 Found 46 hits with Last Name = 'velentza' and Initial = 'a'
Found 46 hits with Last Name = 'velentza' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM4779

(CHEMBL31965 | CHEMBL545315 | CI-1033 | Canertinib ...)Show SMILES Fc1ccc(Nc2ncnc3cc(OCCCN4CCOCC4)c(NC(=O)C=C)cc23)cc1Cl Show InChI InChI=1S/C24H25ClFN5O3/c1-2-23(32)30-21-13-17-20(14-22(21)34-9-3-6-31-7-10-33-11-8-31)27-15-28-24(17)29-16-4-5-19(26)18(25)12-16/h2,4-5,12-15H,1,3,6-11H2,(H,30,32)(H,27,28,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of EGFR (unknown origin) |
Bioorg Med Chem Lett 18: 5916-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.062
BindingDB Entry DOI: 10.7270/Q2M0457X |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-4
(Homo sapiens (Human)) | BDBM4567

(4-anilinoquinazoline deriv. 2 | BMC163482 Compound...)Show InChI InChI=1S/C17H13BrN4O/c1-2-16(23)21-13-6-7-15-14(9-13)17(20-10-19-15)22-12-5-3-4-11(18)8-12/h2-10H,1H2,(H,21,23)(H,19,20,22) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of HER4 (unknown origin) |
Bioorg Med Chem Lett 18: 5916-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.062
BindingDB Entry DOI: 10.7270/Q2M0457X |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-4
(Homo sapiens (Human)) | BDBM4779

(CHEMBL31965 | CHEMBL545315 | CI-1033 | Canertinib ...)Show SMILES Fc1ccc(Nc2ncnc3cc(OCCCN4CCOCC4)c(NC(=O)C=C)cc23)cc1Cl Show InChI InChI=1S/C24H25ClFN5O3/c1-2-23(32)30-21-13-17-20(14-22(21)34-9-3-6-31-7-10-33-11-8-31)27-15-28-24(17)29-16-4-5-19(26)18(25)12-16/h2,4-5,12-15H,1,3,6-11H2,(H,30,32)(H,27,28,29) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of HER4 (unknown origin) |
Bioorg Med Chem Lett 18: 5916-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.062
BindingDB Entry DOI: 10.7270/Q2M0457X |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM4567

(4-anilinoquinazoline deriv. 2 | BMC163482 Compound...)Show InChI InChI=1S/C17H13BrN4O/c1-2-16(23)21-13-6-7-15-14(9-13)17(20-10-19-15)22-12-5-3-4-11(18)8-12/h2-10H,1H2,(H,21,23)(H,19,20,22) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of HER2 (unknown origin) |
Bioorg Med Chem Lett 18: 5916-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.062
BindingDB Entry DOI: 10.7270/Q2M0457X |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Blk
(Homo sapiens (Human)) | BDBM4779

(CHEMBL31965 | CHEMBL545315 | CI-1033 | Canertinib ...)Show SMILES Fc1ccc(Nc2ncnc3cc(OCCCN4CCOCC4)c(NC(=O)C=C)cc23)cc1Cl Show InChI InChI=1S/C24H25ClFN5O3/c1-2-23(32)30-21-13-17-20(14-22(21)34-9-3-6-31-7-10-33-11-8-31)27-15-28-24(17)29-16-4-5-19(26)18(25)12-16/h2,4-5,12-15H,1,3,6-11H2,(H,30,32)(H,27,28,29) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Blk (unknown origin) expressed in mouse BAF3 cells assessed as cytotoxicity |
Bioorg Med Chem Lett 18: 5916-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.062
BindingDB Entry DOI: 10.7270/Q2M0457X |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM4779

(CHEMBL31965 | CHEMBL545315 | CI-1033 | Canertinib ...)Show SMILES Fc1ccc(Nc2ncnc3cc(OCCCN4CCOCC4)c(NC(=O)C=C)cc23)cc1Cl Show InChI InChI=1S/C24H25ClFN5O3/c1-2-23(32)30-21-13-17-20(14-22(21)34-9-3-6-31-7-10-33-11-8-31)27-15-28-24(17)29-16-4-5-19(26)18(25)12-16/h2,4-5,12-15H,1,3,6-11H2,(H,30,32)(H,27,28,29) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of HER2 (unknown origin) |
Bioorg Med Chem Lett 18: 5916-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.062
BindingDB Entry DOI: 10.7270/Q2M0457X |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Blk
(Homo sapiens (Human)) | BDBM4779

