

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50319300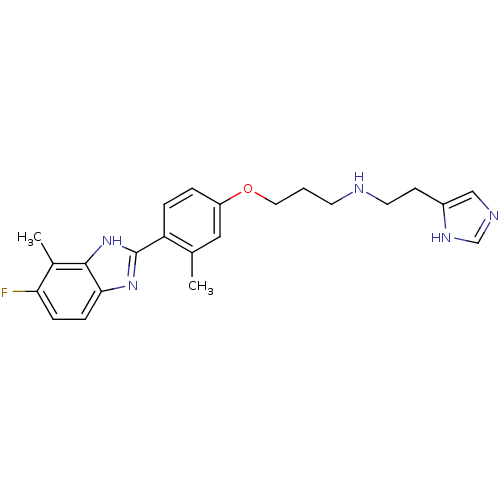 (CHEMBL1083162 | N-(2-(1H-imidazol-4-yl)ethyl)-3-(4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human recombinant histamine H4 receptor | Bioorg Med Chem Lett 20: 3367-71 (2010) Article DOI: 10.1016/j.bmcl.2010.04.017 BindingDB Entry DOI: 10.7270/Q23J3D4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM22904 ((2R)-1-(1H-imidazol-5-yl)propan-2-amine | (R)-alph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity to the human histamine H3 receptor | J Med Chem 46: 3957-60 (2003) Article DOI: 10.1021/jm0341047 BindingDB Entry DOI: 10.7270/Q2QJ7J1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50356884 (CHEMBL1915540) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development, LLC Curated by ChEMBL | Assay Description Binding affinity to human histamine H4 receptor | J Med Chem 58: 7119-27 (2015) Article DOI: 10.1021/acs.jmedchem.5b00516 BindingDB Entry DOI: 10.7270/Q20G3MZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Rattus norvegicus (rat)) | BDBM22566 (5-chloro-2-[(4-methylpiperazin-1-yl)carbonyl]-1H-i...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity of compound towards rat histamine H4 receptor | J Med Chem 46: 3957-60 (2003) Article DOI: 10.1021/jm0341047 BindingDB Entry DOI: 10.7270/Q2QJ7J1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50179351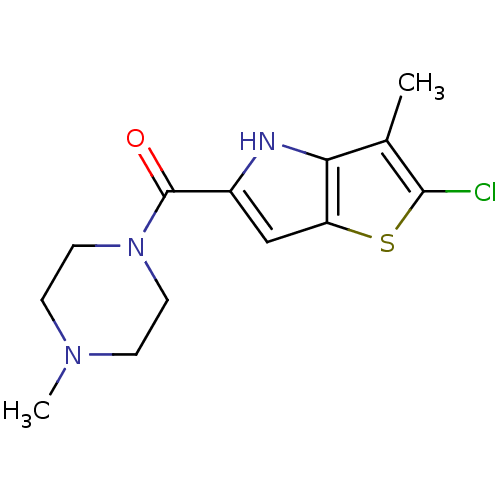 ((2-chloro-3-methyl-4H-thieno[3,2-b]pyrrol-5-yl)(4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]histamine from recombinant human histamine H4 receptor in SK-N-MC cells | J Med Chem 48: 8289-98 (2005) Article DOI: 10.1021/jm0502081 BindingDB Entry DOI: 10.7270/Q2C24W00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM315434 (US10172856, Example 86) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description Receptor binding was assessed using isolated plasma membranes from SKNMC neuroblastoma cell lines stably expressing recombinant human H4 receptor. To... | US Patent US10172856 (2019) BindingDB Entry DOI: 10.7270/Q2BV7JQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Rattus norvegicus (rat)) | BDBM50133004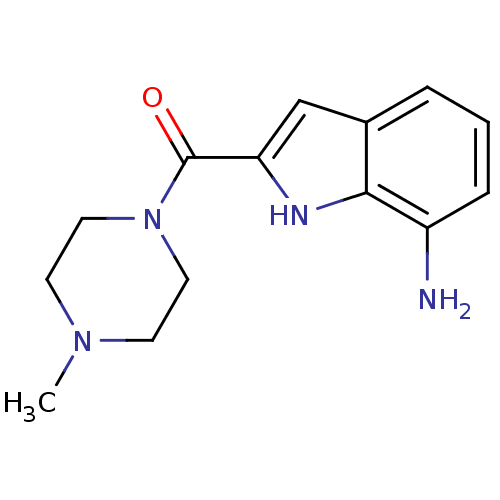 ((7-Amino-1H-indol-2-yl)-(4-methyl-piperazin-1-yl)-...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity of compound towards rat histamine H4 receptor | J Med Chem 46: 3957-60 (2003) Article DOI: 10.1021/jm0341047 BindingDB Entry DOI: 10.7270/Q2QJ7J1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50319302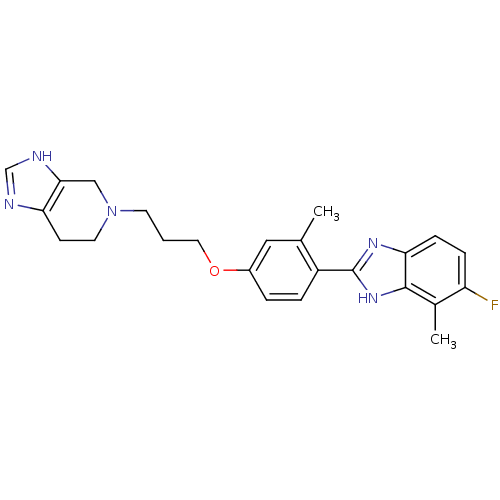 (5-(3-(4-(5-fluoro-4-methyl-1H-benzo[d]imidazol-2-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human recombinant histamine H4 receptor | Bioorg Med Chem Lett 20: 3367-71 (2010) Article DOI: 10.1016/j.bmcl.2010.04.017 BindingDB Entry DOI: 10.7270/Q23J3D4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM22566 (5-chloro-2-[(4-methylpiperazin-1-yl)carbonyl]-1H-i...