 Found 4509 hits with Last Name = 'verner' and Initial = 'e'
Found 4509 hits with Last Name = 'verner' and Initial = 'e' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Coagulation factor X
(Homo sapiens (Human)) | BDBM50101882
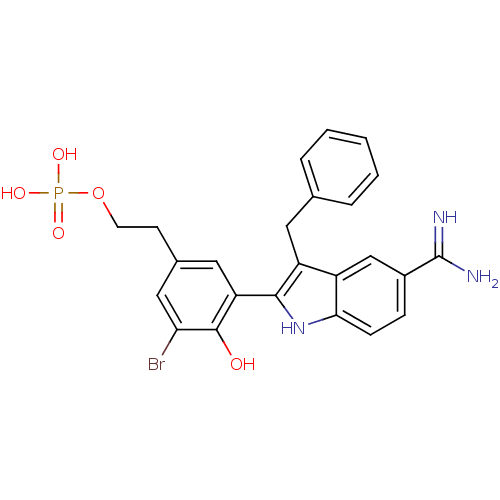
(CHEMBL53829 | Phosphoric acid mono-{2-[3-(3-benzyl...)Show SMILES NC(=N)c1ccc2[nH]c(c(Cc3ccccc3)c2c1)-c1cc(CCOP(O)(O)=O)cc(Br)c1O Show InChI InChI=1S/C24H23BrN3O5P/c25-20-12-15(8-9-33-34(30,31)32)11-19(23(20)29)22-18(10-14-4-2-1-3-5-14)17-13-16(24(26)27)6-7-21(17)28-22/h1-7,11-13,28-29H,8-10H2,(H3,26,27)(H2,30,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Coagulation factor X in human plasma |
Bioorg Med Chem Lett 11: 1797-800 (2001)
BindingDB Entry DOI: 10.7270/Q20K27TW |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50101871

(3-[3-(3-Benzyl-5-carbamimidoyl-1H-indol-2-yl)-5-br...)Show SMILES NC(=N)c1ccc2[nH]c(c(Cc3ccccc3)c2c1)-c1cc(CCC(O)=O)cc(Br)c1O Show InChI InChI=1S/C25H22BrN3O3/c26-20-12-15(6-9-22(30)31)11-19(24(20)32)23-18(10-14-4-2-1-3-5-14)17-13-16(25(27)28)7-8-21(17)29-23/h1-5,7-8,11-13,29,32H,6,9-10H2,(H3,27,28)(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Coagulation factor X in human plasma |
Bioorg Med Chem Lett 11: 1797-800 (2001)
BindingDB Entry DOI: 10.7270/Q20K27TW |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50101881
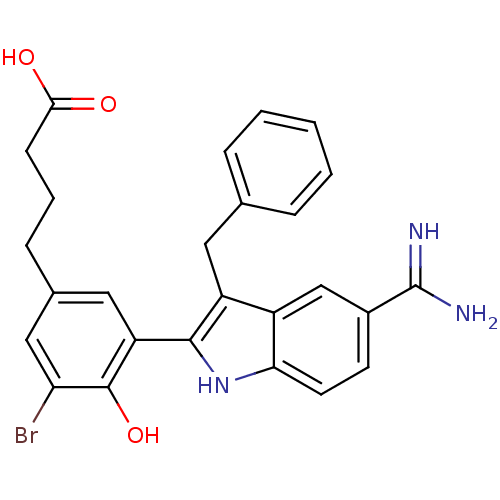
(4-[3-(3-Benzyl-5-carbamimidoyl-1H-indol-2-yl)-5-br...)Show SMILES NC(=N)c1ccc2[nH]c(c(Cc3ccccc3)c2c1)-c1cc(CCCC(O)=O)cc(Br)c1O Show InChI InChI=1S/C26H24BrN3O3/c27-21-13-16(7-4-8-23(31)32)12-20(25(21)33)24-19(11-15-5-2-1-3-6-15)18-14-17(26(28)29)9-10-22(18)30-24/h1-3,5-6,9-10,12-14,30,33H,4,7-8,11H2,(H3,28,29)(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Coagulation factor X in human plasma |
Bioorg Med Chem Lett 11: 1797-800 (2001)
BindingDB Entry DOI: 10.7270/Q20K27TW |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50176251

(2-((6-chloro-1H-benzo[d]imidazol-2-yl)methyl)-1H-b...)Show SMILES NC(=N)c1ccc2nc(Cc3nc4ccc(Cl)cc4[nH]3)[nH]c2c1 Show InChI InChI=1S/C16H13ClN6/c17-9-2-4-11-13(6-9)23-15(21-11)7-14-20-10-3-1-8(16(18)19)5-12(10)22-14/h1-6H,7H2,(H3,18,19)(H,20,22)(H,21,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Binding affinity to FXa in presence of Zn2+ |
Bioorg Med Chem Lett 16: 710-3 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.023
BindingDB Entry DOI: 10.7270/Q2VM4BSJ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50176252
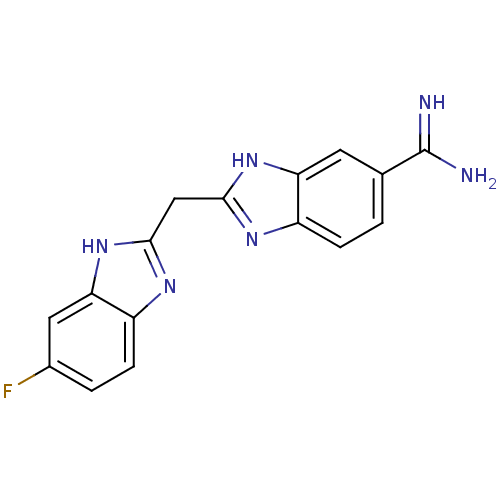
(2-((6-fluoro-1H-benzo[d]imidazol-2-yl)methyl)-1H-b...)Show SMILES NC(=N)c1ccc2nc(Cc3nc4ccc(F)cc4[nH]3)[nH]c2c1 Show InChI InChI=1S/C16H13FN6/c17-9-2-4-11-13(6-9)23-15(21-11)7-14-20-10-3-1-8(16(18)19)5-12(10)22-14/h1-6H,7H2,(H3,18,19)(H,20,22)(H,21,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Binding affinity to FXa in presence of Zn2+ |
Bioorg Med Chem Lett 16: 710-3 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.023
BindingDB Entry DOI: 10.7270/Q2VM4BSJ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM16303
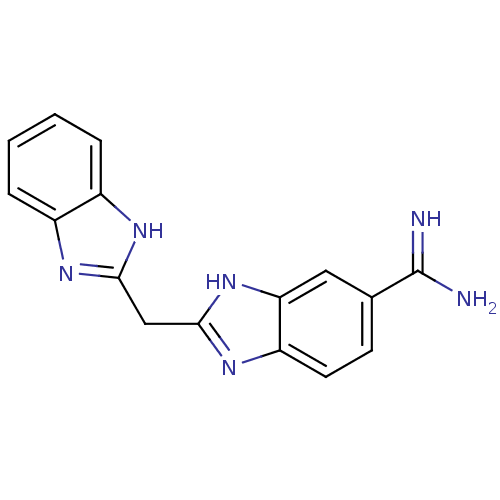
(2-(1H-1,3-benzodiazol-2-ylmethyl)-1H-1,3-benzodiaz...)Show InChI InChI=1S/C16H14N6/c17-16(18)9-5-6-12-13(7-9)22-15(21-12)8-14-19-10-3-1-2-4-11(10)20-14/h1-7H,8H2,(H3,17,18)(H,19,20)(H,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Binding affinity to thrombin in presence of Zn2+ |
Bioorg Med Chem Lett 16: 710-3 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.023
BindingDB Entry DOI: 10.7270/Q2VM4BSJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM16303

