 Found 610 hits with Last Name = 'van den eynde' and Initial = 'b'
Found 610 hits with Last Name = 'van den eynde' and Initial = 'b' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM82247

(8-Chloro-3-methyl-5-phenyl-2,3,4,5-tetrahydro-1H-b...)Show InChI InChI=1S/C17H18ClNO/c1-19-8-7-13-9-16(18)17(20)10-14(13)15(11-19)12-5-3-2-4-6-12/h2-6,9-10,15,20H,7-8,11H2,1H3/t15-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Namur
Curated by ChEMBL
| Assay Description
Displacement of [3H]SCH23390 from human dopamine D1 receptor |
J Med Chem 54: 5320-34 (2011)
Article DOI: 10.1021/jm2006782
BindingDB Entry DOI: 10.7270/Q2RF5W11 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM10755

(14C-5-hydroxy tryptamine creatinine disulfate | 2-...)Show InChI InChI=1S/C10H12N2O/c11-4-3-7-6-12-10-2-1-8(13)5-9(7)10/h1-2,5-6,12-13H,3-4,11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Namur
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT7 receptor |
J Med Chem 54: 5320-34 (2011)
Article DOI: 10.1021/jm2006782
BindingDB Entry DOI: 10.7270/Q2RF5W11 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM21393

(7-(dipropylamino)-5,6,7,8-tetrahydronaphthalen-1-o...)Show InChI InChI=1S/C16H25NO/c1-3-10-17(11-4-2)14-9-8-13-6-5-7-16(18)15(13)12-14/h5-7,14,18H,3-4,8-12H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Namur
Curated by ChEMBL
| Assay Description
Displacement of [3H]8-OH-DPAT from human 5HT1A receptor |
J Med Chem 54: 5320-34 (2011)
Article DOI: 10.1021/jm2006782
BindingDB Entry DOI: 10.7270/Q2RF5W11 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM21395

(3-(2-(4-(4-Fluorobenzoyl)piperidinol)ethyl)-2,4(1H...)Show SMILES Fc1ccc(cc1)C(=O)C1CCN(CCn2c(=O)[nH]c3ccccc3c2=O)CC1 Show InChI InChI=1S/C22H22FN3O3/c23-17-7-5-15(6-8-17)20(27)16-9-11-25(12-10-16)13-14-26-21(28)18-3-1-2-4-19(18)24-22(26)29/h1-8,16H,9-14H2,(H,24,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Namur
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from human 5HT2A receptor |
J Med Chem 54: 5320-34 (2011)
Article DOI: 10.1021/jm2006782
BindingDB Entry DOI: 10.7270/Q2RF5W11 |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1A
(Homo sapiens (Human)) | BDBM9019

(CHEMBL45 | Melatonin | N-[2-(5-methoxy-1H-indol-3-...)Show InChI InChI=1S/C13H16N2O2/c1-9(16)14-6-5-10-8-15-13-4-3-11(17-2)7-12(10)13/h3-4,7-8,15H,5-6H2,1-2H3,(H,14,16) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Namur
Curated by ChEMBL
| Assay Description
Displacement of [125I]2-iodomelatonin from human MT1 receptor |
J Med Chem 54: 5320-34 (2011)
Article DOI: 10.1021/jm2006782
BindingDB Entry DOI: 10.7270/Q2RF5W11 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50366495

