

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50042235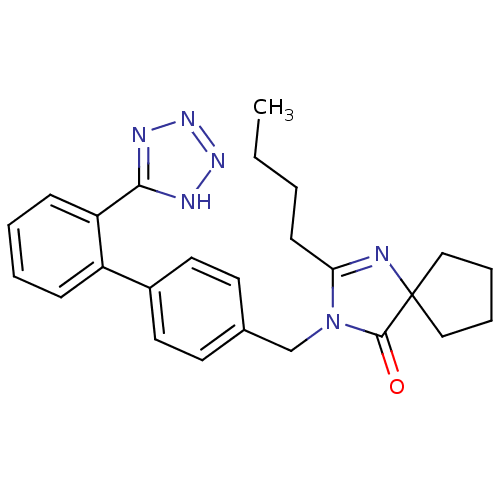 (2-butyl-3-{[2'-(1H-tetrazol-5-yl)[1,1'-biphenyl]-4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | >0.000100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity towards human Endothelin A receptor | J Med Chem 45: 3829-35 (2002) BindingDB Entry DOI: 10.7270/Q2Z60NDM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50091105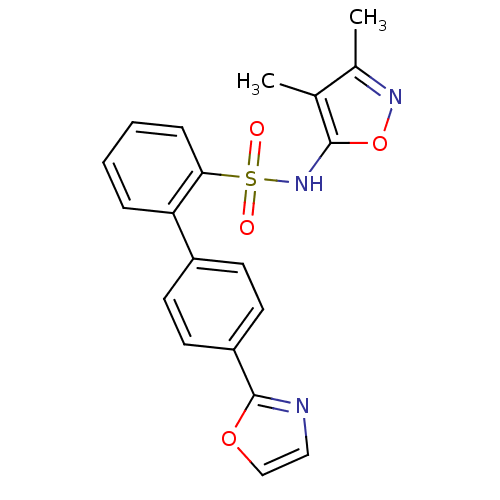 (4''-Oxazol-2-yl-biphenyl-2-sulfonic acid (3,4-dime...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | >0.000100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity towards rat Angiotensin II receptor, type 1 | J Med Chem 45: 3829-35 (2002) BindingDB Entry DOI: 10.7270/Q2Z60NDM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50091105 (4''-Oxazol-2-yl-biphenyl-2-sulfonic acid (3,4-dime...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | >0.000100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity towards human Angiotensin II receptor, type 1 | J Med Chem 45: 3829-35 (2002) BindingDB Entry DOI: 10.7270/Q2Z60NDM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM412656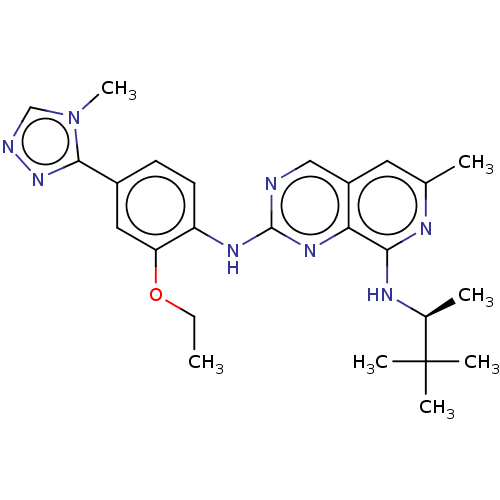 (US10399974, Example 54) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | Article PubMed | 0.0470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of full length human N-terminal 6xHis-tagged MPS1 expressed in baculovirus expression system using 5FAM-H236 peptide as substrate after 60... | J Med Chem 61: 8226-8240 (2018) Article DOI: 10.1021/acs.jmedchem.8b00690 BindingDB Entry DOI: 10.7270/Q2J105S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM241208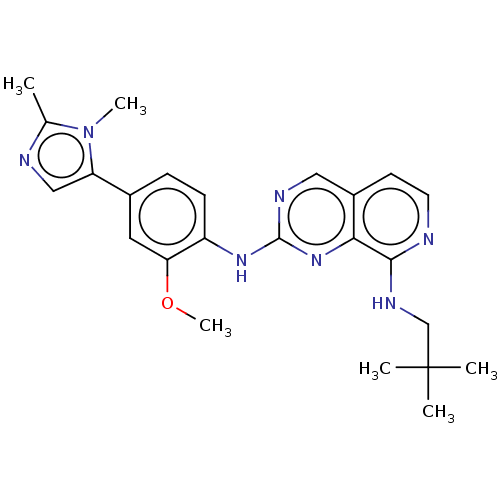 (US11046688, Example 50 | US9409907, 50) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of full length human N-terminal 6xHis-tagged MPS1 expressed in baculovirus expression system using 5FAM-H236 peptide as substrate after 60... | J Med Chem 61: 8226-8240 (2018) Article DOI: 10.1021/acs.jmedchem.8b00690 BindingDB Entry DOI: 10.7270/Q2J105S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM50464039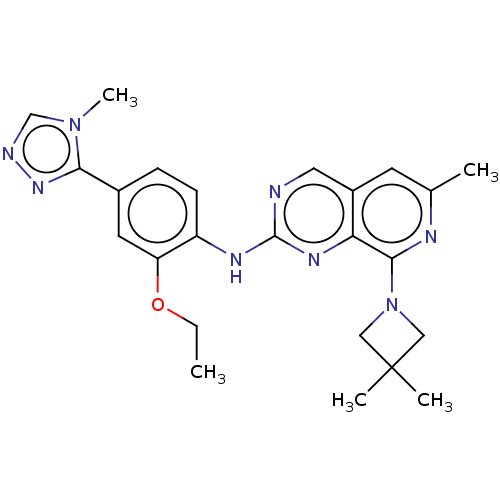 (CHEMBL4245639) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0880 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of full length human N-terminal 6xHis-tagged MPS1 expressed in baculovirus expression system using 5FAM-H236 peptide as substrate after 60... | J Med Chem 61: 8226-8240 (2018) Article DOI: 10.1021/acs.jmedchem.8b00690 