

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50202775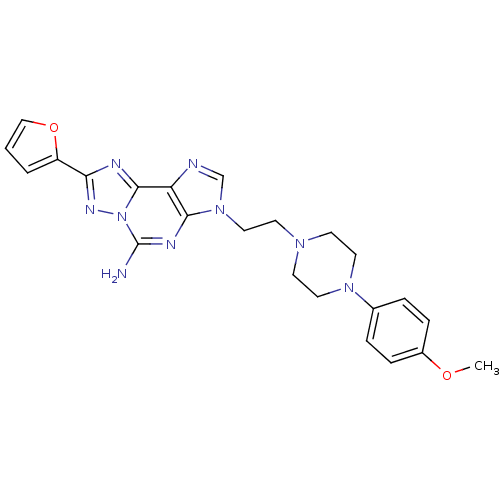 (8-(furan-2-yl)-3-(2-(4-(4-methoxyphenyl)piperazin-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human adenosine A2A receptor | Bioorg Med Chem Lett 17: 1659-62 (2007) Article DOI: 10.1016/j.bmcl.2006.12.104 BindingDB Entry DOI: 10.7270/Q2RF5TPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50202777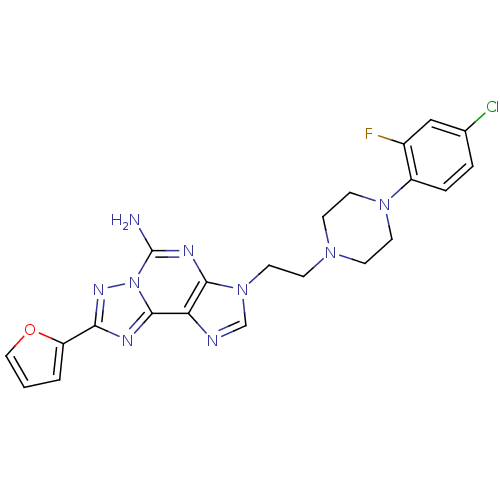 (3-(2-(4-(4-chloro-2-fluorophenyl)piperazin-1-yl)et...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human adenosine A2A receptor | Bioorg Med Chem Lett 17: 1659-62 (2007) Article DOI: 10.1016/j.bmcl.2006.12.104 BindingDB Entry DOI: 10.7270/Q2RF5TPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50202788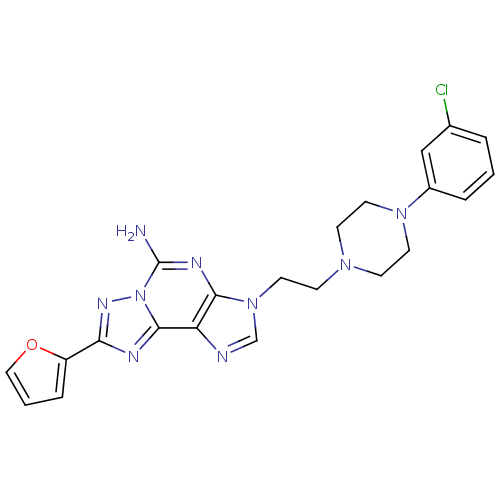 (3-(2-(4-(3-chlorophenyl)piperazin-1-yl)ethyl)-8-(f...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human adenosine A2A receptor | Bioorg Med Chem Lett 17: 1659-62 (2007) Article DOI: 10.1016/j.bmcl.2006.12.104 BindingDB Entry DOI: 10.7270/Q2RF5TPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50202771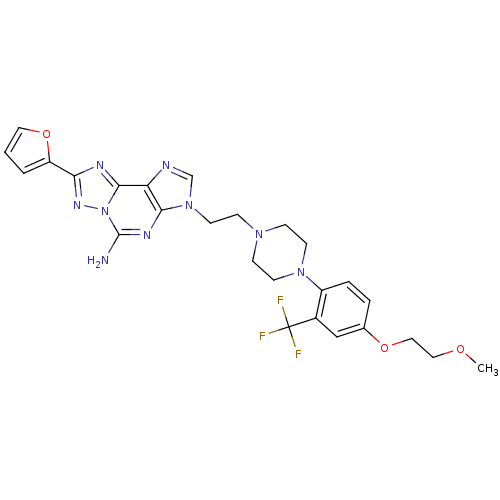 (8-(furan-2-yl)-3-(2-(4-(4-(2-methoxyethoxy)-2-(tri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human adenosine A2A receptor | Bioorg Med Chem Lett 17: 1659-62 (2007) Article DOI: 10.1016/j.bmcl.2006.12.104 BindingDB Entry DOI: 10.7270/Q2RF5TPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM21398 (4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Scios Nova Inc. Curated by ChEMBL | Assay Description In vitro binding affinity towards dopamine receptor D2 in rat striatal membranes by [3H]-sulpiride displacement. | J Med Chem 36: 3073-6 (1993) BindingDB Entry DOI: 10.7270/Q2RF5T2N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM21398 (4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Scios Nova Inc. Curated by ChEMBL | Assay Description Binding affinity against dopamine receptor D2 from rat corpus striatum by using radioligand [3H]-sulpiride | J Med Chem 36: 1488-95 (1993) BindingDB Entry DOI: 10.7270/Q2KD1X0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 2 (5-HT2) receptor (RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50001775 ((ritanserin)6-(2-{4-[Bis-(4-fluoro-phenyl)-methyle...