((+)butaclamol | CHEMBL1255588)Show SMILES CC(C)(C)[C@@]1(O)CCN2C[C@@H]3c4ccccc4CCc4cccc([C@H]2C1)c34 |r| Show InChI InChI=1S/C25H31NO/c1-24(2,3)25(27)13-14-26-16-21-19-9-5-4-7-17(19)11-12-18-8-6-10-20(23(18)21)22(26)15-25/h4-10,21-22,27H,11-16H2,1-3H3/t21-,22-,25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Namur
Curated by ChEMBL
| Assay Description
Displacement of [3H]methyl-spiperone from human dopamine D2s receptor |
J Med Chem 54: 5320-34 (2011)
Article DOI: 10.1021/jm2006782
BindingDB Entry DOI: 10.7270/Q2RF5W11 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50010859
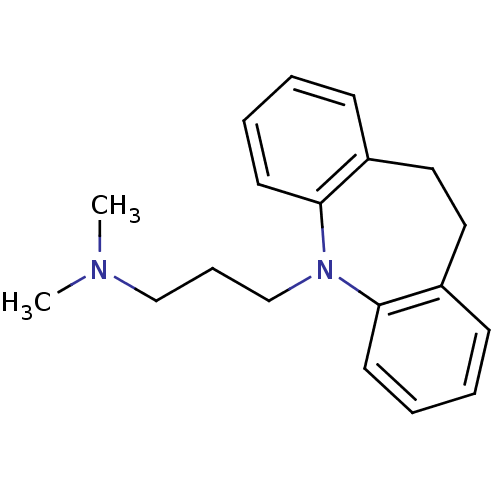
(CHEMBL11 | IMIPRAMINE HYDROCHLORIDE | IMIPRAMINE P...)Show InChI InChI=1S/C19H24N2/c1-20(2)14-7-15-21-18-10-5-3-8-16(18)12-13-17-9-4-6-11-19(17)21/h3-6,8-11H,7,12-15H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 0.770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Namur
Curated by ChEMBL
| Assay Description
Displacement of [3H]imipramine from human 5HT transporter |
J Med Chem 54: 5320-34 (2011)
Article DOI: 10.1021/jm2006782
BindingDB Entry DOI: 10.7270/Q2RF5W11 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM28582
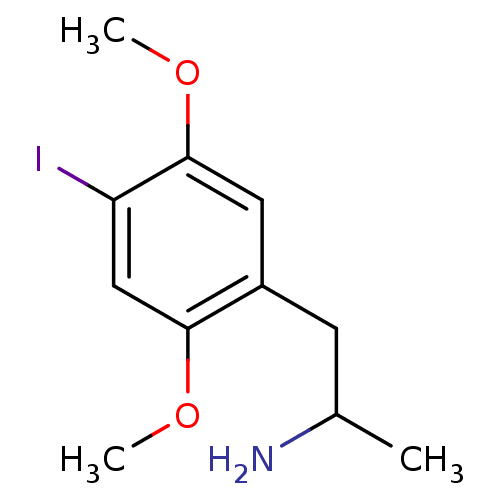
(1-(4-iodo-2,5-dimethoxyphenyl)propan-2-amine | CHE...)Show InChI InChI=1S/C11H16INO2/c1-7(13)4-8-5-11(15-3)9(12)6-10(8)14-2/h5-7H,4,13H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Namur
Curated by ChEMBL
| Assay Description
Displacement of [125I](+/-)-DOI from human 5HT2B receptor |
J Med Chem 54: 5320-34 (2011)
Article DOI: 10.1021/jm2006782
BindingDB Entry DOI: 10.7270/Q2RF5W11 |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Homo sapiens (Human)) | BDBM50005534
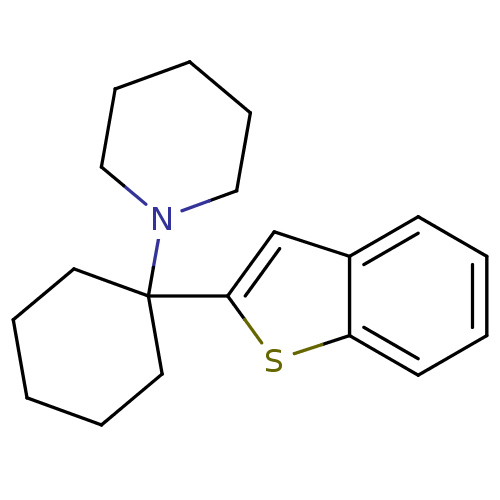
(1-(1-(benzo[b]thiophen-2-yl)cyclohexyl)piperidine ...)Show InChI InChI=1S/C19H25NS/c1-5-11-19(12-6-1,20-13-7-2-8-14-20)18-15-16-9-3-4-10-17(16)21-18/h3-4,9-10,15H,1-2,5-8,11-14H2 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Namur
Curated by ChEMBL
| Assay Description
Displacement of [3H]BTCP from human DA transporter |
J Med Chem 54: 5320-34 (2011)
Article DOI: 10.1021/jm2006782
BindingDB Entry DOI: 10.7270/Q2RF5W11 |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50176062
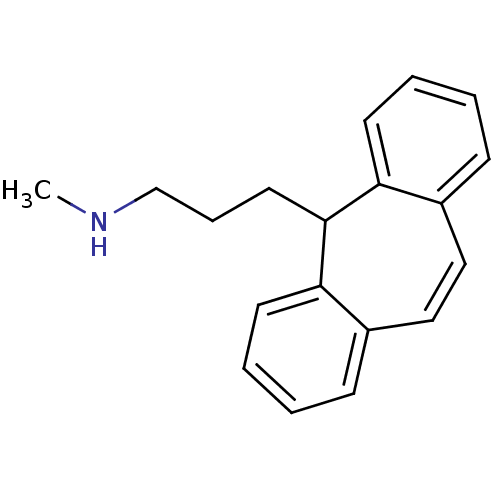
(3-(5H-dibenzo[a,d][7]annulen-5-yl)-N-methylpropan-...)Show InChI InChI=1S/C19H21N/c1-20-14-6-11-19-17-9-4-2-7-15(17)12-13-16-8-3-5-10-18(16)19/h2-5,7-10,12-13,19-20H,6,11,14H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
| DrugBank
Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Namur
Curated by ChEMBL
| Assay Description
Displacement of [3H]nisoxetine from human NE transporter |
J Med Chem 54: 5320-34 (2011)
Article DOI: 10.1021/jm2006782
BindingDB Entry DOI: 10.7270/Q2RF5W11 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM82561
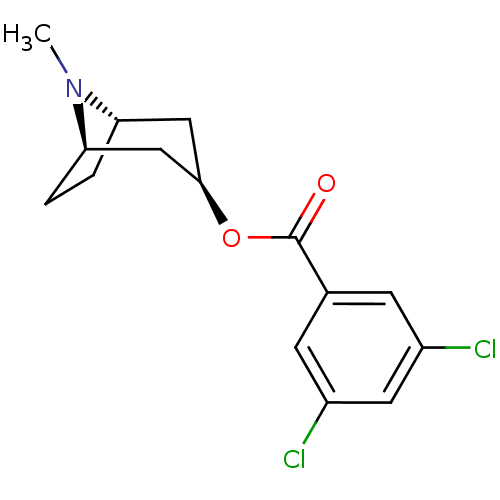
(CAS_40796-97-2 | TROPANYL 3,5-DICHLOROBENZOATE | T...)Show SMILES CN1[C@H]2CC[C@@H]1C[C@@H](C2)OC(=O)c1cc(Cl)cc(Cl)c1 |r| Show InChI InChI=1S/C15H17Cl2NO2/c1-18-12-2-3-13(18)8-14(7-12)20-15(19)9-4-10(16)6-11(17)5-9/h4-6,12-14H,2-3,7-8H2,1H3/t12-,13+,14+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Namur
Curated by ChEMBL
| Assay Description
Displacement of [3H]BRL43694 from human 5HT3 receptor |
J Med Chem 54: 5320-34 (2011)
Article DOI: 10.1021/jm2006782
BindingDB Entry DOI: 10.7270/Q2RF5W11 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Homo sapiens (Human)) | BDBM10755

(14C-5-hydroxy tryptamine creatinine disulfate | 2-...)Show InChI InChI=1S/C10H12N2O/c11-4-3-7-6-12-10-2-1-8(13)5-9(7)10/h1-2,5-6,12-13H,3-4,11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Namur
Curated by ChEMBL
| Assay Description
Displacement of [125I]CYP from human 5HT1B receptor |
J Med Chem 54: 5320-34 (2011)
Article DOI: 10.1021/jm2006782
BindingDB Entry DOI: 10.7270/Q2RF5W11 |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM100152
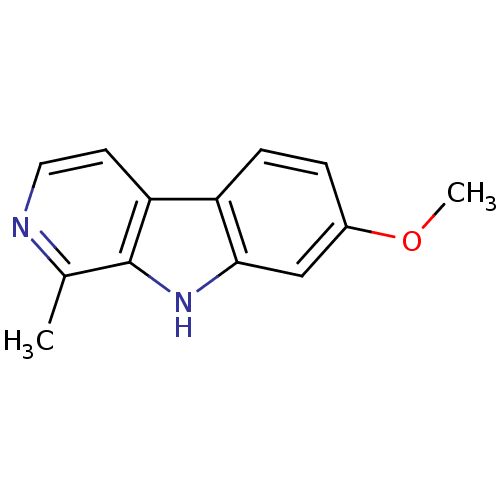
(7-methoxy-1-methyl-9H-beta-carboline;hydrochloride...)Show InChI InChI=1S/C13H12N2O/c1-8-13-11(5-6-14-8)10-4-3-9(16-2)7-12(10)15-13/h3-7,15H,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Namur
Curated by ChEMBL
| Assay Description
Inhibition of MAO-A |
J Med Chem 54: 5320-34 (2011)
Article DOI: 10.1021/jm2006782
BindingDB Entry DOI: 10.7270/Q2RF5W11 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Amine oxidase [flavin-containing] B
(Homo sapiens (Human)) | BDBM11022
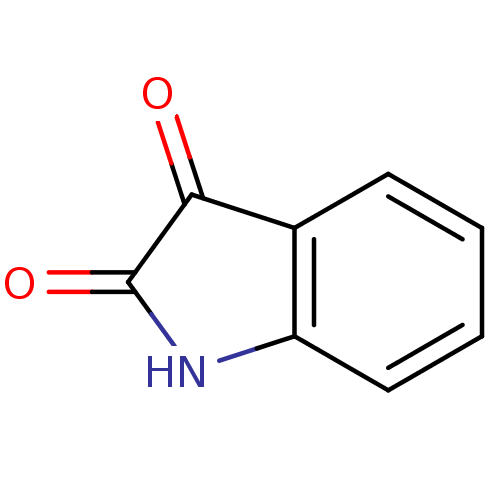
(2,3-dihydro-1H-indole-2,3-dione | CHEMBL326294 | I...)Show InChI InChI=1S/C8H5NO2/c10-7-5-3-1-2-4-6(5)9-8(7)11/h1-4H,(H,9,10,11) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Namur
Curated by ChEMBL
| Assay Description
Inhibition of MAO-B |
J Med Chem 54: 5320-34 (2011)
Article DOI: 10.1021/jm2006782
BindingDB Entry DOI: 10.7270/Q2RF5W11 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM10755

