 Found 194 hits with Last Name = 'ullrich' and Initial = 'jw'
Found 194 hits with Last Name = 'ullrich' and Initial = 'jw' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50074024
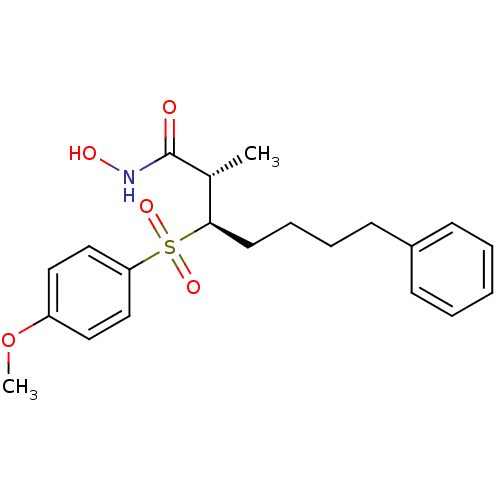
((2S,3R)-3-(4-Methoxy-benzenesulfonyl)-2-methyl-7-p...)Show SMILES COc1ccc(cc1)S(=O)(=O)[C@H](CCCCc1ccccc1)[C@@H](C)C(=O)NO Show InChI InChI=1S/C21H27NO5S/c1-16(21(23)22-24)20(11-7-6-10-17-8-4-3-5-9-17)28(25,26)19-14-12-18(27-2)13-15-19/h3-5,8-9,12-16,20,24H,6-7,10-11H2,1-2H3,(H,22,23)/t16-,20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloproteinase-2 (MMP-2), gelatinase-A |
J Med Chem 42: 541-4 (1999)
Article DOI: 10.1021/jm980567e
BindingDB Entry DOI: 10.7270/Q2VQ31VC |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50074027
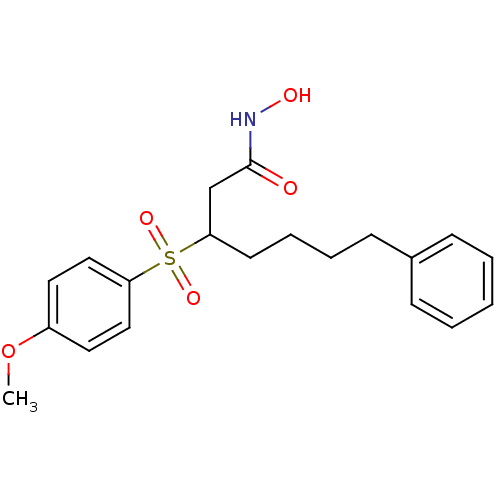
(3-(4-Methoxy-benzenesulfonyl)-7-phenyl-heptanoic a...)Show SMILES COc1ccc(cc1)S(=O)(=O)C(CCCCc1ccccc1)CC(=O)NO Show InChI InChI=1S/C20H25NO5S/c1-26-17-11-13-18(14-12-17)27(24,25)19(15-20(22)21-23)10-6-5-9-16-7-3-2-4-8-16/h2-4,7-8,11-14,19,23H,5-6,9-10,15H2,1H3,(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloproteinase-2 (MMP-2), gelatinase-A |
J Med Chem 42: 541-4 (1999)
Article DOI: 10.1021/jm980567e
BindingDB Entry DOI: 10.7270/Q2VQ31VC |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50074023
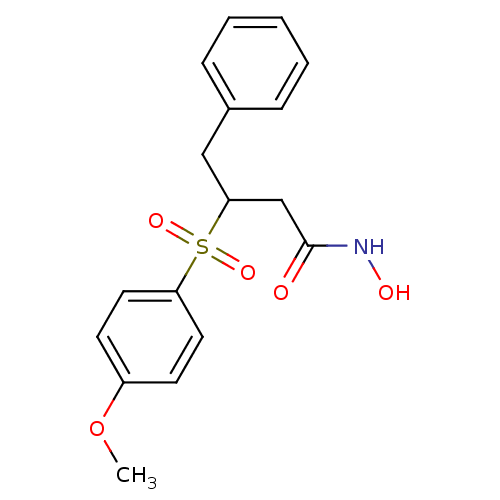
(CHEMBL153469 | N-Hydroxy-3-(4-methoxy-benzenesulfo...)Show InChI InChI=1S/C17H19NO5S/c1-23-14-7-9-15(10-8-14)24(21,22)16(12-17(19)18-20)11-13-5-3-2-4-6-13/h2-10,16,20H,11-12H2,1H3,(H,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloproteinase-2 (MMP-2), gelatinase-A |
J Med Chem 42: 541-4 (1999)
Article DOI: 10.1021/jm980567e
BindingDB Entry DOI: 10.7270/Q2VQ31VC |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50074021
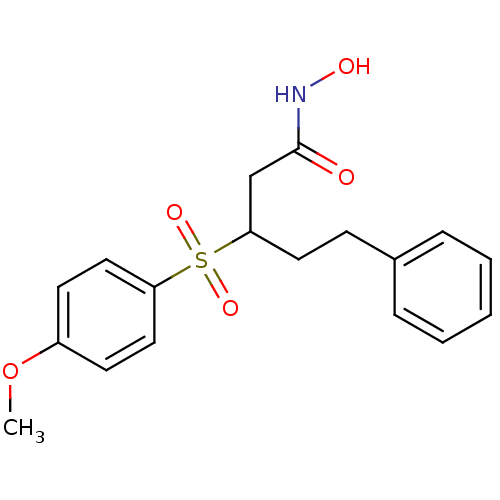
(3-(4-Methoxy-benzenesulfonyl)-5-phenyl-pentanoic a...)Show InChI InChI=1S/C18H21NO5S/c1-24-15-8-11-16(12-9-15)25(22,23)17(13-18(20)19-21)10-7-14-5-3-2-4-6-14/h2-6,8-9,11-12,17,21H,7,10,13H2,1H3,(H,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloproteinase-2 (MMP-2), gelatinase-A |
J Med Chem 42: 541-4 (1999)
Article DOI: 10.1021/jm980567e
BindingDB Entry DOI: 10.7270/Q2VQ31VC |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50074022
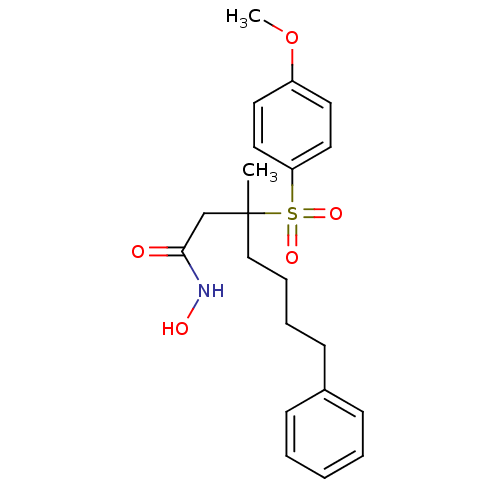
(3-(4-Methoxy-benzenesulfonyl)-3-methyl-7-phenyl-he...)Show SMILES COc1ccc(cc1)S(=O)(=O)C(C)(CCCCc1ccccc1)CC(=O)NO Show InChI InChI=1S/C21H27NO5S/c1-21(16-20(23)22-24,15-7-6-10-17-8-4-3-5-9-17)28(25,26)19-13-11-18(27-2)12-14-19/h3-5,8-9,11-14,24H,6-7,10,15-16H2,1-2H3,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloproteinase-2 (MMP-2), gelatinase-A |
J Med Chem 42: 541-4 (1999)
Article DOI: 10.1021/jm980567e
BindingDB Entry DOI: 10.7270/Q2VQ31VC |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50074028
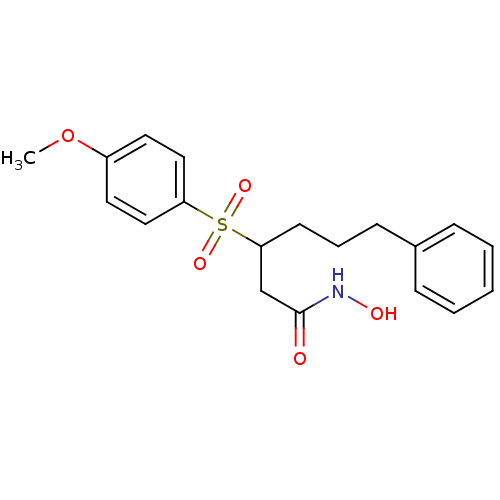
(3-(4-Methoxy-benzenesulfonyl)-6-phenyl-hexanoic ac...)Show SMILES COc1ccc(cc1)S(=O)(=O)C(CCCc1ccccc1)CC(=O)NO Show InChI InChI=1S/C19H23NO5S/c1-25-16-10-12-17(13-11-16)26(23,24)18(14-19(21)20-22)9-5-8-15-6-3-2-4-7-15/h2-4,6-7,10-13,18,22H,5,8-9,14H2,1H3,(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloproteinase-2 (MMP-2), gelatinase-A |
J Med Chem 42: 541-4 (1999)
Article DOI: 10.1021/jm980567e
BindingDB Entry DOI: 10.7270/Q2VQ31VC |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50074026
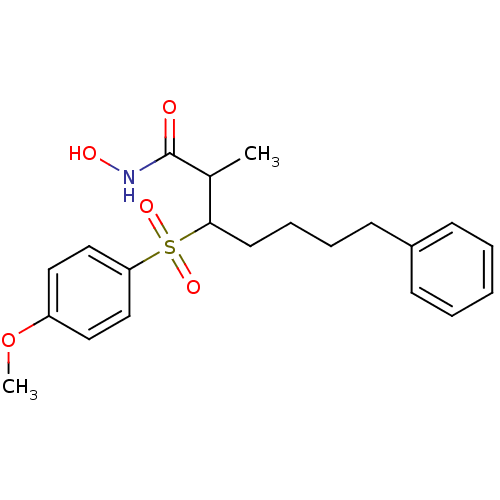
(3-(4-Methoxy-benzenesulfonyl)-2-methyl-7-phenyl-he...)Show SMILES COc1ccc(cc1)S(=O)(=O)C(CCCCc1ccccc1)C(C)C(=O)NO Show InChI InChI=1S/C21H27NO5S/c1-16(21(23)22-24)20(11-7-6-10-17-8-4-3-5-9-17)28(25,26)19-14-12-18(27-2)13-15-19/h3-5,8-9,12-16,20,24H,6-7,10-11H2,1-2H3,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloproteinase-2 (MMP-2), gelatinase-A |
J Med Chem 42: 541-4 (1999)
Article DOI: 10.1021/jm980567e
BindingDB Entry DOI: 10.7270/Q2VQ31VC |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50074021

