

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cannabinoid receptor 2 (Homo sapiens (Human)) | CHEMBL5266788 | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL CHEMBL PC cid PC sid UniChem Similars | 0.0750 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description In vitro antagonism of the 5-HT-3 receptor determined by inhibition of 5-HT-induced depolarization of the isolated rat vagus nerve. | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50094037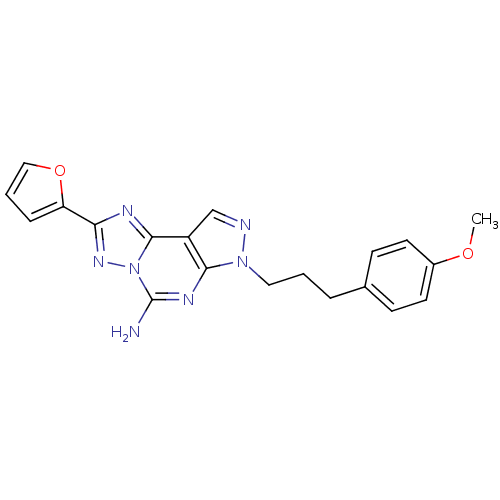 (2-Furan-2-yl-7-[3-(4-methoxy-phenyl)-propyl]-7H-py...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human adenosine A2A receptor expressed in Flp-In-CHO cell membrane assessed as inhibition of NECA-induced cAMP accumulation in... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02169 BindingDB Entry DOI: 10.7270/Q23200R3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50369584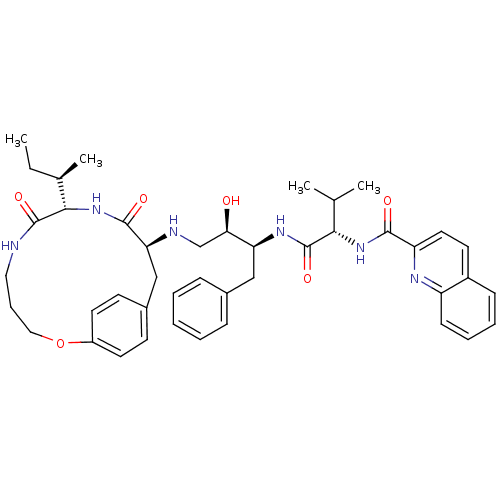 (CHEMBL1790230) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Tested for inhibition of synthetic HIV-1 protease by using fluorometric assay | J Med Chem 47: 1641-51 (2004) Article DOI: 10.1021/jm030337m BindingDB Entry DOI: 10.7270/Q2JD4XJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50369584 (CHEMBL1790230) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibitory constant against HIV-1 protease | J Med Chem 43: 3495-504 (2000) BindingDB Entry DOI: 10.7270/Q2KH0P1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [491-589,Q7K,L33I,C67B,C95B] (Human immunodeficiency virus type 1) | BDBM798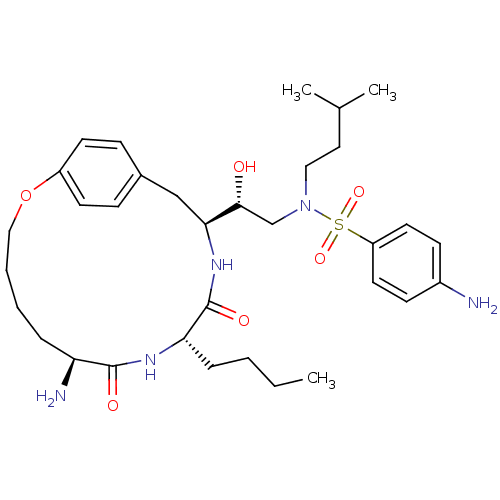 ((2R)-2-[(7S,10S,13S)-7-amino-10-butyl-8,11-dioxo-2...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.310 | -56.5 | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
University of Queensland | Assay Description Inhibition constants were determined by a fluorometric assay. Ki values were calculated from either Dixon plots or Henderson plots in cases where the... | J Med Chem 45: 371-81 (2002) Article DOI: 10.1021/jm010414i BindingDB Entry DOI: 10.7270/Q2FQ9TSJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [491-589,Q7K,L33I,C67B,C95B] (Human immunodeficiency virus type 1) | BDBM797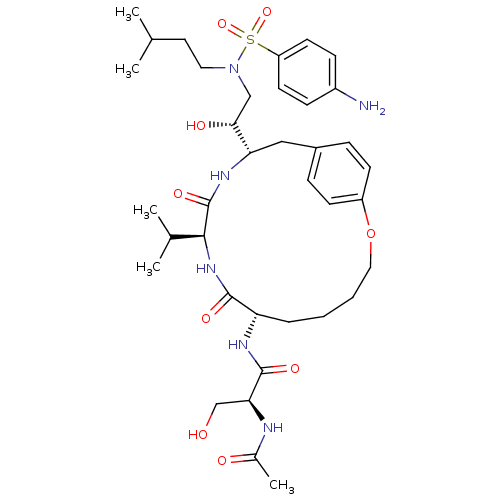 ((2S)-N-[(7S,10S,13S)-13-[(1R)-2-[(4-aminobenzene)(...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.340 | -56.2 | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
