 Found 406 hits with Last Name = 'usmani' and Initial = 'k'
Found 406 hits with Last Name = 'usmani' and Initial = 'k' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50041691
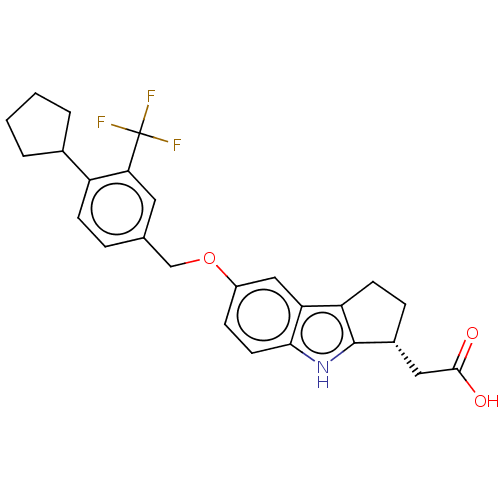
(CHEMBL3358920 | US11149292, Compound (R)-2-(7-(4-c...)Show SMILES OC(=O)C[C@H]1CCc2c1[nH]c1ccc(OCc3ccc(C4CCCC4)c(c3)C(F)(F)F)cc21 |r| Show InChI InChI=1S/C26H26F3NO3/c27-26(28,29)22-11-15(5-8-19(22)16-3-1-2-4-16)14-33-18-7-10-23-21(13-18)20-9-6-17(12-24(31)32)25(20)30-23/h5,7-8,10-11,13,16-17,30H,1-4,6,9,12,14H2,(H,31,32)/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Induction of internalization of HA-tagged human S1P1 receptor expressed in CHO cells |
ACS Med Chem Lett 5: 1313-7 (2014)
Article DOI: 10.1021/ml500389m
BindingDB Entry DOI: 10.7270/Q29W0H4C |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50041691

(CHEMBL3358920 | US11149292, Compound (R)-2-(7-(4-c...)Show SMILES OC(=O)C[C@H]1CCc2c1[nH]c1ccc(OCc3ccc(C4CCCC4)c(c3)C(F)(F)F)cc21 |r| Show InChI InChI=1S/C26H26F3NO3/c27-26(28,29)22-11-15(5-8-19(22)16-3-1-2-4-16)14-33-18-7-10-23-21(13-18)20-9-6-17(12-24(31)32)25(20)30-23/h5,7-8,10-11,13,16-17,30H,1-4,6,9,12,14H2,(H,31,32)/t17-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human adenosine A3 receptor |
ACS Med Chem Lett 5: 1313-7 (2014)
Article DOI: 10.1021/ml500389m
BindingDB Entry DOI: 10.7270/Q29W0H4C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50041982
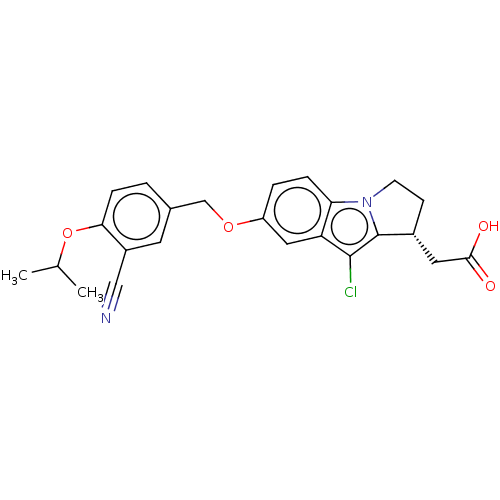
(CHEMBL3359522)Show SMILES CC(C)Oc1ccc(COc2ccc3n4CC[C@H](CC(O)=O)c4c(Cl)c3c2)cc1C#N |r| Show InChI InChI=1S/C24H23ClN2O4/c1-14(2)31-21-6-3-15(9-17(21)12-26)13-30-18-4-5-20-19(11-18)23(25)24-16(10-22(28)29)7-8-27(20)24/h3-6,9,11,14,16H,7-8,10,13H2,1-2H3,(H,28,29)/t16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
ACS Med Chem Lett 5: 1334-9 (2014)
Article DOI: 10.1021/ml500422m
BindingDB Entry DOI: 10.7270/Q2XK8H6S |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50041691

(CHEMBL3358920 | US11149292, Compound (R)-2-(7-(4-c...)Show SMILES OC(=O)C[C@H]1CCc2c1[nH]c1ccc(OCc3ccc(C4CCCC4)c(c3)C(F)(F)F)cc21 |r| Show InChI InChI=1S/C26H26F3NO3/c27-26(28,29)22-11-15(5-8-19(22)16-3-1-2-4-16)14-33-18-7-10-23-21(13-18)20-9-6-17(12-24(31)32)25(20)30-23/h5,7-8,10-11,13,16-17,30H,1-4,6,9,12,14H2,(H,31,32)/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]astemizole from human ERG |
ACS Med Chem Lett 5: 1313-7 (2014)
Article DOI: 10.1021/ml500389m
BindingDB Entry DOI: 10.7270/Q29W0H4C |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50041691

(CHEMBL3358920 | US11149292, Compound (R)-2-(7-(4-c...)Show SMILES OC(=O)C[C@H]1CCc2c1[nH]c1ccc(OCc3ccc(C4CCCC4)c(c3)C(F)(F)F)cc21 |r| Show InChI InChI=1S/C26H26F3NO3/c27-26(28,29)22-11-15(5-8-19(22)16-3-1-2-4-16)14-33-18-7-10-23-21(13-18)20-9-6-17(12-24(31)32)25(20)30-23/h5,7-8,10-11,13,16-17,30H,1-4,6,9,12,14H2,(H,31,32)/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by patch clamp assay |
ACS Med Chem Lett 5: 1313-7 (2014)
Article DOI: 10.1021/ml500389m
BindingDB Entry DOI: 10.7270/Q29W0H4C |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50040961
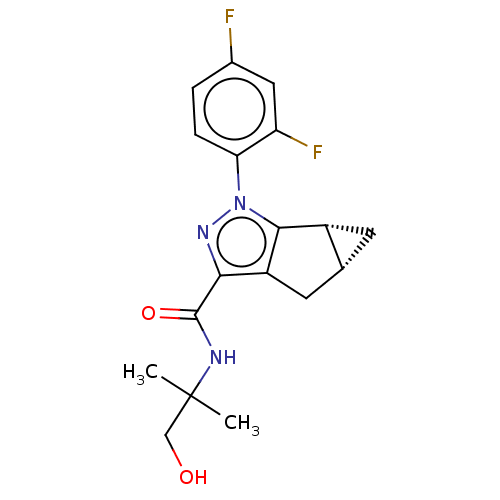
(CHEMBL3354952)Show SMILES [H][C@]12C[C@@]1([H])c1c(C2)c(nn1-c1ccc(F)cc1F)C(=O)NC(C)(C)CO |r| Show InChI InChI=1S/C18H19F2N3O2/c1-18(2,8-24)21-17(25)15-12-6-9-5-11(9)16(12)23(22-15)14-4-3-10(19)7-13(14)20/h3-4,7,9,11,24H,5-6,8H2,1-2H3,(H,21,25)/t9-,11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human ERG channel by patch clamp technique |
Bioorg Med Chem Lett 25: 322-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.11.040
BindingDB Entry DOI: 10.7270/Q21J9CDS |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50386859
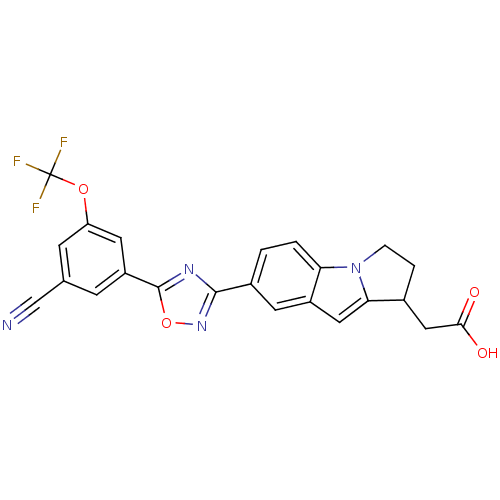
(CHEMBL2048293)Show SMILES OC(=O)CC1CCn2c1cc1cc(ccc21)-c1noc(n1)-c1cc(OC(F)(F)F)cc(c1)C#N Show InChI InChI=1S/C23H15F3N4O4/c24-23(25,26)33-17-6-12(11-27)5-16(8-17)22-28-21(29-34-22)14-1-2-18-15(7-14)9-19-13(10-20(31)32)3-4-30(18)19/h1-2,5-9,13H,3-4,10H2,(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by astemizole assay |
Bioorg Med Chem Lett 22: 4404-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.129
BindingDB Entry DOI: 10.7270/Q2FJ2HVB |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50386859