(14C-5-hydroxy tryptamine creatinine disulfate | 2-...)Show InChI InChI=1S/C10H12N2O/c11-4-3-7-6-12-10-2-1-8(13)5-9(7)10/h1-2,5-6,12-13H,3-4,11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 84 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Namur
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor |
J Med Chem 54: 5320-34 (2011)
Article DOI: 10.1021/jm2006782
BindingDB Entry DOI: 10.7270/Q2RF5W11 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM34233

(2-Phenyl-benzo[d]isoselenazol-3-one | 2-Phenyl-ben...)Show InChI InChI=1S/C13H9NOSe/c15-13-11-8-4-5-9-12(11)16-14(13)10-6-2-1-3-7-10/h1-9H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113892
BindingDB Entry DOI: 10.7270/Q22N56BN |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50013811
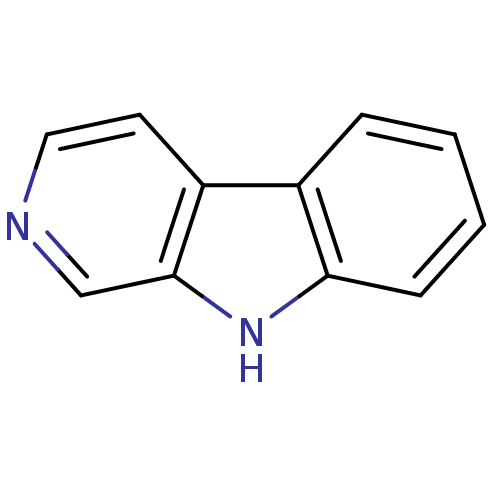
(9H-beta-Carboline | 9H-pyrido[3,4-b]indole | CHEMB...)Show InChI InChI=1S/C11H8N2/c1-2-4-10-8(3-1)9-5-6-12-7-11(9)13-10/h1-7,13H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Namur
Curated by ChEMBL
| Assay Description
Inhibition of IDO |
Bioorg Med Chem 19: 1550-61 (2011)
Article DOI: 10.1016/j.bmc.2010.12.032
BindingDB Entry DOI: 10.7270/Q27M087F |
More data for this
Ligand-Target Pair | |
Beta-1 adrenergic receptor
(Homo sapiens (Human)) | BDBM25753
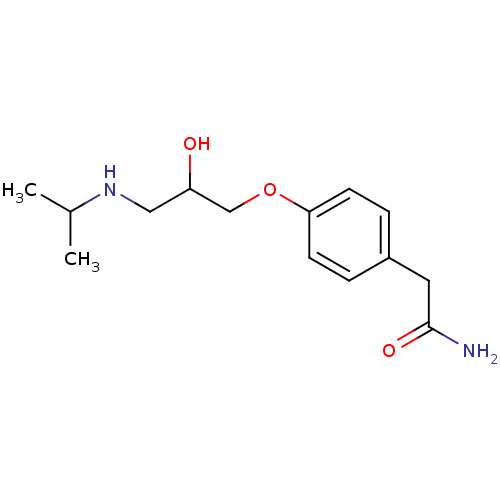
(2-{4-[2-hydroxy-3-(propan-2-ylamino)propoxy]phenyl...)Show InChI InChI=1S/C14H22N2O3/c1-10(2)16-8-12(17)9-19-13-5-3-11(4-6-13)7-14(15)18/h3-6,10,12,16-17H,7-9H2,1-2H3,(H2,15,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Namur
Curated by ChEMBL
| Assay Description
Displacement of [3H](-)-CGP12177 from human adrenergic beta1 receptor |
J Med Chem 54: 5320-34 (2011)
Article DOI: 10.1021/jm2006782
BindingDB Entry DOI: 10.7270/Q2RF5W11 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 5A
(Homo sapiens (Human)) | BDBM10755

(14C-5-hydroxy tryptamine creatinine disulfate | 2-...)Show InChI InChI=1S/C10H12N2O/c11-4-3-7-6-12-10-2-1-8(13)5-9(7)10/h1-2,5-6,12-13H,3-4,11H2 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Namur
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT5A receptor |
J Med Chem 54: 5320-34 (2011)
Article DOI: 10.1021/jm2006782
BindingDB Entry DOI: 10.7270/Q2RF5W11 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM21975
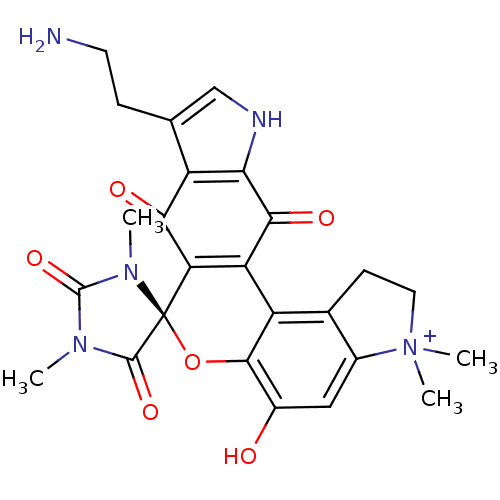
((4S)-16'-(2-aminoethyl)-9'-hydroxy-1,3,6',6'-tetra...)Show SMILES CN1C(=O)N(C)[C@@]2(Oc3c(O)cc4c(CC[N+]4(C)C)c3C3=C2C(=O)c2c(CCN)c[nH]c2C3=O)C1=O |r,c:22| Show InChI InChI=1S/C25H25N5O6/c1-28-23(34)25(29(2)24(28)35)18-17(21(33)19-15(20(18)32)11(5-7-26)10-27-19)16-12-6-8-30(3,4)13(12)9-14(31)22(16)36-25/h9-10H,5-8,26H2,1-4H3,(H-,27,31,32,33)/p+1/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Namur
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 (unknown origin) |
Bioorg Med Chem Lett 23: 47-54 (2012)
Article DOI: 10.1016/j.bmcl.2012.11.036
BindingDB Entry DOI: 10.7270/Q2JM2C03 |
More data for this
Ligand-Target Pair | |
Tryptophan 2,3-dioxygenase
(Homo sapiens (Human)) | BDBM50289137

