

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N-lysine methyltransferase KMT5A (Homo sapiens (Human)) | BDBM50201571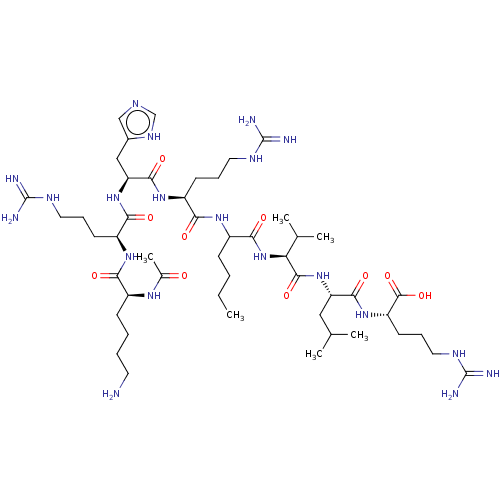 (CHEMBL3934996) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Competitive inhibition of human SETD8 (186 to 352 residues) using biotin-labeled H4K20 (1 to 24 residues) as substrate after 1 hr in presence of 3H-S... | ACS Med Chem Lett 7: 1102-1106 (2016) Article DOI: 10.1021/acsmedchemlett.6b00303 BindingDB Entry DOI: 10.7270/Q2319XVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase KMT5A (Homo sapiens (Human)) | BDBM50051116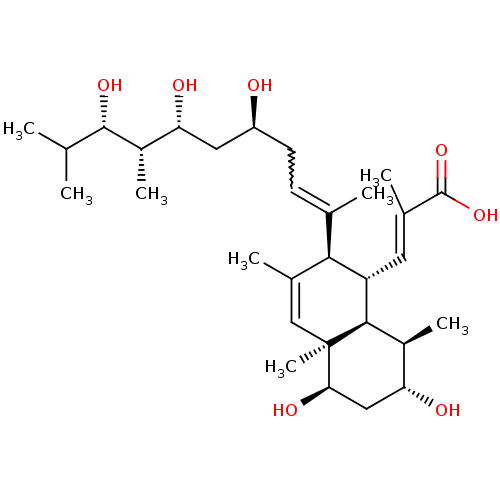 (CHEMBL3318284) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Competitive inhibition of SETD8 (unknown origin) using biotin-labeled H4 (1 to 24 residues) as substrate after 1 hr in presence of varying levels of ... | ACS Med Chem Lett 7: 1102-1106 (2016) Article DOI: 10.1021/acsmedchemlett.6b00303 BindingDB Entry DOI: 10.7270/Q2319XVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM92963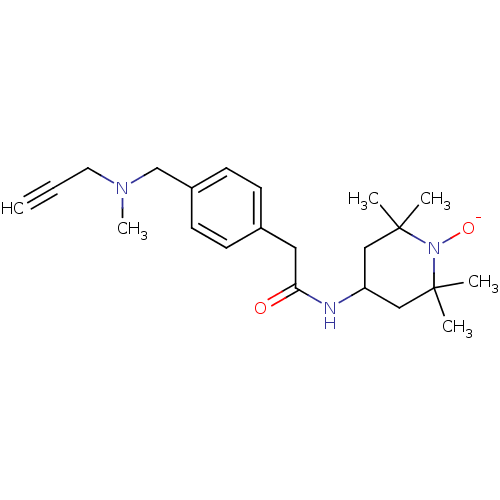 (ParSL) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University | Assay Description Competitive inhibition assay using human and rat MAOs. | Biochemistry 47: 526-36 (2008) Article DOI: 10.1021/bi7019707 BindingDB Entry DOI: 10.7270/Q24M934H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM92963 (ParSL) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.07E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University | Assay Description Competitive inhibition assay using human and rat MAOs. | Biochemistry 47: 526-36 (2008) Article DOI: 10.1021/bi7019707 BindingDB Entry DOI: 10.7270/Q24M934H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM92963 (ParSL) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.51E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University | Assay Description Competitive inhibition assay using human and rat MAOs. | Biochemistry 47: 526-36 (2008) Article DOI: 10.1021/bi7019707 BindingDB Entry DOI: 10.7270/Q24M934H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 4A (Homo sapiens (Human)) | BDBM50457513 (CHEMBL4212908) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Inhibition of H3(1-10)K4me3 peptide binding to thrombin cleavable N-terminal 6His tagged and 13C-IVLM labeled human KDM4A tandem TUDOR domain (897 to... | Bioorg Med Chem Lett 28: 1708-1713 (2018) Article DOI: 10.1016/j.bmcl.2018.04.050 BindingDB Entry DOI: 10.7270/Q2WM1H1X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM92963 (ParSL) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.12E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University | Assay Description Competitive inhibition assay using human and rat MAOs. | Biochemistry 47: 526-36 (2008) Article DOI: 10.1021/bi7019707 BindingDB Entry DOI: 10.7270/Q24M934H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase KMT5B (Homo sapiens (Human)) | BDBM223981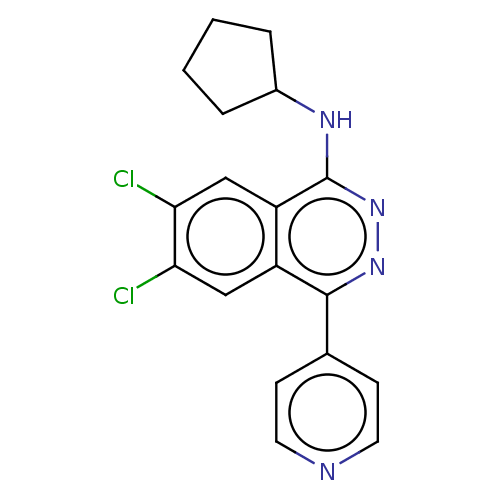 (6,7-Dichloro-N-cyclopentyl-4-(pyridin-4-yl)phthala...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | 8.0 | 25 |