(CHEMBL2048293)Show SMILES OC(=O)CC1CCn2c1cc1cc(ccc21)-c1noc(n1)-c1cc(OC(F)(F)F)cc(c1)C#N Show InChI InChI=1S/C23H15F3N4O4/c24-23(25,26)33-17-6-12(11-27)5-16(8-17)22-28-21(29-34-22)14-1-2-18-15(7-14)9-19-13(10-20(31)32)3-4-30(18)19/h1-2,5-9,13H,3-4,10H2,(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 |
Bioorg Med Chem Lett 22: 4404-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.129
BindingDB Entry DOI: 10.7270/Q2FJ2HVB |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50386859

(CHEMBL2048293)Show SMILES OC(=O)CC1CCn2c1cc1cc(ccc21)-c1noc(n1)-c1cc(OC(F)(F)F)cc(c1)C#N Show InChI InChI=1S/C23H15F3N4O4/c24-23(25,26)33-17-6-12(11-27)5-16(8-17)22-28-21(29-34-22)14-1-2-18-15(7-14)9-19-13(10-20(31)32)3-4-30(18)19/h1-2,5-9,13H,3-4,10H2,(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 |
Bioorg Med Chem Lett 22: 4404-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.129
BindingDB Entry DOI: 10.7270/Q2FJ2HVB |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50386859

(CHEMBL2048293)Show SMILES OC(=O)CC1CCn2c1cc1cc(ccc21)-c1noc(n1)-c1cc(OC(F)(F)F)cc(c1)C#N Show InChI InChI=1S/C23H15F3N4O4/c24-23(25,26)33-17-6-12(11-27)5-16(8-17)22-28-21(29-34-22)14-1-2-18-15(7-14)9-19-13(10-20(31)32)3-4-30(18)19/h1-2,5-9,13H,3-4,10H2,(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 |
Bioorg Med Chem Lett 22: 4404-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.129
BindingDB Entry DOI: 10.7270/Q2FJ2HVB |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50386859

(CHEMBL2048293)Show SMILES OC(=O)CC1CCn2c1cc1cc(ccc21)-c1noc(n1)-c1cc(OC(F)(F)F)cc(c1)C#N Show InChI InChI=1S/C23H15F3N4O4/c24-23(25,26)33-17-6-12(11-27)5-16(8-17)22-28-21(29-34-22)14-1-2-18-15(7-14)9-19-13(10-20(31)32)3-4-30(18)19/h1-2,5-9,13H,3-4,10H2,(H,31,32) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
Bioorg Med Chem Lett 22: 4404-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.129
BindingDB Entry DOI: 10.7270/Q2FJ2HVB |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50386859

(CHEMBL2048293)Show SMILES OC(=O)CC1CCn2c1cc1cc(ccc21)-c1noc(n1)-c1cc(OC(F)(F)F)cc(c1)C#N Show InChI InChI=1S/C23H15F3N4O4/c24-23(25,26)33-17-6-12(11-27)5-16(8-17)22-28-21(29-34-22)14-1-2-18-15(7-14)9-19-13(10-20(31)32)3-4-30(18)19/h1-2,5-9,13H,3-4,10H2,(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
Bioorg Med Chem Lett 22: 4404-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.129
BindingDB Entry DOI: 10.7270/Q2FJ2HVB |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50106206

(CHEMBL3598099)Show SMILES C[C@@H](OC(=O)N1CCC(CC1)O[C@H]1CC[C@@H](CC1)Oc1cnc(cn1)S(C)(=O)=O)C(F)(F)F |r,wU:15.19,wD:12.12,1.0,(-2.36,10.63,;-1.3,10.01,;-1.31,8.47,;-2.65,7.7,;-3.71,8.33,;-2.65,6.16,;-3.99,5.4,;-4,3.86,;-2.66,3.08,;-1.33,3.85,;-1.32,5.39,;-2.67,1.54,;-1.33,.77,;-1.33,-.77,;,-1.54,;1.33,-.77,;1.33,.77,;,1.54,;2.67,-1.54,;4,-.77,;4,.77,;5.33,1.55,;6.67,.78,;6.67,-.76,;5.34,-1.53,;8,1.56,;9.06,2.18,;9.07,.95,;7.99,2.79,;.04,10.77,;.05,12.01,;1.1,10.15,;1.11,11.38,)| Show InChI InChI=1S/C20H28F3N3O6S/c1-13(20(21,22)23)30-19(27)26-9-7-16(8-10-26)31-14-3-5-15(6-4-14)32-17-11-25-18(12-24-17)33(2,28)29/h11-16H,3-10H2,1-2H3/t13-,14-,15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by patch clamp assay |
Bioorg Med Chem Lett 25: 3034-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.102
BindingDB Entry DOI: 10.7270/Q2SB47JS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50041691

