 Found 3740 hits with Last Name = 'ung' and Initial = 't'
Found 3740 hits with Last Name = 'ung' and Initial = 't' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50537072

(CHEMBL440072)Show SMILES C[C@@H](O)[C@H]1NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](CCCCN)NC(=O)[C@@H](CSSC[C@H](NC(=O)[C@H](CO)NC1=O)C(O)=O)NC(=O)CNC(=O)[C@H](C)N Show InChI InChI=1S/C63H88N16O16S2/c1-34(66)53(84)69-30-51(83)70-48-32-96-97-33-49(63(94)95)78-60(91)47(31-80)77-62(93)52(35(2)81)79-55(86)42(22-12-14-24-65)71-58(89)45(27-38-29-68-40-20-10-9-19-39(38)40)75-57(88)44(26-37-17-7-4-8-18-37)73-56(87)43(25-36-15-5-3-6-16-36)74-59(90)46(28-50(67)82)76-54(85)41(72-61(48)92)21-11-13-23-64/h3-10,15-20,29,34-35,41-49,52,68,80-81H,11-14,21-28,30-33,64-66H2,1-2H3,(H2,67,82)(H,69,84)(H,70,83)(H,71,89)(H,72,92)(H,73,87)(H,74,90)(H,75,88)(H,76,85)(H,77,93)(H,78,91)(H,79,86)(H,94,95)/t34-,35+,41+,42-,43+,44-,45+,46-,47-,48+,49-,52+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tarveda Therapeutics
Curated by ChEMBL
| Assay Description
Displacement of [125I]somatostatin from human SSTR2 expressed in CHO-K1 cell membranes after 240 mins |
J Med Chem 62: 2708-2719 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02036
BindingDB Entry DOI: 10.7270/Q2NK3JJ9 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50537063

(CHEMBL4590517)Show SMILES [H][C@@]12O[C@]1(C)[C@H](CC(=O)N(C)c1cc(C\C(C)=C\C=C\[C@@H](OC)[C@@]3(O)C[C@]([H])(OC(=O)N3)[C@H]2C)cc(OC)c1Cl)OC(=O)[C@H](C)N(C)C(=O)CCSSC[C@H](NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@H](N)Cc2ccccc2)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N[C@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCCN)C(=O)N[C@@]([H])([C@@H](C)O)C(=O)N1)C(O)=O |r,t:17,19| Show InChI InChI=1S/C83H108ClN13O21S4/c1-44-18-17-24-65(115-9)83(113)39-64(116-81(112)95-83)45(2)71-82(5,118-71)66(38-68(101)97(7)62-35-50(32-44)36-63(114-8)69(62)84)117-80(111)46(3)96(6)67(100)29-31-119-120-43-61(79(109)110)93-77(107)60-42-122-121-41-59(91-72(102)54(86)33-48-19-11-10-12-20-48)76(106)89-57(34-49-25-27-52(99)28-26-49)74(104)90-58(37-51-40-87-55-22-14-13-21-53(51)55)75(105)88-56(23-15-16-30-85)73(103)94-70(47(4)98)78(108)92-60/h10-14,17-22,24-28,35-36,40,45-47,54,56-61,64-66,70-71,87,98-99,113H,15-16,23,29-34,37-39,41-43,85-86H2,1-9H3,(H,88,105)(H,89,106)(H,90,104)(H,91,102)(H,92,108)(H,93,107)(H,94,103)(H,95,112)(H,109,110)/b24-17+,44-18+/t45-,46+,47-,54-,56+,57+,58-,59+,60+,61+,64+,65-,66+,70+,71+,82-,83+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tarveda Therapeutics
Curated by ChEMBL
| Assay Description
Displacement of [125I]somatostatin from human SSTR2 expressed in CHO-K1 cell membranes after 240 mins |
J Med Chem 62: 2708-2719 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02036
BindingDB Entry DOI: 10.7270/Q2NK3JJ9 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50537077

(CHEMBL4550617)Show SMILES [H][C@@]12O[C@]1(C)[C@H](CC(=O)N(C)c1cc(C\C(C)=C\C=C\[C@@H](OC)[C@@]3(O)C[C@]([H])(OC(=O)N3)[C@H]2C)cc(OC)c1Cl)OC(=O)[C@H](C)N(C)C(=O)CCSSCCOc1ccc(C[C@@H](N)C(=O)N[C@H]2CSSC[C@H](NC(=O)[C@@]([H])(NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc3c[nH]c4ccccc34)NC(=O)[C@H](Cc3ccc(O)cc3)NC2=O)[C@@H](C)O)C(=O)N[C@@H]([C@@H](C)O)C(O)=O)cc1 |r,t:17,19| Show InChI InChI=1S/C86H114ClN13O23S4/c1-45-16-15-20-67(119-10)86(117)41-66(121-84(116)98-86)46(2)74-85(6,123-74)68(40-70(105)100(8)64-37-52(34-45)38-65(118-9)71(64)87)122-83(115)47(3)99(7)69(104)29-32-124-125-33-31-120-55-27-23-50(24-28-55)35-57(89)75(106)94-62-43-126-127-44-63(80(111)97-73(49(5)102)82(113)114)95-81(112)72(48(4)101)96-76(107)59(19-13-14-30-88)91-78(109)61(39-53-42-90-58-18-12-11-17-56(53)58)93-77(108)60(92-79(62)110)36-51-21-25-54(103)26-22-51/h11-12,15-18,20-28,37-38,42,46-49,57,59-63,66-68,72-74,90,101-103,117H,13-14,19,29-36,39-41,43-44,88-89H2,1-10H3,(H,91,109)(H,92,110)(H,93,108)(H,94,106)(H,95,112)(H,96,107)(H,97,111)(H,98,116)(H,113,114)/b20-15+,45-16+/t46-,47+,48-,49-,57-,59+,60+,61-,62+,63+,66+,67-,68+,72+,73+,74+,85-,86+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tarveda Therapeutics
Curated by ChEMBL
| Assay Description
Displacement of [125I]somatostatin from human SSTR2 expressed in CHO-K1 cell membranes after 240 mins |
J Med Chem 62: 2708-2719 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02036
BindingDB Entry DOI: 10.7270/Q2NK3JJ9 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50537069
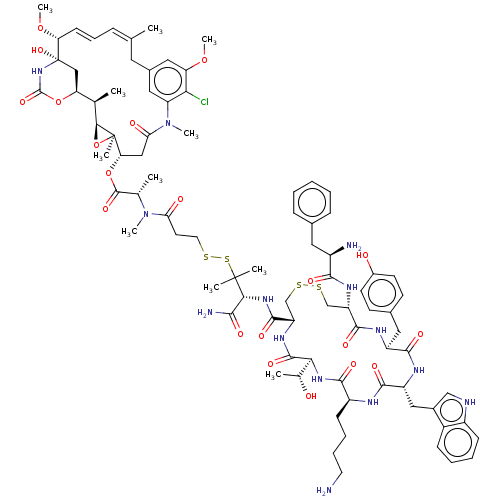
(CHEMBL4584764)Show SMILES [H][C@@]12O[C@]1(C)[C@H](CC(=O)N(C)c1cc(C\C(C)=C\C=C\[C@@H](OC)[C@@]3(O)C[C@]([H])(OC(=O)N3)[C@H]2C)cc(OC)c1Cl)OC(=O)[C@H](C)N(C)C(=O)CCSSC(C)(C)[C@H](NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@H](N)Cc2ccccc2)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N[C@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCCN)C(=O)N[C@@]([H])([C@@H](C)O)C(=O)N1)C(N)=O |r,t:17,19| Show InChI InChI=1S/C85H113ClN14O20S4/c1-45-20-19-26-65(117-11)85(115)41-64(118-82(114)98-85)46(2)72-84(7,120-72)66(40-68(104)100(9)62-37-51(34-45)38-63(116-10)69(62)86)119-81(113)47(3)99(8)67(103)31-33-121-124-83(5,6)71(73(89)105)97-79(111)61-44-123-122-43-60(94-74(106)55(88)35-49-21-13-12-14-22-49)78(110)92-58(36-50-27-29-53(102)30-28-50)76(108)93-59(39-52-42-90-56-24-16-15-23-54(52)56)77(109)91-57(25-17-18-32-87)75(107)96-70(48(4)101)80(112)95-61/h12-16,19-24,26-30,37-38,42,46-48,55,57-61,64-66,70-72,90,101-102,115H,17-18,25,31-36,39-41,43-44,87-88H2,1-11H3,(H2,89,105)(H,91,109)(H,92,110)(H,93,108)(H,94,106)(H,95,112)(H,96,107)(H,97,111)(H,98,114)/b26-19+,45-20+/t46-,47+,48-,55-,57+,58+,59-,60+,61+,64+,65-,66+,70+,71-,72+,84-,85+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tarveda Therapeutics
Curated by ChEMBL
| Assay Description
Displacement of [125I]somatostatin from human SSTR2 expressed in CHO-K1 cell membranes after 240 mins |
J Med Chem 62: 2708-2719 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02036
BindingDB Entry DOI: 10.7270/Q2NK3JJ9 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50537066

(CHEMBL4541310)Show SMILES [H][C@@]12O[C@]1(C)[C@H](CC(=O)N(C)c1cc(C\C(C)=C\C=C\[C@@H](OC)[C@@]3(O)C[C@]([H])(OC(=O)N3)[C@H]2C)cc(OC)c1Cl)OC(=O)[C@H](C)N(C)C(=O)CCSSCCCN[C@H](Cc1ccccc1)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@@]([H])(NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccc(O)cc2)NC1=O)[C@@H](C)O)C(=O)N[C@@H]([C@@H](C)O)C(O)=O |r,t:17,19| Show InChI InChI=1S/C87H116ClN13O22S4/c1-47-20-18-26-68(120-10)87(118)43-67(121-85(117)99-87)48(2)75-86(6,123-75)69(42-71(106)101(8)65-39-54(36-47)40-66(119-9)72(65)88)122-84(116)49(3)100(7)70(105)31-35-125-124-34-19-33-90-60(37-52-21-12-11-13-22-52)77(108)95-63-45-126-127-46-64(81(112)98-74(51(5)103)83(114)115)96-82(113)73(50(4)102)97-76(107)59(25-16-17-32-89)92-79(110)62(41-55-44-91-58-24-15-14-23-57(55)58)94-78(109)61(93-80(63)111)38-53-27-29-56(104)30-28-53/h11-15,18,20-24,26-30,39-40,44,48-51,59-64,67-69,73-75,90-91,102-104,118H,16-17,19,25,31-38,41-43,45-46,89H2,1-10H3,(H,92,110)(H,93,111)(H,94,109)(H,95,108)(H,96,113)(H,97,107)(H,98,112)(H,99,117)(H,114,115)/b26-18+,47-20+/t48-,49+,50-,51-,59+,60-,61+,62-,63+,64+,67+,68-,69+,73+,74+,75+,86-,87+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tarveda Therapeutics
Curated by ChEMBL
| Assay Description
Displacement of [125I]somatostatin from human SSTR2 expressed in CHO-K1 cell membranes after 240 mins |
J Med Chem 62: 2708-2719 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02036
BindingDB Entry DOI: 10.7270/Q2NK3JJ9 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50537076

