

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Liver carboxylesterase 1 (Homo sapiens (Human)) | BDBM195572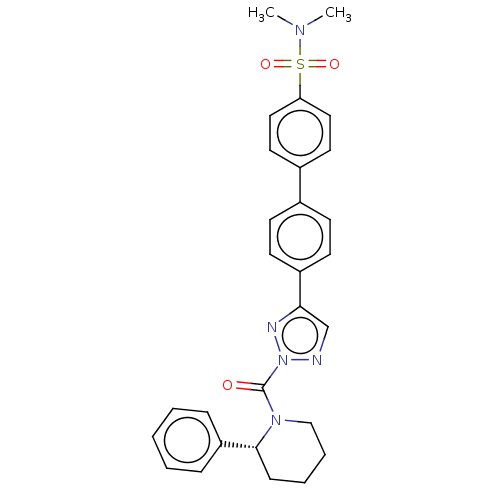 ((R)-N,N-dimethyl-4'-(2-(2-phenylpiperidine-1-c...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <5 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Pfizer Inc. | Assay Description For determination of IC50 values for CES1 inhibitors, the reactions were carried out in 1.5 mL microcentrifuge tubes in a total reaction volume of 25... | ACS Chem Biol 11: 2529-40 (2016) Article DOI: 10.1021/acschembio.6b00266 BindingDB Entry DOI: 10.7270/Q24X56K5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase 1 (Homo sapiens (Human)) | BDBM195575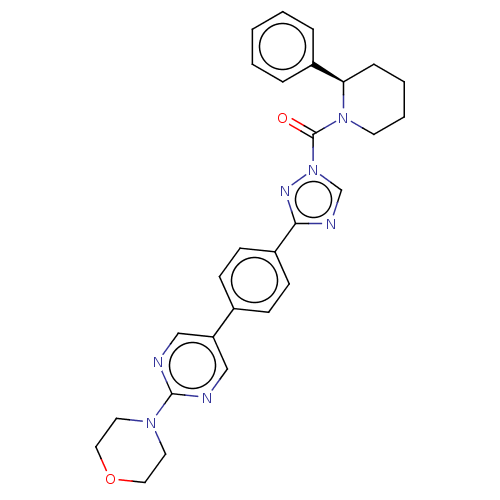 ((R)-(3-(4-(2-morpholinopyrimidin-5-yl)phenyl)-1H-1...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Pfizer Inc. | Assay Description For determination of IC50 values for CES1 inhibitors, the reactions were carried out in 1.5 mL microcentrifuge tubes in a total reaction volume of 25... | ACS Chem Biol 11: 2529-40 (2016) Article DOI: 10.1021/acschembio.6b00266 BindingDB Entry DOI: 10.7270/Q24X56K5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase 1 (Homo sapiens (Human)) | BDBM195574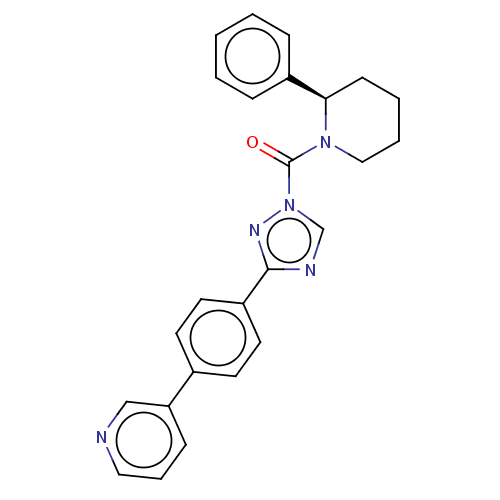 ((R)-(2-phenylpiperidin-1-yl)(3-(4-(pyridin-3-yl)ph...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Pfizer Inc. | Assay Description For determination of IC50 values for CES1 inhibitors, the reactions were carried out in 1.5 mL microcentrifuge tubes in a total reaction volume of 25... | ACS Chem Biol 11: 2529-40 (2016) Article DOI: 10.1021/acschembio.6b00266 BindingDB Entry DOI: 10.7270/Q24X56K5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase 1 (Homo sapiens (Human)) | BDBM195570 ((S)-4'-(2-(2-phenylpiperidine-1-carbonyl)-2H-1...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <5 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Pfizer Inc. | Assay Description For determination of IC50 values for CES1 inhibitors, the reactions were carried out in 1.5 mL microcentrifuge tubes in a total reaction volume of 25... | ACS Chem Biol 11: 2529-40 (2016) Article DOI: 10.1021/acschembio.6b00266 BindingDB Entry DOI: 10.7270/Q24X56K5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophospholipase-like protein 1 (Homo sapiens (Human)) | BDBM195581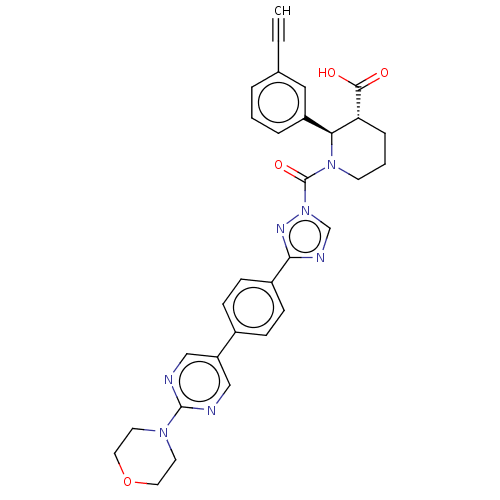 ((2R,3R)-2-(3-ethynylphenyl)-1-(3-(4-(2-morpholinop...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Pfizer Inc. | Assay Description For determination of IC50 values for LYPLAL1 inhibitors, the reactions were carried out in 1.5 mL microcentrifuge tubes in a total reaction volume of... | ACS Chem Biol 11: 2529-40 (2016) Article DOI: 10.1021/acschembio.6b00266 BindingDB Entry DOI: 10.7270/Q24X56K5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase 1 (Homo sapiens (Human)) | BDBM195571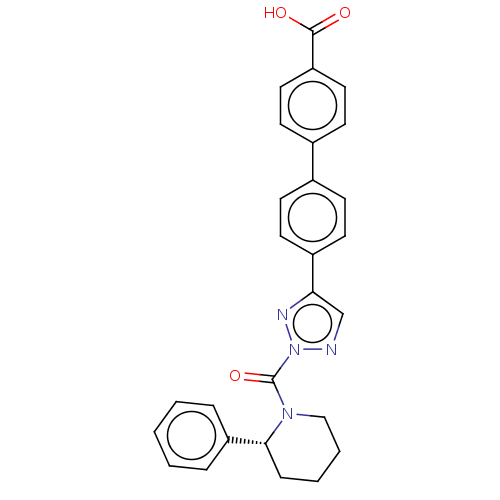 ((R)-4'-(2-(2-phenylpiperidine-1-carbonyl)-2H-1...