(2-(1H-1,3-benzodiazol-2-ylmethyl)-1H-1,3-benzodiaz...)Show InChI InChI=1S/C16H14N6/c17-16(18)9-5-6-12-13(7-9)22-15(21-12)8-14-19-10-3-1-2-4-11(10)20-14/h1-7H,8H2,(H3,17,18)(H,19,20)(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Binding affinity to FXa in presence of Zn2+ |
Bioorg Med Chem Lett 16: 710-3 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.023
BindingDB Entry DOI: 10.7270/Q2VM4BSJ |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 2
(Homo sapiens (Human)) | BDBM286984
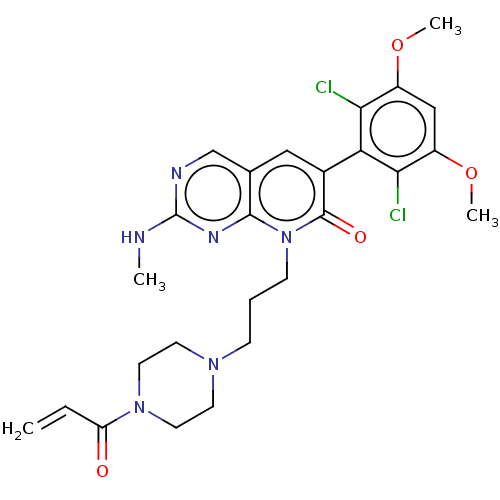
(8-(3-(4-acryloylpiperazin-1-yl)propyl)-6-(2,6-dich...)Show SMILES CNc1ncc2cc(-c3c(Cl)c(OC)cc(OC)c3Cl)c(=O)n(CCCN3CCN(CC3)C(=O)C=C)c2n1 |(-6.67,-.77,;-6.67,.77,;-5.33,1.54,;-5.33,3.08,;-4,3.85,;-2.67,3.08,;-1.33,3.85,;,3.08,;1.33,3.85,;1.33,5.39,;,6.16,;2.67,6.16,;2.67,7.7,;1.33,8.47,;4,5.39,;4,3.85,;5.33,3.08,;6.67,3.85,;2.67,3.08,;2.67,1.54,;,1.54,;1.33,.77,;-1.33,.77,;-1.33,-.77,;,-1.54,;,-3.08,;1.33,-3.85,;1.33,-5.39,;2.67,-6.16,;4,-5.39,;4,-3.85,;2.67,-3.08,;5.33,-6.16,;6.67,-5.39,;5.33,-7.7,;6.67,-8.47,;-2.67,1.54,;-4,.77,)| Show InChI InChI=1S/C26H30Cl2N6O4/c1-5-20(35)33-11-9-32(10-12-33)7-6-8-34-24-16(15-30-26(29-2)31-24)13-17(25(34)36)21-22(27)18(37-3)14-19(38-4)23(21)28/h5,13-15H,1,6-12H2,2-4H3,(H,29,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Principia Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Irreversible inhibition of human FGFR2 preincubated with enzyme followed by peptide substrate addition by caliper capillary electrophoresis method |
J Med Chem 60: 6516-6527 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00360
BindingDB Entry DOI: 10.7270/Q2C53P3H |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Bos taurus (bovine)) | BDBM14338
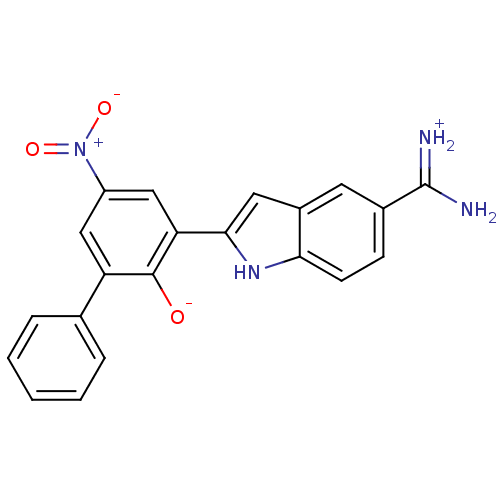
(2-{5-[amino(iminiumyl)methyl]-1H-indol-2-yl}-4-nit...)Show SMILES NC(=[NH2+])c1ccc2[nH]c(cc2c1)-c1cc(cc(-c2ccccc2)c1[O-])[N+]([O-])=O Show InChI InChI=1S/C21H16N4O3/c22-21(23)13-6-7-18-14(8-13)9-19(24-18)17-11-15(25(27)28)10-16(20(17)26)12-4-2-1-3-5-12/h1-11,24,26H,(H3,22,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
| Assay Description
Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... |
J Mol Biol 329: 93-120 (2003)
Article DOI: 10.1016/s0022-2836(03)00399-1
BindingDB Entry DOI: 10.7270/Q2R78CGQ |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM286984

(8-(3-(4-acryloylpiperazin-1-yl)propyl)-6-(2,6-dich...)Show SMILES CNc1ncc2cc(-c3c(Cl)c(OC)cc(OC)c3Cl)c(=O)n(CCCN3CCN(CC3)C(=O)C=C)c2n1 |(-6.67,-.77,;-6.67,.77,;-5.33,1.54,;-5.33,3.08,;-4,3.85,;-2.67,3.08,;-1.33,3.85,;,3.08,;1.33,3.85,;1.33,5.39,;,6.16,;2.67,6.16,;2.67,7.7,;1.33,8.47,;4,5.39,;4,3.85,;5.33,3.08,;6.67,3.85,;2.67,3.08,;2.67,1.54,;,1.54,;1.33,.77,;-1.33,.77,;-1.33,-.77,;,-1.54,;,-3.08,;1.33,-3.85,;1.33,-5.39,;2.67,-6.16,;4,-5.39,;4,-3.85,;2.67,-3.08,;5.33,-6.16,;6.67,-5.39,;5.33,-7.7,;6.67,-8.47,;-2.67,1.54,;-4,.77,)| Show InChI InChI=1S/C26H30Cl2N6O4/c1-5-20(35)33-11-9-32(10-12-33)7-6-8-34-24-16(15-30-26(29-2)31-24)13-17(25(34)36)21-22(27)18(37-3)14-19(38-4)23(21)28/h5,13-15H,1,6-12H2,2-4H3,(H,29,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Principia Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Irreversible inhibition of human FGFR1 using 5-FAM-KKKKEEIYFFF-NH2 as substrate preincubated with enzyme followed by peptide substrate addition by ca... |
J Med Chem 60: 6516-6527 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00360
BindingDB Entry DOI: 10.7270/Q2C53P3H |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50176257
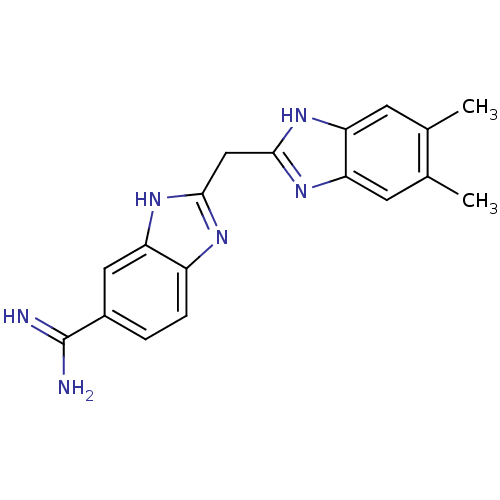
(2-((5,6-dimethyl-1H-benzo[d]imidazol-2-yl)methyl)-...)Show SMILES Cc1cc2nc(Cc3nc4ccc(cc4[nH]3)C(N)=N)[nH]c2cc1C Show InChI InChI=1S/C18H18N6/c1-9-5-13-14(6-10(9)2)23-17(22-13)8-16-21-12-4-3-11(18(19)20)7-15(12)24-16/h3-7H,8H2,1-2H3,(H3,19,20)(H,21,24)(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Binding affinity to FXa in presence of Zn2+ |
Bioorg Med Chem Lett 16: 710-3 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.023
BindingDB Entry DOI: 10.7270/Q2VM4BSJ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50101885
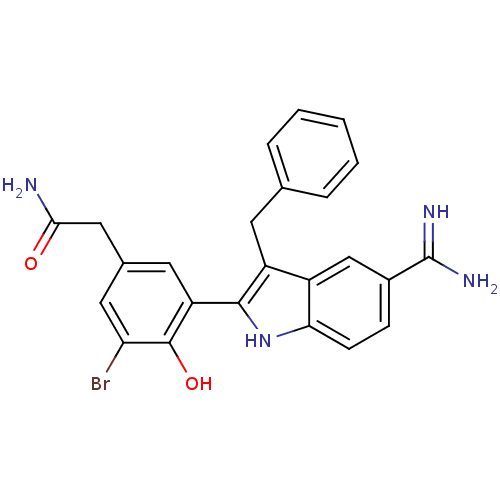
(2-[3-(3-Benzyl-5-carbamimidoyl-1H-indol-2-yl)-5-br...)Show SMILES NC(=O)Cc1cc(Br)c(O)c(c1)-c1[nH]c2ccc(cc2c1Cc1ccccc1)C(N)=N Show InChI InChI=1S/C24H21BrN4O2/c25-19-10-14(11-21(26)30)9-18(23(19)31)22-17(8-13-4-2-1-3-5-13)16-12-15(24(27)28)6-7-20(16)29-22/h1-7,9-10,12,29,31H,8,11H2,(H2,26,30)(H3,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Coagulation factor X in human plasma |
Bioorg Med Chem Lett 11: 1797-800 (2001)
BindingDB Entry DOI: 10.7270/Q20K27TW |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50101883
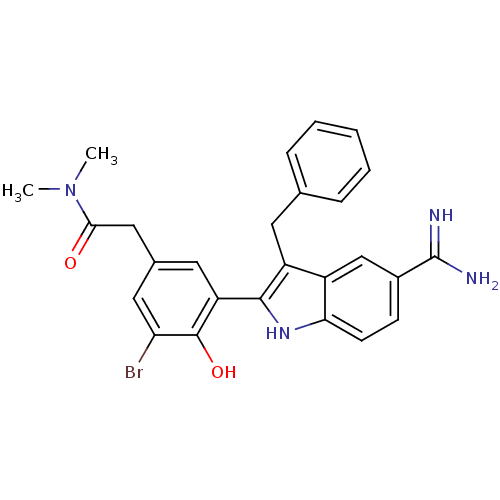
(2-[3-(3-Benzyl-5-carbamimidoyl-1H-indol-2-yl)-5-br...)Show SMILES CN(C)C(=O)Cc1cc(Br)c(O)c(c1)-c1[nH]c2ccc(cc2c1Cc1ccccc1)C(N)=N Show InChI InChI=1S/C26H25BrN4O2/c1-31(2)23(32)13-16-11-20(25(33)21(27)12-16)24-19(10-15-6-4-3-5-7-15)18-14-17(26(28)29)8-9-22(18)30-24/h3-9,11-12,14,30,33H,10,13H2,1-2H3,(H3,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Coagulation factor X in human plasma |
Bioorg Med Chem Lett 11: 1797-800 (2001)
BindingDB Entry DOI: 10.7270/Q20K27TW |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 3
(Homo sapiens (Human)) | BDBM286984