(CHEMBL4564727)Show SMILES [H][C@@]12O[C@]1(C)[C@H](CC(=O)N(C)c1cc(C\C(C)=C\C=C\[C@@H](OC)[C@@]3(O)C[C@]([H])(OC(=O)N3)[C@H]2C)cc(OC)c1Cl)OC(=O)[C@H](C)N(C)C(=O)CCSSC[C@@H](CO)NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@H](N)Cc2ccccc2)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N[C@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCCN)C(=O)N[C@@]([H])([C@@H](C)O)C(=O)N1 |r,t:17,19| Show InChI InChI=1S/C83H110ClN13O20S4/c1-45-18-17-24-66(114-9)83(112)39-65(115-81(111)95-83)46(2)72-82(5,117-72)67(38-69(102)97(7)63-35-51(32-45)36-64(113-8)70(63)84)116-80(110)47(3)96(6)68(101)29-31-118-119-42-53(41-98)88-77(107)61-43-120-121-44-62(92-73(103)56(86)33-49-19-11-10-12-20-49)78(108)90-59(34-50-25-27-54(100)28-26-50)75(105)91-60(37-52-40-87-57-22-14-13-21-55(52)57)76(106)89-58(23-15-16-30-85)74(104)94-71(48(4)99)79(109)93-61/h10-14,17-22,24-28,35-36,40,46-48,53,56,58-62,65-67,71-72,87,98-100,112H,15-16,23,29-34,37-39,41-44,85-86H2,1-9H3,(H,88,107)(H,89,106)(H,90,108)(H,91,105)(H,92,103)(H,93,109)(H,94,104)(H,95,111)/b24-17+,45-18+/t46-,47+,48-,53-,56-,58+,59+,60-,61+,62+,65+,66-,67+,71+,72+,82-,83+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tarveda Therapeutics
Curated by ChEMBL
| Assay Description
Displacement of [125I]somatostatin from human SSTR2 expressed in CHO-K1 cell membranes after 240 mins |
J Med Chem 62: 2708-2719 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02036
BindingDB Entry DOI: 10.7270/Q2NK3JJ9 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50537061
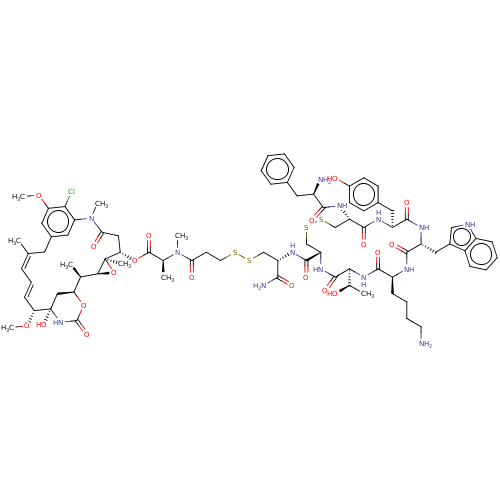
(CHEMBL4527856)Show SMILES [H][C@@]12O[C@]1(C)[C@H](CC(=O)N(C)c1cc(C\C(C)=C\C=C\[C@@H](OC)[C@@]3(O)C[C@]([H])(OC(=O)N3)[C@H]2C)cc(OC)c1Cl)OC(=O)[C@H](C)N(C)C(=O)CCSSC[C@H](NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@H](N)Cc2ccccc2)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N[C@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCCN)C(=O)N[C@@]([H])([C@@H](C)O)C(=O)N1)C(N)=O |r,t:17,19| Show InChI InChI=1S/C83H109ClN14O20S4/c1-44-18-17-24-65(115-9)83(113)39-64(116-81(112)96-83)45(2)71-82(5,118-71)66(38-68(102)98(7)62-35-50(32-44)36-63(114-8)69(62)84)117-80(111)46(3)97(6)67(101)29-31-119-120-41-59(72(87)103)92-78(109)61-43-122-121-42-60(93-73(104)54(86)33-48-19-11-10-12-20-48)77(108)90-57(34-49-25-27-52(100)28-26-49)75(106)91-58(37-51-40-88-55-22-14-13-21-53(51)55)76(107)89-56(23-15-16-30-85)74(105)95-70(47(4)99)79(110)94-61/h10-14,17-22,24-28,35-36,40,45-47,54,56-61,64-66,70-71,88,99-100,113H,15-16,23,29-34,37-39,41-43,85-86H2,1-9H3,(H2,87,103)(H,89,107)(H,90,108)(H,91,106)(H,92,109)(H,93,104)(H,94,110)(H,95,105)(H,96,112)/b24-17+,44-18+/t45-,46+,47-,54-,56+,57+,58-,59+,60+,61+,64+,65-,66+,70+,71+,82-,83+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tarveda Therapeutics
Curated by ChEMBL
| Assay Description
Displacement of [125I]somatostatin from human SSTR2 expressed in CHO-K1 cell membranes after 240 mins |
J Med Chem 62: 2708-2719 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02036
BindingDB Entry DOI: 10.7270/Q2NK3JJ9 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50537068
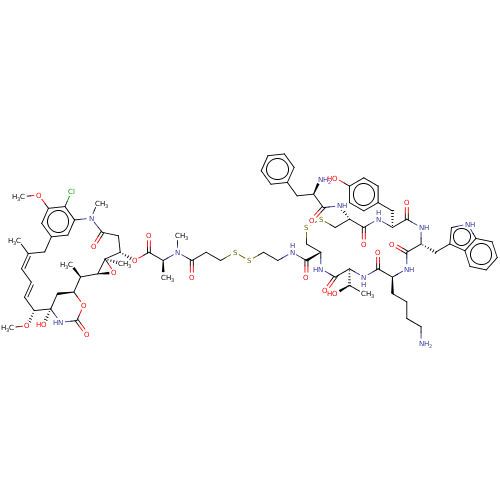
(CHEMBL4592483)Show SMILES [H][C@@]12O[C@]1(C)[C@H](CC(=O)N(C)c1cc(C\C(C)=C\C=C\[C@@H](OC)[C@@]3(O)C[C@]([H])(OC(=O)N3)[C@H]2C)cc(OC)c1Cl)OC(=O)[C@H](C)N(C)C(=O)CCSSCCNC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@H](N)Cc2ccccc2)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N[C@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCCN)C(=O)N[C@@]([H])([C@@H](C)O)C(=O)N1 |r,t:17,19| Show InChI InChI=1S/C82H108ClN13O19S4/c1-45-18-17-24-65(112-9)82(110)41-64(113-80(109)94-82)46(2)71-81(5,115-71)66(40-68(100)96(7)62-37-51(34-45)38-63(111-8)69(62)83)114-79(108)47(3)95(6)67(99)29-32-116-117-33-31-86-73(102)60-43-118-119-44-61(91-72(101)55(85)35-49-19-11-10-12-20-49)77(106)89-58(36-50-25-27-53(98)28-26-50)75(104)90-59(39-52-42-87-56-22-14-13-21-54(52)56)76(105)88-57(23-15-16-30-84)74(103)93-70(48(4)97)78(107)92-60/h10-14,17-22,24-28,37-38,42,46-48,55,57-61,64-66,70-71,87,97-98,110H,15-16,23,29-36,39-41,43-44,84-85H2,1-9H3,(H,86,102)(H,88,105)(H,89,106)(H,90,104)(H,91,101)(H,92,107)(H,93,103)(H,94,109)/b24-17+,45-18+/t46-,47+,48-,55-,57+,58+,59-,60+,61+,64+,65-,66+,70+,71+,81-,82+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tarveda Therapeutics
Curated by ChEMBL
| Assay Description
Displacement of [125I]somatostatin from human SSTR2 expressed in CHO-K1 cell membranes after 240 mins |
J Med Chem 62: 2708-2719 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02036
BindingDB Entry DOI: 10.7270/Q2NK3JJ9 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50537070

(CHEMBL4581874)Show SMILES [H][C@@]12O[C@]1(C)[C@H](CC(=O)N(C)c1cc(C\C(C)=C\C=C\[C@@H](OC)[C@@]3(O)C[C@]([H])(OC(=O)N3)[C@H]2C)cc(OC)c1Cl)OC(=O)[C@H](C)N(C)C(=O)CCC(C)(C)SSC[C@H](NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@H](N)Cc2ccccc2)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N[C@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCCN)C(=O)N[C@@]([H])([C@@H](C)O)C(=O)N1)C(N)=O |r,t:17,19| Show InChI InChI=1S/C86H115ClN14O20S4/c1-46-20-19-26-67(118-11)86(116)41-66(119-83(115)99-86)47(2)73-85(7,121-73)68(40-70(105)101(9)64-37-52(34-46)38-65(117-10)71(64)87)120-82(114)48(3)100(8)69(104)31-32-84(5,6)125-124-43-61(74(90)106)95-80(112)63-45-123-122-44-62(96-75(107)56(89)35-50-21-13-12-14-22-50)79(111)93-59(36-51-27-29-54(103)30-28-51)77(109)94-60(39-53-42-91-57-24-16-15-23-55(53)57)78(110)92-58(25-17-18-33-88)76(108)98-72(49(4)102)81(113)97-63/h12-16,19-24,26-30,37-38,42,47-49,56,58-63,66-68,72-73,91,102-103,116H,17-18,25,31-36,39-41,43-45,88-89H2,1-11H3,(H2,90,106)(H,92,110)(H,93,111)(H,94,109)(H,95,112)(H,96,107)(H,97,113)(H,98,108)(H,99,115)/b26-19+,46-20+/t47-,48+,49-,56-,58+,59+,60-,61+,62+,63+,66+,67-,68+,72+,73+,85-,86+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0750 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tarveda Therapeutics
Curated by ChEMBL
| Assay Description
Displacement of [125I]somatostatin from human SSTR2 expressed in CHO-K1 cell membranes after 240 mins |
J Med Chem 62: 2708-2719 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02036
BindingDB Entry DOI: 10.7270/Q2NK3JJ9 |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [501-599]
(Human immunodeficiency virus type 1) | BDBM854
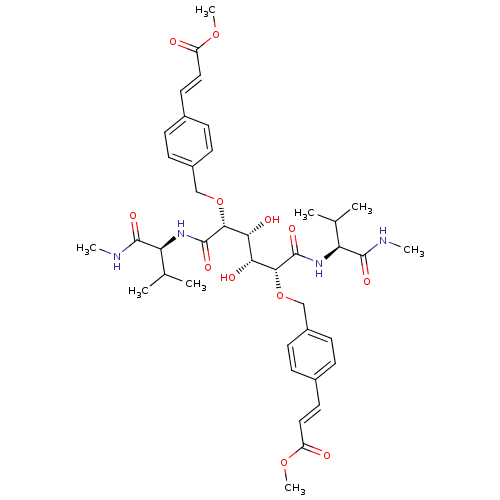
(C2-Symmetric inhibitor 12 | CHEMBL127045 | N1,N6-B...)Show SMILES CNC(=O)[C@@H](NC(=O)[C@H](OCc1ccc(\C=C\C(=O)OC)cc1)[C@H](O)[C@@H](O)[C@@H](OCc1ccc(\C=C\C(=O)OC)cc1)C(=O)N[C@@H](C(C)C)C(=O)NC)C(C)C |r| Show InChI InChI=1S/C40H54N4O12/c1-23(2)31(37(49)41-5)43-39(51)35(55-21-27-13-9-25(10-14-27)17-19-29(45)53-7)33(47)34(48)36(40(52)44-32(24(3)4)38(50)42-6)56-22-28-15-11-26(12-16-28)18-20-30(46)54-8/h9-20,23-24,31-36,47-48H,21-22H2,1-8H3,(H,41,49)(H,42,50)(H,43,51)(H,44,52)/b19-17+,20-18+/t31-,32-,33+,34+,35+,36+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0900 | -58.3 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Uppsala University
| Assay Description
Ki values were determined by using a fluorescent substrate (DABCYL-gamma-Abu-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Gln-EDANS). All incubations were performed a... |
J Med Chem 42: 3835-44 (1999)
Article DOI: 10.1021/jm9910371
BindingDB Entry DOI: 10.7270/Q2T151VX |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM851
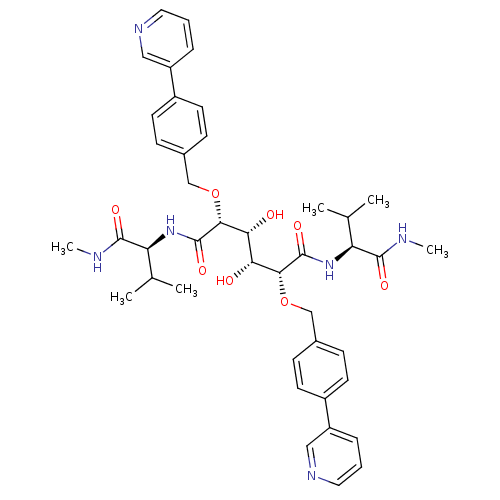
((2R,3R,4R,5R)-3,4-dihydroxy-N,N'-bis[(1S)-2-methyl...)Show SMILES CNC(=O)[C@@H](NC(=O)[C@H](OCc1ccc(cc1)-c1cccnc1)[C@H](O)[C@@H](O)[C@@H](OCc1ccc(cc1)-c1cccnc1)C(=O)N[C@@H](C(C)C)C(=O)NC)C(C)C |r| Show InChI InChI=1S/C42H52N6O8/c1-25(2)33(39(51)43-5)47-41(53)37(55-23-27-11-15-29(16-12-27)31-9-7-19-45-21-31)35(49)36(50)38(42(54)48-34(26(3)4)40(52)44-6)56-24-28-13-17-30(18-14-28)32-10-8-20-46-22-32/h7-22,25-26,33-38,49-50H,23-24H2,1-6H3,(H,43,51)(H,44,52)(H,47,53)(H,48,54)/t33-,34-,35+,36+,37+,38+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.100 | -58.0 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Uppsala University
| Assay Description
A fluorimetric assay was used to determine the effects of the inhibitors on HIV-1 protease. This assay used an internally quenched fluorescent peptid... |
Eur J Biochem 270: 1746-58 (2003)
Article DOI: 10.1046/j.1432-1033.2003.03533.x
BindingDB Entry DOI: 10.7270/Q2NZ85WX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dimer of Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM12218