BindingDB Entry DOI: 10.7270/Q2J105S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM241333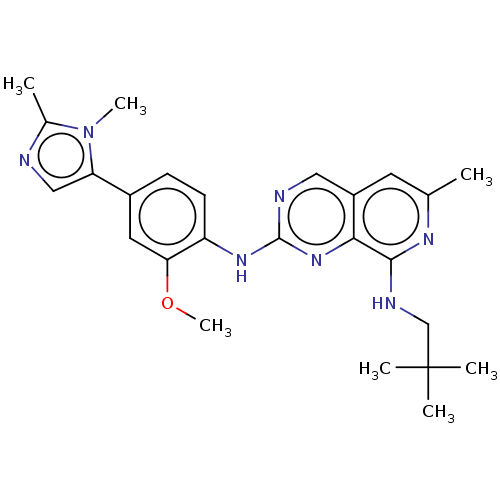 (US10479788, Example 177 | US11046688, Example 177 ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of full length human N-terminal 6xHis-tagged MPS1 expressed in baculovirus expression system using 5FAM-H236 peptide as substrate after 60... | J Med Chem 61: 8226-8240 (2018) Article DOI: 10.1021/acs.jmedchem.8b00690 BindingDB Entry DOI: 10.7270/Q2J105S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM412611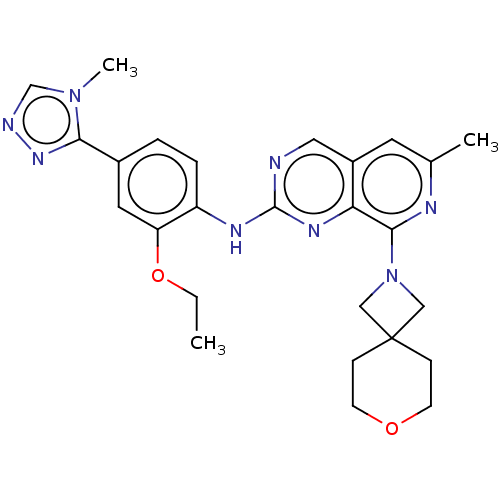 (N-(2-ethoxy-4-(4-methyl-4H-1,2,4-triazol-3-yl)phen...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of full length human N-terminal 6xHis-tagged MPS1 expressed in baculovirus expression system using 5FAM-H236 peptide as substrate after 60... | J Med Chem 61: 8226-8240 (2018) Article DOI: 10.1021/acs.jmedchem.8b00690 BindingDB Entry DOI: 10.7270/Q2J105S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM241338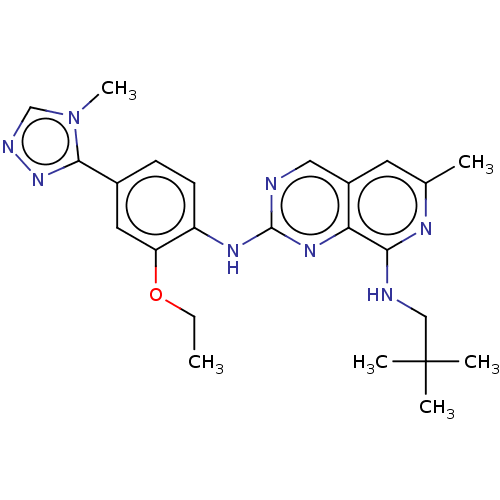 (US10479788, Example 182 | US11046688, Example 182 ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of full length human N-terminal 6xHis-tagged MPS1 expressed in baculovirus expression system using 5FAM-H236 peptide as substrate after 60... | J Med Chem 61: 8226-8240 (2018) Article DOI: 10.1021/acs.jmedchem.8b00690 BindingDB Entry DOI: 10.7270/Q2J105S9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM412614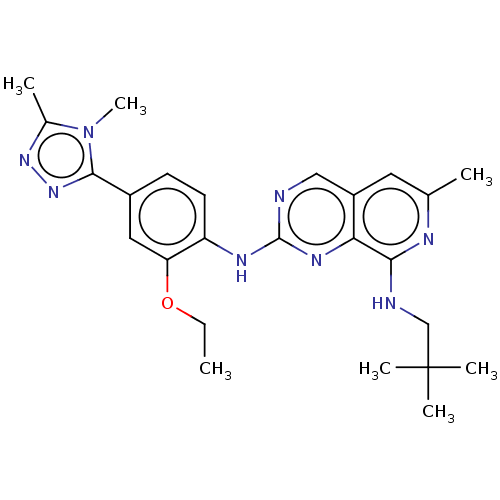 (N2-(4-(4,5-dimethyl-4H-1,2,4-triazol-3-yl)-2-ethox...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of full length human N-terminal 6xHis-tagged MPS1 expressed in baculovirus expression system using 5FAM-H236 peptide as substrate after 60... | J Med Chem 61: 8226-8240 (2018) Article DOI: 10.1021/acs.jmedchem.8b00690 BindingDB Entry DOI: 10.7270/Q2J105S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM50464037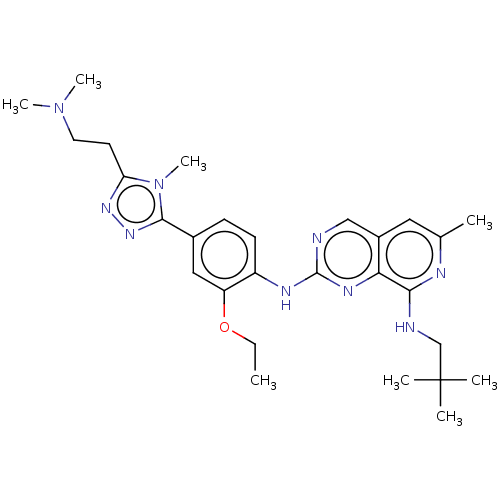 (CHEMBL4240502) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of full length human N-terminal 6xHis-tagged MPS1 expressed in baculovirus expression system using 5FAM-H236 peptide as substrate after 60... | J Med Chem 61: 8226-8240 (2018) Article DOI: 10.1021/acs.jmedchem.8b00690 BindingDB Entry DOI: 10.7270/Q2J105S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM241335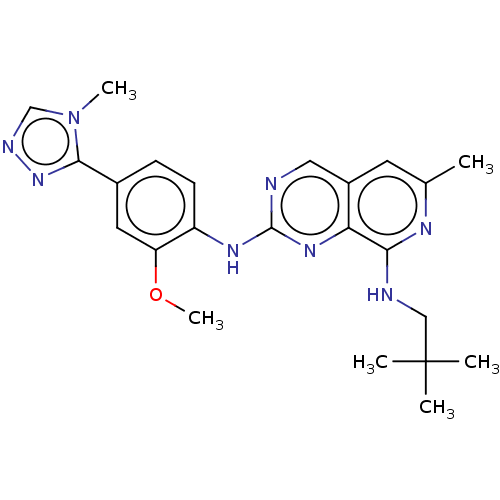 (US9409907, 179) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of full length human N-terminal 6xHis-tagged MPS1 