 Found 109 hits with Last Name = 'vadivelan' and Initial = 's'
Found 109 hits with Last Name = 'vadivelan' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50293305
(4-(2,4-dichlorophenyl)-5-(1H-imidazol-2-yl)-N-(2-(...)Show SMILES [O-][N+](=O)c1ccc(NCCNc2ncc(-c3ncc[nH]3)c(n2)-c2ccc(Cl)cc2Cl)nc1 Show InChI InChI=1S/C20H16Cl2N8O2/c21-12-1-3-14(16(22)9-12)18-15(19-24-6-7-25-19)11-28-20(29-18)26-8-5-23-17-4-2-13(10-27-17)30(31)32/h1-4,6-7,9-11H,5,8H2,(H,23,27)(H,24,25)(H,26,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Road
Curated by ChEMBL
| Assay Description
Inhibition of human GSK3beta |
Eur J Med Chem 44: 2361-71 (2009)
Article DOI: 10.1016/j.ejmech.2008.08.012
BindingDB Entry DOI: 10.7270/Q2057FZX |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50293306

(3-(5-methoxybenzofuran-7-yl)-4-(1-(2-(4-methylpipe...)Show SMILES COc1cc(C2=C(C(=O)NC2=O)c2cn(CCN3CCN(C)CC3)c3ccccc23)c2occc2c1 |t:5| Show InChI InChI=1S/C28H28N4O4/c1-30-8-10-31(11-9-30)12-13-32-17-22(20-5-3-4-6-23(20)32)25-24(27(33)29-28(25)34)21-16-19(35-2)15-18-7-14-36-26(18)21/h3-7,14-17H,8-13H2,1-2H3,(H,29,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Road
Curated by ChEMBL
| Assay Description
Inhibition of human GSK3beta |
Eur J Med Chem 44: 2361-71 (2009)
Article DOI: 10.1016/j.ejmech.2008.08.012
BindingDB Entry DOI: 10.7270/Q2057FZX |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50483581

(CHEMBL1689301)Show SMILES CC1=C(Sc2cccc(c2)C(F)(F)F)C(COCCO)C(=O)NC1=O |c:1| Show InChI InChI=1S/C16H16F3NO4S/c1-9-13(12(8-24-6-5-21)15(23)20-14(9)22)25-11-4-2-3-10(7-11)16(17,18)19/h2-4,7,12,21H,5-6,8H2,1H3,(H,20,22,23) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a |
GVK Biosciences Private Limited
Curated by ChEMBL
| Assay Description
Inhibition of Human immunodeficiency virus 1 reverse transcriptase |
Eur J Med Chem 46: 851-9 (2011)
Article DOI: 10.1016/j.ejmech.2010.12.022
BindingDB Entry DOI: 10.7270/Q2HD7ZGX |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50150699

(3-(9-fluoro-2-(piperidine-1-carbonyl)-1,2,3,4-tetr...)Show SMILES Fc1cc2CN(CCn3cc(C4=C(C(=O)NC4=O)c4cnc5ccccn45)c(c1)c23)C(=O)N1CCCCC1 |t:11| Show InChI InChI=1S/C28H25FN6O3/c29-18-12-17-15-34(28(38)32-7-3-1-4-8-32)11-10-33-16-20(19(13-18)25(17)33)23-24(27(37)31-26(23)36)21-14-30-22-6-2-5-9-35(21)22/h2,5-6,9,12-14,16H,1,3-4,7-8,10-11,15H2,(H,31,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Road
Curated by ChEMBL
| Assay Description
Inhibition of human GSK3beta |
Eur J Med Chem 44: 2361-71 (2009)
Article DOI: 10.1016/j.ejmech.2008.08.012
BindingDB Entry DOI: 10.7270/Q2057FZX |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50150701
(7-(4-(H-imidazo[1,2-a]pyridin-3-yl)-2,5-dioxo-2,5-...)Show SMILES CN(C)C(=O)N1CCn2cc(C3=C(C(=O)NC3=O)c3cnc4ccccn34)c3cccc(C1)c23 |t:11| Show InChI InChI=1S/C25H22N6O3/c1-28(2)25(34)30-11-10-29-14-17(16-7-5-6-15(13-30)22(16)29)20-21(24(33)27-23(20)32)18-12-26-19-8-3-4-9-31(18)19/h3-9,12,14H,10-11,13H2,1-2H3,(H,27,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Road
Curated by ChEMBL
| Assay Description
Inhibition of human GSK3beta |
Eur J Med Chem 44: 2361-71 (2009)
Article DOI: 10.1016/j.ejmech.2008.08.012
BindingDB Entry DOI: 10.7270/Q2057FZX |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50483589
(CHEMBL1689302)Show SMILES CC1=C(Sc2cccc(F)c2)C(COCCO)C(=O)NC1=O |c:1| Show InChI InChI=1S/C15H16FNO4S/c1-9-13(22-11-4-2-3-10(16)7-11)12(8-21-6-5-18)15(20)17-14(9)19/h2-4,7,12,18H,5-6,8H2,1H3,(H,17,19,20) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
GVK Biosciences Private Limited
Curated by ChEMBL
| Assay Description
Inhibition of Human immunodeficiency virus 1 reverse transcriptase |
Eur J Med Chem 46: 851-9 (2011)
Article DOI: 10.1016/j.ejmech.2010.12.022
BindingDB Entry DOI: 10.7270/Q2HD7ZGX |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50293307
