

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Thymidine kinase 2, mitochondrial (Homo sapiens (Human)) | BDBM50314845 (3'-(4-Chlorophenyl-1,2,3-triazol-1-yl)-3'-deoxy-be...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ghent University Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant mitochondrial thymidine kinase 2 using ATP as substrate by Lineweaver-Burk plotting | J Med Chem 53: 2902-12 (2010) Article DOI: 10.1021/jm901532h BindingDB Entry DOI: 10.7270/Q26D5T47 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase 2, mitochondrial (Homo sapiens (Human)) | BDBM50314848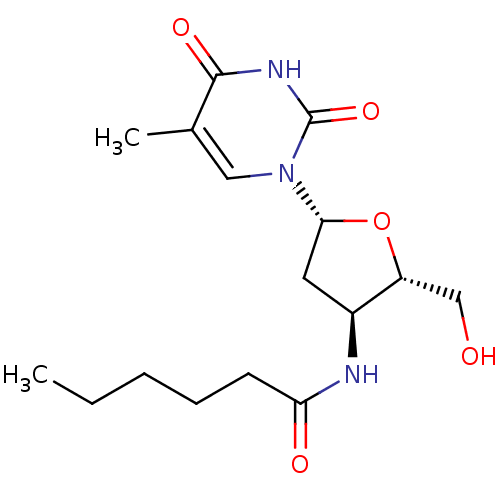 (3'-hexanoylamino-3'-deoxythymidine | CHEMBL1089836) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ghent University Curated by ChEMBL | Assay Description Inhibition of thymidine kinase 2 | J Med Chem 53: 2902-12 (2010) Article DOI: 10.1021/jm901532h BindingDB Entry DOI: 10.7270/Q26D5T47 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate kinase (Mycobacterium tuberculosis) | BDBM50359625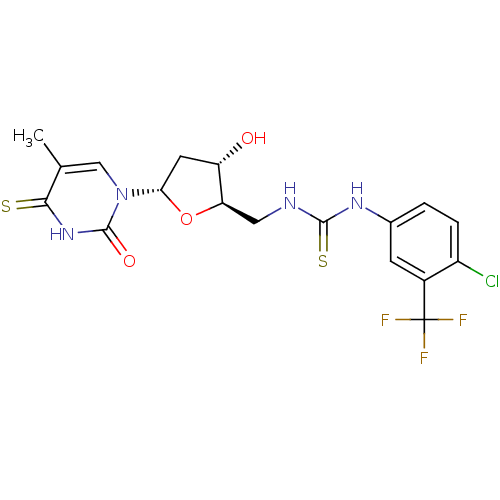 (CHEMBL1928435) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ghent University Curated by ChEMBL | Assay Description Competitive inhibition of Mycobacterium tuberculosis TMPK by Lineweaver-Burk analysis | Bioorg Med Chem 19: 7603-11 (2011) Article DOI: 10.1016/j.bmc.2011.10.021 BindingDB Entry DOI: 10.7270/Q2JH3MMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase 2, mitochondrial (Homo sapiens (Human)) | BDBM50314845 (3'-(4-Chlorophenyl-1,2,3-triazol-1-yl)-3'-deoxy-be...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ghent University Curated by ChEMBL | Assay Description Uncompetitive inhibition of human recombinant mitochondrial thymidine kinase 2 using thymidine as substrate by Lineweaver-Burk plotting | J Med Chem 53: 2902-12 (2010) Article DOI: 10.1021/jm901532h BindingDB Entry DOI: 10.7270/Q26D5T47 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate kinase (Mycobacterium tuberculosis) | BDBM50314839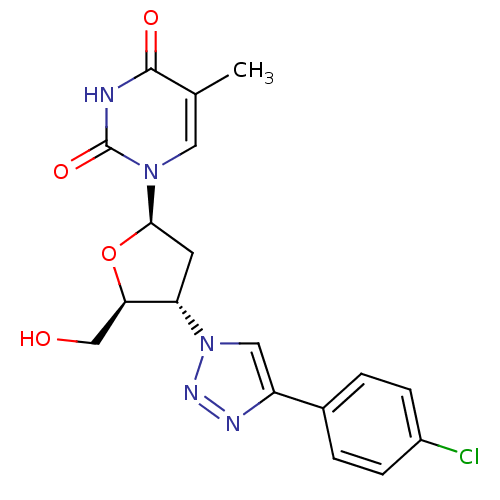 (3'-(4-Chlorophenyl-1,2,3-triazol-1-yl)-3'-deoxy-be...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ghent University Curated by ChEMBL | Assay Description Competitive inhibition of Mycobacterium tuberculosis TMPK by Lineweaver-Burk analysis | Bioorg Med Chem 19: 7603-11 (2011) Article DOI: 10.1016/j.bmc.2011.10.021 BindingDB Entry DOI: 10.7270/Q2JH3MMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate kinase (Mycobacterium tuberculosis) | BDBM50359621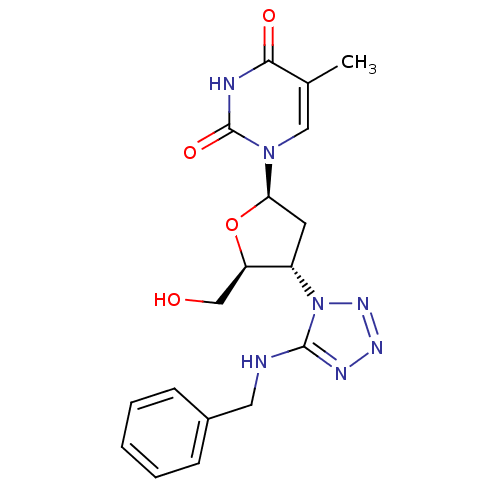 (CHEMBL1928431) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ghent University Curated by ChEMBL | Assay Description Competitive inhibition of Mycobacterium tuberculosis TMPK by Lineweaver-Burk analysis | Bioorg Med Chem 19: 7603-11 (2011) Article DOI: 10.1016/j.bmc.2011.10.021 BindingDB Entry DOI: 10.7270/Q2JH3MMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate kinase (Mycobacterium tuberculosis) | BDBM50314837 (3'-(4-Benzyl-1,2,3-triazol-1-yl)-3'-deoxy-beta-D-t...