 Found 1643 hits with Last Name = 'vernier' and Initial = 'w'
Found 1643 hits with Last Name = 'vernier' and Initial = 'w' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM4779
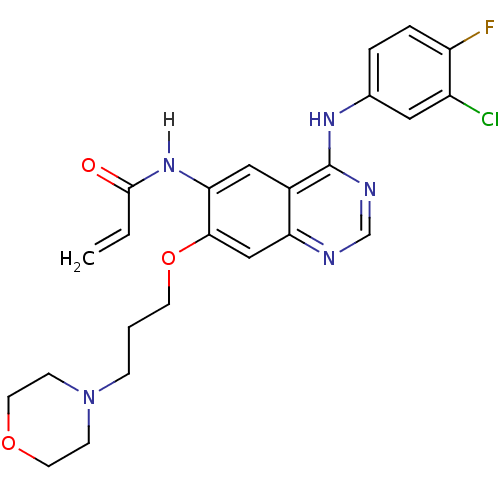
(CHEMBL31965 | CHEMBL545315 | CI-1033 | Canertinib ...)Show SMILES Fc1ccc(Nc2ncnc3cc(OCCCN4CCOCC4)c(NC(=O)C=C)cc23)cc1Cl Show InChI InChI=1S/C24H25ClFN5O3/c1-2-23(32)30-21-13-17-20(14-22(21)34-9-3-6-31-7-10-33-11-8-31)27-15-28-24(17)29-16-4-5-19(26)18(25)12-16/h2,4-5,12-15H,1,3,6-11H2,(H,30,32)(H,27,28,29) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Reversible binding affinity to human EGFR L858R/ T790M double mutant expressed in baculovirus by fluorometric analysis |
J Med Chem 59: 2005-24 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01633
BindingDB Entry DOI: 10.7270/Q2KS6TDD |
More data for this
Ligand-Target Pair | |
3-phosphoinositide-dependent protein kinase 1
(Homo sapiens (Human)) | BDBM50361649
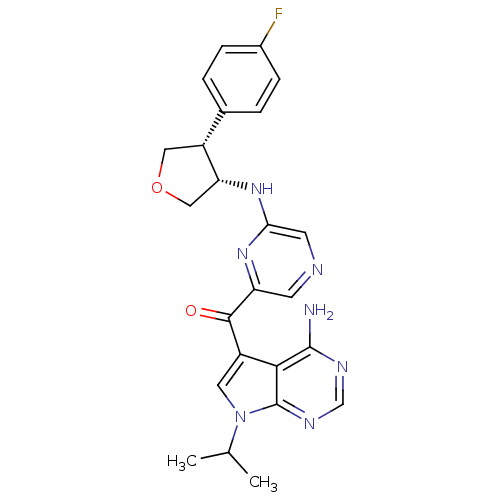
(CHEMBL1938415)Show SMILES CC(C)n1cc(C(=O)c2cncc(N[C@@H]3COC[C@@H]3c3ccc(F)cc3)n2)c2c(N)ncnc12 |r| Show InChI InChI=1S/C24H24FN7O2/c1-13(2)32-9-16(21-23(26)28-12-29-24(21)32)22(33)18-7-27-8-20(30-18)31-19-11-34-10-17(19)14-3-5-15(25)6-4-14/h3-9,12-13,17,19H,10-11H2,1-2H3,(H,30,31)(H2,26,28,29)/t17-,19-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDK1-mediated AKT1 phosphorylation at T308 in human H460 cells after 2 hrs by ELISA |
J Med Chem 54: 8490-500 (2011)
Article DOI: 10.1021/jm201019k
BindingDB Entry DOI: 10.7270/Q23N23TV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
3-phosphoinositide-dependent protein kinase 1
(Homo sapiens (Human)) | BDBM50361648
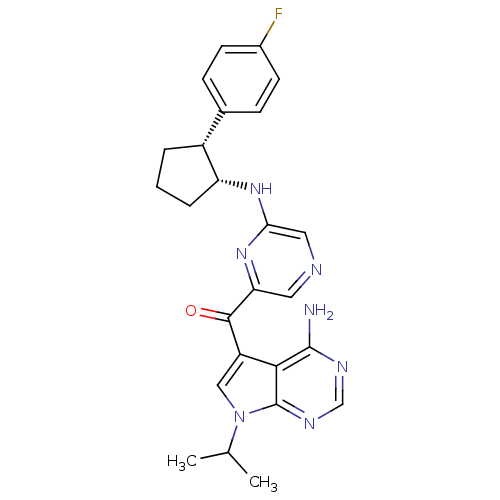
(CHEMBL1940246)Show SMILES CC(C)n1cc(C(=O)c2cncc(N[C@@H]3CCC[C@@H]3c3ccc(F)cc3)n2)c2c(N)ncnc12 |r| Show InChI InChI=1S/C25H26FN7O/c1-14(2)33-12-18(22-24(27)29-13-30-25(22)33)23(34)20-10-28-11-21(32-20)31-19-5-3-4-17(19)15-6-8-16(26)9-7-15/h6-14,17,19H,3-5H2,1-2H3,(H,31,32)(H2,27,29,30)/t17-,19-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDK1-mediated AKT1 phosphorylation at T308 in human H460 cells after 2 hrs by ELISA |
J Med Chem 54: 8490-500 (2011)
Article DOI: 10.1021/jm201019k
BindingDB Entry DOI: 10.7270/Q23N23TV |
More data for this
Ligand-Target Pair | |
3-phosphoinositide-dependent protein kinase 1
(Homo sapiens (Human)) | BDBM50361642
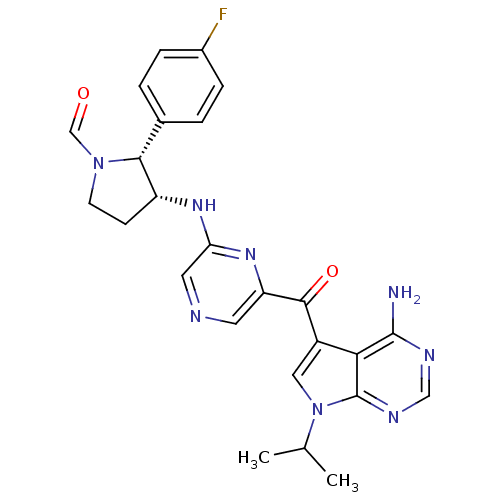
(CHEMBL1940251)Show SMILES CC(C)n1cc(C(=O)c2cncc(N[C@@H]3CCN(C=O)[C@@H]3c3ccc(F)cc3)n2)c2c(N)ncnc12 |r| Show InChI InChI=1S/C25H25FN8O2/c1-14(2)34-11-17(21-24(27)29-12-30-25(21)34)23(36)19-9-28-10-20(32-19)31-18-7-8-33(13-35)22(18)15-3-5-16(26)6-4-15/h3-6,9-14,18,22H,7-8H2,1-2H3,(H,31,32)(H2,27,29,30)/t18-,22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human His-tagged PDK1 catalytic domain using Ac-Sox-PKTFCGTPEYLAPEVRREPRILSEEEQEMFRDFDYIAD-NH2 as substrate by fluorescence... |
J Med Chem 54: 8490-500 (2011)
Article DOI: 10.1021/jm201019k
BindingDB Entry DOI: 10.7270/Q23N23TV |
More data for this
Ligand-Target Pair | |
3-phosphoinositide-dependent protein kinase 1
(Homo sapiens (Human)) | BDBM50361641
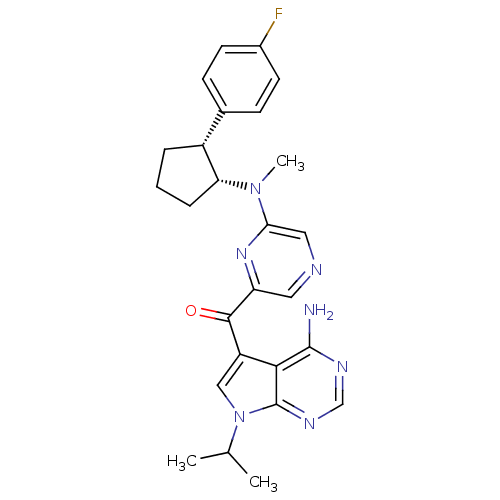
(CHEMBL1940247)Show SMILES CC(C)n1cc(C(=O)c2cncc(n2)N(C)[C@@H]2CCC[C@@H]2c2ccc(F)cc2)c2c(N)ncnc12 |r| Show InChI InChI=1S/C26H28FN7O/c1-15(2)34-13-19(23-25(28)30-14-31-26(23)34)24(35)20-11-29-12-22(32-20)33(3)21-6-4-5-18(21)16-7-9-17(27)10-8-16/h7-15,18,21H,4-6H2,1-3H3,(H2,28,30,31)/t18-,21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDK1-mediated AKT1 phosphorylation at T308 in human H460 cells after 2 hrs by ELISA |
J Med Chem 54: 8490-500 (2011)
Article DOI: 10.1021/jm201019k
BindingDB Entry DOI: 10.7270/Q23N23TV |
More data for this
Ligand-Target Pair | |
3-phosphoinositide-dependent protein kinase 1
(Homo sapiens (Human)) | BDBM50361652
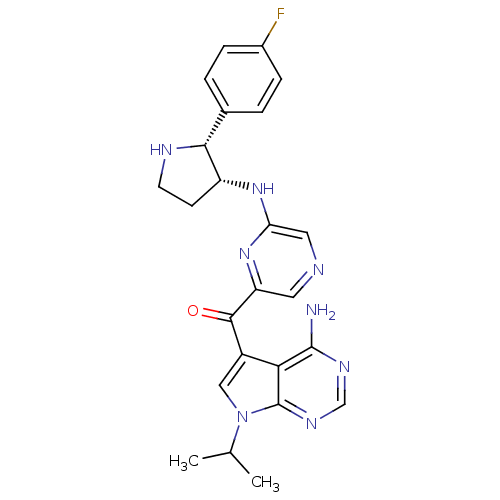
(CHEMBL1940250)Show SMILES CC(C)n1cc(C(=O)c2cncc(N[C@@H]3CCN[C@@H]3c3ccc(F)cc3)n2)c2c(N)ncnc12 |r| Show InChI InChI=1S/C24H25FN8O/c1-13(2)33-11-16(20-23(26)29-12-30-24(20)33)22(34)18-9-27-10-19(32-18)31-17-7-8-28-21(17)14-3-5-15(25)6-4-14/h3-6,9-13,17,21,28H,7-8H2,1-2H3,(H,31,32)(H2,26,29,30)/t17-,21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDK1-mediated AKT1 phosphorylation at T308 in human H460 cells after 2 hrs by ELISA |
J Med Chem 54: 8490-500 (2011)
Article DOI: 10.1021/jm201019k
BindingDB Entry DOI: 10.7270/Q23N23TV |
More data for this
Ligand-Target Pair | |
3-phosphoinositide-dependent protein kinase 1
(Homo sapiens (Human)) | BDBM50361650
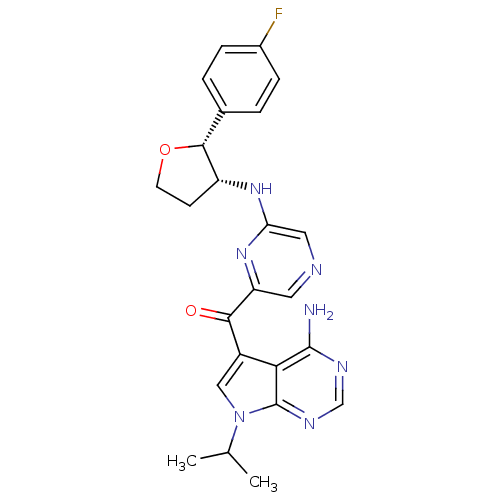
(CHEMBL1940248)Show SMILES CC(C)n1cc(C(=O)c2cncc(N[C@@H]3CCO[C@@H]3c3ccc(F)cc3)n2)c2c(N)ncnc12 |r| Show InChI InChI=1S/C24H24FN7O2/c1-13(2)32-11-16(20-23(26)28-12-29-24(20)32)21(33)18-9-27-10-19(31-18)30-17-7-8-34-22(17)14-3-5-15(25)6-4-14/h3-6,9-13,17,22H,7-8H2,1-2H3,(H,30,31)(H2,26,28,29)/t17-,22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDK1-mediated AKT1 phosphorylation at T308 in human H460 cells after 2 hrs by ELISA |
J Med Chem 54: 8490-500 (2011)
Article DOI: 10.1021/jm201019k
BindingDB Entry DOI: 10.7270/Q23N23TV |
More data for this
Ligand-Target Pair | |
3-phosphoinositide-dependent protein kinase 1
(Homo sapiens (Human)) | BDBM50361643
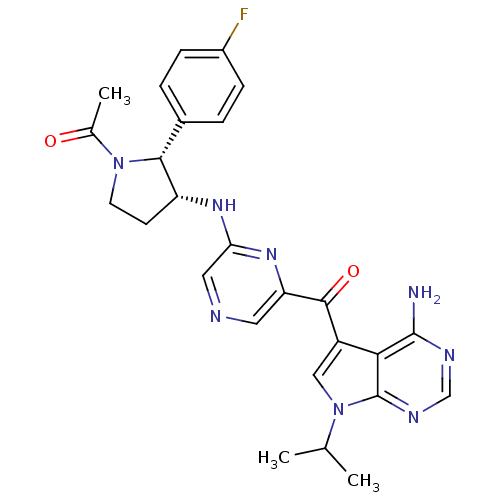