(6-Fluoro-3-((E)-2-pyridin-3-yl-vinyl)-1H-indole | ...)Show InChI InChI=1S/C15H11FN2/c16-13-5-6-14-12(10-18-15(14)8-13)4-3-11-2-1-7-17-9-11/h1-10,18H/b4-3+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 880 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Namur
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant TDO expressed in Escherichia coli BL21 using L-tryptophan as substrate by measuring conversion of N-formy... |
J Med Chem 54: 5320-34 (2011)
Article DOI: 10.1021/jm2006782
BindingDB Entry DOI: 10.7270/Q2RF5W11 |
More data for this
Ligand-Target Pair | |
Tryptophan 2,3-dioxygenase
(Homo sapiens (Human)) | BDBM50350248
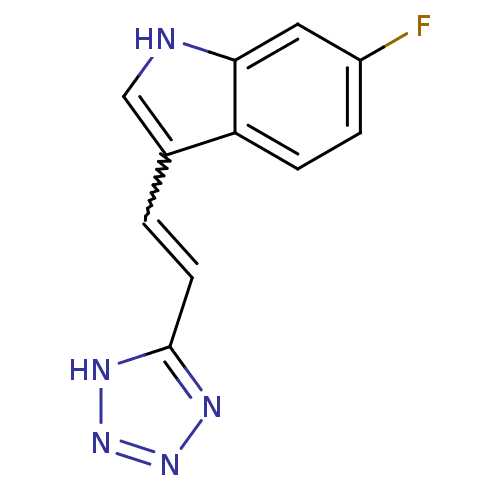
(CHEMBL1812545)Show InChI InChI=1S/C11H8FN5/c12-8-2-3-9-7(6-13-10(9)5-8)1-4-11-14-16-17-15-11/h1-6,13H,(H,14,15,16,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Namur
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant TDO expressed in Escherichia coli BL21 using L-tryptophan as substrate by measuring conversion of N-formy... |
J Med Chem 54: 5320-34 (2011)
Article DOI: 10.1021/jm2006782
BindingDB Entry DOI: 10.7270/Q2RF5W11 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50241727

((S)-2-amino-3-(1-methyl-1H-indol-3-yl)propanoic ac...)Show InChI InChI=1S/C12H14N2O2/c1-14-7-8(6-10(13)12(15)16)9-4-2-3-5-11(9)14/h2-5,7,10H,6,13H2,1H3,(H,15,16)/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Namur
Curated by ChEMBL
| Assay Description
Competitive inhibition of histidine-tagged human recombinant IDO expressed in bacterial strain BL21 AI using L-Trptophan as substrate measured at 490... |
Eur J Med Chem 46: 3058-65 (2011)
Article DOI: 10.1016/j.ejmech.2011.02.049
BindingDB Entry DOI: 10.7270/Q2F19029 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM24813
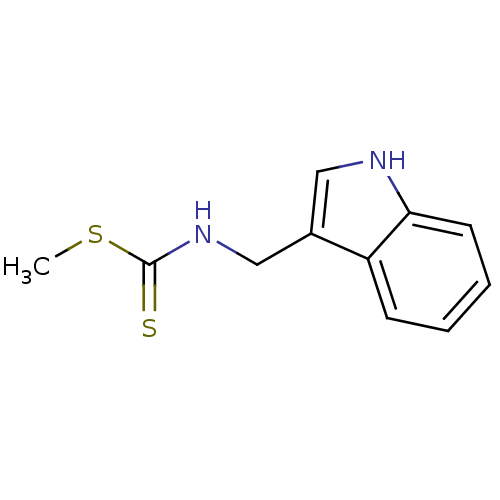
(Brassinin, 1 | N-(1H-indol-3-ylmethyl)(methylsulfa...)Show InChI InChI=1S/C11H12N2S2/c1-15-11(14)13-7-8-6-12-10-5-3-2-4-9(8)10/h2-6,12H,7H2,1H3,(H,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Namur
Curated by ChEMBL
| Assay Description
Inhibition of IDO |
Bioorg Med Chem 19: 1550-61 (2011)
Article DOI: 10.1016/j.bmc.2010.12.032
BindingDB Entry DOI: 10.7270/Q27M087F |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50241727

((S)-2-amino-3-(1-methyl-1H-indol-3-yl)propanoic ac...)Show InChI InChI=1S/C12H14N2O2/c1-14-7-8(6-10(13)12(15)16)9-4-2-3-5-11(9)14/h2-5,7,10H,6,13H2,1H3,(H,15,16)/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Cancer Research
Curated by ChEMBL
| Assay Description
Inhibition of IDO by cell-free assay |
J Med Chem 53: 1172-89 (2010)
Article DOI: 10.1021/jm9014718
BindingDB Entry DOI: 10.7270/Q2BC40G9 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50428658
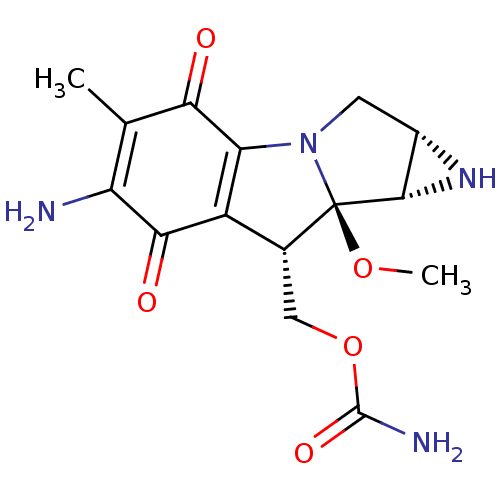
(MITOMYCIN | Mitomycin C | Mitosol | Mitozytrex | M...)Show SMILES CO[C@]12[C@H]3N[C@H]3CN1C1=C([C@H]2COC(N)=O)C(=O)C(N)=C(C)C1=O |r,c:10,t:22| Show InChI InChI=1S/C15H18N4O5/c1-5-9(16)12(21)8-6(4-24-14(17)22)15(23-2)13-7(18-13)3-19(15)10(8)11(5)20/h6-7,13,18H,3-4,16H2,1-2H3,(H2,17,22)/t6-,7+,13+,15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Namur
Curated by ChEMBL
| Assay Description
Uncompetitive inhibition of hexahistidyl-tagged human IDO1 |
Bioorg Med Chem Lett 23: 47-54 (2012)
Article DOI: 10.1016/j.bmcl.2012.11.036
BindingDB Entry DOI: 10.7270/Q2JM2C03 |
More data for this
Ligand-Target Pair | |
Tryptophan 2,3-dioxygenase
(Homo sapiens (Human)) | BDBM50350249
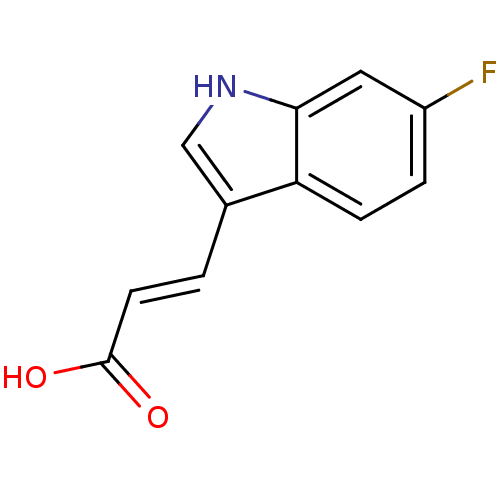
(CHEMBL1812547)Show InChI InChI=1S/C11H8FNO2/c12-8-2-3-9-7(1-4-11(14)15)6-13-10(9)5-8/h1-6,13H,(H,14,15)/b4-1+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.24E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Namur
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant TDO expressed in Escherichia coli BL21 using L-tryptophan as substrate by measuring conversion of N-formy... |
J Med Chem 54: 5320-34 (2011)
Article DOI: 10.1021/jm2006782
BindingDB Entry DOI: 10.7270/Q2RF5W11 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50241727