(CHEMBL3358920 | US11149292, Compound (R)-2-(7-(4-c...)Show SMILES OC(=O)C[C@H]1CCc2c1[nH]c1ccc(OCc3ccc(C4CCCC4)c(c3)C(F)(F)F)cc21 |r| Show InChI InChI=1S/C26H26F3NO3/c27-26(28,29)22-11-15(5-8-19(22)16-3-1-2-4-16)14-33-18-7-10-23-21(13-18)20-9-6-17(12-24(31)32)25(20)30-23/h5,7-8,10-11,13,16-17,30H,1-4,6,9,12,14H2,(H,31,32)/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 (unknown origin) |
ACS Med Chem Lett 5: 1313-7 (2014)
Article DOI: 10.1021/ml500389m
BindingDB Entry DOI: 10.7270/Q29W0H4C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50041983
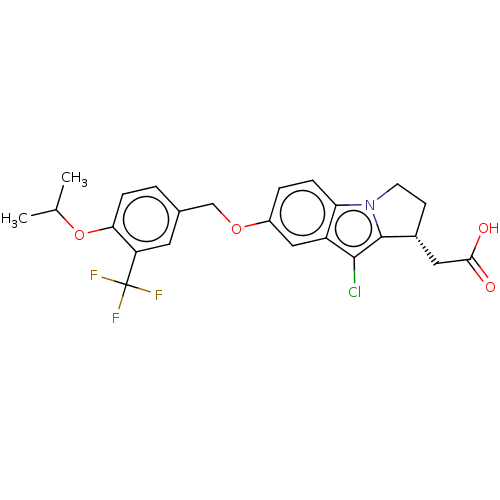
(CHEMBL3359523)Show SMILES CC(C)Oc1ccc(COc2ccc3n4CC[C@H](CC(O)=O)c4c(Cl)c3c2)cc1C(F)(F)F |r| Show InChI InChI=1S/C24H23ClF3NO4/c1-13(2)33-20-6-3-14(9-18(20)24(26,27)28)12-32-16-4-5-19-17(11-16)22(25)23-15(10-21(30)31)7-8-29(19)23/h3-6,9,11,13,15H,7-8,10,12H2,1-2H3,(H,30,31)/t15-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
ACS Med Chem Lett 5: 1334-9 (2014)
Article DOI: 10.1021/ml500422m
BindingDB Entry DOI: 10.7270/Q2XK8H6S |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50041983

(CHEMBL3359523)Show SMILES CC(C)Oc1ccc(COc2ccc3n4CC[C@H](CC(O)=O)c4c(Cl)c3c2)cc1C(F)(F)F |r| Show InChI InChI=1S/C24H23ClF3NO4/c1-13(2)33-20-6-3-14(9-18(20)24(26,27)28)12-32-16-4-5-19-17(11-16)22(25)23-15(10-21(30)31)7-8-29(19)23/h3-6,9,11,13,15H,7-8,10,12H2,1-2H3,(H,30,31)/t15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 (unknown origin) |
ACS Med Chem Lett 5: 1334-9 (2014)
Article DOI: 10.1021/ml500422m
BindingDB Entry DOI: 10.7270/Q2XK8H6S |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50041983

(CHEMBL3359523)Show SMILES CC(C)Oc1ccc(COc2ccc3n4CC[C@H](CC(O)=O)c4c(Cl)c3c2)cc1C(F)(F)F |r| Show InChI InChI=1S/C24H23ClF3NO4/c1-13(2)33-20-6-3-14(9-18(20)24(26,27)28)12-32-16-4-5-19-17(11-16)22(25)23-15(10-21(30)31)7-8-29(19)23/h3-6,9,11,13,15H,7-8,10,12H2,1-2H3,(H,30,31)/t15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 (unknown origin) |
ACS Med Chem Lett 5: 1334-9 (2014)
Article DOI: 10.1021/ml500422m
BindingDB Entry DOI: 10.7270/Q2XK8H6S |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50041983

(CHEMBL3359523)Show SMILES CC(C)Oc1ccc(COc2ccc3n4CC[C@H](CC(O)=O)c4c(Cl)c3c2)cc1C(F)(F)F |r| Show InChI InChI=1S/C24H23ClF3NO4/c1-13(2)33-20-6-3-14(9-18(20)24(26,27)28)12-32-16-4-5-19-17(11-16)22(25)23-15(10-21(30)31)7-8-29(19)23/h3-6,9,11,13,15H,7-8,10,12H2,1-2H3,(H,30,31)/t15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
ACS Med Chem Lett 5: 1334-9 (2014)
Article DOI: 10.1021/ml500422m
BindingDB Entry DOI: 10.7270/Q2XK8H6S |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50041691

(CHEMBL3358920 | US11149292, Compound (R)-2-(7-(4-c...)Show SMILES OC(=O)C[C@H]1CCc2c1[nH]c1ccc(OCc3ccc(C4CCCC4)c(c3)C(F)(F)F)cc21 |r| Show InChI InChI=1S/C26H26F3NO3/c27-26(28,29)22-11-15(5-8-19(22)16-3-1-2-4-16)14-33-18-7-10-23-21(13-18)20-9-6-17(12-24(31)32)25(20)30-23/h5,7-8,10-11,13,16-17,30H,1-4,6,9,12,14H2,(H,31,32)/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 (unknown origin) |
ACS Med Chem Lett 5: 1313-7 (2014)
Article DOI: 10.1021/ml500389m
BindingDB Entry DOI: 10.7270/Q29W0H4C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50041983

(CHEMBL3359523)Show SMILES CC(C)Oc1ccc(COc2ccc3n4CC[C@H](CC(O)=O)c4c(Cl)c3c2)cc1C(F)(F)F |r| Show InChI InChI=1S/C24H23ClF3NO4/c1-13(2)33-20-6-3-14(9-18(20)24(26,27)28)12-32-16-4-5-19-17(11-16)22(25)23-15(10-21(30)31)7-8-29(19)23/h3-6,9,11,13,15H,7-8,10,12H2,1-2H3,(H,30,31)/t15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
ACS Med Chem Lett 5: 1334-9 (2014)
Article DOI: 10.1021/ml500422m
BindingDB Entry DOI: 10.7270/Q2XK8H6S |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50041691

(CHEMBL3358920 | US11149292, Compound (R)-2-(7-(4-c...)Show SMILES OC(=O)C[C@H]1CCc2c1[nH]c1ccc(OCc3ccc(C4CCCC4)c(c3)C(F)(F)F)cc21 |r| Show InChI InChI=1S/C26H26F3NO3/c27-26(28,29)22-11-15(5-8-19(22)16-3-1-2-4-16)14-33-18-7-10-23-21(13-18)20-9-6-17(12-24(31)32)25(20)30-23/h5,7-8,10-11,13,16-17,30H,1-4,6,9,12,14H2,(H,31,32)/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
ACS Med Chem Lett 5: 1313-7 (2014)
Article DOI: 10.1021/ml500389m
BindingDB Entry DOI: 10.7270/Q29W0H4C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50041691

(CHEMBL3358920 | US11149292, Compound (R)-2-(7-(4-c...)Show SMILES OC(=O)C[C@H]1CCc2c1[nH]c1ccc(OCc3ccc(C4CCCC4)c(c3)C(F)(F)F)cc21 |r| Show InChI InChI=1S/C26H26F3NO3/c27-26(28,29)22-11-15(5-8-19(22)16-3-1-2-4-16)14-33-18-7-10-23-21(13-18)20-9-6-17(12-24(31)32)25(20)30-23/h5,7-8,10-11,13,16-17,30H,1-4,6,9,12,14H2,(H,31,32)/t17-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
ACS Med Chem Lett 5: 1313-7 (2014)
Article DOI: 10.1021/ml500389m
BindingDB Entry DOI: 10.7270/Q29W0H4C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50041691

(CHEMBL3358920 | US11149292, Compound (R)-2-(7-(4-c...)Show SMILES OC(=O)C[C@H]1CCc2c1[nH]c1ccc(OCc3ccc(C4CCCC4)c(c3)C(F)(F)F)cc21 |r| Show InChI InChI=1S/C26H26F3NO3/c27-26(28,29)22-11-15(5-8-19(22)16-3-1-2-4-16)14-33-18-7-10-23-21(13-18)20-9-6-17(12-24(31)32)25(20)30-23/h5,7-8,10-11,13,16-17,30H,1-4,6,9,12,14H2,(H,31,32)/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
ACS Med Chem Lett 5: 1313-7 (2014)
Article DOI: 10.1021/ml500389m
BindingDB Entry DOI: 10.7270/Q29W0H4C |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50386859

