 Found 27 hits with Last Name = 'valliant' and Initial = 'jf'
Found 27 hits with Last Name = 'valliant' and Initial = 'jf' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50160305
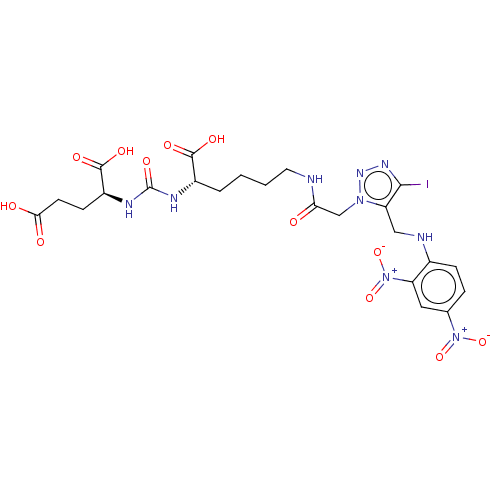
(CHEMBL3786632)Show SMILES OC(=O)CC[C@H](NC(=O)N[C@@H](CCCCNC(=O)Cn1nnc(I)c1CNc1ccc(cc1[N+]([O-])=O)[N+]([O-])=O)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C23H28IN9O12/c24-20-17(10-26-13-5-4-12(32(42)43)9-16(13)33(44)45)31(30-29-20)11-18(34)25-8-2-1-3-14(21(37)38)27-23(41)28-15(22(39)40)6-7-19(35)36/h4-5,9,14-15,26H,1-3,6-8,10-11H2,(H,25,34)(H,35,36)(H,37,38)(H,39,40)(H2,27,28,41)/t14-,15-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University
Curated by ChEMBL
| Assay Description
Displacement of [125I]23 from PSMA in human LNCAP cells |
J Med Chem 59: 2660-73 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01881
BindingDB Entry DOI: 10.7270/Q2X068XP |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50297675
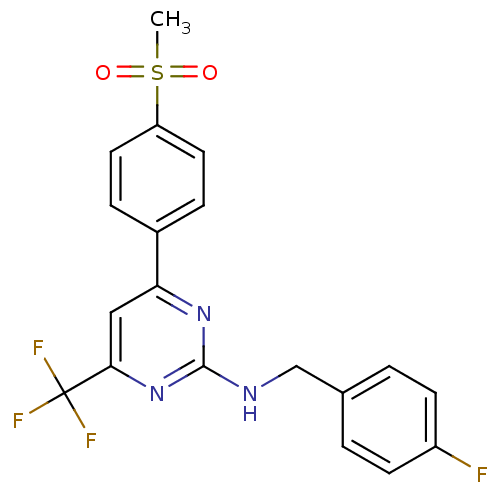
(CHEMBL551148 | N-(4-fluorobenzyl)-4-(4-(methylsulf...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1cc(nc(NCc2ccc(F)cc2)n1)C(F)(F)F Show InChI InChI=1S/C19H15F4N3O2S/c1-29(27,28)15-8-4-13(5-9-15)16-10-17(19(21,22)23)26-18(25-16)24-11-12-2-6-14(20)7-3-12/h2-10H,11H2,1H3,(H,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human COX-2 preincubated for 5 mins followed by addition of arachidonic acid as substrate measured after 2 mins by fluoresc... |
Bioorg Med Chem Lett 26: 1516-20 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.029
BindingDB Entry DOI: 10.7270/Q2377BKC |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM220398