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]histamine from recombinant human histamine H4 receptor in SK-N-MC cells | J Med Chem 48: 8289-98 (2005) Article DOI: 10.1021/jm0502081 BindingDB Entry DOI: 10.7270/Q2C24W00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM315466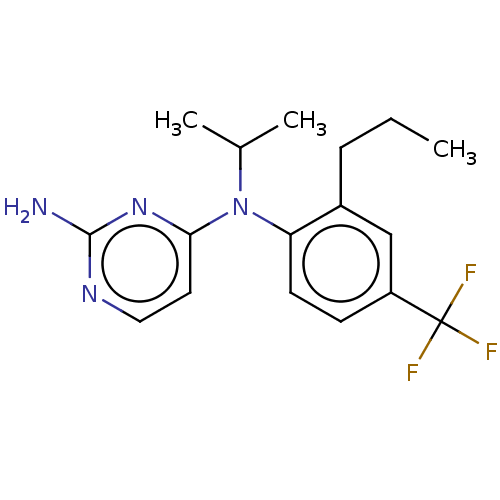 (US10172856, Example 115) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description Receptor binding was assessed using isolated plasma membranes from SKNMC neuroblastoma cell lines stably expressing recombinant human H4 receptor. To... | US Patent US10172856 (2019) BindingDB Entry DOI: 10.7270/Q2BV7JQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM22566 (5-chloro-2-[(4-methylpiperazin-1-yl)carbonyl]-1H-i...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]- histamine from the recombinant human histamine H4 receptor | J Med Chem 46: 3957-60 (2003) Article DOI: 10.1021/jm0341047 BindingDB Entry DOI: 10.7270/Q2QJ7J1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM22566 (5-chloro-2-[(4-methylpiperazin-1-yl)carbonyl]-1H-i...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development, LLC Curated by ChEMBL | Assay Description Binding affinity to human histamine H4 receptor | J Med Chem 58: 7119-27 (2015) Article DOI: 10.1021/acs.jmedchem.5b00516 BindingDB Entry DOI: 10.7270/Q20G3MZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50121205 (CHEBI:18295 | Histamine) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid PDB UniChem Patents | PDB Article PubMed | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development, LLC Curated by ChEMBL | Assay Description Binding affinity to human histamine H4 receptor | J Med Chem 58: 7119-27 (2015) Article DOI: 10.1021/acs.jmedchem.5b00516 BindingDB Entry DOI: 10.7270/Q20G3MZK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50133018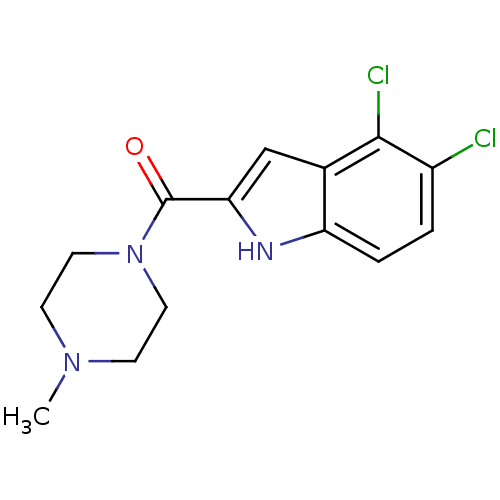 ((4,5-Dichloro-1H-indol-2-yl)-(4-methyl-piperazin-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]- histamine from the recombinant human histamine H4 receptor | J Med Chem 46: 3957-60 (2003) Article DOI: 10.1021/jm0341047 BindingDB Entry DOI: 10.7270/Q2QJ7J1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50133018 ((4,5-Dichloro-1H-indol-2-yl)-(4-methyl-piperazin-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]histamine from recombinant human histamine H4 receptor in SK-N-MC cells | J Med Chem 