(CHEMBL1940252)Show SMILES CC(C)n1cc(C(=O)c2cncc(N[C@@H]3CCN([C@@H]3c3ccc(F)cc3)C(C)=O)n2)c2c(N)ncnc12 |r| Show InChI InChI=1S/C26H27FN8O2/c1-14(2)35-12-18(22-25(28)30-13-31-26(22)35)24(37)20-10-29-11-21(33-20)32-19-8-9-34(15(3)36)23(19)16-4-6-17(27)7-5-16/h4-7,10-14,19,23H,8-9H2,1-3H3,(H,32,33)(H2,28,30,31)/t19-,23-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human His-tagged PDK1 catalytic domain using Ac-Sox-PKTFCGTPEYLAPEVRREPRILSEEEQEMFRDFDYIAD-NH2 as substrate by fluorescence... |
J Med Chem 54: 8490-500 (2011)
Article DOI: 10.1021/jm201019k
BindingDB Entry DOI: 10.7270/Q23N23TV |
More data for this
Ligand-Target Pair | |
3-phosphoinositide-dependent protein kinase 1
(Homo sapiens (Human)) | BDBM50361644
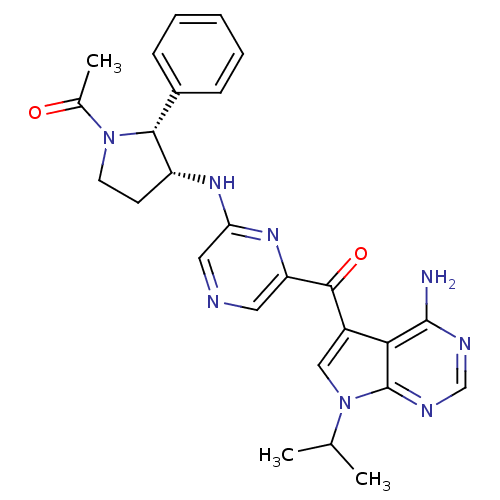
(CHEMBL1940253)Show SMILES CC(C)n1cc(C(=O)c2cncc(N[C@@H]3CCN([C@@H]3c3ccccc3)C(C)=O)n2)c2c(N)ncnc12 |r| Show InChI InChI=1S/C26H28N8O2/c1-15(2)34-13-18(22-25(27)29-14-30-26(22)34)24(36)20-11-28-12-21(32-20)31-19-9-10-33(16(3)35)23(19)17-7-5-4-6-8-17/h4-8,11-15,19,23H,9-10H2,1-3H3,(H,31,32)(H2,27,29,30)/t19-,23-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human His-tagged PDK1 catalytic domain using Ac-Sox-PKTFCGTPEYLAPEVRREPRILSEEEQEMFRDFDYIAD-NH2 as substrate by fluorescence... |
J Med Chem 54: 8490-500 (2011)
Article DOI: 10.1021/jm201019k
BindingDB Entry DOI: 10.7270/Q23N23TV |
More data for this
Ligand-Target Pair | |
3-phosphoinositide-dependent protein kinase 1
(Homo sapiens (Human)) | BDBM50361653
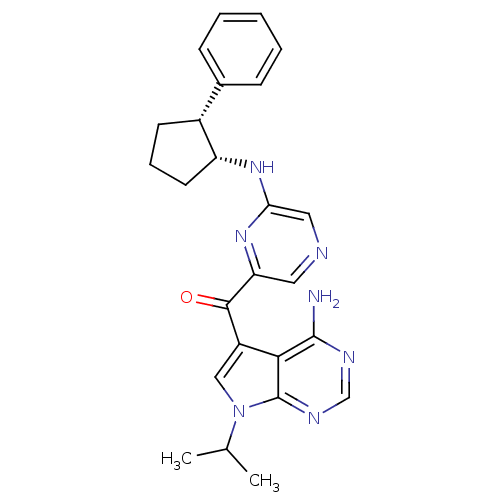
(CHEMBL1940245)Show SMILES CC(C)n1cc(C(=O)c2cncc(N[C@@H]3CCC[C@@H]3c3ccccc3)n2)c2c(N)ncnc12 |r| Show InChI InChI=1S/C25H27N7O/c1-15(2)32-13-18(22-24(26)28-14-29-25(22)32)23(33)20-11-27-12-21(31-20)30-19-10-6-9-17(19)16-7-4-3-5-8-16/h3-5,7-8,11-15,17,19H,6,9-10H2,1-2H3,(H,30,31)(H2,26,28,29)/t17-,19-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDK1-mediated AKT1 phosphorylation at T308 in human H460 cells after 2 hrs by ELISA |
J Med Chem 54: 8490-500 (2011)
Article DOI: 10.1021/jm201019k
BindingDB Entry DOI: 10.7270/Q23N23TV |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50159347
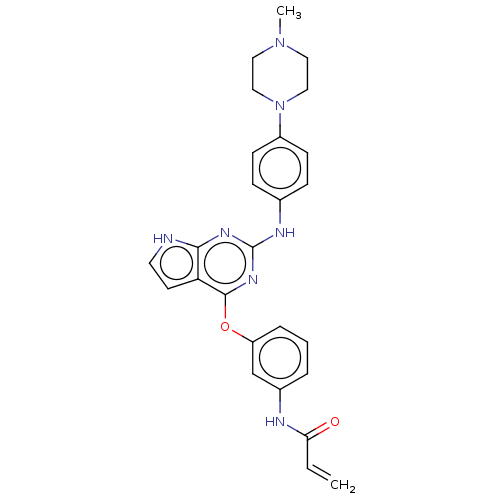
(CHEMBL3787662 | US9586965, Cpd 1)Show SMILES CN1CCN(CC1)c1ccc(Nc2nc(Oc3cccc(NC(=O)C=C)c3)c3cc[nH]c3n2)cc1 Show InChI InChI=1S/C26H27N7O2/c1-3-23(34)28-19-5-4-6-21(17-19)35-25-22-11-12-27-24(22)30-26(31-25)29-18-7-9-20(10-8-18)33-15-13-32(2)14-16-33/h3-12,17H,1,13-16H2,2H3,(H,28,34)(H2,27,29,30,31) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Reversible binding affinity to human EGFR L858R/ T790M double mutant expressed in baculovirus by fluorometric analysis |
J Med Chem 59: 2005-24 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01633
BindingDB Entry DOI: 10.7270/Q2KS6TDD |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50159358
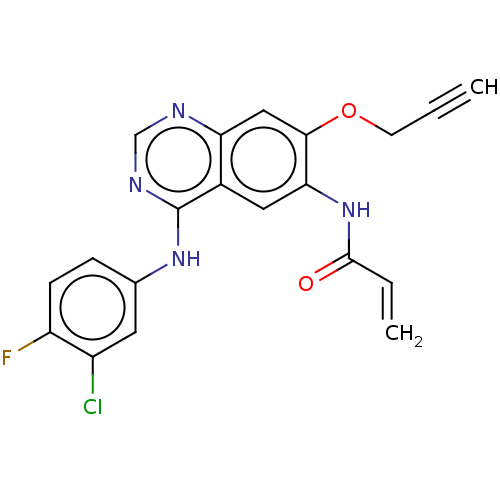
(CHEMBL3787386)Show SMILES Fc1ccc(Nc2ncnc3cc(OCC#C)c(NC(=O)C=C)cc23)cc1Cl Show InChI InChI=1S/C20H14ClFN4O2/c1-3-7-28-18-10-16-13(9-17(18)26-19(27)4-2)20(24-11-23-16)25-12-5-6-15(22)14(21)8-12/h1,4-6,8-11H,2,7H2,(H,26,27)(H,23,24,25) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Reversible binding affinity to human EGFR L858R/ T790M double mutant expressed in baculovirus by fluorometric analysis |
J Med Chem 59: 2005-24 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01633
BindingDB Entry DOI: 10.7270/Q2KS6TDD |
More data for this
Ligand-Target Pair | |
3-phosphoinositide-dependent protein kinase 1
(Homo sapiens (Human)) | BDBM50361651
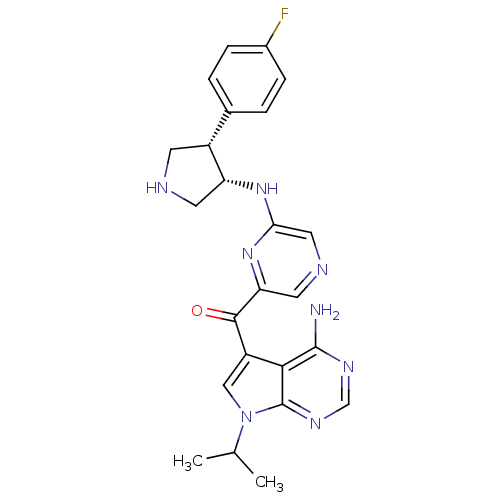
(CHEMBL1940249)Show SMILES CC(C)n1cc(C(=O)c2cncc(N[C@@H]3CNC[C@@H]3c3ccc(F)cc3)n2)c2c(N)ncnc12 |r| Show InChI InChI=1S/C24H25FN8O/c1-13(2)33-11-17(21-23(26)29-12-30-24(21)33)22(34)19-9-28-10-20(32-19)31-18-8-27-7-16(18)14-3-5-15(25)6-4-14/h3-6,9-13,16,18,27H,7-8H2,1-2H3,(H,31,32)(H2,26,29,30)/t16-,18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDK1-mediated AKT1 phosphorylation at T308 in human H460 cells after 2 hrs by ELISA |
J Med Chem 54: 8490-500 (2011)
Article DOI: 10.1021/jm201019k
BindingDB Entry DOI: 10.7270/Q23N23TV |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50159352
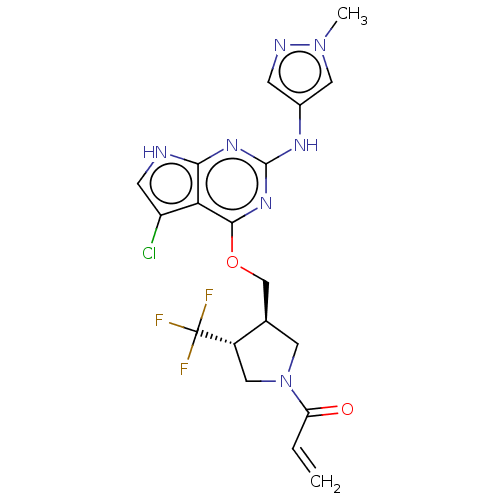
(CHEMBL3786802)Show SMILES Cn1cc(Nc2nc(OC[C@H]3CN(C[C@@H]3C(F)(F)F)C(=O)C=C)c3c(Cl)c[nH]c3n2)cn1 |r| Show InChI InChI=1S/C19H19ClF3N7O2/c1-3-14(31)30-6-10(12(8-30)19(21,22)23)9-32-17-15-13(20)5-24-16(15)27-18(28-17)26-11-4-25-29(2)7-11/h3-5,7,10,12H,1,6,8-9H2,2H3,(H2,24,26,27,28)/t10-,12+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Reversible binding affinity to human EGFR L858R/ T790M double mutant expressed in baculovirus by fluorometric analysis |
J Med Chem 59: 2005-24 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01633
BindingDB Entry DOI: 10.7270/Q2KS6TDD |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50159349
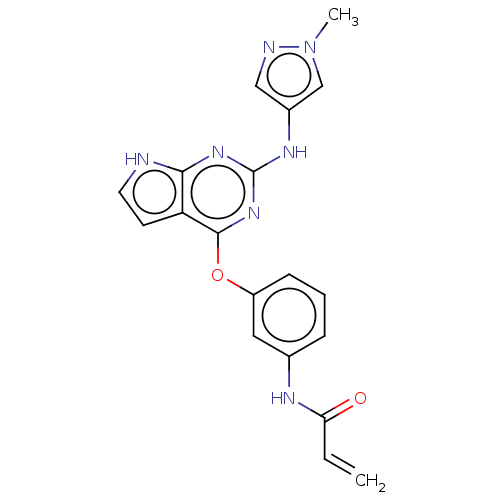
(CHEMBL3786962)Show SMILES Cn1cc(Nc2nc(Oc3cccc(NC(=O)C=C)c3)c3cc[nH]c3n2)cn1 Show InChI InChI=1S/C19H17N7O2/c1-3-16(27)22-12-5-4-6-14(9-12)28-18-15-7-8-20-17(15)24-19(25-18)23-13-10-21-26(2)11-13/h3-11H,1H2,2H3,(H,22,27)(H2,20,23,24,25) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Reversible binding affinity to human EGFR L858R/ T790M double mutant expressed in baculovirus by fluorometric analysis |
J Med Chem 59: 2005-24 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01633
BindingDB Entry DOI: 10.7270/Q2KS6TDD |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50159353