AbbVie | Assay Description Experiments were performed in triplicate at room temperature with 1 h incubation of 10 μl reaction mixture in buffer of 20 mM Tris-HCl, pH 8.0, ... | Nat Chem Biol 13: 317-324 (2017) Article DOI: 10.1038/nchembio.2282 BindingDB Entry DOI: 10.7270/Q2X065XF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic (Homo sapiens (Human)) | BDBM50506590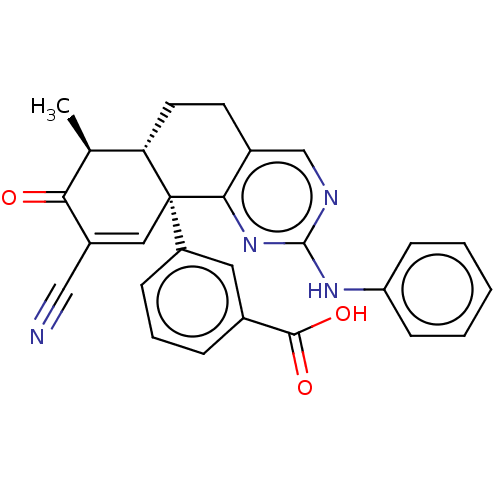 (CHEMBL4593992) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of C-terminal 8-His tagged wild type human IDH1 (1 to 414 residues) expressed in Escherichia coli BL21(DE3)-T1R preincubated for 15 mins u... | J Med Chem 61: 6647-6657 (2018) Article DOI: 10.1021/acs.jmedchem.8b00305 BindingDB Entry DOI: 10.7270/Q2RJ4NR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic (Homo sapiens (Human)) | BDBM50506594 (CHEMBL4556998) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of C-terminal 8-His tagged wild type human IDH1 (1 to 414 residues) expressed in Escherichia coli BL21(DE3)-T1R preincubated for 15 mins u... | J Med Chem 61: 6647-6657 (2018) Article DOI: 10.1021/acs.jmedchem.8b00305 BindingDB Entry DOI: 10.7270/Q2RJ4NR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic (Homo sapiens (Human)) | BDBM50506592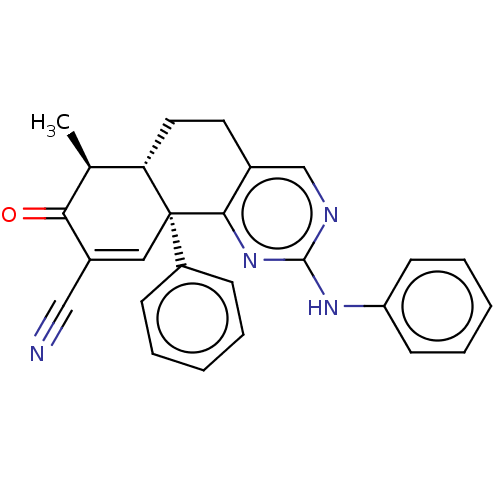 (CHEMBL4535154) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of C-terminal 8-His tagged wild type human IDH1 (1 to 414 residues) expressed in Escherichia coli BL21(DE3)-T1R preincubated for 15 mins u... | J Med Chem 61: 6647-6657 (2018) Article DOI: 10.1021/acs.jmedchem.8b00305 BindingDB Entry DOI: 10.7270/Q2RJ4NR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic (Homo sapiens (Human)) | BDBM50506585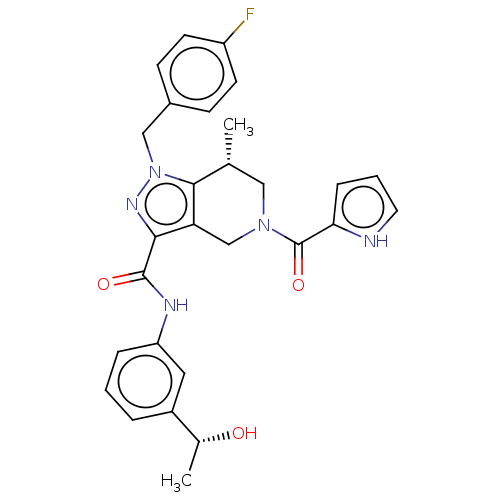 (CHEMBL4515358) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of C-terminal 8-His tagged wild type human IDH1 (1 to 414 residues) expressed in Escherichia coli BL21(DE3)-T1R preincubated for 15 mins u... | J Med Chem 61: 6647-6657 (2018) Article DOI: 10.1021/acs.jmedchem.8b00305 BindingDB Entry DOI: 10.7270/Q2RJ4NR6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone-lysine N-methyltransferase KMT5C (Homo sapiens (Human)) | BDBM223981 (6,7-Dichloro-N-cyclopentyl-4-(pyridin-4-yl)phthala...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 144 | n/a | n/a | n/a | n/a | 8.0 | 25 |