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <5 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Pfizer Inc. | Assay Description For determination of IC50 values for CES1 inhibitors, the reactions were carried out in 1.5 mL microcentrifuge tubes in a total reaction volume of 25... | ACS Chem Biol 11: 2529-40 (2016) Article DOI: 10.1021/acschembio.6b00266 BindingDB Entry DOI: 10.7270/Q24X56K5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophospholipase-like protein 1 (Homo sapiens (Human)) | BDBM195580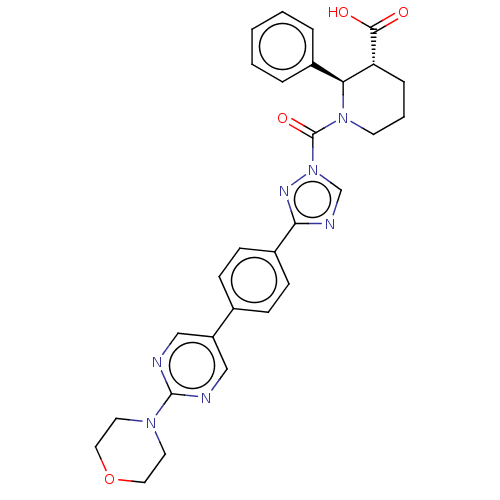 ((2R,3R)-1-(3-(4-(2-morpholinopyrimidin-5-yl)phenyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Pfizer Inc. | Assay Description For determination of IC50 values for LYPLAL1 inhibitors, the reactions were carried out in 1.5 mL microcentrifuge tubes in a total reaction volume of... | ACS Chem Biol 11: 2529-40 (2016) Article DOI: 10.1021/acschembio.6b00266 BindingDB Entry DOI: 10.7270/Q24X56K5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophospholipase-like protein 1 (Homo sapiens (Human)) | BDBM195575 ((R)-(3-(4-(2-morpholinopyrimidin-5-yl)phenyl)-1H-1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Pfizer Inc. | Assay Description For determination of IC50 values for LYPLAL1 inhibitors, the reactions were carried out in 1.5 mL microcentrifuge tubes in a total reaction volume of... | ACS Chem Biol 11: 2529-40 (2016) Article DOI: 10.1021/acschembio.6b00266 BindingDB Entry DOI: 10.7270/Q24X56K5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase 1 (Homo sapiens (Human)) | BDBM195573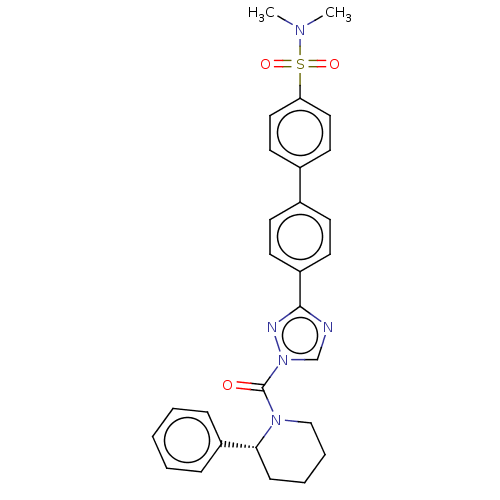 ((R)-4'-(1-(2-phenylpiperidine-1-carbonyl)-1H-1...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Pfizer Inc. | Assay Description For determination of IC50 values for CES1 inhibitors, the reactions were carried out in 1.5 mL microcentrifuge tubes in a total reaction volume of 25... | ACS Chem Biol 11: 2529-40 (2016) Article DOI: 10.1021/acschembio.6b00266 BindingDB Entry DOI: 10.7270/Q24X56K5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane-bound transcription factor site-1 protease (Homo sapiens (Human)) | BDBM50216194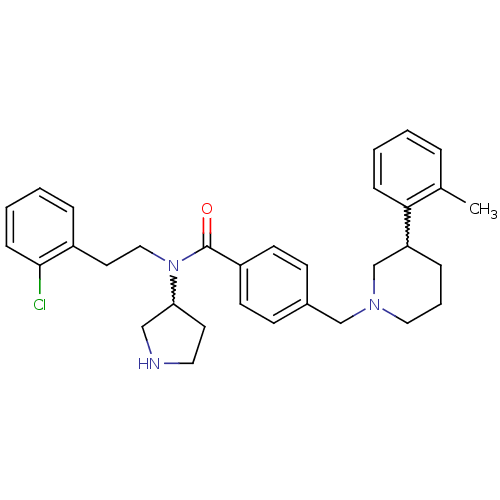 (CHEMBL391935 | N-(2-chlorophenethyl)-N-(pyrrolidin...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-Groton Laboratories Curated by ChEMBL | Assay Description Inhibition of human S1P expressed in CHOK1 cells | Bioorg Med Chem Lett 17: 4411-4 (2007) Article DOI: 10.1016/j.bmcl.2007.06.031 BindingDB Entry DOI: 10.7270/Q26M36JG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophospholipase-like protein 1 (Homo sapiens (Human)) | BDBM195578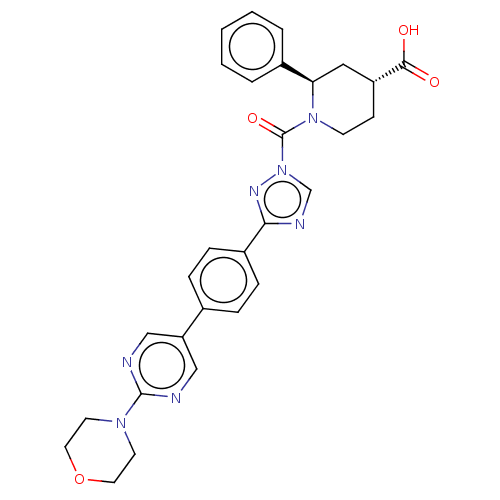 ((2R, 4R)-1-(3-(4-(2-morpholinopyrimidin-5-yl)pheny...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Pfizer Inc. | Assay Description For determination of IC50 values for LYPLAL1 inhibitors, the reactions were carried out in 1.5 mL microcentrifuge tubes in a total reaction volume of... | ACS Chem Biol 11: 2529-40 (2016) Article DOI: 10.1021/acschembio.6b00266 BindingDB Entry DOI: 10.7270/Q24X56K5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophospholipase-like protein 1 (Homo sapiens (Human)) | BDBM195572 ((R)-N,N-dimethyl-4'-(2-(2-phenylpiperidine-1-c...