI=1S/C21H18N4O/c1-25-19(26)21(24-20(25)22,17-10-12-23-13-11-17)18-9-5-8-16(14-18)15-6-3-2-4-7-15/h2-14H,1H3,(H2,22,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 by FRET assay |
Bioorg Med Chem Lett 20: 2326-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.136
BindingDB Entry DOI: 10.7270/Q28C9WDF |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50372266
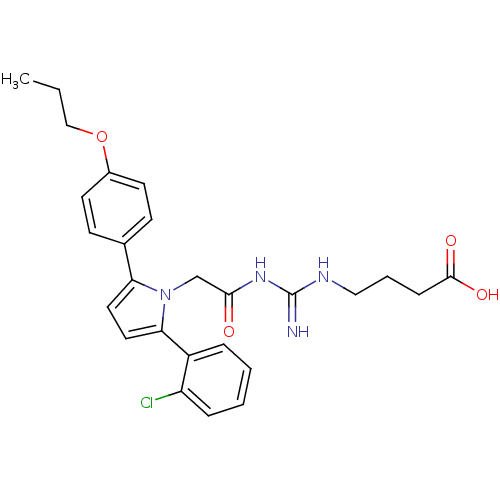
(CHEMBL273186)Show SMILES CCCOc1ccc(cc1)-c1ccc(-c2ccccc2Cl)n1CC(=O)NC(=N)NCCCC(O)=O Show InChI InChI=1S/C26H29ClN4O4/c1-2-16-35-19-11-9-18(10-12-19)22-13-14-23(20-6-3-4-7-21(20)27)31(22)17-24(32)30-26(28)29-15-5-8-25(33)34/h3-4,6-7,9-14H,2,5,8,15-17H2,1H3,(H,33,34)(H3,28,29,30,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 |
Bioorg Med Chem Lett 18: 767-71 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.043
BindingDB Entry DOI: 10.7270/Q27S7PK8 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data