(CHEMBL2048293)Show SMILES OC(=O)CC1CCn2c1cc1cc(ccc21)-c1noc(n1)-c1cc(OC(F)(F)F)cc(c1)C#N Show InChI InChI=1S/C23H15F3N4O4/c24-23(25,26)33-17-6-12(11-27)5-16(8-17)22-28-21(29-34-22)14-1-2-18-15(7-14)9-19-13(10-20(31)32)3-4-30(18)19/h1-2,5-9,13H,3-4,10H2,(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >4.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by patch clamp assay |
Bioorg Med Chem Lett 22: 4404-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.129
BindingDB Entry DOI: 10.7270/Q2FJ2HVB |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50106206

(CHEMBL3598099)Show SMILES C[C@@H](OC(=O)N1CCC(CC1)O[C@H]1CC[C@@H](CC1)Oc1cnc(cn1)S(C)(=O)=O)C(F)(F)F |r,wU:15.19,wD:12.12,1.0,(-2.36,10.63,;-1.3,10.01,;-1.31,8.47,;-2.65,7.7,;-3.71,8.33,;-2.65,6.16,;-3.99,5.4,;-4,3.86,;-2.66,3.08,;-1.33,3.85,;-1.32,5.39,;-2.67,1.54,;-1.33,.77,;-1.33,-.77,;,-1.54,;1.33,-.77,;1.33,.77,;,1.54,;2.67,-1.54,;4,-.77,;4,.77,;5.33,1.55,;6.67,.78,;6.67,-.76,;5.34,-1.53,;8,1.56,;9.06,2.18,;9.07,.95,;7.99,2.79,;.04,10.77,;.05,12.01,;1.1,10.15,;1.11,11.38,)| Show InChI InChI=1S/C20H28F3N3O6S/c1-13(20(21,22)23)30-19(27)26-9-7-16(8-10-26)31-14-3-5-15(6-4-14)32-17-11-25-18(12-24-17)33(2,28)29/h11-16H,3-10H2,1-2H3/t13-,14-,15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]-Astemizole from human ERG |
Bioorg Med Chem Lett 25: 3034-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.102
BindingDB Entry DOI: 10.7270/Q2SB47JS |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50040961

(CHEMBL3354952)Show SMILES [H][C@]12C[C@@]1([H])c1c(C2)c(nn1-c1ccc(F)cc1F)C(=O)NC(C)(C)CO |r| Show InChI InChI=1S/C18H19F2N3O2/c1-18(2,8-24)21-17(25)15-12-6-9-5-11(9)16(12)23(22-15)14-4-3-10(19)7-13(14)20/h3-4,7,9,11,24H,5-6,8H2,1-2H3,(H,21,25)/t9-,11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]Astemizole from human ERG channel |
Bioorg Med Chem Lett 25: 322-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.11.040
BindingDB Entry DOI: 10.7270/Q21J9CDS |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50040960
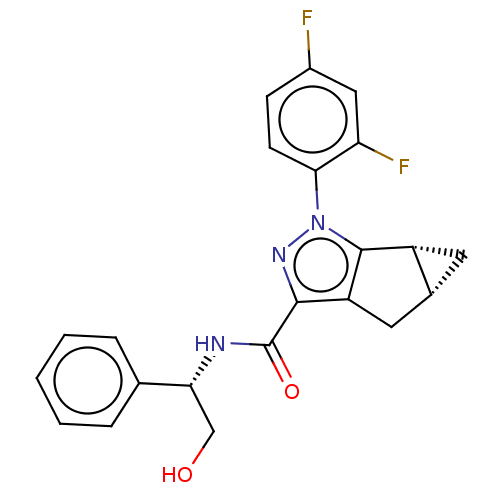
(CHEMBL3354956)Show SMILES [H][C@]12C[C@@]1([H])c1c(C2)c(nn1-c1ccc(F)cc1F)C(=O)N[C@H](CO)c1ccccc1 |r| Show InChI InChI=1S/C22H19F2N3O2/c23-14-6-7-19(17(24)10-14)27-21-15-8-13(15)9-16(21)20(26-27)22(29)25-18(11-28)12-4-2-1-3-5-12/h1-7,10,13,15,18,28H,8-9,11H2,(H,25,29)/t13-,15-,18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inverse agonist activity at rat recombinant CB1 receptor expressed in CHOK1 cells assessed as cAMP accumulation by HTRF method |
Bioorg Med Chem Lett 25: 322-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.11.040
BindingDB Entry DOI: 10.7270/Q21J9CDS |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50040962
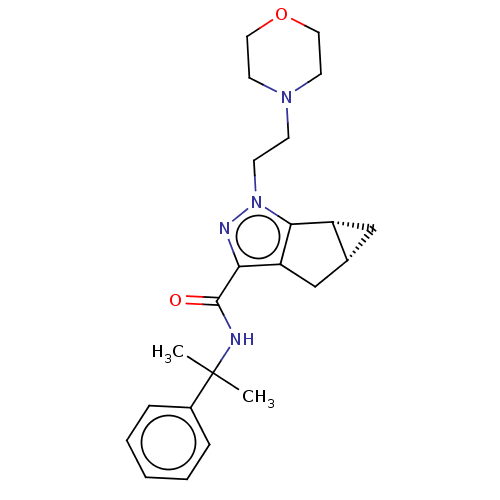
(CHEMBL3354940)Show SMILES [H][C@]12C[C@@]1([H])c1c(C2)c(nn1CCN1CCOCC1)C(=O)NC(C)(C)c1ccccc1 |r| Show InChI InChI=1S/C23H30N4O2/c1-23(2,17-6-4-3-5-7-17)24-22(28)20-19-15-16-14-18(16)21(19)27(25-20)9-8-26-10-12-29-13-11-26/h3-7,16,18H,8-15H2,1-2H3,(H,24,28)/t16-,18-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 19 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant CB1 receptor expressed in CHOK1 cells assessed as cAMP accumulation by HTRF method |
Bioorg Med Chem Lett 25: 322-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.11.040
BindingDB Entry DOI: 10.7270/Q21J9CDS |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50040963

(CHEMBL3354939)Show SMILES [H][C@]12C[C@@]1([H])c1c(C2)c(nn1CCC1CCOCC1)C(=O)NC(C)(C)c1ccccc1 |r| Show InChI InChI=1S/C24H31N3O2/c1-24(2,18-6-4-3-5-7-18)25-23(28)21-20-15-17-14-19(17)22(20)27(26-21)11-8-16-9-12-29-13-10-16/h3-7,16-17,19H,8-15H2,1-2H3,(H,25,28)/t17-,19-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant CB1 receptor expressed in CHOK1 cells assessed as cAMP accumulation by HTRF method |
Bioorg Med Chem Lett 25: 322-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.11.040
BindingDB Entry DOI: 10.7270/Q21J9CDS |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50040964