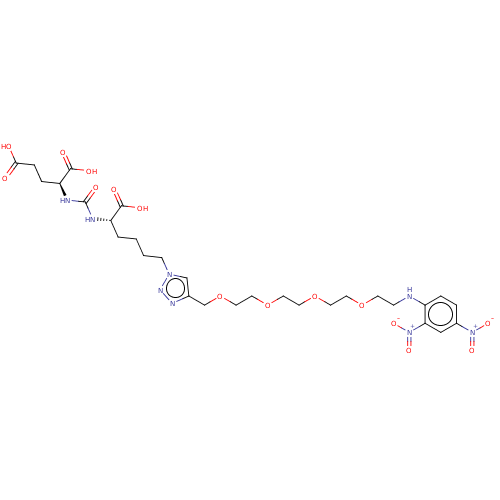
(US9296708, 4, ARM-P4)Show SMILES OC(=O)CC[C@H](NC(=O)N[C@@H](CCCCn1cc(COCCOCCOCCOCCNc2ccc(cc2[N+]([O-])=O)[N+]([O-])=O)nn1)C(O)=O)C(O)=O Show InChI InChI=1S/C29H42N8O15/c38-26(39)7-6-24(28(42)43)32-29(44)31-23(27(40)41)3-1-2-9-35-18-20(33-34-35)19-52-16-15-51-14-13-50-12-11-49-10-8-30-22-5-4-21(36(45)46)17-25(22)37(47)48/h4-5,17-18,23-24,30H,1-3,6-16,19H2,(H,38,39)(H,40,41)(H,42,43)(H2,31,32,44)/t23-,24-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University
Curated by ChEMBL
| Assay Description
Displacement of [125I]23 from PSMA in human LNCAP cells |
J Med Chem 59: 2660-73 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01881
BindingDB Entry DOI: 10.7270/Q2X068XP |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50160294
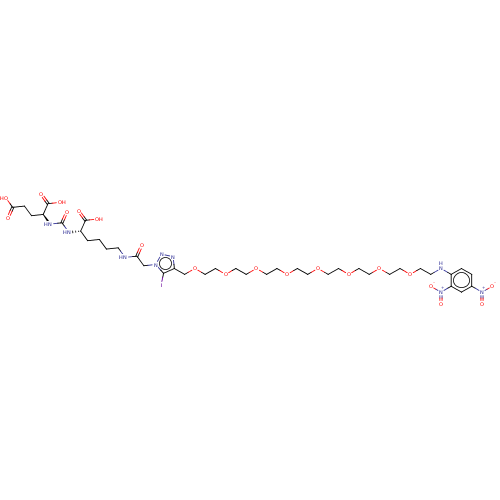
(CHEMBL3785709)Show SMILES OC(=O)CC[C@H](NC(=O)N[C@@H](CCCCNC(=O)Cn1nnc(COCCOCCOCCOCCOCCOCCOCCOCCNc2ccc(cc2[N+]([O-])=O)[N+]([O-])=O)c1I)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C39H60IN9O20/c40-36-32(45-46-47(36)26-34(50)42-8-2-1-3-30(37(53)54)43-39(57)44-31(38(55)56)6-7-35(51)52)27-69-24-23-68-22-21-67-20-19-66-18-17-65-16-15-64-14-13-63-12-11-62-10-9-41-29-5-4-28(48(58)59)25-33(29)49(60)61/h4-5,25,30-31,41H,1-3,6-24,26-27H2,(H,42,50)(H,51,52)(H,53,54)(H,55,56)(H2,43,44,57)/t30-,31-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University
Curated by ChEMBL
| Assay Description
Displacement of [125I]23 from PSMA in human LNCAP cells |
J Med Chem 59: 2660-73 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01881
BindingDB Entry DOI: 10.7270/Q2X068XP |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50160293