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Scios Nova Inc. Curated by ChEMBL | Assay Description In vitro binding affinity towards 5-hydroxytryptamine 2 receptor in rat striatal membranes by [3H]ketanserin displacement. | J Med Chem 36: 3073-6 (1993) BindingDB Entry DOI: 10.7270/Q2RF5T2N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50202779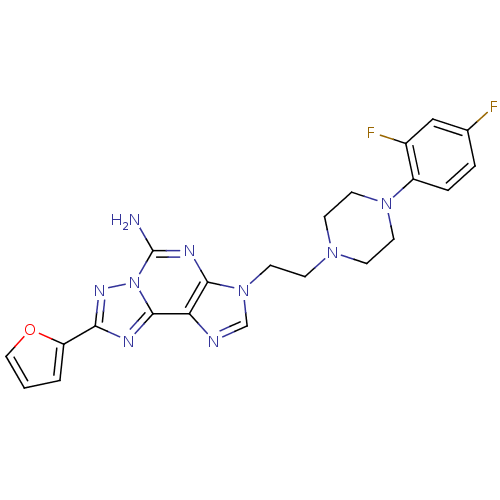 (3-(2-(4-(2,4-difluorophenyl)piperazin-1-yl)ethyl)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human adenosine A2A receptor | Bioorg Med Chem Lett 17: 1659-62 (2007) Article DOI: 10.1016/j.bmcl.2006.12.104 BindingDB Entry DOI: 10.7270/Q2RF5TPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50202790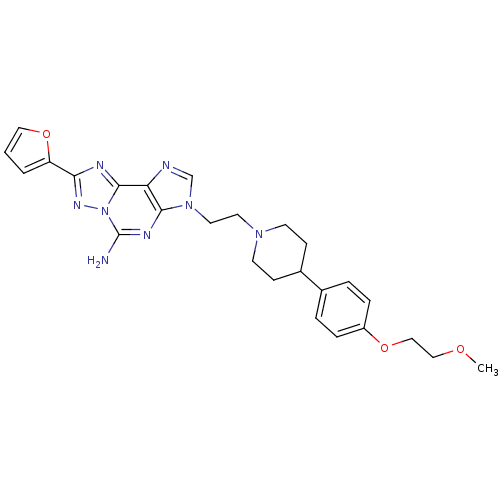 (8-(furan-2-yl)-3-(2-(4-(4-(2-methoxyethoxy)phenyl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human adenosine A2A receptor | Bioorg Med Chem Lett 17: 1659-62 (2007) Article DOI: 10.1016/j.bmcl.2006.12.104 BindingDB Entry DOI: 10.7270/Q2RF5TPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50036649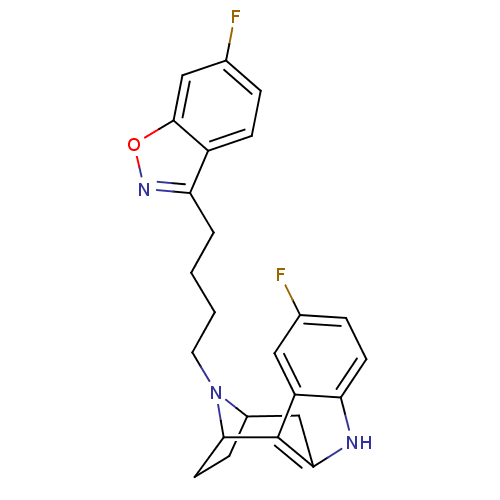 ((7R,10S)-1-(6-fluorobenzo[d]isoxazol-3-yl)-4-[5-fl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Scios Nova Inc. Curated by ChEMBL | Assay Description In vitro binding affinity towards dopamine receptor D2 in rat striatal membranes by [3H]-sulpiride displacement. | J Med Chem 36: 3073-6 (1993) BindingDB Entry DOI: 10.7270/Q2RF5T2N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50036649 ((7R,10S)-1-(6-fluorobenzo[d]isoxazol-3-yl)-4-[5-fl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Scios Nova Inc. Curated by ChEMBL | Assay Description In vitro binding affinity towards dopamine receptor D2 in rat striatal membranes by [3H]-sulpiride displacement. | J Med Chem 36: 3073-6 (1993) BindingDB Entry DOI: 10.7270/Q2RF5T2N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 2 (5-HT2) receptor (RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50036648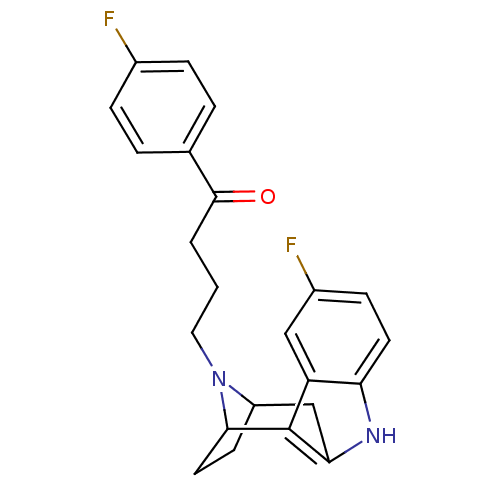 ((7R,10S)-4-[5-fluoro-9,15-diazatetracyclo[10.2.1.0...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Scios Nova Inc. Curated by ChEMBL | Assay Description In vitro binding affinity towards 5-hydroxytryptamine 2 receptor in rat striatal membranes by [3H]ketanserin displacement. | J Med Chem 36: 3073-6 (1993) BindingDB Entry DOI: 10.7270/Q2RF5T2N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 7 (Homo sapiens (Human)) | BDBM10882 (6-ethoxy-1,3-benzothiazole-2-sulfonamide | CHEMBL1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human cytosolic carbonic anhydrase 7 preincubated for 15 mins by stopped flow CO2 hydration assay | Bioorg Med Chem 21: 5168-74 (2013) Article DOI: 10.1016/j.bmc.2013.06.035 BindingDB Entry