(8-(3-(4-acryloylpiperazin-1-yl)propyl)-6-(2,6-dich...)Show SMILES CNc1ncc2cc(-c3c(Cl)c(OC)cc(OC)c3Cl)c(=O)n(CCCN3CCN(CC3)C(=O)C=C)c2n1 |(-6.67,-.77,;-6.67,.77,;-5.33,1.54,;-5.33,3.08,;-4,3.85,;-2.67,3.08,;-1.33,3.85,;,3.08,;1.33,3.85,;1.33,5.39,;,6.16,;2.67,6.16,;2.67,7.7,;1.33,8.47,;4,5.39,;4,3.85,;5.33,3.08,;6.67,3.85,;2.67,3.08,;2.67,1.54,;,1.54,;1.33,.77,;-1.33,.77,;-1.33,-.77,;,-1.54,;,-3.08,;1.33,-3.85,;1.33,-5.39,;2.67,-6.16,;4,-5.39,;4,-3.85,;2.67,-3.08,;5.33,-6.16,;6.67,-5.39,;5.33,-7.7,;6.67,-8.47,;-2.67,1.54,;-4,.77,)| Show InChI InChI=1S/C26H30Cl2N6O4/c1-5-20(35)33-11-9-32(10-12-33)7-6-8-34-24-16(15-30-26(29-2)31-24)13-17(25(34)36)21-22(27)18(37-3)14-19(38-4)23(21)28/h5,13-15H,1,6-12H2,2-4H3,(H,29,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Principia Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Irreversible inhibition of human FGFR3 preincubated with enzyme followed by peptide substrate addition by caliper capillary electrophoresis method |
J Med Chem 60: 6516-6527 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00360
BindingDB Entry DOI: 10.7270/Q2C53P3H |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM14352
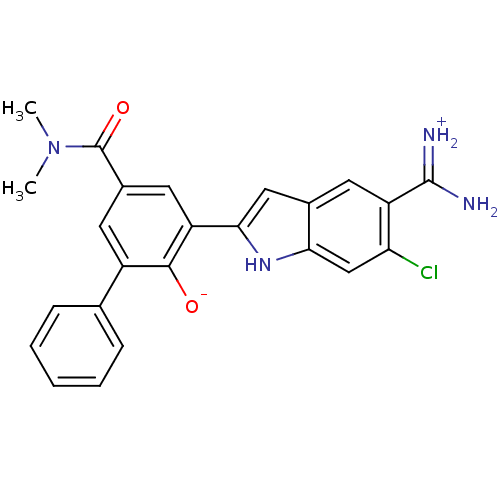
(2-{5-[amino(iminiumyl)methyl]-6-chloro-1H-indol-2-...)Show SMILES CN(C)C(=O)c1cc(-c2cc3cc(C(N)=[NH2+])c(Cl)cc3[nH]2)c([O-])c(c1)-c1ccccc1 Show InChI InChI=1S/C24H21ClN4O2/c1-29(2)24(31)15-9-16(13-6-4-3-5-7-13)22(30)18(10-15)21-11-14-8-17(23(26)27)19(25)12-20(14)28-21/h3-12,28,30H,1-2H3,(H3,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
| Assay Description
Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... |
J Mol Biol 329: 93-120 (2003)
Article DOI: 10.1016/s0022-2836(03)00399-1
BindingDB Entry DOI: 10.7270/Q2R78CGQ |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM14351

(2-{5-[amino(iminiumyl)methyl]-6-chloro-1H-indol-2-...)Show SMILES COc1cc(-c2cc3cc(C(N)=[NH2+])c(Cl)cc3[nH]2)c([O-])c(c1)-c1ccccc1 Show InChI InChI=1S/C22H18ClN3O2/c1-28-14-9-15(12-5-3-2-4-6-12)21(27)17(10-14)20-8-13-7-16(22(24)25)18(23)11-19(13)26-20/h2-11,26-27H,1H3,(H3,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
| Assay Description
Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... |
J Mol Biol 329: 93-120 (2003)
Article DOI: 10.1016/s0022-2836(03)00399-1
BindingDB Entry DOI: 10.7270/Q2R78CGQ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50176254