(CHEMBL3786463)Show SMILES OC(=O)CC[C@H](NC(=O)N[C@@H](CCCCNC(=O)Cn1nnc(COCCOCCOCCOCCNc2ccc(cc2[N+]([O-])=O)[N+]([O-])=O)c1I)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C31H44IN9O16/c32-28-24(19-57-16-15-56-14-13-55-12-11-54-10-9-33-21-5-4-20(40(50)51)17-25(21)41(52)53)37-38-39(28)18-26(42)34-8-2-1-3-22(29(45)46)35-31(49)36-23(30(47)48)6-7-27(43)44/h4-5,17,22-23,33H,1-3,6-16,18-19H2,(H,34,42)(H,43,44)(H,45,46)(H,47,48)(H2,35,36,49)/t22-,23-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University
Curated by ChEMBL
| Assay Description
Displacement of [125I]23 from PSMA in human LNCAP cells |
J Med Chem 59: 2660-73 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01881
BindingDB Entry DOI: 10.7270/Q2X068XP |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50160295
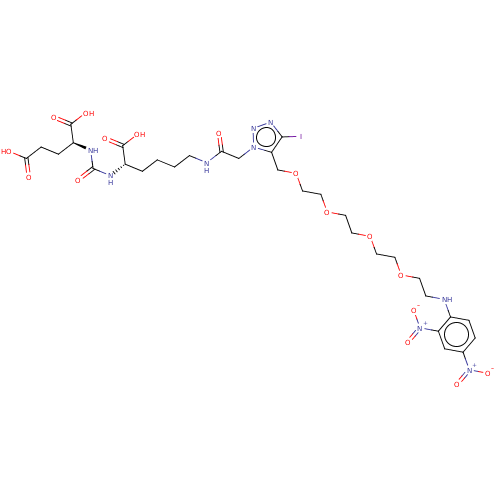
(CHEMBL3785786)Show SMILES OC(=O)CC[C@H](NC(=O)N[C@@H](CCCCNC(=O)Cn1nnc(I)c1COCCOCCOCCOCCNc1ccc(cc1[N+]([O-])=O)[N+]([O-])=O)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C31H44IN9O16/c32-28-25(19-57-16-15-56-14-13-55-12-11-54-10-9-33-21-5-4-20(40(50)51)17-24(21)41(52)53)39(38-37-28)18-26(42)34-8-2-1-3-22(29(45)46)35-31(49)36-23(30(47)48)6-7-27(43)44/h4-5,17,22-23,33H,1-3,6-16,18-19H2,(H,34,42)(H,43,44)(H,45,46)(H,47,48)(H2,35,36,49)/t22-,23-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University
Curated by ChEMBL
| Assay Description
Displacement of [125I]23 from PSMA in human LNCAP cells |
J Med Chem 59: 2660-73 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01881
BindingDB Entry DOI: 10.7270/Q2X068XP |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM220400
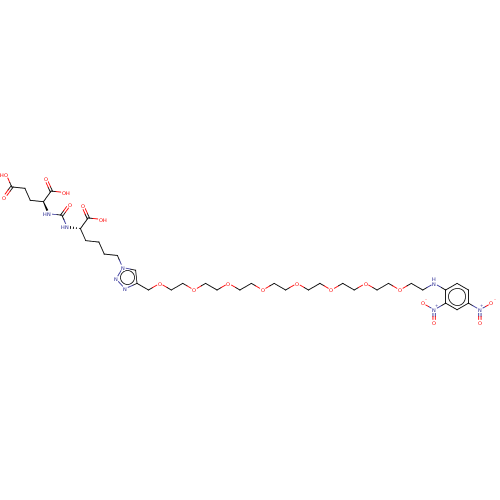
(US9296708, 6, ARM-P8)Show SMILES OC(=O)CC[C@H](NC(=O)N[C@@H](CCCCn1cc(COCCOCCOCCOCCOCCOCCOCCOCCNc2ccc(cc2[N+]([O-])=O)[N+]([O-])=O)nn1)C(O)=O)C(O)=O Show InChI InChI=1S/C37H58N8O19/c46-34(47)7-6-32(36(50)51)40-37(52)39-31(35(48)49)3-1-2-9-43-26-28(41-42-43)27-64-24-23-63-22-21-62-20-19-61-18-17-60-16-15-59-14-13-58-12-11-57-10-8-38-30-5-4-29(44(53)54)25-33(30)45(55)56/h4-5,25-26,31-32,38H,1-3,6-24,27H2,(H,46,47)(H,48,49)(H,50,51)(H2,39,40,52)/t31-,32-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University
Curated by ChEMBL
| Assay Description
Displacement of [125I]23 from PSMA in human LNCAP cells |
J Med Chem 59: 2660-73 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01881
BindingDB Entry DOI: 10.7270/Q2X068XP |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50160292