DOI: 10.7270/Q2QV3QGV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 7 (Homo sapiens (Human)) | BDBM10882 (6-ethoxy-1,3-benzothiazole-2-sulfonamide | CHEMBL1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human cytosolic carbonic anhydrase 7 preincubated for 15 mins at room temperature followed by 72 hrs at 4 degC by stopped flow CO2 hydr... | Bioorg Med Chem 21: 5973-82 (2013) Article DOI: 10.1016/j.bmc.2013.07.044 BindingDB Entry DOI: 10.7270/Q25Q5026 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50202769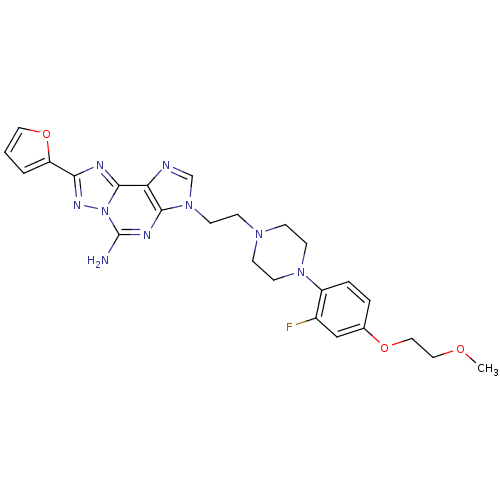 (3-(2-(4-(2-fluoro-4-(2-methoxyethoxy)phenyl)pipera...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human adenosine A2A receptor | Bioorg Med Chem Lett 17: 1659-62 (2007) Article DOI: 10.1016/j.bmcl.2006.12.104 BindingDB Entry DOI: 10.7270/Q2RF5TPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A/2B/2C (Mus musculus (Mouse)) | BDBM50001775 ((ritanserin)6-(2-{4-[Bis-(4-fluoro-phenyl)-methyle...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Scios Nova Inc. Curated by ChEMBL | Assay Description Binding affinity against 5-hydroxytryptamine 2 receptor from rat cortical synaptosomal membrane using radioligand [3H]ketanserin. | J Med Chem 36: 1488-95 (1993) BindingDB Entry DOI: 10.7270/Q2KD1X0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A/2B/2C (Mus musculus (Mouse)) | BDBM50046475 (4-[9,15-diazatetracyclo[10.2.1.02,10.03,8]pentadec...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Scios Nova Inc. Curated by ChEMBL | Assay Description Binding affinity against 5-hydroxytryptamine 2 receptor from rat cortical synaptosomal membrane using radioligand [3H]ketanserin. | J Med Chem 36: 1488-95 (1993) BindingDB Entry DOI: 10.7270/Q2KD1X0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A/2B/2C (Mus musculus (Mouse)) | BDBM50047457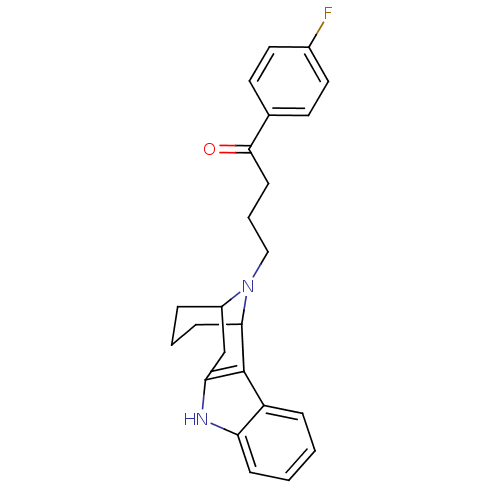 (4-[9,16-diazatetracyclo[10.3.1.02,10.03,8]hexadeca...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Scios Nova Inc. Curated by ChEMBL | Assay Description Binding affinity against 5-hydroxytryptamine 2 receptor from rat cortical synaptosomal membrane using radioligand [3H]ketanserin. | J Med Chem 36: 1488-95 (1993) BindingDB Entry DOI: 10.7270/Q2KD1X0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50202773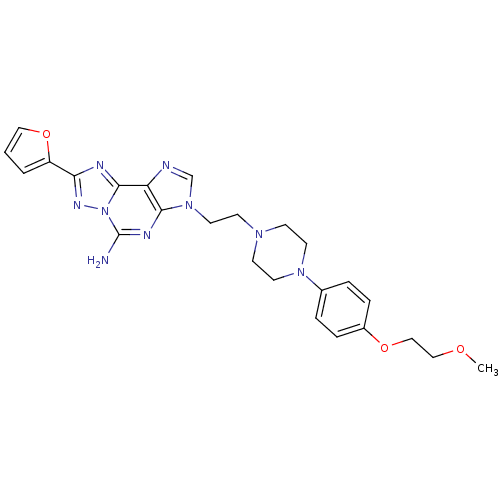 (8-(furan-2-yl)-3-(2-(4-(4-(2-methoxyethoxy)phenyl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human adenosine A2A receptor | Bioorg Med Chem Lett 17: 1659-62 (2007) Article DOI: 10.1016/j.bmcl.2006.12.104 BindingDB Entry DOI: 10.7270/Q2RF5TPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 7 (Homo sapiens (Human)) | BDBM10887 (Sulfamate 7 | Topiramate (TPM) | US11535599, Examp...