((2R,3R,4R,5R)-N-benzyl-2,5-bis(benzyloxy)-3,4-dihy...)Show SMILES O[C@H]([C@@H](O)[C@@H](OCc1ccccc1)C(=O)N[C@@H]1[C@H](O)Cc2ccccc12)[C@@H](OCc1ccccc1)C(=O)NCc1ccccc1 |r| Show InChI InChI=1S/C36H38N2O7/c39-29-20-27-18-10-11-19-28(27)30(29)38-36(43)34(45-23-26-16-8-3-9-17-26)32(41)31(40)33(44-22-25-14-6-2-7-15-25)35(42)37-21-24-12-4-1-5-13-24/h1-19,29-34,39-41H,20-23H2,(H,37,42)(H,38,43)/t29-,30+,31-,32-,33-,34-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.100 | -58.0 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Uppsala University
| Assay Description
A fluorimetric assay was used to determine the effects of the inhibitors on HIV-1 protease. This assay used an internally quenched fluorescent peptid... |
Eur J Biochem 270: 1746-58 (2003)
Article DOI: 10.1046/j.1432-1033.2003.03533.x
BindingDB Entry DOI: 10.7270/Q2NZ85WX |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50537074

(CHEMBL4556000)Show SMILES [H][C@@]12O[C@]1(C)[C@H](CC(=O)N(C)c1cc(C\C(C)=C\C=C\[C@@H](OC)[C@@]3(O)C[C@]([H])(OC(=O)N3)[C@H]2C)cc(OC)c1Cl)OC(=O)[C@H](C)N(C)C(=O)CCSSCCC(=O)NCCCC[C@@H]1N(C)C(=O)[C@H](Cc2ccccc2)NC(=O)[C@@]([H])(NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccc(O)cc2)NC1=O)[C@@H](C)O |r,t:17,19| Show InChI InChI=1S/C84H111ClN12O19S2/c1-48-21-20-28-67(113-10)84(111)46-66(114-82(110)94-84)49(2)74-83(5,116-74)68(45-71(102)96(7)64-42-54(39-48)43-65(112-9)72(64)85)115-81(109)50(3)95(6)70(101)34-38-118-117-37-33-69(100)87-36-19-17-27-63-78(106)91-60(40-53-29-31-56(99)32-30-53)76(104)90-61(44-55-47-88-58-25-15-14-24-57(55)58)77(105)89-59(26-16-18-35-86)75(103)93-73(51(4)98)79(107)92-62(80(108)97(63)8)41-52-22-12-11-13-23-52/h11-15,20-25,28-32,42-43,47,49-51,59-63,66-68,73-74,88,98-99,111H,16-19,26-27,33-41,44-46,86H2,1-10H3,(H,87,100)(H,89,105)(H,90,104)(H,91,106)(H,92,107)(H,93,103)(H,94,110)/b28-20+,48-21+/t49-,50+,51-,59+,60+,61-,62+,63+,66+,67-,68+,73+,74+,83-,84+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tarveda Therapeutics
Curated by ChEMBL
| Assay Description
Displacement of [125I]somatostatin from human SSTR2 expressed in CHO-K1 cell membranes after 240 mins |
J Med Chem 62: 2708-2719 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02036
BindingDB Entry DOI: 10.7270/Q2NK3JJ9 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50537062

(CHEMBL4549303)Show SMILES [H][C@@]12O[C@]1(C)[C@H](CC(=O)N(C)c1cc(C\C(C)=C\C=C\[C@@H](OC)[C@@]3(O)C[C@]([H])(OC(=O)N3)[C@H]2C)cc(OC)c1Cl)OC(=O)[C@H](C)N(C)C(=O)CCSSCCCN[C@H](Cc1ccccc1)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@@]([H])(NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC1=O)[C@@H](C)O)C(=O)N[C@H](CO)[C@@H](C)O |r,t:17,19| Show InChI InChI=1S/C87H118ClN13O20S4/c1-49-23-21-31-70(118-10)87(116)44-69(119-85(115)99-87)50(2)76-86(6,121-76)71(43-73(106)101(8)67-40-56(37-49)41-68(117-9)74(67)88)120-84(114)51(3)100(7)72(105)32-36-123-122-35-22-34-90-61(38-54-24-13-11-14-25-54)78(108)96-65-47-124-125-48-66(82(112)95-64(46-102)52(4)103)97-83(113)75(53(5)104)98-77(107)60(30-19-20-33-89)92-80(110)63(42-57-45-91-59-29-18-17-28-58(57)59)94-79(109)62(93-81(65)111)39-55-26-15-12-16-27-55/h11-18,21,23-29,31,40-41,45,50-53,60-66,69-71,75-76,90-91,102-104,116H,19-20,22,30,32-39,42-44,46-48,89H2,1-10H3,(H,92,110)(H,93,111)(H,94,109)(H,95,112)(H,96,108)(H,97,113)(H,98,107)(H,99,115)/b31-21+,49-23+/t50-,51+,52-,53-,60+,61-,62+,63-,64-,65+,66+,69+,70-,71+,75+,76+,86-,87+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tarveda Therapeutics
Curated by ChEMBL
| Assay Description
Displacement of [125I]somatostatin from human SSTR2 expressed in CHO-K1 cell membranes after 240 mins |
J Med Chem 62: 2708-2719 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02036
BindingDB Entry DOI: 10.7270/Q2NK3JJ9 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50537067
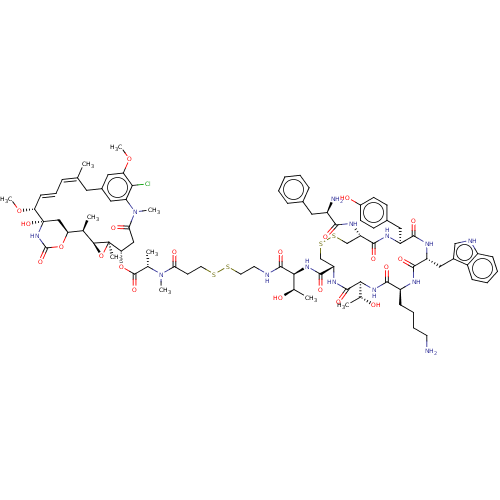
(CHEMBL4532058)Show SMILES [H][C@@]12O[C@]1(C)[C@H](CC(=O)N(C)c1cc(C\C(C)=C\C=C\[C@@H](OC)[C@@]3(O)C[C@]([H])(OC(=O)N3)[C@H]2C)cc(OC)c1Cl)OC(=O)[C@H](C)N(C)C(=O)CCSSCCNC(=O)[C@@H](NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@H](N)Cc2ccccc2)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N[C@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCCN)C(=O)N[C@@]([H])([C@@H](C)O)C(=O)N1)[C@@H](C)O |r,t:17,19| Show InChI InChI=1S/C86H115ClN14O21S4/c1-46-19-18-25-67(119-10)86(117)42-66(120-84(116)99-86)47(2)74-85(6,122-74)68(41-70(106)101(8)64-38-53(35-46)39-65(118-9)71(64)87)121-83(115)48(3)100(7)69(105)30-33-123-124-34-32-90-81(113)72(49(4)102)97-80(112)63-45-126-125-44-62(95-75(107)57(89)36-51-20-12-11-13-21-51)79(111)93-60(37-52-26-28-55(104)29-27-52)77(109)94-61(40-54-43-91-58-23-15-14-22-56(54)58)78(110)92-59(24-16-17-31-88)76(108)98-73(50(5)103)82(114)96-63/h11-15,18-23,25-29,38-39,43,47-50,57,59-63,66-68,72-74,91,102-104,117H,16-17,24,30-37,40-42,44-45,88-89H2,1-10H3,(H,90,113)(H,92,110)(H,93,111)(H,94,109)(H,95,107)(H,96,114)(H,97,112)(H,98,108)(H,99,116)/b25-18+,46-19+/t47-,48+,49-,50-,57-,59+,60+,61-,62+,63+,66+,67-,68+,72+,73+,74+,85-,86+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tarveda Therapeutics
Curated by ChEMBL
| Assay Description
Displacement of [125I]somatostatin from human SSTR2 expressed in CHO-K1 cell membranes after 240 mins |
J Med Chem 62: 2708-2719 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02036
BindingDB Entry DOI: 10.7270/Q2NK3JJ9 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50537064
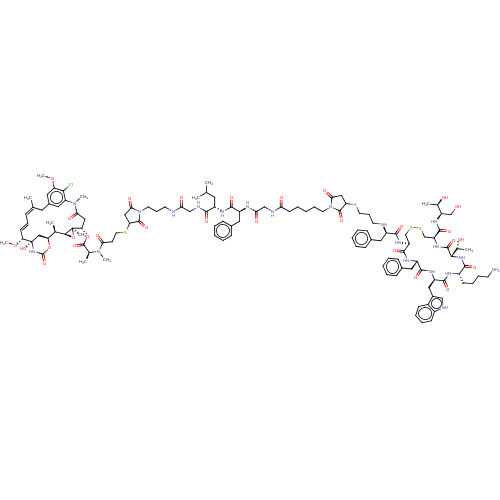
(CHEMBL4563111)Show SMILES [H][C@@]12O[C@]1(C)[C@H](CC(=O)N(C)c1cc(C\C(C)=C\C=C\[C@@H](OC)[C@@]3(O)C[C@]([H])(OC(=O)N3)[C@H]2C)cc(OC)c1Cl)OC(=O)[C@H](C)N(C)C(=O)CCSC1CC(=O)N(CCCNC(=O)CNC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc2ccccc2)NC(=O)CNC(=O)CCCCCN2C(=O)CC(SCCCN[C@H](Cc3ccccc3)C(=O)N[C@H]3CSSC[C@H](NC(=O)[C@@]([H])(NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc4c[nH]c5ccccc45)NC(=O)[C@H](Cc4ccccc4)NC3=O)[C@@H](C)O)C(=O)N[C@H](CO)[C@@H](C)O)C2=O)C1=O |r,t:17,19| Show InChI InChI=1S/C123H167ClN20O29S4/c1-70(2)52-85(109(155)130-65-100(149)127-46-30-49-144-105(154)61-96(119(144)165)175-51-44-102(151)141(9)73(5)120(166)172-98-62-103(152)142(10)92-57-79(58-93(169-11)106(92)124)53-71(3)32-29-42-97(170-12)123(168)63-94(171-121(167)140-123)72(4)108-122(98,8)173-108)133-112(158)86(55-77-35-19-14-20-36-77)131-101(150)66-129-99(148)43-23-16-28-48-143-104(153)60-95(118(143)164)174-50-31-47-126-84(54-76-33-17-13-18-34-76)111(157)137-90-68-176-177-69-91(116(162)136-89(67-145)74(6)146)138-117(163)107(75(7)147)139-110(156)83(41-26-27-45-125)132-114(160)88(59-80-64-128-82-40-25-24-39-81(80)82)135-113(159)87(134-115(90)161)56-78-37-21-15-22-38-78/h13-15,17-22,24-25,29,32-40,42,57-58,64,70,72-75,83-91,94-98,107-108,126,128,145-147,168H,16,23,26-28,30-31,41,43-56,59-63,65-69,125H2,1-12H3,(H,127,149)(H,129,148)(H,130,155)(H,131,150)(H,132,160)(H,133,158)(H,134,161)(H,135,159)(H,136,162)(H,137,157)(H,138,163)(H,139,156)(H,140,167)/b42-29+,71-32+/t72-,73+,74-,75-,83+,84-,85+,86+,87+,88-,89-,90+,91+,94+,95?,96?,97-,98+,107+,108+,122-,123+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tarveda Therapeutics
Curated by ChEMBL
| Assay Description
Displacement of [125I]somatostatin from human SSTR2 expressed in CHO-K1 cell membranes after 240 mins |
J Med Chem 62: 2708-2719 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02036
BindingDB Entry DOI: 10.7270/Q2NK3JJ9 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50537075