(CHEMBL31965 | CHEMBL545315 | CI-1033 | Canertinib ...)Show SMILES Fc1ccc(Nc2ncnc3cc(OCCCN4CCOCC4)c(NC(=O)C=C)cc23)cc1Cl Show InChI InChI=1S/C24H25ClFN5O3/c1-2-23(32)30-21-13-17-20(14-22(21)34-9-3-6-31-7-10-33-11-8-31)27-15-28-24(17)29-16-4-5-19(26)18(25)12-16/h2,4-5,12-15H,1,3,6-11H2,(H,30,32)(H,27,28,29) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Blk (unknown origin) |
Bioorg Med Chem Lett 18: 5916-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.062
BindingDB Entry DOI: 10.7270/Q2M0457X |
More data for this
Ligand-Target Pair | |
Cytoplasmic tyrosine-protein kinase BMX
(Homo sapiens (Human)) | BDBM4779

(CHEMBL31965 | CHEMBL545315 | CI-1033 | Canertinib ...)Show SMILES Fc1ccc(Nc2ncnc3cc(OCCCN4CCOCC4)c(NC(=O)C=C)cc23)cc1Cl Show InChI InChI=1S/C24H25ClFN5O3/c1-2-23(32)30-21-13-17-20(14-22(21)34-9-3-6-31-7-10-33-11-8-31)27-15-28-24(17)29-16-4-5-19(26)18(25)12-16/h2,4-5,12-15H,1,3,6-11H2,(H,30,32)(H,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Bmx (unknown origin) expressed in mouse BAF3 cells assessed as cytotoxicity |
Bioorg Med Chem Lett 18: 5916-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.062
BindingDB Entry DOI: 10.7270/Q2M0457X |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-3
(Homo sapiens (Human)) | BDBM50585538
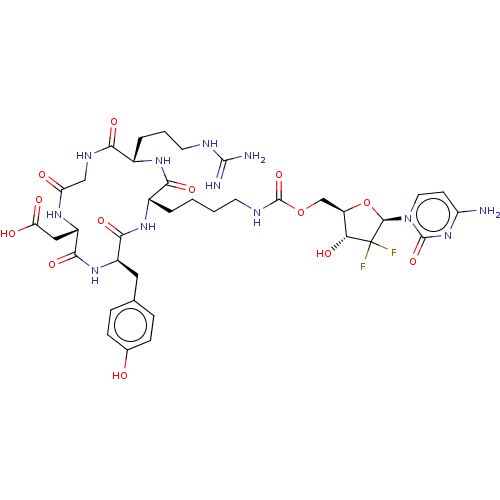
(CHEMBL5087579)Show SMILES NC(=N)NCCC[C@@H]1NC(=O)[C@H](CCCCNC(=O)OC[C@H]2O[C@@H](n3ccc(N)nc3=O)C(F)(F)[C@@H]2O)NC(=O)[C@@H](Cc2ccc(O)cc2)NC(=O)[C@H](CC(O)=O)NC(=O)CNC1=O |r| | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of alphavbeta3 integrin receptor-mediated cell adhesion to vitronectin in human A549 cells preincubated for 30 mins followed by VN substra... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01468
BindingDB Entry DOI: 10.7270/Q2SN0DVG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM4779

(CHEMBL31965 | CHEMBL545315 | CI-1033 | Canertinib ...)Show SMILES Fc1ccc(Nc2ncnc3cc(OCCCN4CCOCC4)c(NC(=O)C=C)cc23)cc1Cl Show InChI InChI=1S/C24H25ClFN5O3/c1-2-23(32)30-21-13-17-20(14-22(21)34-9-3-6-31-7-10-33-11-8-31)27-15-28-24(17)29-16-4-5-19(26)18(25)12-16/h2,4-5,12-15H,1,3,6-11H2,(H,30,32)(H,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 185 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Btk (unknown origin) |
Bioorg Med Chem Lett 18: 5916-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.062
BindingDB Entry DOI: 10.7270/Q2M0457X |
More data for this
Ligand-Target Pair | |
Breakpoint cluster region protein/Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM13530

(4-[(4-methylpiperazin-1-yl)methyl]-N-[4-methyl-3-[...)Show SMILES CN1CCN(Cc2ccc(cc2)C(=O)Nc2ccc(C)c(Nc3nccc(n3)-c3cccnc3)c2)CC1 Show InChI InChI=1S/C29H31N7O/c1-21-5-10-25(18-27(21)34-29-31-13-11-26(33-29)24-4-3-12-30-19-24)32-28(37)23-8-6-22(7-9-23)20-36-16-14-35(2)15-17-36/h3-13,18-19H,14-17,20H2,1-2H3,(H,32,37)(H,31,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 204 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Inhibition of Bcr-abl tyrosine phosphorylation in mouse BA/F3ells p210 Bcr-abl after 90 mins by ELISA |
Nat Chem Biol 2: 95-102 (2006)
Article DOI: 10.1038/nchembio760
BindingDB Entry DOI: 10.7270/Q2H41RNN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Integrin alpha-V/beta-3
(Homo sapiens (Human)) | BDBM50585538