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ghent University Curated by ChEMBL | Assay Description Competitive inhibition of Mycobacterium tuberculosis TMPK by Lineweaver-Burk analysis | Bioorg Med Chem 19: 7603-11 (2011) Article DOI: 10.1016/j.bmc.2011.10.021 BindingDB Entry DOI: 10.7270/Q2JH3MMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate kinase (Mycobacterium tuberculosis) | BDBM50359618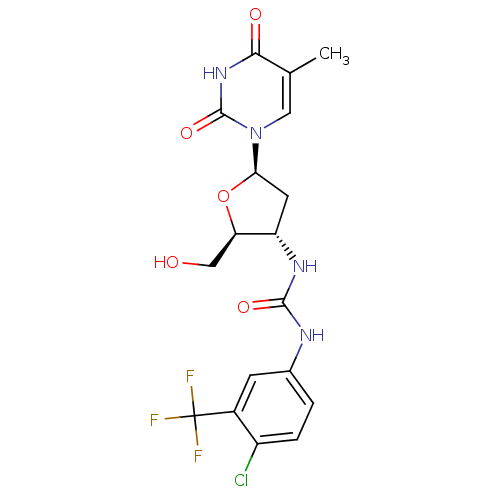 (CHEMBL1928428) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ghent University Curated by ChEMBL | Assay Description Competitive inhibition of Mycobacterium tuberculosis TMPK by Lineweaver-Burk analysis | Bioorg Med Chem 19: 7603-11 (2011) Article DOI: 10.1016/j.bmc.2011.10.021 BindingDB Entry DOI: 10.7270/Q2JH3MMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate kinase (Mycobacterium tuberculosis) | BDBM50314836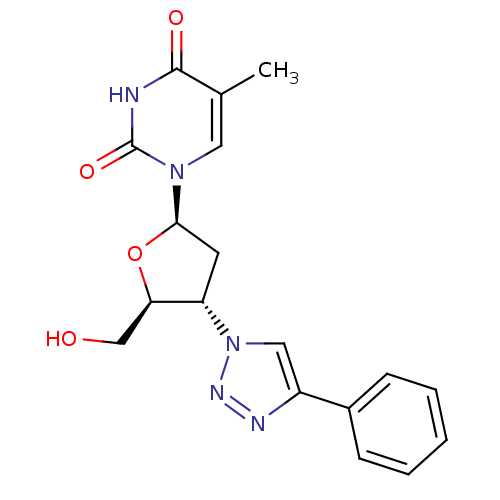 (3'-(4-Phenyl-1,2,3-triazol-1-yl)-3'-deoxythymidine...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ghent University Curated by ChEMBL | Assay Description Competitive inhibition of Mycobacterium tuberculosis TMPK by Lineweaver-Burk analysis | Bioorg Med Chem 19: 7603-11 (2011) Article DOI: 10.1016/j.bmc.2011.10.021 BindingDB Entry DOI: 10.7270/Q2JH3MMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate kinase (Mycobacterium tuberculosis) | BDBM50359627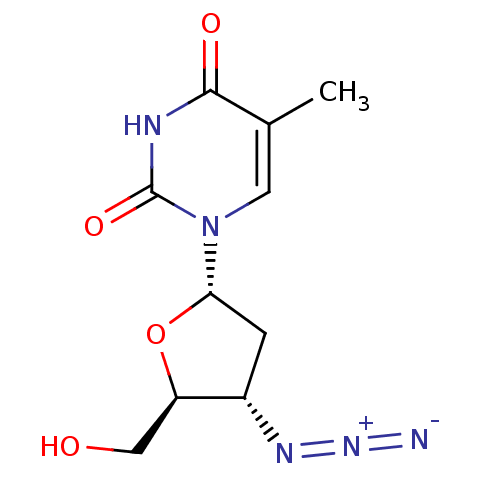 (CHEMBL1253612) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ghent University Curated by ChEMBL | Assay Description Competitive inhibition of Mycobacterium tuberculosis TMPK by Lineweaver-Burk analysis | Bioorg Med Chem 19: 7603-11 (2011) Article DOI: 10.1016/j.bmc.2011.10.021 BindingDB Entry DOI: 10.7270/Q2JH3MMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate kinase (Mycobacterium tuberculosis) | BDBM50359626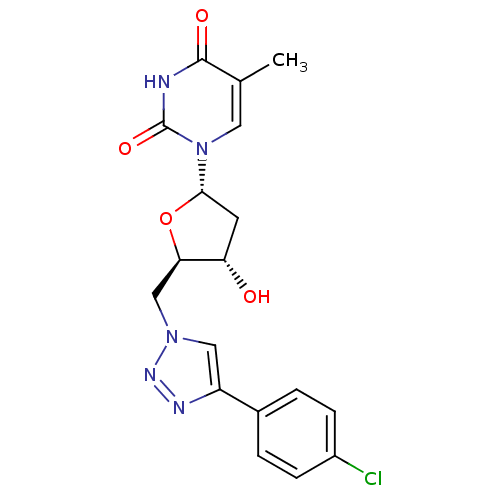 (CHEMBL1928436) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ghent University Curated by ChEMBL | Assay Description Competitive inhibition of Mycobacterium tuberculosis TMPK by Lineweaver-Burk analysis | Bioorg Med Chem 19: 7603-11 (2011) Article DOI: 10.1016/j.bmc.2011.10.021 BindingDB Entry DOI: 10.7270/Q2JH3MMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate kinase (Mycobacterium tuberculosis) | BDBM50314850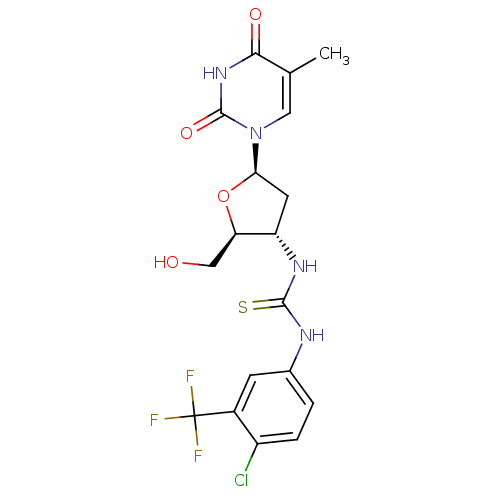 (1-(4-chloro-3-(trifluoromethyl)phenyl)-3-((2S,3S,5...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ghent University Curated by ChEMBL | Assay Description Competitive inhibition of Mycobacterium tuberculosis TMPK by Lineweaver-Burk analysis | Bioorg Med Chem 19: 7603-11 (2011) Article DOI: 10.1016/j.bmc.2011.10.021 BindingDB Entry DOI: 10.7270/Q2JH3MMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate kinase (Mycobacterium tuberculosis) | BDBM50118628 ((R)-1-((R)-4-Azido-5-methylphosphate-tetrahydro-fu...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ghent University Curated by ChEMBL | Assay Description Competitive inhibition of Mycobacterium tuberculosis TMPK expressed in Escherichia coli NM554 by coupled spectrophotometric assay | Bioorg Med Chem 19: 7603-11 (2011) Article DOI: 10.1016/j.bmc.2011.10.021 BindingDB Entry DOI: 10.7270/Q2JH3MMD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Thymidine kinase 2, mitochondrial (Homo sapiens (Human)) | BDBM50206491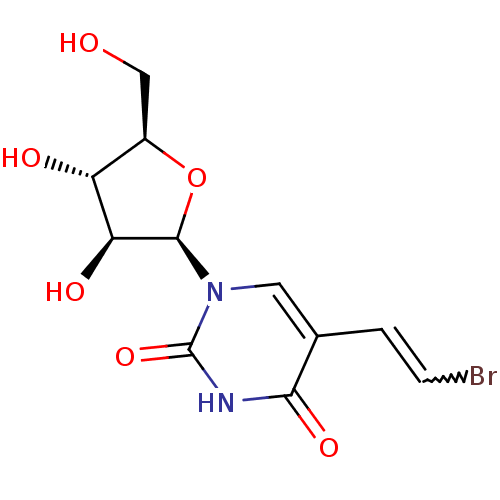 (1-beta-D-arabinofuranosyl-5-(2-bromovinyl)uracil |...