AbbVie | Assay Description Experiments were performed in triplicate at room temperature with 1 h incubation of 10 μl reaction mixture in buffer of 20 mM Tris-HCl, pH 8.0, ... | Nat Chem Biol 13: 317-324 (2017) Article DOI: 10.1038/nchembio.2282 BindingDB Entry DOI: 10.7270/Q2X065XF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic (Homo sapiens (Human)) | BDBM50506593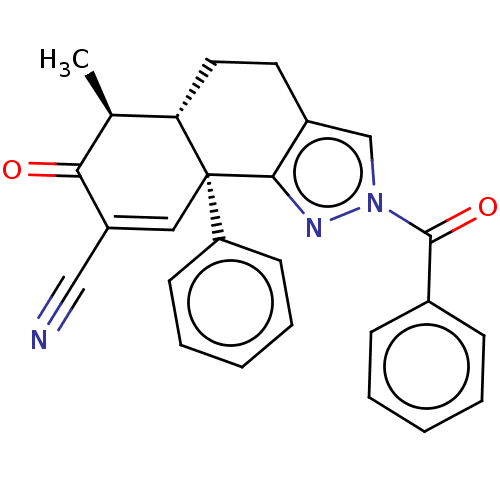 (CHEMBL4540440) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of C-terminal 8-His tagged wild type human IDH1 (1 to 414 residues) expressed in Escherichia coli BL21(DE3)-T1R preincubated for 15 mins u... | J Med Chem 61: 6647-6657 (2018) Article DOI: 10.1021/acs.jmedchem.8b00305 BindingDB Entry DOI: 10.7270/Q2RJ4NR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic (Homo sapiens (Human)) | BDBM50506584 (CHEMBL221360) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of C-terminal 8-His tagged wild type human IDH1 (1 to 414 residues) expressed in Escherichia coli BL21(DE3)-T1R preincubated for 15 mins u... | J Med Chem 61: 6647-6657 (2018) Article DOI: 10.1021/acs.jmedchem.8b00305 BindingDB Entry DOI: 10.7270/Q2RJ4NR6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| N-lysine methyltransferase KMT5A (Homo sapiens (Human)) | BDBM50201571 (CHEMBL3934996) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Inhibition of human SETD8 (186 to 352 residues) using biotin-labeled H4K20 (1 to 24 residues) as substrate after 1 hr in presence of 3H-SAM by scinti... | ACS Med Chem Lett 7: 1102-1106 (2016) Article DOI: 10.1021/acsmedchemlett.6b00303 BindingDB Entry DOI: 10.7270/Q2319XVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic (Homo sapiens (Human)) | BDBM50506591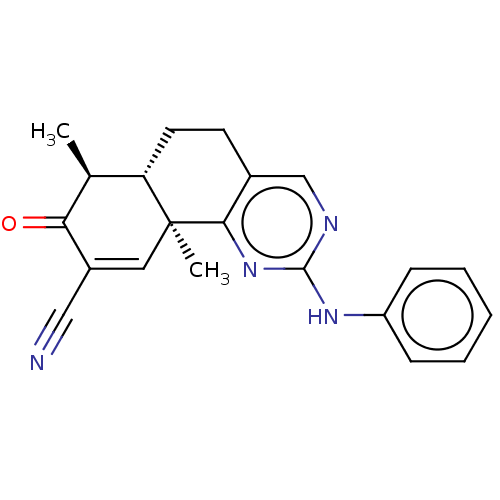 (CHEMBL4548727) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of C-terminal 8-His tagged wild type human IDH1 (1 to 414 residues) expressed in Escherichia coli BL21(DE3)-T1R preincubated for 15 mins u... | J Med Chem 61: 6647-6657 (2018) Article DOI: 10.1021/acs.jmedchem.8b00305 BindingDB Entry DOI: 10.7270/Q2RJ4NR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase KMT5B (Homo sapiens (Human)) | BDBM223980 (6,7-Dichloro-N-cyclopentylquinolin-4-amine (2)) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 590 | n/a | n/a | n/a | n/a | 8.0 | 25 |
AbbVie | Assay Description Experiments were performed in triplicate at room temperature with 1 h incubation of 10 μl reaction mixture in buffer of 20 mM Tris-HCl, pH 8.0, ... | Nat Chem Biol 13: 317-324 (2017) Article DOI: 10.1038/nchembio.2282 BindingDB Entry DOI: 10.7270/Q2X065XF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic (Homo sapiens (Human)) | BDBM195601 (GSK321 | US11311527, Cpd ID GSK321 | US11376246, C...