expressed in baculovirus expression system using 5FAM-H236 peptide as substrate after 60... | J Med Chem 61: 8226-8240 (2018) Article DOI: 10.1021/acs.jmedchem.8b00690 BindingDB Entry DOI: 10.7270/Q2J105S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM50464040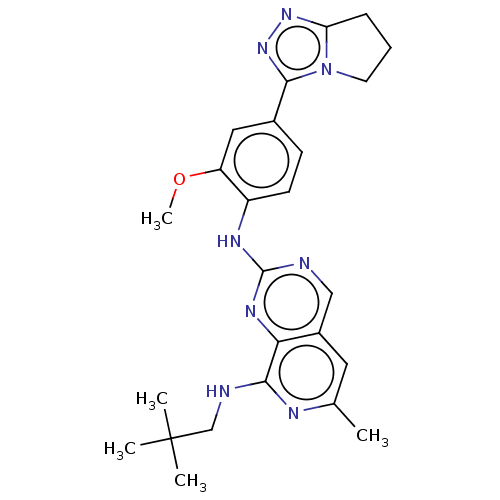 (CHEMBL4251352) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of full length human N-terminal 6xHis-tagged MPS1 expressed in baculovirus expression system using 5FAM-H236 peptide as substrate after 60... | J Med Chem 61: 8226-8240 (2018) Article DOI: 10.1021/acs.jmedchem.8b00690 BindingDB Entry DOI: 10.7270/Q2J105S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM241235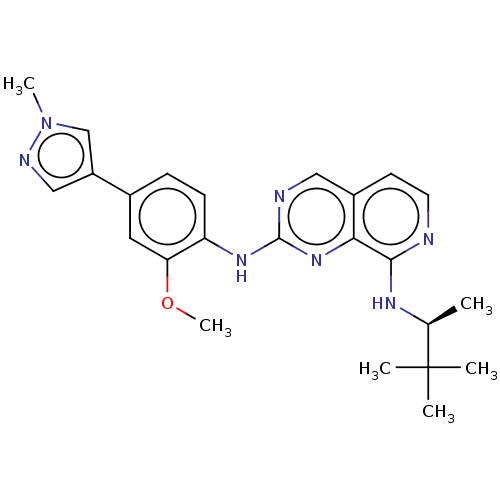 (US10479788, Example 77 | US11046688, Example 77 | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of human full length MPS1 expressed in recombinant baculovirus infected Sf9 insect cells using 5FAM-DHTGFLTEYVATRCONH2 as substrate after ... | J Med Chem 59: 3671-88 (2016) Article DOI: 10.1021/acs.jmedchem.5b01811 BindingDB Entry DOI: 10.7270/Q2DZ0B6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM412610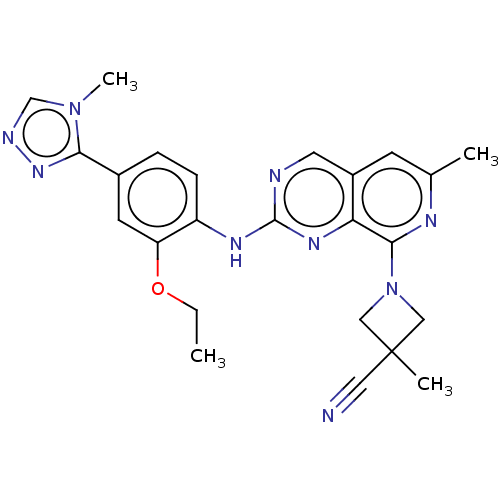 (1-(2-((2-ethoxy-4-(4-methyl-4H-1,2,4-triazol-3-yl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of full length human N-terminal 6xHis-tagged MPS1 expressed in baculovirus expression system using 5FAM-H236 peptide as substrate after 60... | J Med Chem 61: 8226-8240 (2018) Article DOI: 10.1021/acs.jmedchem.8b00690 BindingDB Entry DOI: 10.7270/Q2J105S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM50464038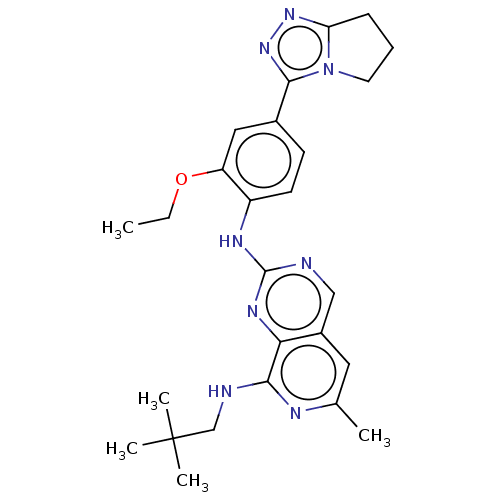 (CHEMBL4250961) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of full length human N-terminal 6xHis-tagged MPS1 expressed in baculovirus expression system using 5FAM-H236 peptide as substrate after 60... | J Med Chem 61: 8226-8240 (2018) Article DOI: 10.1021/acs.jmedchem.8b00690 BindingDB Entry DOI: 10.7270/Q2J105S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM241226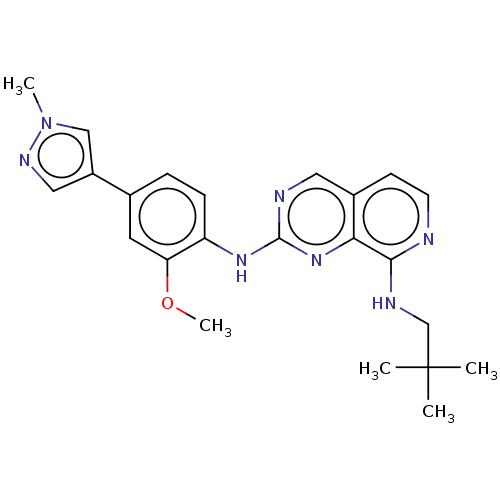 (US10479788, Example 68 | US11046688, Example 68 | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of full length human N-terminal 6xHis-tagged MPS1 expressed in baculovirus expression system using 5FAM-H236 peptide as substrate after 60... | J Med Chem 61: 8226-8240 (2018) Article DOI: 10.1021/acs.jmedchem.8b00690 BindingDB Entry DOI: 10.7270/Q2J105S9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM241226 (US10479788, Example 68 | US11046688, Example 