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ghent University Curated by ChEMBL | Assay Description Inhibition of thymidine kinase 2 | J Med Chem 53: 2902-12 (2010) Article DOI: 10.1021/jm901532h BindingDB Entry DOI: 10.7270/Q26D5T47 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate kinase (Mycobacterium tuberculosis) | BDBM50359622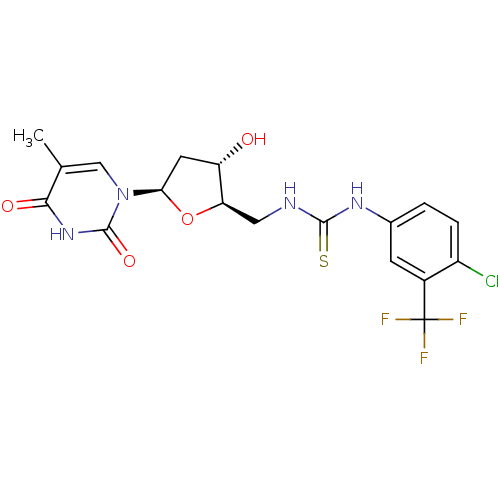 (CHEMBL1928432) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 1.45E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ghent University Curated by ChEMBL | Assay Description Competitive inhibition of Mycobacterium tuberculosis TMPK by Lineweaver-Burk analysis | Bioorg Med Chem 19: 7603-11 (2011) Article DOI: 10.1016/j.bmc.2011.10.021 BindingDB Entry DOI: 10.7270/Q2JH3MMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate kinase (Mycobacterium tuberculosis) | BDBM50359619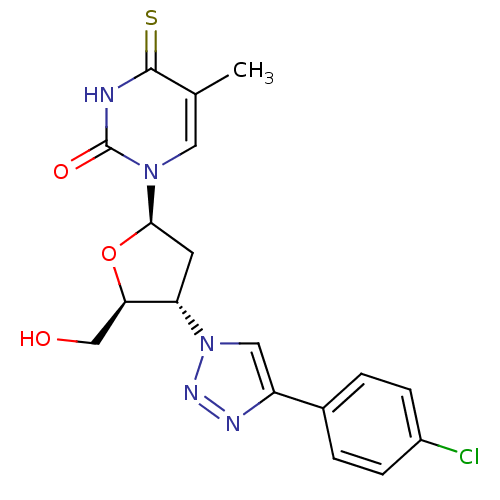 (CHEMBL1928429) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ghent University Curated by ChEMBL | Assay Description Competitive inhibition of Mycobacterium tuberculosis TMPK by Lineweaver-Burk analysis | Bioorg Med Chem 19: 7603-11 (2011) Article DOI: 10.1016/j.bmc.2011.10.021 BindingDB Entry DOI: 10.7270/Q2JH3MMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate kinase (Mycobacterium tuberculosis) | BDBM50223817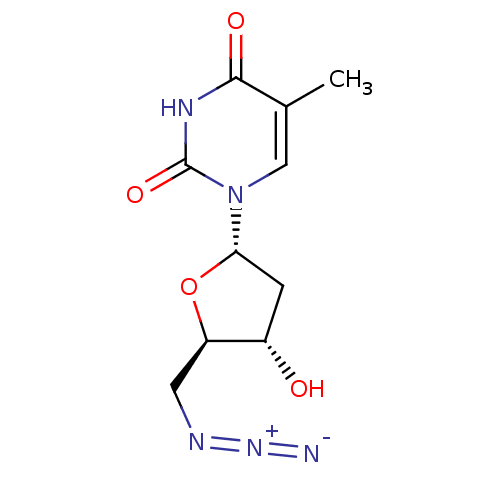 (5'-azido-5'-deoxy-alpha-D-thymidine | CHEMBL235305) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.65E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ghent University Curated by ChEMBL | Assay Description Competitive inhibition of Mycobacterium tuberculosis TMPK by Lineweaver-Burk analysis | Bioorg Med Chem 19: 7603-11 (2011) Article DOI: 10.1016/j.bmc.2011.10.021 BindingDB Entry DOI: 10.7270/Q2JH3MMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate kinase (Mycobacterium tuberculosis) | BDBM50002692 ((AZT) 1-(4-Azido-5-hydroxymethyl-tetrahydro-furan-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ghent University Curated by ChEMBL | Assay Description Competitive inhibition of Mycobacterium tuberculosis TMPK by Lineweaver-Burk analysis | Bioorg Med Chem 19: 7603-11 (2011) Article DOI: 10.1016/j.bmc.2011.10.021 BindingDB Entry DOI: 10.7270/Q2JH3MMD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Thymidylate kinase (Mycobacterium tuberculosis) | BDBM50359628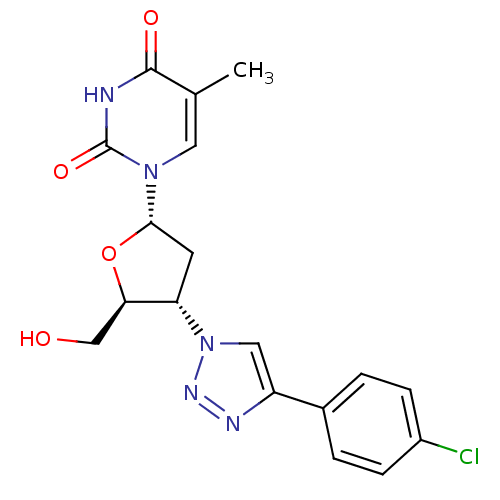 (CHEMBL1928437) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ghent University Curated by ChEMBL | Assay Description Competitive inhibition of Mycobacterium tuberculosis TMPK by Lineweaver-Burk analysis | Bioorg Med Chem 19: 7603-11 (2011) Article DOI: 10.1016/j.bmc.2011.10.021 BindingDB Entry DOI: 10.7270/Q2JH3MMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate kinase (Mycobacterium tuberculosis) | BDBM50359620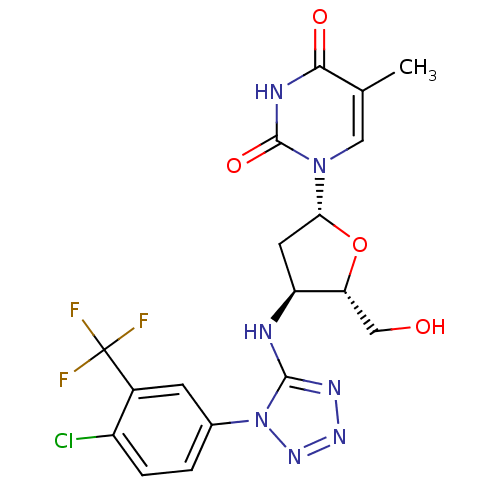 (CHEMBL1928430) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ghent University Curated by ChEMBL | Assay Description Competitive inhibition