(3-(4-Methoxy-benzenesulfonyl)-5-phenyl-pentanoic a...)Show InChI InChI=1S/C18H21NO5S/c1-24-15-8-11-16(12-9-15)25(22,23)17(13-18(20)19-21)10-7-14-5-3-2-4-6-14/h2-6,8-9,11-12,17,21H,7,10,13H2,1H3,(H,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloproteinase-3 (MMP-3), stromelysin-1 |
J Med Chem 42: 541-4 (1999)
Article DOI: 10.1021/jm980567e
BindingDB Entry DOI: 10.7270/Q2VQ31VC |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50074023

(CHEMBL153469 | N-Hydroxy-3-(4-methoxy-benzenesulfo...)Show InChI InChI=1S/C17H19NO5S/c1-23-14-7-9-15(10-8-14)24(21,22)16(12-17(19)18-20)11-13-5-3-2-4-6-13/h2-10,16,20H,11-12H2,1H3,(H,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloproteinase-3 (MMP-3), stromelysin-1 |
J Med Chem 42: 541-4 (1999)
Article DOI: 10.1021/jm980567e
BindingDB Entry DOI: 10.7270/Q2VQ31VC |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50074024

((2S,3R)-3-(4-Methoxy-benzenesulfonyl)-2-methyl-7-p...)Show SMILES COc1ccc(cc1)S(=O)(=O)[C@H](CCCCc1ccccc1)[C@@H](C)C(=O)NO Show InChI InChI=1S/C21H27NO5S/c1-16(21(23)22-24)20(11-7-6-10-17-8-4-3-5-9-17)28(25,26)19-14-12-18(27-2)13-15-19/h3-5,8-9,12-16,20,24H,6-7,10-11H2,1-2H3,(H,22,23)/t16-,20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloproteinase-3 (MMP-3), stromelysin-1 |
J Med Chem 42: 541-4 (1999)
Article DOI: 10.1021/jm980567e
BindingDB Entry DOI: 10.7270/Q2VQ31VC |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50074026

(3-(4-Methoxy-benzenesulfonyl)-2-methyl-7-phenyl-he...)Show SMILES COc1ccc(cc1)S(=O)(=O)C(CCCCc1ccccc1)C(C)C(=O)NO Show InChI InChI=1S/C21H27NO5S/c1-16(21(23)22-24)20(11-7-6-10-17-8-4-3-5-9-17)28(25,26)19-14-12-18(27-2)13-15-19/h3-5,8-9,12-16,20,24H,6-7,10-11H2,1-2H3,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloproteinase-3 (MMP-3), stromelysin-1 |
J Med Chem 42: 541-4 (1999)
Article DOI: 10.1021/jm980567e
BindingDB Entry DOI: 10.7270/Q2VQ31VC |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50074028

(3-(4-Methoxy-benzenesulfonyl)-6-phenyl-hexanoic ac...)Show SMILES COc1ccc(cc1)S(=O)(=O)C(CCCc1ccccc1)CC(=O)NO Show InChI InChI=1S/C19H23NO5S/c1-25-16-10-12-17(13-11-16)26(23,24)18(14-19(21)20-22)9-5-8-15-6-3-2-4-7-15/h2-4,6-7,10-13,18,22H,5,8-9,14H2,1H3,(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloproteinase-3 (MMP-3), stromelysin-1 |
J Med Chem 42: 541-4 (1999)
Article DOI: 10.1021/jm980567e
BindingDB Entry DOI: 10.7270/Q2VQ31VC |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50074027

(3-(4-Methoxy-benzenesulfonyl)-7-phenyl-heptanoic a...)Show SMILES COc1ccc(cc1)S(=O)(=O)C(CCCCc1ccccc1)CC(=O)NO Show InChI InChI=1S/C20H25NO5S/c1-26-17-11-13-18(14-12-17)27(24,25)19(15-20(22)21-23)10-6-5-9-16-7-3-2-4-8-16/h2-4,7-8,11-14,19,23H,5-6,9-10,15H2,1H3,(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloproteinase-3 (MMP-3), stromelysin-1 |
J Med Chem 42: 541-4 (1999)
Article DOI: 10.1021/jm980567e
BindingDB Entry DOI: 10.7270/Q2VQ31VC |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50074022