68 | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of human full length MPS1 expressed in recombinant baculovirus infected Sf9 insect cells using 5FAM-DHTGFLTEYVATRCONH2 as substrate after ... | J Med Chem 59: 3671-88 (2016) Article DOI: 10.1021/acs.jmedchem.5b01811 BindingDB Entry DOI: 10.7270/Q2DZ0B6T | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50125339 (3-[2'-(3,4-Dimethyl-isoxazol-5-ylsulfamoyl)-2-meth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity towards Angiotensin II receptor, type 1 in rat aortic smooth muscle cells using 0.2 nM [125I]-labeled Sar-Ile-angiotensin II | Bioorg Med Chem Lett 13: 1093-6 (2003) BindingDB Entry DOI: 10.7270/Q2NG4PZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM241207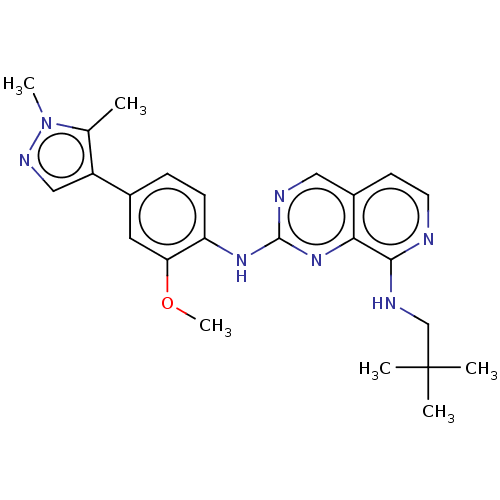 (US11046688, Example 49 | US9409907, 49) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of full length human N-terminal 6xHis-tagged MPS1 expressed in baculovirus expression system using 5FAM-H236 peptide as substrate after 60... | J Med Chem 61: 8226-8240 (2018) Article DOI: 10.1021/acs.jmedchem.8b00690 BindingDB Entry DOI: 10.7270/Q2J105S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50042235 (2-butyl-3-{[2'-(1H-tetrazol-5-yl)[1,1'-biphenyl]-4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity towards rat Angiotensin II receptor, type 1 | J Med Chem 45: 3829-35 (2002) BindingDB Entry DOI: 10.7270/Q2Z60NDM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM412658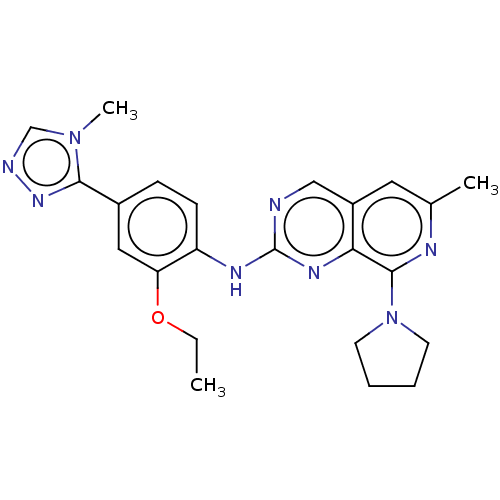 (US10399974, Example 56) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | Article PubMed | 0.820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of full length human N-terminal 6xHis-tagged MPS1 expressed in baculovirus expression system using 5FAM-H236 peptide as substrate after 60... | J Med Chem 61: 8226-8240 (2018) Article DOI: 10.1021/acs.jmedchem.8b00690 BindingDB Entry DOI: 10.7270/Q2J105S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50125343 (2'-(3,3-Dimethyl-2-oxo-pyrrolidin-1-ylmethyl)-4'-(...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity towards human ETA receptor expressed in CHO-K1 cells in the presence of 0.05 nM [125I]-labeled endothelin 1 | Bioorg Med Chem Lett 13: 1093-6 (2003) BindingDB Entry DOI: 10.7270/Q2NG4PZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50042235 (2-butyl-3-{[2'-(1H-tetrazol-5-yl)[1,1'-biphenyl]-4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity towards human Angiotensin II receptor, type 1 | J Med Chem 45: 