of Mycobacterium tuberculosis TMPK by Lineweaver-Burk analysis | Bioorg Med Chem 19: 7603-11 (2011) Article DOI: 10.1016/j.bmc.2011.10.021 BindingDB Entry DOI: 10.7270/Q2JH3MMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate kinase (Mycobacterium tuberculosis) | BDBM50359623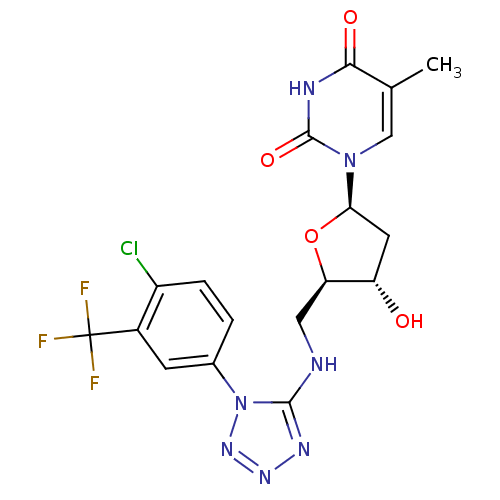 (CHEMBL1928433) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ghent University Curated by ChEMBL | Assay Description Competitive inhibition of Mycobacterium tuberculosis TMPK by Lineweaver-Burk analysis | Bioorg Med Chem 19: 7603-11 (2011) Article DOI: 10.1016/j.bmc.2011.10.021 BindingDB Entry DOI: 10.7270/Q2JH3MMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate kinase (Homo sapiens (Human)) | BDBM50359618 (CHEMBL1928428) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ghent University Curated by ChEMBL | Assay Description Competitive inhibition of human TMPK by Lineweaver-Burk analysis | Bioorg Med Chem 19: 7603-11 (2011) Article DOI: 10.1016/j.bmc.2011.10.021 BindingDB Entry DOI: 10.7270/Q2JH3MMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate kinase (Mycobacterium tuberculosis) | BDBM50359624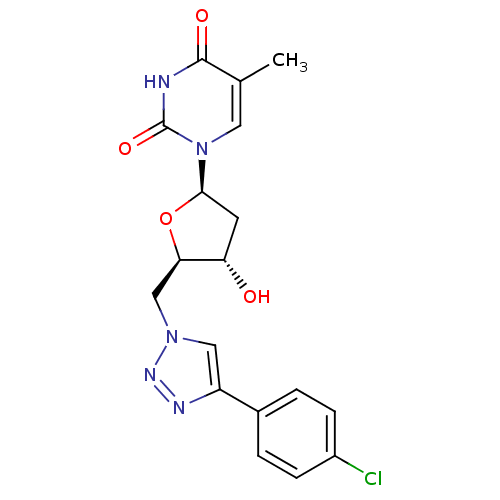 (CHEMBL1926703) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.01E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ghent University Curated by ChEMBL | Assay Description Competitive inhibition of Mycobacterium tuberculosis TMPK by Lineweaver-Burk analysis | Bioorg Med Chem 19: 7603-11 (2011) Article DOI: 10.1016/j.bmc.2011.10.021 BindingDB Entry DOI: 10.7270/Q2JH3MMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1-deoxy-D-xylulose 5-phosphate reductoisomerase (Escherichia coli) | BDBM50153713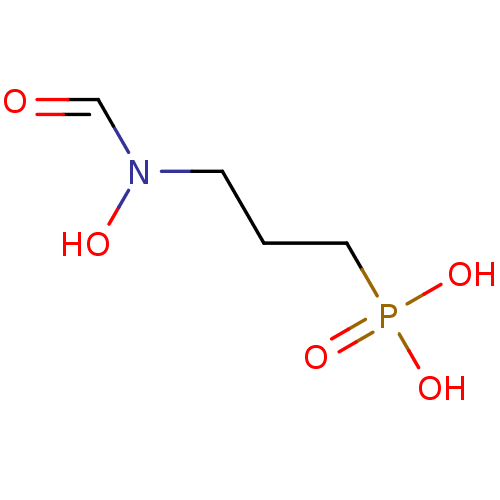 (3-(N-hydroxyformamido)propylphosphonic acid | 3-[F...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Ghent University Curated by ChEMBL | Assay Description Inhibition of recombinant Escherichia coli DXR | Bioorg Med Chem Lett 16: 1888-91 (2006) Article DOI: 10.1016/j.bmcl.2005.12.082 BindingDB Entry DOI: 10.7270/Q2FJ2GB6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 1-deoxy-D-xylulose 5-phosphate reductoisomerase (Escherichia coli) | BDBM50181153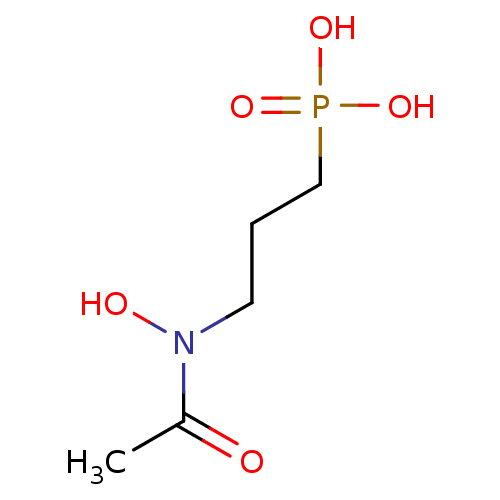 (3-(N-hydroxyacetamido)propylphosphonic acid | 3-(N...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Ghent University Curated by ChEMBL | Assay Description Inhibition of recombinant Escherichia coli DXR | Bioorg Med Chem Lett 16: 1888-91 (2006) Article DOI: 10.1016/j.bmcl.2005.12.082 BindingDB Entry DOI: 10.7270/Q2FJ2GB6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Thymidine kinase 2, mitochondrial (Homo sapiens (Human)) | BDBM50314845 (3'-(4-Chlorophenyl-1,2,3-triazol-1-yl)-3'-deoxy-be...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Ghent University Curated by ChEMBL | Assay Description Inhibition of human recombinant mitochondrial thymidine kinase 2 assessed as inhibition of [methyl-3H]dThd phosphorylation after 30 mins by scintilla... | J Med Chem 53: 2902-12 (2010) Article DOI: 10.1021/jm901532h BindingDB Entry DOI: 10.7270/Q26D5T47 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase 2, mitochondrial (Homo sapiens (Human)) | BDBM50314840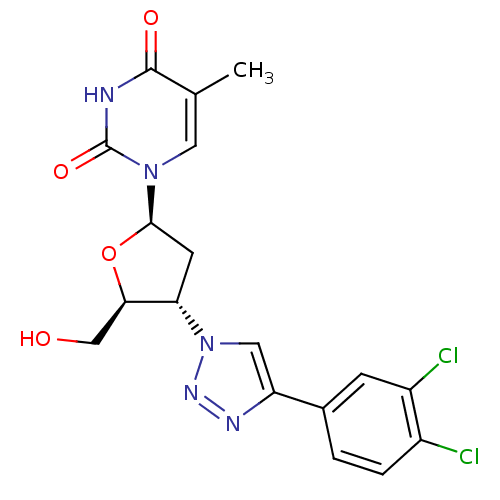 (3'-Deoxy-3'-(4-(3,4-dichlorophenyl)-1,2,3-triazol-...