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 970 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of C-terminal 8-His tagged wild type human IDH1 (1 to 414 residues) expressed in Escherichia coli BL21(DE3)-T1R preincubated for 15 mins u... | J Med Chem 61: 6647-6657 (2018) Article DOI: 10.1021/acs.jmedchem.8b00305 BindingDB Entry DOI: 10.7270/Q2RJ4NR6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone-lysine N-methyltransferase KMT5C (Homo sapiens (Human)) | BDBM223980 (6,7-Dichloro-N-cyclopentylquinolin-4-amine (2)) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | 8.0 | 25 |
AbbVie | Assay Description Experiments were performed in triplicate at room temperature with 1 h incubation of 10 μl reaction mixture in buffer of 20 mM Tris-HCl, pH 8.0, ... | Nat Chem Biol 13: 317-324 (2017) Article DOI: 10.1038/nchembio.2282 BindingDB Entry DOI: 10.7270/Q2X065XF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic (Homo sapiens (Human)) | BDBM50506589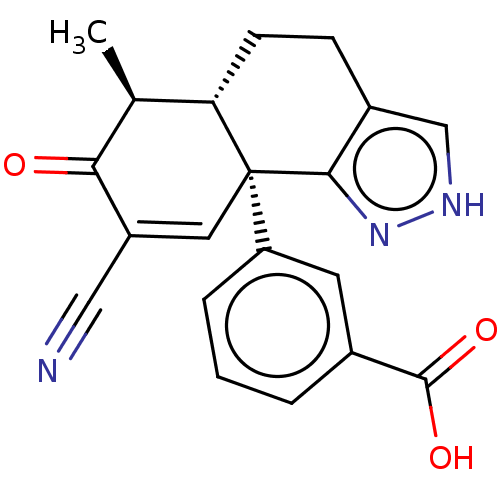 (CHEMBL4549554) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of C-terminal 8-His tagged wild type human IDH1 (1 to 414 residues) expressed in Escherichia coli BL21(DE3)-T1R preincubated for 15 mins u... | J Med Chem 61: 6647-6657 (2018) Article DOI: 10.1021/acs.jmedchem.8b00305 BindingDB Entry DOI: 10.7270/Q2RJ4NR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic (Homo sapiens (Human)) | BDBM50506587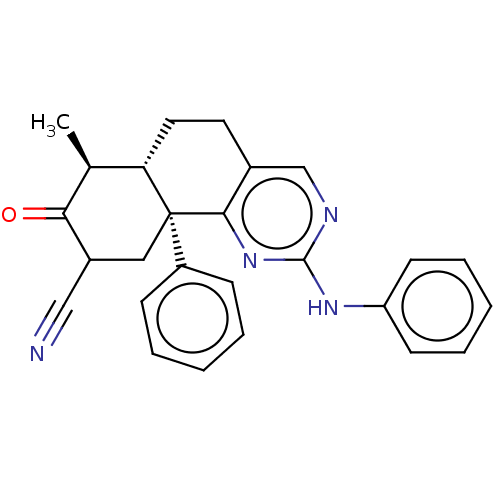 (CHEMBL4551778) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 1.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of C-terminal 8-His tagged wild type human IDH1 (1 to 414 residues) expressed in Escherichia coli BL21(DE3)-T1R preincubated for 15 mins u... | J Med Chem 61: 6647-6657 (2018) Article DOI: 10.1021/acs.jmedchem.8b00305 BindingDB Entry DOI: 10.7270/Q2RJ4NR6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone-lysine N-methyltransferase KMT5C (Homo sapiens (Human)) | BDBM223979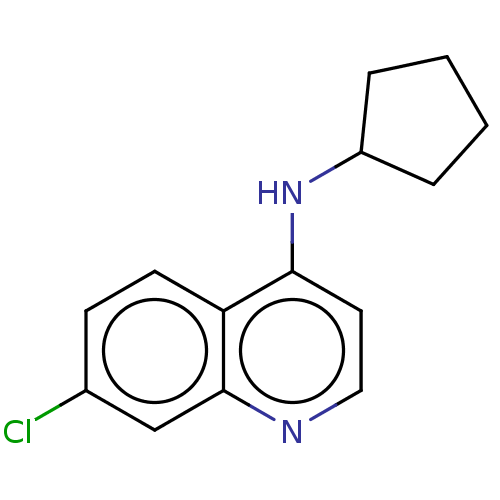 (7-Chloro-N-cyclopentylquinolin-4-amine (1)) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a | 8.0 | 25 |
AbbVie | Assay Description Experiments were performed in triplicate at room temperature with 1 h incubation of 10 μl reaction mixture in buffer of 20 mM Tris-HCl, pH 8.0, ... | Nat Chem Biol 13: 317-324 (2017) Article DOI: 10.1038/nchembio.2282 BindingDB Entry DOI: 10.7270/Q2X065XF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase KMT5B (Homo sapiens (Human)) | BDBM223979 (7-Chloro-N-cyclopentylquinolin-4-amine (1)) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.90E+3 | n/a | n/a | n/a | n/a | 8.0 | 25 |