(CHEMBL5087579)Show SMILES NC(=N)NCCC[C@@H]1NC(=O)[C@H](CCCCNC(=O)OC[C@H]2O[C@@H](n3ccc(N)nc3=O)C(F)(F)[C@@H]2O)NC(=O)[C@@H](Cc2ccc(O)cc2)NC(=O)[C@H](CC(O)=O)NC(=O)CNC1=O |r| | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of alphavbeta3 integrin receptor-mediated cell adhesion to vitronectin in human WM 266-4 cells preincubated for 30 mins followed by VN sub... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01468
BindingDB Entry DOI: 10.7270/Q2SN0DVG |
More data for this
Ligand-Target Pair | |
Breakpoint cluster region protein/Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50325999
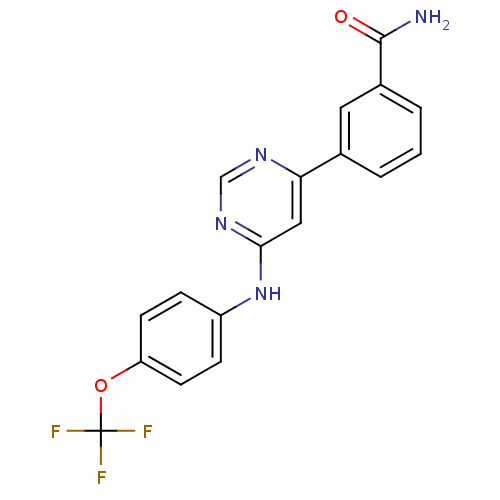
(3-(6-(4-(trifluoromethoxy)phenylamino)pyrimidin-4-...)Show SMILES NC(=O)c1cccc(c1)-c1cc(Nc2ccc(OC(F)(F)F)cc2)ncn1 Show InChI InChI=1S/C18H13F3N4O2/c19-18(20,21)27-14-6-4-13(5-7-14)25-16-9-15(23-10-24-16)11-2-1-3-12(8-11)17(22)26/h1-10H,(H2,22,26)(H,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 267 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Inhibition of Bcr-abl tyrosine phosphorylation in mouse BA/F3ells p210 Bcr-abl after 90 mins by ELISA |
Nat Chem Biol 2: 95-102 (2006)
Article DOI: 10.1038/nchembio760
BindingDB Entry DOI: 10.7270/Q2H41RNN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytoplasmic tyrosine-protein kinase BMX
(Homo sapiens (Human)) | BDBM4567

(4-anilinoquinazoline deriv. 2 | BMC163482 Compound...)Show InChI InChI=1S/C17H13BrN4O/c1-2-16(23)21-13-6-7-15-14(9-13)17(20-10-19-15)22-12-5-3-4-11(18)8-12/h2-10H,1H2,(H,21,23)(H,19,20,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 303 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Bmx (unknown origin) expressed in mouse BAF3 cells assessed as cytotoxicity |
Bioorg Med Chem Lett 18: 5916-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.062
BindingDB Entry DOI: 10.7270/Q2M0457X |
More data for this
Ligand-Target Pair | |
Cytoplasmic tyrosine-protein kinase BMX
(Homo sapiens (Human)) | BDBM4567

(4-anilinoquinazoline deriv. 2 | BMC163482 Compound...)Show InChI InChI=1S/C17H13BrN4O/c1-2-16(23)21-13-6-7-15-14(9-13)17(20-10-19-15)22-12-5-3-4-11(18)8-12/h2-10H,1H2,(H,21,23)(H,19,20,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 324 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of wild type Bmx (unknown origin) expressed in mouse BAF3 cells assessed as cytotoxicity |
Bioorg Med Chem Lett 18: 5916-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.062
BindingDB Entry DOI: 10.7270/Q2M0457X |
More data for this
Ligand-Target Pair | |
Cytoplasmic tyrosine-protein kinase BMX
(Homo sapiens (Human)) | BDBM4779

(CHEMBL31965 | CHEMBL545315 | CI-1033 | Canertinib ...)Show SMILES Fc1ccc(Nc2ncnc3cc(OCCCN4CCOCC4)c(NC(=O)C=C)cc23)cc1Cl Show InChI InChI=1S/C24H25ClFN5O3/c1-2-23(32)30-21-13-17-20(14-22(21)34-9-3-6-31-7-10-33-11-8-31)27-15-28-24(17)29-16-4-5-19(26)18(25)12-16/h2,4-5,12-15H,1,3,6-11H2,(H,30,32)(H,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 586 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Bmx (unknown origin) |
Bioorg Med Chem Lett 18: 5916-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.062
BindingDB Entry DOI: 10.7270/Q2M0457X |
More data for this
Ligand-Target Pair | |
Cytoplasmic tyrosine-protein kinase BMX
(Homo sapiens (Human)) | BDBM4567