(CHEMBL3787220)Show SMILES Cn1cc(Nc2nc(OC[C@H]3CN(C[C@@H]3F)C(=O)C=C)c3c(Cl)c[nH]c3n2)cn1 |r| Show InChI InChI=1S/C18H19ClFN7O2/c1-3-14(28)27-6-10(13(20)8-27)9-29-17-15-12(19)5-21-16(15)24-18(25-17)23-11-4-22-26(2)7-11/h3-5,7,10,13H,1,6,8-9H2,2H3,(H2,21,23,24,25)/t10-,13+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Reversible binding affinity to human EGFR L858R/ T790M double mutant expressed in baculovirus by fluorometric analysis |
J Med Chem 59: 2005-24 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01633
BindingDB Entry DOI: 10.7270/Q2KS6TDD |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50159356
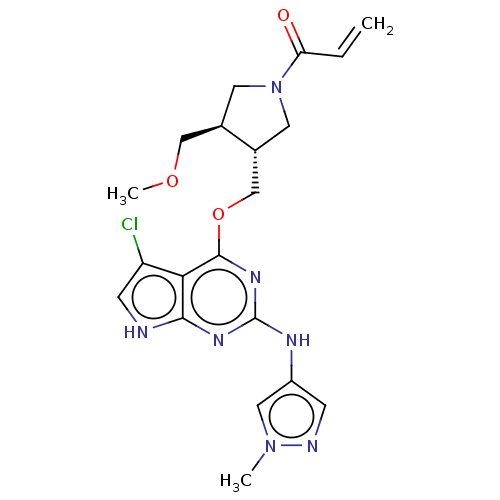
(CHEMBL3786523)Show SMILES COC[C@H]1CN(C[C@@H]1COc1nc(Nc2cnn(C)c2)nc2[nH]cc(Cl)c12)C(=O)C=C |r| Show InChI InChI=1S/C20H24ClN7O3/c1-4-16(29)28-7-12(10-30-3)13(8-28)11-31-19-17-15(21)6-22-18(17)25-20(26-19)24-14-5-23-27(2)9-14/h4-6,9,12-13H,1,7-8,10-11H2,2-3H3,(H2,22,24,25,26)/t12-,13-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Reversible binding affinity to human EGFR L858R/ T790M double mutant expressed in baculovirus by fluorometric analysis |
J Med Chem 59: 2005-24 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01633
BindingDB Entry DOI: 10.7270/Q2KS6TDD |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50159360
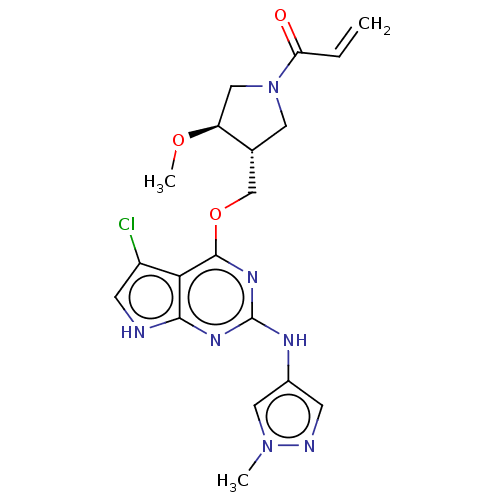
(CHEMBL3786098)Show SMILES CO[C@H]1CN(C[C@@H]1COc1nc(Nc2cnn(C)c2)nc2[nH]cc(Cl)c12)C(=O)C=C |r| Show InChI InChI=1S/C19H22ClN7O3/c1-4-15(28)27-7-11(14(9-27)29-3)10-30-18-16-13(20)6-21-17(16)24-19(25-18)23-12-5-22-26(2)8-12/h4-6,8,11,14H,1,7,9-10H2,2-3H3,(H2,21,23,24,25)/t11-,14+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Reversible binding affinity to human EGFR L858R/ T790M double mutant expressed in baculovirus by fluorometric analysis |
J Med Chem 59: 2005-24 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01633
BindingDB Entry DOI: 10.7270/Q2KS6TDD |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50159354
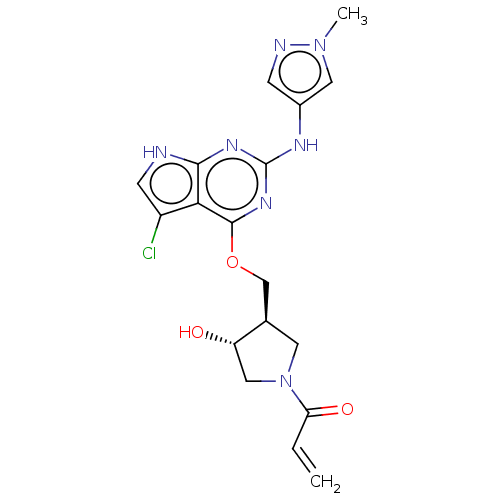
(CHEMBL3786220)Show SMILES Cn1cc(Nc2nc(OC[C@H]3CN(C[C@@H]3O)C(=O)C=C)c3c(Cl)c[nH]c3n2)cn1 |r| Show InChI InChI=1S/C18H20ClN7O3/c1-3-14(28)26-6-10(13(27)8-26)9-29-17-15-12(19)5-20-16(15)23-18(24-17)22-11-4-21-25(2)7-11/h3-5,7,10,13,27H,1,6,8-9H2,2H3,(H2,20,22,23,24)/t10-,13+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Reversible binding affinity to human EGFR L858R/ T790M double mutant expressed in baculovirus by fluorometric analysis |
J Med Chem 59: 2005-24 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01633
BindingDB Entry DOI: 10.7270/Q2KS6TDD |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50159351
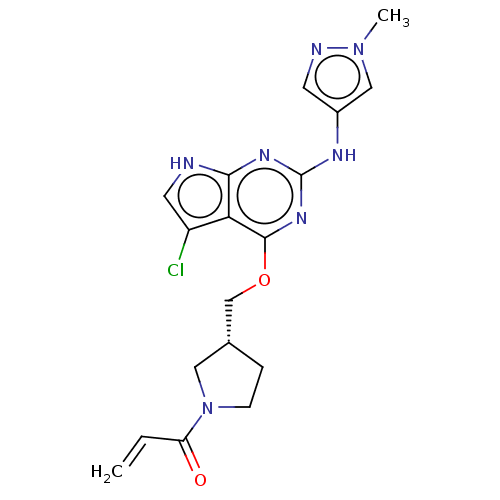
(CHEMBL3786858)Show SMILES Cn1cc(Nc2nc(OC[C@@H]3CCN(C3)C(=O)C=C)c3c(Cl)c[nH]c3n2)cn1 |r| Show InChI InChI=1S/C18H20ClN7O2/c1-3-14(27)26-5-4-11(8-26)10-28-17-15-13(19)7-20-16(15)23-18(24-17)22-12-6-21-25(2)9-12/h3,6-7,9,11H,1,4-5,8,10H2,2H3,(H2,20,22,23,24)/t11-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Reversible binding affinity to human EGFR L858R/ T790M double mutant expressed in baculovirus by fluorometric analysis |
J Med Chem 59: 2005-24 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01633
BindingDB Entry DOI: 10.7270/Q2KS6TDD |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50159359
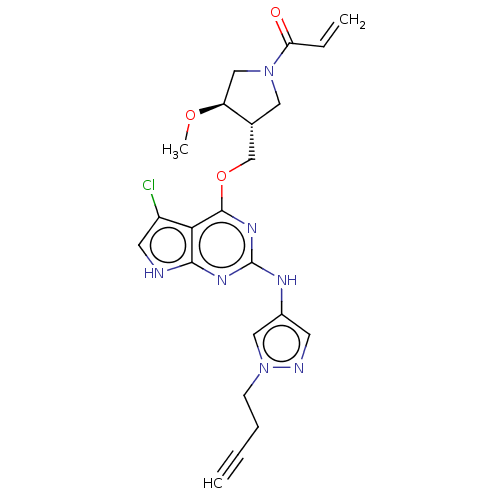
(CHEMBL3786812)Show SMILES CO[C@H]1CN(C[C@@H]1COc1nc(Nc2cnn(CCC#C)c2)nc2[nH]cc(Cl)c12)C(=O)C=C |r| Show InChI InChI=1S/C22H24ClN7O3/c1-4-6-7-30-11-15(8-25-30)26-22-27-20-19(16(23)9-24-20)21(28-22)33-13-14-10-29(18(31)5-2)12-17(14)32-3/h1,5,8-9,11,14,17H,2,6-7,10,12-13H2,3H3,(H2,24,26,27,28)/t14-,17+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Reversible binding affinity to human EGFR L858R/ T790M double mutant expressed in baculovirus by fluorometric analysis |
J Med Chem 59: 2005-24 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01633
BindingDB Entry DOI: 10.7270/Q2KS6TDD |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50361651

