

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50000788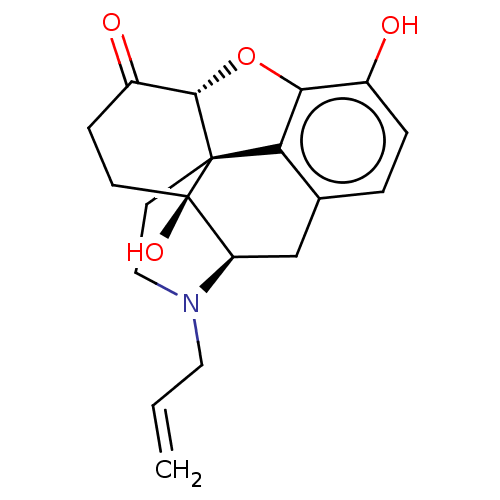 ((morphine)4-methyl-(1S,5R,13R,14S,17R)-12-oxa-4-az...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [3H]-naloxone from rat mu opioid receptor expressed in HEK cells after 60 mins | Bioorg Med Chem 22: 4694-703 (2014) Article DOI: 10.1016/j.bmc.2014.07.012 BindingDB Entry DOI: 10.7270/Q2H133NH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50005429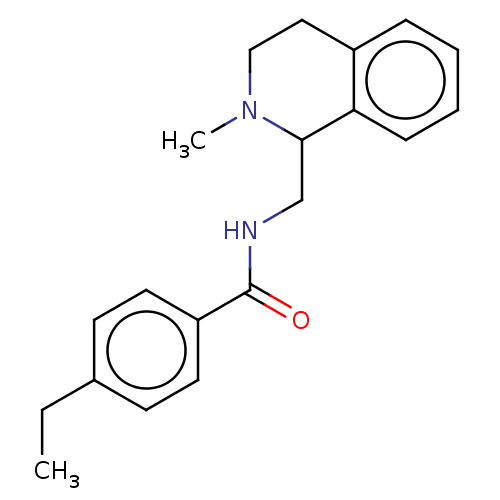 (CHEMBL4070288) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [3H]naloxone from recombinant human MOR expressed in HEK cell membranes incubated for 60 mins | Eur J Med Chem 126: 202-217 (2017) Article DOI: 10.1016/j.ejmech.2016.09.003 BindingDB Entry DOI: 10.7270/Q2RB76TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50007985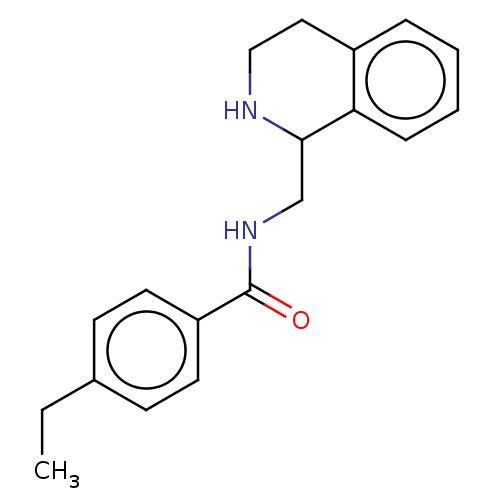 (CHEMBL4097865) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Agonist activity at human MOR expressed in HEK293 cells assessed as inhibition of forskolin-induced adenylyl cyclase-mediated cAMP accumulation after... | Eur J Med Chem 126: 202-217 (2017) Article DOI: 10.1016/j.ejmech.2016.09.003 BindingDB Entry DOI: 10.7270/Q2RB76TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50042064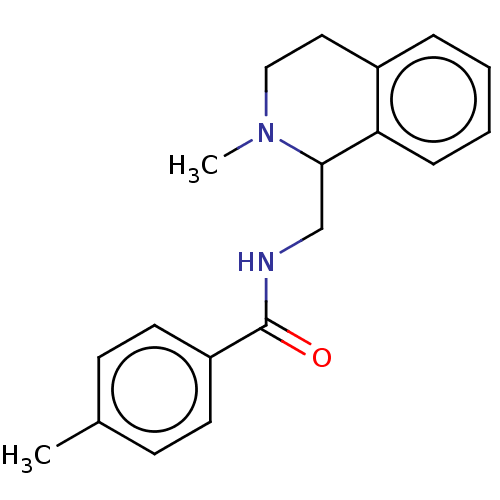 (CHEMBL4097466) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [3H]naloxone from recombinant human MOR expressed in HEK cell membranes incubated for 60 mins | Eur J Med Chem 126: 202-217 (2017) Article DOI: 10.1016/j.ejmech.2016.09.003 BindingDB Entry DOI: 10.7270/Q2RB76TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50015426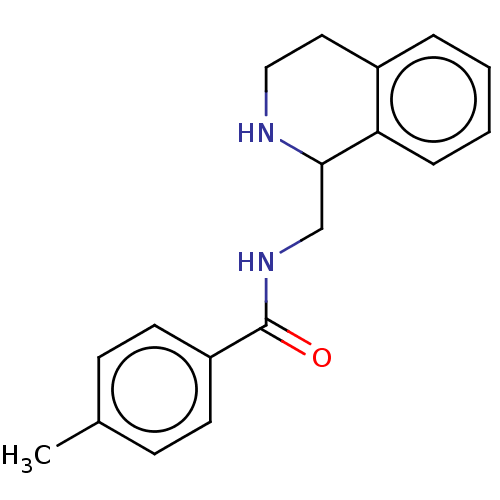 (CHEMBL4070750) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [3H]naloxone from recombinant human MOR expressed in HEK cell membranes incubated for 60 mins | Eur J Med Chem 126: 202-217 (2017) Article DOI: 10.1016/j.ejmech.2016.09.003 BindingDB Entry DOI: 10.7270/Q2RB76TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50009239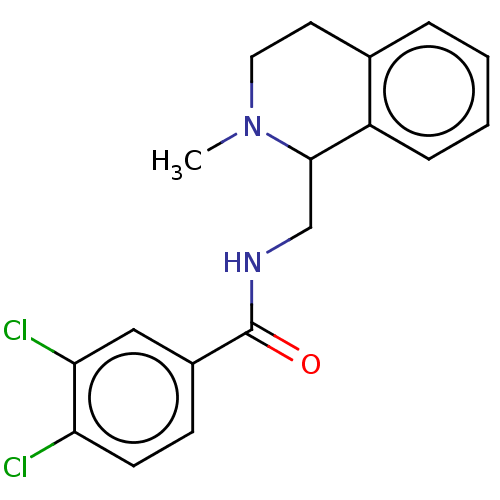 (CHEMBL4092125) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [3H]naloxone from recombinant human MOR expressed in HEK cell membranes incubated for 60 mins | Eur J Med Chem 126: 202-217 (2017) Article DOI: 10.1016/j.ejmech.2016.09.003 BindingDB Entry DOI: 10.7270/Q2RB76TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50008523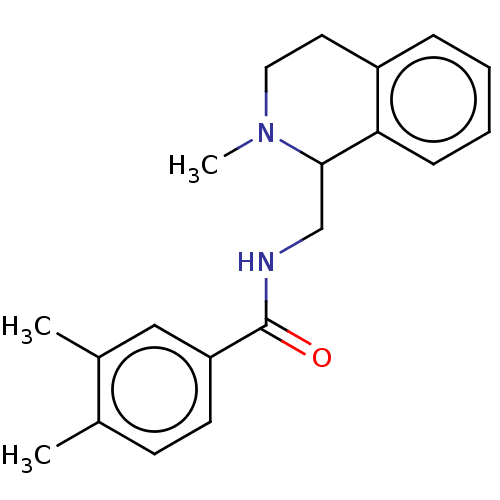 (CHEMBL4071332) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [3H]naloxone