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human cytosolic carbonic anhydrase 7 preincubated for 15 mins at room temperature followed by 72 hrs at 4 degC by stopped flow CO2 hydr... | Bioorg Med Chem 21: 5973-82 (2013) Article DOI: 10.1016/j.bmc.2013.07.044 BindingDB Entry DOI: 10.7270/Q25Q5026 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50202783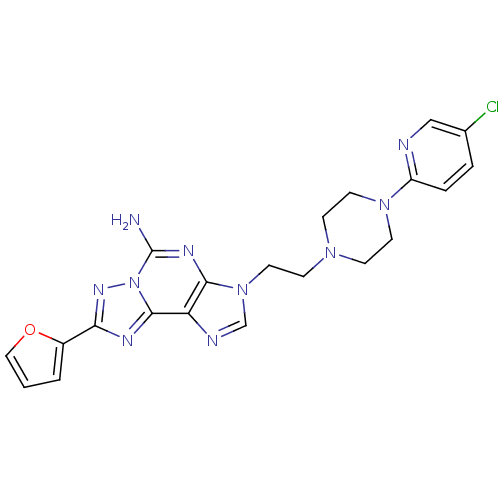 (3-(2-(4-(5-chloropyridin-2-yl)piperazin-1-yl)ethyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human adenosine A2A receptor | Bioorg Med Chem Lett 17: 1659-62 (2007) Article DOI: 10.1016/j.bmcl.2006.12.104 BindingDB Entry DOI: 10.7270/Q2RF5TPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 7 (Homo sapiens (Human)) | BDBM10887 (Sulfamate 7 | Topiramate (TPM) | US11535599, Examp...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human cytosolic carbonic anhydrase 7 preincubated for 15 mins by stopped flow CO2 hydration assay | Bioorg Med Chem 21: 5168-74 (2013) Article DOI: 10.1016/j.bmc.2013.06.035 BindingDB Entry DOI: 10.7270/Q2QV3QGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50202766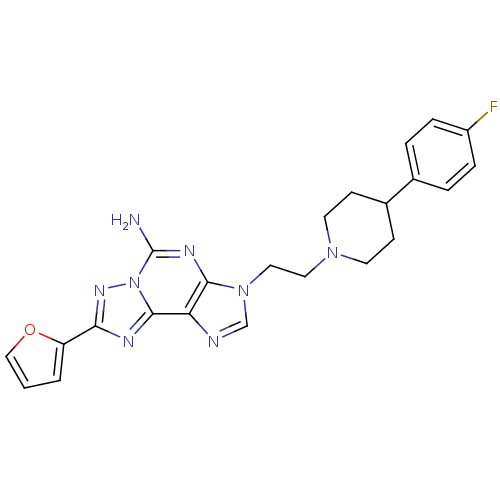 (3-(2-(4-(4-fluorophenyl)piperidin-1-yl)ethyl)-8-(f...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human adenosine A2A receptor | Bioorg Med Chem Lett 17: 1659-62 (2007) Article DOI: 10.1016/j.bmcl.2006.12.104 BindingDB Entry DOI: 10.7270/Q2RF5TPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50202767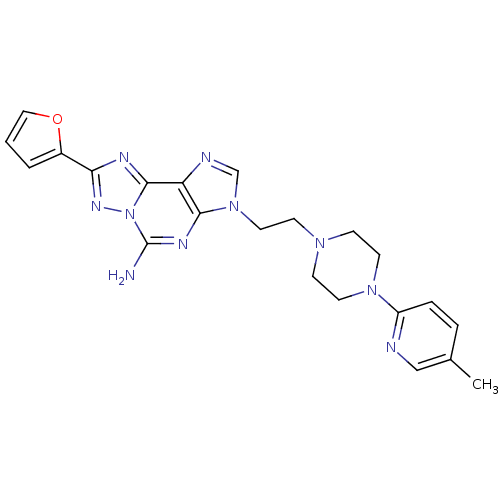 (8-(furan-2-yl)-3-(2-(4-(5-methylpyridin-2-yl)piper...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human adenosine A2A receptor | Bioorg Med Chem Lett 17: 1659-62 (2007) Article DOI: 10.1016/j.bmcl.2006.12.104 BindingDB Entry DOI: 10.7270/Q2RF5TPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 7 (Homo sapiens (Human)) | BDBM50492936 (CHEMBL2414441) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human cytosolic carbonic anhydrase 7 preincubated for 15 mins by stopped flow CO2 hydration assay | Bioorg Med Chem 21: 5168-74 (2013) Article DOI: 10.1016/j.bmc.2013.06.035 BindingDB Entry DOI: 10.7270/Q2QV3QGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50047436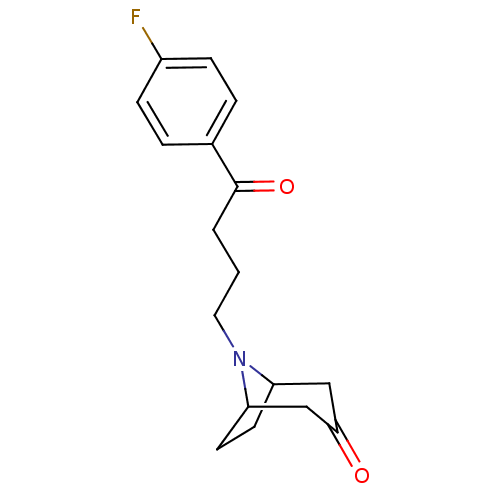 (8-[4-(4-Fluoro-phenyl)-4-oxo-butyl]-8-aza-bicyclo[...