(4-anilinoquinazoline deriv. 2 | BMC163482 Compound...)Show InChI InChI=1S/C17H13BrN4O/c1-2-16(23)21-13-6-7-15-14(9-13)17(20-10-19-15)22-12-5-3-4-11(18)8-12/h2-10H,1H2,(H,21,23)(H,19,20,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Bmx (unknown origin) |
Bioorg Med Chem Lett 18: 5916-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.062
BindingDB Entry DOI: 10.7270/Q2M0457X |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-3
(Homo sapiens (Human)) | BDBM50390492
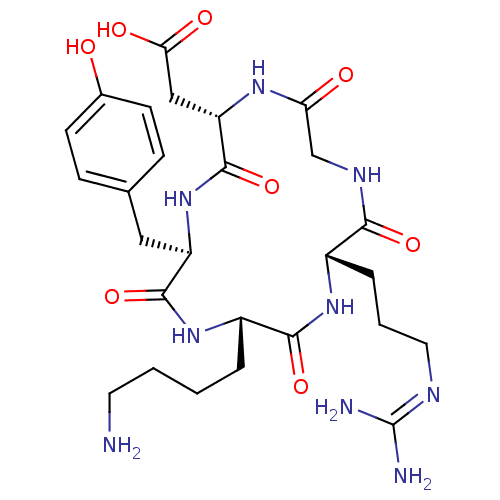
(CHEMBL2071605)Show SMILES [#7]-[#6]-[#6]-[#6]-[#6]-[#6@@H]-1-[#7]-[#6](=O)-[#6@@H](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6]-1=O |r| Show InChI InChI=1S/C27H41N9O8/c28-10-2-1-4-18-24(42)34-17(5-3-11-31-27(29)30)23(41)32-14-21(38)33-20(13-22(39)40)26(44)36-19(25(43)35-18)12-15-6-8-16(37)9-7-15/h6-9,17-20,37H,1-5,10-14,28H2,(H,32,41)(H,33,38)(H,34,42)(H,35,43)(H,36,44)(H,39,40)(H4,29,30,31)/t17-,18-,19+,20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of alphavbeta3 integrin receptor-mediated cell adhesion to vitronectin in human WM 266-4 cells preincubated for 30 mins followed by VN sub... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01468
BindingDB Entry DOI: 10.7270/Q2SN0DVG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM4779

(CHEMBL31965 | CHEMBL545315 | CI-1033 | Canertinib ...)Show SMILES Fc1ccc(Nc2ncnc3cc(OCCCN4CCOCC4)c(NC(=O)C=C)cc23)cc1Cl Show InChI InChI=1S/C24H25ClFN5O3/c1-2-23(32)30-21-13-17-20(14-22(21)34-9-3-6-31-7-10-33-11-8-31)27-15-28-24(17)29-16-4-5-19(26)18(25)12-16/h2,4-5,12-15H,1,3,6-11H2,(H,30,32)(H,27,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of JAK3 (unknown origin) expressed in mouse BAF3 cells assessed as cytotoxicity |
Bioorg Med Chem Lett 18: 5916-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.062
BindingDB Entry DOI: 10.7270/Q2M0457X |
More data for this
Ligand-Target Pair | |
O94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139
(Homo sapiens (Human)) | BDBM50469778
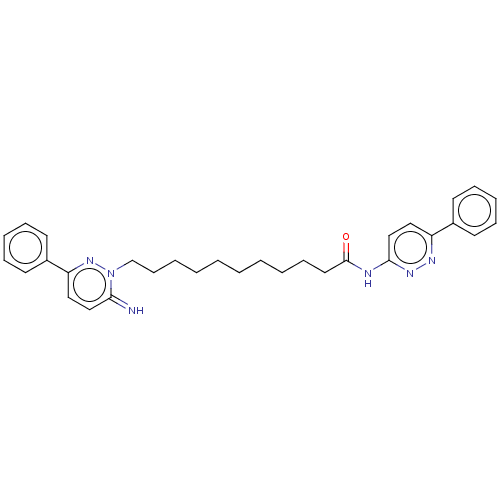
(CHEMBL116963)Show SMILES N=c1ccc(nn1CCCCCCCCCCC(=O)Nc1ccc(nn1)-c1ccccc1)-c1ccccc1 Show InChI InChI=1S/C31H36N6O/c32-29-22-20-28(26-17-11-8-12-18-26)36-37(29)24-14-6-4-2-1-3-5-13-19-31(38)33-30-23-21-27(34-35-30)25-15-9-7-10-16-25/h7-12,15-18,20-23,32H,1-6,13-14,19,24H2,(H,33,35,38) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase C |
Bioorg Med Chem Lett 13: 3465-70 (2003)
Article DOI: 10.1016/s0960-894x(03)00733-9
BindingDB Entry DOI: 10.7270/Q2V69N9R |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM4567

(4-anilinoquinazoline deriv. 2 | BMC163482 Compound...)Show InChI InChI=1S/C17H13BrN4O/c1-2-16(23)21-13-6-7-15-14(9-13)17(20-10-19-15)22-12-5-3-4-11(18)8-12/h2-10H,1H2,(H,21,23)(H,19,20,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.14E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of JAK3 (unknown origin) |
Bioorg Med Chem Lett 18: 5916-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.062
BindingDB Entry DOI: 10.7270/Q2M0457X |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM4779