from recombinant human MOR expressed in HEK cell membranes incubated for 60 mins | Eur J Med Chem 126: 202-217 (2017) Article DOI: 10.1016/j.ejmech.2016.09.003 BindingDB Entry DOI: 10.7270/Q2RB76TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50015433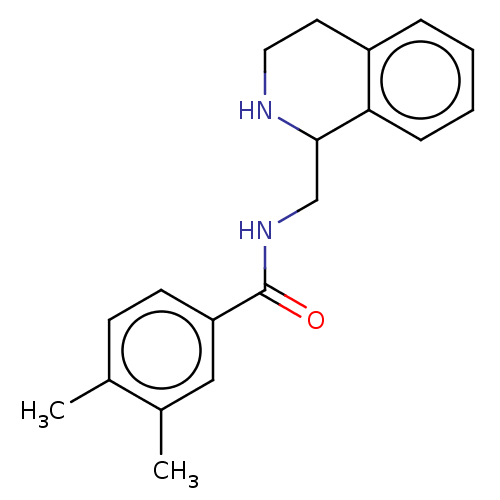 (CHEMBL4105599) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [3H]naloxone from recombinant human MOR expressed in HEK cell membranes incubated for 60 mins | Eur J Med Chem 126: 202-217 (2017) Article DOI: 10.1016/j.ejmech.2016.09.003 BindingDB Entry DOI: 10.7270/Q2RB76TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50008003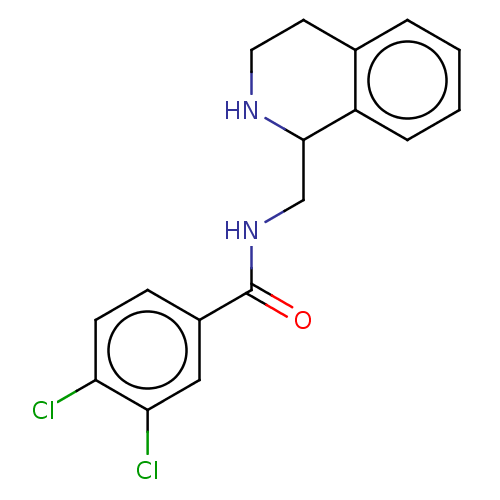 (CHEMBL4065924) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [3H]naloxone from recombinant human MOR expressed in HEK cell membranes incubated for 60 mins | Eur J Med Chem 126: 202-217 (2017) Article DOI: 10.1016/j.ejmech.2016.09.003 BindingDB Entry DOI: 10.7270/Q2RB76TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50055967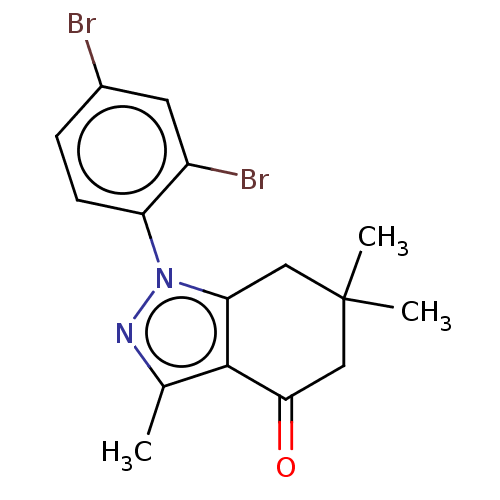 (CHEMBL3325714) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [3H]-naloxone from rat mu opioid receptor expressed in HEK cells after 60 mins | Bioorg Med Chem 22: 4694-703 (2014) Article DOI: 10.1016/j.bmc.2014.07.012 BindingDB Entry DOI: 10.7270/Q2H133NH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50055966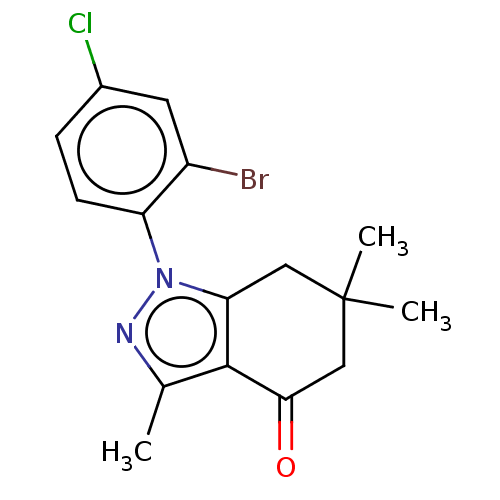 (CHEMBL3325715) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [3H]-naloxone from rat mu opioid receptor expressed in HEK cells after 60 mins | Bioorg Med Chem 22: 4694-703 (2014) Article DOI: 10.1016/j.bmc.2014.07.012 BindingDB Entry DOI: 10.7270/Q2H133NH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50055965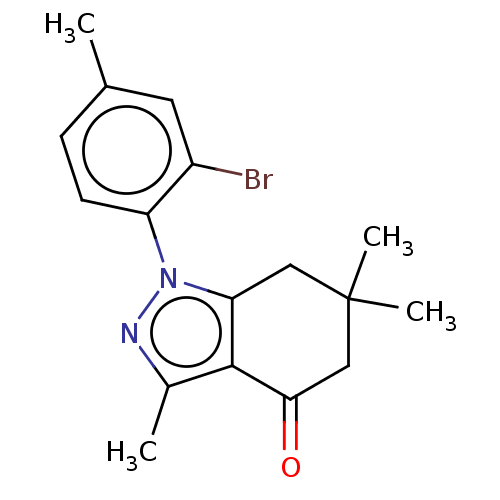 (CHEMBL3325716) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [3H]-naloxone from rat mu opioid receptor expressed in HEK cells after 60 mins | Bioorg Med Chem 22: 4694-703 (2014) Article DOI: 10.1016/j.bmc.2014.07.012 BindingDB Entry DOI: 10.7270/Q2H133NH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50055969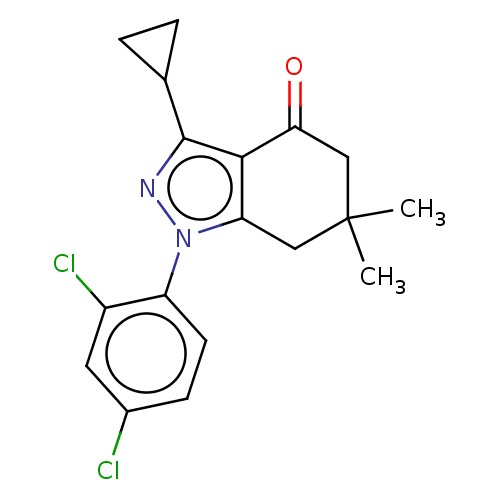 (CHEMBL3325719) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [3H]-naloxone from rat mu opioid receptor expressed in HEK cells after 60 mins | Bioorg Med Chem 22: 4694-703 (2014) Article DOI: 10.1016/j.bmc.2014.07.012 BindingDB Entry DOI: 10.7270/Q2H133NH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50055973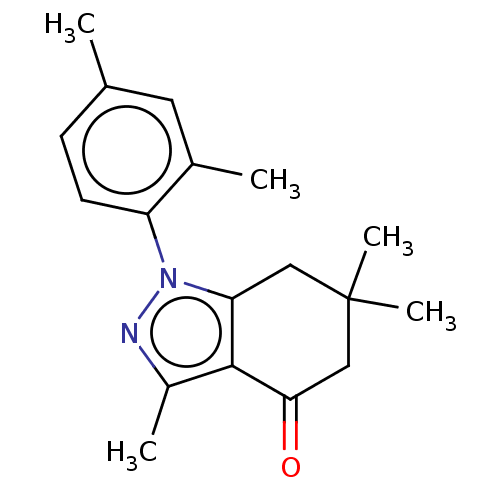 (CHEMBL3325707) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.93E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [3H]-naloxone from rat mu opioid receptor expressed in HEK cells after 60 mins | Bioorg Med Chem 22: 4694-703 (2014) Article DOI: 10.1016/j.bmc.2014.07.012 BindingDB Entry DOI: 10.7270/Q2H133NH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM21973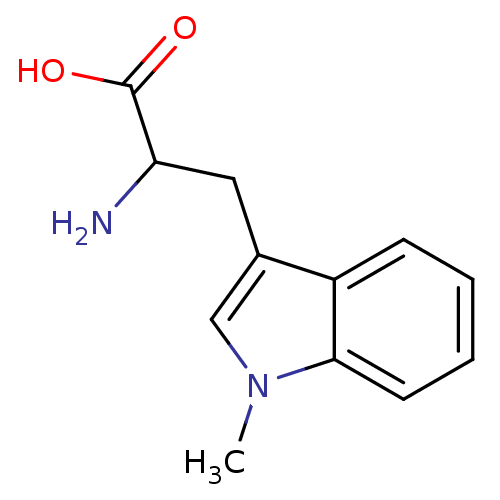 (1-Methyltryptophan, 1 | 2-amino-3-(1-methyl-1H-ind...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 3.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of human recombinant IDO using L-tryptophan as substrate | Bioorg Med Chem Lett 24: 3403-6 (2014) Article DOI: 10.1016/j.bmcl.2014.05.084 BindingDB Entry DOI: 10.7270/Q23R0VH6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50055972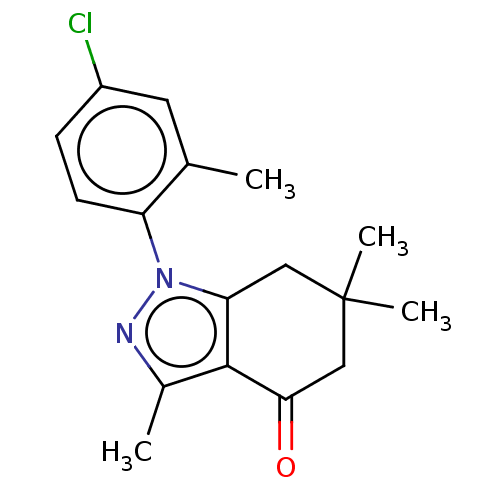 (CHEMBL3325710) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [3H]-naloxone from rat mu opioid receptor expressed in HEK cells after 60 mins | Bioorg Med Chem 22: 4694-703 (2014) Article DOI: 10.1016/j.bmc.2014.07.012 BindingDB Entry DOI: 10.7270/Q2H133NH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50055964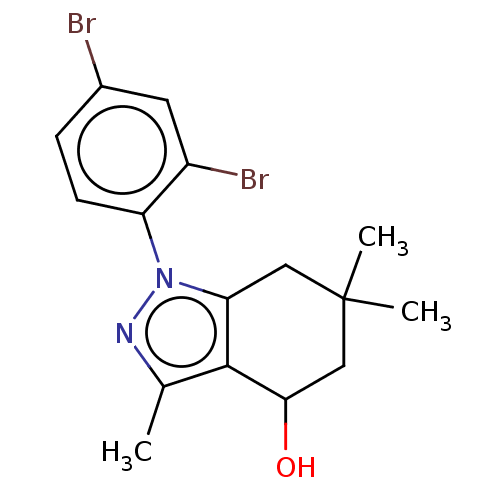 (CHEMBL3325841) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [3H]-naloxone from rat mu opioid receptor expressed in HEK cells after 60 mins | Bioorg Med Chem 22: 4694-703 (2014) Article DOI: 10.1016/j.bmc.2014.07.012 BindingDB Entry DOI: 10.7270/Q2H133NH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50000296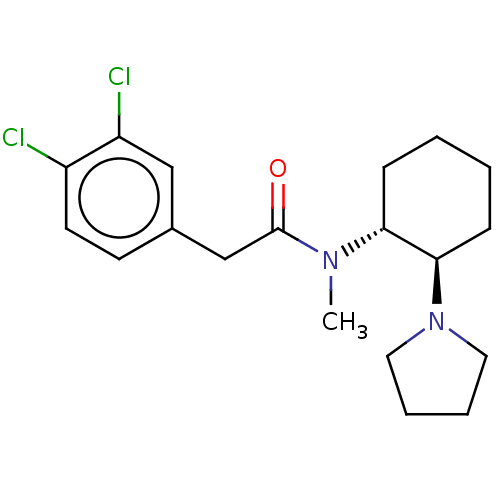 (CHEMBL441765 | CHEMBL482811 | U-50488H | US1149237...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Agonist activity at human kappa opioid receptor expressed in HEK293 cells assessed as inhibition of forskolin-induced cAMP production after 30 mins b... | Bioorg Med Chem 22: 4694-703 (2014) Article DOI: 10.1016/j.bmc.2014.07.012 BindingDB Entry DOI: 10.7270/Q2H133NH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM21008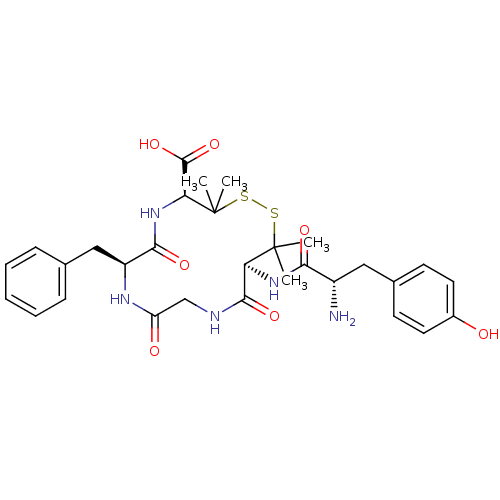 ((4S,7S,13S)-13-[(2S)-2-amino-3-(4-hydroxyphenyl)pr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Agonist activity at human DOR expressed in HEK293 cells assessed as inhibition of forskolin-induced adenylyl cyclase-mediated cAMP accumulation after... | Eur J Med Chem 126: 202-217 (2017) Article DOI: 10.1016/j.ejmech.2016.09.003 BindingDB Entry DOI: 10.7270/Q2RB76TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50001683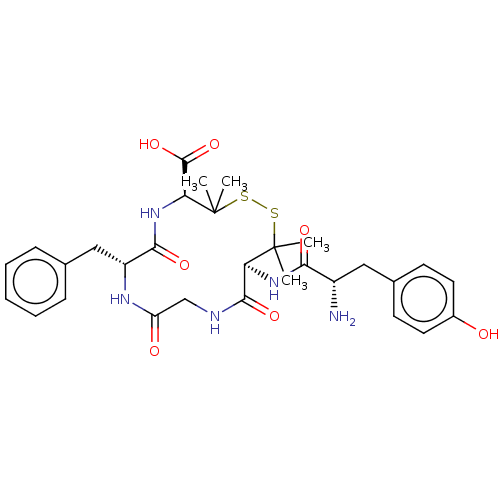 (13-[2-Amino-3-(4-hydroxy-phenyl)-propionylamino]-7...