University of Queensland | Assay Description Inhibition constants were determined by a fluorometric assay. Ki values were calculated from either Dixon plots or Henderson plots in cases where the... | J Med Chem 45: 371-81 (2002) Article DOI: 10.1021/jm010414i BindingDB Entry DOI: 10.7270/Q2FQ9TSJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50581950 (CHEMBL4204703) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human adenosine A1 receptor expressed in Flp-In-CHO cell membrane assessed as inhibition of NECA-induced cAMP accumulation in ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02169 BindingDB Entry DOI: 10.7270/Q23200R3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50142990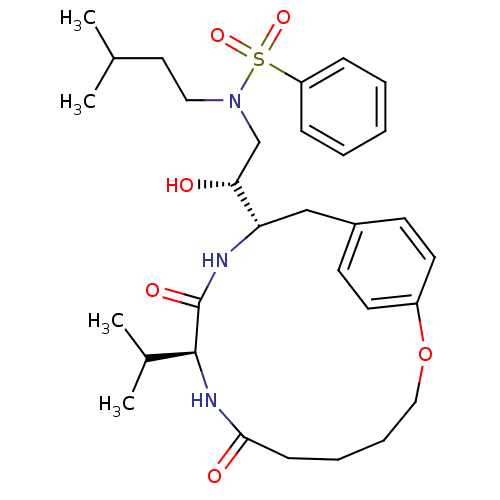 (CHEMBL288836 | N-[(R)-2-Hydroxy-2-((9S,12S)-9-isop...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Tested for inhibition of synthetic HIV-1 protease by using fluorometric assay | J Med Chem 47: 1641-51 (2004) Article DOI: 10.1021/jm030337m BindingDB Entry DOI: 10.7270/Q2JD4XJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50581950 (CHEMBL4204703) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human adenosine A1 receptor expressed in Flp-In-CHO cell membrane assessed as inhibition of forskolin-stimulated cAMP accumula... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02169 BindingDB Entry DOI: 10.7270/Q23200R3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50581955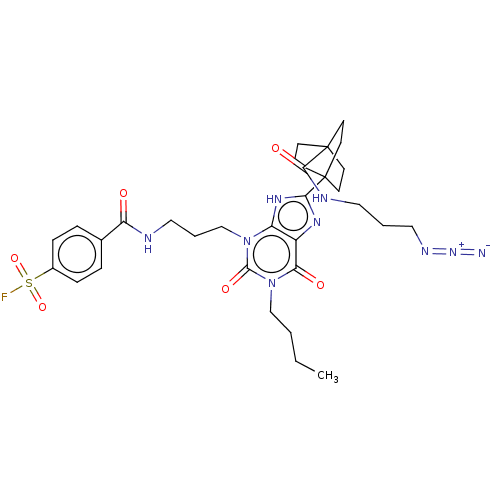 (CHEMBL5073669) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human adenosine A1 receptor expressed in Flp-In-CHO cell membrane assessed as inhibition of forskolin-stimulated cAMP accumula... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02169 BindingDB Entry DOI: 10.7270/Q23200R3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50581950 (CHEMBL4204703) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.562 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H] DPCPX from human A1 adenosine receptor expressed in Flp-In-CHO cell membrane incubated for 4 hrs by microbeta scintillation coun... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02169 BindingDB Entry DOI: 10.7270/Q23200R3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50581950 (CHEMBL4204703) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H] DPCPX from human A1 adenosine receptor expressed in Flp-In-CHO cell membrane incubated for 4 hrs by microbeta scintillation coun... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02169 BindingDB Entry DOI: 10.7270/Q23200R3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50581957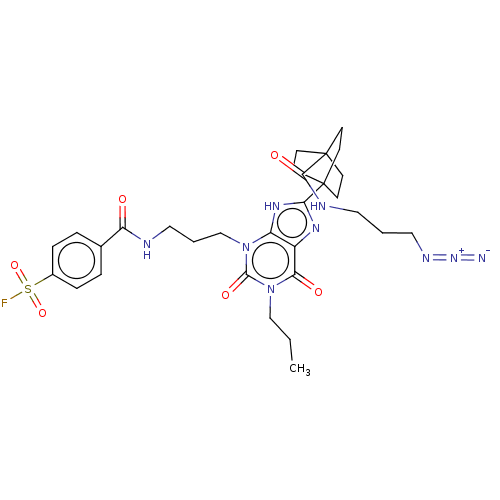 (CHEMBL5080823) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human adenosine A1 receptor expressed in Flp-In-CHO cell membrane assessed as inhibition of forskolin-stimulated cAMP accumula... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02169 BindingDB Entry DOI: 10.7270/Q23200R3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50092152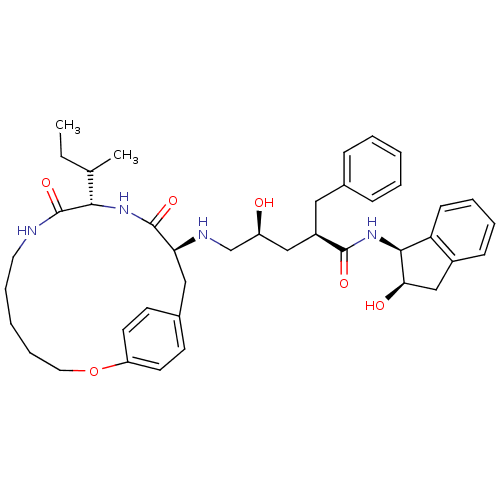 (2-Benzyl-5-(10-sec-butyl-9,12-dioxo-2-oxa-8,11-dia...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibitory constant against HIV-1 protease | J Med Chem 43: 3495-504 (2000) BindingDB Entry DOI: 10.7270/Q2KH0P1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50370377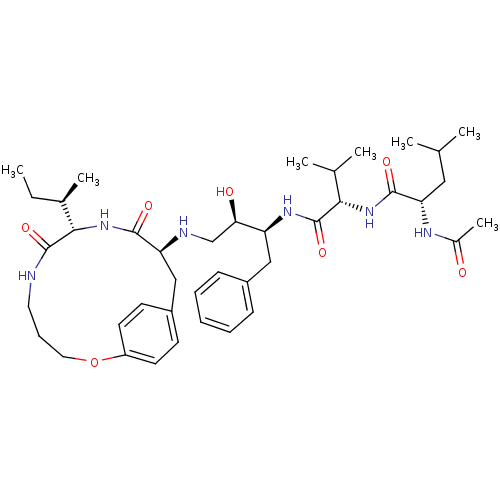 (CHEMBL1790231) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Tested for inhibition of synthetic HIV-1 protease by using fluorometric assay | J Med Chem 47: 1641-51 (2004) Article DOI: 10.1021/jm030337m BindingDB Entry DOI: 10.7270/Q2JD4XJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50581964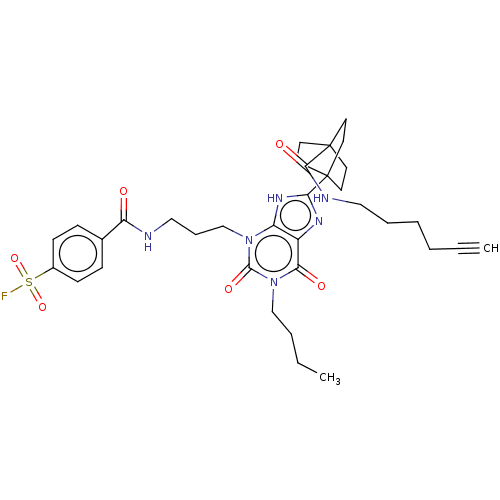 (CHEMBL5092788) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human adenosine A1 receptor expressed in Flp-In-CHO cell membrane assessed as inhibition of forskolin-stimulated cAMP accumula... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02169 BindingDB Entry DOI: 10.7270/Q23200R3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | CHEMBL5273987 | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL CHEMBL PC cid PC sid UniChem | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description In vitro antagonism of the 5-HT-3 receptor determined by inhibition of 5-HT-induced depolarization of the isolated rat vagus nerve. | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [491-589,Q7K,L33I,C67B,C95B] (Human immunodeficiency virus type 1) | BDBM795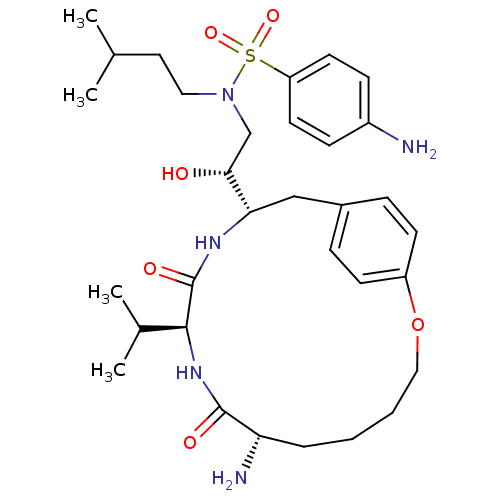 ((2R)-2-[(7S,10S,13S)-7-amino-8,11-dioxo-10-(propan...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.900 | -53.7 | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
University of Queensland | Assay Description Inhibition constants were determined by a fluorometric assay. Ki values were calculated from either Dixon plots or Henderson plots in cases where the... | J Med Chem 45: 371-81 (2002) Article DOI: 10.1021/jm010414i BindingDB Entry DOI: 10.7270/Q2FQ9TSJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50370379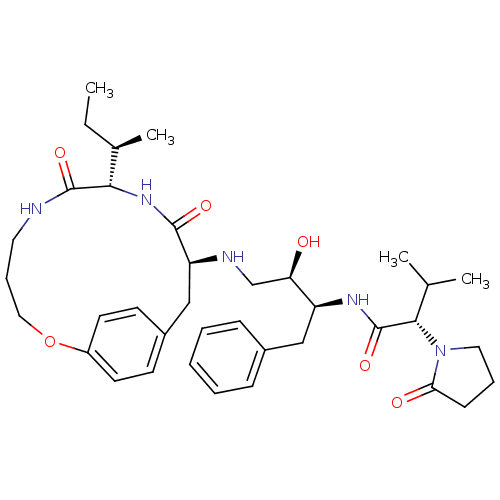 (CHEMBL1790229) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Tested for inhibition of synthetic HIV-1 protease by using fluorometric assay | J Med Chem 47: 1641-51 (2004) Article DOI: 10.1021/jm030337m BindingDB Entry DOI: 10.7270/Q2JD4XJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50142978 ((S)-11-{(S)-2-[Benzenesulfonyl-(3-methyl-butyl)-am...