(CHEMBL3354938)Show SMILES [H][C@]12C[C@@]1([H])c1c(C2)c(nn1CC1CCOCC1)C(=O)NC(C)(C)c1ccccc1 |r| Show InChI InChI=1S/C23H29N3O2/c1-23(2,17-6-4-3-5-7-17)24-22(27)20-19-13-16-12-18(16)21(19)26(25-20)14-15-8-10-28-11-9-15/h3-7,15-16,18H,8-14H2,1-2H3,(H,24,27)/t16-,18-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant CB1 receptor expressed in CHOK1 cells assessed as cAMP accumulation by HTRF method |
Bioorg Med Chem Lett 25: 322-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.11.040
BindingDB Entry DOI: 10.7270/Q21J9CDS |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50040965
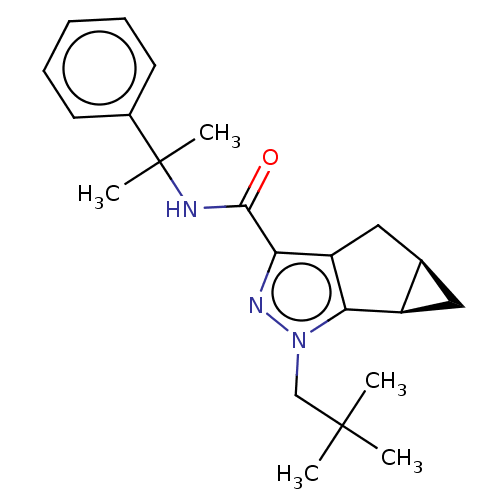
(CHEMBL3354937)Show SMILES [H][C@]12C[C@@]1([H])c1c(C2)c(nn1CC(C)(C)C)C(=O)NC(C)(C)c1ccccc1 |r| Show InChI InChI=1S/C22H29N3O/c1-21(2,3)13-25-19-16-11-14(16)12-17(19)18(24-25)20(26)23-22(4,5)15-9-7-6-8-10-15/h6-10,14,16H,11-13H2,1-5H3,(H,23,26)/t14-,16-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 7.40 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant CB1 receptor expressed in CHOK1 cells assessed as cAMP accumulation by HTRF method |
Bioorg Med Chem Lett 25: 322-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.11.040
BindingDB Entry DOI: 10.7270/Q21J9CDS |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50040966

(CHEMBL3354936)Show SMILES [H][C@]12C[C@@]1([H])c1c(C2)c(nn1C(C)C)C(=O)NC(C)(C)c1ccccc1 |r| Show InChI InChI=1S/C20H25N3O/c1-12(2)23-18-15-10-13(15)11-16(18)17(22-23)19(24)21-20(3,4)14-8-6-5-7-9-14/h5-9,12-13,15H,10-11H2,1-4H3,(H,21,24)/t13-,15-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 51 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant CB1 receptor expressed in CHOK1 cells assessed as cAMP accumulation by HTRF method |
Bioorg Med Chem Lett 25: 322-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.11.040
BindingDB Entry DOI: 10.7270/Q21J9CDS |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50040967

(CHEMBL3354935)Show SMILES [H][C@]12C[C@@]1([H])c1c(C2)c(nn1C(C)(C)C)C(=O)NC(C)(C)c1ccccc1 |r| Show InChI InChI=1S/C21H27N3O/c1-20(2,3)24-18-15-11-13(15)12-16(18)17(23-24)19(25)22-21(4,5)14-9-7-6-8-10-14/h6-10,13,15H,11-12H2,1-5H3,(H,22,25)/t13-,15-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 86 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant CB1 receptor expressed in CHOK1 cells assessed as cAMP accumulation by HTRF method |
Bioorg Med Chem Lett 25: 322-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.11.040
BindingDB Entry DOI: 10.7270/Q21J9CDS |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50040968

(CHEMBL3352843)Show SMILES [H][C@]12C[C@@]1([H])c1c(C2)c(nn1-c1ccncn1)C(=O)NC(C)(C)CO |r| Show InChI InChI=1S/C16H19N5O2/c1-16(2,7-22)19-15(23)13-11-6-9-5-10(9)14(11)21(20-13)12-3-4-17-8-18-12/h3-4,8-10,22H,5-7H2,1-2H3,(H,19,23)/t9-,10-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 420 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant CB2 receptor expressed in CHOK1 cells assessed as cAMP accumulation by HTRF method |
Bioorg Med Chem Lett 25: 322-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.11.040
BindingDB Entry DOI: 10.7270/Q21J9CDS |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50040969
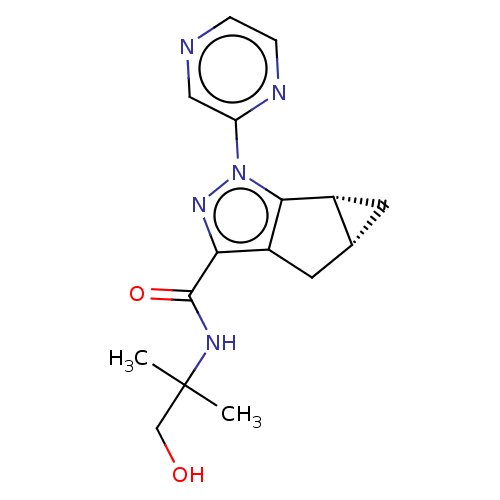
(CHEMBL3354976)Show SMILES [H][C@]12C[C@@]1([H])c1c(C2)c(nn1-c1cnccn1)C(=O)NC(C)(C)CO |r| Show InChI InChI=1S/C16H19N5O2/c1-16(2,8-22)19-15(23)13-11-6-9-5-10(9)14(11)21(20-13)12-7-17-3-4-18-12/h3-4,7,9-10,22H,5-6,8H2,1-2H3,(H,19,23)/t9-,10-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 25 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant CB2 receptor expressed in CHOK1 cells assessed as cAMP accumulation by HTRF method |
Bioorg Med Chem Lett 25: 322-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.11.040
BindingDB Entry DOI: 10.7270/Q21J9CDS |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50040970