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Agonist activity at human delta opioid receptor expressed in HEK293 cells assessed as inhibition of forskolin-induced cAMP production after 30 mins b... | Bioorg Med Chem 22: 4694-703 (2014) Article DOI: 10.1016/j.bmc.2014.07.012 BindingDB Entry DOI: 10.7270/Q2H133NH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50000296 (CHEMBL441765 | CHEMBL482811 | U-50488H | US1149237...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Agonist activity at human KOR expressed in HEK293 cells assessed as inhibition of forskolin-induced adenylyl cyclase-mediated cAMP accumulation after... | Eur J Med Chem 126: 202-217 (2017) Article DOI: 10.1016/j.ejmech.2016.09.003 BindingDB Entry DOI: 10.7270/Q2RB76TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM21015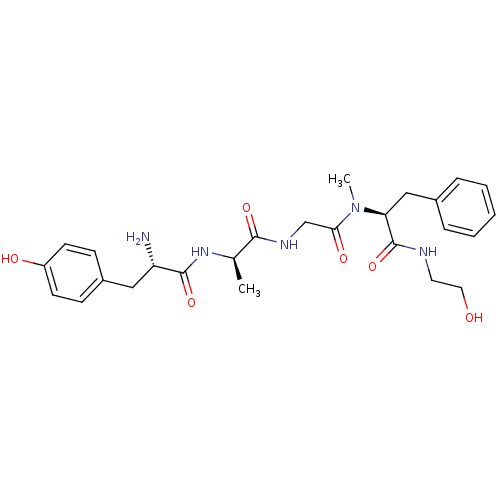 ((2S)-2-{2-[(2R)-2-[(2S)-2-amino-3-(4-hydroxyphenyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Agonist activity at human mu opioid receptor expressed in HEK293 cells assessed as inhibition of forskolin-induced cAMP production after 30 mins by H... | Bioorg Med Chem 22: 4694-703 (2014) Article DOI: 10.1016/j.bmc.2014.07.012 BindingDB Entry DOI: 10.7270/Q2H133NH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM21015 ((2S)-2-{2-[(2R)-2-[(2S)-2-amino-3-(4-hydroxyphenyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Agonist activity at human MOR expressed in HEK293 cells assessed as inhibition of forskolin-induced adenylyl cyclase-mediated cAMP accumulation after... | Eur J Med Chem 126: 202-217 (2017) Article DOI: 10.1016/j.ejmech.2016.09.003 BindingDB Entry DOI: 10.7270/Q2RB76TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50520460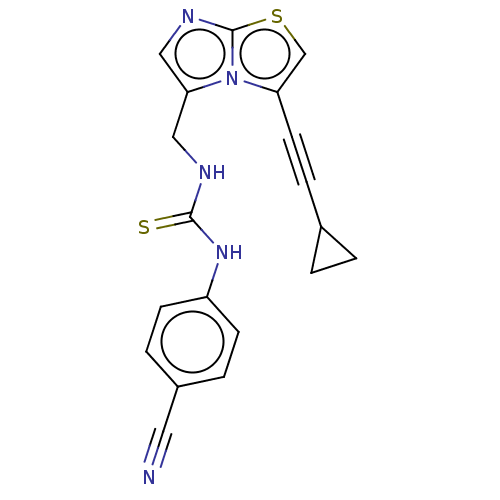 (CHEMBL4549501) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of recombinant full-length N-terminal His-tagged human IDO1 (1 to 403 residues) expressed in Escherichia coli BL21(DE3) cells | J Med Chem 63: 1642-1659 (2020) Article DOI: 10.1021/acs.jmedchem.9b01549 BindingDB Entry DOI: 10.7270/Q26113QV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50138819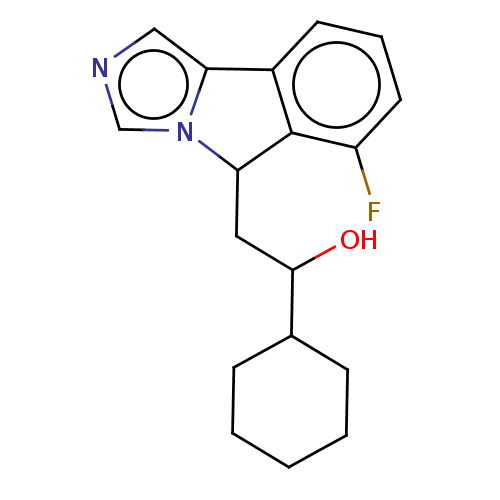 (CHEMBL3753837 | US10233190, Example 1357) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of recombinant human IDO1 assessed as conversion of N-formylkynurenine to kynurenine incubated for 1 hr by fluorescence analysis | J Med Chem 59: 282-93 (2016) Article DOI: 10.1021/acs.jmedchem.5b01390 BindingDB Entry DOI: 10.7270/Q28917QW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50584094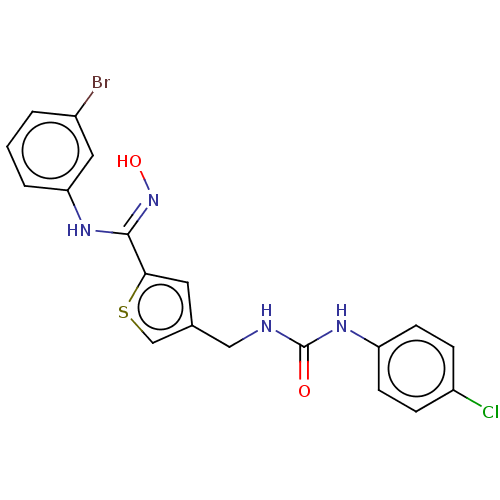 (CHEMBL5077002) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human IDO1 protein assessed as reduction in N-formylkynurenine formation using L-tryptophan as substrate by methylene blue reagent base... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.114043 BindingDB Entry DOI: 10.7270/Q2SX6J39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50520450 (CHEMBL4436159) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of recombinant full-length N-terminal His-tagged human IDO1 (1 to 403 residues) expressed in Escherichia coli BL21(DE3) cells | J Med Chem 63: 1642-1659 (2020) Article DOI: 10.1021/acs.jmedchem.9b01549 BindingDB Entry DOI: 10.7270/Q26113QV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM50127174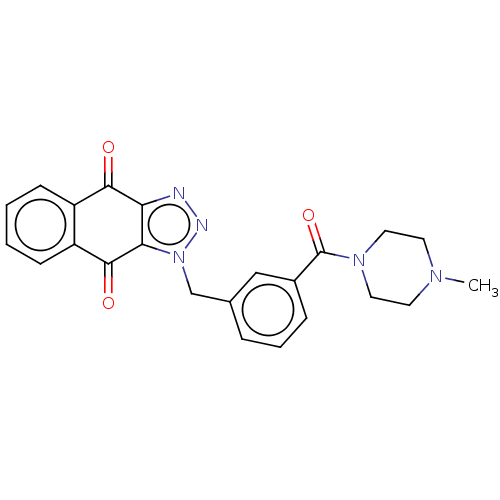 (CHEMBL3628599) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of recombinant human TDO expressed in Escherichia coli incubated for 1 hr measured by fluorescence assay | J Med Chem 58: 7807-19 (2015) Article DOI: 10.1021/acs.jmedchem.5b00921 BindingDB Entry DOI: 10.7270/Q2JS9S7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50584075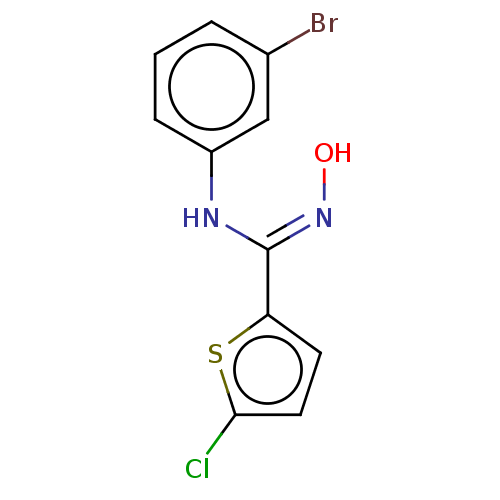 (CHEMBL5076745) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human IDO1 protein assessed as reduction in N-formylkynurenine formation using L-tryptophan as substrate by methylene blue reagent base... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.114043 BindingDB Entry DOI: 10.7270/Q2SX6J39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50145168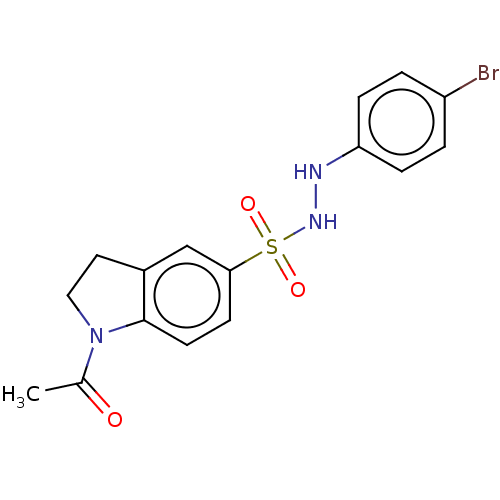 (CHEMBL3764319) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of human Indoleamine 2,3-dioxygenase using L-tryptophan as substrate by emission fluorescence analysis | J Med Chem 59: 419-30 (2016) Article DOI: 10.1021/acs.jmedchem.5b01640 BindingDB Entry DOI: 10.7270/Q2FF3V69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50145157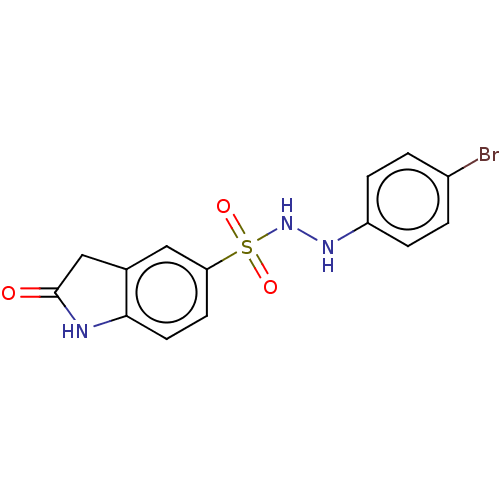 (CHEMBL3763166) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of human Indoleamine 2,3-dioxygenase using L-tryptophan as substrate by emission fluorescence analysis | J Med Chem 59: 419-30 (2016) Article DOI: 10.1021/acs.jmedchem.5b01640 BindingDB Entry DOI: 10.7270/Q2FF3V69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50584093 (CHEMBL5087302) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human IDO1 protein assessed as reduction in N-formylkynurenine formation using L-tryptophan as substrate by methylene blue reagent base... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.114043 BindingDB Entry DOI: 10.7270/Q2SX6J39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50126144 (CHEMBL3629569 | US10155972, Compound NewLink 1 | U...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of recombinant human IDO1 assessed as conversion of N-formylkynurenine to kynurenine incubated for 1 hr by fluorescence analysis | J Med Chem 59: 282-93 (2016) Article DOI: 10.1021/acs.jmedchem.5b01390 BindingDB Entry DOI: 10.7270/Q28917QW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50003238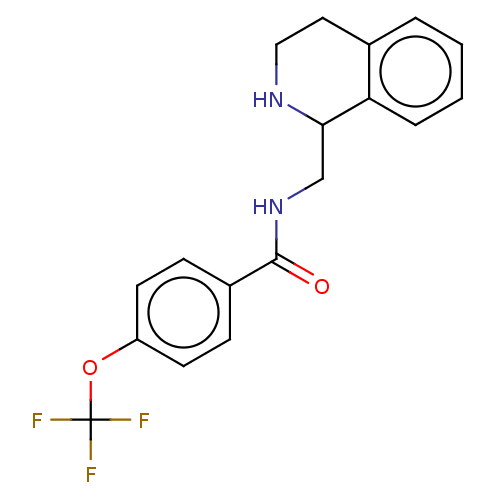 (CHEMBL4082299) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Agonist activity at human DOR expressed in HEK293 cells assessed as inhibition of forskolin-induced adenylyl cyclase-mediated cAMP accumulation after... | Eur J Med Chem 126: 202-217 (2017) Article DOI: 10.1016/j.ejmech.2016.09.003 BindingDB Entry DOI: 10.7270/Q2RB76TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50013629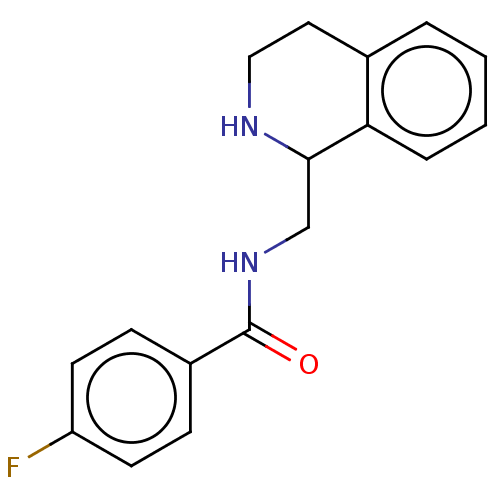 (CHEMBL4062124) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Agonist activity at human DOR expressed in HEK293 cells assessed as inhibition of forskolin-induced adenylyl cyclase-mediated cAMP accumulation after... | Eur J Med Chem 126: 202-217 (2017) Article DOI: 10.1016/j.ejmech.2016.09.003 BindingDB Entry DOI: 10.7270/Q2RB76TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50520449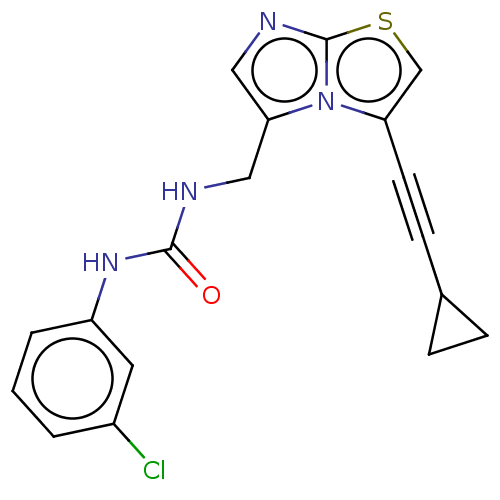 (CHEMBL4459210) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of recombinant full-length N-terminal His-tagged human IDO1 (1 to 403 residues) expressed in Escherichia coli BL21(DE3) cells | J Med Chem 63: 1642-1659 (2020) Article DOI: 10.1021/acs.jmedchem.9b01549 BindingDB Entry DOI: 10.7270/Q26113QV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50046105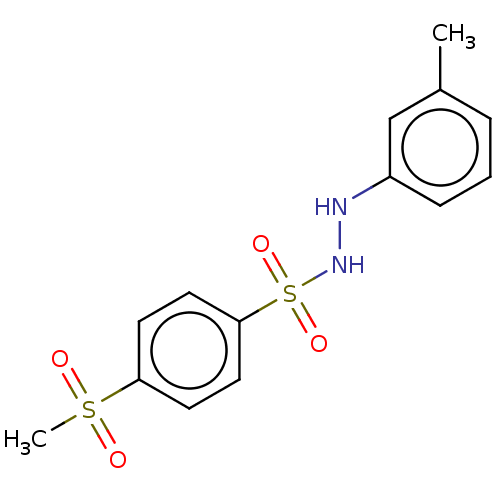 (CHEMBL3310843) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of human recombinant IDO expressed in Escherichia coli using N-formylkynurenine as substrate incubated for 1 hr prior to NaOH addition mea... | Bioorg Med Chem Lett 24: 3403-6 (2014) Article DOI: 10.1016/j.bmcl.2014.05.084 BindingDB Entry DOI: 10.7270/Q23R0VH6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50145166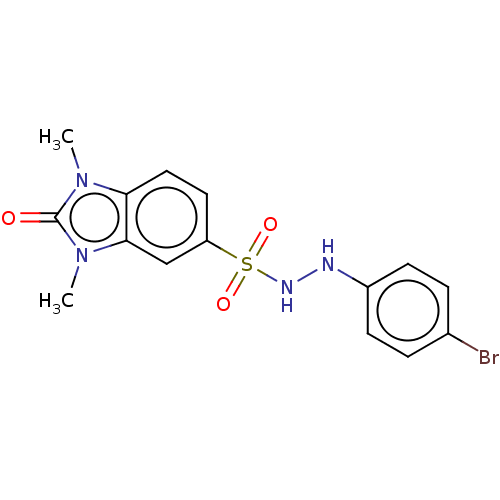 (CHEMBL3763493) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of human Indoleamine 2,3-dioxygenase using L-tryptophan as substrate by emission fluorescence analysis | J Med Chem 59: 