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Pfizer Inc. | Assay Description For determination of IC50 values for LYPLAL1 inhibitors, the reactions were carried out in 1.5 mL microcentrifuge tubes in a total reaction volume of... | ACS Chem Biol 11: 2529-40 (2016) Article DOI: 10.1021/acschembio.6b00266 BindingDB Entry DOI: 10.7270/Q24X56K5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50078322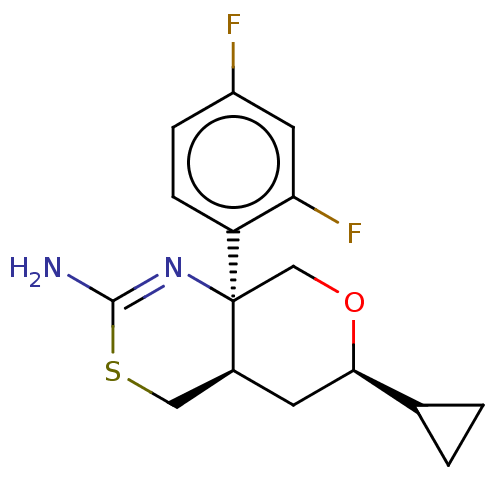 (CHEMBL3414710) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Eurofarma Laboratorios S.A. Curated by ChEMBL | Assay Description Inhibition of BACE1 in human H4 cells overexpressing APP695 assessed as sAPPbeta level after 18 hrs by ELISA | J Med Chem 58: 2678-702 (2015) Article DOI: 10.1021/jm501833t BindingDB Entry DOI: 10.7270/Q2W37Z1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50078321 (CHEMBL3414711 | US9260455, 7) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Eurofarma Laboratorios S.A. Curated by ChEMBL | Assay Description Inhibition of BACE1 in human H4 cells overexpressing APP695 assessed as sAPPbeta level after 18 hrs by ELISA | J Med Chem 58: 2678-702 (2015) Article DOI: 10.1021/jm501833t BindingDB Entry DOI: 10.7270/Q2W37Z1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane-bound transcription factor site-1 protease (Homo sapiens (Human)) | BDBM50216201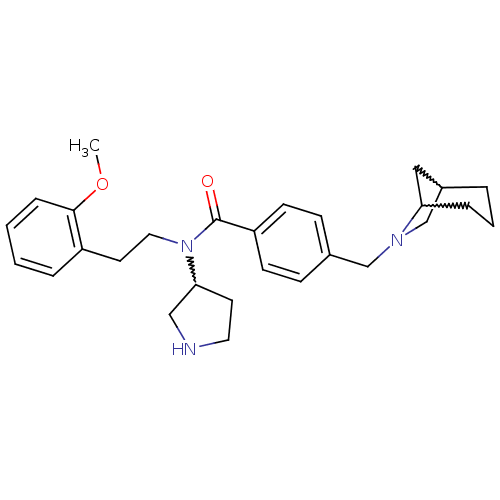 (CHEMBL233987 | N-(2-methoxyphenethyl)-4-(6-aza-bic...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-Groton Laboratories Curated by ChEMBL | Assay Description Inhibition of human S1P expressed in CHOK1 cells | Bioorg Med Chem Lett 17: 4411-4 (2007) Article DOI: 10.1016/j.bmcl.2007.06.031 BindingDB Entry DOI: 10.7270/Q26M36JG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50078324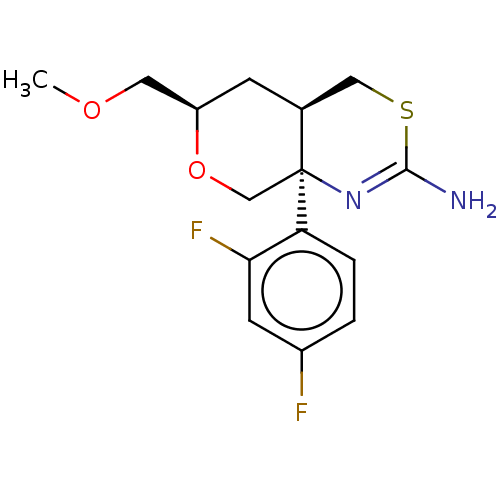 (CHEMBL3414708 | US9260455, 8) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Eurofarma Laboratorios S.A. Curated by ChEMBL | Assay Description Inhibition of BACE1 in human H4 cells overexpressing APP695 assessed as sAPPbeta level after 18 hrs by ELISA | J Med Chem 58: 2678-702 (2015) Article DOI: 10.1021/jm501833t BindingDB Entry DOI: 10.7270/Q2W37Z1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophospholipase-like protein 1 (Homo sapiens (Human)) | BDBM195574 ((R)-(2-phenylpiperidin-1-yl)(3-(4-(pyridin-3-yl)ph...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Pfizer Inc. | Assay Description For determination of IC50 values for LYPLAL1 inhibitors, the reactions were carried out in 1.5 mL microcentrifuge tubes in a total reaction volume of... | ACS Chem Biol 11: 2529-40 (2016) Article DOI: 10.1021/acschembio.6b00266 BindingDB Entry DOI: 10.7270/Q24X56K5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane-bound transcription factor site-1 protease (Homo sapiens (Human)) | BDBM50216180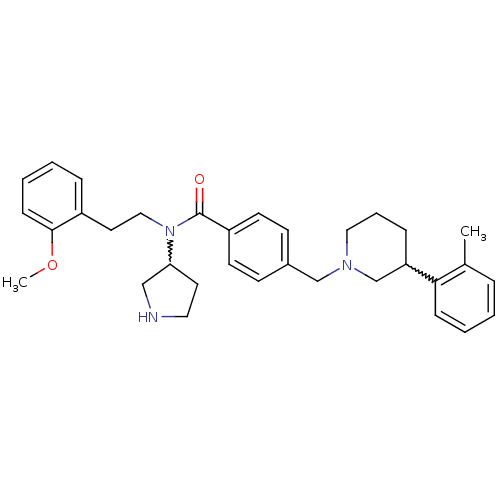 (CHEMBL233799 | N-(2-methoxyphenethyl)-N-(pyrrolidi...