(2-((5,7-dichloro-1H-benzo[d]imidazol-2-yl)methyl)-...)Show SMILES NC(=N)c1ccc2nc(Cc3nc4cc(Cl)cc(Cl)c4[nH]3)[nH]c2c1 Show InChI InChI=1S/C16H12Cl2N6/c17-8-4-9(18)15-12(5-8)23-14(24-15)6-13-21-10-2-1-7(16(19)20)3-11(10)22-13/h1-5H,6H2,(H3,19,20)(H,21,22)(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Binding affinity to FXa in presence of Zn2+ |
Bioorg Med Chem Lett 16: 710-3 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.023
BindingDB Entry DOI: 10.7270/Q2VM4BSJ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50176250
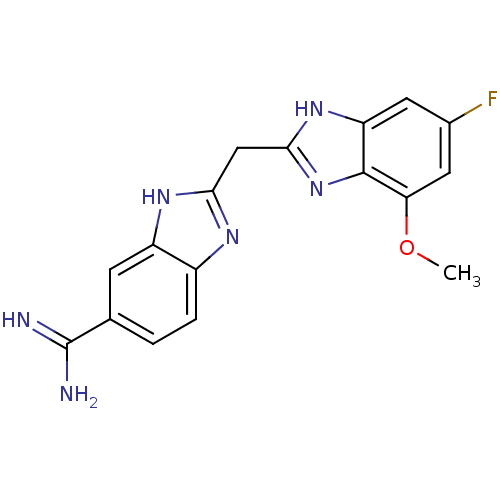
(2-((5-fluoro-7-methoxy-1H-benzo[d]imidazol-2-yl)me...)Show SMILES COc1cc(F)cc2[nH]c(Cc3nc4ccc(cc4[nH]3)C(N)=N)nc12 Show InChI InChI=1S/C17H15FN6O/c1-25-13-6-9(18)5-12-16(13)24-15(23-12)7-14-21-10-3-2-8(17(19)20)4-11(10)22-14/h2-6H,7H2,1H3,(H3,19,20)(H,21,22)(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Binding affinity to FXa in presence of Zn2+ |
Bioorg Med Chem Lett 16: 710-3 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.023
BindingDB Entry DOI: 10.7270/Q2VM4BSJ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50101880
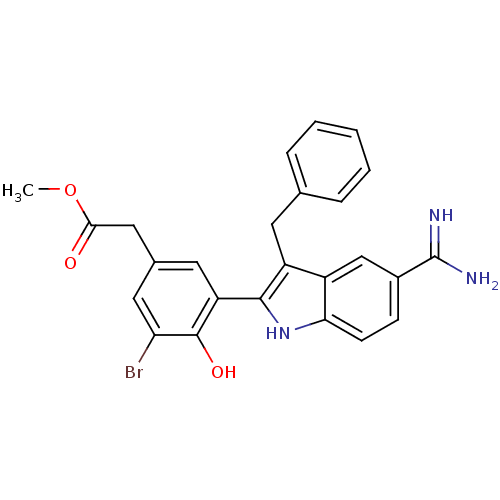
(CHEMBL416127 | [3-(3-Benzyl-5-carbamimidoyl-1H-ind...)Show SMILES COC(=O)Cc1cc(Br)c(O)c(c1)-c1[nH]c2ccc(cc2c1Cc1ccccc1)C(N)=N Show InChI InChI=1S/C25H22BrN3O3/c1-32-22(30)12-15-10-19(24(31)20(26)11-15)23-18(9-14-5-3-2-4-6-14)17-13-16(25(27)28)7-8-21(17)29-23/h2-8,10-11,13,29,31H,9,12H2,1H3,(H3,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Coagulation factor X in human plasma |
Bioorg Med Chem Lett 11: 1797-800 (2001)
BindingDB Entry DOI: 10.7270/Q20K27TW |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50101879
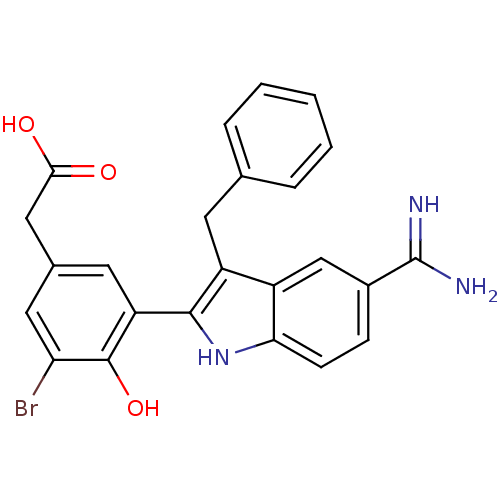
(CHEMBL50924 | [3-(3-Benzyl-5-carbamimidoyl-1H-indo...)Show SMILES NC(=N)c1ccc2[nH]c(c(Cc3ccccc3)c2c1)-c1cc(CC(O)=O)cc(Br)c1O Show InChI InChI=1S/C24H20BrN3O3/c25-19-10-14(11-21(29)30)9-18(23(19)31)22-17(8-13-4-2-1-3-5-13)16-12-15(24(26)27)6-7-20(16)28-22/h1-7,9-10,12,28,31H,8,11H2,(H3,26,27)(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Coagulation factor X in human plasma |
Bioorg Med Chem Lett 11: 1797-800 (2001)
BindingDB Entry DOI: 10.7270/Q20K27TW |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM14354
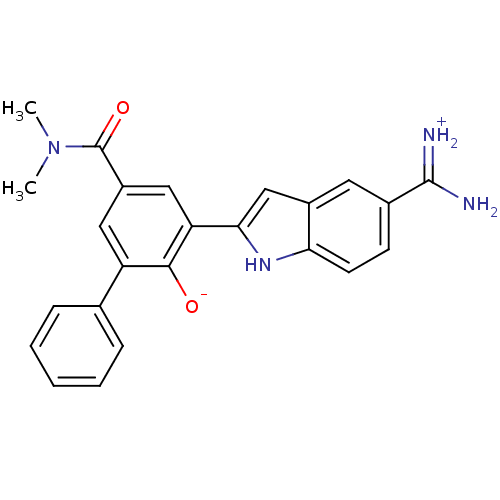
(2-{5-[amino(iminiumyl)methyl]-1H-indol-2-yl}-4-(di...)Show SMILES CN(C)C(=O)c1cc(-c2cc3cc(ccc3[nH]2)C(N)=[NH2+])c([O-])c(c1)-c1ccccc1 Show InChI InChI=1S/C24H22N4O2/c1-28(2)24(30)17-11-18(14-6-4-3-5-7-14)22(29)19(12-17)21-13-16-10-15(23(25)26)8-9-20(16)27-21/h3-13,27,29H,1-2H3,(H3,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
| Assay Description
Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... |
J Mol Biol 329: 93-120 (2003)
Article DOI: 10.1016/s0022-2836(03)00399-1
BindingDB Entry DOI: 10.7270/Q2R78CGQ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50101870
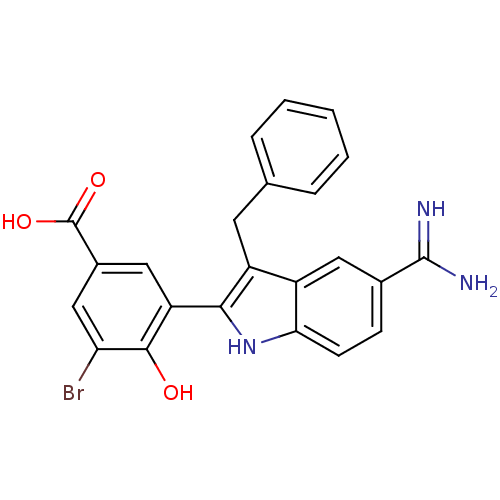
(3-(3-Benzyl-5-carbamimidoyl-1H-indol-2-yl)-5-bromo...)Show SMILES NC(=N)c1ccc2[nH]c(c(Cc3ccccc3)c2c1)-c1cc(cc(Br)c1O)C(O)=O Show InChI InChI=1S/C23H18BrN3O3/c24-18-11-14(23(29)30)10-17(21(18)28)20-16(8-12-4-2-1-3-5-12)15-9-13(22(25)26)6-7-19(15)27-20/h1-7,9-11,27-28H,8H2,(H3,25,26)(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Coagulation factor X in human plasma |
Bioorg Med Chem Lett 11: 1797-800 (2001)
BindingDB Entry DOI: 10.7270/Q20K27TW |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM24621
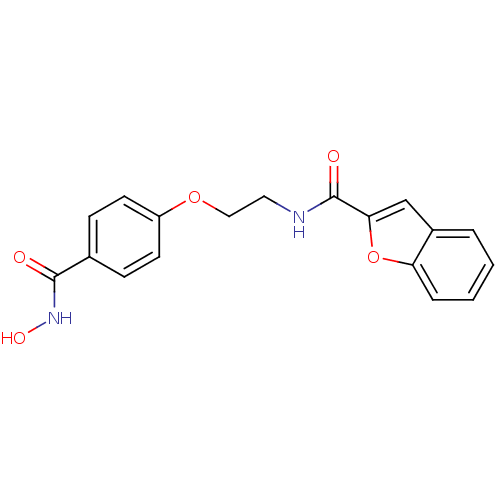
(CG-003 | N-{2-[4-(hydroxycarbamoyl)phenoxy]ethyl}-...)Show InChI InChI=1S/C18H16N2O5/c21-17(20-23)12-5-7-14(8-6-12)24-10-9-19-18(22)16-11-13-3-1-2-4-15(13)25-16/h1-8,11,23H,9-10H2,(H,19,22)(H,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 4 | -47.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera Genomics
| Assay Description
HDAC activity was measured using a continuous trypsin-coupled assay. For inhibitor characterization, measurements were done using 96-well assay plat... |
Mol Cancer Ther 5: 1309-17 (2006)
Article DOI: 10.1158/1535-7163.MCT-05-0442
BindingDB Entry DOI: 10.7270/Q2DF6PJJ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM24620
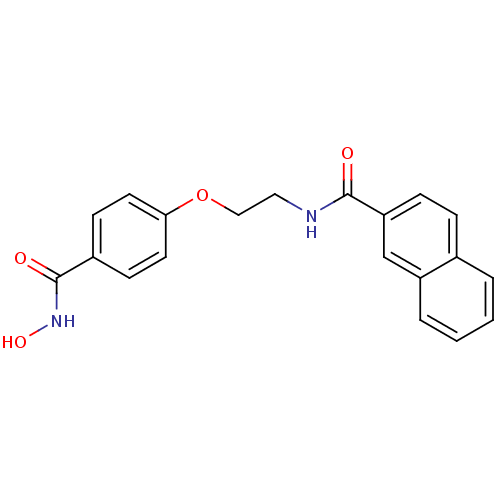
(CG-002 | N-{2-[4-(hydroxycarbamoyl)phenoxy]ethyl}n...)Show InChI InChI=1S/C20H18N2O4/c23-19(17-6-5-14-3-1-2-4-16(14)13-17)21-11-12-26-18-9-7-15(8-10-18)20(24)22-25/h1-10,13,25H,11-12H2,(H,21,23)(H,22,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | -46.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera Genomics
| Assay Description
HDAC activity was measured using a continuous trypsin-coupled assay. For inhibitor characterization, measurements were done using 96-well assay plat... |
Mol Cancer Ther 5: 1309-17 (2006)
Article DOI: 10.1158/1535-7163.MCT-05-0442
BindingDB Entry DOI: 10.7270/Q2DF6PJJ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50176256