((S)-2-amino-3-(1-methyl-1H-indol-3-yl)propanoic ac...)Show InChI InChI=1S/C12H14N2O2/c1-14-7-8(6-10(13)12(15)16)9-4-2-3-5-11(9)14/h2-5,7,10H,6,13H2,1H3,(H,15,16)/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Namur
Curated by ChEMBL
| Assay Description
Inhibition of IDO |
Bioorg Med Chem 19: 1550-61 (2011)
Article DOI: 10.1016/j.bmc.2010.12.032
BindingDB Entry DOI: 10.7270/Q27M087F |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50350250
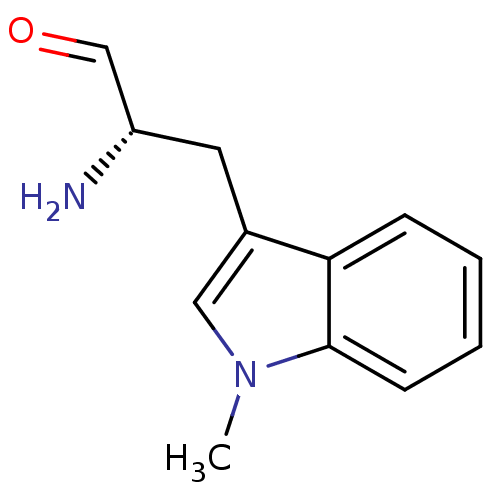
(CHEMBL1812660)Show InChI InChI=1S/C12H14N2O/c1-14-7-9(6-10(13)8-15)11-4-2-3-5-12(11)14/h2-5,7-8,10H,6,13H2,1H3/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Namur
Curated by ChEMBL
| Assay Description
Inhibition of IDO |
J Med Chem 54: 5320-34 (2011)
Article DOI: 10.1021/jm2006782
BindingDB Entry DOI: 10.7270/Q2RF5W11 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50336434
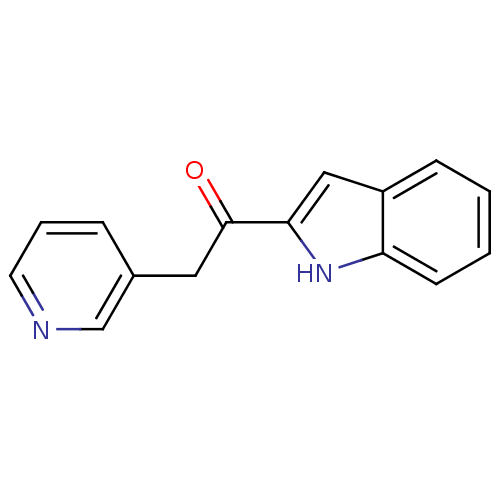
(1-(1H-Indol-2-yl)-2-pyridin-3-yl-ethanone | CHEMBL...)Show InChI InChI=1S/C15H12N2O/c18-15(8-11-4-3-7-16-10-11)14-9-12-5-1-2-6-13(12)17-14/h1-7,9-10,17H,8H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Namur
Curated by ChEMBL
| Assay Description
Uncompetitive inhibition of histidine-tagged human recombinant IDO expressed in bacterial strain BL21 AI using L-Trptophan as substrate measured at 4... |
Eur J Med Chem 46: 3058-65 (2011)
Article DOI: 10.1016/j.ejmech.2011.02.049
BindingDB Entry DOI: 10.7270/Q2F19029 |
More data for this
Ligand-Target Pair | |
Tryptophan 2,3-dioxygenase
(Homo sapiens (Human)) | BDBM21974
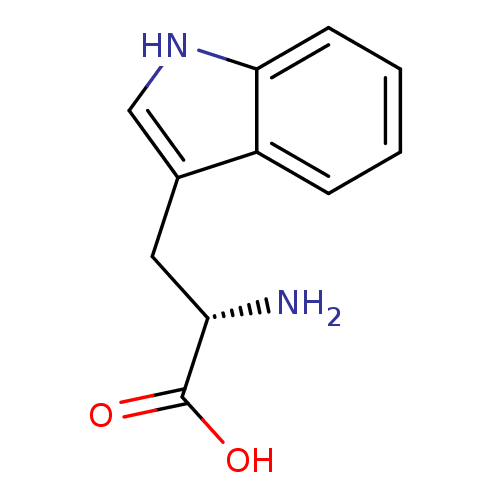
((2S)-2-amino-3-(1H-indol-3-yl)propanoic acid | CHE...)Show InChI InChI=1S/C11H12N2O2/c12-9(11(14)15)5-7-6-13-10-4-2-1-3-8(7)10/h1-4,6,9,13H,5,12H2,(H,14,15)/t9-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 1.15E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Namur
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant TDO expressed in Escherichia coli BL21 using L-tryptophan as substrate by measuring conversion of N-formy... |
J Med Chem 54: 5320-34 (2011)
Article DOI: 10.1021/jm2006782
BindingDB Entry DOI: 10.7270/Q2RF5W11 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50562516
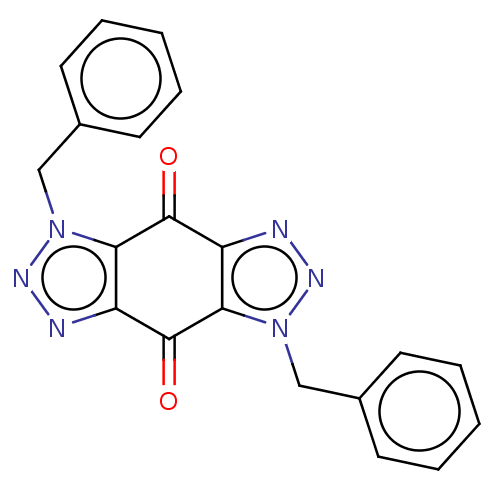
(CHEMBL4746602)Show SMILES O=C1c2nnn(Cc3ccccc3)c2C(=O)c2nnn(Cc3ccccc3)c12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127910
BindingDB Entry DOI: 10.7270/Q2M90DF3 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Mus musculus) | BDBM50391363
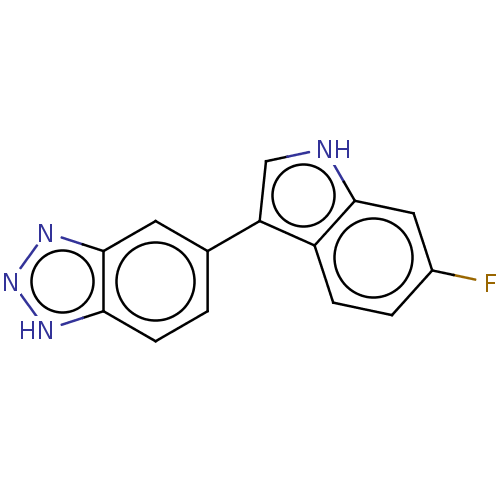
(CHEMBL2148074)Show InChI InChI=1S/C8H6ClN3O/c9-5-1-2-8(13)6(3-5)7-4-10-12-11-7/h1-4,13H,(H,10,11,12) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig Center for Cancer Research of the University of Lausanne
Curated by ChEMBL
| Assay Description
Inhibition of mouse IDO1 in P815 clone 6 cells by HPLC analysis |
J Med Chem 55: 5270-90 (2012)
Article DOI: 10.1021/jm300260v
BindingDB Entry DOI: 10.7270/Q27H1KNW |
More data for this
Ligand-Target Pair | |
Tryptophan 2,3-dioxygenase
(Homo sapiens (Human)) | BDBM50567017
(CHEMBL4862796) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human TDO2 expressed in mouse P815B cells assessed as kynurenine concentration formation using L-tryptophan as substrate incubated for ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00323
BindingDB Entry DOI: 10.7270/Q2GM8C2N |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Mus musculus) | BDBM50391358