(CHEMBL549953 | N-(4-(ethylsulfonamido)phenethyl)-2...)Show SMILES CCS(=O)(=O)Nc1ccc(CCNC(=O)c2ccc(O)c3nc([nH]c23)-c2ccc(F)cc2Cl)cc1 Show InChI InChI=1S/C24H22ClFN4O4S/c1-2-35(33,34)30-16-6-3-14(4-7-16)11-12-27-24(32)18-9-10-20(31)22-21(18)28-23(29-22)17-8-5-15(26)13-19(17)25/h3-10,13,30-31H,2,11-12H2,1H3,(H,27,32)(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Road
Curated by ChEMBL
| Assay Description
Inhibition of human GSK3beta |
Eur J Med Chem 44: 2361-71 (2009)
Article DOI: 10.1016/j.ejmech.2008.08.012
BindingDB Entry DOI: 10.7270/Q2057FZX |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50483582
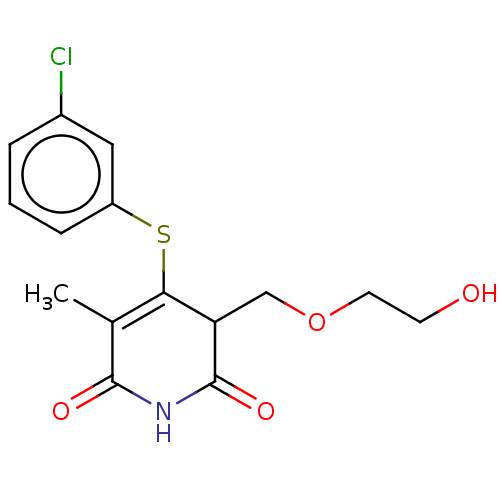
(CHEMBL1689303)Show SMILES CC1=C(Sc2cccc(Cl)c2)C(COCCO)C(=O)NC1=O |c:1| Show InChI InChI=1S/C15H16ClNO4S/c1-9-13(22-11-4-2-3-10(16)7-11)12(8-21-6-5-18)15(20)17-14(9)19/h2-4,7,12,18H,5-6,8H2,1H3,(H,17,19,20) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
GVK Biosciences Private Limited
Curated by ChEMBL
| Assay Description
Inhibition of Human immunodeficiency virus 1 reverse transcriptase |
Eur J Med Chem 46: 851-9 (2011)
Article DOI: 10.1016/j.ejmech.2010.12.022
BindingDB Entry DOI: 10.7270/Q2HD7ZGX |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM3175

(3-[1-[3-(Amidinothio)propyl]-3-indolyl]-4-(1-methy...)Show SMILES Cn1cc(C2=C(C(=O)NC2=O)c2cn(CCCSC(N)=N)c3ccccc23)c2ccccc12 |t:4| Show InChI InChI=1S/C25H23N5O2S/c1-29-13-17(15-7-2-4-9-19(15)29)21-22(24(32)28-23(21)31)18-14-30(11-6-12-33-25(26)27)20-10-5-3-8-16(18)20/h2-5,7-10,13-14H,6,11-12H2,1H3,(H3,26,27)(H,28,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Road
Curated by ChEMBL
| Assay Description
Inhibition of human GSK3beta |
Eur J Med Chem 44: 2361-71 (2009)
Article DOI: 10.1016/j.ejmech.2008.08.012
BindingDB Entry DOI: 10.7270/Q2057FZX |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50483597
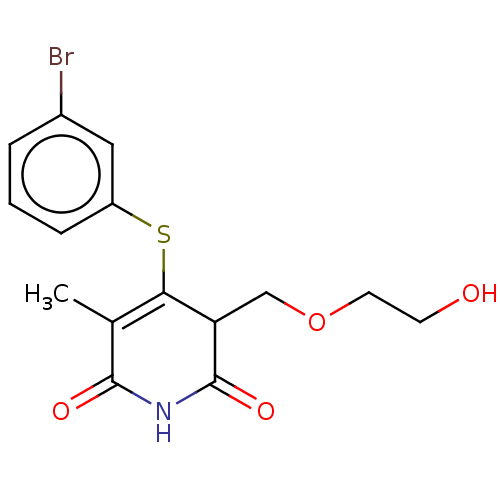
(CHEMBL1689304)Show SMILES CC1=C(Sc2cccc(Br)c2)C(COCCO)C(=O)NC1=O |c:1| Show InChI InChI=1S/C15H16BrNO4S/c1-9-13(22-11-4-2-3-10(16)7-11)12(8-21-6-5-18)15(20)17-14(9)19/h2-4,7,12,18H,5-6,8H2,1H3,(H,17,19,20) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
GVK Biosciences Private Limited
Curated by ChEMBL
| Assay Description
Inhibition of Human immunodeficiency virus 1 reverse transcriptase |
Eur J Med Chem 46: 851-9 (2011)
Article DOI: 10.1016/j.ejmech.2010.12.022
BindingDB Entry DOI: 10.7270/Q2HD7ZGX |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50483583
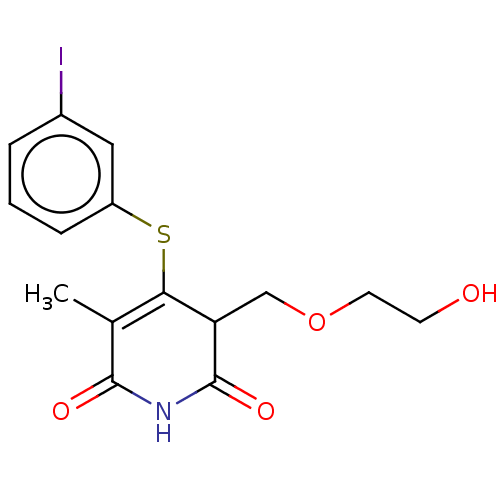
(CHEMBL1689305)Show SMILES CC1=C(Sc2cccc(I)c2)C(COCCO)C(=O)NC1=O |c:1| Show InChI InChI=1S/C15H16INO4S/c1-9-13(22-11-4-2-3-10(16)7-11)12(8-21-6-5-18)15(20)17-14(9)19/h2-4,7,12,18H,5-6,8H2,1H3,(H,17,19,20) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
GVK Biosciences Private Limited
Curated by ChEMBL
| Assay Description
Inhibition of Human immunodeficiency virus 1 reverse transcriptase |
Eur J Med Chem 46: 851-9 (2011)
Article DOI: 10.1016/j.ejmech.2010.12.022
BindingDB Entry DOI: 10.7270/Q2HD7ZGX |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM8579
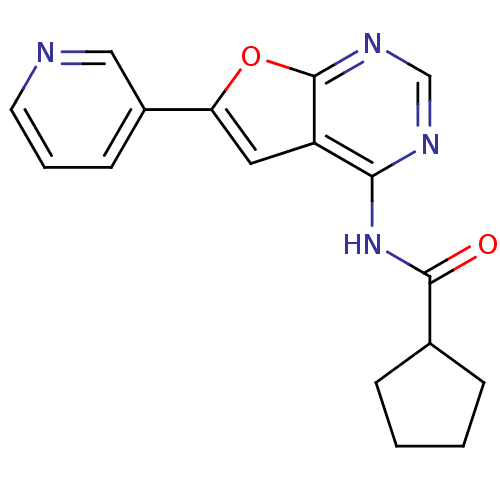
(4-Acylamino-6-arylfuro[2,3-d]pyrimidine 24 | N-[6-...)Show InChI InChI=1S/C17H16N4O2/c22-16(11-4-1-2-5-11)21-15-13-8-14(12-6-3-7-18-9-12)23-17(13)20-10-19-15/h3,6-11H,1-2,4-5H2,(H,19,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Road
Curated by ChEMBL
| Assay Description
Inhibition of human GSK3beta |
Eur J Med Chem 44: 2361-71 (2009)
Article DOI: 10.1016/j.ejmech.2008.08.012
BindingDB Entry DOI: 10.7270/Q2057FZX |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50483595

(CHEMBL1689306)Show SMILES CC1=C(Sc2cccc(c2)[N+]([O-])=O)C(COCCO)C(=O)NC1=O |c:1| Show InChI InChI=1S/C15H16N2O6S/c1-9-13(24-11-4-2-3-10(7-11)17(21)22)12(8-23-6-5-18)15(20)16-14(9)19/h2-4,7,12,18H,5-6,8H2,1H3,(H,16,19,20) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
GVK Biosciences Private Limited
Curated by ChEMBL
| Assay Description