(3-(4-Methoxy-benzenesulfonyl)-3-methyl-7-phenyl-he...)Show SMILES COc1ccc(cc1)S(=O)(=O)C(C)(CCCCc1ccccc1)CC(=O)NO Show InChI InChI=1S/C21H27NO5S/c1-21(16-20(23)22-24,15-7-6-10-17-8-4-3-5-9-17)28(25,26)19-13-11-18(27-2)12-14-19/h3-5,8-9,11-14,24H,6-7,10,15-16H2,1-2H3,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloproteinase-3 (MMP-3), stromelysin-1 |
J Med Chem 42: 541-4 (1999)
Article DOI: 10.1021/jm980567e
BindingDB Entry DOI: 10.7270/Q2VQ31VC |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50074023

(CHEMBL153469 | N-Hydroxy-3-(4-methoxy-benzenesulfo...)Show InChI InChI=1S/C17H19NO5S/c1-23-14-7-9-15(10-8-14)24(21,22)16(12-17(19)18-20)11-13-5-3-2-4-6-13/h2-10,16,20H,11-12H2,1H3,(H,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloproteinase-1 (MMP-1), fibroblast collagenase. |
J Med Chem 42: 541-4 (1999)
Article DOI: 10.1021/jm980567e
BindingDB Entry DOI: 10.7270/Q2VQ31VC |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50074026

(3-(4-Methoxy-benzenesulfonyl)-2-methyl-7-phenyl-he...)Show SMILES COc1ccc(cc1)S(=O)(=O)C(CCCCc1ccccc1)C(C)C(=O)NO Show InChI InChI=1S/C21H27NO5S/c1-16(21(23)22-24)20(11-7-6-10-17-8-4-3-5-9-17)28(25,26)19-14-12-18(27-2)13-15-19/h3-5,8-9,12-16,20,24H,6-7,10-11H2,1-2H3,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloproteinase-1 (MMP-1), fibroblast collagenase. |
J Med Chem 42: 541-4 (1999)
Article DOI: 10.1021/jm980567e
BindingDB Entry DOI: 10.7270/Q2VQ31VC |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50074024

((2S,3R)-3-(4-Methoxy-benzenesulfonyl)-2-methyl-7-p...)Show SMILES COc1ccc(cc1)S(=O)(=O)[C@H](CCCCc1ccccc1)[C@@H](C)C(=O)NO Show InChI InChI=1S/C21H27NO5S/c1-16(21(23)22-24)20(11-7-6-10-17-8-4-3-5-9-17)28(25,26)19-14-12-18(27-2)13-15-19/h3-5,8-9,12-16,20,24H,6-7,10-11H2,1-2H3,(H,22,23)/t16-,20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloproteinase-1 (MMP-1), fibroblast collagenase. |
J Med Chem 42: 541-4 (1999)
Article DOI: 10.1021/jm980567e
BindingDB Entry DOI: 10.7270/Q2VQ31VC |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50074028

(3-(4-Methoxy-benzenesulfonyl)-6-phenyl-hexanoic ac...)Show SMILES COc1ccc(cc1)S(=O)(=O)C(CCCc1ccccc1)CC(=O)NO Show InChI InChI=1S/C19H23NO5S/c1-25-16-10-12-17(13-11-16)26(23,24)18(14-19(21)20-22)9-5-8-15-6-3-2-4-7-15/h2-4,6-7,10-13,18,22H,5,8-9,14H2,1H3,(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloproteinase-1 (MMP-1), fibroblast collagenase. |
J Med Chem 42: 541-4 (1999)
Article DOI: 10.1021/jm980567e
BindingDB Entry DOI: 10.7270/Q2VQ31VC |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50074022

(3-(4-Methoxy-benzenesulfonyl)-3-methyl-7-phenyl-he...)Show SMILES COc1ccc(cc1)S(=O)(=O)C(C)(CCCCc1ccccc1)CC(=O)NO Show InChI InChI=1S/C21H27NO5S/c1-21(16-20(23)22-24,15-7-6-10-17-8-4-3-5-9-17)28(25,26)19-13-11-18(27-2)12-14-19/h3-5,8-9,11-14,24H,6-7,10,15-16H2,1-2H3,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloproteinase-1 (MMP-1), fibroblast collagenase. |
J Med Chem 42: 541-4 (1999)
Article DOI: 10.1021/jm980567e
BindingDB Entry DOI: 10.7270/Q2VQ31VC |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50074021

(3-(4-Methoxy-benzenesulfonyl)-5-phenyl-pentanoic a...)Show InChI InChI=1S/C18H21NO5S/c1-24-15-8-11-16(12-9-15)25(22,23)17(13-18(20)19-21)10-7-14-5-3-2-4-6-14/h2-6,8-9,11-12,17,21H,7,10,13H2,1H3,(H,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloproteinase-1 (MMP-1), fibroblast collagenase. |
J Med Chem 42: 541-4 (1999)
Article DOI: 10.1021/jm980567e
BindingDB Entry DOI: 10.7270/Q2VQ31VC |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50074027

(3-(4-Methoxy-benzenesulfonyl)-7-phenyl-heptanoic a...)Show SMILES COc1ccc(cc1)S(=O)(=O)C(CCCCc1ccccc1)CC(=O)NO Show InChI InChI=1S/C20H25NO5S/c1-26-17-11-13-18(14-12-17)27(24,25)19(15-20(22)21-23)10-6-5-9-16-7-3-2-4-8-16/h2-4,7-8,11-14,19,23H,5-6,9-10,15H2,1H3,(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloproteinase-1 (MMP-1), fibroblast collagenase. |
J Med Chem 42: 541-4 (1999)
Article DOI: 10.1021/jm980567e
BindingDB Entry DOI: 10.7270/Q2VQ31VC |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50074025
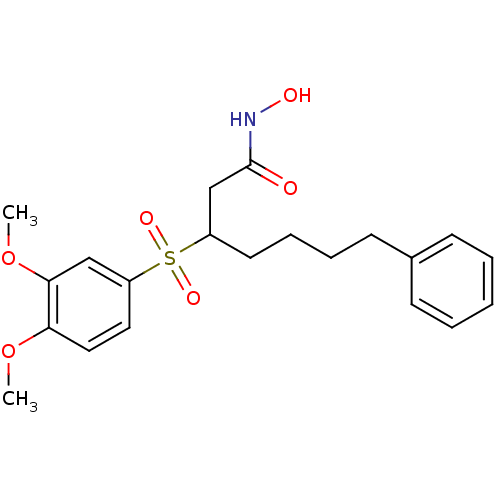
(3-(3,4-Dimethoxy-benzenesulfonyl)-7-phenyl-heptano...)Show SMILES COc1ccc(cc1OC)S(=O)(=O)C(CCCCc1ccccc1)CC(=O)NO Show InChI InChI=1S/C21H27NO6S/c1-27-19-13-12-18(14-20(19)28-2)29(25,26)17(15-21(23)22-24)11-7-6-10-16-8-4-3-5-9-16/h3-5,8-9,12-14,17,24H,6-7,10-11,15H2,1-2H3,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloproteinase-2 (MMP-2), gelatinase-A |
J Med Chem 42: 541-4 (1999)
Article DOI: 10.1021/jm980567e
BindingDB Entry DOI: 10.7270/Q2VQ31VC |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50074025