(CHEMBL4548228)Show SMILES [H][C@@]12O[C@]1(C)[C@H](CC(=O)N(C)c1cc(C\C(C)=C\C=C\[C@@H](OC)[C@@]3(O)C[C@]([H])(OC(=O)N3)[C@H]2C)cc(OC)c1Cl)OC(=O)[C@H](C)N(C)C(=O)CCSC1CC(=O)N(CC(=O)NC[C@H](NC(=O)[C@@H]2CSSC[C@H](NC(=O)[C@H](N)Cc3ccccc3)C(=O)N[C@@H](Cc3ccc(O)cc3)C(=O)N[C@H](Cc3c[nH]c4ccccc34)C(=O)N[C@@H](CCCCN)C(=O)N[C@@]([H])([C@@H](C)O)C(=O)N2)C(N)=O)C1=O |r,t:17,19| Show InChI InChI=1S/C89H115ClN16O23S3/c1-46-18-17-24-68(126-9)89(124)40-66(127-87(123)103-89)47(2)76-88(5,129-76)69(39-72(111)105(7)64-35-52(32-46)36-65(125-8)74(64)90)128-86(122)48(3)104(6)71(110)29-31-130-67-38-73(112)106(85(67)121)43-70(109)95-42-61(77(93)113)99-83(119)63-45-132-131-44-62(100-78(114)56(92)33-50-19-11-10-12-20-50)82(118)97-59(34-51-25-27-54(108)28-26-51)80(116)98-60(37-53-41-94-57-22-14-13-21-55(53)57)81(117)96-58(23-15-16-30-91)79(115)102-75(49(4)107)84(120)101-63/h10-14,17-22,24-28,35-36,41,47-49,56,58-63,66-69,75-76,94,107-108,124H,15-16,23,29-34,37-40,42-45,91-92H2,1-9H3,(H2,93,113)(H,95,109)(H,96,117)(H,97,118)(H,98,116)(H,99,119)(H,100,114)(H,101,120)(H,102,115)(H,103,123)/b24-17+,46-18+/t47-,48+,49-,56-,58+,59+,60-,61+,62+,63+,66+,67?,68-,69+,75+,76+,88-,89+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tarveda Therapeutics
Curated by ChEMBL
| Assay Description
Displacement of [125I]somatostatin from human SSTR2 expressed in CHO-K1 cell membranes after 240 mins |
J Med Chem 62: 2708-2719 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02036
BindingDB Entry DOI: 10.7270/Q2NK3JJ9 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50537071

(CHEMBL4581646)Show SMILES [H][C@]1(CCCN1C(=O)C[C@@H](OC)[C@H]([C@@H](C)CC)N(C)C(=O)[C@@H](NC(=O)[C@H](C(C)C)N(C)C(=O)OCCSSC[C@H](NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@H](N)Cc2ccccc2)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N[C@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCCN)C(=O)N[C@@]([H])([C@@H](C)O)C(=O)N1)C(N)=O)C(C)C)[C@H](OC)[C@@H](C)C(=O)N[C@H](C)[C@@H](O)c1ccccc1 |r| Show InChI InChI=1S/C90H132N16O19S4/c1-14-52(6)76(71(123-12)45-72(109)106-39-25-33-70(106)78(124-13)53(7)80(112)95-54(8)77(110)58-28-19-16-20-29-58)104(10)89(121)73(50(2)3)102-88(120)75(51(4)5)105(11)90(122)125-40-41-126-127-47-67(79(93)111)99-86(118)69-49-129-128-48-68(100-81(113)62(92)42-56-26-17-15-18-27-56)85(117)97-65(43-57-34-36-60(108)37-35-57)83(115)98-66(44-59-46-94-63-31-22-21-30-61(59)63)84(116)96-64(32-23-24-38-91)82(114)103-74(55(9)107)87(119)101-69/h15-22,26-31,34-37,46,50-55,62,64-71,73-78,94,107-108,110H,14,23-25,32-33,38-45,47-49,91-92H2,1-13H3,(H2,93,111)(H,95,112)(H,96,116)(H,97,117)(H,98,115)(H,99,118)(H,100,113)(H,101,119)(H,102,120)(H,103,114)/t52-,53+,54+,55+,62+,64-,65-,66+,67-,68-,69-,70-,71+,73-,74-,75-,76-,77+,78+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tarveda Therapeutics
Curated by ChEMBL
| Assay Description
Displacement of [125I]somatostatin from human SSTR2 expressed in CHO-K1 cell membranes after 240 mins |
J Med Chem 62: 2708-2719 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02036
BindingDB Entry DOI: 10.7270/Q2NK3JJ9 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50537065
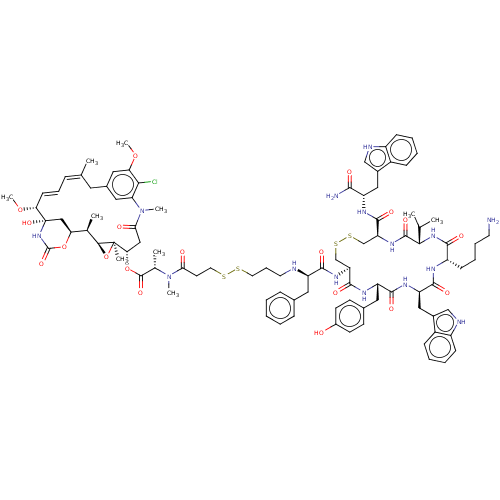
(CHEMBL4537192)Show SMILES [H][C@@]12O[C@]1(C)[C@H](CC(=O)N(C)c1cc(C\C(C)=C\C=C\[C@@H](OC)[C@@]3(O)C[C@]([H])(OC(=O)N3)[C@H]2C)cc(OC)c1Cl)OC(=O)[C@H](C)N(C)C(=O)CCSSCCCN[C@H](Cc1ccccc1)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@@H](NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccc(O)cc2)NC1=O)C(C)C)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(N)=O |r,t:17,19| Show InChI InChI=1S/C95H122ClN15O19S4/c1-53(2)82-91(122)107-73(89(120)103-68(84(98)115)45-60-49-100-65-27-16-14-25-63(60)65)52-134-133-51-72(90(121)104-70(42-58-31-33-62(112)34-32-58)87(118)105-71(46-61-50-101-66-28-17-15-26-64(61)66)88(119)102-67(85(116)108-82)29-18-19-36-97)106-86(117)69(41-57-23-12-11-13-24-57)99-37-21-38-131-132-39-35-79(113)110(7)56(5)92(123)129-78-47-80(114)111(8)74-43-59(44-75(126-9)81(74)96)40-54(3)22-20-30-77(127-10)95(125)48-76(128-93(124)109-95)55(4)83-94(78,6)130-83/h11-17,20,22-28,30-34,43-44,49-50,53,55-56,67-73,76-78,82-83,99-101,112,125H,18-19,21,29,35-42,45-48,51-52,97H2,1-10H3,(H2,98,115)(H,102,119)(H,103,120)(H,104,121)(H,105,118)(H,106,117)(H,107,122)(H,108,116)(H,109,124)/b30-20+,54-22+/t55-,56+,67+,68+,69-,70+,71-,72+,73+,76+,77-,78+,82+,83+,94-,95+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tarveda Therapeutics
Curated by ChEMBL
| Assay Description
Displacement of [125I]somatostatin from human SSTR2 expressed in CHO-K1 cell membranes after 240 mins |
J Med Chem 62: 2708-2719 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02036
BindingDB Entry DOI: 10.7270/Q2NK3JJ9 |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50213021
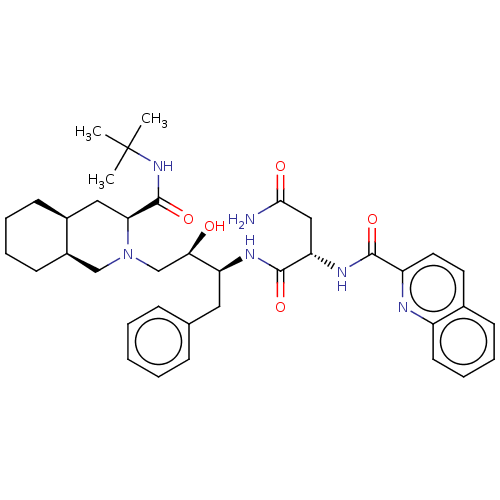
(CHEBI:63621 | Fortovase | Invirase | Ro-31-8959 | ...)Show SMILES [H][C@@]12CCCC[C@]1([H])CN(C[C@@H](O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(N)=O)NC(=O)c1ccc3ccccc3n1)[C@@H](C2)C(=O)NC(C)(C)C |r| Show InChI InChI=1S/C38H50N6O5/c1-38(2,3)43-37(49)32-20-26-14-7-8-15-27(26)22-44(32)23-33(45)30(19-24-11-5-4-6-12-24)41-36(48)31(21-34(39)46)42-35(47)29-18-17-25-13-9-10-16-28(25)40-29/h4-6,9-13,16-18,26-27,30-33,45H,7-8,14-15,19-23H2,1-3H3,(H2,39,46)(H,41,48)(H,42,47)(H,43,49)/t26-,27+,30-,31-,32-,33+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease expressed in Escherichia coli by fluorometric assay |
J Med Chem 53: 607-15 (2010)
Article DOI: 10.1021/jm901165g
BindingDB Entry DOI: 10.7270/Q29Z97R7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM19854