Inhibition of Human immunodeficiency virus 1 reverse transcriptase |
Eur J Med Chem 46: 851-9 (2011)
Article DOI: 10.1016/j.ejmech.2010.12.022
BindingDB Entry DOI: 10.7270/Q2HD7ZGX |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50132317
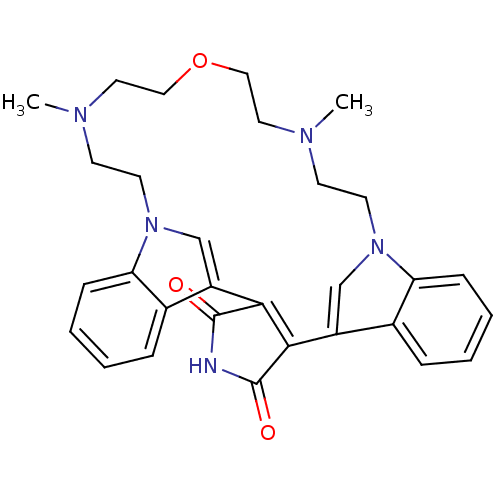
(17,23-dimethyl-20-oxa-4,14,17,23,26-pentaazahexacy...)Show SMILES CN1CCOCCN(C)CCn2cc(C3=C(C(=O)NC3=O)c3cn(CC1)c1ccccc31)c1ccccc21 |t:14| Show InChI InChI=1S/C30H33N5O3/c1-32-11-13-34-19-23(21-7-3-5-9-25(21)34)27-28(30(37)31-29(27)36)24-20-35(26-10-6-4-8-22(24)26)14-12-33(2)16-18-38-17-15-32/h3-10,19-20H,11-18H2,1-2H3,(H,31,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Road
Curated by ChEMBL
| Assay Description
Inhibition of human GSK3beta |
Eur J Med Chem 44: 2361-71 (2009)
Article DOI: 10.1016/j.ejmech.2008.08.012
BindingDB Entry DOI: 10.7270/Q2057FZX |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50483584
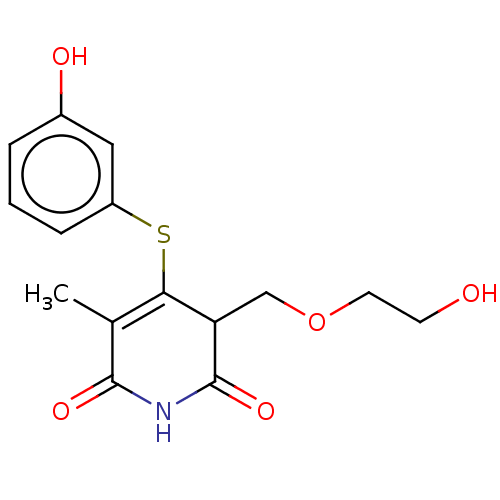
(CHEMBL1689307)Show SMILES CC1=C(Sc2cccc(O)c2)C(COCCO)C(=O)NC1=O |c:1| Show InChI InChI=1S/C15H17NO5S/c1-9-13(22-11-4-2-3-10(18)7-11)12(8-21-6-5-17)15(20)16-14(9)19/h2-4,7,12,17-18H,5-6,8H2,1H3,(H,16,19,20) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
GVK Biosciences Private Limited
Curated by ChEMBL
| Assay Description
Inhibition of Human immunodeficiency virus 1 reverse transcriptase |
Eur J Med Chem 46: 851-9 (2011)
Article DOI: 10.1016/j.ejmech.2010.12.022
BindingDB Entry DOI: 10.7270/Q2HD7ZGX |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50293308
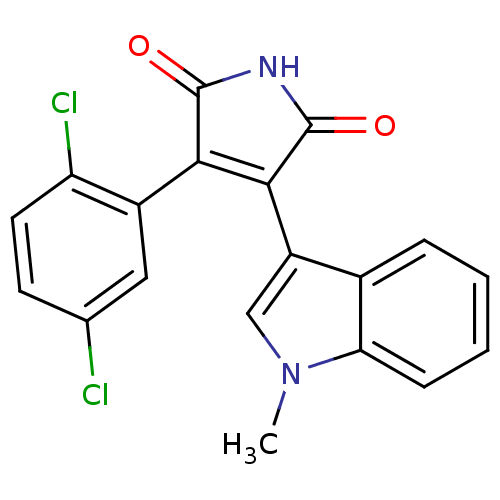
(3-(2,5-dichlorophenyl)-4-(1-methyl-1H-indol-3-yl)-...)Show SMILES Cn1cc(C2=C(C(=O)NC2=O)c2cc(Cl)ccc2Cl)c2ccccc12 |t:4| Show InChI InChI=1S/C19H12Cl2N2O2/c1-23-9-13(11-4-2-3-5-15(11)23)17-16(18(24)22-19(17)25)12-8-10(20)6-7-14(12)21/h2-9H,1H3,(H,22,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Road
Curated by ChEMBL
| Assay Description
Inhibition of human GSK3beta |
Eur J Med Chem 44: 2361-71 (2009)
Article DOI: 10.1016/j.ejmech.2008.08.012
BindingDB Entry DOI: 10.7270/Q2057FZX |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM7492
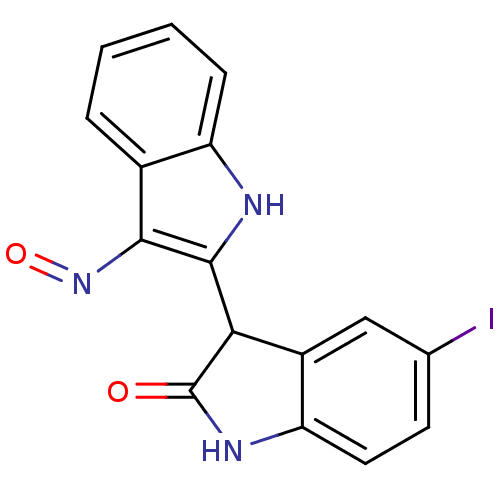
(3-[(2Z,3E)-3-(hydroxyimino)-2,3-dihydro-1H-indol-2...)Show InChI InChI=1S/C16H10IN3O2/c17-8-5-6-12-10(7-8)13(16(21)19-12)15-14(20-22)9-3-1-2-4-11(9)18-15/h1-7,13,18H,(H,19,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Road
Curated by ChEMBL
| Assay Description
Inhibition of human GSK3beta |
Eur J Med Chem 44: 2361-71 (2009)
Article DOI: 10.1016/j.ejmech.2008.08.012
BindingDB Entry DOI: 10.7270/Q2057FZX |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM7491
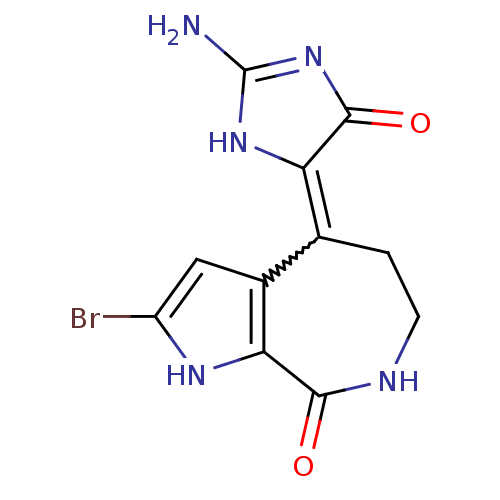
((4Z)-4-(2-amino-5-oxo-3,5-dihydro-4H-imidazol-4-yl...)Show SMILES NC1=NC(=O)C(N1)=C1CCNC(=O)c2[nH]c(Br)cc12 |w:7.19,t:1| Show InChI InChI=1S/C11H10BrN5O2/c12-6-3-5-4(7-10(19)17-11(13)16-7)1-2-14-9(18)8(5)15-6/h3,15H,1-2H2,(H,14,18)(H3,13,16,17,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Road
Curated by ChEMBL
| Assay Description
Inhibition of human GSK3beta |
Eur J Med Chem 44: 2361-71 (2009)
Article DOI: 10.1016/j.ejmech.2008.08.012
BindingDB Entry DOI: 10.7270/Q2057FZX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ZAP-70
(Homo sapiens (Human)) | BDBM50300382

(4-(4-(5-chloro-4-(2-(propylsulfonyl)phenylamino)py...)Show SMILES CCCS(=O)(=O)c1ccccc1Nc1nc(Nc2ccc(cc2OC)N2CCN(CC2)C(=O)NC)ncc1Cl Show InChI InChI=1S/C26H32ClN7O4S/c1-4-15-39(36,37)23-8-6-5-7-21(23)30-24-19(27)17-29-25(32-24)31-20-10-9-18(16-22(20)38-3)33-11-13-34(14-12-33)26(35)28-2/h5-10,16-17H,4,11-15H2,1-3H3,(H,28,35)(H2,29,30,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GVK Biosciences Pvt. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human GST-fused ZAP-70 expressed in Sf9 cells |
Eur J Med Chem 44: 4793-800 (2009)
Article DOI: 10.1016/j.ejmech.2009.07.018