(CHEMBL1940249)Show SMILES CC(C)n1cc(C(=O)c2cncc(N[C@@H]3CNC[C@@H]3c3ccc(F)cc3)n2)c2c(N)ncnc12 |r| Show InChI InChI=1S/C24H25FN8O/c1-13(2)33-11-17(21-23(26)29-12-30-24(21)33)22(34)19-9-28-10-20(32-19)31-18-8-27-7-16(18)14-3-5-15(25)6-4-14/h3-6,9-13,16,18,27H,7-8H2,1-2H3,(H,31,32)(H2,26,29,30)/t16-,18-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged AKT1 using 5FAM-GRPRTSSFAEGCONH2 as substrate by fluorescence based assay |
J Med Chem 54: 8490-500 (2011)
Article DOI: 10.1021/jm201019k
BindingDB Entry DOI: 10.7270/Q23N23TV |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50159348
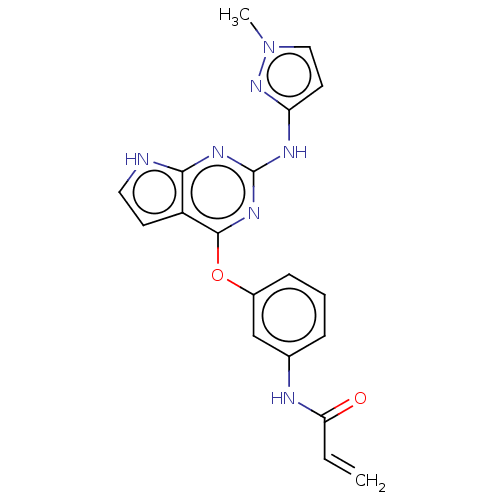
(CHEMBL3786453)Show SMILES Cn1ccc(Nc2nc(Oc3cccc(NC(=O)C=C)c3)c3cc[nH]c3n2)n1 Show InChI InChI=1S/C19H17N7O2/c1-3-16(27)21-12-5-4-6-13(11-12)28-18-14-7-9-20-17(14)23-19(24-18)22-15-8-10-26(2)25-15/h3-11H,1H2,2H3,(H,21,27)(H2,20,22,23,24,25) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Reversible binding affinity to human EGFR L858R/ T790M double mutant expressed in baculovirus by fluorometric analysis |
J Med Chem 59: 2005-24 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01633
BindingDB Entry DOI: 10.7270/Q2KS6TDD |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50159355
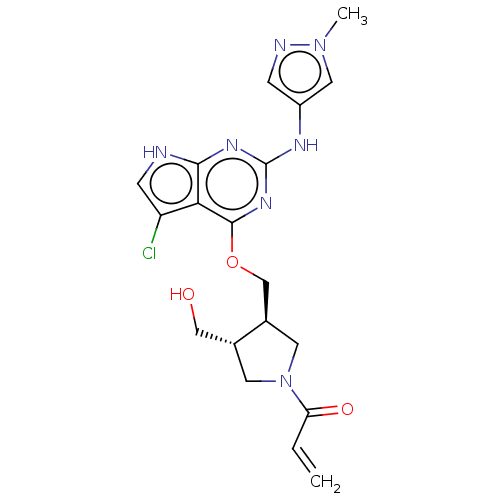
(CHEMBL3786647)Show SMILES Cn1cc(Nc2nc(OC[C@H]3CN(C[C@@H]3CO)C(=O)C=C)c3c(Cl)c[nH]c3n2)cn1 |r| Show InChI InChI=1S/C19H22ClN7O3/c1-3-15(29)27-6-11(9-28)12(7-27)10-30-18-16-14(20)5-21-17(16)24-19(25-18)23-13-4-22-26(2)8-13/h3-5,8,11-12,28H,1,6-7,9-10H2,2H3,(H2,21,23,24,25)/t11-,12-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Reversible binding affinity to human EGFR L858R/ T790M double mutant expressed in baculovirus by fluorometric analysis |
J Med Chem 59: 2005-24 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01633
BindingDB Entry DOI: 10.7270/Q2KS6TDD |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50159357
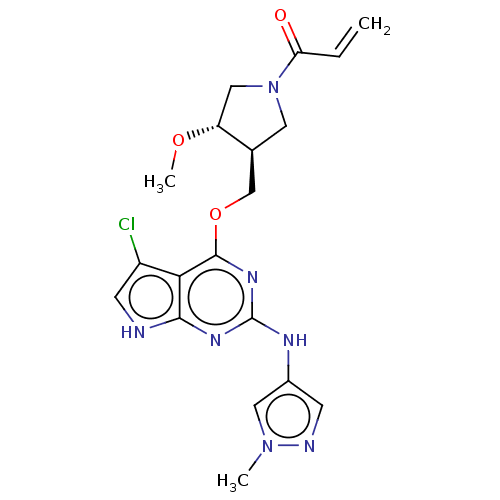
(CHEMBL3787132)Show SMILES CO[C@@H]1CN(C[C@H]1COc1nc(Nc2cnn(C)c2)nc2[nH]cc(Cl)c12)C(=O)C=C |r| Show InChI InChI=1S/C19H22ClN7O3/c1-4-15(28)27-7-11(14(9-27)29-3)10-30-18-16-13(20)6-21-17(16)24-19(25-18)23-12-5-22-26(2)8-12/h4-6,8,11,14H,1,7,9-10H2,2-3H3,(H2,21,23,24,25)/t11-,14+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Reversible binding affinity to human EGFR L858R/ T790M double mutant expressed in baculovirus by fluorometric analysis |
J Med Chem 59: 2005-24 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01633
BindingDB Entry DOI: 10.7270/Q2KS6TDD |
More data for this
Ligand-Target Pair | |
3-phosphoinositide-dependent protein kinase 1
(Homo sapiens (Human)) | BDBM50361647