3829-35 (2002) BindingDB Entry DOI: 10.7270/Q2Z60NDM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50125362 (3-[2'-(3,4-Dimethyl-isoxazol-5-ylsulfamoyl)-2-ethy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity towards Angiotensin II receptor, type 1 in rat aortic smooth muscle cells using 0.2 nM [125I]-labeled Sar-Ile-angiotensin II | Bioorg Med Chem Lett 13: 1093-6 (2003) BindingDB Entry DOI: 10.7270/Q2NG4PZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM241213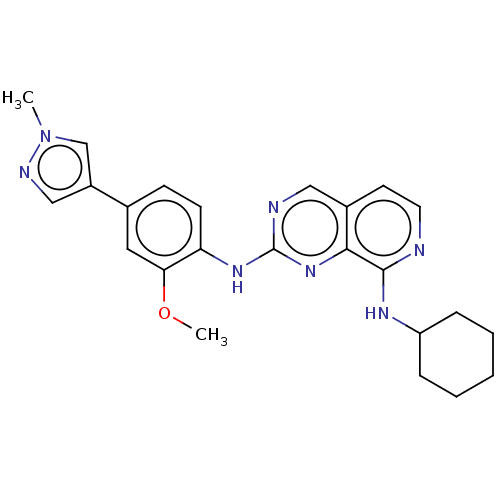 (US10479788, Example 55 | US11046688, Example 55 | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of human full length MPS1 expressed in recombinant baculovirus infected Sf9 insect cells using 5FAM-DHTGFLTEYVATRCONH2 as substrate after ... | J Med Chem 59: 3671-88 (2016) Article DOI: 10.1021/acs.jmedchem.5b01811 BindingDB Entry DOI: 10.7270/Q2DZ0B6T | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50091105 (4''-Oxazol-2-yl-biphenyl-2-sulfonic acid (3,4-dime...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity towards human Endothelin A receptor | J Med Chem 45: 3829-35 (2002) BindingDB Entry DOI: 10.7270/Q2Z60NDM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50117910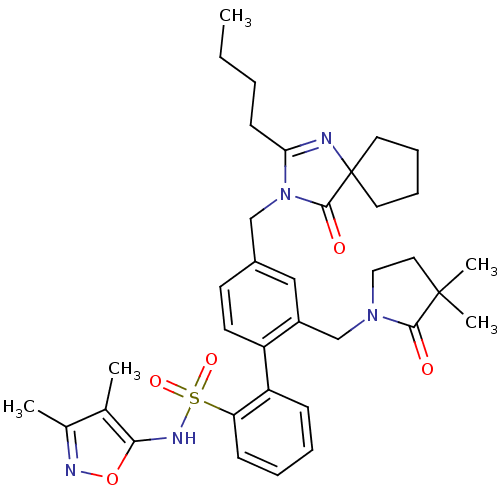 (4''-(2-Butyl-4-oxo-1,3-diaza-spiro[4.4]non-1-en-3-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity towards human Endothelin A receptor | J Med Chem 45: 3829-35 (2002) BindingDB Entry DOI: 10.7270/Q2Z60NDM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50125352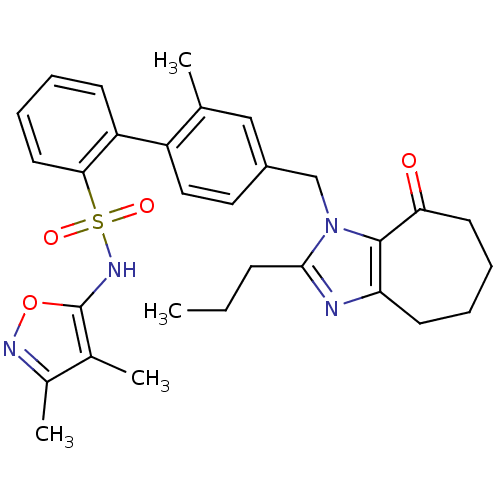 (2'-Methyl-4'-(8-oxo-2-propyl-5,6,7,8-tetrahydro-4H...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity towards Angiotensin II receptor, type 1 in rat aortic smooth muscle cells using 0.2 nM [125I]-labeled Sar-Ile-angiotensin II | Bioorg Med Chem Lett 13: 1093-6 (2003) BindingDB Entry DOI: 10.7270/Q2NG4PZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50125356 (3-[2'-(3,4-Dimethyl-isoxazol-5-ylsulfamoyl)-2-(2-f...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity towards Angiotensin II receptor, type 1 in rat aortic smooth muscle cells using 0.2 nM [125I]-labeled Sar-Ile-angiotensin II | Bioorg Med Chem Lett 13: 1093-6 (2003) BindingDB Entry DOI: 10.7270/Q2NG4PZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50117910 (4''-(2-Butyl-4-oxo-1,3-diaza-spiro[4.4]non-1-en-3-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity towards human ETA receptor expressed in CHO-K1 cells in the presence of 0.05 nM [125I]-labeled