BindingDB Entry DOI: 10.7270/Q25X290Z |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ZAP-70
(Homo sapiens (Human)) | BDBM50300401
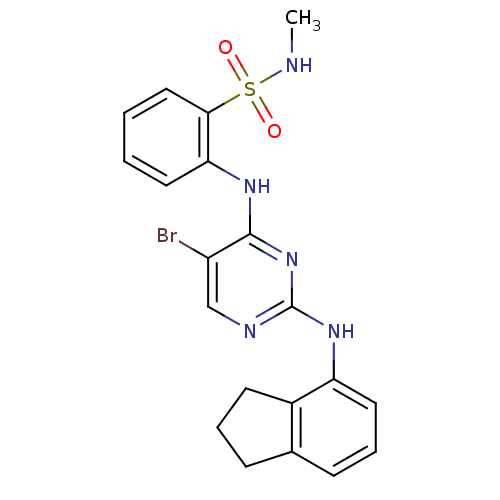
(2-(5-bromo-2-(2,3-dihydro-1H-inden-4-ylamino)pyrim...)Show SMILES CNS(=O)(=O)c1ccccc1Nc1nc(Nc2cccc3CCCc23)ncc1Br Show InChI InChI=1S/C20H20BrN5O2S/c1-22-29(27,28)18-11-3-2-9-17(18)24-19-15(21)12-23-20(26-19)25-16-10-5-7-13-6-4-8-14(13)16/h2-3,5,7,9-12,22H,4,6,8H2,1H3,(H2,23,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GVK Biosciences Pvt. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human GST-fused ZAP-70 expressed in Sf9 cells |
Eur J Med Chem 44: 4793-800 (2009)
Article DOI: 10.1016/j.ejmech.2009.07.018
BindingDB Entry DOI: 10.7270/Q25X290Z |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50059889

((staurosporine)3-methoxy-2-methyl-4-methylamino-(2...)Show SMILES CN[C@@H]1CC2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20?,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Road
Curated by ChEMBL
| Assay Description
Inhibition of human GSK3beta |
Eur J Med Chem 44: 2361-71 (2009)
Article DOI: 10.1016/j.ejmech.2008.08.012
BindingDB Entry DOI: 10.7270/Q2057FZX |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50483593
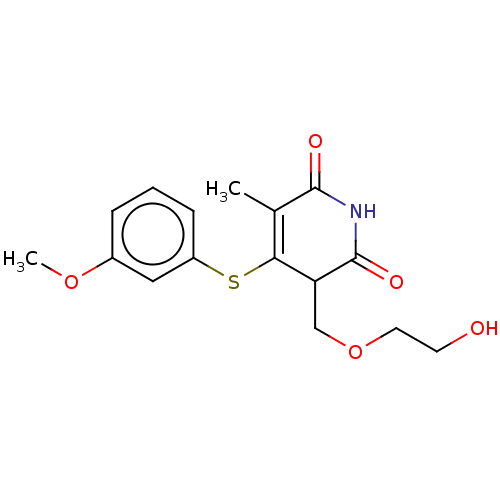
(CHEMBL1687958)Show SMILES COc1cccc(SC2=C(C)C(=O)NC(=O)C2COCCO)c1 |c:8| Show InChI InChI=1S/C16H19NO5S/c1-10-14(23-12-5-3-4-11(8-12)21-2)13(9-22-7-6-18)16(20)17-15(10)19/h3-5,8,13,18H,6-7,9H2,1-2H3,(H,17,19,20) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
GVK Biosciences Private Limited
Curated by ChEMBL
| Assay Description
Inhibition of Human immunodeficiency virus 1 reverse transcriptase |
Eur J Med Chem 46: 851-9 (2011)
Article DOI: 10.1016/j.ejmech.2010.12.022
BindingDB Entry DOI: 10.7270/Q2HD7ZGX |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM7393
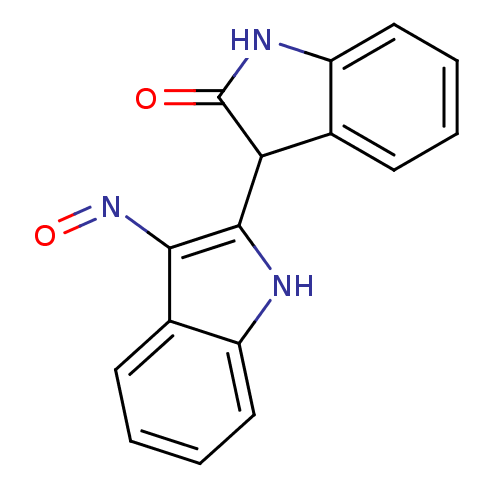
(3-[(2Z,3E)-3-(hydroxyimino)-2,3-dihydro-1H-indol-2...)Show InChI InChI=1S/C16H11N3O2/c20-16-13(9-5-1-3-7-11(9)18-16)15-14(19-21)10-6-2-4-8-12(10)17-15/h1-8,13,17H,(H,18,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Road
Curated by ChEMBL
| Assay Description
Inhibition of human GSK3beta |
Eur J Med Chem 44: 2361-71 (2009)
Article DOI: 10.1016/j.ejmech.2008.08.012
BindingDB Entry DOI: 10.7270/Q2057FZX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50483585
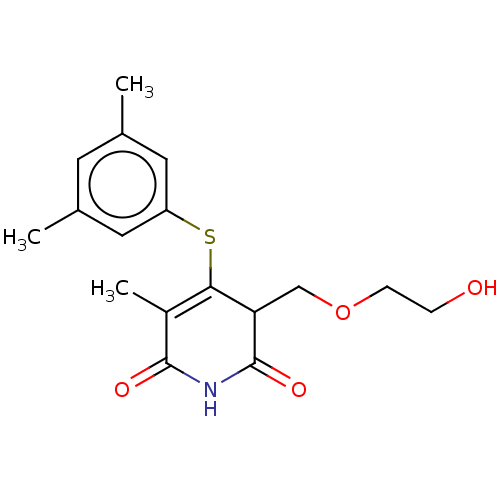
(CHEMBL1689308)Show SMILES CC1=C(Sc2cc(C)cc(C)c2)C(COCCO)C(=O)NC1=O |c:1| Show InChI InChI=1S/C17H21NO4S/c1-10-6-11(2)8-13(7-10)23-15-12(3)16(20)18-17(21)14(15)9-22-5-4-19/h6-8,14,19H,4-5,9H2,1-3H3,(H,18,20,21) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
GVK Biosciences Private Limited
Curated by ChEMBL
| Assay Description
Inhibition of Human immunodeficiency virus 1 reverse transcriptase |
Eur J Med Chem 46: 851-9 (2011)
Article DOI: 10.1016/j.ejmech.2010.12.022
BindingDB Entry DOI: 10.7270/Q2HD7ZGX |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50483586
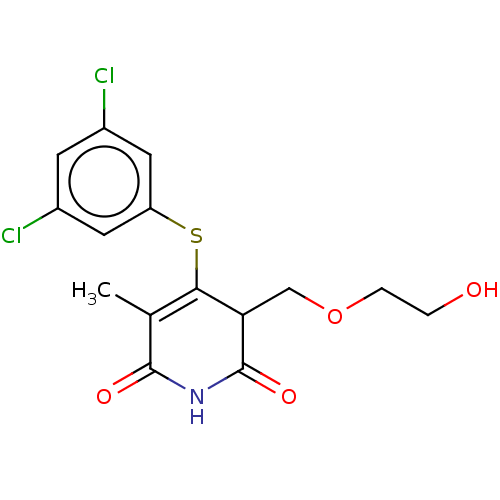
(CHEMBL1689309)Show SMILES CC1=C(Sc2cc(Cl)cc(Cl)c2)C(COCCO)C(=O)NC1=O |c:1| Show InChI InChI=1S/C15H15Cl2NO4S/c1-8-13(23-11-5-9(16)4-10(17)6-11)12(7-22-3-2-19)15(21)18-14(8)20/h4-6,12,19H,2-3,7H2,1H3,(H,18,20,21) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
GVK Biosciences Private Limited
Curated by ChEMBL
| Assay Description
Inhibition of Human immunodeficiency virus 1 reverse transcriptase |
Eur J Med Chem 46: 851-9 (2011)
Article DOI: 10.1016/j.ejmech.2010.12.022
BindingDB Entry DOI: 10.7270/Q2HD7ZGX |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50293309
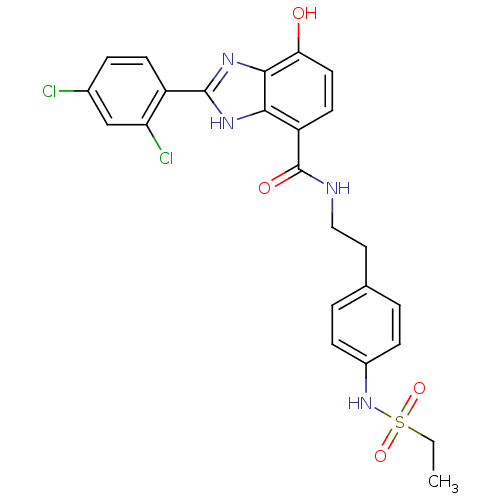
(CHEMBL559559 | N-(4-(ethylsulfonamido)phenethyl)-2...)Show SMILES CCS(=O)(=O)Nc1ccc(CCNC(=O)c2ccc(O)c3nc([nH]c23)-c2ccc(Cl)cc2Cl)cc1 Show InChI InChI=1S/C24H22Cl2N4O4S/c1-2-35(33,34)30-16-6-3-14(4-7-16)11-12-27-24(32)18-9-10-20(31)22-21(18)28-23(29-22)17-8-5-15(25)13-19(17)26/h3-10,13,30-31H,2,11-12H2,1H3,(H,27,32)(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Road