(3-(3,4-Dimethoxy-benzenesulfonyl)-7-phenyl-heptano...)Show SMILES COc1ccc(cc1OC)S(=O)(=O)C(CCCCc1ccccc1)CC(=O)NO Show InChI InChI=1S/C21H27NO6S/c1-27-19-13-12-18(14-20(19)28-2)29(25,26)17(15-21(23)22-24)11-7-6-10-16-8-4-3-5-9-16/h3-5,8-9,12-14,17,24H,6-7,10-11,15H2,1-2H3,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloproteinase-1 (MMP-1), fibroblast collagenase. |
J Med Chem 42: 541-4 (1999)
Article DOI: 10.1021/jm980567e
BindingDB Entry DOI: 10.7270/Q2VQ31VC |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50074025

(3-(3,4-Dimethoxy-benzenesulfonyl)-7-phenyl-heptano...)Show SMILES COc1ccc(cc1OC)S(=O)(=O)C(CCCCc1ccccc1)CC(=O)NO Show InChI InChI=1S/C21H27NO6S/c1-27-19-13-12-18(14-20(19)28-2)29(25,26)17(15-21(23)22-24)11-7-6-10-16-8-4-3-5-9-16/h3-5,8-9,12-14,17,24H,6-7,10-11,15H2,1-2H3,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloproteinase-3 (MMP-3), stromelysin-1 |
J Med Chem 42: 541-4 (1999)
Article DOI: 10.1021/jm980567e
BindingDB Entry DOI: 10.7270/Q2VQ31VC |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM18627

((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...)Show SMILES [H][C@@]12CC[C@@](O)(C#CC)[C@@]1(C)C[C@@H](C1=C3CCC(=O)C=C3CC[C@@]21[H])c1ccc(cc1)N(C)C |r,c:14,20| Show InChI InChI=1S/C29H35NO2/c1-5-15-29(32)16-14-26-24-12-8-20-17-22(31)11-13-23(20)27(24)25(18-28(26,29)2)19-6-9-21(10-7-19)30(3)4/h6-7,9-10,17,24-26,32H,8,11-14,16,18H2,1-4H3/t24-,25+,26-,28-,29-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Progesterone receptor antagonist activity based on its ability to block progesterone induced alkaline phosphatase in the human breast cancer cell lin... |
Bioorg Med Chem Lett 12: 3487-90 (2002)
BindingDB Entry DOI: 10.7270/Q2R210RC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50305075
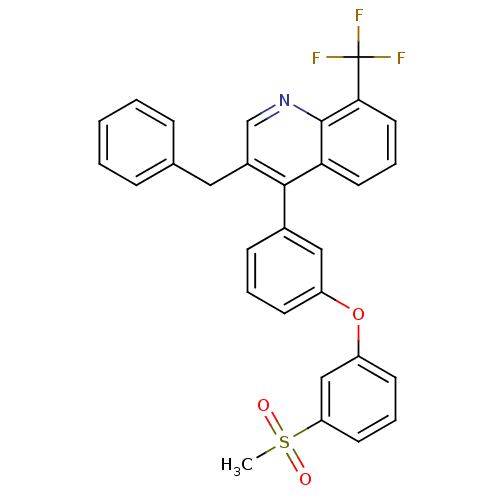
(3-benzyl-4-(3-(3-(methylsulfonyl)phenoxy)phenyl)-8...)Show SMILES CS(=O)(=O)c1cccc(Oc2cccc(c2)-c2c(Cc3ccccc3)cnc3c(cccc23)C(F)(F)F)c1 Show InChI InChI=1S/C30H22F3NO3S/c1-38(35,36)25-13-6-12-24(18-25)37-23-11-5-10-21(17-23)28-22(16-20-8-3-2-4-9-20)19-34-29-26(28)14-7-15-27(29)30(31,32)33/h2-15,17-19H,16H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.920 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]T0901317 from human recombinant LXRalpha-LBD |
Bioorg Med Chem Lett 20: 209-12 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.132
BindingDB Entry DOI: 10.7270/Q2KW5G4B |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50305077