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Scios Nova Inc. Curated by ChEMBL | Assay Description Binding affinity against dopamine receptor D2 from rat corpus striatum by using radioligand [3H]-sulpiride | J Med Chem 36: 1488-95 (1993) BindingDB Entry DOI: 10.7270/Q2KD1X0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50202789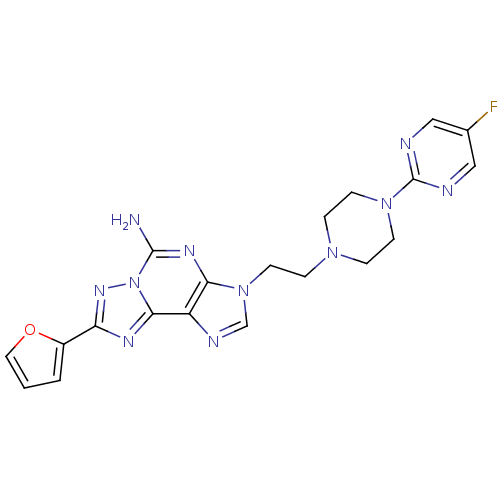 (3-(2-(4-(5-fluoropyrimidin-2-yl)piperazin-1-yl)eth...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human adenosine A2A receptor | Bioorg Med Chem Lett 17: 1659-62 (2007) Article DOI: 10.1016/j.bmcl.2006.12.104 BindingDB Entry DOI: 10.7270/Q2RF5TPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50202772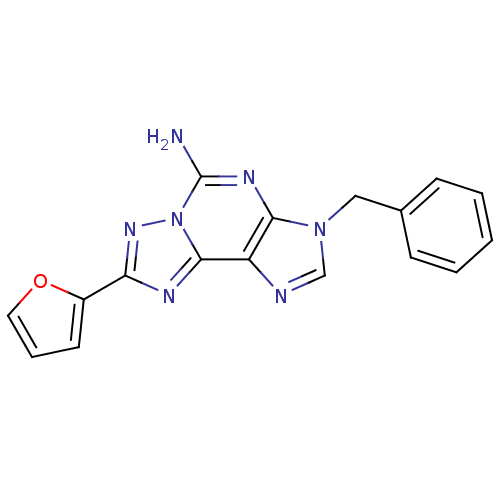 (3-benzyl-8-(furan-2-yl)-3H-[1,2,4]triazolo[1,5-g]p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human adenosine A2A receptor | Bioorg Med Chem Lett 17: 1659-62 (2007) Article DOI: 10.1016/j.bmcl.2006.12.104 BindingDB Entry DOI: 10.7270/Q2RF5TPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50495579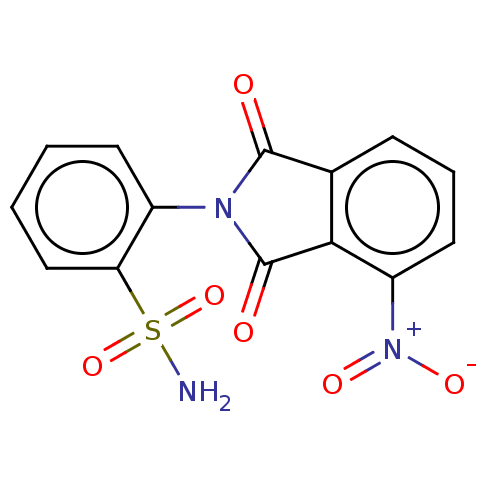 (CHEMBL3113924) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human cytosolic carbonic anhydrase 2 by stopped-flow CO2 hydration assay | Bioorg Med Chem 22: 1586-95 (2014) Article DOI: 10.1016/j.bmc.2014.01.031 BindingDB Entry DOI: 10.7270/Q2F47S3J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50202765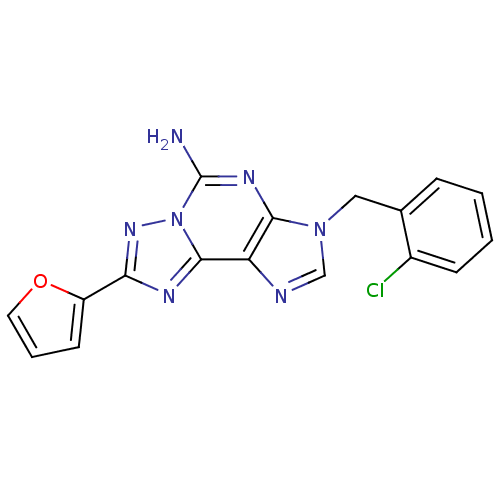 (3-(2-chlorobenzyl)-8-(furan-2-yl)-3H-[1,2,4]triazo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human adenosine A2A receptor | Bioorg Med Chem Lett 17: 1659-62 (2007) Article DOI: 10.1016/j.bmcl.2006.12.104 BindingDB Entry DOI: 10.7270/Q2RF5TPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50036647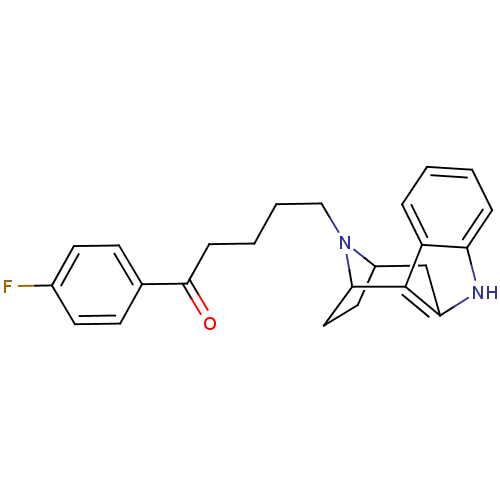 ((7R,10S)-5-[9,15-diazatetracyclo[10.2.1.02,10.03,8...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Scios Nova Inc. Curated by ChEMBL | Assay Description In vitro binding affinity towards dopamine receptor D2 in rat striatal membranes by [3H]-sulpiride displacement. | J Med Chem 36: 3073-6 (1993) BindingDB Entry DOI: 10.7270/Q2RF5T2N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50202782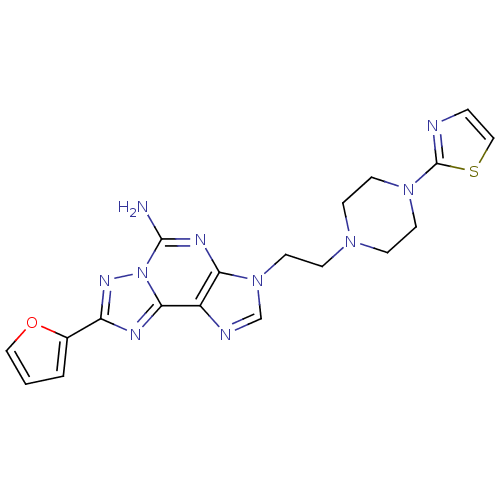 (8-(furan-2-yl)-3-(2-(4-(thiazol-2-yl)piperazin-1-y...