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-Groton Laboratories Curated by ChEMBL | Assay Description Inhibition of human S1P expressed in CHOK1 cells | Bioorg Med Chem Lett 17: 4411-4 (2007) Article DOI: 10.1016/j.bmcl.2007.06.031 BindingDB Entry DOI: 10.7270/Q26M36JG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50078349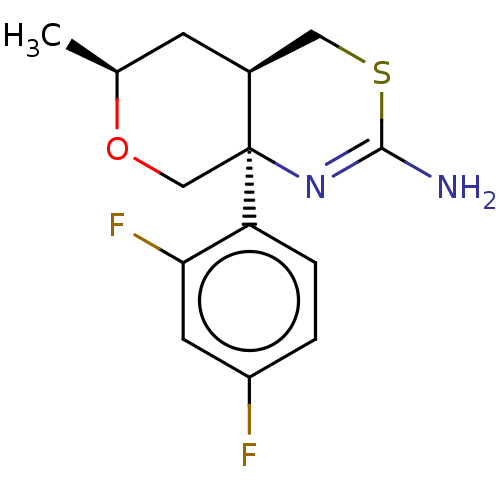 (CHEMBL3414707 | US9260455, 3) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Eurofarma Laboratorios S.A. Curated by ChEMBL | Assay Description Inhibition of BACE1 in human H4 cells overexpressing APP695 assessed as sAPPbeta level after 18 hrs by ELISA | J Med Chem 58: 2678-702 (2015) Article DOI: 10.1021/jm501833t BindingDB Entry DOI: 10.7270/Q2W37Z1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophospholipase-like protein 1 (Homo sapiens (Human)) | BDBM195573 ((R)-4'-(1-(2-phenylpiperidine-1-carbonyl)-1H-1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Pfizer Inc. | Assay Description For determination of IC50 values for LYPLAL1 inhibitors, the reactions were carried out in 1.5 mL microcentrifuge tubes in a total reaction volume of... | ACS Chem Biol 11: 2529-40 (2016) Article DOI: 10.1021/acschembio.6b00266 BindingDB Entry DOI: 10.7270/Q24X56K5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophospholipase-like protein 1 (Homo sapiens (Human)) | BDBM195571 ((R)-4'-(2-(2-phenylpiperidine-1-carbonyl)-2H-1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 59 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Pfizer Inc. | Assay Description For determination of IC50 values for LYPLAL1 inhibitors, the reactions were carried out in 1.5 mL microcentrifuge tubes in a total reaction volume of... | ACS Chem Biol 11: 2529-40 (2016) Article DOI: 10.1021/acschembio.6b00266 BindingDB Entry DOI: 10.7270/Q24X56K5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50078323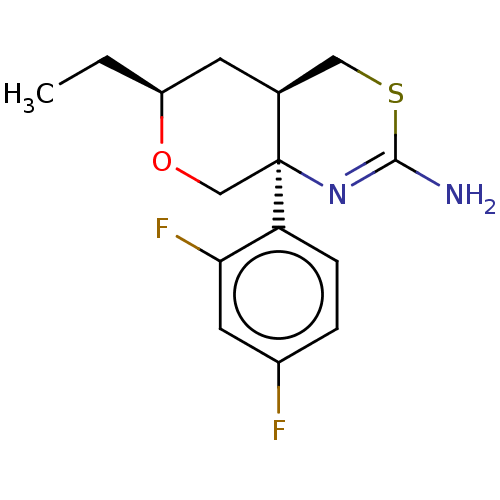 (CHEMBL3414709) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
Eurofarma Laboratorios S.A. Curated by ChEMBL | Assay Description Inhibition of BACE1 in human H4 cells overexpressing APP695 assessed as sAPPbeta level after 18 hrs by ELISA | J Med Chem 58: 2678-702 (2015) Article DOI: 10.1021/jm501833t BindingDB Entry DOI: 10.7270/Q2W37Z1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50078320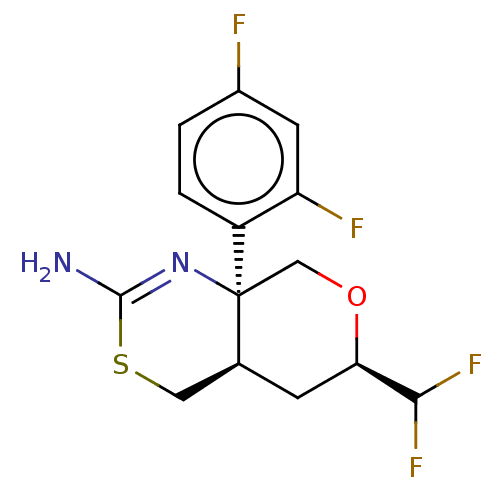 (CHEMBL3414700 | US9260455, 4) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
Eurofarma Laboratorios S.A. Curated by ChEMBL | Assay Description Inhibition of BACE1 in human H4 cells overexpressing APP695 assessed as sAPPbeta level after 18 hrs by ELISA | J Med Chem 58: 2678-702 (2015) Article DOI: 10.1021/jm501833t BindingDB Entry DOI: 10.7270/Q2W37Z1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM142394 (US8933221, 4) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
Eurofarma Laboratorios S.A. Curated by ChEMBL | Assay Description Inhibition of BACE1 in human H4 cells overexpressing APP695 assessed as sAPPbeta level after 18 hrs by ELISA | J Med Chem 58: 2678-702 (2015) Article DOI: 10.1021/jm501833t BindingDB Entry DOI: 10.7270/Q2W37Z1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane-bound transcription factor site-1 protease (Homo sapiens (Human)) | BDBM50216199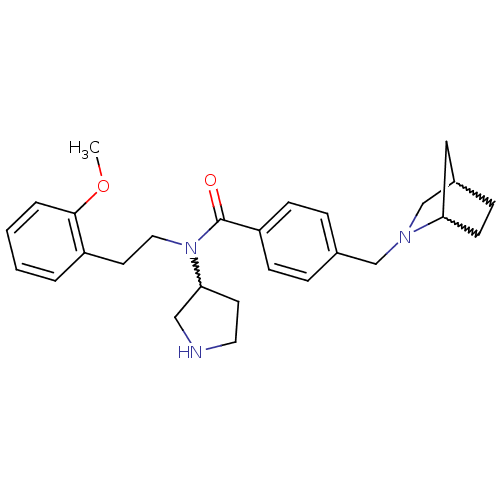 (4-(2-aza-bicyclo[2.2.1]heptan-2-ylmethyl)-N-(2-met...