(3-methyl-4-(3-(3-(methylsulfonyl)phenoxy)phenyl)-8...)Show SMILES Cc1cnc2c(cccc2c1-c1cccc(Oc2cccc(c2)S(C)(=O)=O)c1)C(F)(F)F Show InChI InChI=1S/C24H18F3NO3S/c1-15-14-28-23-20(10-5-11-21(23)24(25,26)27)22(15)16-6-3-7-17(12-16)31-18-8-4-9-19(13-18)32(2,29)30/h3-14H,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]T0901317 from human recombinant LXRalpha-LBD |
Bioorg Med Chem Lett 20: 209-12 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.132
BindingDB Entry DOI: 10.7270/Q2KW5G4B |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50305072
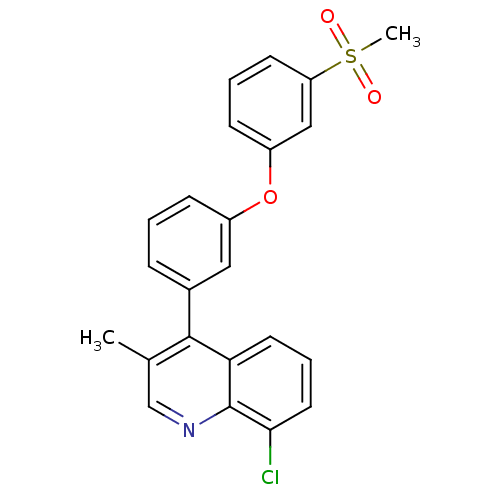
(8-chloro-3-methyl-4-(3-(3-(methylsulfonyl)phenoxy)...)Show SMILES Cc1cnc2c(Cl)cccc2c1-c1cccc(Oc2cccc(c2)S(C)(=O)=O)c1 Show InChI InChI=1S/C23H18ClNO3S/c1-15-14-25-23-20(10-5-11-21(23)24)22(15)16-6-3-7-17(12-16)28-18-8-4-9-19(13-18)29(2,26)27/h3-14H,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]T0901317 from human recombinant LXRalpha-LBD |
Bioorg Med Chem Lett 20: 209-12 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.132
BindingDB Entry DOI: 10.7270/Q2KW5G4B |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50305073
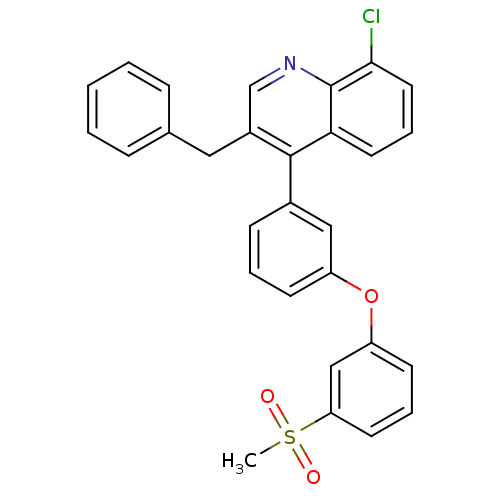
(3-benzyl-8-chloro-4-(3-(3-(methylsulfonyl)phenoxy)...)Show SMILES CS(=O)(=O)c1cccc(Oc2cccc(c2)-c2c(Cc3ccccc3)cnc3c(Cl)cccc23)c1 Show InChI InChI=1S/C29H22ClNO3S/c1-35(32,33)25-13-6-12-24(18-25)34-23-11-5-10-21(17-23)28-22(16-20-8-3-2-4-9-20)19-31-29-26(28)14-7-15-27(29)30/h2-15,17-19H,16H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]T0901317 from human recombinant LXRalpha-LBD |
Bioorg Med Chem Lett 20: 209-12 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.132
BindingDB Entry DOI: 10.7270/Q2KW5G4B |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50305064
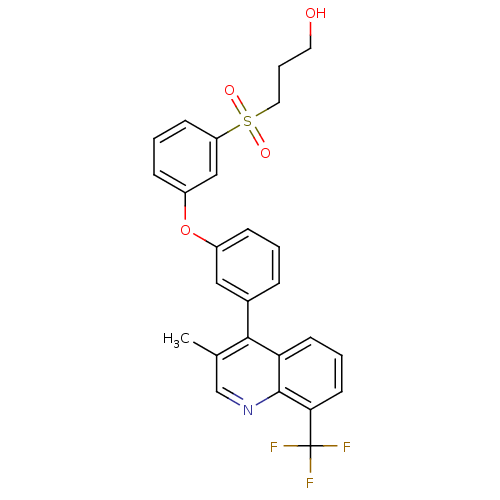
(3-(3-(3-(3-methyl-8-(trifluoromethyl)quinolin-4-yl...)Show SMILES Cc1cnc2c(cccc2c1-c1cccc(Oc2cccc(c2)S(=O)(=O)CCCO)c1)C(F)(F)F Show InChI InChI=1S/C26H22F3NO4S/c1-17-16-30-25-22(10-4-11-23(25)26(27,28)29)24(17)18-6-2-7-19(14-18)34-20-8-3-9-21(15-20)35(32,33)13-5-12-31/h2-4,6-11,14-16,31H,5,12-13H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]T0901317 from human recombinant LXRalpha-LBD |
Bioorg Med Chem Lett 20: 209-12 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.132
BindingDB Entry DOI: 10.7270/Q2KW5G4B |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50317735
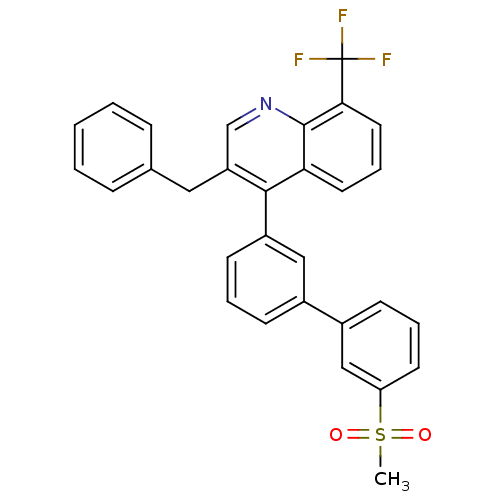
(3-benzyl-4-(3'-(methylsulfonyl)biphenyl-3-yl)-8-(t...)Show SMILES CS(=O)(=O)c1cccc(c1)-c1cccc(c1)-c1c(Cc2ccccc2)cnc2c(cccc12)C(F)(F)F Show InChI InChI=1S/C30H22F3NO2S/c1-37(35,36)25-13-6-11-22(18-25)21-10-5-12-23(17-21)28-24(16-20-8-3-2-4-9-20)19-34-29-26(28)14-7-15-27(29)30(31,32)33/h2-15,17-19H,16H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]T0901317 from LXRbeta ligand binding domain |
Bioorg Med Chem Lett 20: 2903-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.031
BindingDB Entry DOI: 10.7270/Q2TD9XJZ |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50305070