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Tested for inhibition of synthetic HIV-1 protease by using fluorometric assay | J Med Chem 47: 1641-51 (2004) Article DOI: 10.1021/jm030337m BindingDB Entry DOI: 10.7270/Q2JD4XJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50142965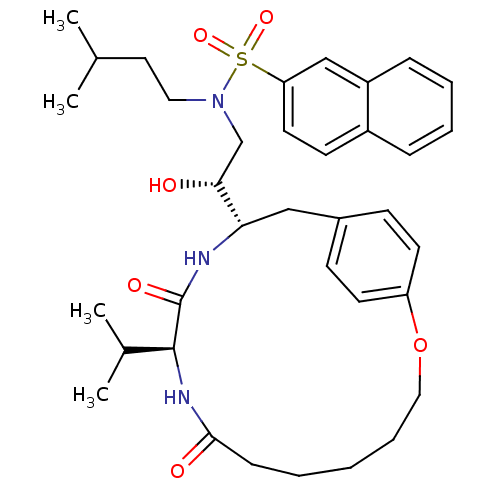 (CHEMBL296937 | Naphthalene-2-sulfonic acid [(S)-2-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Tested for inhibition of synthetic HIV-1 protease by using fluorometric assay | J Med Chem 47: 1641-51 (2004) Article DOI: 10.1021/jm030337m BindingDB Entry DOI: 10.7270/Q2JD4XJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50086884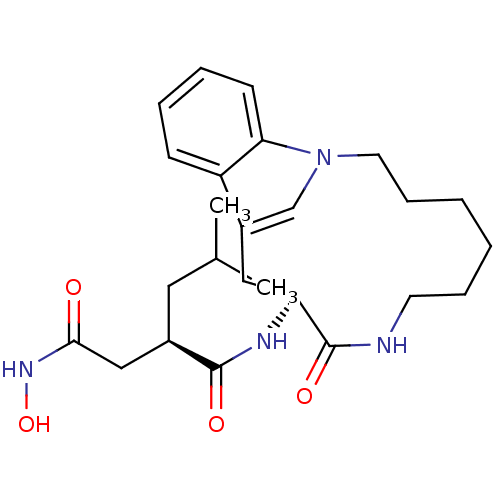 ((R)-N*4*-Hydroxy-2-isobutyl-N*1*-((S)-9-oxo-1,8-di...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland Curated by ChEMBL | Assay Description binding affinity towards HIV-1 Protease enzyme | J Med Chem 43: 1271-81 (2001) BindingDB Entry DOI: 10.7270/Q23779F5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50086880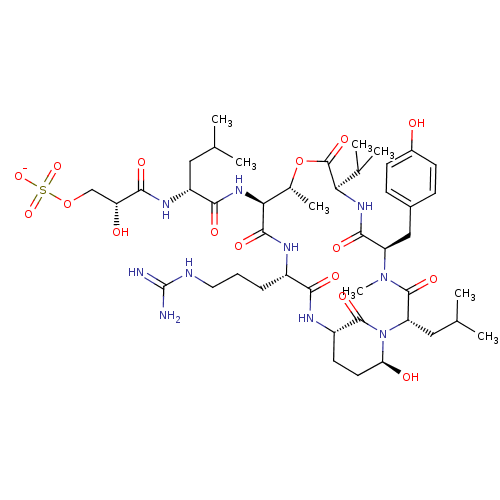 (Proteolytic Enzyme inhibitor) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland Curated by ChEMBL | Assay Description binding affinity towards HIV-1 Protease enzyme | J Med Chem 43: 1271-81 (2001) BindingDB Entry DOI: 10.7270/Q23779F5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50268232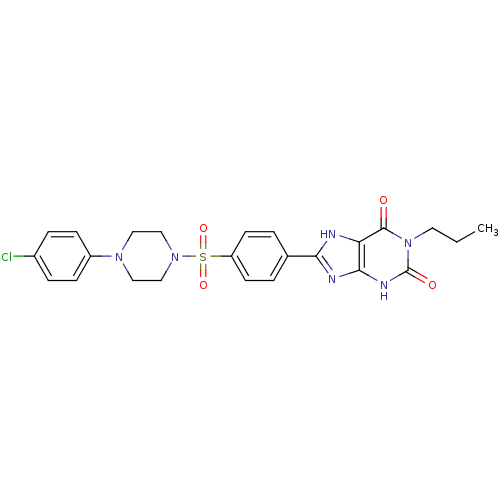 (8-(4-(4-(4-Chlorophenyl)piperazine-1-sulfonyl)phen...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human adenosine A2B receptor expressed in Flp-In-CHO cell membrane assessed as inhibition of NECA-induced cAMP accumulation in... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02169 BindingDB Entry DOI: 10.7270/Q23200R3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50581960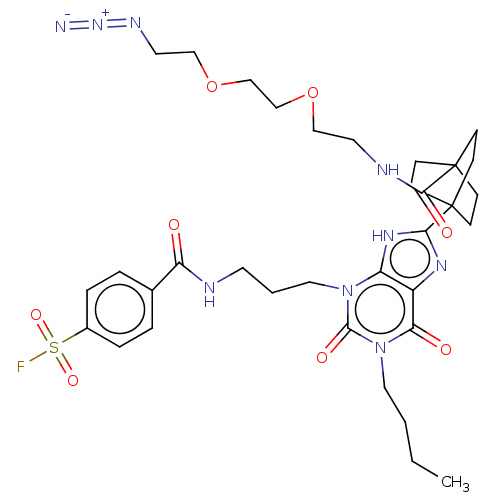 (CHEMBL5074992) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human adenosine A1 receptor expressed in Flp-In-CHO cell membrane assessed as inhibition of forskolin-stimulated cAMP accumula... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02169 BindingDB Entry DOI: 10.7270/Q23200R3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50581964 (CHEMBL5092788) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H] DPCPX from human A1 adenosine receptor expressed in Flp-In-CHO cell membrane incubated for 4 hrs by microbeta scintillation coun... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02169 BindingDB Entry DOI: 10.7270/Q23200R3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phenylethanolamine N-methyltransferase (Homo sapiens (Human)) | BDBM13014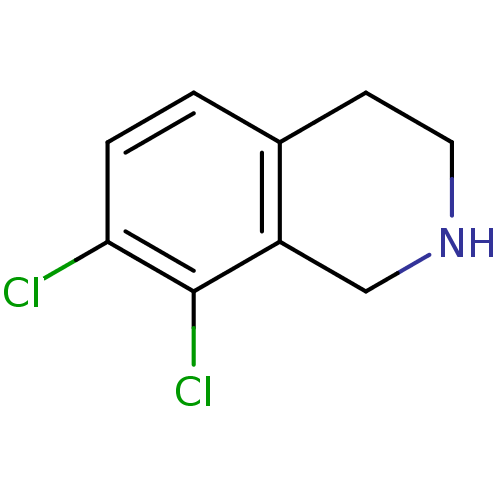 (7,8-Dichloro-1,2,3,4-tetrahydro-isoquinoline; hydr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | 1.55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Binding affinity to human wild type his-tagged PNMT | J Med Chem 48: 7243-52 (2005) Article DOI: 10.1021/jm050568o BindingDB Entry DOI: 10.7270/Q2QC049M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50581964 (CHEMBL5092788) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H] DPCPX from human A1 adenosine receptor expressed in Flp-In-CHO cell membrane incubated for 4 hrs by microbeta scintillation coun... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02169 BindingDB Entry DOI: 10.7270/Q23200R3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50142986 (CHEMBL295464 | N-[(S)-2-(R)-Hydroxy-2-((S)-10-isop...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Tested for inhibition of synthetic HIV-1 protease by using fluorometric assay | J Med Chem 47: 1641-51 (2004) Article DOI: 10.1021/jm030337m BindingDB Entry DOI: 10.7270/Q2JD4XJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50092151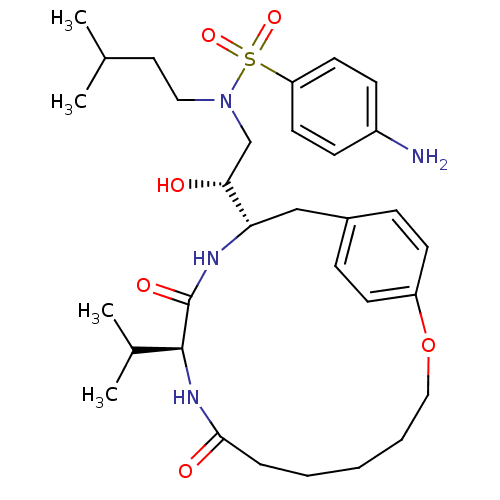 ((10S,13S,1'R)-13-[1'-HYDROXY-2'-(N-P-AMINOBENZENES...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Tested for inhibition of synthetic HIV-1 protease by using fluorometric assay | J Med Chem 47: 1641-51 (2004) Article DOI: 10.1021/jm030337m BindingDB Entry DOI: 10.7270/Q2JD4XJK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50092151 ((10S,13S,1'R)-13-[1'-HYDROXY-2'-(N-P-AMINOBENZENES...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibitory constant against HIV-1 protease | J Med Chem 43: 3495-504 (2000) BindingDB Entry DOI: 10.7270/Q2KH0P1S | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [491-589,Q7K,L33I,C67B,C95B] (Human immunodeficiency virus type 1) | BDBM796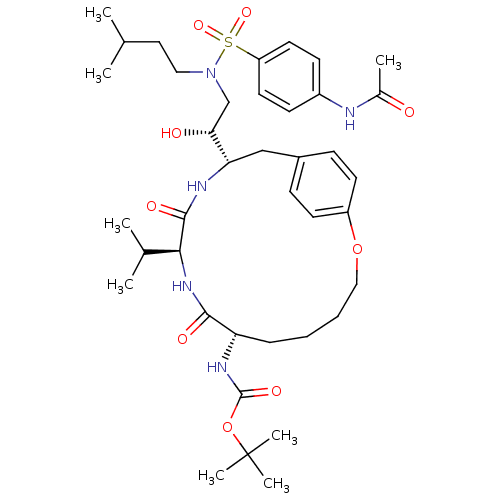 (Macrocycle-containing Compound 9b | tert-butyl N-[...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2 | -51.6 | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