419-30 (2016) Article DOI: 10.1021/acs.jmedchem.5b01640 BindingDB Entry DOI: 10.7270/Q2FF3V69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM50127173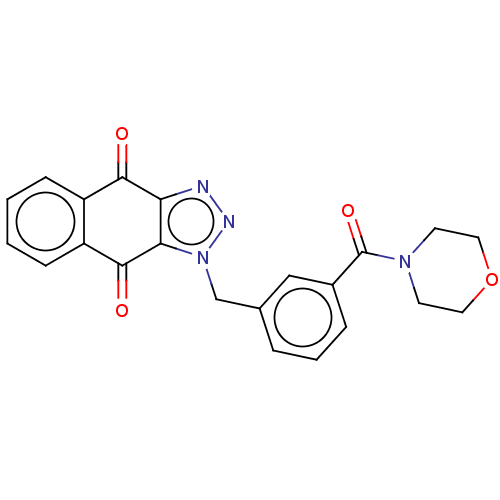 (CHEMBL3628598) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of recombinant human TDO expressed in Escherichia coli incubated for 1 hr measured by fluorescence assay | J Med Chem 58: 7807-19 (2015) Article DOI: 10.1021/acs.jmedchem.5b00921 BindingDB Entry DOI: 10.7270/Q2JS9S7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM80319 (MLS001171649 | N-[4-(m-toluidinosulfamoyl)phenyl]a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of human recombinant IDO expressed in Escherichia coli using N-formylkynurenine as substrate incubated for 1 hr prior to NaOH addition mea... | Bioorg Med Chem Lett 24: 3403-6 (2014) Article DOI: 10.1016/j.bmcl.2014.05.084 BindingDB Entry DOI: 10.7270/Q23R0VH6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50009239 (CHEMBL4092125) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Agonist activity at human MOR expressed in HEK293 cells assessed as inhibition of forskolin-induced adenylyl cyclase-mediated cAMP accumulation after... | Eur J Med Chem 126: 202-217 (2017) Article DOI: 10.1016/j.ejmech.2016.09.003 BindingDB Entry DOI: 10.7270/Q2RB76TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50007981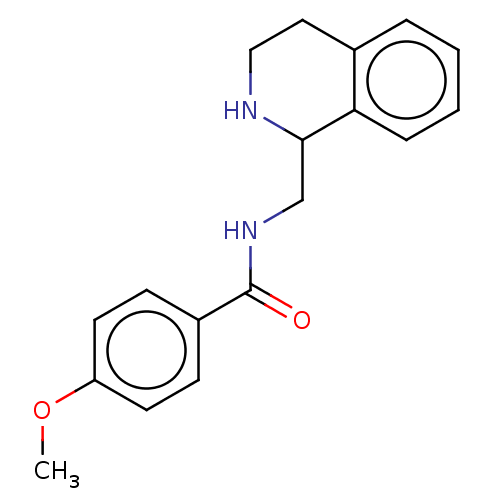 (CHEMBL4100317) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Agonist activity at human KOR expressed in HEK293 cells assessed as inhibition of forskolin-induced adenylyl cyclase-mediated cAMP accumulation after... | Eur J Med Chem 126: 202-217 (2017) Article DOI: 10.1016/j.ejmech.2016.09.003 BindingDB Entry DOI: 10.7270/Q2RB76TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50046106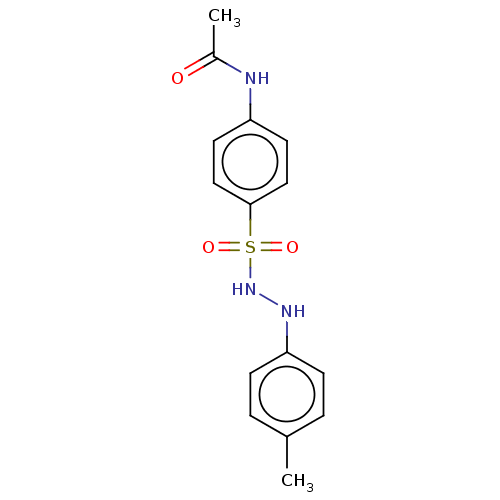 (CHEMBL3310847) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of human recombinant IDO expressed in Escherichia coli using N-formylkynurenine as substrate incubated for 1 hr prior to NaOH addition mea... | Bioorg Med Chem Lett 24: 3403-6 (2014) Article DOI: 10.1016/j.bmcl.2014.05.084 BindingDB Entry DOI: 10.7270/Q23R0VH6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50145159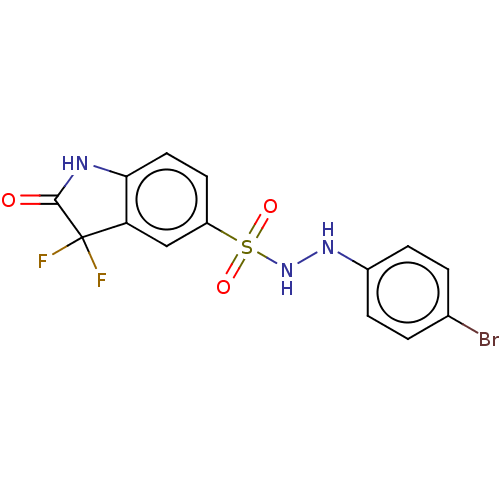 (CHEMBL3765205) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of human Indoleamine 2,3-dioxygenase using L-tryptophan as substrate by emission fluorescence analysis | J Med Chem 59: 419-30 (2016) Article DOI: 10.1021/acs.jmedchem.5b01640 BindingDB Entry DOI: 