48: 8289-98 (2005) Article DOI: 10.1021/jm0502081 BindingDB Entry DOI: 10.7270/Q2C24W00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Mus musculus (mouse)) | BDBM50006789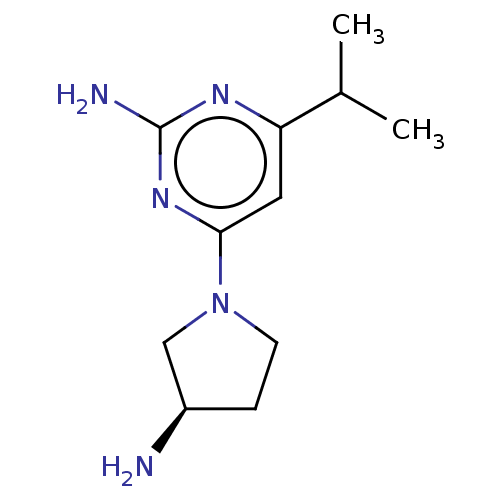 (CHEMBL3236549) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development, LLC Curated by ChEMBL | Assay Description Displacement of [3H]histamine from mouse recombinant histamine H4 receptor | J Med Chem 58: 7119-27 (2015) Article DOI: 10.1021/acs.jmedchem.5b00516 BindingDB Entry DOI: 10.7270/Q20G3MZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50319295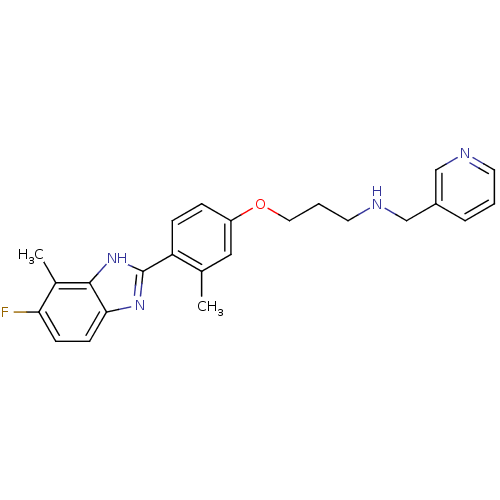 (3-(4-(5-fluoro-4-methyl-1H-benzo[d]imidazol-2-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human recombinant histamine H4 receptor | Bioorg Med Chem Lett 20: 3367-71 (2010) Article DOI: 10.1016/j.bmcl.2010.04.017 BindingDB Entry DOI: 10.7270/Q23J3D4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine receptor H4 (GUINEA PIG) | BDBM50121205 (CHEBI:18295 | Histamine) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid PDB UniChem Patents | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development, LLC Curated by ChEMBL | Assay Description Binding affinity to guinea pig histamine H4 receptor | J Med Chem 58: 7119-27 (2015) Article DOI: 10.1021/acs.jmedchem.5b00516 BindingDB Entry DOI: 10.7270/Q20G3MZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM315348 (US10172856, Example 1) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description Receptor binding was assessed using isolated plasma membranes from SKNMC neuroblastoma cell lines stably expressing recombinant human H4 receptor. To... | US Patent US10172856 (2019) BindingDB Entry DOI: 10.7270/Q2BV7JQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50356884 (CHEMBL1915540) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development, LLC Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human histamine H4 receptor expressed in HEK293T cells | J Med Chem 58: 7119-27 (2015) Article DOI: 10.1021/acs.jmedchem.5b00516 BindingDB Entry DOI: 10.7270/Q20G3MZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50132999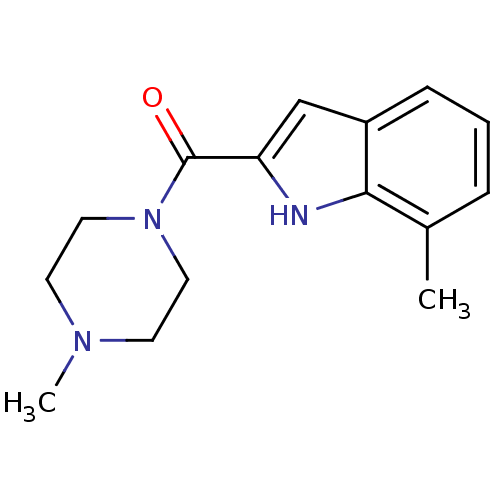 ((7-Methyl-1H-indol-2-yl)-(4-methyl-piperazin-1-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]histamine from recombinant human histamine H4 receptor in SK-N-MC cells | J Med Chem 48: 8289-98 (2005) Article DOI: 10.1021/jm0502081 BindingDB Entry DOI: 10.7270/Q2C24W00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50179340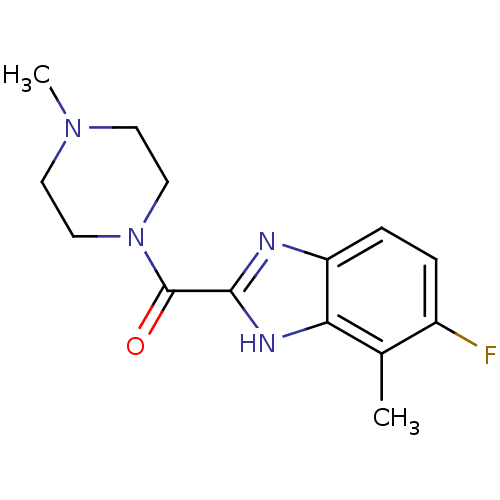 ((5-fluoro-4-methyl-1H-benzoimidazol-2-yl)(4-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]histamine from recombinant