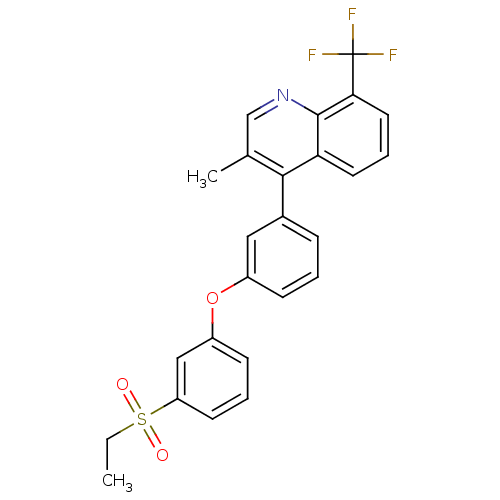
(4-(3-(3-(ethylsulfonyl)phenoxy)phenyl)-3-methyl-8-...)Show SMILES CCS(=O)(=O)c1cccc(Oc2cccc(c2)-c2c(C)cnc3c(cccc23)C(F)(F)F)c1 Show InChI InChI=1S/C25H20F3NO3S/c1-3-33(30,31)20-10-5-9-19(14-20)32-18-8-4-7-17(13-18)23-16(2)15-29-24-21(23)11-6-12-22(24)25(26,27)28/h4-15H,3H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]T0901317 from human recombinant LXRalpha-LBD |
Bioorg Med Chem Lett 20: 209-12 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.132
BindingDB Entry DOI: 10.7270/Q2KW5G4B |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50317744
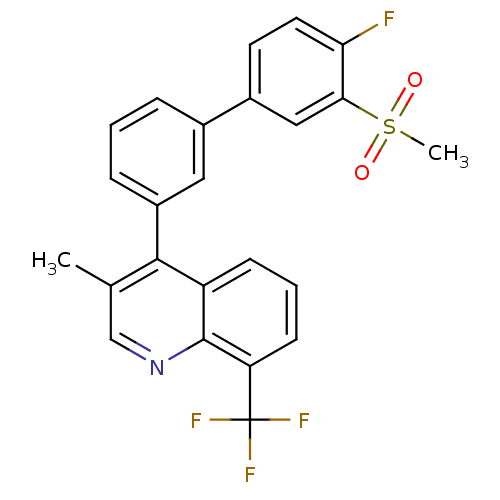
(4-(4'-fluoro-3'-(methylsulfonyl)biphenyl-3-yl)-3-m...)Show SMILES Cc1cnc2c(cccc2c1-c1cccc(c1)-c1ccc(F)c(c1)S(C)(=O)=O)C(F)(F)F Show InChI InChI=1S/C24H17F4NO2S/c1-14-13-29-23-18(7-4-8-19(23)24(26,27)28)22(14)17-6-3-5-15(11-17)16-9-10-20(25)21(12-16)32(2,30)31/h3-13H,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]T0901317 from LXRbeta ligand binding domain |
Bioorg Med Chem Lett 20: 2903-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.031
BindingDB Entry DOI: 10.7270/Q2TD9XJZ |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50305068
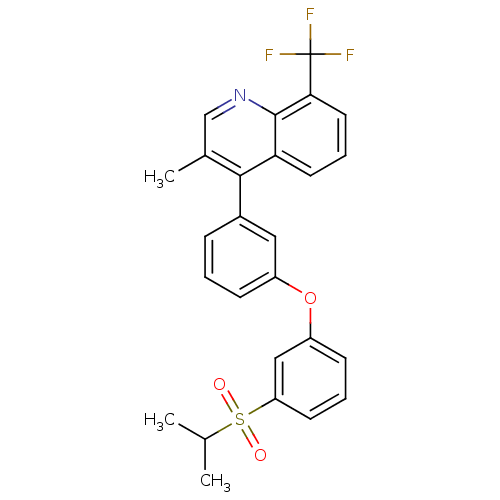
(4-(3-(3-(isopropylsulfonyl)phenoxy)phenyl)-3-methy...)Show SMILES CC(C)S(=O)(=O)c1cccc(Oc2cccc(c2)-c2c(C)cnc3c(cccc23)C(F)(F)F)c1 Show InChI InChI=1S/C26H22F3NO3S/c1-16(2)34(31,32)21-10-5-9-20(14-21)33-19-8-4-7-18(13-19)24-17(3)15-30-25-22(24)11-6-12-23(25)26(27,28)29/h4-16H,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]T0901317 from human recombinant LXRalpha-LBD |
Bioorg Med Chem Lett 20: 209-12 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.132
BindingDB Entry DOI: 10.7270/Q2KW5G4B |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50317733
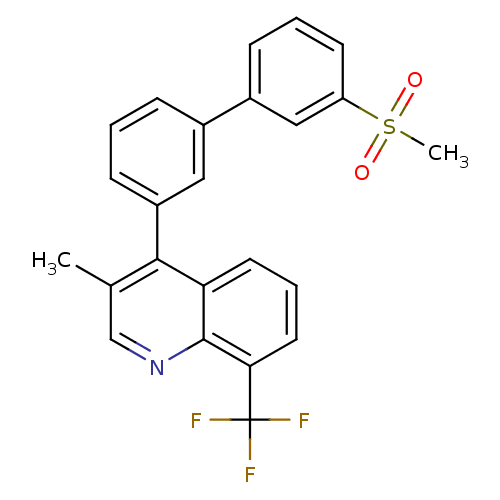
(3-methyl-4-(3'-(methylsulfonyl)biphenyl-3-yl)-8-(t...)Show SMILES Cc1cnc2c(cccc2c1-c1cccc(c1)-c1cccc(c1)S(C)(=O)=O)C(F)(F)F Show InChI InChI=1S/C24H18F3NO2S/c1-15-14-28-23-20(10-5-11-21(23)24(25,26)27)22(15)18-8-3-6-16(12-18)17-7-4-9-19(13-17)31(2,29)30/h3-14H,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]T0901317 from LXRbeta ligand binding domain |
Bioorg Med Chem Lett 20: 2903-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.031
BindingDB Entry DOI: 10.7270/Q2TD9XJZ |
More data for this
Ligand-Target Pair | |
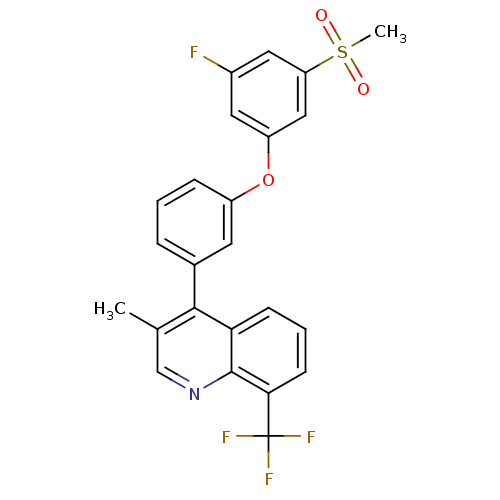
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50305060
(4-(3-(3-fluoro-5-(methylsulfonyl)phenoxy)phenyl)-3...)Show SMILES Cc1cnc2c(cccc2c1-c1cccc(Oc2cc(F)cc(c2)S(C)(=O)=O)c1)C(F)(F)F Show InChI InChI=1S/C24H17F4NO3S/c1-14-13-29-23-20(7-4-8-21(23)24(26,27)28)22(14)15-5-3-6-17(9-15)32-18-10-16(25)11-19(12-18)33(2,30)31/h3-13H,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]T0901317 from human recombinant LXRalpha-LBD |
Bioorg Med Chem Lett 20: 209-12 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.132
BindingDB Entry DOI: 10.7270/Q2KW5G4B |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50305075