(2-((5,6-difluoro-1H-benzo[d]imidazol-2-yl)methyl)-...)Show SMILES NC(=N)c1ccc2nc(Cc3nc4cc(F)c(F)cc4[nH]3)[nH]c2c1 Show InChI InChI=1S/C16H12F2N6/c17-8-4-12-13(5-9(8)18)24-15(23-12)6-14-21-10-2-1-7(16(19)20)3-11(10)22-14/h1-5H,6H2,(H3,19,20)(H,21,22)(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Binding affinity to FXa in presence of Zn2+ |
Bioorg Med Chem Lett 16: 710-3 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.023
BindingDB Entry DOI: 10.7270/Q2VM4BSJ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50176255
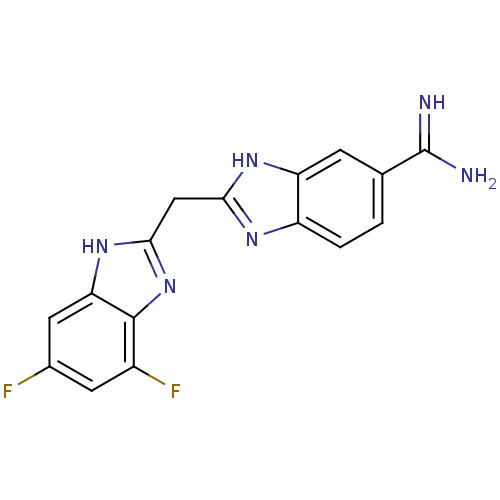
(2-((5,7-difluoro-1H-benzo[d]imidazol-2-yl)methyl)-...)Show SMILES NC(=N)c1ccc2nc(Cc3nc4c(F)cc(F)cc4[nH]3)[nH]c2c1 Show InChI InChI=1S/C16H12F2N6/c17-8-4-9(18)15-12(5-8)23-14(24-15)6-13-21-10-2-1-7(16(19)20)3-11(10)22-13/h1-5H,6H2,(H3,19,20)(H,21,22)(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Binding affinity to FXa in presence of Zn2+ |
Bioorg Med Chem Lett 16: 710-3 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.023
BindingDB Entry DOI: 10.7270/Q2VM4BSJ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM24622

(3-[(dimethylamino)methyl]-N-{2-[4-(hydroxycarbamoy...)Show SMILES CN(C)Cc1c(oc2ccccc12)C(=O)NCCOc1ccc(cc1)C(=O)NO Show InChI InChI=1S/C21H23N3O5/c1-24(2)13-17-16-5-3-4-6-18(16)29-19(17)21(26)22-11-12-28-15-9-7-14(8-10-15)20(25)23-27/h3-10,27H,11-13H2,1-2H3,(H,22,26)(H,23,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7 | -46.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera Genomics
| Assay Description
HDAC activity was measured using a continuous trypsin-coupled assay. For inhibitor characterization, measurements were done using 96-well assay plat... |
Mol Cancer Ther 5: 1309-17 (2006)
Article DOI: 10.1158/1535-7163.MCT-05-0442
BindingDB Entry DOI: 10.7270/Q2DF6PJJ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM13940
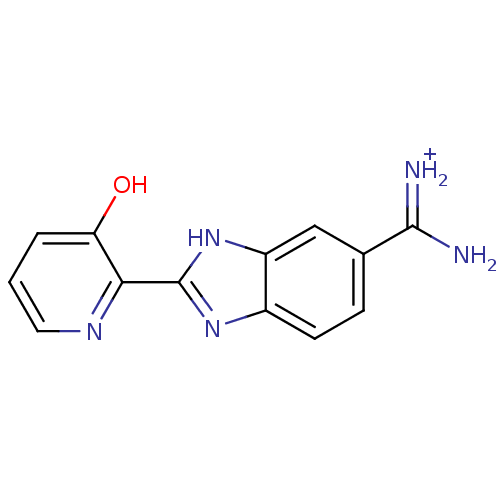
(2-(3-HYDROXY-PYRIDIN-2-YL)-1H-BENZOIMIDAZOLE-5-CAR...)Show InChI InChI=1S/C13H11N5O/c14-12(15)7-3-4-8-9(6-7)18-13(17-8)11-10(19)2-1-5-16-11/h1-6,19H,(H3,14,15)(H,17,18)/p+1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Corporation
| Assay Description
Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... |
J Mol Biol 307: 1451-86 (2001)
Article DOI: 10.1006/jmbi.2001.4516
BindingDB Entry DOI: 10.7270/Q2FX77PG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM14142
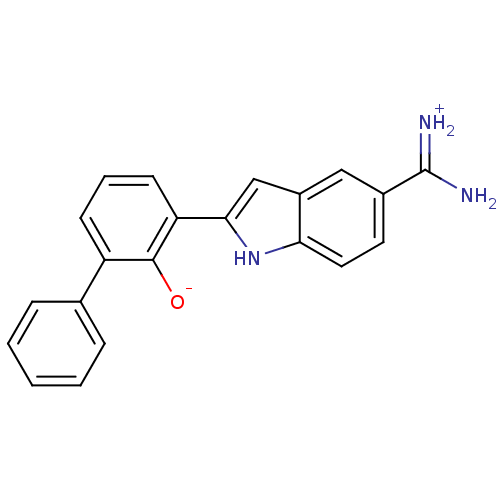
(2-{5-[amino(iminiumyl)methyl]-1H-indol-2-yl}-6-phe...)Show SMILES NC(=[NH2+])c1ccc2[nH]c(cc2c1)-c1cccc(-c2ccccc2)c1[O-] Show InChI InChI=1S/C21H17N3O/c22-21(23)14-9-10-18-15(11-14)12-19(24-18)17-8-4-7-16(20(17)25)13-5-2-1-3-6-13/h1-12,24-25H,(H3,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| DrugBank
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 8 | -45.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Axys Pharmaceutical
| Assay Description
Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... |
Chem Biol 8: 1107-21 (2001)
Article DOI: 10.1016/S1074-5521(01)00084-9
BindingDB Entry DOI: 10.7270/Q2S75DKN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50102780