human histamine H4 receptor in SK-N-MC cells | J Med Chem 48: 8289-98 (2005) Article DOI: 10.1021/jm0502081 BindingDB Entry DOI: 10.7270/Q2C24W00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM315377 (US10172856, Example 30) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description Receptor binding was assessed using isolated plasma membranes from SKNMC neuroblastoma cell lines stably expressing recombinant human H4 receptor. To... | US Patent US10172856 (2019) BindingDB Entry DOI: 10.7270/Q2BV7JQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM315465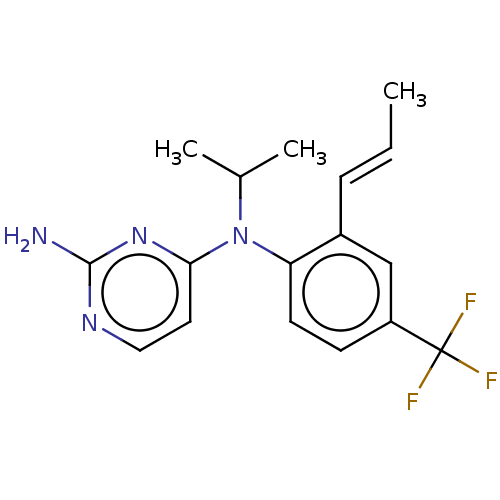 (US10172856, Example 114) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description Receptor binding was assessed using isolated plasma membranes from SKNMC neuroblastoma cell lines stably expressing recombinant human H4 receptor. To... | US Patent US10172856 (2019) BindingDB Entry DOI: 10.7270/Q2BV7JQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50132999 ((7-Methyl-1H-indol-2-yl)-(4-methyl-piperazin-1-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]- histamine from the recombinant human histamine H4 receptor | J Med Chem 46: 3957-60 (2003) Article DOI: 10.1021/jm0341047 BindingDB Entry DOI: 10.7270/Q2QJ7J1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM315471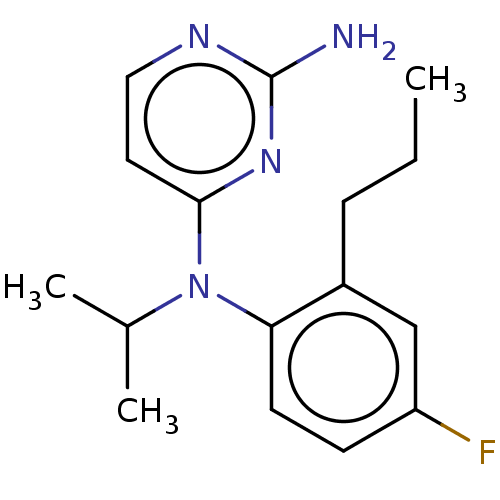 (US10172856, Example 119) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description Receptor binding was assessed using isolated plasma membranes from SKNMC neuroblastoma cell lines stably expressing recombinant human H4 receptor. To... | US Patent US10172856 (2019) BindingDB Entry DOI: 10.7270/Q2BV7JQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM315386 (US10172856, Example 38) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description Receptor binding was assessed using isolated plasma membranes from SKNMC neuroblastoma cell lines stably expressing recombinant human H4 receptor. To... | US Patent US10172856 (2019) BindingDB Entry DOI: 10.7270/Q2BV7JQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50133005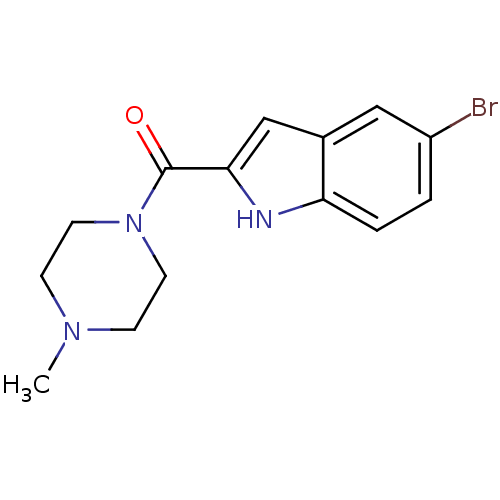 ((5-Bromo-1H-indol-2-yl)-(4-methyl-piperazin-1-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]histamine from recombinant human histamine H4 receptor in SK-N-MC cells | J Med Chem 48: 8289-98 (2005) Article DOI: 10.1021/jm0502081 BindingDB Entry DOI: 10.7270/Q2C24W00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50133004 ((7-Amino-1H-indol-2-yl)-(4-methyl-piperazin-1-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]histamine from recombinant human histamine H4 receptor in SK-N-MC cells | J Med Chem 48: 8289-98 (2005) Article DOI: 10.1021/jm0502081 