AbbVie | Assay Description Experiments were performed in triplicate at room temperature with 1 h incubation of 10 μl reaction mixture in buffer of 20 mM Tris-HCl, pH 8.0, ... | Nat Chem Biol 13: 317-324 (2017) Article DOI: 10.1038/nchembio.2282 BindingDB Entry DOI: 10.7270/Q2X065XF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase KMT5A (Homo sapiens (Human)) | BDBM50051116 (CHEMBL3318284) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Inhibition of SETD8 (unknown origin) using biotin-labeled H4 (1 to 24 residues) as substrate after 1 hr in presence of [3H]SAM by scintillation proxi... | ACS Med Chem Lett 7: 1102-1106 (2016) Article DOI: 10.1021/acsmedchemlett.6b00303 BindingDB Entry DOI: 10.7270/Q2319XVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic (Homo sapiens (Human)) | BDBM50506586 (CHEMBL4459125) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of C-terminal 8-His tagged wild type human IDH1 (1 to 414 residues) expressed in Escherichia coli BL21(DE3)-T1R preincubated for 15 mins u... | J Med Chem 61: 6647-6657 (2018) Article DOI: 10.1021/acs.jmedchem.8b00305 BindingDB Entry DOI: 10.7270/Q2RJ4NR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase KMT5C (Homo sapiens (Human)) | BDBM223982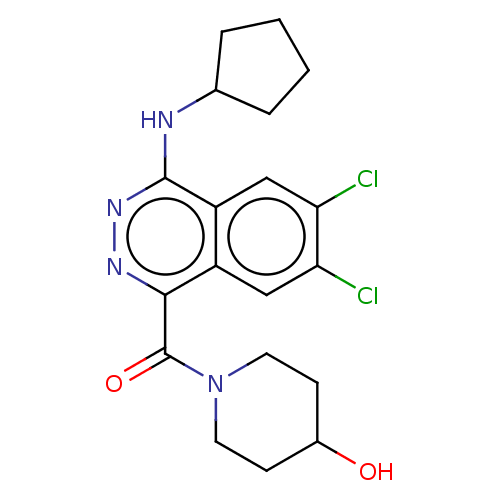 ((6,7-Dichloro-4-(cyclopentylamino)phthalazin-1-yl)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | 8.0 | 25 |
AbbVie | Assay Description Experiments were performed in triplicate at room temperature with 1 h incubation of 10 μl reaction mixture in buffer of 20 mM Tris-HCl, pH 8.0, ... | Nat Chem Biol 13: 317-324 (2017) Article DOI: 10.1038/nchembio.2282 BindingDB Entry DOI: 10.7270/Q2X065XF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic (Homo sapiens (Human)) | BDBM50506588 (CHEMBL4467995) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of C-terminal 8-His tagged wild type human IDH1 (1 to 414 residues) expressed in Escherichia coli BL21(DE3)-T1R preincubated for 15 mins u... | J Med Chem 61: 6647-6657 (2018) Article DOI: 10.1021/acs.jmedchem.8b00305 BindingDB Entry DOI: 10.7270/Q2RJ4NR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic (Homo sapiens (Human)) | BDBM50506583 (CHEMBL4562890) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of C-terminal 8-His tagged wild type human IDH1 (1 to 414 residues) expressed in Escherichia coli BL21(DE3)-T1R preincubated for 15 mins u... | J Med Chem 61: 6647-6657 (2018) Article DOI: 10.1021/acs.jmedchem.8b00305 BindingDB Entry DOI: 10.7270/Q2RJ4NR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase KMT5B (Homo sapiens (Human)) | BDBM223982 ((6,7-Dichloro-4-(cyclopentylamino)phthalazin-1-yl)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | 8.0 | 25 |