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Ghent University Curated by ChEMBL | Assay Description Inhibition of human recombinant mitochondrial thymidine kinase 2 assessed as inhibition of [methyl-3H]dThd phosphorylation after 30 mins by scintilla... | J Med Chem 53: 2902-12 (2010) Article DOI: 10.1021/jm901532h BindingDB Entry DOI: 10.7270/Q26D5T47 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase 2, mitochondrial (Homo sapiens (Human)) | BDBM50314839 (3'-(4-Chlorophenyl-1,2,3-triazol-1-yl)-3'-deoxy-be...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Ghent University Curated by ChEMBL | Assay Description Inhibition of human recombinant mitochondrial thymidine kinase 2 assessed as inhibition of [methyl-3H]dThd phosphorylation after 30 mins by scintilla... | J Med Chem 53: 2902-12 (2010) Article DOI: 10.1021/jm901532h BindingDB Entry DOI: 10.7270/Q26D5T47 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1-deoxy-D-xylulose 5-phosphate reductoisomerase (Escherichia coli) | BDBM50181154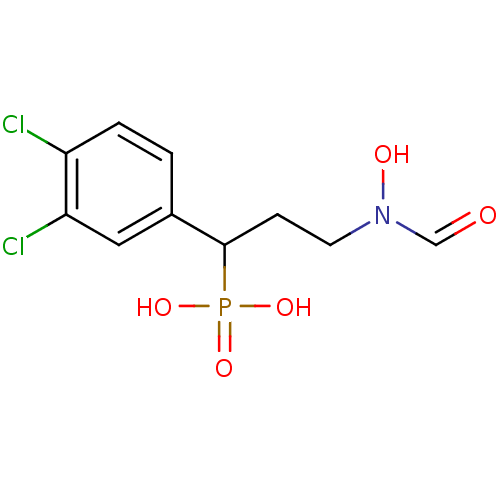 (1-(3,4-dichlorophenyl)-3-(N-hydroxyformamido)propy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
Ghent University Curated by ChEMBL | Assay Description Inhibition of recombinant Escherichia coli DXR | Bioorg Med Chem Lett 16: 1888-91 (2006) Article DOI: 10.1016/j.bmcl.2005.12.082 BindingDB Entry DOI: 10.7270/Q2FJ2GB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1-deoxy-D-xylulose 5-phosphate reductoisomerase (Escherichia coli) | BDBM50181152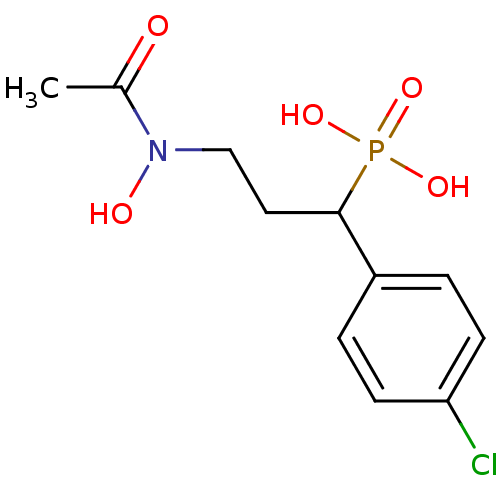 (1-(4-chlorophenyl)-3-(N-hydroxyacetamido)propylpho...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 99 | n/a | n/a | n/a | n/a | n/a | n/a |
Ghent University Curated by ChEMBL | Assay Description Inhibition of recombinant Escherichia coli DXR | Bioorg Med Chem Lett 16: 1888-91 (2006) Article DOI: 10.1016/j.bmcl.2005.12.082 BindingDB Entry DOI: 10.7270/Q2FJ2GB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1-deoxy-D-xylulose 5-phosphate reductoisomerase (Escherichia coli) | BDBM50181148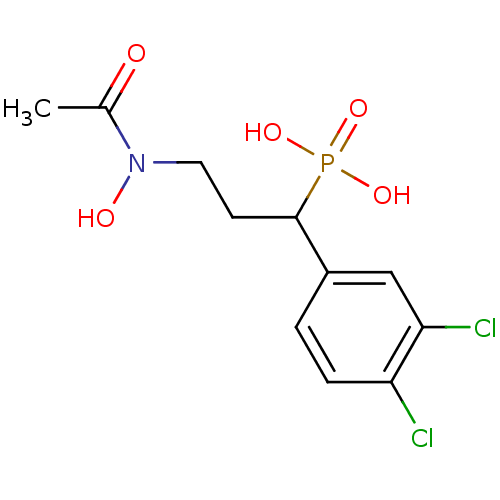 (1-(3,4-dichlorophenyl)-3-(N-hydroxyacetamido)propy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 119 | n/a | n/a | n/a | n/a | n/a | n/a |
Ghent University Curated by ChEMBL | Assay Description Inhibition of recombinant Escherichia coli DXR | Bioorg Med Chem Lett 16: 1888-91 (2006) Article DOI: 10.1016/j.bmcl.2005.12.082 BindingDB Entry DOI: 10.7270/Q2FJ2GB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase 2, mitochondrial (Homo sapiens (Human)) | BDBM50314838 (3'-(4-Cyclopentylmethyl-1,2,3-triazol-1-yl)-3'-deo...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Ghent University Curated by ChEMBL | Assay Description Inhibition of human recombinant mitochondrial thymidine kinase 2 assessed as inhibition of [methyl-3H]dThd phosphorylation after 30 mins by scintilla... | J Med Chem 53: 2902-12 (2010) Article DOI: 10.1021/jm901532h BindingDB Entry DOI: 10.7270/Q26D5T47 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase 2, mitochondrial (Homo sapiens (Human)) | BDBM50314850 (1-(4-chloro-3-(trifluoromethyl)phenyl)-3-((2S,3S,5...