Curated by ChEMBL
| Assay Description
Inhibition of human GSK3beta |
Eur J Med Chem 44: 2361-71 (2009)
Article DOI: 10.1016/j.ejmech.2008.08.012
BindingDB Entry DOI: 10.7270/Q2057FZX |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50483574

(CHEMBL1689310)Show SMILES CC(C)C1=C(Sc2ccccc2)C(COCCO)C(=O)NC1=O |c:3| Show InChI InChI=1S/C17H21NO4S/c1-11(2)14-15(23-12-6-4-3-5-7-12)13(10-22-9-8-19)16(20)18-17(14)21/h3-7,11,13,19H,8-10H2,1-2H3,(H,18,20,21) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
GVK Biosciences Private Limited
Curated by ChEMBL
| Assay Description
Inhibition of Human immunodeficiency virus 1 reverse transcriptase |
Eur J Med Chem 46: 851-9 (2011)
Article DOI: 10.1016/j.ejmech.2010.12.022
BindingDB Entry DOI: 10.7270/Q2HD7ZGX |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM8296
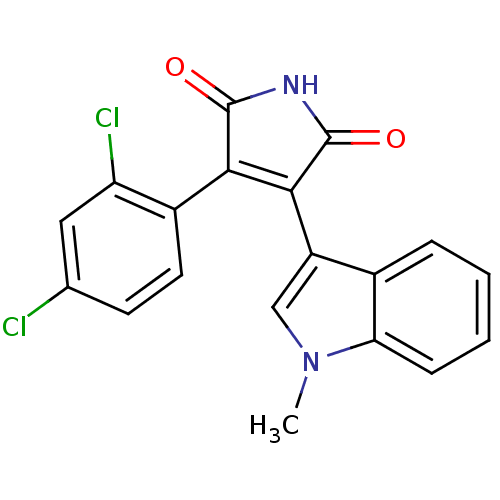
(3-(2,4-dichlorophenyl)-4-(1-methyl-1H-indol-3-yl)-...)Show SMILES Cn1cc(C2=C(C(=O)NC2=O)c2ccc(Cl)cc2Cl)c2ccccc12 |t:4| Show InChI InChI=1S/C19H12Cl2N2O2/c1-23-9-13(11-4-2-3-5-15(11)23)17-16(18(24)22-19(17)25)12-7-6-10(20)8-14(12)21/h2-9H,1H3,(H,22,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Road
Curated by ChEMBL
| Assay Description
Inhibition of human GSK3beta |
Eur J Med Chem 44: 2361-71 (2009)
Article DOI: 10.1016/j.ejmech.2008.08.012
BindingDB Entry DOI: 10.7270/Q2057FZX |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50293310
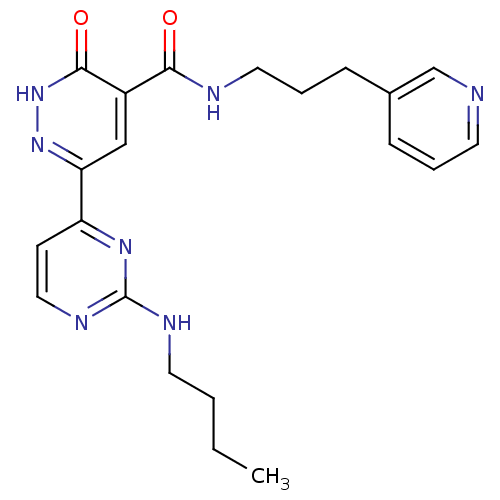
(6-(2-(butylamino)pyrimidin-4-yl)-3-oxo-N-(3-(pyrid...)Show SMILES CCCCNc1nccc(n1)-c1cc(C(=O)NCCCc2cccnc2)c(=O)[nH]n1 Show InChI InChI=1S/C21H25N7O2/c1-2-3-10-24-21-25-12-8-17(26-21)18-13-16(20(30)28-27-18)19(29)23-11-5-7-15-6-4-9-22-14-15/h4,6,8-9,12-14H,2-3,5,7,10-11H2,1H3,(H,23,29)(H,28,30)(H,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Road
Curated by ChEMBL
| Assay Description
Inhibition of human GSK3beta |
Eur J Med Chem 44: 2361-71 (2009)
Article DOI: 10.1016/j.ejmech.2008.08.012
BindingDB Entry DOI: 10.7270/Q2057FZX |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50293311
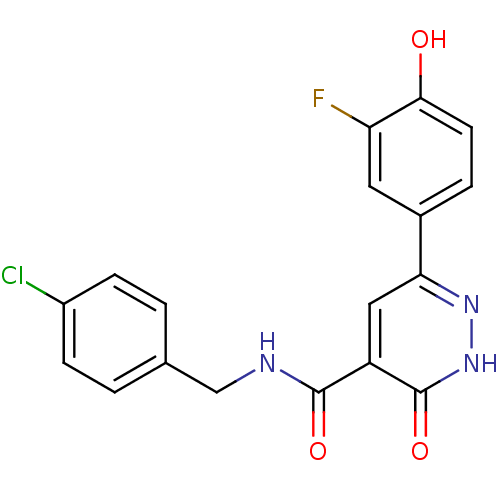
(CHEMBL540245 | N-(4-chlorobenzyl)-6-(3-fluoro-4-hy...)Show SMILES Oc1ccc(cc1F)-c1cc(C(=O)NCc2ccc(Cl)cc2)c(=O)[nH]n1 Show InChI InChI=1S/C18H13ClFN3O3/c19-12-4-1-10(2-5-12)9-21-17(25)13-8-15(22-23-18(13)26)11-3-6-16(24)14(20)7-11/h1-8,24H,9H2,(H,21,25)(H,23,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Road
Curated by ChEMBL
| Assay Description
Inhibition of human GSK3beta |
Eur J Med Chem 44: 2361-71 (2009)
Article DOI: 10.1016/j.ejmech.2008.08.012
BindingDB Entry DOI: 10.7270/Q2057FZX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ZAP-70
(Homo sapiens (Human)) | BDBM50300383
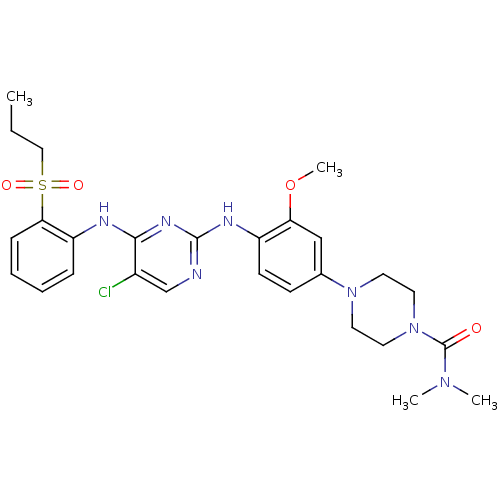
(4-(4-(5-chloro-4-(2-(propylsulfonyl)phenylamino)py...)Show SMILES CCCS(=O)(=O)c1ccccc1Nc1nc(Nc2ccc(cc2OC)N2CCN(CC2)C(=O)N(C)C)ncc1Cl Show InChI InChI=1S/C27H34ClN7O4S/c1-5-16-40(37,38)24-9-7-6-8-22(24)30-25-20(28)18-29-26(32-25)31-21-11-10-19(17-23(21)39-4)34-12-14-35(15-13-34)27(36)33(2)3/h6-11,17-18H,5,12-16H2,1-4H3,(H2,29,30,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
GVK Biosciences Pvt. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human GST-fused ZAP-70 expressed in Sf9 cells |
Eur J Med Chem 44: 4793-800 (2009)
Article DOI: 10.1016/j.ejmech.2009.07.018
BindingDB Entry DOI: 10.7270/Q25X290Z |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50293312

(CHEMBL562089 | N-(4-(2-morpholinoacetamido)pheneth...)Show SMILES Oc1ccc(C(=O)NCCc2ccc(NC(=O)CN3CCOCC3)cc2)c2[nH]c(nc12)-c1ccc(F)cc1 Show InChI InChI=1S/C28H28FN5O4/c29-20-5-3-19(4-6-20)27-32-25-22(9-10-23(35)26(25)33-27)28(37)30-12-11-18-1-7-21(8-2-18)31-24(36)17-34-13-15-38-16-14-34/h1-10,35H,11-17H2,(H,30,37)(H,31,36)(H,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Road