10.7270/Q2FF3V69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50145164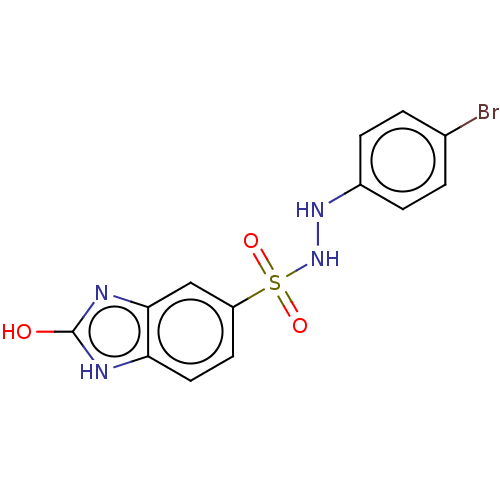 (CHEMBL3765698) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of human Indoleamine 2,3-dioxygenase using L-tryptophan as substrate by emission fluorescence analysis | J Med Chem 59: 419-30 (2016) Article DOI: 10.1021/acs.jmedchem.5b01640 BindingDB Entry DOI: 10.7270/Q2FF3V69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50046107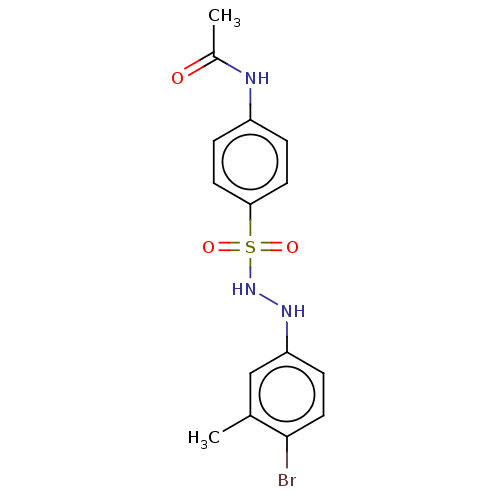 (CHEMBL3310860) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of human recombinant IDO expressed in Escherichia coli using N-formylkynurenine as substrate incubated for 1 hr prior to NaOH addition mea... | Bioorg Med Chem Lett 24: 3403-6 (2014) Article DOI: 10.1016/j.bmcl.2014.05.084 BindingDB Entry DOI: 10.7270/Q23R0VH6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50046109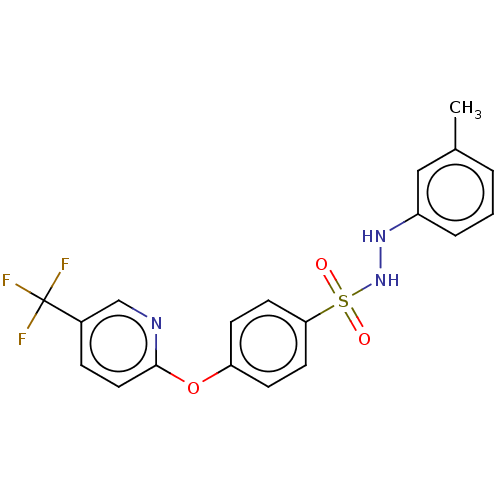 (CHEMBL3310845) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of human recombinant IDO expressed in Escherichia coli using N-formylkynurenine as substrate incubated for 1 hr prior to NaOH addition mea... | Bioorg Med Chem Lett 24: 3403-6 (2014) Article DOI: 10.1016/j.bmcl.2014.05.084 BindingDB Entry DOI: 10.7270/Q23R0VH6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50046108 (CHEMBL3310844) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of human recombinant IDO expressed in Escherichia coli using N-formylkynurenine as substrate incubated for 1 hr prior to NaOH addition mea... | Bioorg Med Chem Lett 24: 3403-6 (2014) Article DOI: 10.1016/j.bmcl.2014.05.084 BindingDB Entry DOI: 10.7270/Q23R0VH6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM50127164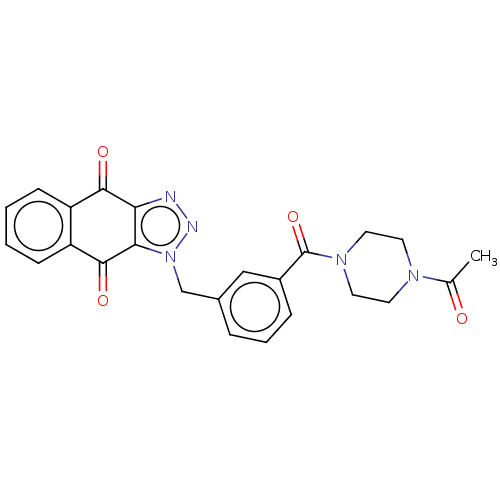 (CHEMBL3628597) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of recombinant human TDO expressed in Escherichia coli incubated for 1 hr measured by fluorescence assay | J Med Chem 58: 7807-19 (2015) Article DOI: 10.1021/acs.jmedchem.5b00921 BindingDB Entry DOI: 10.7270/Q2JS9S7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50046110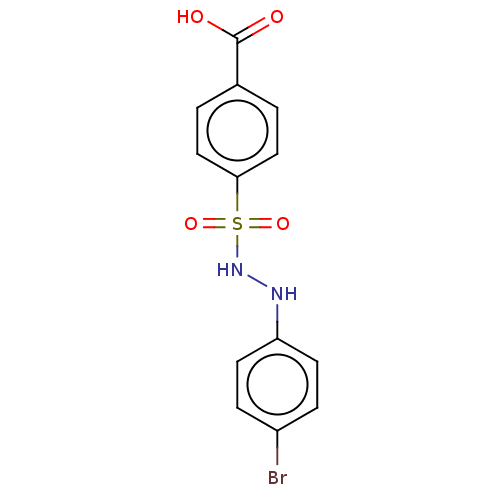 (CHEMBL3310981) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of human recombinant IDO expressed in Escherichia coli using N-formylkynurenine as substrate incubated for 1 hr prior to NaOH addition mea... | Bioorg Med Chem Lett 24: 3403-6 (2014) Article DOI: 10.1016/j.bmcl.2014.05.084 BindingDB Entry DOI: 10.7270/Q23R0VH6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 417 total ) | Next | Last >> |