(CHEMBL1940244)Show SMILES CC(C)n1cc(C(=O)c2cncc(NCCc3cccnc3)n2)c2c(N)ncnc12 Show InChI InChI=1S/C21H22N8O/c1-13(2)29-11-15(18-20(22)26-12-27-21(18)29)19(30)16-9-24-10-17(28-16)25-7-5-14-4-3-6-23-8-14/h3-4,6,8-13H,5,7H2,1-2H3,(H,25,28)(H2,22,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDK1-mediated AKT1 phosphorylation at T308 in human H460 cells after 2 hrs by ELISA |
J Med Chem 54: 8490-500 (2011)
Article DOI: 10.1021/jm201019k
BindingDB Entry DOI: 10.7270/Q23N23TV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50159350
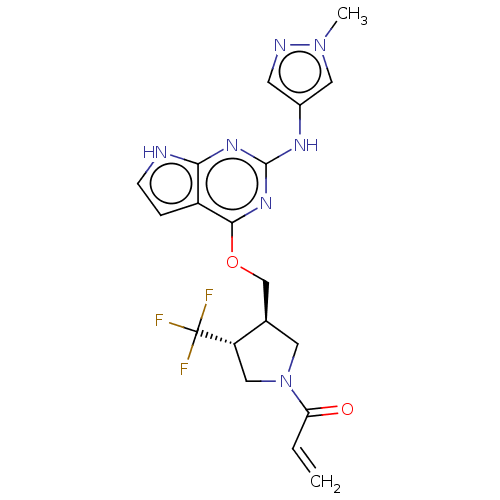
(CHEMBL3785393)Show SMILES Cn1cc(Nc2nc(OC[C@H]3CN(C[C@@H]3C(F)(F)F)C(=O)C=C)c3cc[nH]c3n2)cn1 |r| Show InChI InChI=1S/C19H20F3N7O2/c1-3-15(30)29-7-11(14(9-29)19(20,21)22)10-31-17-13-4-5-23-16(13)26-18(27-17)25-12-6-24-28(2)8-12/h3-6,8,11,14H,1,7,9-10H2,2H3,(H2,23,25,26,27)/t11-,14+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Reversible binding affinity to human EGFR L858R/ T790M double mutant expressed in baculovirus by fluorometric analysis |
J Med Chem 59: 2005-24 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01633
BindingDB Entry DOI: 10.7270/Q2KS6TDD |
More data for this
Ligand-Target Pair | |
3-phosphoinositide-dependent protein kinase 1
(Homo sapiens (Human)) | BDBM50361646
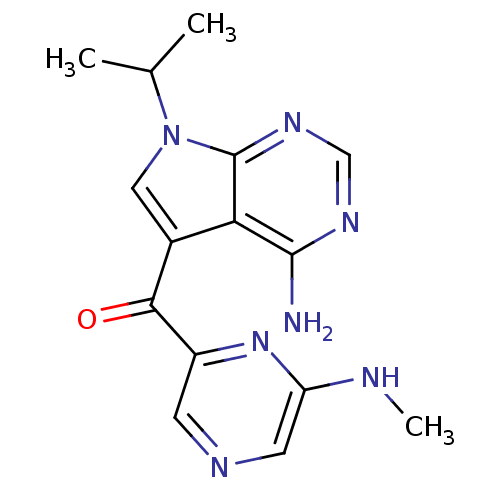
(CHEMBL1940243)Show InChI InChI=1S/C15H17N7O/c1-8(2)22-6-9(12-14(16)19-7-20-15(12)22)13(23)10-4-18-5-11(17-3)21-10/h4-8H,1-3H3,(H,17,21)(H2,16,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDK1-mediated AKT1 phosphorylation at T308 in human H460 cells after 2 hrs by ELISA |
J Med Chem 54: 8490-500 (2011)
Article DOI: 10.1021/jm201019k
BindingDB Entry DOI: 10.7270/Q23N23TV |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50361652