Curated by ChEMBL
| Assay Description
Inhibition of human GSK3beta |
Eur J Med Chem 44: 2361-71 (2009)
Article DOI: 10.1016/j.ejmech.2008.08.012
BindingDB Entry DOI: 10.7270/Q2057FZX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ZAP-70
(Homo sapiens (Human)) | BDBM50300384

(2-(5-chloro-2-(4-(3-(dimethylamino)-2,2-dimethylpr...)Show SMILES COc1cc(OCC(C)(C)CN(C)C)ccc1Nc1ncc(Cl)c(Nc2ccccc2S(=O)(=O)NC(C)C)n1 Show InChI InChI=1S/C27H37ClN6O4S/c1-18(2)33-39(35,36)24-11-9-8-10-22(24)30-25-20(28)15-29-26(32-25)31-21-13-12-19(14-23(21)37-7)38-17-27(3,4)16-34(5)6/h8-15,18,33H,16-17H2,1-7H3,(H2,29,30,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
GVK Biosciences Pvt. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human GST-fused ZAP-70 expressed in Sf9 cells |
Eur J Med Chem 44: 4793-800 (2009)
Article DOI: 10.1016/j.ejmech.2009.07.018
BindingDB Entry DOI: 10.7270/Q25X290Z |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50483587

(CHEMBL1689312)Show SMILES CC(C)C1=C(Sc2cc(C)cc(C)c2)C(COCCO)C(=O)NC1=O |c:3| Show InChI InChI=1S/C19H25NO4S/c1-11(2)16-17(25-14-8-12(3)7-13(4)9-14)15(10-24-6-5-21)18(22)20-19(16)23/h7-9,11,15,21H,5-6,10H2,1-4H3,(H,20,22,23) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
GVK Biosciences Private Limited
Curated by ChEMBL
| Assay Description
Inhibition of Human immunodeficiency virus 1 reverse transcriptase |
Eur J Med Chem 46: 851-9 (2011)
Article DOI: 10.1016/j.ejmech.2010.12.022
BindingDB Entry DOI: 10.7270/Q2HD7ZGX |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Homo sapiens (Human)) | BDBM50297422

(1-(3-(2,6-dihydroxyphenyl)-5-m-tolyl-4,5-dihydro-1...)Show SMILES CC(=O)N1N=C(CC1c1cccc(C)c1)c1c(O)cccc1O |c:4| Show InChI InChI=1S/C18H18N2O3/c1-11-5-3-6-13(9-11)15-10-14(19-20(15)12(2)21)18-16(22)7-4-8-17(18)23/h3-9,15,22-23H,10H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
GVK Biosciences Pvt. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of MAOB |
Eur J Med Chem 44: 3584-90 (2009)
Article DOI: 10.1016/j.ejmech.2009.02.031
BindingDB Entry DOI: 10.7270/Q2Z60P4F |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50293313
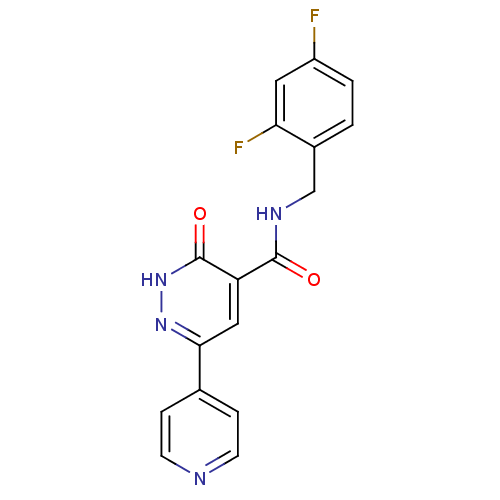
(CHEMBL557354 | N-(2,4-difluorobenzyl)-3-oxo-6-(pyr...)Show SMILES Fc1ccc(CNC(=O)c2cc(n[nH]c2=O)-c2ccncc2)c(F)c1 Show InChI InChI=1S/C17H12F2N4O2/c18-12-2-1-11(14(19)7-12)9-21-16(24)13-8-15(22-23-17(13)25)10-3-5-20-6-4-10/h1-8H,9H2,(H,21,24)(H,23,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Road
Curated by ChEMBL
| Assay Description
Inhibition of human GSK3beta |
Eur J Med Chem 44: 2361-71 (2009)
Article DOI: 10.1016/j.ejmech.2008.08.012
BindingDB Entry DOI: 10.7270/Q2057FZX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ZAP-70
(Homo sapiens (Human)) | BDBM50300385
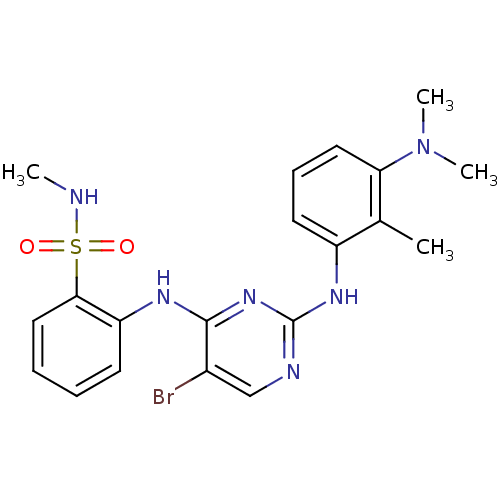
(2-(5-bromo-2-(3-(dimethylamino)-2-methylphenylamin...)Show SMILES CNS(=O)(=O)c1ccccc1Nc1nc(Nc2cccc(N(C)C)c2C)ncc1Br Show InChI InChI=1S/C20H23BrN6O2S/c1-13-15(9-7-10-17(13)27(3)4)25-20-23-12-14(21)19(26-20)24-16-8-5-6-11-18(16)30(28,29)22-2/h5-12,22H,1-4H3,(H2,23,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
GVK Biosciences Pvt. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human GST-fused ZAP-70 expressed in Sf9 cells |
Eur J Med Chem 44: 4793-800 (2009)
Article DOI: 10.1016/j.ejmech.2009.07.018
BindingDB Entry DOI: 10.7270/Q25X290Z |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50130725

(3-(3-Chloro-4-hydroxy-phenylamino)-4-(2-nitro-phen...)Show SMILES Oc1ccc(NC2=C(C(=O)NC2=O)c2ccccc2[N+]([O-])=O)cc1Cl |t:6| Show InChI InChI=1S/C16H10ClN3O5/c17-10-7-8(5-6-12(10)21)18-14-13(15(22)19-16(14)23)9-3-1-2-4-11(9)20(24)25/h1-7,21H,(H2,18,19,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Road
Curated by ChEMBL
| Assay Description
Inhibition of human GSK3beta |
Eur J Med Chem 44: 2361-71 (2009)
Article DOI: 10.1016/j.ejmech.2008.08.012