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Ghent University Curated by ChEMBL | Assay Description Inhibition of human recombinant mitochondrial thymidine kinase 2 assessed as inhibition of [methyl-3H]dThd phosphorylation after 30 mins by scintilla... | J Med Chem 53: 2902-12 (2010) Article DOI: 10.1021/jm901532h BindingDB Entry DOI: 10.7270/Q26D5T47 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1-deoxy-D-xylulose 5-phosphate reductoisomerase (Escherichia coli) | BDBM50181150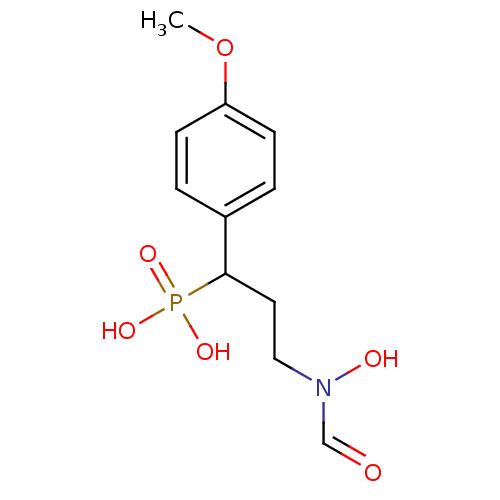 (3-(N-hydroxyformamido)-1-(4-methoxyphenyl)propylph...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 156 | n/a | n/a | n/a | n/a | n/a | n/a |
Ghent University Curated by ChEMBL | Assay Description Inhibition of recombinant Escherichia coli DXR | Bioorg Med Chem Lett 16: 1888-91 (2006) Article DOI: 10.1016/j.bmcl.2005.12.082 BindingDB Entry DOI: 10.7270/Q2FJ2GB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase 2, mitochondrial (Homo sapiens (Human)) | BDBM50314835 (3'-(4-Butyl-1,2,3-triazol-1-yl)-3'-deoxy-beta-D-th...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Ghent University Curated by ChEMBL | Assay Description Inhibition of human recombinant mitochondrial thymidine kinase 2 assessed as inhibition of [methyl-3H]dThd phosphorylation after 30 mins by scintilla... | J Med Chem 53: 2902-12 (2010) Article DOI: 10.1021/jm901532h BindingDB Entry DOI: 10.7270/Q26D5T47 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase 2, mitochondrial (Homo sapiens (Human)) | BDBM50314844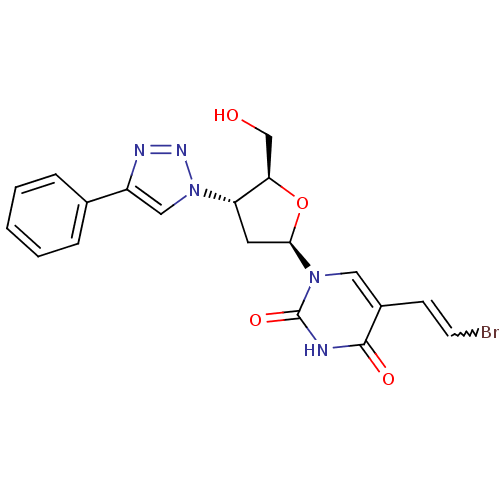 (3'-Deoxy-3'-(4-phenyl-1,2,3-triazol-1-yl)-beta-(E)...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Ghent University Curated by ChEMBL | Assay Description Inhibition of human recombinant mitochondrial thymidine kinase 2 assessed as inhibition of [methyl-3H]dThd phosphorylation after 30 mins by scintilla... | J Med Chem 53: 2902-12 (2010) Article DOI: 10.1021/jm901532h BindingDB Entry DOI: 10.7270/Q26D5T47 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase 2, mitochondrial (Homo sapiens (Human)) | BDBM50314837 (3'-(4-Benzyl-1,2,3-triazol-1-yl)-3'-deoxy-beta-D-t...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Ghent University Curated by ChEMBL | Assay Description Inhibition of human recombinant mitochondrial thymidine kinase 2 assessed as inhibition of [methyl-3H]dThd phosphorylation after 30 mins by scintilla... | J Med Chem 53: 2902-12 (2010) Article DOI: 10.1021/jm901532h BindingDB Entry DOI: 10.7270/Q26D5T47 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1-deoxy-D-xylulose 5-phosphate reductoisomerase (Escherichia coli) | BDBM50181147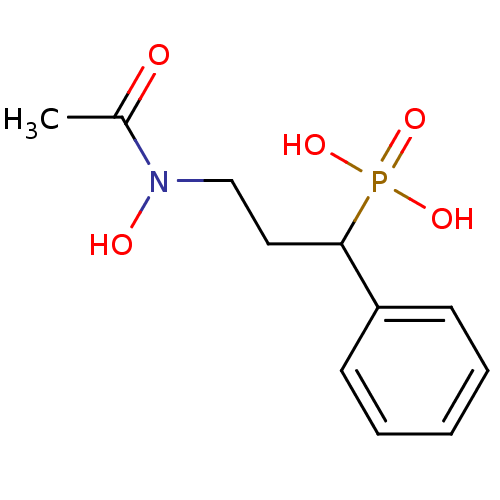 (3-(N-hydroxyacetamido)-1-phenylpropylphosphonic ac...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 311 | n/a | n/a | n/a | n/a | n/a | n/a |