(CHEMBL2147989)Show InChI InChI=1S/C8H6ClN3/c9-7-3-1-2-6(4-7)8-5-10-12-11-8/h1-5H,(H,10,11,12) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig Center for Cancer Research of the University of Lausanne
Curated by ChEMBL
| Assay Description
Inhibition of mouse IDO1 in P815 clone 6 cells by HPLC analysis |
J Med Chem 55: 5270-90 (2012)
Article DOI: 10.1021/jm300260v
BindingDB Entry DOI: 10.7270/Q27H1KNW |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Mus musculus) | BDBM50391359
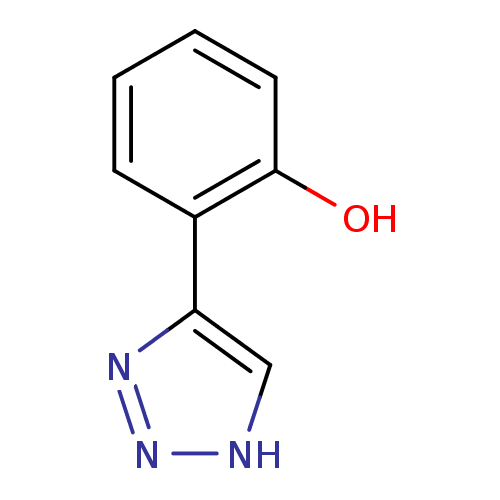
(CHEMBL2147998)Show InChI InChI=1S/C8H7N3O/c12-8-4-2-1-3-6(8)7-5-9-11-10-7/h1-5,12H,(H,9,10,11) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig Center for Cancer Research of the University of Lausanne
Curated by ChEMBL
| Assay Description
Inhibition of mouse IDO1 in P815 clone 6 cells by HPLC analysis |
J Med Chem 55: 5270-90 (2012)
Article DOI: 10.1021/jm300260v
BindingDB Entry DOI: 10.7270/Q27H1KNW |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Mus musculus) | BDBM17467