(CHEMBL426819 | CRA-013783/L-006235 | N-{1-[(cyanom...)Show SMILES CN1CCN(CC1)c1nc(cs1)-c1ccc(cc1)C(=O)NC1(CCCCC1)C(=O)NCC#N Show InChI InChI=1S/C24H30N6O2S/c1-29-13-15-30(16-14-29)23-27-20(17-33-23)18-5-7-19(8-6-18)21(31)28-24(9-3-2-4-10-24)22(32)26-12-11-25/h5-8,17H,2-4,9-10,12-16H2,1H3,(H,26,32)(H,28,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM358

((2R,3R,4R,5R)-2,5-bis(benzyloxy)-3,4-dihydroxy-N,N...)Show SMILES O[C@H]([C@@H](O)[C@@H](OCc1ccccc1)C(=O)N[C@@H]1[C@H](O)Cc2ccccc12)[C@@H](OCc1ccccc1)C(=O)N[C@@H]1[C@H](O)Cc2ccccc12 |r| Show InChI InChI=1S/C38H40N2O8/c41-29-19-25-15-7-9-17-27(25)31(29)39-37(45)35(47-21-23-11-3-1-4-12-23)33(43)34(44)36(48-22-24-13-5-2-6-14-24)38(46)40-32-28-18-10-8-16-26(28)20-30(32)42/h1-18,29-36,41-44H,19-22H2,(H,39,45)(H,40,46)/t29-,30-,31+,32+,33-,34-,35-,36-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.200 | -56.3 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Uppsala University
| Assay Description
A fluorimetric assay was used to determine the effects of the inhibitors on HIV-1 protease. This assay used an internally quenched fluorescent peptid... |
Eur J Biochem 270: 1746-58 (2003)
Article DOI: 10.1046/j.1432-1033.2003.03533.x
BindingDB Entry DOI: 10.7270/Q2NZ85WX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50066918

((2R,3R,4R,5R)-2,5-Bis-benzyloxy-3,4-dihydroxy-hexa...)Show SMILES O[C@H]([C@@H](O)[C@@H](OCc1ccccc1)C(=O)N[C@@H]1[C@H](O)Cc2ccccc12)[C@@H](OCc1ccccc1)C(=O)N[C@H]1[C@@H](O)Cc2ccccc12 Show InChI InChI=1S/C38H40N2O8/c41-29-19-25-15-7-9-17-27(25)31(29)39-37(45)35(47-21-23-11-3-1-4-12-23)33(43)34(44)36(48-22-24-13-5-2-6-14-24)38(46)40-32-28-18-10-8-16-26(28)20-30(32)42/h1-18,29-36,41-44H,19-22H2,(H,39,45)(H,40,46)/t29-,30+,31+,32-,33-,34-,35-,36-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Linköping University
Curated by ChEMBL
| Assay Description
Inhibitory activity against purified HIV-1 protease expressed in E. coli in sensitive fluorometric assay |
J Med Chem 41: 3782-92 (1998)
Article DOI: 10.1021/jm970777b
BindingDB Entry DOI: 10.7270/Q2PK0F93 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50418366

(CHEMBL1774023)Show SMILES Clc1ccc(C[C@@H](NC(=O)Cc2ccc(Cl)cc2)C(=O)N[C@@H]2C[C@@H]3CC[C@H](C2)N3)cc1 |r| Show InChI InChI=1S/C24H27Cl2N3O2/c25-17-5-1-15(2-6-17)11-22(29-23(30)12-16-3-7-18(26)8-4-16)24(31)28-21-13-19-9-10-20(14-21)27-19/h1-8,19-22,27H,9-14H2,(H,28,31)(H,29,30)/t19-,20+,21+,22-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.234 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MSD
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from human V1A receptor expressed in CHO cells |
Bioorg Med Chem Lett 21: 3163-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.096
BindingDB Entry DOI: 10.7270/Q2K64KB0 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM50410611
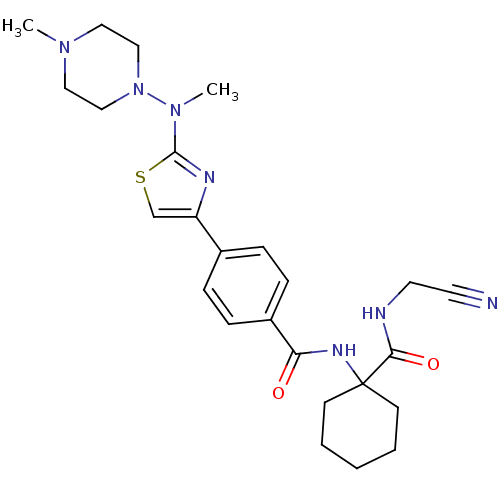
(CHEMBL414669)Show SMILES CN(N1CCN(C)CC1)c1nc(cs1)-c1ccc(cc1)C(=O)NC1(CCCCC1)C(=O)NCC#N Show InChI InChI=1S/C25H33N7O2S/c1-30-14-16-32(17-15-30)31(2)24-28-21(18-35-24)19-6-8-20(9-7-19)22(33)29-25(10-4-3-5-11-25)23(34)27-13-12-26/h6-9,18H,3-5,10-11,13-17H2,1-2H3,(H,27,34)(H,29,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50537078