(3-benzyl-4-(3-(3-(methylsulfonyl)phenoxy)phenyl)-8...)Show SMILES CS(=O)(=O)c1cccc(Oc2cccc(c2)-c2c(Cc3ccccc3)cnc3c(cccc23)C(F)(F)F)c1 Show InChI InChI=1S/C30H22F3NO3S/c1-38(35,36)25-13-6-12-24(18-25)37-23-11-5-10-21(17-23)28-22(16-20-8-3-2-4-9-20)19-34-29-26(28)14-7-15-27(29)30(31,32)33/h2-15,17-19H,16H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]T0901317 from human recombinant LXRbeta-LBD |
Bioorg Med Chem Lett 20: 209-12 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.132
BindingDB Entry DOI: 10.7270/Q2KW5G4B |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50305077

(3-methyl-4-(3-(3-(methylsulfonyl)phenoxy)phenyl)-8...)Show SMILES Cc1cnc2c(cccc2c1-c1cccc(Oc2cccc(c2)S(C)(=O)=O)c1)C(F)(F)F Show InChI InChI=1S/C24H18F3NO3S/c1-15-14-28-23-20(10-5-11-21(23)24(25,26)27)22(15)16-6-3-7-17(12-16)31-18-8-4-9-19(13-18)32(2,29)30/h3-14H,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]T0901317 from human recombinant LXRbeta-LBD |
Bioorg Med Chem Lett 20: 209-12 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.132
BindingDB Entry DOI: 10.7270/Q2KW5G4B |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50317737
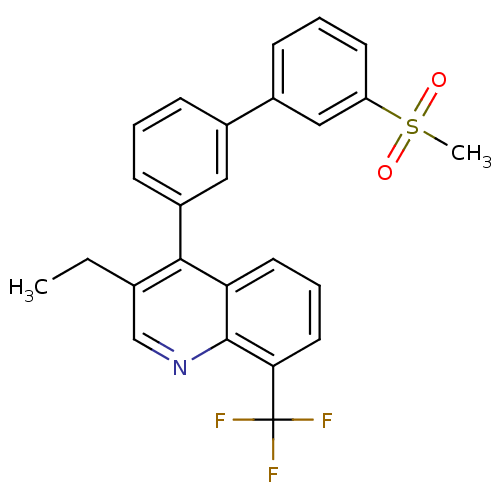
(3-ethyl-4-(3'-(methylsulfonyl)biphenyl-3-yl)-8-(tr...)Show SMILES CCc1cnc2c(cccc2c1-c1cccc(c1)-c1cccc(c1)S(C)(=O)=O)C(F)(F)F Show InChI InChI=1S/C25H20F3NO2S/c1-3-16-15-29-24-21(11-6-12-22(24)25(26,27)28)23(16)19-9-4-7-17(13-19)18-8-5-10-20(14-18)32(2,30)31/h4-15H,3H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]T0901317 from LXRbeta ligand binding domain |
Bioorg Med Chem Lett 20: 2903-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.031
BindingDB Entry DOI: 10.7270/Q2TD9XJZ |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50305067
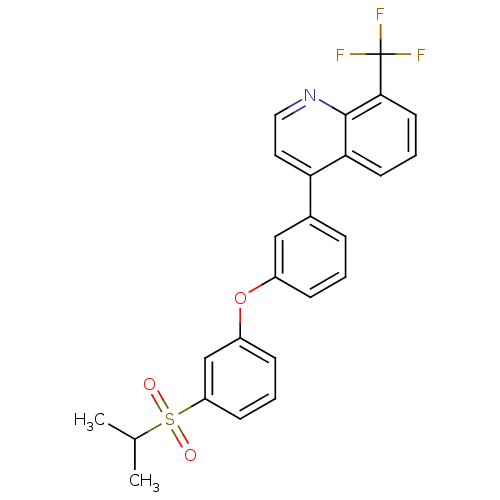
(4-(3-(3-(isopropylsulfonyl)phenoxy)phenyl)-8-(trif...)Show SMILES CC(C)S(=O)(=O)c1cccc(Oc2cccc(c2)-c2ccnc3c(cccc23)C(F)(F)F)c1 Show InChI InChI=1S/C25H20F3NO3S/c1-16(2)33(30,31)20-9-4-8-19(15-20)32-18-7-3-6-17(14-18)21-12-13-29-24-22(21)10-5-11-23(24)25(26,27)28/h3-16H,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]T0901317 from human recombinant LXRalpha-LBD |
Bioorg Med Chem Lett 20: 209-12 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.132
BindingDB Entry DOI: 10.7270/Q2KW5G4B |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50305069
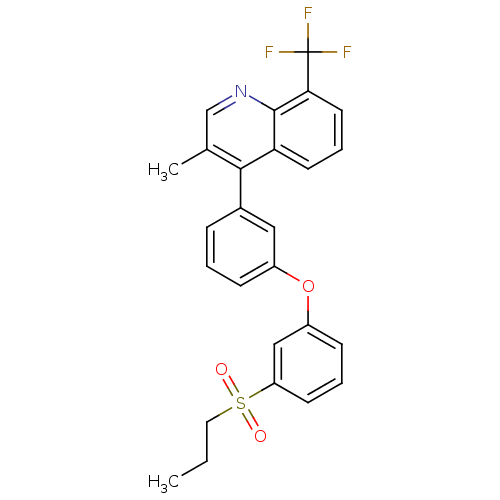
(3-methyl-4-(3-(3-(propylsulfonyl)phenoxy)phenyl)-8...)Show SMILES CCCS(=O)(=O)c1cccc(Oc2cccc(c2)-c2c(C)cnc3c(cccc23)C(F)(F)F)c1 Show InChI InChI=1S/C26H22F3NO3S/c1-3-13-34(31,32)21-10-5-9-20(15-21)33-19-8-4-7-18(14-19)24-17(2)16-30-25-22(24)11-6-12-23(25)26(27,28)29/h4-12,14-16H,3,13H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]T0901317 from human recombinant LXRalpha-LBD |
Bioorg Med Chem Lett 20: 209-12 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.132
BindingDB Entry DOI: 10.7270/Q2KW5G4B |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
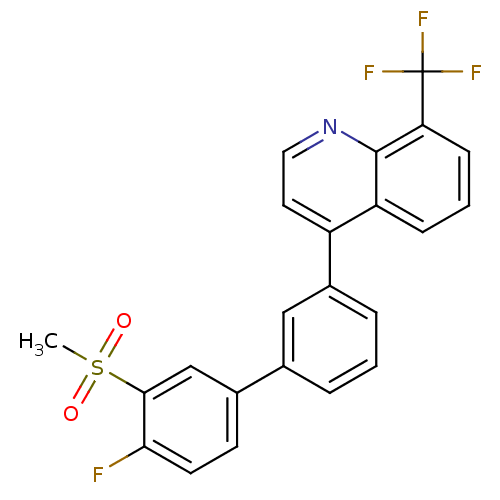
(Homo sapiens (Human)) | BDBM50317746
(4-(4'-fluoro-3'-(methylsulfonyl)biphenyl-3-yl)-8-(...)Show SMILES CS(=O)(=O)c1cc(ccc1F)-c1cccc(c1)-c1ccnc2c(cccc12)C(F)(F)F Show InChI InChI=1S/C23H15F4NO2S/c1-31(29,30)21-13-15(8-9-20(21)24)14-4-2-5-16(12-14)17-10-11-28-22-18(17)6-3-7-19(22)23(25,26)27/h2-13H,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]T0901317 from LXRbeta ligand binding domain |
Bioorg Med Chem Lett 20: 2903-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.031
BindingDB Entry DOI: 10.7270/Q2TD9XJZ |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
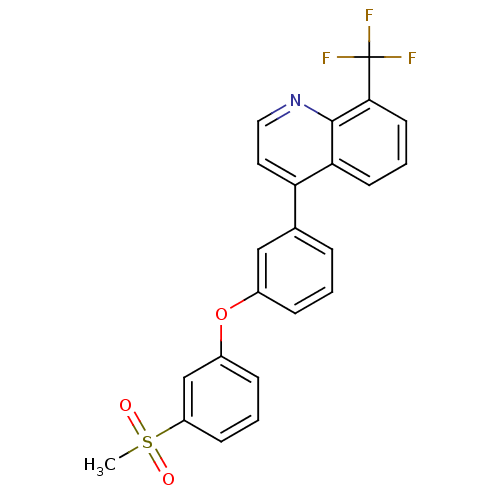
(Homo sapiens (Human)) | BDBM50305074
(4-(3-(3-(methylsulfonyl)phenoxy)phenyl)-8-(trifluo...)Show SMILES CS(=O)(=O)c1cccc(Oc2cccc(c2)-c2ccnc3c(cccc23)C(F)(F)F)c1 Show InChI InChI=1S/C23H16F3NO3S/c1-31(28,29)18-8-3-7-17(14-18)30-16-6-2-5-15(13-16)19-11-12-27-22-20(19)9-4-10-21(22)23(24,25)26/h2-14H,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]T0901317 from human recombinant LXRalpha-LBD |
Bioorg Med Chem Lett 20: 209-12 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.132
BindingDB Entry DOI: 10.7270/Q2KW5G4B |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
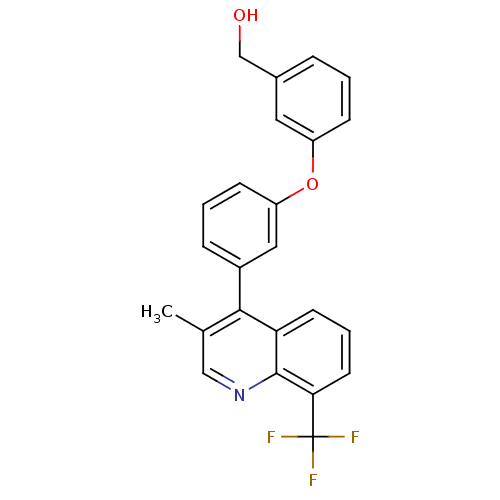
(Homo sapiens (Human)) | BDBM35094
(biarylether alcohol quinoline, 5f)Show SMILES Cc1cnc2c(cccc2c1-c1cccc(Oc2cccc(CO)c2)c1)C(F)(F)F Show InChI InChI=1S/C24H18F3NO2/c1-15-13-28-23-20(9-4-10-21(23)24(25,26)27)22(15)17-6-3-8-19(12-17)30-18-7-2-5-16(11-18)14-29/h2-13,29H,14H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]T0901317 from human recombinant LXRalpha-LBD |
Bioorg Med Chem Lett 20: 209-12 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.132
BindingDB Entry DOI: 10.7270/Q2KW5G4B |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50305059
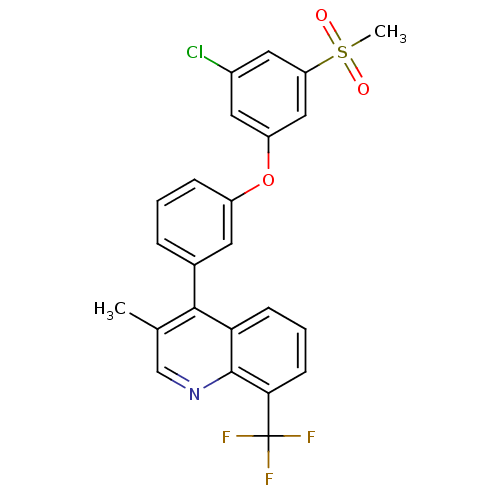
(4-(3-(3-chloro-5-(methylsulfonyl)phenoxy)phenyl)-3...)Show SMILES Cc1cnc2c(cccc2c1-c1cccc(Oc2cc(Cl)cc(c2)S(C)(=O)=O)c1)C(F)(F)F Show InChI InChI=1S/C24H17ClF3NO3S/c1-14-13-29-23-20(7-4-8-21(23)24(26,27)28)22(14)15-5-3-6-17(9-15)32-18-10-16(25)11-19(12-18)33(2,30)31/h3-13H,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]T0901317 from human recombinant LXRalpha-LBD |
Bioorg Med Chem Lett 20: 209-12 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.132
BindingDB Entry DOI: 10.7270/Q2KW5G4B |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM17292