(CHEMBL1940250)Show SMILES CC(C)n1cc(C(=O)c2cncc(N[C@@H]3CCN[C@@H]3c3ccc(F)cc3)n2)c2c(N)ncnc12 |r| Show InChI InChI=1S/C24H25FN8O/c1-13(2)33-11-16(20-23(26)29-12-30-24(20)33)22(34)18-9-27-10-19(32-18)31-17-7-8-28-21(17)14-3-5-15(25)6-4-14/h3-6,9-13,17,21,28H,7-8H2,1-2H3,(H,31,32)(H2,26,29,30)/t17-,21-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged AKT1 using 5FAM-GRPRTSSFAEGCONH2 as substrate by fluorescence based assay |
J Med Chem 54: 8490-500 (2011)
Article DOI: 10.1021/jm201019k
BindingDB Entry DOI: 10.7270/Q23N23TV |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Mus musculus (Mouse)) | BDBM50361642

(CHEMBL1940251)Show SMILES CC(C)n1cc(C(=O)c2cncc(N[C@@H]3CCN(C=O)[C@@H]3c3ccc(F)cc3)n2)c2c(N)ncnc12 |r| Show InChI InChI=1S/C25H25FN8O2/c1-14(2)34-11-17(21-24(27)29-12-30-25(21)34)23(36)19-9-28-10-20(32-19)31-18-7-8-33(13-35)22(18)15-3-5-16(26)6-4-15/h3-6,9-14,18,22H,7-8H2,1-2H3,(H,31,32)(H2,27,29,30)/t18-,22-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mouse PI3Kalpha after 30 mins by fluorescence polarization assay |
J Med Chem 54: 8490-500 (2011)
Article DOI: 10.1021/jm201019k
BindingDB Entry DOI: 10.7270/Q23N23TV |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Mus musculus (Mouse)) | BDBM50361653