(2-(2-Hydroxy-3-phenyl-phenyl)-1H-indole-5-carboxam...)Show SMILES NC(=N)c1ccc2[nH]c(cc2c1)-c1cccc(-c2ccccc2)c1O Show InChI InChI=1S/C21H17N3O/c22-21(23)14-9-10-18-15(11-14)12-19(24-18)17-8-4-7-16(20(17)25)13-5-2-1-3-6-13/h1-12,24-25H,(H3,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Human Serine Protease Urokinase Plasminogen Activator |
J Med Chem 44: 2753-71 (2001)
BindingDB Entry DOI: 10.7270/Q2RX9BC7 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50115874
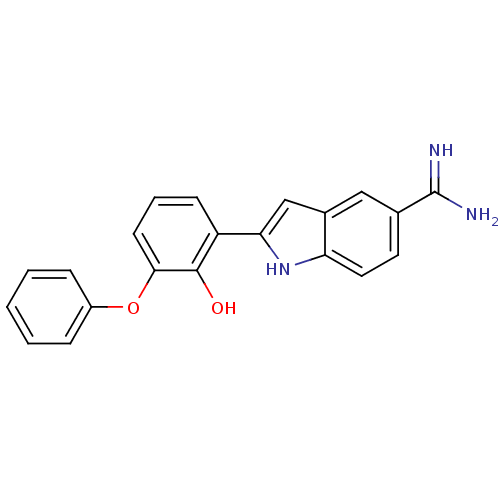
(2-(2-Hydroxy-3-phenoxy-phenyl)-1H-indole-5-carboxa...)Show SMILES NC(=N)c1ccc2[nH]c(cc2c1)-c1cccc(Oc2ccccc2)c1O Show InChI InChI=1S/C21H17N3O2/c22-21(23)13-9-10-17-14(11-13)12-18(24-17)16-7-4-8-19(20(16)25)26-15-5-2-1-3-6-15/h1-12,24-25H,(H3,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of urokinase-type plasminogen activator |
Bioorg Med Chem Lett 12: 2019-22 (2002)
BindingDB Entry DOI: 10.7270/Q237782T |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50102780

(2-(2-Hydroxy-3-phenyl-phenyl)-1H-indole-5-carboxam...)Show SMILES NC(=N)c1ccc2[nH]c(cc2c1)-c1cccc(-c2ccccc2)c1O Show InChI InChI=1S/C21H17N3O/c22-21(23)14-9-10-18-15(11-14)12-19(24-18)17-8-4-7-16(20(17)25)13-5-2-1-3-6-13/h1-12,24-25H,(H3,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
The compound was tested for inhibition of human urokinase type plasminogen activator (microPa) |
J Med Chem 44: 3856-71 (2001)
BindingDB Entry DOI: 10.7270/Q22R3QXR |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM24622

(3-[(dimethylamino)methyl]-N-{2-[4-(hydroxycarbamoy...)Show SMILES CN(C)Cc1c(oc2ccccc12)C(=O)NCCOc1ccc(cc1)C(=O)NO Show InChI InChI=1S/C21H23N3O5/c1-24(2)13-17-16-5-3-4-6-18(16)29-19(17)21(26)22-11-12-28-15-9-7-14(8-10-15)20(25)23-27/h3-10,27H,11-13H2,1-2H3,(H,22,26)(H,23,25) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8.20 | -45.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera Genomics
| Assay Description
HDAC activity was measured using a continuous trypsin-coupled assay. For inhibitor characterization, measurements were done using 96-well assay plat... |
Mol Cancer Ther 5: 1309-17 (2006)
Article DOI: 10.1158/1535-7163.MCT-05-0442
BindingDB Entry DOI: 10.7270/Q2DF6PJJ |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50106240
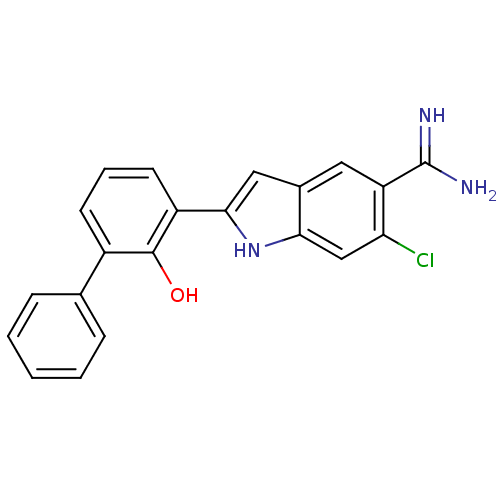
(6-Chloro-2-(2-hydroxy-biphenyl-3-yl)-1H-indole-5-c...)Show SMILES NC(=N)c1cc2cc([nH]c2cc1Cl)-c1cccc(-c2ccccc2)c1O Show InChI InChI=1S/C21H16ClN3O/c22-17-11-18-13(9-16(17)21(23)24)10-19(25-18)15-8-4-7-14(20(15)26)12-5-2-1-3-6-12/h1-11,25-26H,(H3,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
The compound was tested for inhibition of human urokinase type plasminogen activator (microPa) |
J Med Chem 44: 3856-71 (2001)
BindingDB Entry DOI: 10.7270/Q22R3QXR |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM14152
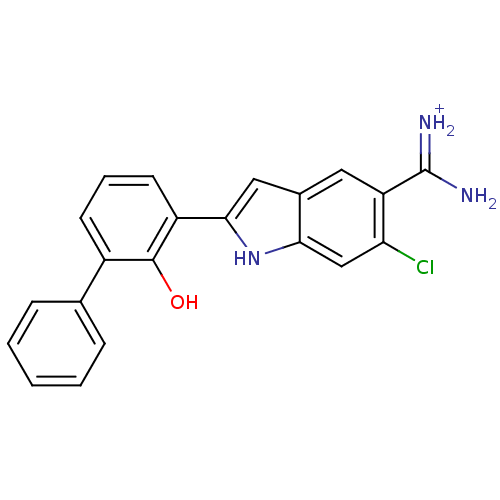
(6-CHLORO-2-(2-HYDROXY-BIPHENYL-3-YL)-1H-INDOLE-5-C...)Show SMILES NC(=[NH2+])c1cc2cc([nH]c2cc1Cl)-c1cccc(-c2ccccc2)c1O Show InChI InChI=1S/C21H16ClN3O/c22-17-11-18-13(9-16(17)21(23)24)10-19(25-18)15-8-4-7-14(20(15)26)12-5-2-1-3-6-12/h1-11,25-26H,(H3,23,24)/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 9 | -45.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Axys Pharmaceutical
| Assay Description
Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... |
Chem Biol 8: 1107-21 (2001)
Article DOI: 10.1016/S1074-5521(01)00084-9
BindingDB Entry DOI: 10.7270/Q2S75DKN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50101867