BindingDB Entry DOI: 10.7270/Q2C24W00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50121205 (CHEBI:18295 | Histamine) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid PDB UniChem Patents | PDB Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development, LLC Curated by ChEMBL | Assay Description Binding affinity to histamine H4 receptor (unknown origin) | J Med Chem 58: 7119-27 (2015) Article DOI: 10.1021/acs.jmedchem.5b00516 BindingDB Entry DOI: 10.7270/Q20G3MZK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM315433 (US10172856, Example 85) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description Receptor binding was assessed using isolated plasma membranes from SKNMC neuroblastoma cell lines stably expressing recombinant human H4 receptor. To... | US Patent US10172856 (2019) BindingDB Entry DOI: 10.7270/Q2BV7JQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM315391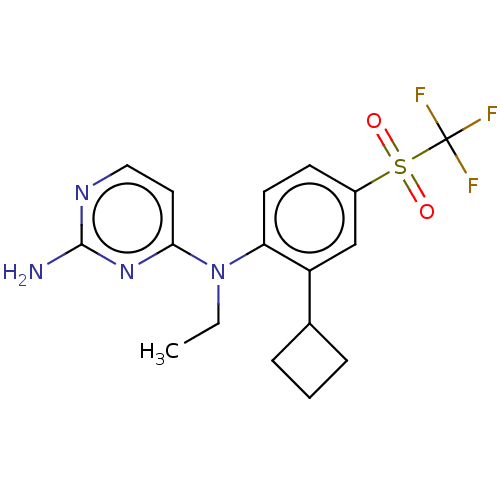 (US10172856, Example 43) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description Receptor binding was assessed using isolated plasma membranes from SKNMC neuroblastoma cell lines stably expressing recombinant human H4 receptor. To... | US Patent US10172856 (2019) BindingDB Entry DOI: 10.7270/Q2BV7JQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50133004 ((7-Amino-1H-indol-2-yl)-(4-methyl-piperazin-1-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]- histamine from the recombinant human histamine H4 receptor | J Med Chem 46: 3957-60 (2003) Article DOI: 10.1021/jm0341047 BindingDB Entry DOI: 10.7270/Q2QJ7J1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50133005 ((5-Bromo-1H-indol-2-yl)-(4-methyl-piperazin-1-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]- histamine from the recombinant human histamine H4 receptor | J Med Chem 46: 3957-60 (2003) Article DOI: 10.1021/jm0341047 BindingDB Entry DOI: 10.7270/Q2QJ7J1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM315370 (US10172856, Example 23) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description Receptor binding was assessed using isolated plasma membranes from SKNMC neuroblastoma cell lines stably expressing recombinant human H4 receptor. To... | US Patent US10172856 (2019) BindingDB Entry DOI: 10.7270/Q2BV7JQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM315366 (US10172856, Example 19) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description Receptor binding was assessed using isolated plasma membranes from SKNMC neuroblastoma cell lines stably expressing recombinant human H4 receptor. To... | US Patent US10172856 (2019) BindingDB Entry DOI: 10.7270/Q2BV7JQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM315372 (US10172856, Example 25) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description Receptor binding was assessed using isolated plasma membranes from SKNMC neuroblastoma cell lines stably expressing recombinant human H4 receptor. To... | US Patent US10172856 (2019) BindingDB Entry DOI: 10.7270/Q2BV7JQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM315353 (US10172856, Example 6) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description Receptor binding was assessed using isolated plasma membranes from SKNMC neuroblastoma cell lines stably expressing recombinant human H4 receptor. To... | US Patent US10172856 (2019) BindingDB Entry DOI: 10.7270/Q2BV7JQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM315381 (US10172856, Example 33) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description Receptor binding was assessed using isolated plasma membranes from SKNMC neuroblastoma cell lines stably expressing recombinant human H4 receptor. To... | US Patent US10172856 (2019) BindingDB Entry DOI: 10.7270/Q2BV7JQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM315458 (US10172856, Example 109) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description Receptor binding was assessed using isolated plasma membranes from SKNMC neuroblastoma cell lines stably expressing recombinant human H4 receptor. To... | US Patent US10172856 (2019) BindingDB Entry DOI: 10.7270/Q2BV7JQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM471715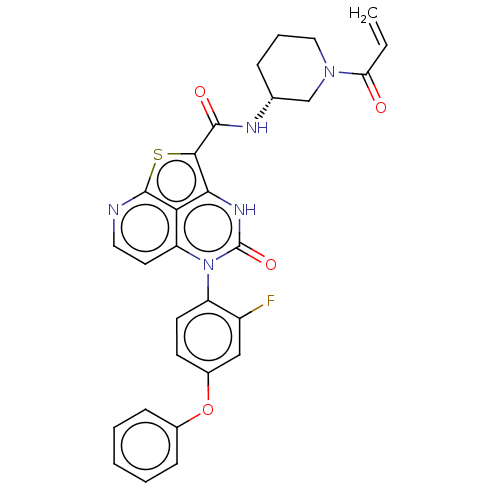 ((R)-N-(1-Acryloylpiperidin-3-yl)-5-(2-fluoro-4-phe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 9.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Covalent inhibition of recombinant human GST-tagged BTK (2 to 659 end residues) expressed in baculovirus expression system assessed as inhibition con... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00044 BindingDB Entry DOI: 10.7270/Q25D8WM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM315399 (US10172856, Example 51) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description Receptor binding was assessed using isolated plasma membranes from SKNMC neuroblastoma cell lines stably expressing recombinant human H4 receptor. To... | US Patent US10172856 (2019) BindingDB Entry DOI: 10.7270/Q2BV7JQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50133011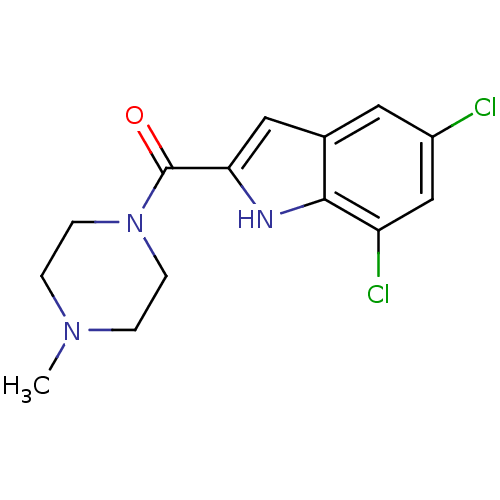 ((5,7-Dichloro-1H-indol-2-yl)-(4-methyl-piperazin-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]- histamine from the recombinant human histamine H4 receptor | J Med Chem 46: 3957-60 (2003) Article DOI: 10.1021/jm0341047 BindingDB Entry DOI: 