endothelin 1 | Bioorg Med Chem Lett 13: 1093-6 (2003) BindingDB Entry DOI: 10.7270/Q2NG4PZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50125359 (3-[2'-(3,4-Dimethyl-isoxazol-5-ylsulfamoyl)-2-meth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity towards Angiotensin II receptor, type 1 in rat aortic smooth muscle cells using 0.2 nM [125I]-labeled Sar-Ile-angiotensin II | Bioorg Med Chem Lett 13: 1093-6 (2003) BindingDB Entry DOI: 10.7270/Q2NG4PZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM241216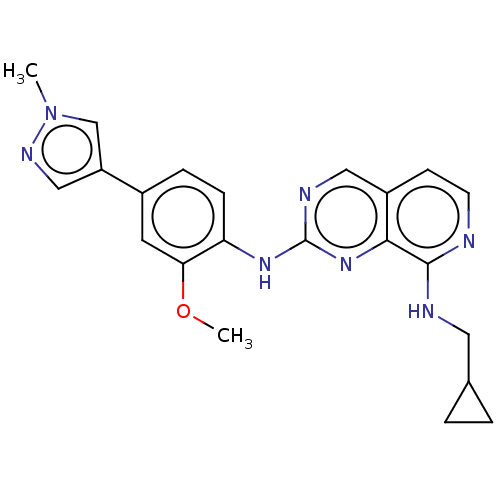 (US10479788, Example 58 | US11046688, Example 58 | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of human full length MPS1 expressed in recombinant baculovirus infected Sf9 insect cells using 5FAM-DHTGFLTEYVATRCONH2 as substrate after ... | J Med Chem 59: 3671-88 (2016) Article DOI: 10.1021/acs.jmedchem.5b01811 BindingDB Entry DOI: 10.7270/Q2DZ0B6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50125350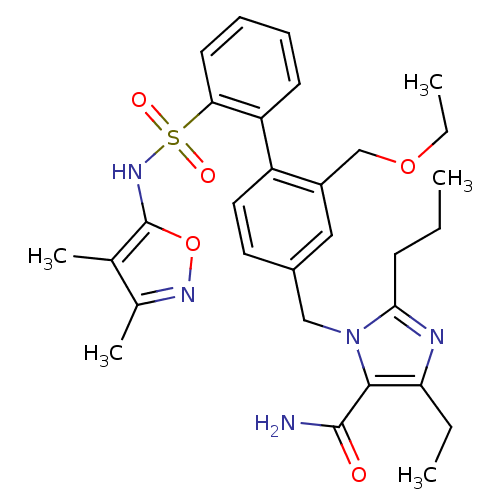 (3-[2'-(3,4-Dimethyl-isoxazol-5-ylsulfamoyl)-2-etho...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity towards Angiotensin II receptor, type 1 in rat aortic smooth muscle cells using 0.2 nM [125I]-labeled Sar-Ile-angiotensin II | Bioorg Med Chem Lett 13: 1093-6 (2003) BindingDB Entry DOI: 10.7270/Q2NG4PZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM241195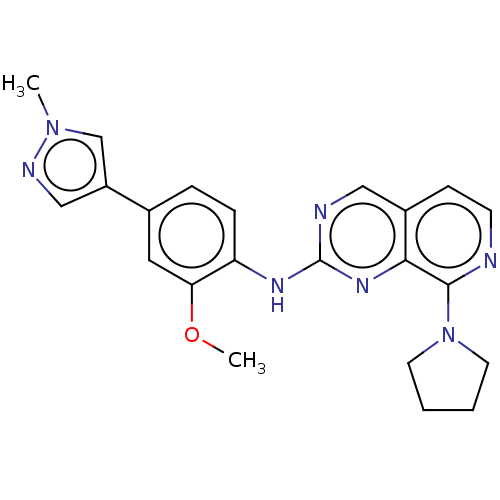 (US10479788, Example 37 | US11046688, Example 37 | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of human full length MPS1 expressed in recombinant baculovirus infected Sf9 insect cells using 5FAM-DHTGFLTEYVATRCONH2 as substrate after ... | J Med Chem 59: 3671-88 (2016) Article DOI: 10.1021/acs.jmedchem.5b01811 BindingDB Entry DOI: 10.7270/Q2DZ0B6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM241223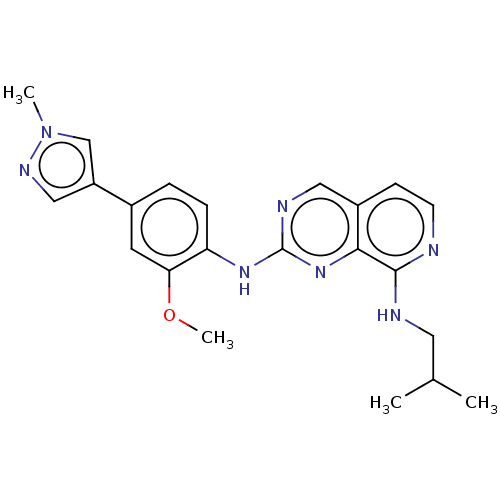 (US10479788, Example 65 | US11046688, Example 65 | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of human full length MPS1 expressed in recombinant baculovirus infected Sf9 insect cells using 5FAM-DHTGFLTEYVATRCONH2 as substrate after ... | J Med Chem 59: 3671-88 (2016) Article DOI: 10.1021/acs.jmedchem.5b01811 BindingDB Entry DOI: 10.7270/Q2DZ0B6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM412644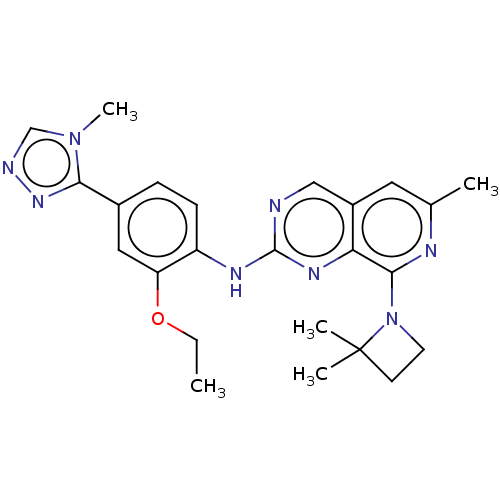 (US10399974, Example 42) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of full length human N-terminal 6xHis-tagged MPS1 expressed in baculovirus expression system using 