(1,2,3-triazole analogue, 23 | 5-(3-bromophenyl)-1H...)Show InChI InChI=1S/C8H6BrN3/c9-7-3-1-2-6(4-7)8-5-10-12-11-8/h1-5H,(H,10,11,12) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig Center for Cancer Research of the University of Lausanne
Curated by ChEMBL
| Assay Description
Inhibition of mouse IDO1 in P815 clone 6 cells by HPLC analysis |
J Med Chem 55: 5270-90 (2012)
Article DOI: 10.1021/jm300260v
BindingDB Entry DOI: 10.7270/Q27H1KNW |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Mus musculus) | BDBM50391365
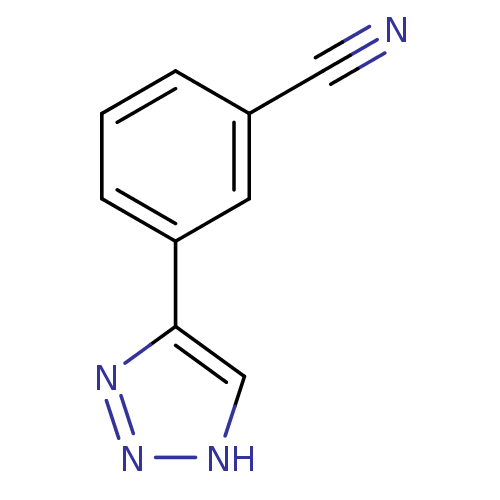
(CHEMBL2147990)Show InChI InChI=1S/C9H6N4/c10-5-7-2-1-3-8(4-7)9-6-11-13-12-9/h1-4,6H,(H,11,12,13) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig Center for Cancer Research of the University of Lausanne
Curated by ChEMBL
| Assay Description
Inhibition of mouse IDO1 in P815 clone 6 cells by HPLC analysis |
J Med Chem 55: 5270-90 (2012)
Article DOI: 10.1021/jm300260v
BindingDB Entry DOI: 10.7270/Q27H1KNW |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Mus musculus) | BDBM50391372
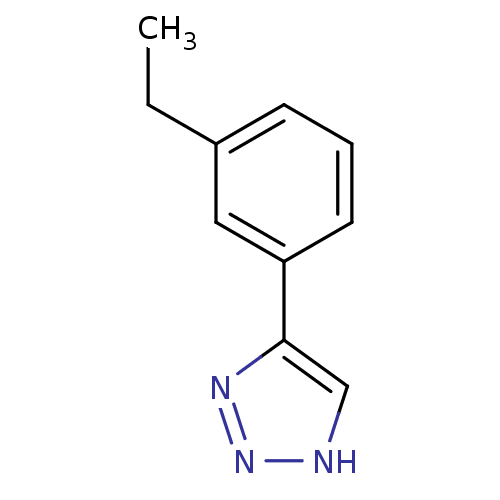
(CHEMBL2147992)Show InChI InChI=1S/C10H11N3/c1-2-8-4-3-5-9(6-8)10-7-11-13-12-10/h3-7H,2H2,1H3,(H,11,12,13) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig Center for Cancer Research of the University of Lausanne
Curated by ChEMBL
| Assay Description
Inhibition of mouse IDO1 in P815 clone 6 cells by HPLC analysis |
J Med Chem 55: 5270-90 (2012)
Article DOI: 10.1021/jm300260v
BindingDB Entry DOI: 10.7270/Q27H1KNW |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Mus musculus) | BDBM50355861
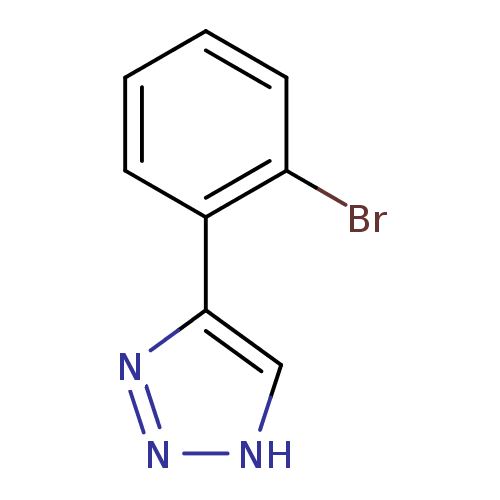
(CHEMBL1909733)Show InChI InChI=1S/C8H6BrN3/c9-7-4-2-1-3-6(7)8-5-10-12-11-8/h1-5H,(H,10,11,12) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig Center for Cancer Research of the University of Lausanne
Curated by ChEMBL
| Assay Description
Inhibition of mouse IDO1 in P815 clone 6 cells by HPLC analysis |
J Med Chem 55: 5270-90 (2012)
Article DOI: 10.1021/jm300260v
BindingDB Entry DOI: 10.7270/Q27H1KNW |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Mus musculus) | BDBM50391360
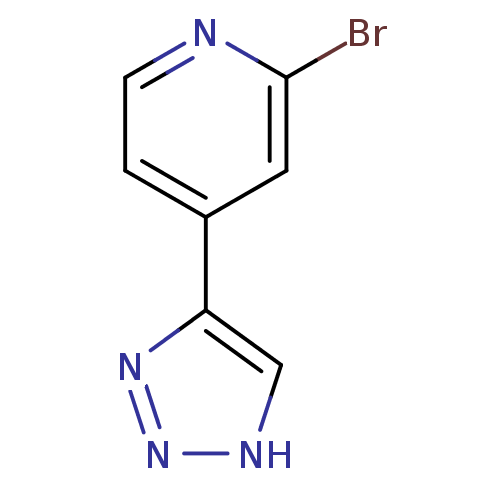
(CHEMBL2147995)Show InChI InChI=1S/C7H5BrN4/c8-7-3-5(1-2-9-7)6-4-10-12-11-6/h1-4H,(H,10,11,12) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig Center for Cancer Research of the University of Lausanne
Curated by ChEMBL
| Assay Description
Inhibition of mouse IDO1 in P815 clone 6 cells by HPLC analysis |
J Med Chem 55: 5270-90 (2012)
Article DOI: 10.1021/jm300260v
BindingDB Entry DOI: 10.7270/Q27H1KNW |
More data for this
Ligand-Target Pair | |
Tryptophan 2,3-dioxygenase
(Homo sapiens (Human)) | BDBM50599381
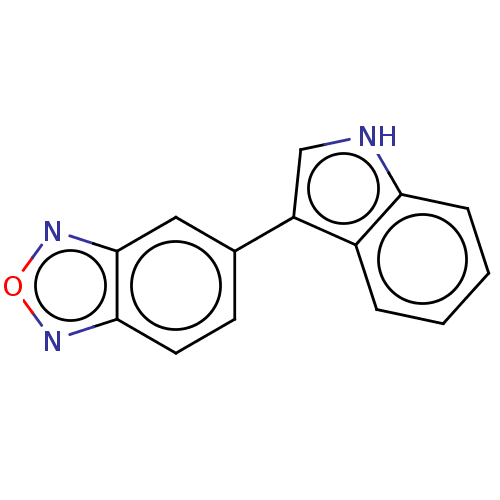
(CHEMBL5169552) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113892
BindingDB Entry DOI: 10.7270/Q22N56BN |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50300305
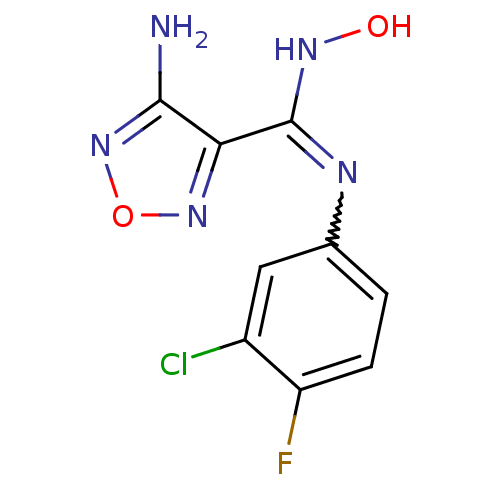
(4-Amino-N-(3-chloro-4-fluorophenyl)-N'-hydroxy-1,2...)Show InChI InChI=1S/C9H7ClFN5O2/c10-5-3-4(1-2-6(5)11)13-9(14-17)7-8(12)16-18-15-7/h1-3,17H,(H2,12,16)(H,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig Center for Cancer Research of the University of Lausanne
Curated by ChEMBL
| Assay Description
Inhibition of human IDO1 transfected in mouse P815B clone-6 cells by HPLC analysis |
J Med Chem 55: 5270-90 (2012)
Article DOI: 10.1021/jm300260v
BindingDB Entry DOI: 10.7270/Q27H1KNW |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Mus musculus) | BDBM50391374
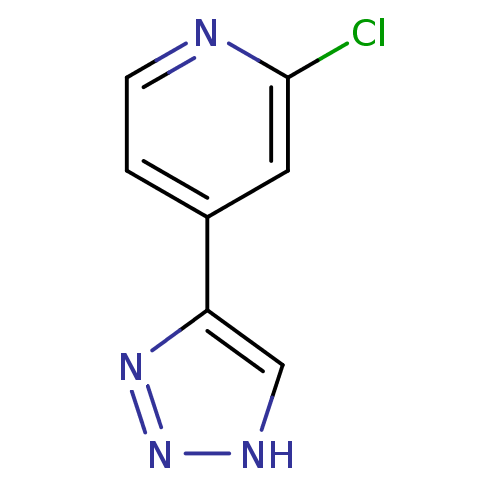
(CHEMBL2146496)Show InChI InChI=1S/C7H5ClN4/c8-7-3-5(1-2-9-7)6-4-10-12-11-6/h1-4H,(H,10,11,12) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig Center for Cancer Research of the University of Lausanne
Curated by ChEMBL
| Assay Description
Inhibition of mouse IDO1 in P815 clone 6 cells by HPLC analysis |
J Med Chem 55: 5270-90 (2012)
Article DOI: 10.1021/jm300260v
BindingDB Entry DOI: 10.7270/Q27H1KNW |
More data for this
Ligand-Target Pair | |
Tryptophan 2,3-dioxygenase
(Homo sapiens (Human)) | BDBM50567007
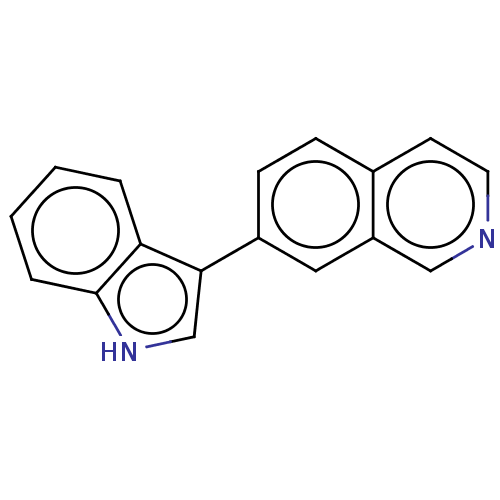
(CHEMBL4852583) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human TDO2 expressed in mouse P815B cells assessed as kynurenine concentration formation using L-tryptophan as substrate incubated for ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00323
BindingDB Entry DOI: 10.7270/Q2GM8C2N |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Mus musculus) | BDBM50391366
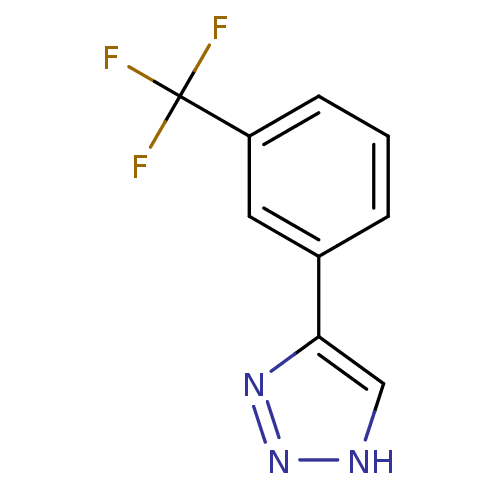
(CHEMBL2147991)Show InChI InChI=1S/C9H6F3N3/c10-9(11,12)7-3-1-2-6(4-7)8-5-13-15-14-8/h1-5H,(H,13,14,15) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig Center for Cancer Research of the University of Lausanne
Curated by ChEMBL
| Assay Description
Inhibition of mouse IDO1 in P815 clone 6 cells by HPLC analysis |
J Med Chem 55: 5270-90 (2012)
Article DOI: 10.1021/jm300260v
BindingDB Entry DOI: 10.7270/Q27H1KNW |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50391363