(CHEMBL3785826)Show SMILES OC(=O)CC[C@H](NC(=O)N[C@@H](CCCCn1nnc(COCCOCCOCCOCCOCCOCCOCCOCCNc2ccc(cc2[N+]([O-])=O)[N+]([O-])=O)c1I)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C37H57IN8O19/c38-34-31(42-43-44(34)9-2-1-3-29(35(49)50)40-37(53)41-30(36(51)52)6-7-33(47)48)26-65-24-23-64-22-21-63-20-19-62-18-17-61-16-15-60-14-13-59-12-11-58-10-8-39-28-5-4-27(45(54)55)25-32(28)46(56)57/h4-5,25,29-30,39H,1-3,6-24,26H2,(H,47,48)(H,49,50)(H,51,52)(H2,40,41,53)/t29-,30-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University
Curated by ChEMBL
| Assay Description
Displacement of [125I]23 from PSMA in human LNCAP cells |
J Med Chem 59: 2660-73 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01881
BindingDB Entry DOI: 10.7270/Q2X068XP |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50160291

(CHEMBL3786511)Show SMILES OC(=O)CC[C@H](NC(=O)N[C@@H](CCCCn1nnc(COCCOCCOCCOCCNc2ccc(cc2[N+]([O-])=O)[N+]([O-])=O)c1I)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C29H41IN8O15/c30-26-23(34-35-36(26)9-2-1-3-21(27(41)42)32-29(45)33-22(28(43)44)6-7-25(39)40)18-53-16-15-52-14-13-51-12-11-50-10-8-31-20-5-4-19(37(46)47)17-24(20)38(48)49/h4-5,17,21-22,31H,1-3,6-16,18H2,(H,39,40)(H,41,42)(H,43,44)(H2,32,33,45)/t21-,22-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University
Curated by ChEMBL
| Assay Description
Displacement of [125I]23 from PSMA in human LNCAP cells |
J Med Chem 59: 2660-73 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01881
BindingDB Entry DOI: 10.7270/Q2X068XP |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM11639

(4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...)Show SMILES Cc1ccc(cc1)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H14F3N3O2S/c1-11-2-4-12(5-3-11)15-10-16(17(18,19)20)22-23(15)13-6-8-14(9-7-13)26(21,24)25/h2-10H,1H3,(H2,21,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta
Curated by ChEMBL
| Assay Description
Inhibition of COX-2 (unknown origin) |
Bioorg Med Chem Lett 26: 1516-20 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.029
BindingDB Entry DOI: 10.7270/Q2377BKC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM11639

(4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...)Show SMILES Cc1ccc(cc1)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H14F3N3O2S/c1-11-2-4-12(5-3-11)15-10-16(17(18,19)20)22-23(15)13-6-8-14(9-7-13)26(21,24)25/h2-10H,1H3,(H2,21,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human COX-2 preincubated for 5 mins followed by addition of arachidonic acid as substrate measured after 2 mins by fluoresc... |
Bioorg Med Chem Lett 26: 1516-20 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.029
BindingDB Entry DOI: 10.7270/Q2377BKC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM17659

((R,S)-2-phosphonomethylpentanedioic acid | 2-(phos...)Show InChI InChI=1S/C6H11O7P/c7-5(8)2-1-4(6(9)10)3-14(11,12)13/h4H,1-3H2,(H,7,8)(H,9,10)(H2,11,12,13) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University
Curated by ChEMBL
| Assay Description
Displacement of [125I]23 from PSMA in human LNCAP cells |
J Med Chem 59: 2660-73 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01881
BindingDB Entry DOI: 10.7270/Q2X068XP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50207446
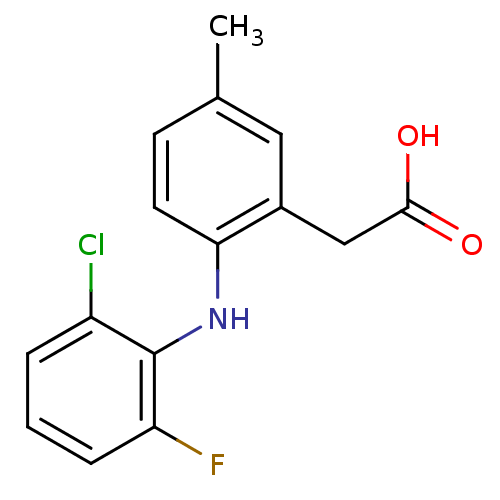
(2-(2-(2-chloro-6-fluorophenylamino)-5-methylphenyl...)Show InChI InChI=1S/C15H13ClFNO2/c1-9-5-6-13(10(7-9)8-14(19)20)18-15-11(16)3-2-4-12(15)17/h2-7,18H,8H2,1H3,(H,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta
Curated by ChEMBL
| Assay Description
Inhibition of COX-2 (unknown origin) |
Bioorg Med Chem Lett 26: 1516-20 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.029
BindingDB Entry DOI: 10.7270/Q2377BKC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50160296