(CHEMBL31965 | CHEMBL545315 | CI-1033 | Canertinib ...)Show SMILES Fc1ccc(Nc2ncnc3cc(OCCCN4CCOCC4)c(NC(=O)C=C)cc23)cc1Cl Show InChI InChI=1S/C24H25ClFN5O3/c1-2-23(32)30-21-13-17-20(14-22(21)34-9-3-6-31-7-10-33-11-8-31)27-15-28-24(17)29-16-4-5-19(26)18(25)12-16/h2,4-5,12-15H,1,3,6-11H2,(H,30,32)(H,27,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.88E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of JAK3 (unknown origin) |
Bioorg Med Chem Lett 18: 5916-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.062
BindingDB Entry DOI: 10.7270/Q2M0457X |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Blk
(Homo sapiens (Human)) | BDBM4567

(4-anilinoquinazoline deriv. 2 | BMC163482 Compound...)Show InChI InChI=1S/C17H13BrN4O/c1-2-16(23)21-13-6-7-15-14(9-13)17(20-10-19-15)22-12-5-3-4-11(18)8-12/h2-10H,1H2,(H,21,23)(H,19,20,22) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.47E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Blk (unknown origin) |
Bioorg Med Chem Lett 18: 5916-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.062
BindingDB Entry DOI: 10.7270/Q2M0457X |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM4779

(CHEMBL31965 | CHEMBL545315 | CI-1033 | Canertinib ...)Show SMILES Fc1ccc(Nc2ncnc3cc(OCCCN4CCOCC4)c(NC(=O)C=C)cc23)cc1Cl Show InChI InChI=1S/C24H25ClFN5O3/c1-2-23(32)30-21-13-17-20(14-22(21)34-9-3-6-31-7-10-33-11-8-31)27-15-28-24(17)29-16-4-5-19(26)18(25)12-16/h2,4-5,12-15H,1,3,6-11H2,(H,30,32)(H,27,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.65E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Itk (unknown origin) |
Bioorg Med Chem Lett 18: 5916-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.062
BindingDB Entry DOI: 10.7270/Q2M0457X |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM4567

(4-anilinoquinazoline deriv. 2 | BMC163482 Compound...)Show InChI InChI=1S/C17H13BrN4O/c1-2-16(23)21-13-6-7-15-14(9-13)17(20-10-19-15)22-12-5-3-4-11(18)8-12/h2-10H,1H2,(H,21,23)(H,19,20,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.83E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Btk (unknown origin) |
Bioorg Med Chem Lett 18: 5916-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.062
BindingDB Entry DOI: 10.7270/Q2M0457X |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-3
(Homo sapiens (Human)) | BDBM50585539
(CHEMBL5091463)Show SMILES NC(=N)NCCC[C@@H]1NC(=O)[C@H](CCCCNC(=O)OCCSSCCOC(=O)OC[C@H]2O[C@@H](n3ccc(N)nc3=O)C(F)(F)[C@@H]2O)NC(=O)[C@@H](Cc2ccc(O)cc2)NC(=O)[C@H](CC(O)=O)NC(=O)CNC1=O |r| | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of alphavbeta3 integrin receptor-mediated cell adhesion to vitronectin in human WM 266-4 cells preincubated for 30 mins followed by VN sub... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01468
BindingDB Entry DOI: 10.7270/Q2SN0DVG |
More data for this
Ligand-Target Pair | |
Myosin light chain kinase, smooth muscle
(Gallus gallus (chicken)) | BDBM50108799

((2-[N -(2-hydroxyethyl)]-N -(4-methoxybenzenesulfo...)Show SMILES COc1ccc(cc1)S(=O)(=O)N(CCO)c1ccccc1CN(C)C\C=C\c1ccc(Cl)cc1 Show InChI InChI=1S/C26H29ClN2O4S/c1-28(17-5-6-21-9-11-23(27)12-10-21)20-22-7-3-4-8-26(22)29(18-19-30)34(31,32)25-15-13-24(33-2)14-16-25/h3-16,30H,17-20H2,1-2H3/b6-5+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
| PubMed
| n/a | n/a | 9.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Myosin Light Chain kinase (MLCK) |
J Med Chem 45: 563-6 (2002)
BindingDB Entry DOI: 10.7270/Q2GH9H8B |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Blk
(Homo sapiens (Human)) | BDBM4567

(4-anilinoquinazoline deriv. 2 | BMC163482 Compound...)Show InChI InChI=1S/C17H13BrN4O/c1-2-16(23)21-13-6-7-15-14(9-13)17(20-10-19-15)22-12-5-3-4-11(18)8-12/h2-10H,1H2,(H,21,23)(H,19,20,22) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Blk (unknown origin) expressed in mouse BAF3 cells assessed as cytotoxicity |
Bioorg Med Chem Lett 18: 5916-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.062
BindingDB Entry DOI: 10.7270/Q2M0457X |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM4567