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 95 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-Groton Laboratories Curated by ChEMBL | Assay Description Inhibition of human S1P expressed in CHOK1 cells | Bioorg Med Chem Lett 17: 4411-4 (2007) Article DOI: 10.1016/j.bmcl.2007.06.031 BindingDB Entry DOI: 10.7270/Q26M36JG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane-bound transcription factor site-1 protease (Homo sapiens (Human)) | BDBM50216188 (CHEMBL233422 | N-(2-methoxyphenethyl)-4-(((1S,2S)-...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-Groton Laboratories Curated by ChEMBL | Assay Description Inhibition of human S1P expressed in CHOK1 cells | Bioorg Med Chem Lett 17: 4411-4 (2007) Article DOI: 10.1016/j.bmcl.2007.06.031 BindingDB Entry DOI: 10.7270/Q26M36JG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane-bound transcription factor site-1 protease (Homo sapiens (Human)) | BDBM50216193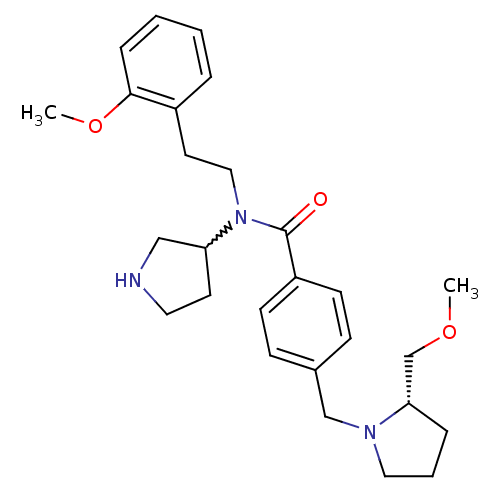 (CHEMBL233609 | N-(2-methoxyphenethyl)-4-(((S)-2-(m...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-Groton Laboratories Curated by ChEMBL | Assay Description Inhibition of human S1P expressed in CHOK1 cells | Bioorg Med Chem Lett 17: 4411-4 (2007) Article DOI: 10.1016/j.bmcl.2007.06.031 BindingDB Entry DOI: 10.7270/Q26M36JG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50078321 (CHEMBL3414711 | US9260455, 7) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Eurofarma Laboratorios S.A. Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) using biotin-GLTNIKTEEISEISYEVEFR-C[Oregon Green]KK-OH as substrate after 3 hrs by fluorescence polarization ass... | J Med Chem 58: 2678-702 (2015) Article DOI: 10.1021/jm501833t BindingDB Entry DOI: 10.7270/Q2W37Z1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane-bound transcription factor site-1 protease (Homo sapiens (Human)) | BDBM50216197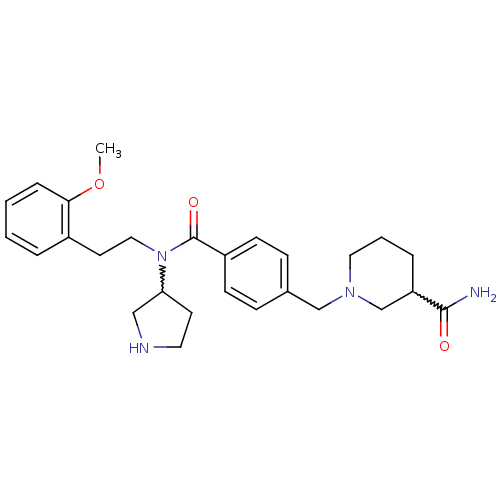 (CHEMBL233610 | N-(2-methoxyphenethyl)-4-(((S)-2-(m...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-Groton Laboratories Curated by ChEMBL | Assay Description Inhibition of human S1P expressed in CHOK1 cells | Bioorg Med Chem Lett 17: 4411-4 (2007) Article DOI: 10.1016/j.bmcl.2007.06.031 BindingDB Entry DOI: 10.7270/Q26M36JG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane-bound transcription factor site-1 protease (Homo sapiens (Human)) | BDBM50216181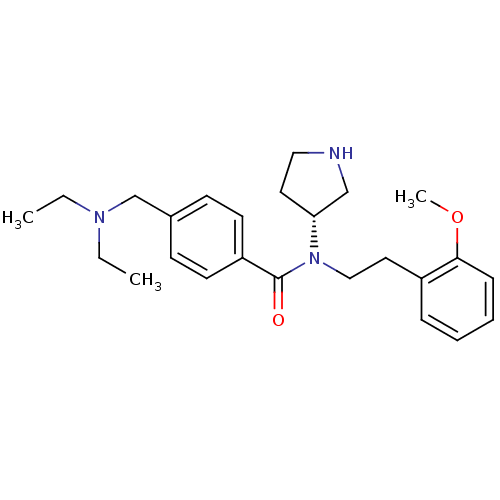 ((R)-N-(2-methoxyphenethyl)-4-((diethylamino)methyl...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-Groton Laboratories Curated by ChEMBL | Assay Description Inhibition of human S1P expressed in CHOK1 cells | Bioorg Med Chem Lett 17: 4411-4 (2007) Article DOI: 10.1016/j.bmcl.2007.06.031 BindingDB Entry DOI: 10.7270/Q26M36JG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane-bound transcription factor site-1 protease (Homo sapiens (Human)) | BDBM50216204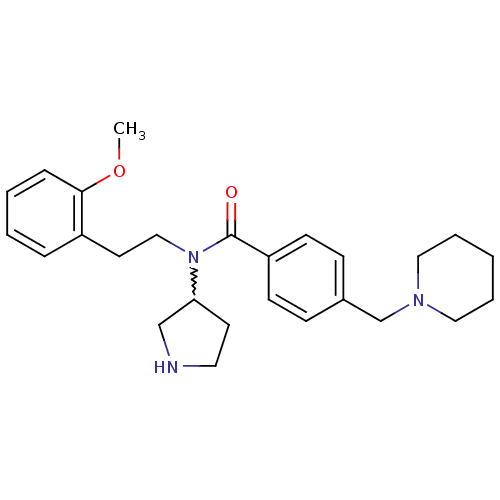 (CHEMBL394028 | N-(2-methoxyphenethyl)-4-(piperidin...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-Groton Laboratories Curated by ChEMBL | Assay Description Inhibition of human S1P expressed in CHOK1 cells | Bioorg Med Chem Lett 17: 4411-4 (2007) Article DOI: 10.1016/j.bmcl.2007.06.031 BindingDB Entry DOI: 10.7270/Q26M36JG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM142369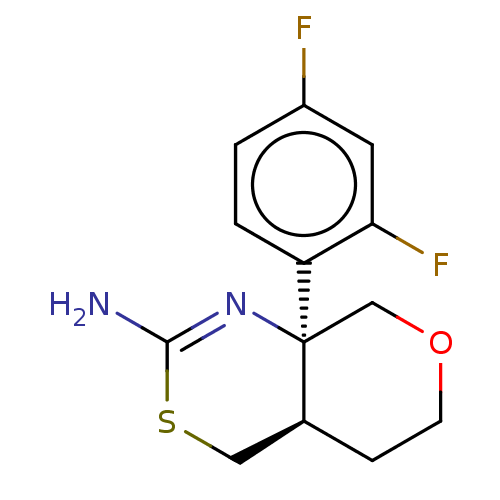 (US8933221, 10) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 265 | n/a | n/a | n/a | n/a | n/a | n/a |