AbbVie | Assay Description Experiments were performed in triplicate at room temperature with 1 h incubation of 10 μl reaction mixture in buffer of 20 mM Tris-HCl, pH 8.0, ... | Nat Chem Biol 13: 317-324 (2017) Article DOI: 10.1038/nchembio.2282 BindingDB Entry DOI: 10.7270/Q2X065XF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 4A (Homo sapiens (Human)) | BDBM50457514 (CHEMBL4204908) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.22E+6 | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Inhibition of NanoLuc-KDM4A-TUDOR domain (unknown origin) binding to Histone H3.3-HaloTag expressed in 293T cells incubated for 18 hrs by Nano-BRET b... | Bioorg Med Chem Lett 28: 1708-1713 (2018) Article DOI: 10.1016/j.bmcl.2018.04.050 BindingDB Entry DOI: 10.7270/Q2WM1H1X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 4A (Homo sapiens (Human)) | BDBM50457513 (CHEMBL4212908) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 1.55E+4 | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Binding affinity to thrombin cleavable N-terminal 6His tagged and 13C-IVLM labeled human KDM4A tandem TUDOR domain (897 to 1011 residues) expressed i... | Bioorg Med Chem Lett 28: 1708-1713 (2018) Article DOI: 10.1016/j.bmcl.2018.04.050 BindingDB Entry DOI: 10.7270/Q2WM1H1X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 4C (Homo sapiens (Human)) | BDBM50457513 (CHEMBL4212908) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 1.29E+5 | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Binding affinity to 6His-tagged and human KDM4C tandem TUDOR domain (874 to 990 residues) expressed in Escherichia coli BL21(DE3)-T1R by ITC method | Bioorg Med Chem Lett 28: 1708-1713 (2018) Article DOI: 10.1016/j.bmcl.2018.04.050 BindingDB Entry DOI: 10.7270/Q2WM1H1X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 4A (Homo sapiens (Human)) | BDBM50457513 (CHEMBL4212908) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 2.27E+4 | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Binding affinity to thrombin cleavable N-terminal 6His tagged and 13C-IVLM labeled human KDM4A tandem TUDOR domain (897 to 1011 residues) expressed i... | Bioorg Med Chem Lett 28: 1708-1713 (2018) Article DOI: 10.1016/j.bmcl.2018.04.050 BindingDB Entry DOI: 10.7270/Q2WM1H1X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 4A (Homo sapiens (Human)) | BDBM50457513 (CHEMBL4212908) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 5.51E+4 | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Binding affinity to thrombin cleavable N-terminal 6His tagged and 13C-IVLM labeled human KDM4A tandem TUDOR domain (897 to 1011 residues) expressed i... | Bioorg Med Chem Lett 28: 1708-1713 (2018) Article DOI: 10.1016/j.bmcl.2018.04.050 BindingDB Entry DOI: 10.7270/Q2WM1H1X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 4A (Homo sapiens (Human)) | BDBM50457515 (CHEMBL4208006) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Binding affinity to thrombin