(CHEMBL1940245)Show SMILES CC(C)n1cc(C(=O)c2cncc(N[C@@H]3CCC[C@@H]3c3ccccc3)n2)c2c(N)ncnc12 |r| Show InChI InChI=1S/C25H27N7O/c1-15(2)32-13-18(22-24(26)28-14-29-25(22)32)23(33)20-11-27-12-21(31-20)30-19-10-6-9-17(19)16-7-4-3-5-8-16/h3-5,7-8,11-15,17,19H,6,9-10H2,1-2H3,(H,30,31)(H2,26,28,29)/t17-,19-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mouse PI3Kalpha after 30 mins by fluorescence polarization assay |
J Med Chem 54: 8490-500 (2011)
Article DOI: 10.1021/jm201019k
BindingDB Entry DOI: 10.7270/Q23N23TV |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Mus musculus (Mouse)) | BDBM50361652

(CHEMBL1940250)Show SMILES CC(C)n1cc(C(=O)c2cncc(N[C@@H]3CCN[C@@H]3c3ccc(F)cc3)n2)c2c(N)ncnc12 |r| Show InChI InChI=1S/C24H25FN8O/c1-13(2)33-11-16(20-23(26)29-12-30-24(20)33)22(34)18-9-27-10-19(32-18)31-17-7-8-28-21(17)14-3-5-15(25)6-4-14/h3-6,9-13,17,21,28H,7-8H2,1-2H3,(H,31,32)(H2,26,29,30)/t17-,21-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mouse PI3Kalpha after 30 mins by fluorescence polarization assay |
J Med Chem 54: 8490-500 (2011)
Article DOI: 10.1021/jm201019k
BindingDB Entry DOI: 10.7270/Q23N23TV |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50361642

(CHEMBL1940251)Show SMILES CC(C)n1cc(C(=O)c2cncc(N[C@@H]3CCN(C=O)[C@@H]3c3ccc(F)cc3)n2)c2c(N)ncnc12 |r| Show InChI InChI=1S/C25H25FN8O2/c1-14(2)34-11-17(21-24(27)29-12-30-25(21)34)23(36)19-9-28-10-20(32-19)31-18-7-8-33(13-35)22(18)15-3-5-16(26)6-4-15/h3-6,9-14,18,22H,7-8H2,1-2H3,(H,31,32)(H2,27,29,30)/t18-,22-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged AKT1 using 5FAM-GRPRTSSFAEGCONH2 as substrate by fluorescence based assay |
J Med Chem 54: 8490-500 (2011)
Article DOI: 10.1021/jm201019k
BindingDB Entry DOI: 10.7270/Q23N23TV |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50361648

(CHEMBL1940246)Show SMILES CC(C)n1cc(C(=O)c2cncc(N[C@@H]3CCC[C@@H]3c3ccc(F)cc3)n2)c2c(N)ncnc12 |r| Show InChI InChI=1S/C25H26FN7O/c1-14(2)33-12-18(22-24(27)29-13-30-25(22)33)23(34)20-10-28-11-21(32-20)31-19-5-3-4-17(19)15-6-8-16(26)9-7-15/h6-14,17,19H,3-5H2,1-2H3,(H,31,32)(H2,27,29,30)/t17-,19-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged AKT1 using 5FAM-GRPRTSSFAEGCONH2 as substrate by fluorescence based assay |
J Med Chem 54: 8490-500 (2011)
Article DOI: 10.1021/jm201019k
BindingDB Entry DOI: 10.7270/Q23N23TV |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50361650

(CHEMBL1940248)Show SMILES CC(C)n1cc(C(=O)c2cncc(N[C@@H]3CCO[C@@H]3c3ccc(F)cc3)n2)c2c(N)ncnc12 |r| Show InChI InChI=1S/C24H24FN7O2/c1-13(2)32-11-16(20-23(26)28-12-29-24(20)32)21(33)18-9-27-10-19(31-18)30-17-7-8-34-22(17)14-3-5-15(25)6-4-14/h3-6,9-13,17,22H,7-8H2,1-2H3,(H,30,31)(H2,26,28,29)/t17-,22-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged AKT1 using 5FAM-GRPRTSSFAEGCONH2 as substrate by fluorescence based assay |
J Med Chem 54: 8490-500 (2011)
Article DOI: 10.1021/jm201019k
BindingDB Entry DOI: 10.7270/Q23N23TV |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Mus musculus (Mouse)) | BDBM50361647

(CHEMBL1940244)Show SMILES CC(C)n1cc(C(=O)c2cncc(NCCc3cccnc3)n2)c2c(N)ncnc12 Show InChI InChI=1S/C21H22N8O/c1-13(2)29-11-15(18-20(22)26-12-27-21(18)29)19(30)16-9-24-10-17(28-16)25-7-5-14-4-3-6-23-8-14/h3-4,6,8-13H,5,7H2,1-2H3,(H,25,28)(H2,22,26,27) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mouse PI3Kalpha after 30 mins by fluorescence polarization assay |
J Med Chem 54: 8490-500 (2011)
Article DOI: 10.1021/jm201019k
BindingDB Entry DOI: 10.7270/Q23N23TV |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50361649

(CHEMBL1938415)Show SMILES CC(C)n1cc(C(=O)c2cncc(N[C@@H]3COC[C@@H]3c3ccc(F)cc3)n2)c2c(N)ncnc12 |r| Show InChI InChI=1S/C24H24FN7O2/c1-13(2)32-9-16(21-23(26)28-12-29-24(21)32)22(33)18-7-27-8-20(30-18)31-19-11-34-10-17(19)14-3-5-15(25)6-4-14/h3-9,12-13,17,19H,10-11H2,1-2H3,(H,30,31)(H2,26,28,29)/t17-,19-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged AKT1 using 5FAM-GRPRTSSFAEGCONH2 as substrate by fluorescence based assay |
J Med Chem 54: 8490-500 (2011)
Article DOI: 10.1021/jm201019k
BindingDB Entry DOI: 10.7270/Q23N23TV |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Mus musculus (Mouse)) | BDBM50361648

(CHEMBL1940246)Show SMILES CC(C)n1cc(C(=O)c2cncc(N[C@@H]3CCC[C@@H]3c3ccc(F)cc3)n2)c2c(N)ncnc12 |r| Show InChI InChI=1S/C25H26FN7O/c1-14(2)33-12-18(22-24(27)29-13-30-25(22)33)23(34)20-10-28-11-21(32-20)31-19-5-3-4-17(19)15-6-8-16(26)9-7-15/h6-14,17,19H,3-5H2,1-2H3,(H,31,32)(H2,27,29,30)/t17-,19-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mouse PI3Kalpha after 30 mins by fluorescence polarization assay |
J Med Chem 54: 8490-500 (2011)
Article DOI: 10.1021/jm201019k
BindingDB Entry DOI: 10.7270/Q23N23TV |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50361643

(CHEMBL1940252)Show SMILES CC(C)n1cc(C(=O)c2cncc(N[C@@H]3CCN([C@@H]3c3ccc(F)cc3)C(C)=O)n2)c2c(N)ncnc12 |r| Show InChI InChI=1S/C26H27FN8O2/c1-14(2)35-12-18(22-25(28)30-13-31-26(22)35)24(37)20-10-29-11-21(33-20)32-19-8-9-34(15(3)36)23(19)16-4-6-17(27)7-5-16/h4-7,10-14,19,23H,8-9H2,1-3H3,(H,32,33)(H2,28,30,31)/t19-,23-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged AKT1 using 5FAM-GRPRTSSFAEGCONH2 as substrate by fluorescence based assay |
J Med Chem 54: 8490-500 (2011)
Article DOI: 10.1021/jm201019k
BindingDB Entry DOI: 10.7270/Q23N23TV |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Mus musculus (Mouse)) | BDBM50361651