Eurofarma Laboratorios S.A. Curated by ChEMBL | Assay Description Inhibition of BACE1 in human H4 cells overexpressing APP695 assessed as sAPPbeta level after 18 hrs by ELISA | J Med Chem 58: 2678-702 (2015) Article DOI: 10.1021/jm501833t BindingDB Entry DOI: 10.7270/Q2W37Z1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50078322 (CHEMBL3414710) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Eurofarma Laboratorios S.A. Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) using biotin-GLTNIKTEEISEISYEVEFR-C[Oregon Green]KK-OH as substrate after 3 hrs by fluorescence polarization ass... | J Med Chem 58: 2678-702 (2015) Article DOI: 10.1021/jm501833t BindingDB Entry DOI: 10.7270/Q2W37Z1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane-bound transcription factor site-1 protease (Homo sapiens (Human)) | BDBM50216206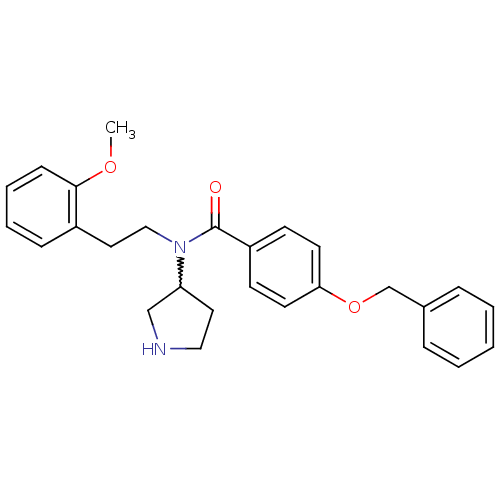 (CHEMBL393989 | N-(2-methoxyphenethyl)-4-(benzyloxy...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-Groton Laboratories Curated by ChEMBL | Assay Description Inhibition of human S1P expressed in CHOK1 cells | Bioorg Med Chem Lett 17: 4411-4 (2007) Article DOI: 10.1016/j.bmcl.2007.06.031 BindingDB Entry DOI: 10.7270/Q26M36JG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50078352 (CHEMBL3414705) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 375 | n/a | n/a | n/a | n/a | n/a | n/a |
Eurofarma Laboratorios S.A. Curated by ChEMBL | Assay Description Inhibition of BACE1 in human H4 cells overexpressing APP695 assessed as sAPPbeta level after 18 hrs by ELISA | J Med Chem 58: 2678-702 (2015) Article DOI: 10.1021/jm501833t BindingDB Entry DOI: 10.7270/Q2W37Z1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane-bound transcription factor site-1 protease (Homo sapiens (Human)) | BDBM50216191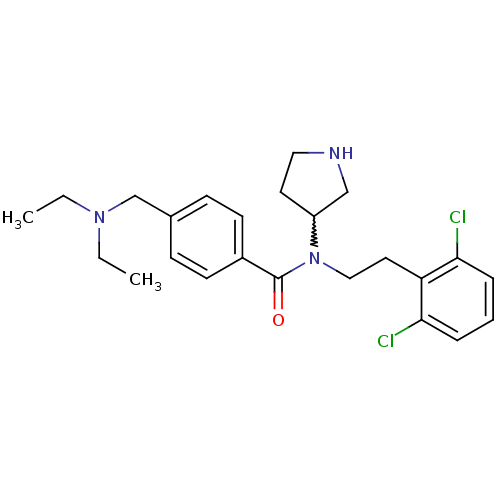 (CHEMBL232587 | N-(2,6-dichlorophenethyl)-4-((dieth...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-Groton Laboratories Curated by ChEMBL | Assay Description Inhibition of human S1P expressed in CHOK1 cells | Bioorg Med Chem Lett 17: 4411-4 (2007) Article DOI: 10.1016/j.bmcl.2007.06.031 BindingDB Entry DOI: 10.7270/Q26M36JG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50078323 (CHEMBL3414709) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
Eurofarma Laboratorios S.A. Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) using biotin-GLTNIKTEEISEISYEVEFR-C[Oregon Green]KK-OH as substrate after 3 hrs by fluorescence polarization ass... | J Med Chem 58: 2678-702 (2015) Article DOI: 10.1021/jm501833t BindingDB Entry DOI: 10.7270/Q2W37Z1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane-bound transcription factor site-1 protease (Homo sapiens (Human)) | BDBM50216187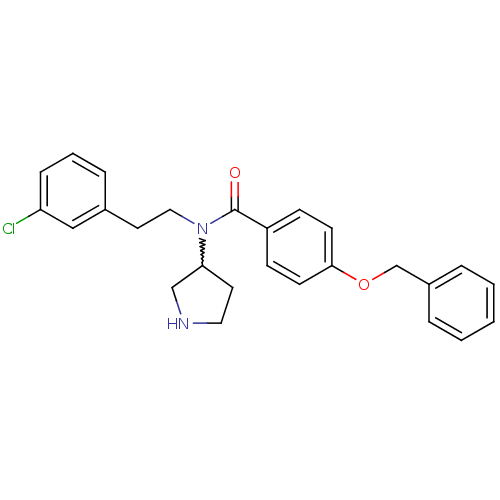 (CHEMBL232777 | N-(3-chlorophenethyl)-4-(benzyloxy)...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-Groton Laboratories Curated by ChEMBL | Assay Description Inhibition of human S1P expressed in CHOK1 cells | Bioorg Med Chem Lett 17: 4411-4 (2007) Article DOI: 10.1016/j.bmcl.2007.06.031 BindingDB Entry DOI: 10.7270/Q26M36JG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane-bound transcription factor site-1 protease (Homo sapiens (Human)) | BDBM50216196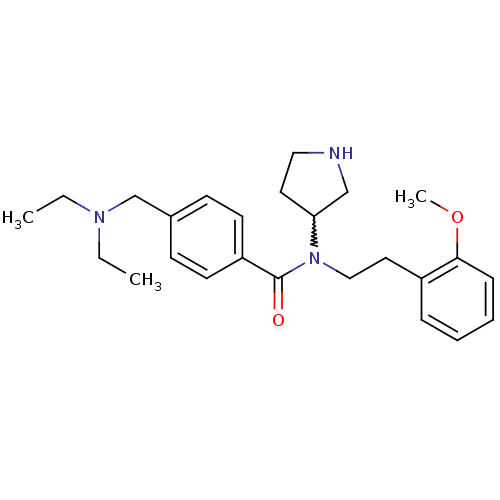 (CHEMBL231776 | N-(2-methoxyphenethyl)-4-((diethyla...