((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@]1([H])c3ccc(O)cc3CC[C@@]21[H] Show InChI InChI=1S/C18H24O2/c1-18-9-8-14-13-5-3-12(19)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3,5,10,14-17,19-20H,2,4,6-9H2,1H3/t14-,15-,16+,17+,18+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human LBD of ERbeta |
Bioorg Med Chem Lett 17: 118-22 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.088
BindingDB Entry DOI: 10.7270/Q2XW4JFX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50305062
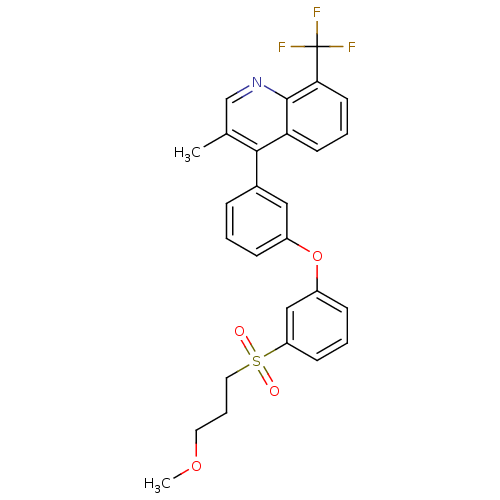
(4-(3-(3-(3-methoxypropylsulfonyl)phenoxy)phenyl)-3...)Show SMILES COCCCS(=O)(=O)c1cccc(Oc2cccc(c2)-c2c(C)cnc3c(cccc23)C(F)(F)F)c1 Show InChI InChI=1S/C27H24F3NO4S/c1-18-17-31-26-23(11-5-12-24(26)27(28,29)30)25(18)19-7-3-8-20(15-19)35-21-9-4-10-22(16-21)36(32,33)14-6-13-34-2/h3-5,7-12,15-17H,6,13-14H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]T0901317 from human recombinant LXRalpha-LBD |
Bioorg Med Chem Lett 20: 209-12 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.132
BindingDB Entry DOI: 10.7270/Q2KW5G4B |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50317742
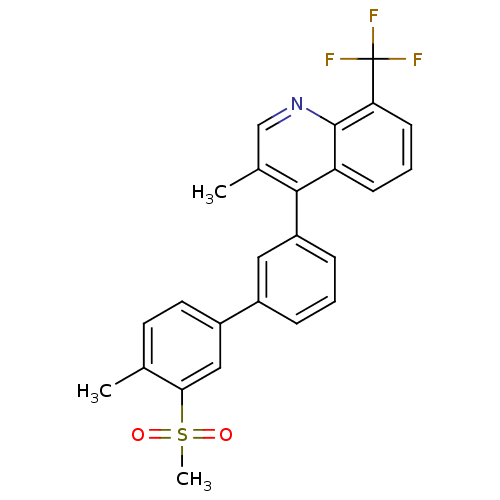
(3-methyl-4-(4'-methyl-3'-(methylsulfonyl)biphenyl-...)Show SMILES Cc1ccc(cc1S(C)(=O)=O)-c1cccc(c1)-c1c(C)cnc2c(cccc12)C(F)(F)F Show InChI InChI=1S/C25H20F3NO2S/c1-15-10-11-18(13-22(15)32(3,30)31)17-6-4-7-19(12-17)23-16(2)14-29-24-20(23)8-5-9-21(24)25(26,27)28/h4-14H,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]T0901317 from LXRbeta ligand binding domain |
Bioorg Med Chem Lett 20: 2903-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.031
BindingDB Entry DOI: 10.7270/Q2TD9XJZ |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50317745
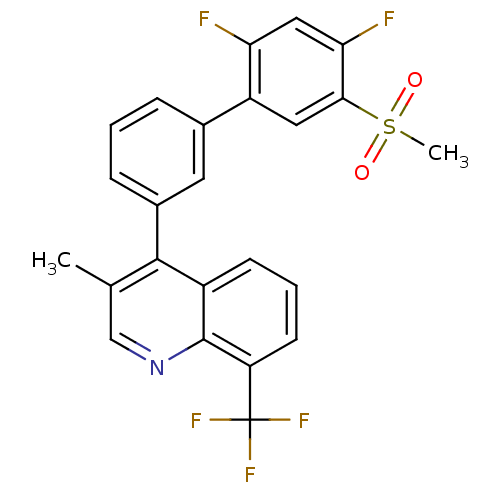
(4-(2',4'-difluoro-5'-(methylsulfonyl)biphenyl-3-yl...)Show SMILES Cc1cnc2c(cccc2c1-c1cccc(c1)-c1cc(c(F)cc1F)S(C)(=O)=O)C(F)(F)F Show InChI InChI=1S/C24H16F5NO2S/c1-13-12-30-23-16(7-4-8-18(23)24(27,28)29)22(13)15-6-3-5-14(9-15)17-10-21(33(2,31)32)20(26)11-19(17)25/h3-12H,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]T0901317 from LXRbeta ligand binding domain |
Bioorg Med Chem Lett 20: 2903-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.031
BindingDB Entry DOI: 10.7270/Q2TD9XJZ |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50305073

(3-benzyl-8-chloro-4-(3-(3-(methylsulfonyl)phenoxy)...)Show SMILES CS(=O)(=O)c1cccc(Oc2cccc(c2)-c2c(Cc3ccccc3)cnc3c(Cl)cccc23)c1 Show InChI InChI=1S/C29H22ClNO3S/c1-35(32,33)25-13-6-12-24(18-25)34-23-11-5-10-21(17-23)28-22(16-20-8-3-2-4-9-20)19-31-29-26(28)14-7-15-27(29)30/h2-15,17-19H,16H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]T0901317 from human recombinant LXRbeta-LBD |
Bioorg Med Chem Lett 20: 209-12 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.132
BindingDB Entry DOI: 10.7270/Q2KW5G4B |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data