(3-Benzyl-2-[3-bromo-2-hydroxy-5-(2H-tetrazol-5-yl)...)Show SMILES NC(=N)c1ccc2[nH]c(c(Cc3ccccc3)c2c1)-c1cc(cc(Br)c1O)-c1nnn[nH]1 Show InChI InChI=1S/C23H18BrN7O/c24-18-11-14(23-28-30-31-29-23)10-17(21(18)32)20-16(8-12-4-2-1-3-5-12)15-9-13(22(25)26)6-7-19(15)27-20/h1-7,9-11,27,32H,8H2,(H3,25,26)(H,28,29,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Coagulation factor X in human plasma |
Bioorg Med Chem Lett 11: 1797-800 (2001)
BindingDB Entry DOI: 10.7270/Q20K27TW |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50101869

(2-[3-(3-Benzyl-5-carbamimidoyl-1H-indol-2-yl)-5-br...)Show SMILES CC(C)(C(O)=O)c1cc(Br)c(O)c(c1)-c1[nH]c2ccc(cc2c1Cc1ccccc1)C(N)=N Show InChI InChI=1S/C26H24BrN3O3/c1-26(2,25(32)33)16-12-19(23(31)20(27)13-16)22-18(10-14-6-4-3-5-7-14)17-11-15(24(28)29)8-9-21(17)30-22/h3-9,11-13,30-31H,10H2,1-2H3,(H3,28,29)(H,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Coagulation factor X in human plasma |
Bioorg Med Chem Lett 11: 1797-800 (2001)
BindingDB Entry DOI: 10.7270/Q20K27TW |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Bos taurus (bovine)) | BDBM14142

(2-{5-[amino(iminiumyl)methyl]-1H-indol-2-yl}-6-phe...)Show SMILES NC(=[NH2+])c1ccc2[nH]c(cc2c1)-c1cccc(-c2ccccc2)c1[O-] Show InChI InChI=1S/C21H17N3O/c22-21(23)14-9-10-18-15(11-14)12-19(24-18)17-8-4-7-16(20(17)25)13-5-2-1-3-6-13/h1-12,24-25H,(H3,22,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| DrugBank
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 11 | -45.0 | n/a | n/a | n/a | n/a | n/a | 7.45 | 22 |
Celera
| Assay Description
Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... |
J Mol Biol 329: 93-120 (2003)
Article DOI: 10.1016/s0022-2836(03)00399-1
BindingDB Entry DOI: 10.7270/Q2R78CGQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50115868
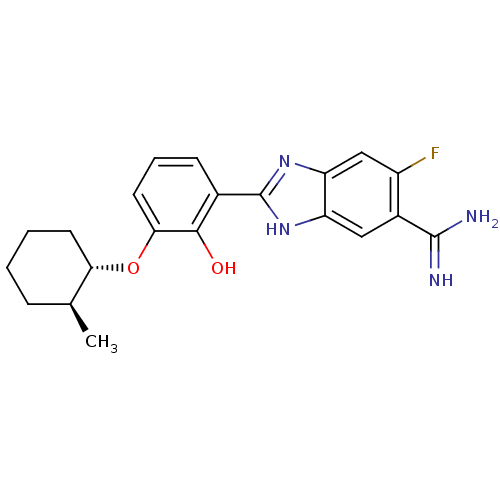
(2-{5-[AMINO(IMINIO)METHYL]-6-FLUORO-1H-BENZIMIDAZO...)Show SMILES C[C@H]1CCCC[C@@H]1Oc1cccc(-c2nc3cc(F)c(cc3[nH]2)C(N)=N)c1O Show InChI InChI=1S/C21H23FN4O2/c1-11-5-2-3-7-17(11)28-18-8-4-6-12(19(18)27)21-25-15-9-13(20(23)24)14(22)10-16(15)26-21/h4,6,8-11,17,27H,2-3,5,7H2,1H3,(H3,23,24)(H,25,26)/t11-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of urokinase-type plasminogen activator |
Bioorg Med Chem Lett 12: 2019-22 (2002)
BindingDB Entry DOI: 10.7270/Q237782T |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM14149
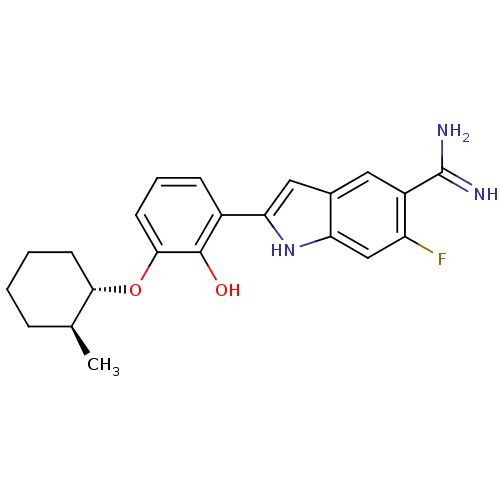
(6-fluoro-2-(2-hydroxy-3-{[(1S,2S)-2-methylcyclohex...)Show SMILES C[C@H]1CCCC[C@@H]1Oc1cccc(-c2cc3cc(C(N)=N)c(F)cc3[nH]2)c1O |r| Show InChI InChI=1S/C22H24FN3O2/c1-12-5-2-3-7-19(12)28-20-8-4-6-14(21(20)27)18-10-13-9-15(22(24)25)16(23)11-17(13)26-18/h4,6,8-12,19,26-27H,2-3,5,7H2,1H3,(H3,24,25)/t12-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 11 | -45.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Axys Pharmaceutical
| Assay Description
Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... |
Chem Biol 8: 1107-21 (2001)
Article DOI: 10.1016/S1074-5521(01)00084-9
BindingDB Entry DOI: 10.7270/Q2S75DKN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine protease 1
(Bos taurus (bovine)) | BDBM14335

(2-{5-[amino(iminiumyl)methyl]-1H-indol-2-yl}-4-chl...)Show SMILES NC(=[NH2+])c1ccc2[nH]c(cc2c1)-c1cc(Cl)cc(-c2ccccc2)c1[O-] Show InChI InChI=1S/C21H16ClN3O/c22-15-10-16(12-4-2-1-3-5-12)20(26)17(11-15)19-9-14-8-13(21(23)24)6-7-18(14)25-19/h1-11,25-26H,(H3,23,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
| Assay Description
Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... |
J Mol Biol 329: 93-120 (2003)
Article DOI: 10.1016/s0022-2836(03)00399-1
BindingDB Entry DOI: 10.7270/Q2R78CGQ |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM14152

(6-CHLORO-2-(2-HYDROXY-BIPHENYL-3-YL)-1H-INDOLE-5-C...)Show SMILES NC(=[NH2+])c1cc2cc([nH]c2cc1Cl)-c1cccc(-c2ccccc2)c1O Show InChI InChI=1S/C21H16ClN3O/c22-17-11-18-13(9-16(17)21(23)24)10-19(25-18)15-8-4-7-14(20(15)26)12-5-2-1-3-6-12/h1-11,25-26H,(H3,23,24)/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
| Assay Description
Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... |
J Mol Biol 329: 93-120 (2003)
Article DOI: 10.1016/s0022-2836(03)00399-1
BindingDB Entry DOI: 10.7270/Q2R78CGQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM14338