5FAM-H236 peptide as substrate after 60... | J Med Chem 61: 8226-8240 (2018) Article DOI: 10.1021/acs.jmedchem.8b00690 BindingDB Entry DOI: 10.7270/Q2J105S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50125339 (3-[2'-(3,4-Dimethyl-isoxazol-5-ylsulfamoyl)-2-meth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity towards human ETA receptor expressed in CHO-K1 cells in the presence of 0.05 nM [125I]-labeled endothelin 1 | Bioorg Med Chem Lett 13: 1093-6 (2003) BindingDB Entry DOI: 10.7270/Q2NG4PZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50125340 (2'-Methoxymethyl-4'-(8-oxo-2-propyl-5,6,7,8-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity towards Angiotensin II receptor, type 1 in rat aortic smooth muscle cells using 0.2 nM [125I]-labeled Sar-Ile-angiotensin II | Bioorg Med Chem Lett 13: 1093-6 (2003) BindingDB Entry DOI: 10.7270/Q2NG4PZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50125345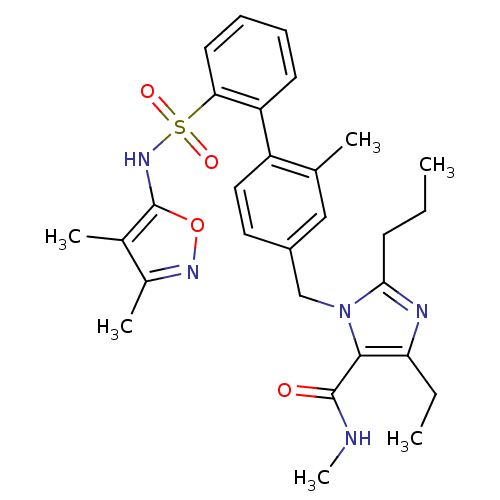 (3-[2'-(3,4-Dimethyl-isoxazol-5-ylsulfamoyl)-2-meth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity towards Angiotensin II receptor, type 1 in rat aortic smooth muscle cells using 0.2 nM [125I]-labeled Sar-Ile-angiotensin II | Bioorg Med Chem Lett 13: 1093-6 (2003) BindingDB Entry DOI: 10.7270/Q2NG4PZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM241234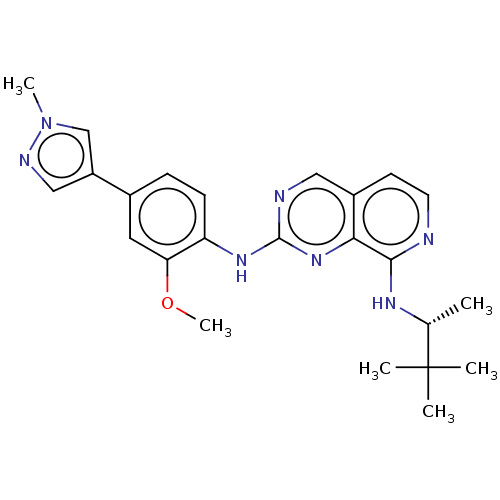 (US10479788, Example 76 | US11046688, Example 76 | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of human full length MPS1 expressed in recombinant baculovirus infected Sf9 insect cells using 5FAM-DHTGFLTEYVATRCONH2 as substrate after ... | J Med Chem 59: 3671-88 (2016) Article DOI: 10.1021/acs.jmedchem.5b01811 BindingDB Entry DOI: 10.7270/Q2DZ0B6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50117911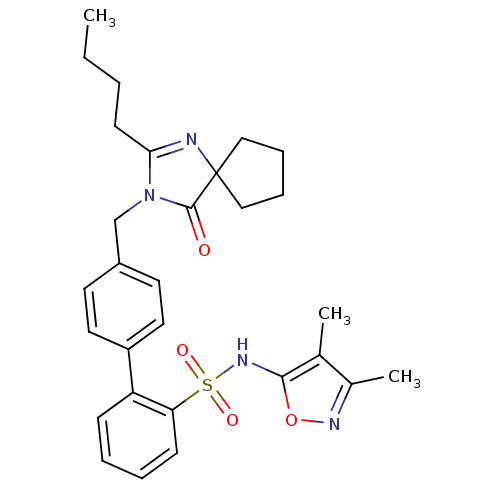 (4'-(2-Butyl-4-oxo-1,3-diaza-spiro[4.4]non-1-en-3-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity towards rat Angiotensin II receptor, type 1 | J Med Chem 45: 3829-35 (2002) BindingDB Entry DOI: 10.7270/Q2Z60NDM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50125345 (3-[2'-(3,4-Dimethyl-isoxazol-5-ylsulfamoyl)-2-meth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity towards human ETA receptor expressed in CHO-K1 cells in the presence of 0.05 nM [125I]-labeled endothelin 1 | Bioorg Med Chem Lett 13: 1093-6 (2003) BindingDB Entry DOI: 10.7270/Q2NG4PZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50125359 (3-[2'-(3,4-Dimethyl-isoxazol-5-ylsulfamoyl)-2-meth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity towards human ETA receptor expressed in CHO-K1 cells in the presence of 0.05 nM [125I]-labeled endothelin 1 | Bioorg Med Chem Lett 13: 1093-6 (2003) BindingDB Entry DOI: 10.7270/Q2NG4PZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50125360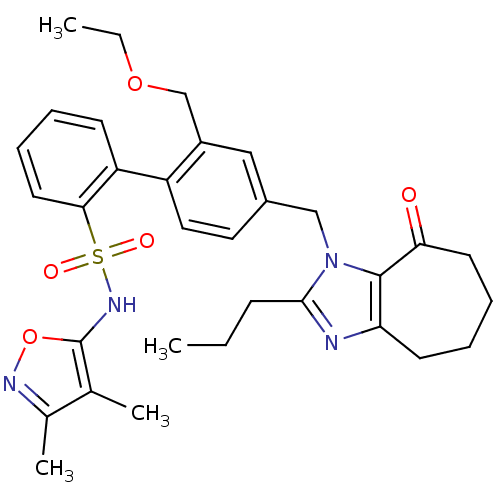 (2'-Ethoxymethyl-4'-(8-oxo-2-propyl-5,6,7,8-tetrahy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity towards Angiotensin II receptor, type 1 in rat aortic smooth muscle cells using 0.2 nM [125I]-labeled Sar-Ile-angiotensin II | Bioorg Med Chem Lett 13: 1093-6 (2003) BindingDB Entry DOI: 10.7270/Q2NG4PZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50117910 (4''-(2-Butyl-4-oxo-1,3-diaza-spiro[4.4]non-1-en-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity towards rat Angiotensin II receptor, type 1 | J Med Chem 45: 3829-35 (2002) BindingDB Entry DOI: 10.7270/Q2Z60NDM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50117910 (4''-(2-Butyl-4-oxo-1,3-diaza-spiro[4.4]non-1-en-3-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity towards Angiotensin II receptor, type 1 in rat aortic smooth muscle cells using 0.2 nM [125I]-labeled Sar-Ile-angiotensin II | Bioorg Med Chem Lett 13: 1093-6 (2003) BindingDB Entry DOI: 10.7270/Q2NG4PZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50125361 (3-[2'-(3,4-Dimethyl-isoxazol-5-ylsulfamoyl)-2-meth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity towards human ETA receptor expressed in CHO-K1 cells in the presence of 0.05 nM [125I]-labeled endothelin 1 | Bioorg Med Chem Lett 13: 1093-6 (2003) BindingDB Entry DOI: 10.7270/Q2NG4PZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50125362 (3-[2'-(3,4-Dimethyl-isoxazol-5-ylsulfamoyl)-2-ethy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity towards human ETA receptor expressed in CHO-K1 cells in the presence of 0.05 nM [125I]-labeled endothelin 1 | Bioorg Med Chem Lett 13: 1093-6 (2003) BindingDB Entry DOI: 10.7270/Q2NG4PZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50125358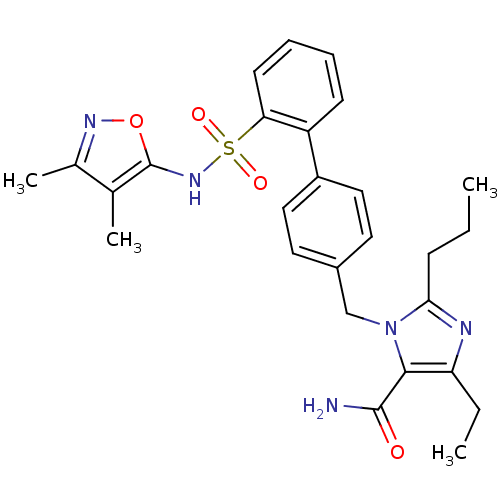 (3-[2'-(3,4-Dimethyl-isoxazol-5-ylsulfamoyl)-biphen...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity towards Angiotensin II receptor, type 1 in rat aortic smooth muscle cells using 0.2 nM [125I]-labeled Sar-Ile-angiotensin II | Bioorg Med Chem Lett 13: 1093-6 (2003) BindingDB Entry DOI: 10.7270/Q2NG4PZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 996 total ) | Next | Last >> |