(CHEMBL3786678)Show SMILES OC(=O)CC[C@H](NC(=O)N[C@@H](CCCCNC(=O)Cn1nnc(I)c1COCCOCCOCCOCCOCCOCCOCCOCCNc1ccc(cc1[N+]([O-])=O)[N+]([O-])=O)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C39H60IN9O20/c40-36-33(47(46-45-36)26-34(50)42-8-2-1-3-30(37(53)54)43-39(57)44-31(38(55)56)6-7-35(51)52)27-69-24-23-68-22-21-67-20-19-66-18-17-65-16-15-64-14-13-63-12-11-62-10-9-41-29-5-4-28(48(58)59)25-32(29)49(60)61/h4-5,25,30-31,41H,1-3,6-24,26-27H2,(H,42,50)(H,51,52)(H,53,54)(H,55,56)(H2,43,44,57)/t30-,31-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 98 | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University
Curated by ChEMBL
| Assay Description
Displacement of [125I]23 from PSMA in human LNCAP cells |
J Med Chem 59: 2660-73 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01881
BindingDB Entry DOI: 10.7270/Q2X068XP |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50160303

(CHEMBL3787537)Show SMILES NCc1c(I)nnn1CC(=O)NCCCC[C@H](NC(=O)N[C@@H](CCC(O)=O)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C17H26IN7O8/c18-14-11(7-19)25(24-23-14)8-12(26)20-6-2-1-3-9(15(29)30)21-17(33)22-10(16(31)32)4-5-13(27)28/h9-10H,1-8,19H2,(H,20,26)(H,27,28)(H,29,30)(H,31,32)(H2,21,22,33)/t9-,10-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 123 | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University
Curated by ChEMBL
| Assay Description
Displacement of [125I]23 from PSMA in human LNCAP cells |
J Med Chem 59: 2660-73 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01881
BindingDB Entry DOI: 10.7270/Q2X068XP |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50154401
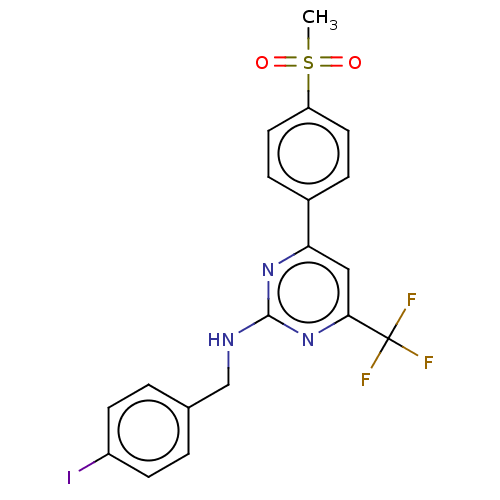
(CHEMBL3774612)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1cc(nc(NCc2ccc(I)cc2)n1)C(F)(F)F Show InChI InChI=1S/C19H15F3IN3O2S/c1-29(27,28)15-8-4-13(5-9-15)16-10-17(19(20,21)22)26-18(25-16)24-11-12-2-6-14(23)7-3-12/h2-10H,11H2,1H3,(H,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 850 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human COX-2 preincubated for 5 mins followed by addition of arachidonic acid as substrate measured after 2 mins by fluoresc... |
Bioorg Med Chem Lett 26: 1516-20 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.029
BindingDB Entry DOI: 10.7270/Q2377BKC |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50029616
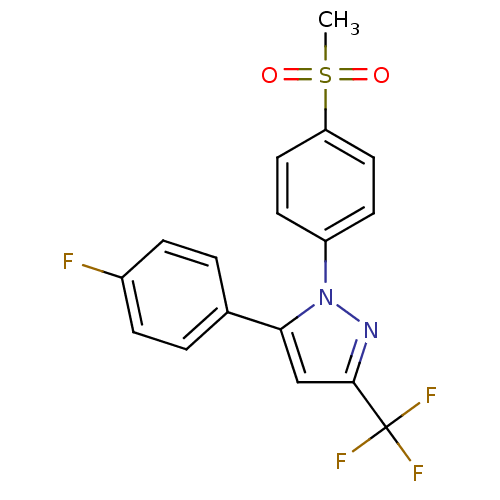
(5-(4-Fluoro-phenyl)-1-(4-methanesulfonyl-phenyl)-3...)Show SMILES CS(=O)(=O)c1ccc(cc1)-n1nc(cc1-c1ccc(F)cc1)C(F)(F)F Show InChI InChI=1S/C17H12F4N2O2S/c1-26(24,25)14-8-6-13(7-9-14)23-15(10-16(22-23)17(19,20)21)11-2-4-12(18)5-3-11/h2-10H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta
Curated by ChEMBL
| Assay Description
Inhibition of COX-2 (unknown origin) |
Bioorg Med Chem Lett 26: 1516-20 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.029
BindingDB Entry DOI: 10.7270/Q2377BKC |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50154400