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 540 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-Groton Laboratories Curated by ChEMBL | Assay Description Inhibition of human S1P expressed in CHOK1 cells | Bioorg Med Chem Lett 17: 4411-4 (2007) Article DOI: 10.1016/j.bmcl.2007.06.031 BindingDB Entry DOI: 10.7270/Q26M36JG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane-bound transcription factor site-1 protease (Homo sapiens (Human)) | BDBM50216183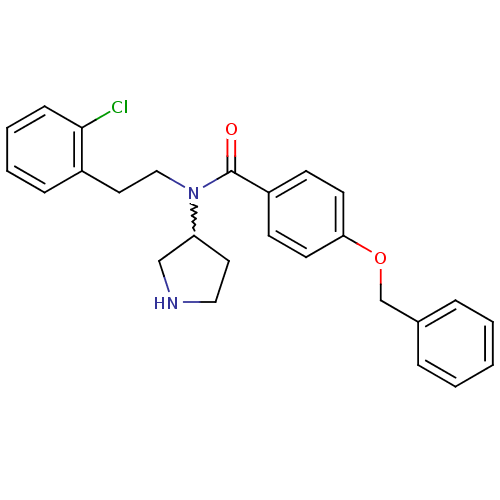 (CHEMBL233573 | N-(2-chlorophenethyl)-4-(benzyloxy)...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 590 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-Groton Laboratories Curated by ChEMBL | Assay Description Inhibition of human S1P expressed in CHOK1 cells | Bioorg Med Chem Lett 17: 4411-4 (2007) Article DOI: 10.1016/j.bmcl.2007.06.031 BindingDB Entry DOI: 10.7270/Q26M36JG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50078320 (CHEMBL3414700 | US9260455, 4) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 670 | n/a | n/a | n/a | n/a | n/a | n/a |
Eurofarma Laboratorios S.A. Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) using biotin-GLTNIKTEEISEISYEVEFR-C[Oregon Green]KK-OH as substrate after 3 hrs by fluorescence polarization ass... | J Med Chem 58: 2678-702 (2015) Article DOI: 10.1021/jm501833t BindingDB Entry DOI: 10.7270/Q2W37Z1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50078349 (CHEMBL3414707 | US9260455, 3) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Eurofarma Laboratorios S.A. Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) using biotin-GLTNIKTEEISEISYEVEFR-C[Oregon Green]KK-OH as substrate after 3 hrs by fluorescence polarization ass... | J Med Chem 58: 2678-702 (2015) Article DOI: 10.1021/jm501833t BindingDB Entry DOI: 10.7270/Q2W37Z1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophospholipase-like protein 1 (Homo sapiens (Human)) | BDBM195576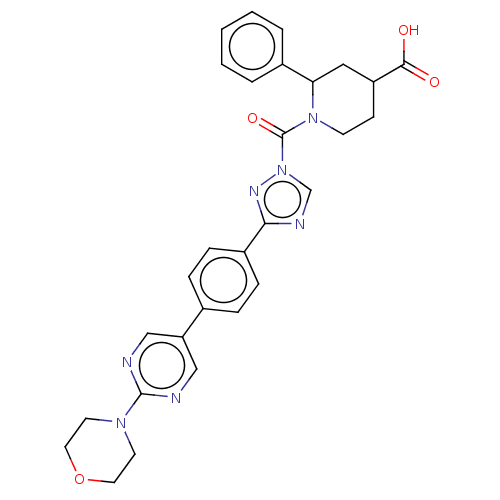 (cis-1-(3-(4-(2-morpholinopyrimidin-5-yl)phenyl)-1H...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 710 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Pfizer Inc. | Assay Description For determination of IC50 values for LYPLAL1 inhibitors, the reactions were carried out in 1.5 mL microcentrifuge tubes in a total reaction volume of... | ACS Chem Biol 11: 2529-40 (2016) Article DOI: 10.1021/acschembio.6b00266 BindingDB Entry DOI: 10.7270/Q24X56K5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane-bound transcription factor site-1 protease (Homo sapiens (Human)) | BDBM50216207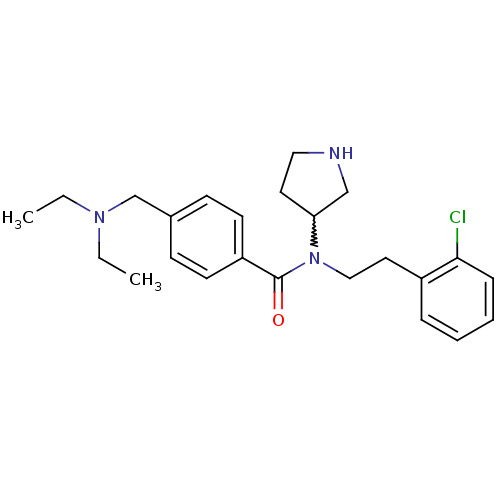 (CHEMBL233572 | N-(2-chlorophenethyl)-4-((diethylam...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 840 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-Groton Laboratories Curated by ChEMBL | Assay Description Inhibition of human S1P expressed in CHOK1 cells | Bioorg Med Chem Lett 17: 4411-4 (2007) Article DOI: 10.1016/j.bmcl.2007.06.031 BindingDB Entry DOI: 10.7270/Q26M36JG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50078351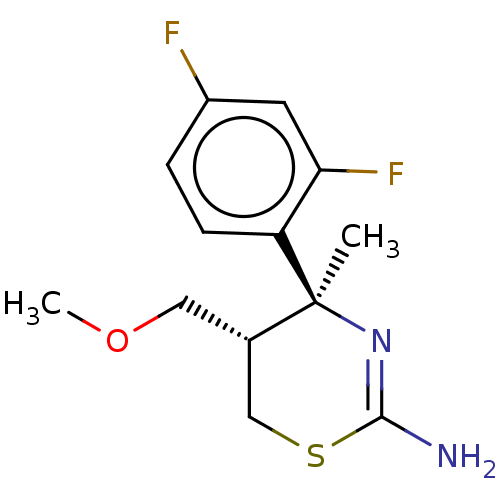 (CHEMBL3414703) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 850 | n/a | n/a | n/a | n/a | n/a | n/a |