(4-anilinoquinazoline deriv. 2 | BMC163482 Compound...)Show InChI InChI=1S/C17H13BrN4O/c1-2-16(23)21-13-6-7-15-14(9-13)17(20-10-19-15)22-12-5-3-4-11(18)8-12/h2-10H,1H2,(H,21,23)(H,19,20,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of JAK3 (unknown origin) expressed in mouse BAF3 cells assessed as cytotoxicity |
Bioorg Med Chem Lett 18: 5916-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.062
BindingDB Entry DOI: 10.7270/Q2M0457X |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase STK11
(Homo sapiens (Human)) | BDBM4779

(CHEMBL31965 | CHEMBL545315 | CI-1033 | Canertinib ...)Show SMILES Fc1ccc(Nc2ncnc3cc(OCCCN4CCOCC4)c(NC(=O)C=C)cc23)cc1Cl Show InChI InChI=1S/C24H25ClFN5O3/c1-2-23(32)30-21-13-17-20(14-22(21)34-9-3-6-31-7-10-33-11-8-31)27-15-28-24(17)29-16-4-5-19(26)18(25)12-16/h2,4-5,12-15H,1,3,6-11H2,(H,30,32)(H,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Lkb1 (unknown origin) |
Bioorg Med Chem Lett 18: 5916-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.062
BindingDB Entry DOI: 10.7270/Q2M0457X |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase STK11
(Homo sapiens (Human)) | BDBM4567

(4-anilinoquinazoline deriv. 2 | BMC163482 Compound...)Show InChI InChI=1S/C17H13BrN4O/c1-2-16(23)21-13-6-7-15-14(9-13)17(20-10-19-15)22-12-5-3-4-11(18)8-12/h2-10H,1H2,(H,21,23)(H,19,20,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Lkb1 (unknown origin) |
Bioorg Med Chem Lett 18: 5916-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.062
BindingDB Entry DOI: 10.7270/Q2M0457X |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM4567

(4-anilinoquinazoline deriv. 2 | BMC163482 Compound...)Show InChI InChI=1S/C17H13BrN4O/c1-2-16(23)21-13-6-7-15-14(9-13)17(20-10-19-15)22-12-5-3-4-11(18)8-12/h2-10H,1H2,(H,21,23)(H,19,20,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Itk (unknown origin) |
Bioorg Med Chem Lett 18: 5916-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.062
BindingDB Entry DOI: 10.7270/Q2M0457X |
More data for this
Ligand-Target Pair | |
O94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139
(Homo sapiens (Human)) | BDBM50469776
(CHEMBL117443)Show SMILES Cc1ccc(=N)n(CCCCCCCCCCC(=O)Nc2ccc(nn2)-c2ccccc2)n1 Show InChI InChI=1S/C26H34N6O/c1-21-16-18-24(27)32(31-21)20-12-7-5-3-2-4-6-11-15-26(33)28-25-19-17-23(29-30-25)22-13-9-8-10-14-22/h8-10,13-14,16-19,27H,2-7,11-12,15,20H2,1H3,(H,28,30,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase C |
Bioorg Med Chem Lett 13: 3465-70 (2003)
Article DOI: 10.1016/s0960-894x(03)00733-9
BindingDB Entry DOI: 10.7270/Q2V69N9R |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-3
(Homo sapiens (Human)) | BDBM50585539

(CHEMBL5091463)Show SMILES NC(=N)NCCC[C@@H]1NC(=O)[C@H](CCCCNC(=O)OCCSSCCOC(=O)OC[C@H]2O[C@@H](n3ccc(N)nc3=O)C(F)(F)[C@@H]2O)NC(=O)[C@@H](Cc2ccc(O)cc2)NC(=O)[C@H](CC(O)=O)NC(=O)CNC1=O |r| | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of alphavbeta3 integrin receptor-mediated cell adhesion to vitronectin in human A549 cells preincubated for 30 mins followed by VN substra... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01468
BindingDB Entry DOI: 10.7270/Q2SN0DVG |
More data for this
Ligand-Target Pair | |
cAMP-dependent protein kinase catalytic subunit gamma
(Homo sapiens (Human)) | BDBM50469778