(CHEMBL4577466)Show SMILES [H][C@]([C@@H](C)CC)([C@@H](CC(=O)N1CCC[C@@]1([H])[C@H](OC)[C@@H](C)C(=O)N[C@H](C)[C@@H](O)c1ccccc1)OC)N(C)C(=O)[C@@H](NC(=O)[C@H](C(C)C)N(C)C(=O)OCCSSCCCN[C@H](Cc1ccccc1)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@@]([H])(NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC1=O)[C@@H](C)O)C(=O)N[C@H](CO)[C@@H](C)O)C(C)C |r| Show InChI InChI=1S/C94H141N15O19S4/c1-15-57(6)81(76(126-13)50-77(113)109-43-29-40-75(109)83(127-14)58(7)84(115)98-59(8)82(114)64-35-23-18-24-36-64)107(11)93(124)78(55(2)3)105-92(123)80(56(4)5)108(12)94(125)128-44-46-130-129-45-30-42-96-69(47-62-31-19-16-20-32-62)86(117)103-73-53-131-132-54-74(90(121)102-72(52-110)60(9)111)104-91(122)79(61(10)112)106-85(116)68(39-27-28-41-95)99-88(119)71(49-65-51-97-67-38-26-25-37-66(65)67)101-87(118)70(100-89(73)120)48-63-33-21-17-22-34-63/h16-26,31-38,51,55-61,68-76,78-83,96-97,110-112,114H,15,27-30,39-50,52-54,95H2,1-14H3,(H,98,115)(H,99,119)(H,100,120)(H,101,118)(H,102,121)(H,103,117)(H,104,122)(H,105,123)(H,106,116)/t57-,58+,59+,60+,61+,68-,69+,70-,71+,72+,73-,74-,75-,76+,78-,79-,80-,81-,82+,83+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tarveda Therapeutics
Curated by ChEMBL
| Assay Description
Displacement of [125I]somatostatin from human SSTR2 expressed in CHO-K1 cell membranes after 240 mins |
J Med Chem 62: 2708-2719 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02036
BindingDB Entry DOI: 10.7270/Q2NK3JJ9 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM50410588
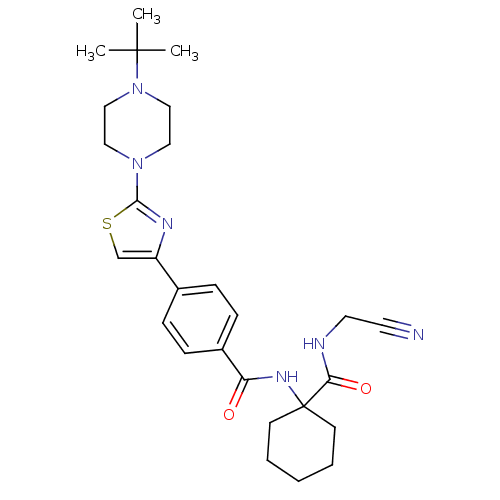
(CHEMBL200708)Show SMILES CC(C)(C)N1CCN(CC1)c1nc(cs1)-c1ccc(cc1)C(=O)NC1(CCCCC1)C(=O)NCC#N Show InChI InChI=1S/C27H36N6O2S/c1-26(2,3)33-17-15-32(16-18-33)25-30-22(19-36-25)20-7-9-21(10-8-20)23(34)31-27(11-5-4-6-12-27)24(35)29-14-13-28/h7-10,19H,4-6,11-12,14-18H2,1-3H3,(H,29,35)(H,31,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50067593
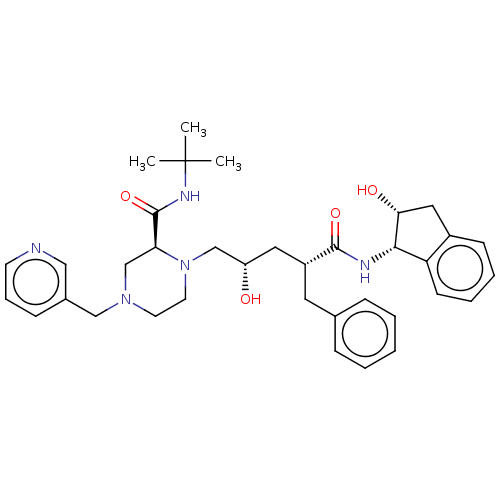
(CHEBI:44032 | Crixivan | Indinavir | L-735524 | MK...)Show SMILES CC(C)(C)NC(=O)[C@@H]1CN(Cc2cccnc2)CCN1C[C@@H](O)C[C@@H](Cc1ccccc1)C(=O)N[C@@H]1[C@H](O)Cc2ccccc12 |r| Show InChI InChI=1S/C36H47N5O4/c1-36(2,3)39-35(45)31-24-40(22-26-12-9-15-37-21-26)16-17-41(31)23-29(42)19-28(18-25-10-5-4-6-11-25)34(44)38-33-30-14-8-7-13-27(30)20-32(33)43/h4-15,21,28-29,31-33,42-43H,16-20,22-24H2,1-3H3,(H,38,44)(H,39,45)/t28-,29+,31+,32-,33+/m1/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease expressed in Escherichia coli by fluorometric assay |
J Med Chem 53: 607-15 (2010)
Article DOI: 10.1021/jm901165g
BindingDB Entry DOI: 10.7270/Q29Z97R7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dimer of Gag-Pol polyprotein [501-599]
(Human immunodeficiency virus type 1) | BDBM845
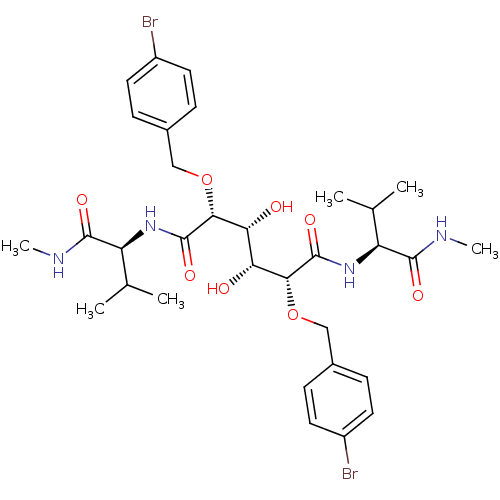
((2R,3R,4R,5R)-2,5-bis[(4-bromophenyl)methoxy]-3,4-...)Show SMILES CNC(=O)[C@@H](NC(=O)[C@H](OCc1ccc(Br)cc1)[C@H](O)[C@@H](O)[C@@H](OCc1ccc(Br)cc1)C(=O)N[C@@H](C(C)C)C(=O)NC)C(C)C |r| Show InChI InChI=1S/C32H44Br2N4O8/c1-17(2)23(29(41)35-5)37-31(43)27(45-15-19-7-11-21(33)12-8-19)25(39)26(40)28(46-16-20-9-13-22(34)14-10-20)32(44)38-24(18(3)4)30(42)36-6/h7-14,17-18,23-28,39-40H,15-16H2,1-6H3,(H,35,41)(H,36,42)(H,37,43)(H,38,44)/t23-,24-,25+,26+,27+,28+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | -55.3 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Uppsala University
| Assay Description
Ki values were determined by using a fluorescent substrate (DABCYL-gamma-Abu-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Gln-EDANS). All incubations were performed a... |
J Med Chem 42: 3835-44 (1999)
Article DOI: 10.1021/jm9910371
BindingDB Entry DOI: 10.7270/Q2T151VX |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [501-599]
(Human immunodeficiency virus type 1) | BDBM855
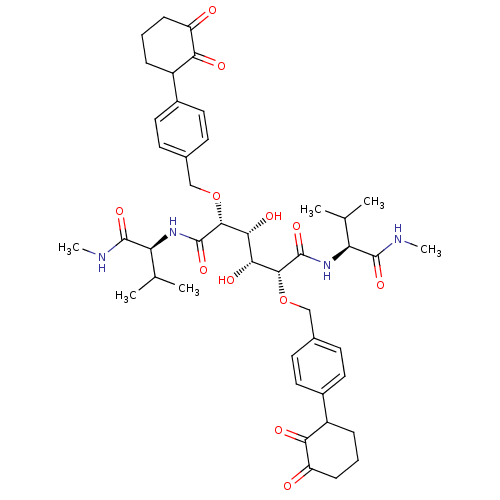
((2R,3R,4R,5R)-3,4-dihydroxy-2,5-bis({[4-(2-hydroxy...)Show SMILES CNC(=O)[C@@H](NC(=O)[C@H](OCc1ccc(cc1)C1CCCC(=O)C1=O)[C@H](O)[C@@H](O)[C@@H](OCc1ccc(cc1)C1CCCC(=O)C1=O)C(=O)N[C@@H](C(C)C)C(=O)NC)C(C)C |r| Show InChI InChI=1S/C44H58N4O12/c1-23(2)33(41(55)45-5)47-43(57)39(59-21-25-13-17-27(18-14-25)29-9-7-11-31(49)35(29)51)37(53)38(54)40(44(58)48-34(24(3)4)42(56)46-6)60-22-26-15-19-28(20-16-26)30-10-8-12-32(50)36(30)52/h13-20,23-24,29-30,33-34,37-40,53-54H,7-12,21-22H2,1-6H3,(H,45,55)(H,46,56)(H,47,57)(H,48,58)/t29?,30?,33-,34-,37+,38+,39+,40+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | -55.3 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Uppsala University
| Assay Description
Ki values were determined by using a fluorescent substrate (DABCYL-gamma-Abu-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Gln-EDANS). All incubations were performed a... |
J Med Chem 42: 3835-44 (1999)
Article DOI: 10.1021/jm9910371
BindingDB Entry DOI: 10.7270/Q2T151VX |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [501-599]
(Human immunodeficiency virus type 1) | BDBM851

((2R,3R,4R,5R)-3,4-dihydroxy-N,N'-bis[(1S)-2-methyl...)Show SMILES CNC(=O)[C@@H](NC(=O)[C@H](OCc1ccc(cc1)-c1cccnc1)[C@H](O)[C@@H](O)[C@@H](OCc1ccc(cc1)-c1cccnc1)C(=O)N[C@@H](C(C)C)C(=O)NC)C(C)C |r| Show InChI InChI=1S/C42H52N6O8/c1-25(2)33(39(51)43-5)47-41(53)37(55-23-27-11-15-29(16-12-27)31-9-7-19-45-21-31)35(49)36(50)38(42(54)48-34(26(3)4)40(52)44-6)56-24-28-13-17-30(18-14-28)32-10-8-20-46-22-32/h7-22,25-26,33-38,49-50H,23-24H2,1-6H3,(H,43,51)(H,44,52)(H,47,53)(H,48,54)/t33-,34-,35+,36+,37+,38+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 0.300 | -55.3 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Uppsala University
| Assay Description
Ki values were determined by using a fluorescent substrate (DABCYL-gamma-Abu-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Gln-EDANS). All incubations were performed a... |
J Med Chem 42: 3835-44 (1999)
Article DOI: 10.1021/jm9910371
BindingDB Entry DOI: 10.7270/Q2T151VX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dimer of Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM587
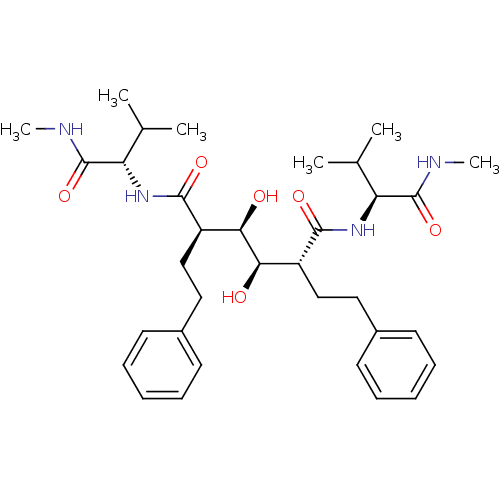
((2R,3R,4R,5R)-3,4-dihydroxy-N,N'-bis[(1S)-2-methyl...)Show SMILES CNC(=O)[C@@H](NC(=O)[C@H](CCc1ccccc1)[C@@H](O)[C@H](O)[C@@H](CCc1ccccc1)C(=O)N[C@@H](C(C)C)C(=O)NC)C(C)C |r| Show InChI InChI=1S/C34H50N4O6/c1-21(2)27(33(43)35-5)37-31(41)25(19-17-23-13-9-7-10-14-23)29(39)30(40)26(20-18-24-15-11-8-12-16-24)32(42)38-28(22(3)4)34(44)36-6/h7-16,21-22,25-30,39-40H,17-20H2,1-6H3,(H,35,43)(H,36,44)(H,37,41)(H,38,42)/t25-,26-,27+,28+,29-,30-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | -54.5 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Stockholm University
| Assay Description
A fluorimetric assay was used to determine the effects of the inhibitors on HIV-1 protease. This assay used an internally quenched fluorescent peptid... |
J Med Chem 44: 3407-16 (2001)
Article DOI: 10.1021/jm0011171
BindingDB Entry DOI: 10.7270/Q2862DND |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [501-599]
(Human immunodeficiency virus type 1) | BDBM348

((2R,3R,4R,5R)-2,5-bis(benzyloxy)-3,4-dihydroxy-N,N...)Show SMILES CNC(=O)[C@@H](NC(=O)[C@H](OCc1ccccc1)[C@H](O)[C@@H](O)[C@@H](OCc1ccccc1)C(=O)N[C@@H](C(C)C)C(=O)NC)C(C)C |r| Show InChI InChI=1S/C32H46N4O8/c1-19(2)23(29(39)33-5)35-31(41)27(43-17-21-13-9-7-10-14-21)25(37)26(38)28(44-18-22-15-11-8-12-16-22)32(42)36-24(20(3)4)30(40)34-6/h7-16,19-20,23-28,37-38H,17-18H2,1-6H3,(H,33,39)(H,34,40)(H,35,41)(H,36,42)/t23-,24-,25+,26+,27+,28+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.400 | -54.5 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Uppsala University
| Assay Description
Ki values were determined by using a fluorescent substrate (DABCYL-gamma-Abu-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Gln-EDANS). All incubations were performed a... |
J Med Chem 42: 3835-44 (1999)
Article DOI: 10.1021/jm9910371
BindingDB Entry DOI: 10.7270/Q2T151VX |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM12216