BindingDB Entry DOI: 10.7270/Q2057FZX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ZAP-70
(Homo sapiens (Human)) | BDBM50300386
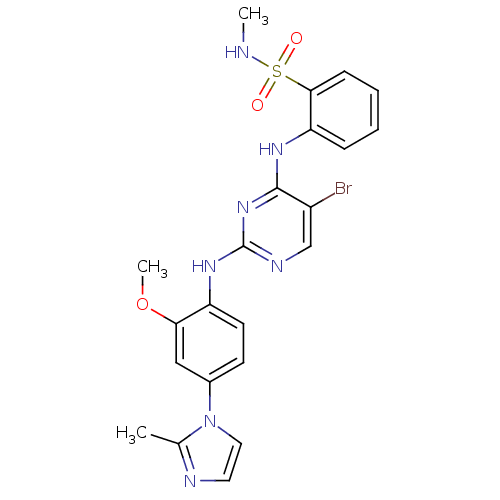
(2-(5-bromo-2-(2-methoxy-4-(2-methyl-1H-imidazol-1-...)Show SMILES CNS(=O)(=O)c1ccccc1Nc1nc(Nc2ccc(cc2OC)-n2ccnc2C)ncc1Br Show InChI InChI=1S/C22H22BrN7O3S/c1-14-25-10-11-30(14)15-8-9-17(19(12-15)33-3)28-22-26-13-16(23)21(29-22)27-18-6-4-5-7-20(18)34(31,32)24-2/h4-13,24H,1-3H3,(H2,26,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
GVK Biosciences Pvt. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human GST-fused ZAP-70 expressed in Sf9 cells |
Eur J Med Chem 44: 4793-800 (2009)
Article DOI: 10.1016/j.ejmech.2009.07.018
BindingDB Entry DOI: 10.7270/Q25X290Z |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50293314
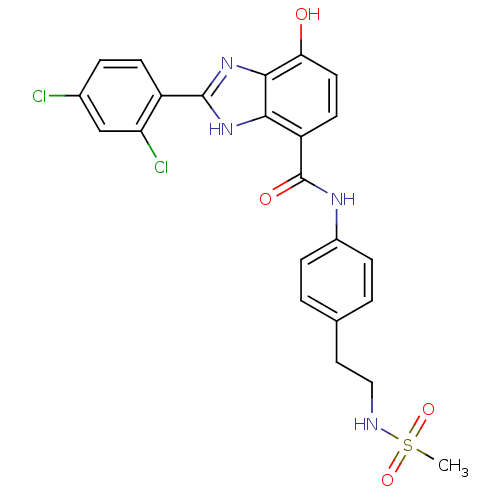
(2-(2,4-dichlorophenyl)-7-hydroxy-N-(4-(2-(methylsu...)Show SMILES CS(=O)(=O)NCCc1ccc(NC(=O)c2ccc(O)c3nc([nH]c23)-c2ccc(Cl)cc2Cl)cc1 Show InChI InChI=1S/C23H20Cl2N4O4S/c1-34(32,33)26-11-10-13-2-5-15(6-3-13)27-23(31)17-8-9-19(30)21-20(17)28-22(29-21)16-7-4-14(24)12-18(16)25/h2-9,12,26,30H,10-11H2,1H3,(H,27,31)(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Road
Curated by ChEMBL
| Assay Description
Inhibition of human GSK3beta |
Eur J Med Chem 44: 2361-71 (2009)
Article DOI: 10.1016/j.ejmech.2008.08.012
BindingDB Entry DOI: 10.7270/Q2057FZX |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Homo sapiens (Human)) | BDBM19187
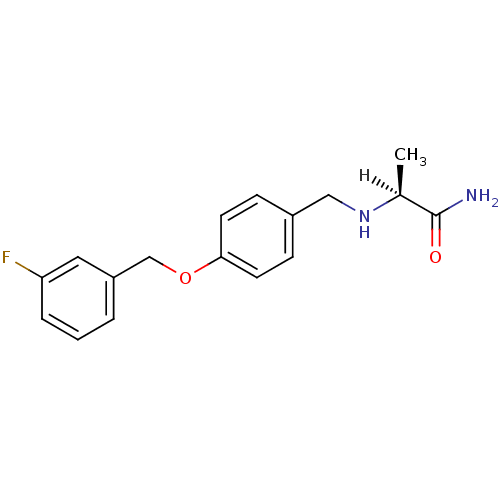
((2S)-2-[({4-[(3-fluorophenyl)methoxy]phenyl}methyl...)Show SMILES [H][C@@](C)(NCc1ccc(OCc2cccc(F)c2)cc1)C(N)=O |r| Show InChI InChI=1S/C17H19FN2O2/c1-12(17(19)21)20-10-13-5-7-16(8-6-13)22-11-14-3-2-4-15(18)9-14/h2-9,12,20H,10-11H2,1H3,(H2,19,21)/t12-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
GVK Biosciences Pvt. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of MAOB |
Eur J Med Chem 44: 3584-90 (2009)
Article DOI: 10.1016/j.ejmech.2009.02.031
BindingDB Entry DOI: 10.7270/Q2Z60P4F |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50483590

(CHEMBL1689311)Show SMILES CCC1=C(Sc2cc(C)cc(C)c2)C(COCCO)C(=O)NC1=O |c:2| Show InChI InChI=1S/C18H23NO4S/c1-4-14-16(24-13-8-11(2)7-12(3)9-13)15(10-23-6-5-20)18(22)19-17(14)21/h7-9,15,20H,4-6,10H2,1-3H3,(H,19,21,22) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
GVK Biosciences Private Limited
Curated by ChEMBL
| Assay Description
Inhibition of Human immunodeficiency virus 1 reverse transcriptase |
Eur J Med Chem 46: 851-9 (2011)
Article DOI: 10.1016/j.ejmech.2010.12.022
BindingDB Entry DOI: 10.7270/Q2HD7ZGX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ZAP-70
(Homo sapiens (Human)) | BDBM50300387
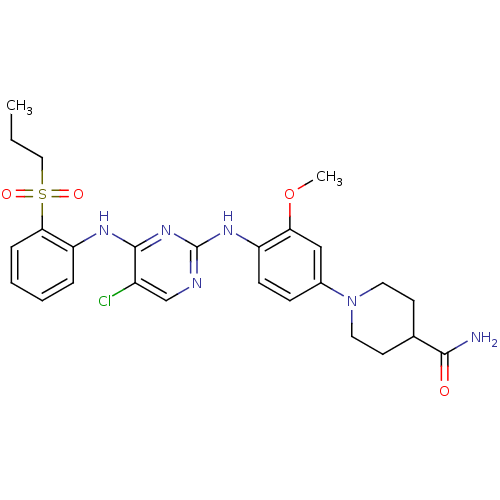
(1-(4-(5-chloro-4-(2-(propylsulfonyl)phenylamino)py...)Show SMILES CCCS(=O)(=O)c1ccccc1Nc1nc(Nc2ccc(cc2OC)N2CCC(CC2)C(N)=O)ncc1Cl Show InChI InChI=1S/C26H31ClN6O4S/c1-3-14-38(35,36)23-7-5-4-6-21(23)30-25-19(27)16-29-26(32-25)31-20-9-8-18(15-22(20)37-2)33-12-10-17(11-13-33)24(28)34/h4-9,15-17H,3,10-14H2,1-2H3,(H2,28,34)(H2,29,30,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
GVK Biosciences Pvt. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human GST-fused ZAP-70 expressed in Sf9 cells |
Eur J Med Chem 44: 4793-800 (2009)
Article DOI: 10.1016/j.ejmech.2009.07.018
BindingDB Entry DOI: 10.7270/Q25X290Z |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50483596
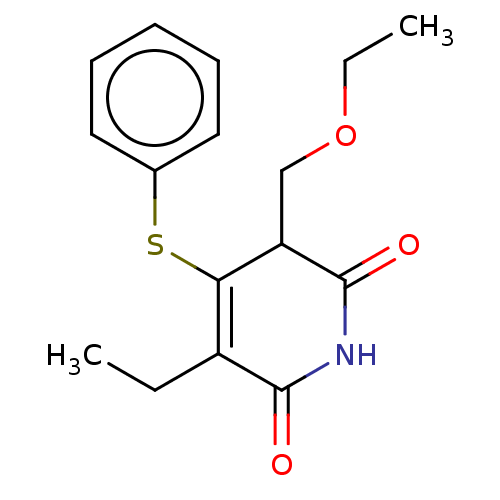
(CHEMBL1689314)Show InChI InChI=1S/C16H19NO3S/c1-3-12-14(21-11-8-6-5-7-9-11)13(10-20-4-2)16(19)17-15(12)18/h5-9,13H,3-4,10H2,1-2H3,(H,17,18,19) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
GVK Biosciences Private Limited
Curated by ChEMBL
| Assay Description
Inhibition of Human immunodeficiency virus 1 reverse transcriptase |
Eur J Med Chem 46: 851-9 (2011)
Article DOI: 10.1016/j.ejmech.2010.12.022