(CHEMBL2148074)Show InChI InChI=1S/C8H6ClN3O/c9-5-1-2-8(13)6(3-5)7-4-10-12-11-7/h1-4,13H,(H,10,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 71 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Ludwig Center for Cancer Research of the University of Lausanne
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant N-terminal His-tagged IDO1 (Ala2 to Gly403) overexpressed in Escherichia coli BL21 at pH 7.4 after 60 mins by HPLC an... |
J Med Chem 55: 5270-90 (2012)
Article DOI: 10.1021/jm300260v
BindingDB Entry DOI: 10.7270/Q27H1KNW |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50391363

(CHEMBL2148074)Show InChI InChI=1S/C8H6ClN3O/c9-5-1-2-8(13)6(3-5)7-4-10-12-11-7/h1-4,13H,(H,10,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig Center for Cancer Research of the University of Lausanne
Curated by ChEMBL
| Assay Description
Inhibition of human IDO1 transfected in mouse P815B clone-6 cells by HPLC analysis |
J Med Chem 55: 5270-90 (2012)
Article DOI: 10.1021/jm300260v
BindingDB Entry DOI: 10.7270/Q27H1KNW |
More data for this
Ligand-Target Pair | |
Tryptophan 2,3-dioxygenase
(Homo sapiens (Human)) | BDBM50599383
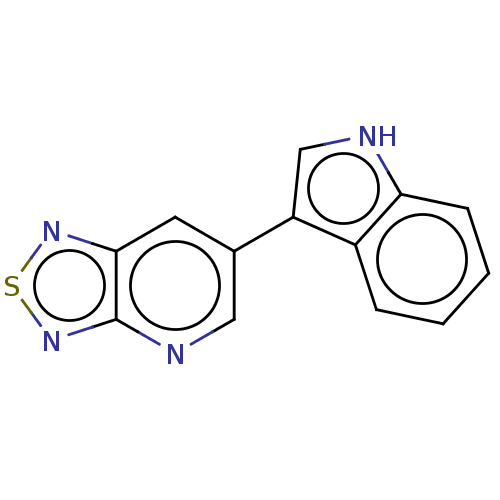
(CHEMBL5201313) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113892
BindingDB Entry DOI: 10.7270/Q22N56BN |
More data for this
Ligand-Target Pair | |
Tryptophan 2,3-dioxygenase
(Homo sapiens (Human)) | BDBM50599384
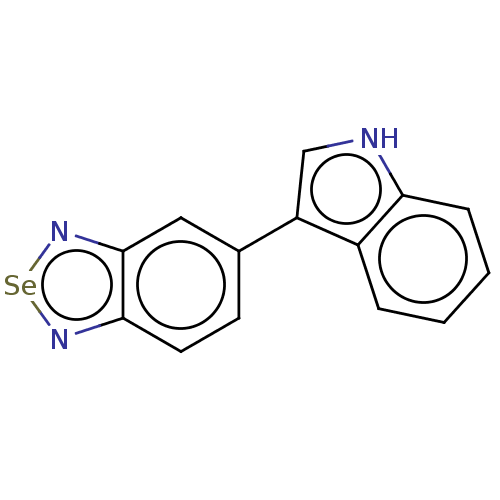
(CHEMBL5185564) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 94 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113892
BindingDB Entry DOI: 10.7270/Q22N56BN |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data