Ghent University Curated by ChEMBL | Assay Description Inhibition of recombinant Escherichia coli DXR | Bioorg Med Chem Lett 16: 1888-91 (2006) Article DOI: 10.1016/j.bmcl.2005.12.082 BindingDB Entry DOI: 10.7270/Q2FJ2GB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase 2, mitochondrial (Homo sapiens (Human)) | BDBM50314836 (3'-(4-Phenyl-1,2,3-triazol-1-yl)-3'-deoxythymidine...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Ghent University Curated by ChEMBL | Assay Description Inhibition of human recombinant mitochondrial thymidine kinase 2 assessed as inhibition of [methyl-3H]dThd phosphorylation after 30 mins by scintilla... | J Med Chem 53: 2902-12 (2010) Article DOI: 10.1021/jm901532h BindingDB Entry DOI: 10.7270/Q26D5T47 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1-deoxy-D-xylulose 5-phosphate reductoisomerase (Escherichia coli) | BDBM50181149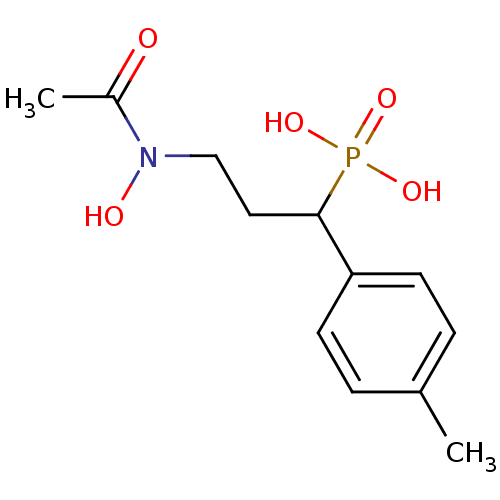 (3-(N-hydroxyacetamido)-1-p-tolylpropylphosphonic a...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 396 | n/a | n/a | n/a | n/a | n/a | n/a |
Ghent University Curated by ChEMBL | Assay Description Inhibition of recombinant Escherichia coli DXR | Bioorg Med Chem Lett 16: 1888-91 (2006) Article DOI: 10.1016/j.bmcl.2005.12.082 BindingDB Entry DOI: 10.7270/Q2FJ2GB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase 2, mitochondrial (Homo sapiens (Human)) | BDBM50314849 (CHEMBL1091872 | N-methyl-4-((8-(5-methyl-2,4-dioxo...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Ghent University Curated by ChEMBL | Assay Description Inhibition of thymidine kinase 2 | J Med Chem 53: 2902-12 (2010) Article DOI: 10.1021/jm901532h BindingDB Entry DOI: 10.7270/Q26D5T47 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1-deoxy-D-xylulose 5-phosphate reductoisomerase (Escherichia coli) | BDBM50181151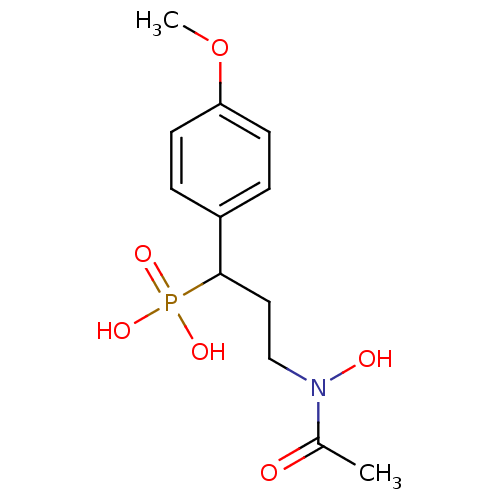 (3-(N-hydroxyacetamido)-1-(4-methoxyphenyl)propylph...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 459 | n/a | n/a | n/a | n/a | n/a | n/a |
Ghent University Curated by ChEMBL | Assay Description Inhibition of recombinant Escherichia coli DXR | Bioorg Med Chem Lett 16: 1888-91 (2006) Article DOI: 10.1016/j.bmcl.2005.12.082 BindingDB Entry DOI: 10.7270/Q2FJ2GB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Deoxynucleoside kinase (Drosophila melanogaster) | BDBM50314845 (3'-(4-Chlorophenyl-1,2,3-triazol-1-yl)-3'-deoxy-be...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
Ghent University Curated by ChEMBL | Assay Description Inhibition of Drosophila melanogaster multifunctional deoxynucleoside kinase assessed as inhibition of [methyl-3H]dThd phosphorylation after 30 mins ... | J Med Chem 53: 2902-12 (2010) Article DOI: 10.1021/jm901532h BindingDB Entry DOI: 10.7270/Q26D5T47 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Deoxynucleoside kinase (Drosophila melanogaster) | BDBM50314840 (3'-Deoxy-3'-(4-(3,4-dichlorophenyl)-1,2,3-triazol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 990 | n/a | n/a | n/a | n/a | n/a | n/a |