BindingDB Entry DOI: 10.7270/Q2HD7ZGX |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50130724
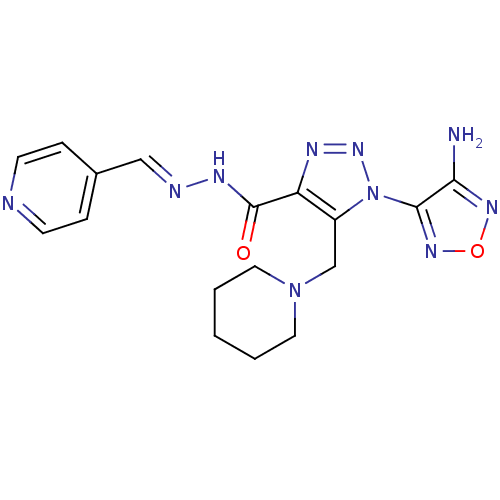
(1-(4-Amino-furazan-3-yl)-5-piperidin-1-ylmethyl-1H...)Show SMILES Nc1nonc1-n1nnc(C(=O)N\N=C\c2ccncc2)c1CN1CCCCC1 Show InChI InChI=1S/C17H20N10O2/c18-15-16(24-29-23-15)27-13(11-26-8-2-1-3-9-26)14(21-25-27)17(28)22-20-10-12-4-6-19-7-5-12/h4-7,10H,1-3,8-9,11H2,(H2,18,23)(H,22,28)/b20-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Road
Curated by ChEMBL
| Assay Description
Inhibition of human GSK3beta |
Eur J Med Chem 44: 2361-71 (2009)
Article DOI: 10.1016/j.ejmech.2008.08.012
BindingDB Entry DOI: 10.7270/Q2057FZX |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50229962
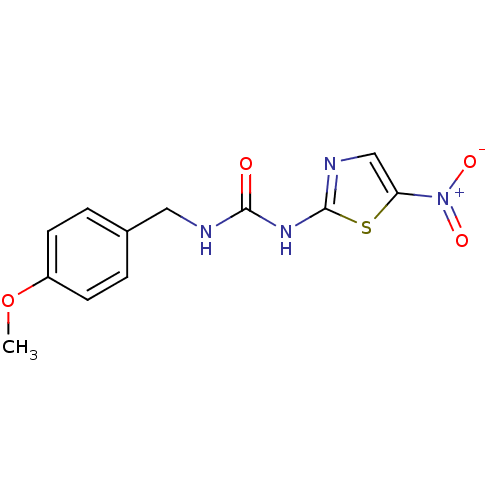
(1-(4-methoxybenzyl)-3-(5-nitrothiazol-2-yl)urea | ...)Show InChI InChI=1S/C12H12N4O4S/c1-20-9-4-2-8(3-5-9)6-13-11(17)15-12-14-7-10(21-12)16(18)19/h2-5,7H,6H2,1H3,(H2,13,14,15,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| MMDB
PDB
Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Road
Curated by ChEMBL
| Assay Description
Inhibition of human GSK3beta |
Eur J Med Chem 44: 2361-71 (2009)
Article DOI: 10.1016/j.ejmech.2008.08.012
BindingDB Entry DOI: 10.7270/Q2057FZX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50483575
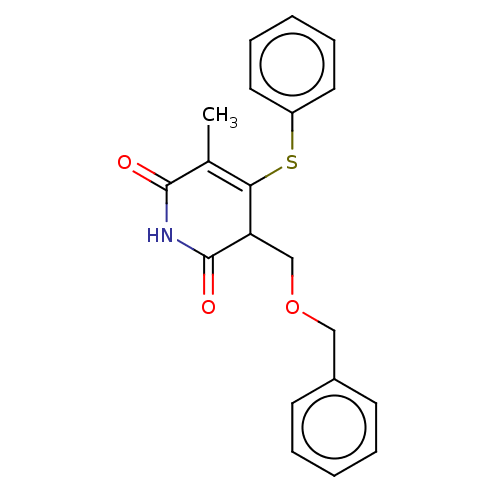
(CHEMBL1689313)Show SMILES CC1=C(Sc2ccccc2)C(COCc2ccccc2)C(=O)NC1=O |c:1| Show InChI InChI=1S/C20H19NO3S/c1-14-18(25-16-10-6-3-7-11-16)17(20(23)21-19(14)22)13-24-12-15-8-4-2-5-9-15/h2-11,17H,12-13H2,1H3,(H,21,22,23) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 111 | n/a | n/a | n/a | n/a | n/a | n/a |
GVK Biosciences Private Limited
Curated by ChEMBL
| Assay Description
Inhibition of Human immunodeficiency virus 1 reverse transcriptase |
Eur J Med Chem 46: 851-9 (2011)
Article DOI: 10.1016/j.ejmech.2010.12.022
BindingDB Entry DOI: 10.7270/Q2HD7ZGX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ZAP-70
(Homo sapiens (Human)) | BDBM50300388
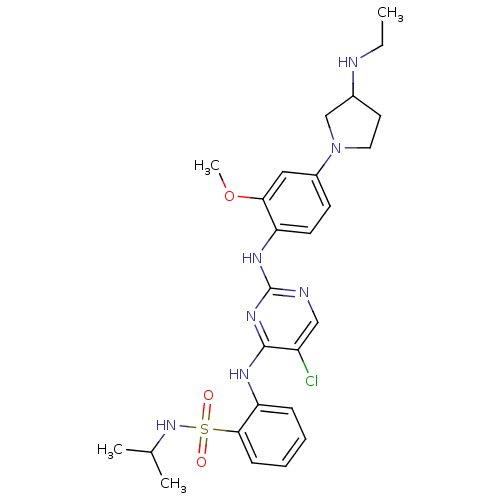
(2-(5-chloro-2-(4-(3-(ethylamino)pyrrolidin-1-yl)-2...)Show SMILES CCNC1CCN(C1)c1ccc(Nc2ncc(Cl)c(Nc3ccccc3S(=O)(=O)NC(C)C)n2)c(OC)c1 Show InChI InChI=1S/C26H34ClN7O3S/c1-5-28-18-12-13-34(16-18)19-10-11-21(23(14-19)37-4)31-26-29-15-20(27)25(32-26)30-22-8-6-7-9-24(22)38(35,36)33-17(2)3/h6-11,14-15,17-18,28,33H,5,12-13,16H2,1-4H3,(H2,29,30,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
GVK Biosciences Pvt. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human GST-fused ZAP-70 expressed in Sf9 cells |
Eur J Med Chem 44: 4793-800 (2009)
Article DOI: 10.1016/j.ejmech.2009.07.018
BindingDB Entry DOI: 10.7270/Q25X290Z |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50483591
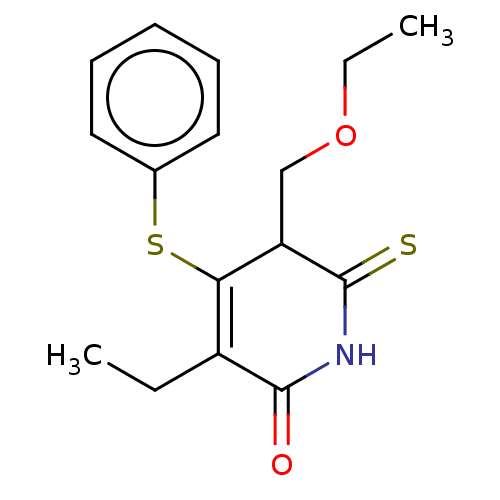
(CHEMBL1689315)Show InChI InChI=1S/C16H19NO2S2/c1-3-12-14(21-11-8-6-5-7-9-11)13(10-19-4-2)16(20)17-15(12)18/h5-9,13H,3-4,10H2,1-2H3,(H,17,18,20) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 137 | n/a | n/a | n/a | n/a | n/a | n/a |
GVK Biosciences Private Limited
Curated by ChEMBL
| Assay Description
Inhibition of Human immunodeficiency virus 1 reverse transcriptase |
Eur J Med Chem 46: 851-9 (2011)
Article DOI: 10.1016/j.ejmech.2010.12.022
BindingDB Entry DOI: 10.7270/Q2HD7ZGX |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50293315
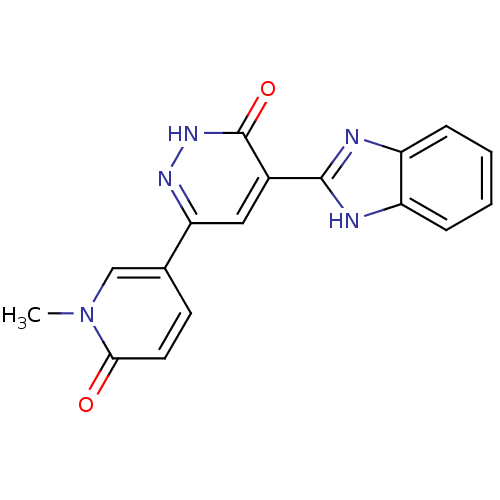
(4-(1H-benzo[d]imidazol-2-yl)-6-(1-methyl-6-oxo-1,6...)Show SMILES Cn1cc(ccc1=O)-c1cc(-c2nc3ccccc3[nH]2)c(=O)[nH]n1 Show InChI InChI=1S/C17H13N5O2/c1-22-9-10(6-7-15(22)23)14-8-11(17(24)21-20-14)16-18-12-4-2-3-5-13(12)19-16/h2-9H,1H3,(H,18,19)(H,21,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Road
Curated by ChEMBL
| Assay Description
Inhibition of human GSK3beta |
Eur J Med Chem 44: 2361-71 (2009)
Article DOI: 10.1016/j.ejmech.2008.08.012
BindingDB Entry DOI: 10.7270/Q2057FZX |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data