((2R,3R,4R,5R)-2,5-bis(benzyloxy)-3,4-dihydroxy-N-[...)Show SMILES CNC(=O)[C@@H](NC(=O)[C@H](OCc1ccccc1)[C@H](O)[C@@H](O)[C@@H](OCc1ccccc1)C(=O)N[C@@H]1[C@H](O)Cc2ccccc12)C(C)C |r| Show InChI InChI=1S/C35H43N3O8/c1-21(2)27(33(42)36-3)37-34(43)31(45-19-22-12-6-4-7-13-22)29(40)30(41)32(46-20-23-14-8-5-9-15-23)35(44)38-28-25-17-11-10-16-24(25)18-26(28)39/h4-17,21,26-32,39-41H,18-20H2,1-3H3,(H,36,42)(H,37,43)(H,38,44)/t26-,27+,28+,29-,30-,31-,32-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.400 | -54.5 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Uppsala University
| Assay Description
A fluorimetric assay was used to determine the effects of the inhibitors on HIV-1 protease. This assay used an internally quenched fluorescent peptid... |
Eur J Biochem 270: 1746-58 (2003)
Article DOI: 10.1046/j.1432-1033.2003.03533.x
BindingDB Entry DOI: 10.7270/Q2NZ85WX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM348

((2R,3R,4R,5R)-2,5-bis(benzyloxy)-3,4-dihydroxy-N,N...)Show SMILES CNC(=O)[C@@H](NC(=O)[C@H](OCc1ccccc1)[C@H](O)[C@@H](O)[C@@H](OCc1ccccc1)C(=O)N[C@@H](C(C)C)C(=O)NC)C(C)C |r| Show InChI InChI=1S/C32H46N4O8/c1-19(2)23(29(39)33-5)35-31(41)27(43-17-21-13-9-7-10-14-21)25(37)26(38)28(44-18-22-15-11-8-12-16-22)32(42)36-24(20(3)4)30(40)34-6/h7-16,19-20,23-28,37-38H,17-18H2,1-6H3,(H,33,39)(H,34,40)(H,35,41)(H,36,42)/t23-,24-,25+,26+,27+,28+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Linköping University
Curated by ChEMBL
| Assay Description
Inhibitory activity against purified HIV-1 protease expressed in E. coli in sensitive fluorometric assay |
J Med Chem 41: 3782-92 (1998)
Article DOI: 10.1021/jm970777b
BindingDB Entry DOI: 10.7270/Q2PK0F93 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM50410609
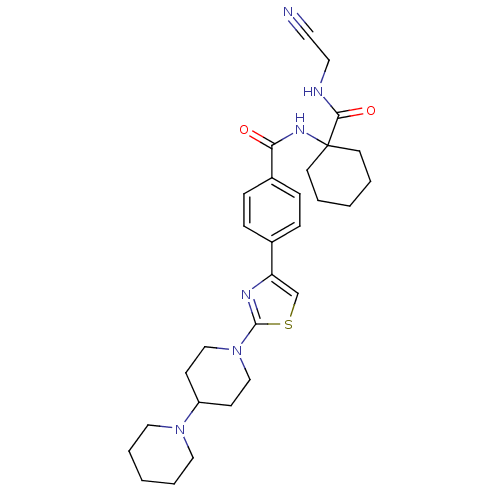
(CHEMBL198798)Show SMILES O=C(NC1(CCCCC1)C(=O)NCC#N)c1ccc(cc1)-c1csc(n1)N1CCC(CC1)N1CCCCC1 Show InChI InChI=1S/C29H38N6O2S/c30-15-16-31-27(37)29(13-3-1-4-14-29)33-26(36)23-9-7-22(8-10-23)25-21-38-28(32-25)35-19-11-24(12-20-35)34-17-5-2-6-18-34/h7-10,21,24H,1-6,11-14,16-20H2,(H,31,37)(H,33,36) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM50410590
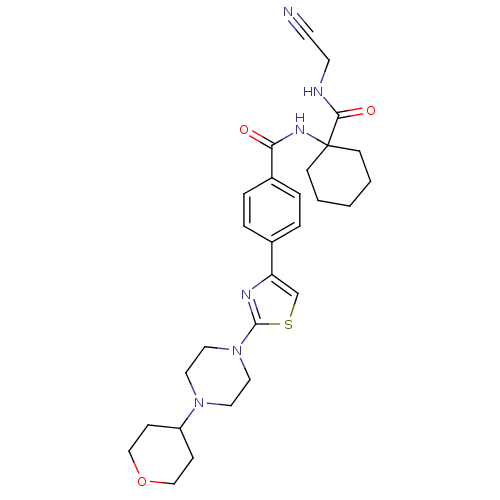
(CHEMBL200543)Show SMILES O=C(NC1(CCCCC1)C(=O)NCC#N)c1ccc(cc1)-c1csc(n1)N1CCN(CC1)C1CCOCC1 Show InChI InChI=1S/C28H36N6O3S/c29-12-13-30-26(36)28(10-2-1-3-11-28)32-25(35)22-6-4-21(5-7-22)24-20-38-27(31-24)34-16-14-33(15-17-34)23-8-18-37-19-9-23/h4-7,20,23H,1-3,8-11,13-19H2,(H,30,36)(H,32,35) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM50410587
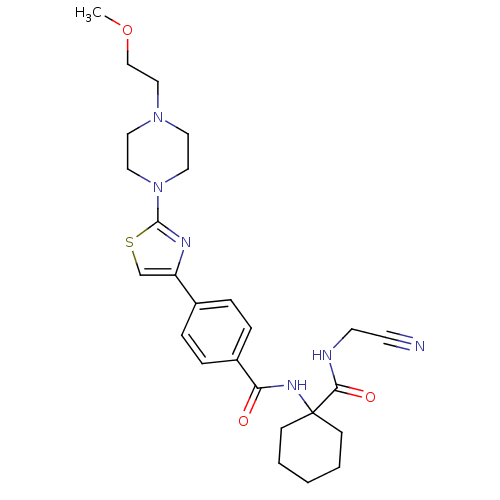
(CHEMBL200602)Show SMILES COCCN1CCN(CC1)c1nc(cs1)-c1ccc(cc1)C(=O)NC1(CCCCC1)C(=O)NCC#N Show InChI InChI=1S/C26H34N6O3S/c1-35-18-17-31-13-15-32(16-14-31)25-29-22(19-36-25)20-5-7-21(8-6-20)23(33)30-26(9-3-2-4-10-26)24(34)28-12-11-27/h5-8,19H,2-4,9-10,12-18H2,1H3,(H,28,34)(H,30,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM19854

(CHEMBL426819 | CRA-013783/L-006235 | N-{1-[(cyanom...)Show SMILES CN1CCN(CC1)c1nc(cs1)-c1ccc(cc1)C(=O)NC1(CCCCC1)C(=O)NCC#N Show InChI InChI=1S/C24H30N6O2S/c1-29-13-15-30(16-14-29)23-27-20(17-33-23)18-5-7-19(8-6-18)21(31)28-24(9-3-2-4-10-24)22(32)26-12-11-25/h5-8,17H,2-4,9-10,12-16H2,1H3,(H,26,32)(H,28,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM13934

(Atazanavir | BMS 232632 | CGP 73547 | CHEMBL1163 |...)Show SMILES COC(=O)N[C@H](C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)CN(Cc1ccc(cc1)-c1ccccn1)NC(=O)[C@@H](NC(=O)OC)C(C)(C)C)C(C)(C)C |r| Show InChI InChI=1S/C38H52N6O7/c1-37(2,3)31(41-35(48)50-7)33(46)40-29(22-25-14-10-9-11-15-25)30(45)24-44(43-34(47)32(38(4,5)6)42-36(49)51-8)23-26-17-19-27(20-18-26)28-16-12-13-21-39-28/h9-21,29-32,45H,22-24H2,1-8H3,(H,40,46)(H,41,48)(H,42,49)(H,43,47)/t29-,30-,31+,32+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease expressed in Escherichia coli by fluorometric assay |
J Med Chem 53: 607-15 (2010)
Article DOI: 10.1021/jm901165g
BindingDB Entry DOI: 10.7270/Q29Z97R7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50067593

(CHEBI:44032 | Crixivan | Indinavir | L-735524 | MK...)Show SMILES CC(C)(C)NC(=O)[C@@H]1CN(Cc2cccnc2)CCN1C[C@@H](O)C[C@@H](Cc1ccccc1)C(=O)N[C@@H]1[C@H](O)Cc2ccccc12 |r| Show InChI InChI=1S/C36H47N5O4/c1-36(2,3)39-35(45)31-24-40(22-26-12-9-15-37-21-26)16-17-41(31)23-29(42)19-28(18-25-10-5-4-6-11-25)34(44)38-33-30-14-8-7-13-27(30)20-32(33)43/h4-15,21,28-29,31-33,42-43H,16-20,22-24H2,1-3H3,(H,38,44)(H,39,45)/t28-,29+,31+,32-,33+/m1/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease expressed in Escherichia coli |
J Med Chem 51: 1053-7 (2008)
Article DOI: 10.1021/jm070680h
BindingDB Entry DOI: 10.7270/Q20Z7629 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50418365
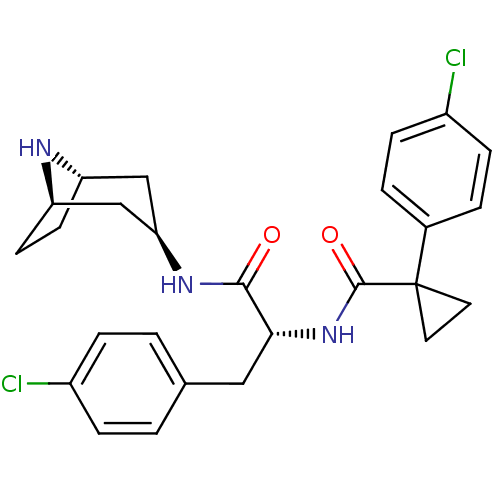
(CHEMBL1774024)Show SMILES Clc1ccc(C[C@@H](NC(=O)C2(CC2)c2ccc(Cl)cc2)C(=O)N[C@@H]2C[C@@H]3CC[C@H](C2)N3)cc1 |r| Show InChI InChI=1S/C26H29Cl2N3O2/c27-18-5-1-16(2-6-18)13-23(24(32)30-22-14-20-9-10-21(15-22)29-20)31-25(33)26(11-12-26)17-3-7-19(28)8-4-17/h1-8,20-23,29H,9-15H2,(H,30,32)(H,31,33)/t20-,21+,22+,23-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.562 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MSD
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from human V1A receptor expressed in CHO cells |
Bioorg Med Chem Lett 21: 3163-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.096
BindingDB Entry DOI: 10.7270/Q2K64KB0 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM50410607
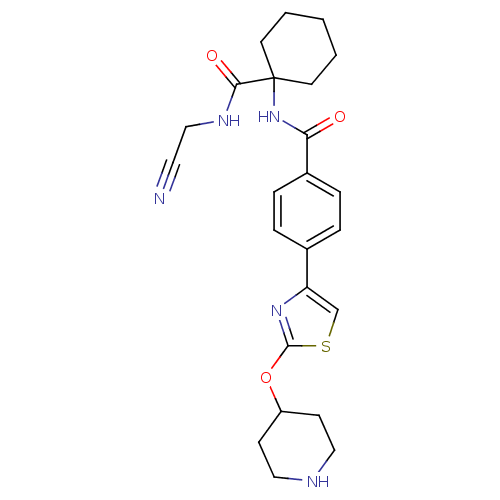
(CHEMBL200744)Show SMILES O=C(NC1(CCCCC1)C(=O)NCC#N)c1ccc(cc1)-c1csc(OC2CCNCC2)n1 Show InChI InChI=1S/C24H29N5O3S/c25-12-15-27-22(31)24(10-2-1-3-11-24)29-21(30)18-6-4-17(5-7-18)20-16-33-23(28-20)32-19-8-13-26-14-9-19/h4-7,16,19,26H,1-3,8-11,13-15H2,(H,27,31)(H,29,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM50410571
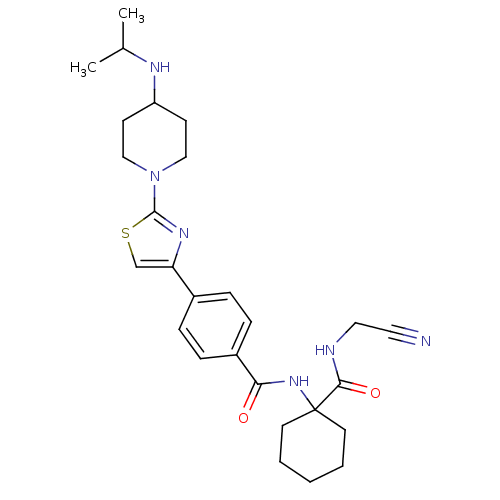
(CHEMBL200287)Show SMILES CC(C)NC1CCN(CC1)c1nc(cs1)-c1ccc(cc1)C(=O)NC1(CCCCC1)C(=O)NCC#N Show InChI InChI=1S/C27H36N6O2S/c1-19(2)30-22-10-16-33(17-11-22)26-31-23(18-36-26)20-6-8-21(9-7-20)24(34)32-27(12-4-3-5-13-27)25(35)29-15-14-28/h6-9,18-19,22,30H,3-5,10-13,15-17H2,1-2H3,(H,29,35)(H,32,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50225074