cleavable N-terminal 6His tagged and 13C-IVLM labeled human KDM4A tandem TUDOR domain (897 to 1011 residues) expressed i... | Bioorg Med Chem Lett 28: 1708-1713 (2018) Article DOI: 10.1016/j.bmcl.2018.04.050 BindingDB Entry DOI: 10.7270/Q2WM1H1X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 4A (Homo sapiens (Human)) | BDBM50457516 (CHEMBL4214213) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Binding affinity to thrombin cleavable N-terminal 6His tagged and 13C-IVLM labeled human KDM4A tandem TUDOR domain (897 to 1011 residues) expressed i... | Bioorg Med Chem Lett 28: 1708-1713 (2018) Article DOI: 10.1016/j.bmcl.2018.04.050 BindingDB Entry DOI: 10.7270/Q2WM1H1X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 4B (Homo sapiens (Human)) | BDBM50457513 (CHEMBL4212908) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 1.27E+5 | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Binding affinity to 6His-tagged and human KDM4B tandem TUDOR domain (916 to 1030 residues) expressed in Escherichia coli BL21(DE3)-T1R by ITC method | Bioorg Med Chem Lett 28: 1708-1713 (2018) Article DOI: 10.1016/j.bmcl.2018.04.050 BindingDB Entry DOI: 10.7270/Q2WM1H1X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 4A (Homo sapiens (Human)) | BDBM50457517 (CHEMBL4208145) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | n/a | 8.00E+4 | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Binding affinity to thrombin cleavable N-terminal 6His-tagged and 13C-IVLM labeled human KDM4A tandem TUDOR domain (897 to 1011 residues) expressed i... | Bioorg Med Chem Lett 28: 1708-1713 (2018) Article DOI: 10.1016/j.bmcl.2018.04.050 BindingDB Entry DOI: 10.7270/Q2WM1H1X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Lysine-specific demethylase 4A (Homo sapiens (Human)) | BDBM50457513 (CHEMBL4212908) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.05E+5 | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Inhibition of NanoLuc-KDM4A-TUDOR domain (unknown origin) binding to Histone H3.3-HaloTag expressed in 293T cells incubated for 18 hrs by Nano-BRET b... | Bioorg Med Chem Lett 28: 1708-1713 (2018) Article DOI: 10.1016/j.bmcl.2018.04.050 BindingDB Entry DOI: 10.7270/Q2WM1H1X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 4A (Homo sapiens (Human)) | BDBM50457513 (CHEMBL4212908) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 8.17E+4 | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Binding affinity to thrombin cleavable N-terminal 6His tagged and 13C-IVLM labeled human KDM4A tandem TUDOR domain (897 to 1011 residues) expressed i... | Bioorg Med Chem Lett 28: 1708-1713 (2018) Article DOI: 10.1016/j.bmcl.2018.04.050 BindingDB Entry DOI: 10.7270/Q2WM1H1X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||