University of Queensland | Assay Description Inhibition constants were determined by a fluorometric assay. Ki values were calculated from either Dixon plots or Henderson plots in cases where the... | J Med Chem 45: 371-81 (2002) Article DOI: 10.1021/jm010414i BindingDB Entry DOI: 10.7270/Q2FQ9TSJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50142974 (4-Amino-N-sec-butyl-N-[(S)-2-(R)-hydroxy-2-((S)-10...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Tested for inhibition of synthetic HIV-1 protease by using fluorometric assay | J Med Chem 47: 1641-51 (2004) Article DOI: 10.1021/jm030337m BindingDB Entry DOI: 10.7270/Q2JD4XJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50581961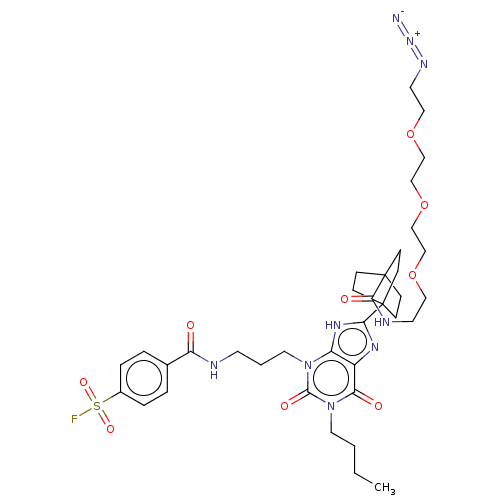 (CHEMBL5088372) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human adenosine A1 receptor expressed in Flp-In-CHO cell membrane assessed as inhibition of forskolin-stimulated cAMP accumula... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02169 BindingDB Entry DOI: 10.7270/Q23200R3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50581959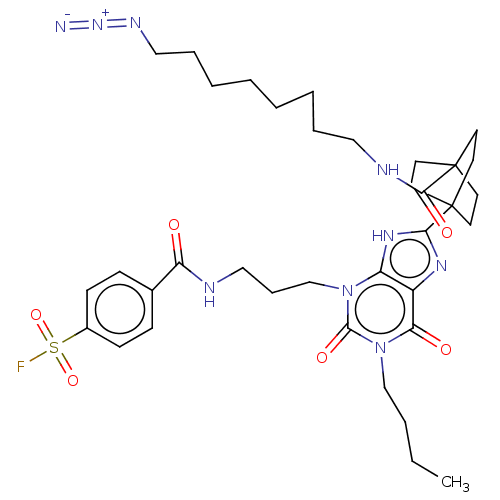 (CHEMBL5076192) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human adenosine A1 receptor expressed in Flp-In-CHO cell membrane assessed as inhibition of forskolin-stimulated cAMP accumula... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02169 BindingDB Entry DOI: 10.7270/Q23200R3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50581957 (CHEMBL5080823) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human adenosine A1 receptor expressed in Flp-In-CHO cell membrane assessed as inhibition of NECA-induced cAMP accumulation in ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02169 BindingDB Entry DOI: 10.7270/Q23200R3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [491-589,Q7K,L33I,C67B,C95B] (Human immunodeficiency virus type 1) | BDBM800 (Macrocycle-containing Compound 9f | N-[(7S,10S,13S...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.80 | -50.8 | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