(CHEMBL116963)Show SMILES N=c1ccc(nn1CCCCCCCCCCC(=O)Nc1ccc(nn1)-c1ccccc1)-c1ccccc1 Show InChI InChI=1S/C31H36N6O/c32-29-22-20-28(26-17-11-8-12-18-26)36-37(29)24-14-6-4-2-1-3-5-13-19-31(38)33-30-23-21-27(34-35-30)25-15-9-7-10-16-25/h7-12,15-18,20-23,32H,1-6,13-14,19,24H2,(H,33,35,38) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase A |
Bioorg Med Chem Lett 13: 3465-70 (2003)
Article DOI: 10.1016/s0960-894x(03)00733-9
BindingDB Entry DOI: 10.7270/Q2V69N9R |
More data for this
Ligand-Target Pair | |
Calcium/calmodulin-dependent protein kinase type II subunit gamma
(Homo sapiens (Human)) | BDBM50074289
((4-Methyl-6-phenyl-pyridazin-3-yl)-(2-morpholin-4-...)Show InChI InChI=1S/C17H22N4O/c1-14-13-16(15-5-3-2-4-6-15)19-20-17(14)18-7-8-21-9-11-22-12-10-21/h2-6,13H,7-12H2,1H3,(H,18,20) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of Calcium/calmodulin-dependent protein kinase type II |
Bioorg Med Chem Lett 13: 3465-70 (2003)
Article DOI: 10.1016/s0960-894x(03)00733-9
BindingDB Entry DOI: 10.7270/Q2V69N9R |
More data for this
Ligand-Target Pair | |
Calcium/calmodulin-dependent protein kinase type II subunit gamma
(Homo sapiens (Human)) | BDBM50469778

(CHEMBL116963)Show SMILES N=c1ccc(nn1CCCCCCCCCCC(=O)Nc1ccc(nn1)-c1ccccc1)-c1ccccc1 Show InChI InChI=1S/C31H36N6O/c32-29-22-20-28(26-17-11-8-12-18-26)36-37(29)24-14-6-4-2-1-3-5-13-19-31(38)33-30-23-21-27(34-35-30)25-15-9-7-10-16-25/h7-12,15-18,20-23,32H,1-6,13-14,19,24H2,(H,33,35,38) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of Calcium/calmodulin-dependent protein kinase type II |
Bioorg Med Chem Lett 13: 3465-70 (2003)
Article DOI: 10.1016/s0960-894x(03)00733-9
BindingDB Entry DOI: 10.7270/Q2V69N9R |
More data for this
Ligand-Target Pair | |
Calcium/calmodulin-dependent protein kinase type II subunit gamma
(Homo sapiens (Human)) | BDBM50469777

(CHEMBL115531)Show SMILES N=c1cc2CCc3ccccc3-c2nn1CCCCCCCCCCC(=O)Nc1ccc(nn1)-c1ccccc1 Show InChI InChI=1S/C33H38N6O/c34-30-24-27-20-19-25-14-11-12-17-28(25)33(27)38-39(30)23-13-6-4-2-1-3-5-10-18-32(40)35-31-22-21-29(36-37-31)26-15-8-7-9-16-26/h7-9,11-12,14-17,21-22,24,34H,1-6,10,13,18-20,23H2,(H,35,37,40) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of Calcium/calmodulin-dependent protein kinase type II |
Bioorg Med Chem Lett 13: 3465-70 (2003)
Article DOI: 10.1016/s0960-894x(03)00733-9
BindingDB Entry DOI: 10.7270/Q2V69N9R |
More data for this
Ligand-Target Pair | |
cAMP-dependent protein kinase catalytic subunit gamma
(Homo sapiens (Human)) | BDBM50469777

(CHEMBL115531)Show SMILES N=c1cc2CCc3ccccc3-c2nn1CCCCCCCCCCC(=O)Nc1ccc(nn1)-c1ccccc1 Show InChI InChI=1S/C33H38N6O/c34-30-24-27-20-19-25-14-11-12-17-28(25)33(27)38-39(30)23-13-6-4-2-1-3-5-10-18-32(40)35-31-22-21-29(36-37-31)26-15-8-7-9-16-26/h7-9,11-12,14-17,21-22,24,34H,1-6,10,13,18-20,23H2,(H,35,37,40) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase A |
Bioorg Med Chem Lett 13: 3465-70 (2003)
Article DOI: 10.1016/s0960-894x(03)00733-9
BindingDB Entry DOI: 10.7270/Q2V69N9R |
More data for this
Ligand-Target Pair | |
cAMP-dependent protein kinase catalytic subunit gamma
(Homo sapiens (Human)) | BDBM50469776

(CHEMBL117443)Show SMILES Cc1ccc(=N)n(CCCCCCCCCCC(=O)Nc2ccc(nn2)-c2ccccc2)n1 Show InChI InChI=1S/C26H34N6O/c1-21-16-18-24(27)32(31-21)20-12-7-5-3-2-4-6-11-15-26(33)28-25-19-17-23(29-30-25)22-13-9-8-10-14-22/h8-10,13-14,16-19,27H,2-7,11-12,15,20H2,1H3,(H,28,30,33) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase A |
Bioorg Med Chem Lett 13: 3465-70 (2003)
Article DOI: 10.1016/s0960-894x(03)00733-9
BindingDB Entry DOI: 10.7270/Q2V69N9R |
More data for this
Ligand-Target Pair | |
cAMP-dependent protein kinase catalytic subunit gamma
(Homo sapiens (Human)) | BDBM50074289