10.7270/Q2QJ7J1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM315470 (US10172856, Example 118) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description Receptor binding was assessed using isolated plasma membranes from SKNMC neuroblastoma cell lines stably expressing recombinant human H4 receptor. To... | US Patent US10172856 (2019) BindingDB Entry DOI: 10.7270/Q2BV7JQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM315435 (US10172856, Example 87) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description Receptor binding was assessed using isolated plasma membranes from SKNMC neuroblastoma cell lines stably expressing recombinant human H4 receptor. To... | US Patent US10172856 (2019) BindingDB Entry DOI: 10.7270/Q2BV7JQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50133011 ((5,7-Dichloro-1H-indol-2-yl)-(4-methyl-piperazin-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]histamine from recombinant human histamine H4 receptor in SK-N-MC cells | J Med Chem 48: 8289-98 (2005) Article DOI: 10.1021/jm0502081 BindingDB Entry DOI: 10.7270/Q2C24W00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Rattus norvegicus (rat)) | BDBM50356884 (CHEMBL1915540) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development, LLC Curated by ChEMBL | Assay Description Displacement of [3H]histamine from rat histamine H4 receptor expressed in HEK293T cells | J Med Chem 58: 7119-27 (2015) Article DOI: 10.1021/acs.jmedchem.5b00516 BindingDB Entry DOI: 10.7270/Q20G3MZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50006789 (CHEMBL3236549) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development, LLC Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human recombinant histamine H4 receptor | J Med Chem 58: 7119-27 (2015) Article DOI: 10.1021/acs.jmedchem.5b00516 BindingDB Entry DOI: 10.7270/Q20G3MZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM315385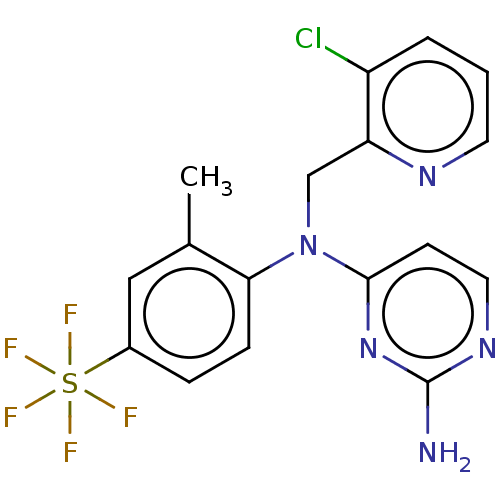 (US10172856, Example 37) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description Receptor binding was assessed using isolated plasma membranes from SKNMC neuroblastoma cell lines stably expressing recombinant human H4 receptor. To... | US Patent US10172856 (2019) BindingDB Entry DOI: 10.7270/Q2BV7JQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM315355 (US10172856, Example 8) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description Receptor binding was assessed using isolated plasma membranes from SKNMC neuroblastoma cell lines stably expressing recombinant human H4 receptor. To... | US Patent US10172856 (2019) BindingDB Entry DOI: 10.7270/Q2BV7JQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 4658 total ) | Next | Last >> |