(2-{5-[amino(iminiumyl)methyl]-1H-indol-2-yl}-4-nit...)Show SMILES NC(=[NH2+])c1ccc2[nH]c(cc2c1)-c1cc(cc(-c2ccccc2)c1[O-])[N+]([O-])=O Show InChI InChI=1S/C21H16N4O3/c22-21(23)13-6-7-18-14(8-13)9-19(24-18)17-11-15(25(27)28)10-16(20(17)26)12-4-2-1-3-5-12/h1-11,24,26H,(H3,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
| Assay Description
Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... |
J Mol Biol 329: 93-120 (2003)
Article DOI: 10.1016/s0022-2836(03)00399-1
BindingDB Entry DOI: 10.7270/Q2R78CGQ |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Bos taurus (bovine)) | BDBM14337
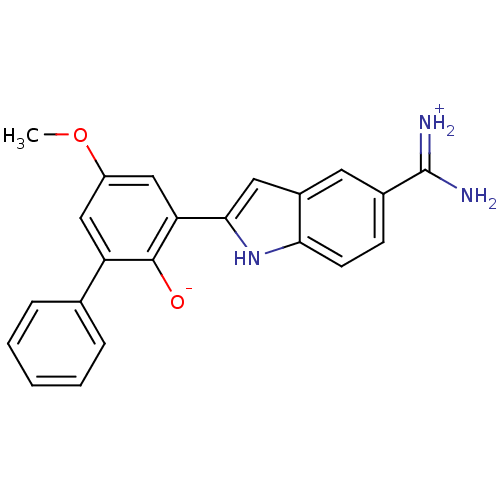
(2-{5-[amino(iminiumyl)methyl]-1H-indol-2-yl}-4-met...)Show SMILES COc1cc(-c2cc3cc(ccc3[nH]2)C(N)=[NH2+])c([O-])c(c1)-c1ccccc1 Show InChI InChI=1S/C22H19N3O2/c1-27-16-11-17(13-5-3-2-4-6-13)21(26)18(12-16)20-10-15-9-14(22(23)24)7-8-19(15)25-20/h2-12,25-26H,1H3,(H3,23,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| DrugBank
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
| Assay Description
Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... |
J Mol Biol 329: 93-120 (2003)
Article DOI: 10.1016/s0022-2836(03)00399-1
BindingDB Entry DOI: 10.7270/Q2R78CGQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50102790

(2-(2-Hydroxy-5-nitro-biphenyl-3-yl)-1H-indole-5-ca...)Show SMILES NC(=N)c1ccc2[nH]c(cc2c1)-c1cc(cc(c1O)-c1ccccc1)[N+]([O-])=O Show InChI InChI=1S/C21H16N4O3/c22-21(23)13-6-7-18-14(8-13)9-19(24-18)17-11-15(25(27)28)10-16(20(17)26)12-4-2-1-3-5-12/h1-11,24,26H,(H3,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Binding affinity against human coagulation factor X |
J Med Chem 44: 2753-71 (2001)
BindingDB Entry DOI: 10.7270/Q2RX9BC7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lyn
(Homo sapiens (Human)) | BDBM1773
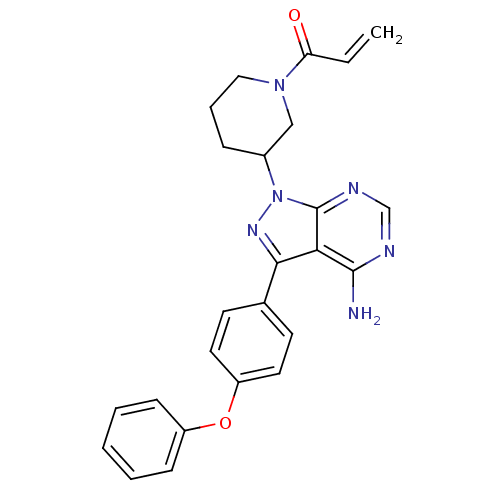
(US10280174, Comparative Compound 1 | US8476284, 4 ...)Show SMILES Nc1ncnc2n(nc(-c3ccc(Oc4ccccc4)cc3)c12)C1CCCN(C1)C(=O)C=C Show InChI InChI=1S/C25H24N6O2/c1-2-21(32)30-14-6-7-18(15-30)31-25-22(24(26)27-16-28-25)23(29-31)17-10-12-20(13-11-17)33-19-8-4-3-5-9-19/h2-5,8-13,16,18H,1,6-7,14-15H2,(H2,26,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| US Patent
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Pharmacyclics, Inc.
US Patent
| Assay Description
Btk kinase activity was determined using a time-resolved fluorescence resonance energy transfer (TR-FRET) methodology. |
US Patent US8476284 (2013)
BindingDB Entry DOI: 10.7270/Q2CJ8C3G |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM14152

(6-CHLORO-2-(2-HYDROXY-BIPHENYL-3-YL)-1H-INDOLE-5-C...)Show SMILES NC(=[NH2+])c1cc2cc([nH]c2cc1Cl)-c1cccc(-c2ccccc2)c1O Show InChI InChI=1S/C21H16ClN3O/c22-17-11-18-13(9-16(17)21(23)24)10-19(25-18)15-8-4-7-14(20(15)26)12-5-2-1-3-6-12/h1-11,25-26H,(H3,23,24)/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
| Assay Description
Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... |
J Mol Biol 344: 527-47 (2004)
Article DOI: 10.1016/j.jmb.2004.09.032
BindingDB Entry DOI: 10.7270/Q2V40SF3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine protease 1
(Bos taurus (bovine)) | BDBM14329

(2-{5-[amino(iminiumyl)methyl]-1H-indol-2-yl}-4-met...)Show SMILES Cc1cc(-c2cc3cc(ccc3[nH]2)C(N)=[NH2+])c([O-])c(c1)-c1ccccc1 Show InChI InChI=1S/C22H19N3O/c1-13-9-17(14-5-3-2-4-6-14)21(26)18(10-13)20-12-16-11-15(22(23)24)7-8-19(16)25-20/h2-12,25-26H,1H3,(H3,23,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 15 | -44.2 | n/a | n/a | n/a | n/a | n/a | 7.6 | 22 |
Celera
| Assay Description
Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... |
J Mol Biol 329: 93-120 (2003)
Article DOI: 10.1016/s0022-2836(03)00399-1
BindingDB Entry DOI: 10.7270/Q2R78CGQ |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM14150
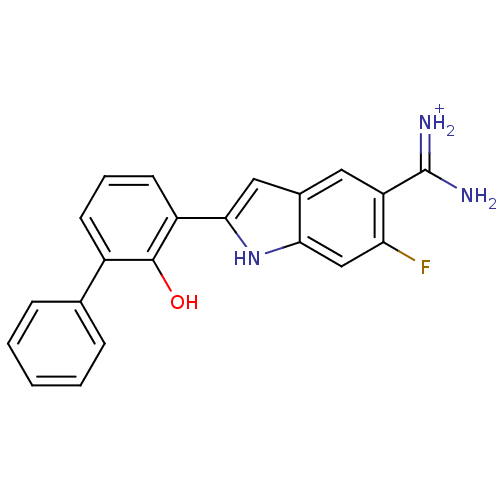
(APC-11417 | CA-12 | CRA-11417 | {amino[6-fluoro-2-...)Show SMILES NC(=[NH2+])c1cc2cc([nH]c2cc1F)-c1cccc(-c2ccccc2)c1O Show InChI InChI=1S/C21H16FN3O/c22-17-11-18-13(9-16(17)21(23)24)10-19(25-18)15-8-4-7-14(20(15)26)12-5-2-1-3-6-12/h1-11,25-26H,(H3,23,24)/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
| Assay Description
Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... |
J Mol Biol 344: 527-47 (2004)
Article DOI: 10.1016/j.jmb.2004.09.032
BindingDB Entry DOI: 10.7270/Q2V40SF3 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM13940

(2-(3-HYDROXY-PYRIDIN-2-YL)-1H-BENZOIMIDAZOLE-5-CAR...)Show InChI InChI=1S/C13H11N5O/c14-12(15)7-3-4-8-9(6-7)18-13(17-8)11-10(19)2-1-5-16-11/h1-6,19H,(H3,14,15)(H,17,18)/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Corporation
| Assay Description
Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... |
J Mol Biol 307: 1451-86 (2001)
Article DOI: 10.1006/jmbi.2001.4516
BindingDB Entry DOI: 10.7270/Q2FX77PG |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data