University of Queensland | Assay Description Inhibition constants were determined by a fluorometric assay. Ki values were calculated from either Dixon plots or Henderson plots in cases where the... | J Med Chem 45: 371-81 (2002) Article DOI: 10.1021/jm010414i BindingDB Entry DOI: 10.7270/Q2FQ9TSJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50581959 (CHEMBL5076192) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human adenosine A3 receptor expressed in Flp-In-CHO cell membrane assessed as inhibition of NECA-induced cAMP accumulation in ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02169 BindingDB Entry DOI: 10.7270/Q23200R3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50142980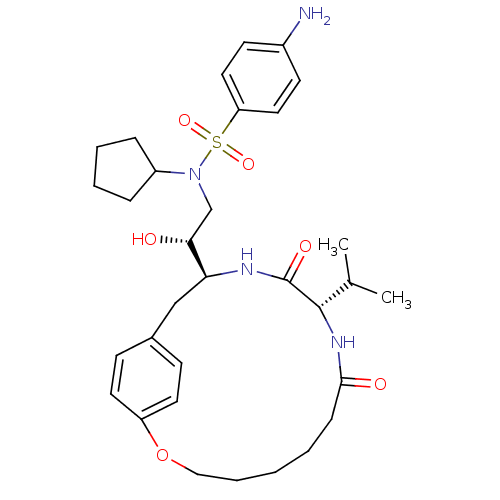 (4-Amino-N-cyclopentyl-N-[(S)-2-(R)-hydroxy-2-((S)-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Tested for inhibition of synthetic HIV-1 protease by using fluorometric assay | J Med Chem 47: 1641-51 (2004) Article DOI: 10.1021/jm030337m BindingDB Entry DOI: 10.7270/Q2JD4XJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [491-589,Q7K,L33I,C67B,C95B] (Human immunodeficiency virus type 1) | BDBM801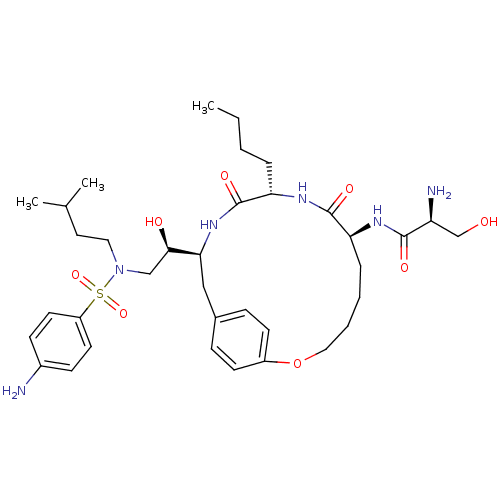 ((2S)-2-amino-N-[(7S,10S,13S)-13-[(1R)-2-[(4-aminob...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3 | -50.6 | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
University of Queensland | Assay Description Inhibition constants were determined by a fluorometric assay. Ki values were calculated from either Dixon plots or Henderson plots in cases where the... | J Med Chem 45: 371-81 (2002) Article DOI: 10.1021/jm010414i BindingDB Entry DOI: 10.7270/Q2FQ9TSJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50369797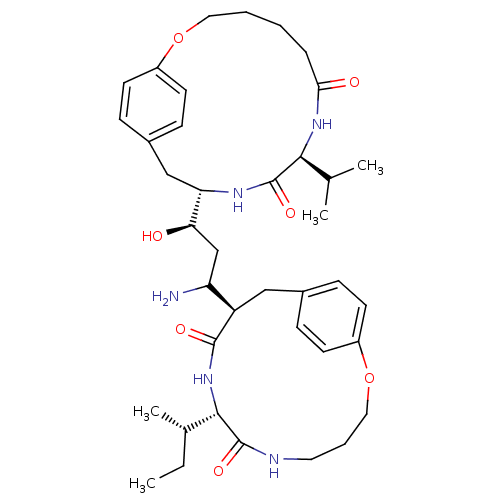 (CHEMBL1794029) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland Curated by ChEMBL | Assay Description binding affinity towards HIV-1 Protease enzyme | J Med Chem 43: 1271-81 (2001) BindingDB Entry DOI: 10.7270/Q23779F5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50142966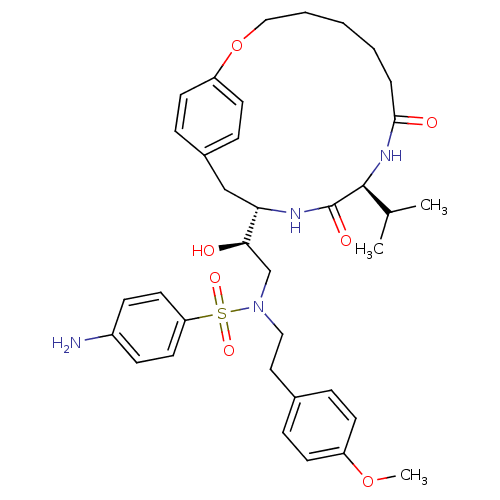 (4-Amino-N-[(S)-2-(R)-hydroxy-2-((S)-10-isopropyl-8...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Tested for inhibition of synthetic HIV-1 protease by using fluorometric assay | J Med Chem 47: 1641-51 (2004) Article DOI: 10.1021/jm030337m BindingDB Entry DOI: 10.7270/Q2JD4XJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50581961 (CHEMBL5088372) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human adenosine A1 receptor expressed in Flp-In-CHO cell membrane assessed as inhibition of NECA-induced cAMP accumulation in ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02169 BindingDB Entry DOI: 10.7270/Q23200R3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50581954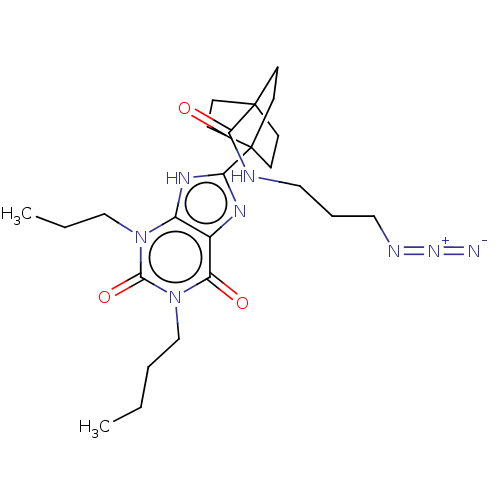 (CHEMBL5087305) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human adenosine A1 receptor expressed in Flp-In-CHO cell membrane assessed as inhibition of forskolin-stimulated cAMP accumula... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02169 BindingDB Entry DOI: 10.7270/Q23200R3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50581957 (CHEMBL5080823) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H] DPCPX from human A1 adenosine receptor expressed in Flp-In-CHO cell membrane incubated for 4 hrs by microbeta scintillation coun... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02169 BindingDB Entry DOI: 10.7270/Q23200R3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50581960 (CHEMBL5074992) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human adenosine A1 receptor expressed in Flp-In-CHO cell membrane assessed as inhibition of NECA-induced cAMP accumulation in ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02169 BindingDB Entry DOI: 10.7270/Q23200R3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50581957 (CHEMBL5080823) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H] DPCPX from human A1 adenosine receptor expressed in Flp-In-CHO cell membrane incubated for 4 hrs by microbeta scintillation coun... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02169 BindingDB Entry DOI: 10.7270/Q23200R3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50581959 (CHEMBL5076192) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H] DPCPX from human A1 adenosine receptor expressed in Flp-In-CHO cell membrane incubated for 4 hrs by microbeta scintillation coun... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02169 BindingDB Entry DOI: 10.7270/Q23200R3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50581959 (CHEMBL5076192) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H] DPCPX from human A1 adenosine receptor expressed in Flp-In-CHO cell membrane incubated for 4 hrs by microbeta scintillation coun... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02169 BindingDB Entry DOI: 10.7270/Q23200R3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50581958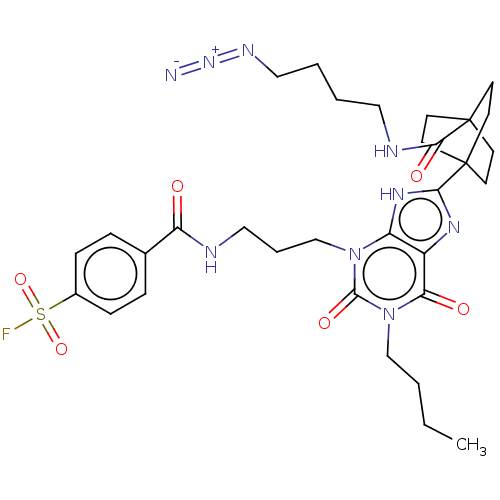 (CHEMBL5089483) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human adenosine A1 receptor expressed in Flp-In-CHO cell membrane assessed as inhibition of NECA-induced cAMP accumulation in ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02169 BindingDB Entry DOI: 10.7270/Q23200R3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 525 total ) | Next | Last >> |