Ghent University Curated by ChEMBL | Assay Description Inhibition of Drosophila melanogaster multifunctional deoxynucleoside kinase assessed as inhibition of [methyl-3H]dThd phosphorylation after 30 mins ... | J Med Chem 53: 2902-12 (2010) Article DOI: 10.1021/jm901532h BindingDB Entry DOI: 10.7270/Q26D5T47 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase 2, mitochondrial (Homo sapiens (Human)) | BDBM50314842 (3'-Deoxy-3'-(5-phenyl-1,2,3-triazol-1-yl)-beta-D-t...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ghent University Curated by ChEMBL | Assay Description Inhibition of human recombinant mitochondrial thymidine kinase 2 assessed as inhibition of [methyl-3H]dThd phosphorylation after 30 mins by scintilla... | J Med Chem 53: 2902-12 (2010) Article DOI: 10.1021/jm901532h BindingDB Entry DOI: 10.7270/Q26D5T47 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase 2, mitochondrial (Homo sapiens (Human)) | BDBM50314841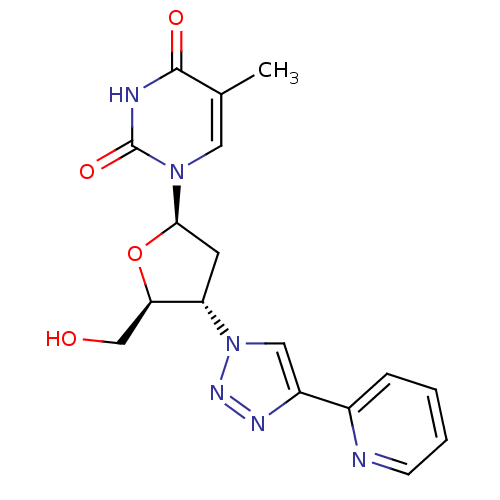 (3'-(4-(2-Pyridine)-1,2,3-triazole-1-yl)-2'-deoxyth...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ghent University Curated by ChEMBL | Assay Description Inhibition of human recombinant mitochondrial thymidine kinase 2 assessed as inhibition of [methyl-3H]dThd phosphorylation after 30 mins by scintilla... | J Med Chem 53: 2902-12 (2010) Article DOI: 10.1021/jm901532h BindingDB Entry DOI: 10.7270/Q26D5T47 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase 2, mitochondrial (Homo sapiens (Human)) | BDBM50118490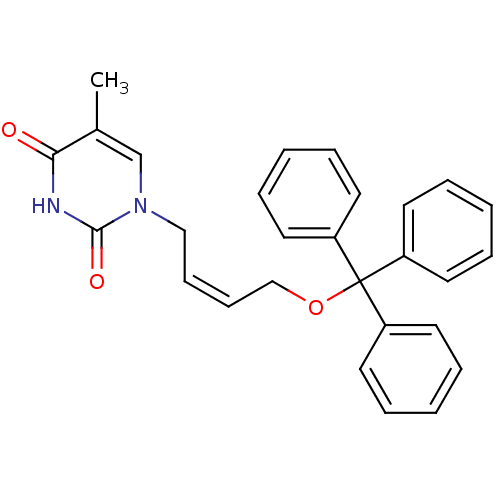 (1-[(Z)-4-(triphenylmethoxy)-2-butenyl]thymine | 5-...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ghent University Curated by ChEMBL | Assay Description Inhibition of thymidine kinase 2 | J Med Chem 53: 2902-12 (2010) Article DOI: 10.1021/jm901532h BindingDB Entry DOI: 10.7270/Q26D5T47 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Deoxynucleoside kinase (Drosophila melanogaster) | BDBM50314839 (3'-(4-Chlorophenyl-1,2,3-triazol-1-yl)-3'-deoxy-be...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ghent University Curated by ChEMBL | Assay Description Inhibition of Drosophila melanogaster multifunctional deoxynucleoside kinase assessed as inhibition of [methyl-3H]dThd phosphorylation after 30 mins ... | J Med Chem 53: 2902-12 (2010) Article DOI: 10.1021/jm901532h BindingDB Entry DOI: 10.7270/Q26D5T47 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Deoxynucleoside kinase (Drosophila melanogaster) | BDBM50314836 (3'-(4-Phenyl-1,2,3-triazol-1-yl)-3'-deoxythymidine...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ghent University Curated by ChEMBL | Assay Description Inhibition of Drosophila melanogaster multifunctional deoxynucleoside kinase assessed as inhibition of [methyl-3H]dThd phosphorylation after 30 mins ... | J Med Chem 53: 2902-12 (2010) Article DOI: 10.1021/jm901532h BindingDB Entry DOI: 10.7270/Q26D5T47 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Deoxynucleoside kinase (Drosophila melanogaster) | BDBM50314844 (3'-Deoxy-3'-(4-phenyl-1,2,3-triazol-1-yl)-beta-(E)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ghent University Curated by ChEMBL | Assay Description Inhibition of Drosophila melanogaster multifunctional deoxynucleoside kinase assessed as inhibition of [methyl-3H]dThd phosphorylation after 30 mins ... | J Med Chem 53: 2902-12 (2010) Article DOI: 10.1021/jm901532h BindingDB Entry DOI: 10.7270/Q26D5T47 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 101 total ) | Next | Last >> |