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human adenosine A2A receptor | Bioorg Med Chem Lett 17: 1659-62 (2007) Article DOI: 10.1016/j.bmcl.2006.12.104 BindingDB Entry DOI: 10.7270/Q2RF5TPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50202787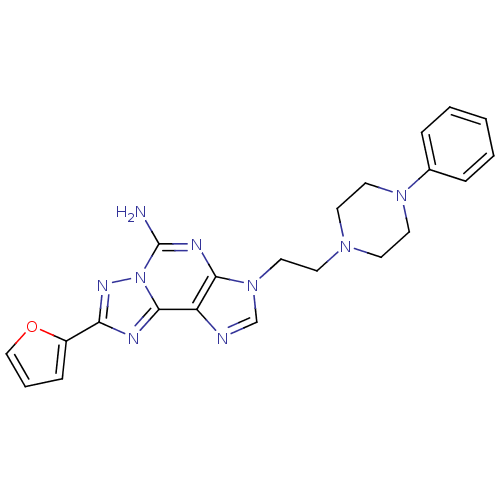 (8-(furan-2-yl)-3-(2-(4-phenylpiperazin-1-yl)ethyl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human adenosine A2A receptor | Bioorg Med Chem Lett 17: 1659-62 (2007) Article DOI: 10.1016/j.bmcl.2006.12.104 BindingDB Entry DOI: 10.7270/Q2RF5TPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A/2B/2C (Mus musculus (Mouse)) | BDBM50036648 ((7R,10S)-4-[5-fluoro-9,15-diazatetracyclo[10.2.1.0...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Scios Nova Inc. Curated by ChEMBL | Assay Description Binding affinity against 5-hydroxytryptamine 2 receptor from rat cortical synaptosomal membrane using radioligand [3H]ketanserin. | J Med Chem 36: 1488-95 (1993) BindingDB Entry DOI: 10.7270/Q2KD1X0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 2 (5-HT2) receptor (RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50036650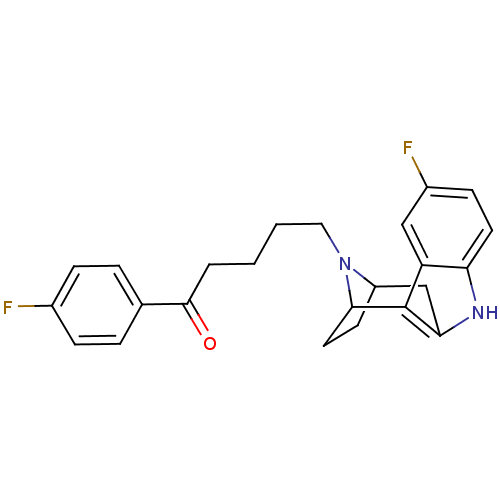 ((7R,10S)-5-[5-fluoro-9,15-diazatetracyclo[10.2.1.0...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.87 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Scios Nova Inc. Curated by ChEMBL | Assay Description In vitro binding affinity towards 5-hydroxytryptamine 2 receptor in rat striatal membranes by [3H]ketanserin displacement. | J Med Chem 36: 3073-6 (1993) BindingDB Entry DOI: 10.7270/Q2RF5T2N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 2 (5-HT2) receptor (RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50036648 ((7R,10S)-4-[5-fluoro-9,15-diazatetracyclo[10.2.1.0...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.96 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Scios Nova Inc. Curated by ChEMBL | Assay Description In vitro binding affinity towards 5-hydroxytryptamine 2 receptor in rat striatal membranes by [3H]ketanserin displacement. | J Med Chem 36: 3073-6 (1993) BindingDB Entry DOI: 10.7270/Q2RF5T2N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM168281 (US9669031, 109 8-chloro-6-(pyrimidin-4-ylamino)spi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00368 BindingDB Entry DOI: 10.7270/Q20C50T2 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 2 (5-HT2) receptor (RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50036648 ((7R,10S)-4-[5-fluoro-9,15-diazatetracyclo[10.2.1.0...