Eurofarma Laboratorios S.A. Curated by ChEMBL | Assay Description Inhibition of BACE1 in human H4 cells overexpressing APP695 assessed as sAPPbeta level after 18 hrs by ELISA | J Med Chem 58: 2678-702 (2015) Article DOI: 10.1021/jm501833t BindingDB Entry DOI: 10.7270/Q2W37Z1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50078324 (CHEMBL3414708 | US9260455, 8) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 850 | n/a | n/a | n/a | n/a | n/a | n/a |
Eurofarma Laboratorios S.A. Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) using biotin-GLTNIKTEEISEISYEVEFR-C[Oregon Green]KK-OH as substrate after 3 hrs by fluorescence polarization ass... | J Med Chem 58: 2678-702 (2015) Article DOI: 10.1021/jm501833t BindingDB Entry DOI: 10.7270/Q2W37Z1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane-bound transcription factor site-1 protease (Homo sapiens (Human)) | BDBM50216208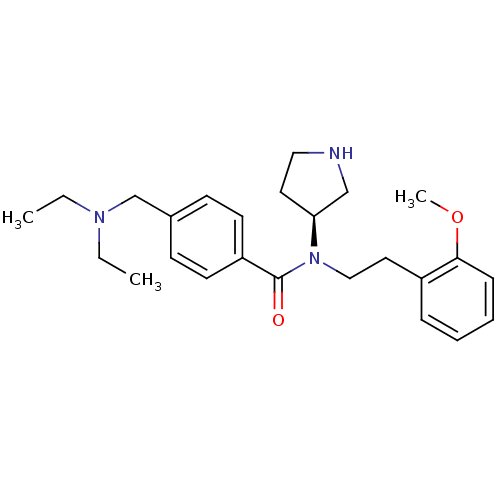 ((S)-N-(2-methoxyphenethyl)-4-((diethylamino)methyl...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 970 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-Groton Laboratories Curated by ChEMBL | Assay Description Inhibition of human S1P expressed in CHOK1 cells | Bioorg Med Chem Lett 17: 4411-4 (2007) Article DOI: 10.1016/j.bmcl.2007.06.031 BindingDB Entry DOI: 10.7270/Q26M36JG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM142394 (US8933221, 4) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eurofarma Laboratorios S.A. Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) using biotin-GLTNIKTEEISEISYEVEFR-C[Oregon Green]KK-OH as substrate after 3 hrs by fluorescence polarization ass... | J Med Chem 58: 2678-702 (2015) Article DOI: 10.1021/jm501833t BindingDB Entry DOI: 10.7270/Q2W37Z1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophospholipase-like protein 1 (Homo sapiens (Human)) | BDBM195579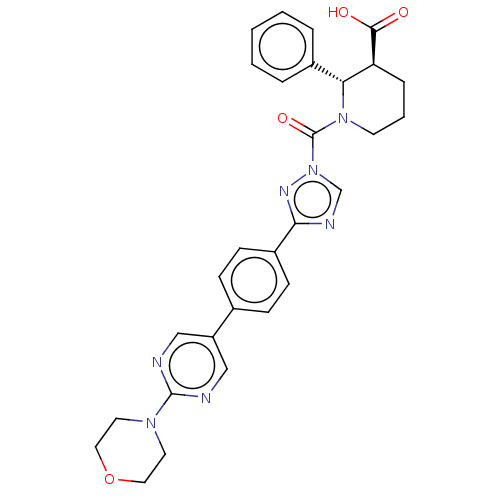 ((2S,3S)-1-(3-(4-(2-morpholinopyrimidin-5-yl)phenyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Pfizer Inc. | Assay Description For determination of IC50 values for LYPLAL1 inhibitors, the reactions were carried out in 1.5 mL microcentrifuge tubes in a total reaction volume of... | ACS Chem Biol 11: 2529-40 (2016) Article DOI: 10.1021/acschembio.6b00266 BindingDB Entry DOI: 10.7270/Q24X56K5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane-bound transcription factor site-1 protease (Homo sapiens (Human)) | BDBM50216186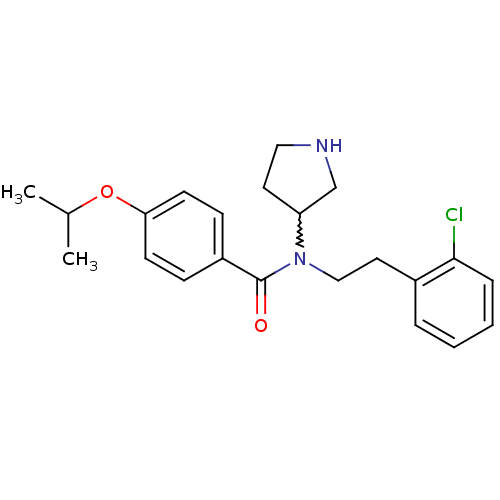 (CHEMBL232577 | N-(2-chlorophenethyl)-4-isopropoxy-...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-Groton Laboratories Curated by ChEMBL | Assay Description Inhibition of human S1P expressed in CHOK1 cells | Bioorg Med Chem Lett 17: 4411-4 (2007) Article DOI: 10.1016/j.bmcl.2007.06.031 BindingDB Entry DOI: 10.7270/Q26M36JG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 90 total ) | Next | Last >> |