(CHEMBL3354975 | US11214548, Compound 269)Show SMILES [H][C@]12C[C@@]1([H])c1c(C2)c(nn1-c1cccnc1)C(=O)NC(C)(C)CO |r| Show InChI InChI=1S/C17H20N4O2/c1-17(2,9-22)19-16(23)14-13-7-10-6-12(10)15(13)21(20-14)11-4-3-5-18-8-11/h3-5,8,10,12,22H,6-7,9H2,1-2H3,(H,19,23)/t10-,12-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 809 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant CB2 receptor expressed in CHOK1 cells assessed as cAMP accumulation by HTRF method |
Bioorg Med Chem Lett 25: 322-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.11.040
BindingDB Entry DOI: 10.7270/Q21J9CDS |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50040971
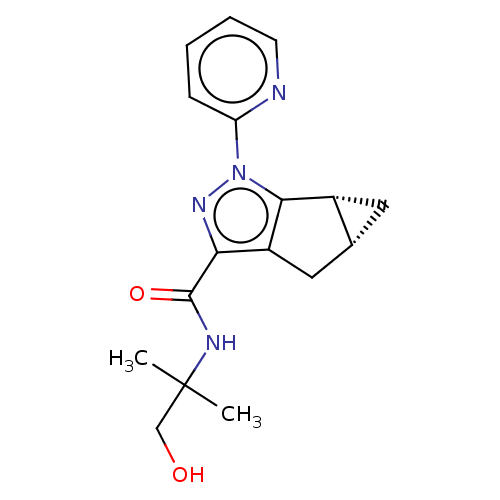
(CHEMBL3354974)Show SMILES [H][C@]12C[C@@]1([H])c1c(C2)c(nn1-c1ccccn1)C(=O)NC(C)(C)CO |r| Show InChI InChI=1S/C17H20N4O2/c1-17(2,9-22)19-16(23)14-12-8-10-7-11(10)15(12)21(20-14)13-5-3-4-6-18-13/h3-6,10-11,22H,7-9H2,1-2H3,(H,19,23)/t10-,11-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 42 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant CB2 receptor expressed in CHOK1 cells assessed as cAMP accumulation by HTRF method |
Bioorg Med Chem Lett 25: 322-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.11.040
BindingDB Entry DOI: 10.7270/Q21J9CDS |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50040972
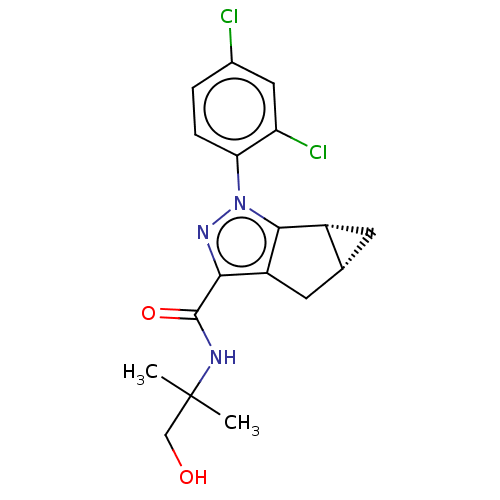
(CHEMBL3354973)Show SMILES [H][C@]12C[C@@]1([H])c1c(C2)c(nn1-c1ccc(Cl)cc1Cl)C(=O)NC(C)(C)CO |r| Show InChI InChI=1S/C18H19Cl2N3O2/c1-18(2,8-24)21-17(25)15-12-6-9-5-11(9)16(12)23(22-15)14-4-3-10(19)7-13(14)20/h3-4,7,9,11,24H,5-6,8H2,1-2H3,(H,21,25)/t9-,11-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 15 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant CB2 receptor expressed in CHOK1 cells assessed as cAMP accumulation by HTRF method |
Bioorg Med Chem Lett 25: 322-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.11.040
BindingDB Entry DOI: 10.7270/Q21J9CDS |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50040973
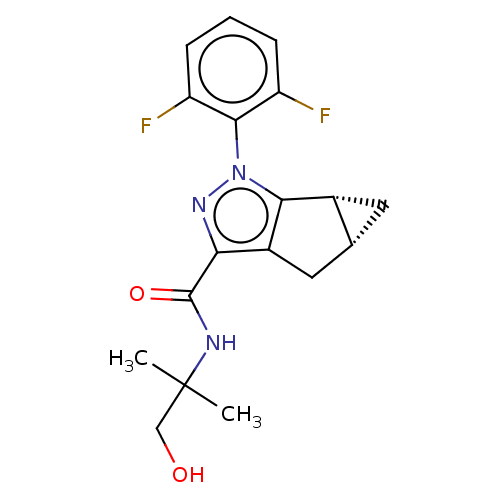
(CHEMBL3354972)Show SMILES [H][C@]12C[C@@]1([H])c1c(C2)c(nn1-c1c(F)cccc1F)C(=O)NC(C)(C)CO |r,wU:1.0,3.4,(21.92,-31.59,;21.53,-33.09,;20.13,-32.44,;20.27,-33.98,;18.93,-33.2,;20.73,-35.45,;22.27,-35.46,;22.76,-34,;22.73,-36.93,;21.48,-37.82,;20.24,-36.9,;18.7,-36.94,;17.97,-38.29,;18.78,-39.6,;16.43,-38.33,;15.62,-37.01,;16.36,-35.65,;17.9,-35.62,;18.65,-34.27,;24.07,-37.69,;24.09,-39.23,;25.4,-36.9,;26.74,-37.66,;27.51,-38.99,;25.97,-38.98,;28.08,-36.88,;29.41,-37.65,)| Show InChI InChI=1S/C18H19F2N3O2/c1-18(2,8-24)21-17(25)14-11-7-9-6-10(9)15(11)23(22-14)16-12(19)4-3-5-13(16)20/h3-5,9-10,24H,6-8H2,1-2H3,(H,21,25)/t9-,10-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.30E+4 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant CB2 receptor expressed in CHOK1 cells assessed as cAMP accumulation by HTRF method |
Bioorg Med Chem Lett 25: 322-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.11.040
BindingDB Entry DOI: 10.7270/Q21J9CDS |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50040974
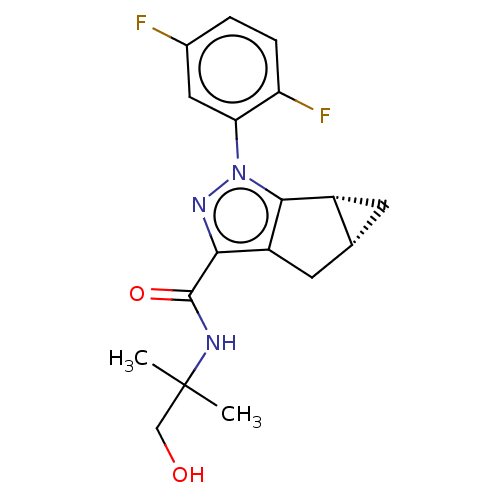
(CHEMBL3354971)Show SMILES [H][C@]12C[C@@]1([H])c1c(C2)c(nn1-c1cc(F)ccc1F)C(=O)NC(C)(C)CO |r| Show InChI InChI=1S/C18H19F2N3O2/c1-18(2,8-24)21-17(25)15-12-6-9-5-11(9)16(12)23(22-15)14-7-10(19)3-4-13(14)20/h3-4,7,9,11,24H,5-6,8H2,1-2H3,(H,21,25)/t9-,11-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant CB2 receptor expressed in CHOK1 cells assessed as cAMP accumulation by HTRF method |