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Scios Nova Inc. Curated by ChEMBL | Assay Description In vitro binding affinity towards 5-hydroxytryptamine 2 receptor in rat striatal membranes by [3H]ketanserin displacement. | J Med Chem 36: 3073-6 (1993) BindingDB Entry DOI: 10.7270/Q2RF5T2N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50202768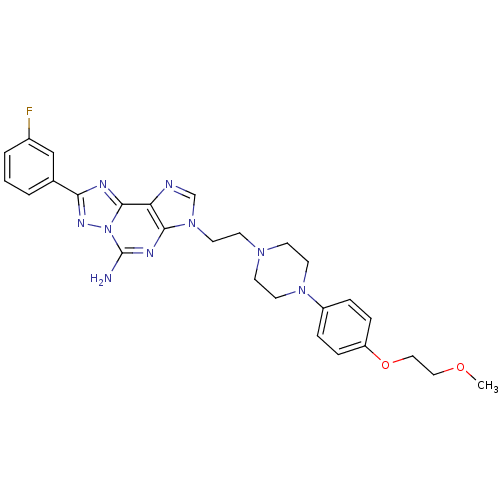 (8-(3-fluorophenyl)-3-(2-(4-(4-(2-methoxyethoxy)phe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human adenosine A2A receptor | Bioorg Med Chem Lett 17: 1659-62 (2007) Article DOI: 10.1016/j.bmcl.2006.12.104 BindingDB Entry DOI: 10.7270/Q2RF5TPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 7 (Homo sapiens (Human)) | BDBM50035674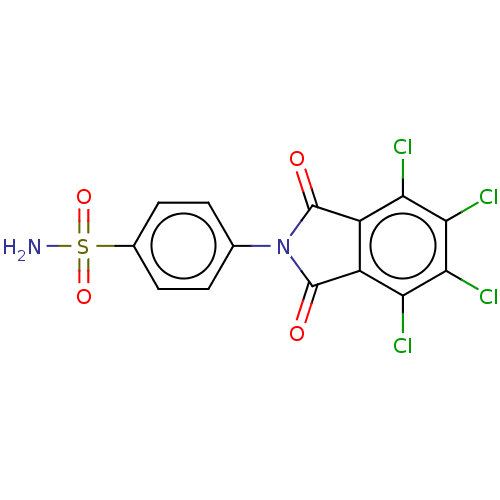 (CHEMBL2377770) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human cytosolic carbonic anhydrase 7 preincubated for 15 mins by stopped flow CO2 hydration assay | Bioorg Med Chem 21: 5168-74 (2013) Article DOI: 10.1016/j.bmc.2013.06.035 BindingDB Entry DOI: 10.7270/Q2QV3QGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 7 (Homo sapiens (Human)) | BDBM50013792 (CHEBI:6822 | METHAZOLAMIDE | Neptazane) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Similars | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human cytosolic carbonic anhydrase 7 preincubated for 15 mins by stopped flow CO2 hydration assay | Bioorg Med Chem 21: 5168-74 (2013) Article DOI: 10.1016/j.bmc.2013.06.035 BindingDB Entry DOI: 10.7270/Q2QV3QGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 7 (Homo sapiens (Human)) | BDBM50013792 (CHEBI:6822 | METHAZOLAMIDE | Neptazane) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Similars | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human cytosolic carbonic anhydrase 7 preincubated for 15 mins at room temperature followed by 72 hrs at 4 degC by stopped flow CO2 hydr... | Bioorg Med Chem 21: 5973-82 (2013) Article DOI: 10.1016/j.bmc.2013.07.044 BindingDB Entry DOI: 10.7270/Q25Q5026 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 2 (5-HT2) receptor (RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50036647 ((7R,10S)-5-[9,15-diazatetracyclo[10.2.1.02,10.03,8...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Scios Nova Inc. Curated by ChEMBL | Assay Description In vitro binding affinity towards 5-hydroxytryptamine 2 receptor in rat striatal membranes by [3H]ketanserin displacement. | J Med Chem 36: 3073-6 (1993) BindingDB Entry DOI: 10.7270/Q2RF5T2N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 7 (Homo sapiens (Human)) | BDBM50492940 (CHEMBL2414443) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human cytosolic carbonic anhydrase 7 preincubated for 15 mins by stopped flow CO2 hydration assay | Bioorg