(CHEMBL3774538)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1cc(nc(OCc2ccc(I)cc2)n1)C(F)(F)F Show InChI InChI=1S/C19H14F3IN2O3S/c1-29(26,27)15-8-4-13(5-9-15)16-10-17(19(20,21)22)25-18(24-16)28-11-12-2-6-14(23)7-3-12/h2-10H,11H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human COX-2 preincubated for 5 mins followed by addition of arachidonic acid as substrate measured after 2 mins by fluoresc... |
Bioorg Med Chem Lett 26: 1516-20 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.029
BindingDB Entry DOI: 10.7270/Q2377BKC |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50154412

(CHEMBL3775406)Show InChI InChI=1S/C15H13FINO2/c1-9-5-6-13(10(7-9)8-14(19)20)18-15-11(16)3-2-4-12(15)17/h2-7,18H,8H2,1H3,(H,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta
Curated by ChEMBL
| Assay Description
Inhibition of COX-2 (unknown origin) |
Bioorg Med Chem Lett 26: 1516-20 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.029
BindingDB Entry DOI: 10.7270/Q2377BKC |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50336971
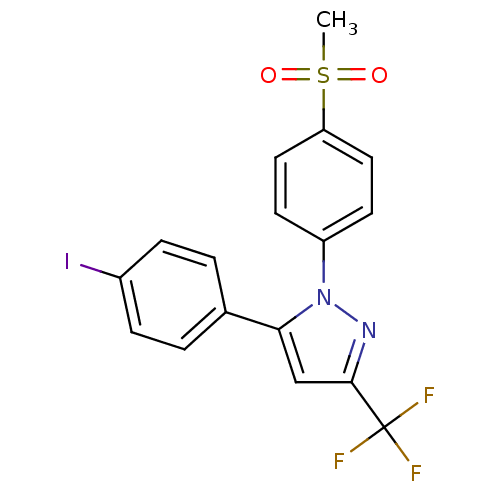
(5-(4-iodophenyl)-1-(4-(methylsulfonyl)phenyl)-3-(t...)Show SMILES CS(=O)(=O)c1ccc(cc1)-n1nc(cc1-c1ccc(I)cc1)C(F)(F)F Show InChI InChI=1S/C17H12F3IN2O2S/c1-26(24,25)14-8-6-13(7-9-14)23-15(10-16(22-23)17(18,19)20)11-2-4-12(21)5-3-11/h2-10H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta
Curated by ChEMBL
| Assay Description
Inhibition of COX-2 (unknown origin) |
Bioorg Med Chem Lett 26: 1516-20 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.029
BindingDB Entry DOI: 10.7270/Q2377BKC |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50154402
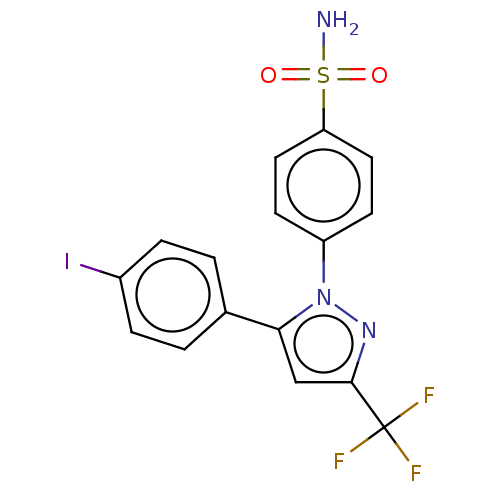
(CHEMBL3775974)Show SMILES NS(=O)(=O)c1ccc(cc1)-n1nc(cc1-c1ccc(I)cc1)C(F)(F)F Show InChI InChI=1S/C16H11F3IN3O2S/c17-16(18,19)15-9-14(10-1-3-11(20)4-2-10)23(22-15)12-5-7-13(8-6-12)26(21,24)25/h1-9H,(H2,21,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta
Curated by ChEMBL
| Assay Description
Inhibition of COX-2 (unknown origin) |
Bioorg Med Chem Lett 26: 1516-20 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.029
BindingDB Entry DOI: 10.7270/Q2377BKC |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50154399