Bioorg Med Chem Lett 25: 322-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.11.040
BindingDB Entry DOI: 10.7270/Q21J9CDS |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50040975
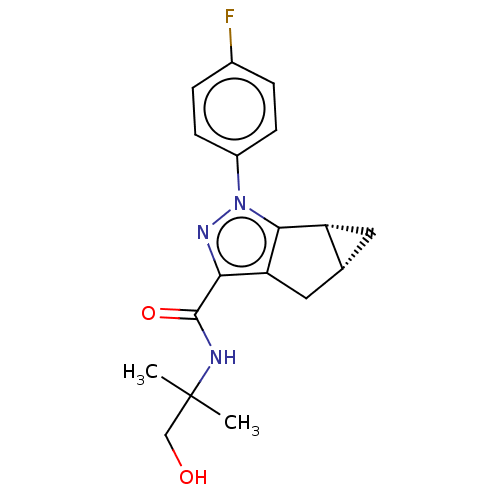
(CHEMBL3354970)Show SMILES [H][C@]12C[C@@]1([H])c1c(C2)c(nn1-c1ccc(F)cc1)C(=O)NC(C)(C)CO |r| Show InChI InChI=1S/C18H20FN3O2/c1-18(2,9-23)20-17(24)15-14-8-10-7-13(10)16(14)22(21-15)12-5-3-11(19)4-6-12/h3-6,10,13,23H,7-9H2,1-2H3,(H,20,24)/t10-,13-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 25 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant CB2 receptor expressed in CHOK1 cells assessed as cAMP accumulation by HTRF method |
Bioorg Med Chem Lett 25: 322-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.11.040
BindingDB Entry DOI: 10.7270/Q21J9CDS |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50040976
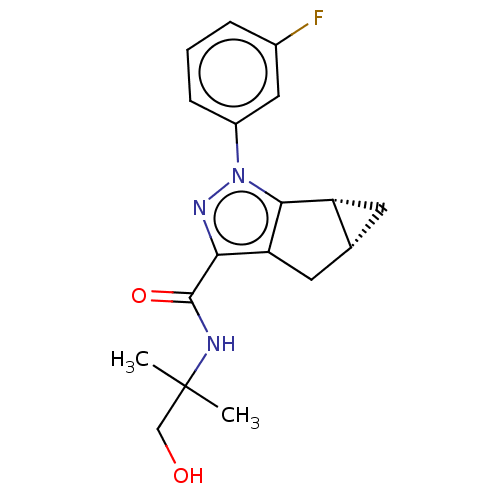
(CHEMBL3354969)Show SMILES [H][C@]12C[C@@]1([H])c1c(C2)c(nn1-c1cccc(F)c1)C(=O)NC(C)(C)CO |r| Show InChI InChI=1S/C18H20FN3O2/c1-18(2,9-23)20-17(24)15-14-7-10-6-13(10)16(14)22(21-15)12-5-3-4-11(19)8-12/h3-5,8,10,13,23H,6-7,9H2,1-2H3,(H,20,24)/t10-,13-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 7.40 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant CB2 receptor expressed in CHOK1 cells assessed as cAMP accumulation by HTRF method |
Bioorg Med Chem Lett 25: 322-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.11.040
BindingDB Entry DOI: 10.7270/Q21J9CDS |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50040977
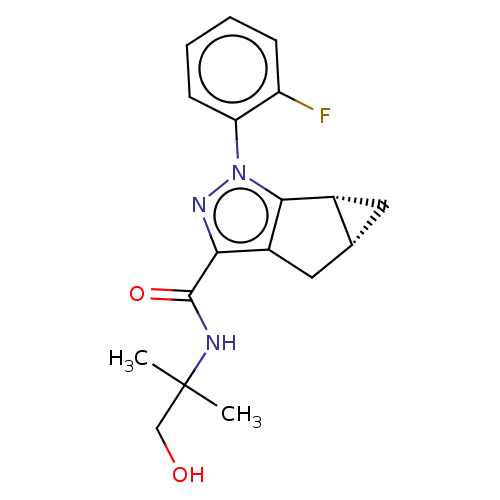
(CHEMBL3354968)Show SMILES [H][C@]12C[C@@]1([H])c1c(C2)c(nn1-c1ccccc1F)C(=O)NC(C)(C)CO |r| Show InChI InChI=1S/C18H20FN3O2/c1-18(2,9-23)20-17(24)15-12-8-10-7-11(10)16(12)22(21-15)14-6-4-3-5-13(14)19/h3-6,10-11,23H,7-9H2,1-2H3,(H,20,24)/t10-,11-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 19 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant CB2 receptor expressed in CHOK1 cells assessed as cAMP accumulation by HTRF method |
Bioorg Med Chem Lett 25: 322-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.11.040
BindingDB Entry DOI: 10.7270/Q21J9CDS |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50040978
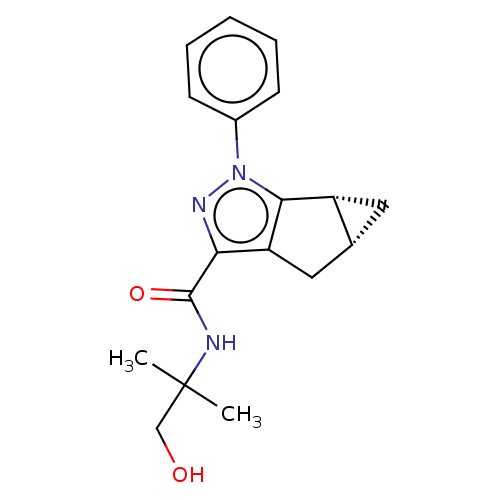
(CHEMBL3354967)Show SMILES [H][C@]12C[C@@]1([H])c1c(C2)c(nn1-c1ccccc1)C(=O)NC(C)(C)CO |r| Show InChI InChI=1S/C18H21N3O2/c1-18(2,10-22)19-17(23)15-14-9-11-8-13(11)16(14)21(20-15)12-6-4-3-5-7-12/h3-7,11,13,22H,8-10H2,1-2H3,(H,19,23)/t11-,13-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 101 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant CB2 receptor expressed in CHOK1 cells assessed as cAMP accumulation by HTRF method |
Bioorg Med Chem Lett 25: 322-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.11.040
BindingDB Entry DOI: 10.7270/Q21J9CDS |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50040979
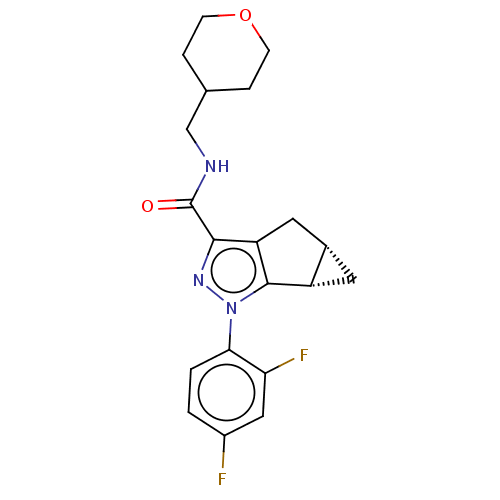
(CHEMBL3354966 | US11214548, Compound 408)Show SMILES [H][C@@]12C[C@]1([H])c1c(C2)c(nn1-c1ccc(F)cc1F)C(=O)NCC1CCOCC1 |r| Show InChI InChI=1S/C20H21F2N3O2/c21-13-1-2-17(16(22)9-13)25-19-14-7-12(14)8-15(19)18(24-25)20(26)23-10-11-3-5-27-6-4-11/h1-2,9,11-12,14H,3-8,10H2,(H,23,26)/t12-,14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 76 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant CB2 receptor expressed in CHOK1 cells assessed as cAMP accumulation by HTRF method |
Bioorg Med Chem Lett 25: 322-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.11.040