((1S,3S,5S)-2-[(S)-2-amino-2-(3-hydroxy-adamantan-1...)Show SMILES N[C@H](C(=O)N1[C@H]2C[C@H]2C[C@H]1C#N)C12CC3CC(CC(O)(C3)C1)C2 |TLB:1:12:15:20.18.17,THB:13:14:17:22.12.21,13:12:15.14.20:17,21:12:15:20.18.17,21:18:15:22.13.12,19:18:15:22.13.12| Show InChI InChI=1S/C18H25N3O2/c19-8-13-2-12-3-14(12)21(13)16(22)15(20)17-4-10-1-11(5-17)7-18(23,6-10)9-17/h10-15,23H,1-7,9,20H2/t10?,11?,12-,13+,14+,15-,17?,18?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 |
Bioorg Med Chem Lett 17: 6476-80 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.090
BindingDB Entry DOI: 10.7270/Q2B56JGM |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [501-599]
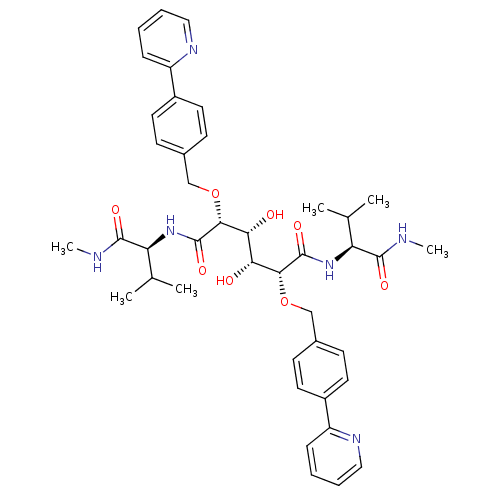
(Human immunodeficiency virus type 1) | BDBM852
((2R,3R,4R,5R)-3,4-dihydroxy-N,N'-bis[(1S)-2-methyl...)Show SMILES CNC(=O)[C@@H](NC(=O)[C@H](OCc1ccc(cc1)-c1ccccn1)[C@H](O)[C@@H](O)[C@@H](OCc1ccc(cc1)-c1ccccn1)C(=O)N[C@@H](C(C)C)C(=O)NC)C(C)C |r| Show InChI InChI=1S/C42H52N6O8/c1-25(2)33(39(51)43-5)47-41(53)37(55-23-27-13-17-29(18-14-27)31-11-7-9-21-45-31)35(49)36(50)38(42(54)48-34(26(3)4)40(52)44-6)56-24-28-15-19-30(20-16-28)32-12-8-10-22-46-32/h7-22,25-26,33-38,49-50H,23-24H2,1-6H3,(H,43,51)(H,44,52)(H,47,53)(H,48,54)/t33-,34-,35+,36+,37+,38+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.600 | -53.5 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Uppsala University
| Assay Description
Ki values were determined by using a fluorescent substrate (DABCYL-gamma-Abu-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Gln-EDANS). All incubations were performed a... |
J Med Chem 42: 3835-44 (1999)
Article DOI: 10.1021/jm9910371
BindingDB Entry DOI: 10.7270/Q2T151VX |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [501-599]
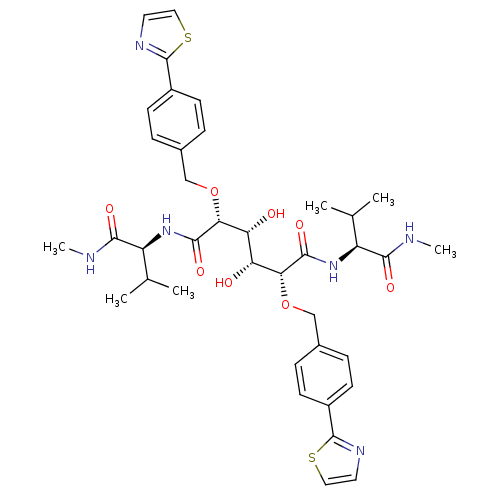
(Human immunodeficiency virus type 1) | BDBM853
((2R,3R,4R,5R)-3,4-dihydroxy-N,N'-bis[(1S)-2-methyl...)Show SMILES CNC(=O)[C@@H](NC(=O)[C@H](OCc1ccc(cc1)-c1nccs1)[C@H](O)[C@@H](O)[C@@H](OCc1ccc(cc1)-c1nccs1)C(=O)N[C@@H](C(C)C)C(=O)NC)C(C)C |r| Show InChI InChI=1S/C38H48N6O8S2/c1-21(2)27(33(47)39-5)43-35(49)31(51-19-23-7-11-25(12-8-23)37-41-15-17-53-37)29(45)30(46)32(36(50)44-28(22(3)4)34(48)40-6)52-20-24-9-13-26(14-10-24)38-42-16-18-54-38/h7-18,21-22,27-32,45-46H,19-20H2,1-6H3,(H,39,47)(H,40,48)(H,43,49)(H,44,50)/t27-,28-,29+,30+,31+,32+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.600 | -53.5 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Uppsala University
| Assay Description
Ki values were determined by using a fluorescent substrate (DABCYL-gamma-Abu-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Gln-EDANS). All incubations were performed a... |
J Med Chem 42: 3835-44 (1999)
Article DOI: 10.1021/jm9910371
BindingDB Entry DOI: 10.7270/Q2T151VX |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM358

((2R,3R,4R,5R)-2,5-bis(benzyloxy)-3,4-dihydroxy-N,N...)Show SMILES O[C@H]([C@@H](O)[C@@H](OCc1ccccc1)C(=O)N[C@@H]1[C@H](O)Cc2ccccc12)[C@@H](OCc1ccccc1)C(=O)N[C@@H]1[C@H](O)Cc2ccccc12 |r| Show InChI InChI=1S/C38H40N2O8/c41-29-19-25-15-7-9-17-27(25)31(29)39-37(45)35(47-21-23-11-3-1-4-12-23)33(43)34(44)36(48-22-24-13-5-2-6-14-24)38(46)40-32-28-18-10-8-16-26(28)20-30(32)42/h1-18,29-36,41-44H,19-22H2,(H,39,45)(H,40,46)/t29-,30-,31+,32+,33-,34-,35-,36-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.600 | -53.5 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Stockholm University
| Assay Description
A fluorimetric assay was used to determine the effects of the inhibitors on HIV-1 protease. This assay used an internally quenched fluorescent peptid... |
J Med Chem 44: 3407-16 (2001)
Article DOI: 10.1021/jm0011171
BindingDB Entry DOI: 10.7270/Q2862DND |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cathepsin K
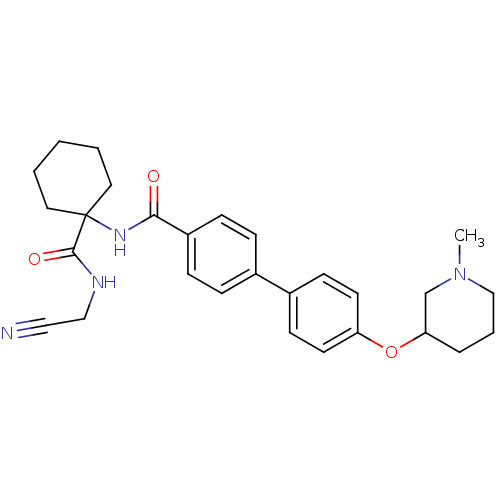
(Oryctolagus cuniculus (rabbit)) | BDBM50410580
(CHEMBL435913)Show SMILES CN1CCCC(C1)Oc1ccc(cc1)-c1ccc(cc1)C(=O)NC1(CCCCC1)C(=O)NCC#N Show InChI InChI=1S/C28H34N4O3/c1-32-19-5-6-25(20-32)35-24-13-11-22(12-14-24)21-7-9-23(10-8-21)26(33)31-28(15-3-2-4-16-28)27(34)30-18-17-29/h7-14,25H,2-6,15-16,18-20H2,1H3,(H,30,34)(H,31,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [501-599]
(Human immunodeficiency virus type 1) | BDBM846
((2R,3R,4R,5R)-3,4-dihydroxy-N,N'-bis[(1S)-2-methyl...)Show SMILES CNC(=O)[C@@H](NC(=O)[C@H](OCc1ccc(cc1)-c1ccccc1)[C@H](O)[C@@H](O)[C@@H](OCc1ccc(cc1)-c1ccccc1)C(=O)N[C@@H](C(C)C)C(=O)NC)C(C)C |r| Show InChI InChI=1S/C44H54N4O8/c1-27(2)35(41(51)45-5)47-43(53)39(55-25-29-17-21-33(22-18-29)31-13-9-7-10-14-31)37(49)38(50)40(44(54)48-36(28(3)4)42(52)46-6)56-26-30-19-23-34(24-20-30)32-15-11-8-12-16-32/h7-24,27-28,35-40,49-50H,25-26H2,1-6H3,(H,45,51)(H,46,52)(H,47,53)(H,48,54)/t35-,36-,37+,38+,39+,40+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.700 | -53.1 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Uppsala University
| Assay Description
Ki values were determined by using a fluorescent substrate (DABCYL-gamma-Abu-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Gln-EDANS). All incubations were performed a... |
J Med Chem 42: 3835-44 (1999)
Article DOI: 10.1021/jm9910371
BindingDB Entry DOI: 10.7270/Q2T151VX |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data


























