(CHEMBL3774963)Show SMILES NS(=O)(=O)c1ccc(cc1)-c1cc(nc(NCc2ccc(I)cc2)n1)C(F)(F)F Show InChI InChI=1S/C18H14F3IN4O2S/c19-18(20,21)16-9-15(12-3-7-14(8-4-12)29(23,27)28)25-17(26-16)24-10-11-1-5-13(22)6-2-11/h1-9H,10H2,(H2,23,27,28)(H,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human COX-2 preincubated for 5 mins followed by addition of arachidonic acid as substrate measured after 2 mins by fluoresc... |
Bioorg Med Chem Lett 26: 1516-20 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.029
BindingDB Entry DOI: 10.7270/Q2377BKC |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM11639

(4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...)Show SMILES Cc1ccc(cc1)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H14F3N3O2S/c1-11-2-4-12(5-3-11)15-10-16(17(18,19)20)22-23(15)13-6-8-14(9-7-13)26(21,24)25/h2-10H,1H3,(H2,21,24,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX-1 preincubated for 5 mins followed by addition of arachidonic acid as substrate measured after 2 mins by fluorescence analysi... |
Bioorg Med Chem Lett 26: 1516-20 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.029
BindingDB Entry DOI: 10.7270/Q2377BKC |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50154401

(CHEMBL3774612)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1cc(nc(NCc2ccc(I)cc2)n1)C(F)(F)F Show InChI InChI=1S/C19H15F3IN3O2S/c1-29(27,28)15-8-4-13(5-9-15)16-10-17(19(20,21)22)26-18(25-16)24-11-12-2-6-14(23)7-3-12/h2-10H,11H2,1H3,(H,24,25,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX-1 preincubated for 5 mins followed by addition of arachidonic acid as substrate measured after 2 mins by fluorescence analysi... |
Bioorg Med Chem Lett 26: 1516-20 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.029
BindingDB Entry DOI: 10.7270/Q2377BKC |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50154400

(CHEMBL3774538)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1cc(nc(OCc2ccc(I)cc2)n1)C(F)(F)F Show InChI InChI=1S/C19H14F3IN2O3S/c1-29(26,27)15-8-4-13(5-9-15)16-10-17(19(20,21)22)25-18(24-16)28-11-12-2-6-14(23)7-3-12/h2-10H,11H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX-1 preincubated for 5 mins followed by addition of arachidonic acid as substrate measured after 2 mins by fluorescence analysi... |
Bioorg Med Chem Lett 26: 1516-20 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.029
BindingDB Entry DOI: 10.7270/Q2377BKC |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50154399

(CHEMBL3774963)Show SMILES NS(=O)(=O)c1ccc(cc1)-c1cc(nc(NCc2ccc(I)cc2)n1)C(F)(F)F Show InChI InChI=1S/C18H14F3IN4O2S/c19-18(20,21)16-9-15(12-3-7-14(8-4-12)29(23,27)28)25-17(26-16)24-10-11-1-5-13(22)6-2-11/h1-9H,10H2,(H2,23,27,28)(H,24,25,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX-1 preincubated for 5 mins followed by addition of arachidonic acid as substrate measured after 2 mins by fluorescence analysi... |
Bioorg Med Chem Lett 26: 1516-20 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.029
BindingDB Entry DOI: 10.7270/Q2377BKC |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50297675

(CHEMBL551148 | N-(4-fluorobenzyl)-4-(4-(methylsulf...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1cc(nc(NCc2ccc(F)cc2)n1)C(F)(F)F Show InChI InChI=1S/C19H15F4N3O2S/c1-29(27,28)15-8-4-13(5-9-15)16-10-17(19(21,22)23)26-18(25-16)24-11-12-2-6-14(20)7-3-12/h2-10H,11H2,1H3,(H,24,25,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX-1 preincubated for 5 mins followed by addition of arachidonic acid as substrate measured after 2 mins by fluorescence analysi... |
Bioorg Med Chem Lett 26: 1516-20 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.029
BindingDB Entry DOI: 10.7270/Q2377BKC |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data