BindingDB Entry DOI: 10.7270/Q21J9CDS |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50040980
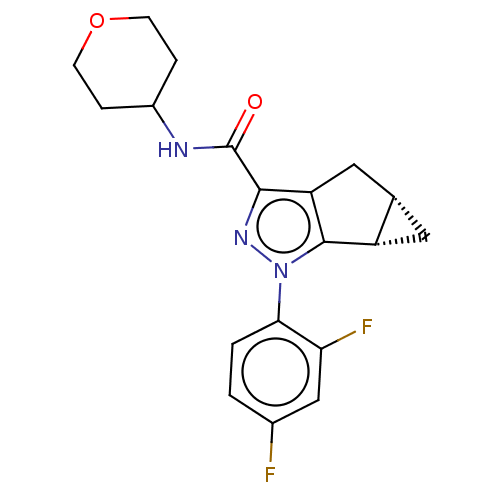
(CHEMBL3354965)Show SMILES [H][C@@]12C[C@]1([H])c1c(C2)c(nn1-c1ccc(F)cc1F)C(=O)NC1CCOCC1 |r| Show InChI InChI=1S/C19H19F2N3O2/c20-11-1-2-16(15(21)9-11)24-18-13-7-10(13)8-14(18)17(23-24)19(25)22-12-3-5-26-6-4-12/h1-2,9-10,12-13H,3-8H2,(H,22,25)/t10-,13-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 39 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant CB2 receptor expressed in CHOK1 cells assessed as cAMP accumulation by HTRF method |
Bioorg Med Chem Lett 25: 322-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.11.040
BindingDB Entry DOI: 10.7270/Q21J9CDS |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50040981
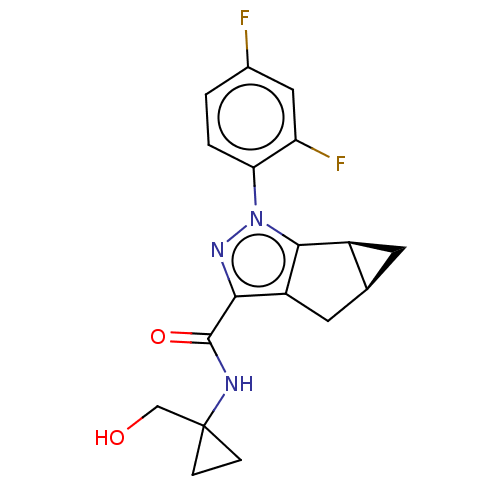
(CHEMBL3354964)Show SMILES [H][C@@]12C[C@]1([H])c1c(C2)c(nn1-c1ccc(F)cc1F)C(=O)NC1(CO)CC1 |r| Show InChI InChI=1S/C18H17F2N3O2/c19-10-1-2-14(13(20)7-10)23-16-11-5-9(11)6-12(16)15(22-23)17(25)21-18(8-24)3-4-18/h1-2,7,9,11,24H,3-6,8H2,(H,21,25)/t9-,11-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 25 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant CB2 receptor expressed in CHOK1 cells assessed as cAMP accumulation by HTRF method |
Bioorg Med Chem Lett 25: 322-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.11.040
BindingDB Entry DOI: 10.7270/Q21J9CDS |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50040982
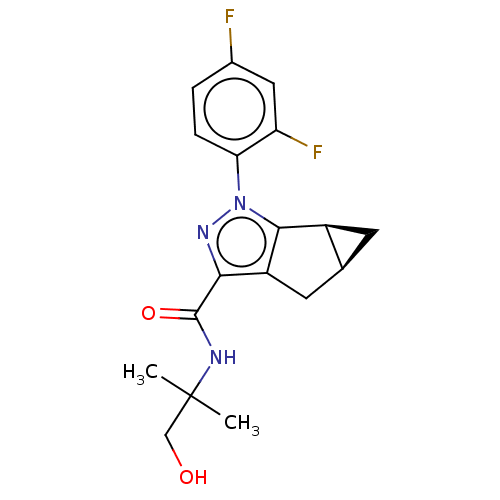
(CHEMBL3354963)Show SMILES [H][C@@]12C[C@]1([H])c1c(C2)c(nn1-c1ccc(F)cc1F)C(=O)NC(C)(C)CO |r| Show InChI InChI=1S/C18H19F2N3O2/c1-18(2,8-24)21-17(25)15-12-6-9-5-11(9)16(12)23(22-15)14-4-3-10(19)7-13(14)20/h3-4,7,9,11,24H,5-6,8H2,1-2H3,(H,21,25)/t9-,11-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 3 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant CB2 receptor expressed in CHOK1 cells assessed as cAMP accumulation by HTRF method |
Bioorg Med Chem Lett 25: 322-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.11.040
BindingDB Entry DOI: 10.7270/Q21J9CDS |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50040983
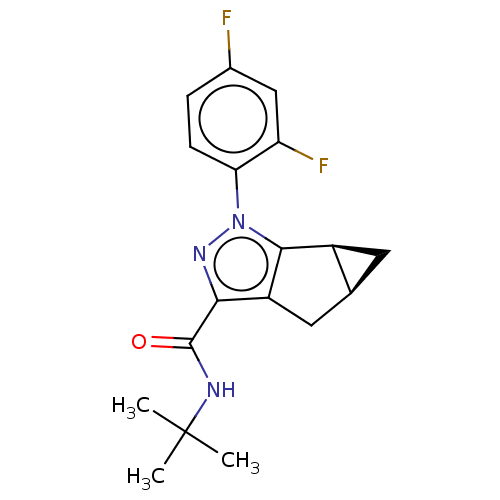
(CHEMBL3354962)Show SMILES [H][C@@]12C[C@]1([H])c1c(C2)c(nn1-c1ccc(F)cc1F)C(=O)NC(C)(C)C |r| Show InChI InChI=1S/C18H19F2N3O/c1-18(2,3)21-17(24)15-12-7-9-6-11(9)16(12)23(22-15)14-5-4-10(19)8-13(14)20/h4-5,8-9,11H,6-7H2,1-3H3,(H,21,24)/t9-,11-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 7.20 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant CB2 receptor expressed in CHOK1 cells assessed as cAMP accumulation by HTRF method |
Bioorg Med Chem Lett 25: 322-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.11.040
BindingDB Entry DOI: 10.7270/Q21J9CDS |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50040984
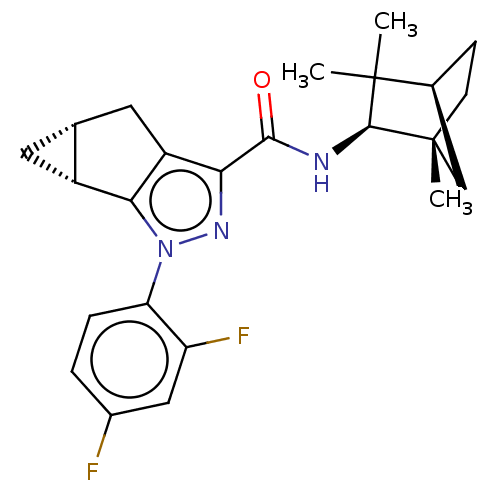
(CHEMBL3354961)Show SMILES [H][C@]12C[C@@]1([H])c1c(C2)c(nn1-c1ccc(F)cc1F)C(=O)N[C@@H]1[C@]2(C)CC[C@@]([H])(C2)C1(C)C |r| Show InChI InChI=1S/C24H27F2N3O/c1-23(2)13-6-7-24(3,11-13)22(23)27-21(30)19-16-9-12-8-15(12)20(16)29(28-19)18-5-4-14(25)10-17(18)26/h4-5,10,12-13,15,22H,6-9,11H2,1-3H3,(H,27,30)/t12-,13+,15-,22+,24-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant CB2 receptor expressed in CHOK1 cells assessed as cAMP accumulation by HTRF method |
Bioorg Med Chem Lett 25: 322-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.11.040
BindingDB Entry DOI: 10.7270/Q21J9CDS |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data