Med Chem 21: 5168-74 (2013) Article DOI: 10.1016/j.bmc.2013.06.035 BindingDB Entry DOI: 10.7270/Q2QV3QGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50492940 (CHEMBL2414443) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human cytosolic carbonic anhydrase 2 preincubated for 15 mins by stopped flow CO2 hydration assay | Bioorg Med Chem 21: 5168-74 (2013) Article DOI: 10.1016/j.bmc.2013.06.035 BindingDB Entry DOI: 10.7270/Q2QV3QGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 7 (Homo sapiens (Human)) | BDBM10880 (AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human cytosolic carbonic anhydrase 7 preincubated for 15 mins at room temperature followed by 72 hrs at 4 degC by stopped flow CO2 hydr... | Bioorg Med Chem 21: 5973-82 (2013) Article DOI: 10.1016/j.bmc.2013.07.044 BindingDB Entry DOI: 10.7270/Q25Q5026 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 7 (Homo sapiens (Human)) | BDBM10880 (AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human cytosolic carbonic anhydrase 7 preincubated for 15 mins by stopped flow CO2 hydration assay | Bioorg Med Chem 21: 5168-74 (2013) Article DOI: 10.1016/j.bmc.2013.06.035 BindingDB Entry DOI: 10.7270/Q2QV3QGV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50036649 ((7R,10S)-1-(6-fluorobenzo[d]isoxazol-3-yl)-4-[5-fl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Scios Nova Inc. Curated by ChEMBL | Assay Description Binding affinity against dopamine receptor D2 from rat corpus striatum by using radioligand [3H]-sulpiride | J Med Chem 36: 1488-95 (1993) BindingDB Entry DOI: 10.7270/Q2KD1X0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 7 (Homo sapiens (Human)) | BDBM10885 ((4R)-4-(ethylamino)-2-(3-methoxypropyl)-1,1-dioxo-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human cytosolic carbonic anhydrase 7 preincubated for 15 mins at room temperature followed by 72 hrs at 4 degC by stopped flow CO2 hydr... | Bioorg Med Chem 21: 5973-82 (2013) Article DOI: 10.1016/j.bmc.2013.07.044 BindingDB Entry DOI: 10.7270/Q25Q5026 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 7 (Homo sapiens (Human)) | BDBM10885 ((4R)-4-(ethylamino)-2-(3-methoxypropyl)-1,1-dioxo-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human cytosolic carbonic anhydrase 7 preincubated for 15 mins by stopped flow CO2 hydration assay | Bioorg Med Chem 21: 5168-74 (2013) Article DOI: 10.1016/j.bmc.2013.06.035 BindingDB Entry DOI: 10.7270/Q2QV3QGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1349 total ) | Next | Last >> |