((4-Methyl-6-phenyl-pyridazin-3-yl)-(2-morpholin-4-...)Show InChI InChI=1S/C17H22N4O/c1-14-13-16(15-5-3-2-4-6-15)19-20-17(14)18-7-8-21-9-11-22-12-10-21/h2-6,13H,7-12H2,1H3,(H,18,20) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase A |
Bioorg Med Chem Lett 13: 3465-70 (2003)
Article DOI: 10.1016/s0960-894x(03)00733-9
BindingDB Entry DOI: 10.7270/Q2V69N9R |
More data for this
Ligand-Target Pair | |
O94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139
(Homo sapiens (Human)) | BDBM50074289

((4-Methyl-6-phenyl-pyridazin-3-yl)-(2-morpholin-4-...)Show InChI InChI=1S/C17H22N4O/c1-14-13-16(15-5-3-2-4-6-15)19-20-17(14)18-7-8-21-9-11-22-12-10-21/h2-6,13H,7-12H2,1H3,(H,18,20) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase C |
Bioorg Med Chem Lett 13: 3465-70 (2003)
Article DOI: 10.1016/s0960-894x(03)00733-9
BindingDB Entry DOI: 10.7270/Q2V69N9R |
More data for this
Ligand-Target Pair | |
O94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139
(Homo sapiens (Human)) | BDBM50469777

(CHEMBL115531)Show SMILES N=c1cc2CCc3ccccc3-c2nn1CCCCCCCCCCC(=O)Nc1ccc(nn1)-c1ccccc1 Show InChI InChI=1S/C33H38N6O/c34-30-24-27-20-19-25-14-11-12-17-28(25)33(27)38-39(30)23-13-6-4-2-1-3-5-10-18-32(40)35-31-22-21-29(36-37-31)26-15-8-7-9-16-26/h7-9,11-12,14-17,21-22,24,34H,1-6,10,13,18-20,23H2,(H,35,37,40) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase C |
Bioorg Med Chem Lett 13: 3465-70 (2003)
Article DOI: 10.1016/s0960-894x(03)00733-9
BindingDB Entry DOI: 10.7270/Q2V69N9R |
More data for this
Ligand-Target Pair | |
Myosin light chain kinase, smooth muscle
(Gallus gallus (chicken)) | BDBM50108800
(11-(3-Imino-5,6-dihydro-3H-benzo[h]cinnolin-2-yl)-...)Show SMILES N=c1cc2CCc3ccccc3-c2nn1CCCCCCCCCCC(=O)N1CCN(CC1)c1ncccn1 Show InChI InChI=1S/C31H41N7O/c32-28-24-26-16-15-25-12-8-9-13-27(25)30(26)35-38(28)19-10-6-4-2-1-3-5-7-14-29(39)36-20-22-37(23-21-36)31-33-17-11-18-34-31/h8-9,11-13,17-18,24,32H,1-7,10,14-16,19-23H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Myosin Light Chain kinase (MLCK) |
J Med Chem 45: 563-6 (2002)
BindingDB Entry DOI: 10.7270/Q2GH9H8B |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-3
(Homo sapiens (Human)) | BDBM50390492

(CHEMBL2071605)Show SMILES [#7]-[#6]-[#6]-[#6]-[#6]-[#6@@H]-1-[#7]-[#6](=O)-[#6@@H](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6]-1=O |r| Show InChI InChI=1S/C27H41N9O8/c28-10-2-1-4-18-24(42)34-17(5-3-11-31-27(29)30)23(41)32-14-21(38)33-20(13-22(39)40)26(44)36-19(25(43)35-18)12-15-6-8-16(37)9-7-15/h6-9,17-20,37H,1-5,10-14,28H2,(H,32,41)(H,33,38)(H,34,42)(H,35,43)(H,36,44)(H,39,40)(H4,29,30,31)/t17-,18-,19+,20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of alphavbeta3 integrin receptor-mediated cell adhesion to vitronectin in human A549 cells preincubated for 30 mins followed by VN substra... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01468
BindingDB Entry DOI: 10.7270/Q2SN0DVG |
More data for this
Ligand-Target Pair | |
Calcium/calmodulin-dependent protein kinase type II subunit gamma
(Homo sapiens (Human)) | BDBM50469776

(CHEMBL117443)Show SMILES Cc1ccc(=N)n(CCCCCCCCCCC(=O)Nc2ccc(nn2)-c2ccccc2)n1 Show InChI InChI=1S/C26H34N6O/c1-21-16-18-24(27)32(31-21)20-12-7-5-3-2-4-6-11-15-26(33)28-25-19-17-23(29-30-25)22-13-9-8-10-14-22/h8-10,13-14,16-19,27H,2-7,11-12,15,20H2,1H3,(H,28,30,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of Calcium/calmodulin-dependent protein kinase type II |
Bioorg Med Chem Lett 13: 3465-70 (2003)
Article DOI: 10.1016/s0960-894x(03)00733-9
BindingDB Entry DOI: 10.7270/Q2V69N9R |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data