(CHEMBL1940249)Show SMILES CC(C)n1cc(C(=O)c2cncc(N[C@@H]3CNC[C@@H]3c3ccc(F)cc3)n2)c2c(N)ncnc12 |r| Show InChI InChI=1S/C24H25FN8O/c1-13(2)33-11-17(21-23(26)29-12-30-24(21)33)22(34)19-9-28-10-20(32-19)31-18-8-27-7-16(18)14-3-5-15(25)6-4-14/h3-6,9-13,16,18,27H,7-8H2,1-2H3,(H,31,32)(H2,26,29,30)/t16-,18-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mouse PI3Kalpha after 30 mins by fluorescence polarization assay |
J Med Chem 54: 8490-500 (2011)
Article DOI: 10.1021/jm201019k
BindingDB Entry DOI: 10.7270/Q23N23TV |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Mus musculus (Mouse)) | BDBM50361650

(CHEMBL1940248)Show SMILES CC(C)n1cc(C(=O)c2cncc(N[C@@H]3CCO[C@@H]3c3ccc(F)cc3)n2)c2c(N)ncnc12 |r| Show InChI InChI=1S/C24H24FN7O2/c1-13(2)32-11-16(20-23(26)28-12-29-24(20)32)21(33)18-9-27-10-19(31-18)30-17-7-8-34-22(17)14-3-5-15(25)6-4-14/h3-6,9-13,17,22H,7-8H2,1-2H3,(H,30,31)(H2,26,28,29)/t17-,22-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mouse PI3Kalpha after 30 mins by fluorescence polarization assay |
J Med Chem 54: 8490-500 (2011)
Article DOI: 10.1021/jm201019k
BindingDB Entry DOI: 10.7270/Q23N23TV |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50361649

(CHEMBL1938415)Show SMILES CC(C)n1cc(C(=O)c2cncc(N[C@@H]3COC[C@@H]3c3ccc(F)cc3)n2)c2c(N)ncnc12 |r| Show InChI InChI=1S/C24H24FN7O2/c1-13(2)32-9-16(21-23(26)28-12-29-24(21)32)22(33)18-7-27-8-20(30-18)31-19-11-34-10-17(19)14-3-5-15(25)6-4-14/h3-9,12-13,17,19H,10-11H2,1-2H3,(H,30,31)(H2,26,28,29)/t17-,19-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged mTOR assessed as phosphorylation at Thr46 in GFP-4E-BP1 substrate by TR-FRET assay |
J Med Chem 54: 8490-500 (2011)
Article DOI: 10.1021/jm201019k
BindingDB Entry DOI: 10.7270/Q23N23TV |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Mus musculus (Mouse)) | BDBM50361649

(CHEMBL1938415)Show SMILES CC(C)n1cc(C(=O)c2cncc(N[C@@H]3COC[C@@H]3c3ccc(F)cc3)n2)c2c(N)ncnc12 |r| Show InChI InChI=1S/C24H24FN7O2/c1-13(2)32-9-16(21-23(26)28-12-29-24(21)32)22(33)18-7-27-8-20(30-18)31-19-11-34-10-17(19)14-3-5-15(25)6-4-14/h3-9,12-13,17,19H,10-11H2,1-2H3,(H,30,31)(H2,26,28,29)/t17-,19-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mouse PI3Kalpha after 30 mins by fluorescence polarization assay |
J Med Chem 54: 8490-500 (2011)
Article DOI: 10.1021/jm201019k
BindingDB Entry DOI: 10.7270/Q23N23TV |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50159361
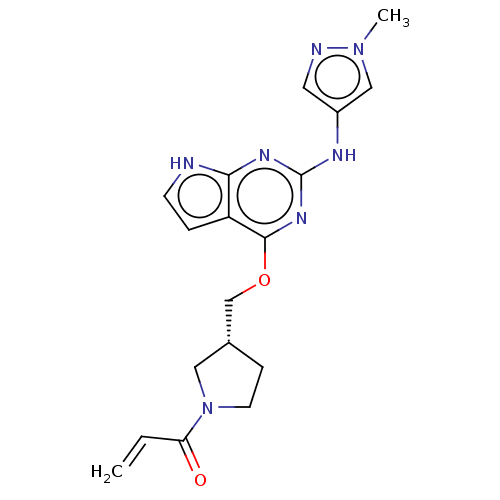
(CHEMBL3787327)Show SMILES Cn1cc(Nc2nc(OC[C@@H]3CCN(C3)C(=O)C=C)c3cc[nH]c3n2)cn1 |r| Show InChI InChI=1S/C18H21N7O2/c1-3-15(26)25-7-5-12(9-25)11-27-17-14-4-6-19-16(14)22-18(23-17)21-13-8-20-24(2)10-13/h3-4,6,8,10,12H,1,5,7,9,11H2,2H3,(H2,19,21,22,23)/t12-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Reversible binding affinity to human EGFR L858R/ T790M double mutant expressed in baculovirus by fluorometric analysis |
J Med Chem 59: 2005-24 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01633
BindingDB Entry DOI: 10.7270/Q2KS6TDD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Mus musculus (Mouse)) | BDBM50361641

(CHEMBL1940247)Show SMILES CC(C)n1cc(C(=O)c2cncc(n2)N(C)[C@@H]2CCC[C@@H]2c2ccc(F)cc2)c2c(N)ncnc12 |r| Show InChI InChI=1S/C26H28FN7O/c1-15(2)34-13-19(23-25(28)30-14-31-26(23)34)24(35)20-11-29-12-22(32-20)33(3)21-6-4-5-18(21)16-7-9-17(27)10-8-16/h7-15,18,21H,4-6H2,1-3H3,(H2,28,30,31)/t18-,21-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mouse PI3Kalpha after 30 mins by fluorescence polarization assay |
J Med Chem 54: 8490-500 (2011)
Article DOI: 10.1021/jm201019k
BindingDB Entry DOI: 10.7270/Q23N23TV |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50361644

(CHEMBL1940253)Show SMILES CC(C)n1cc(C(=O)c2cncc(N[C@@H]3CCN([C@@H]3c3ccccc3)C(C)=O)n2)c2c(N)ncnc12 |r| Show InChI InChI=1S/C26H28N8O2/c1-15(2)34-13-18(22-25(27)29-14-30-26(22)34)24(36)20-11-28-12-21(32-20)31-19-9-10-33(16(3)35)23(19)17-7-5-4-6-8-17/h4-8,11-15,19,23H,9-10H2,1-3H3,(H,31,32)(H2,27,29,30)/t19-,23-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged AKT1 using 5FAM-GRPRTSSFAEGCONH2 as substrate by fluorescence based assay |
J Med Chem 54: 8490-500 (2011)
Article DOI: 10.1021/jm201019k
BindingDB Entry DOI: 10.7270/Q23N23TV |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50361651

(CHEMBL1940249)Show SMILES CC(C)n1cc(C(=O)c2cncc(N[C@@H]3CNC[C@@H]3c3ccc(F)cc3)n2)c2c(N)ncnc12 |r| Show InChI InChI=1S/C24H25FN8O/c1-13(2)33-11-17(21-23(26)29-12-30-24(21)33)22(34)19-9-28-10-20(32-19)31-18-8-27-7-16(18)14-3-5-15(25)6-4-14/h3-6,9-13,16,18,27H,7-8H2,1-2H3,(H,31,32)(H2,26,29,30)/t16-,18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged mTOR assessed as phosphorylation at Thr46 in GFP-4E-BP1 substrate by TR-FRET assay |
J Med Chem 54: 8490-500 (2011)
Article DOI: 10.1021/jm201019k
BindingDB Entry DOI: 10.7270/Q23N23TV |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Mus musculus (Mouse)) | BDBM50361643

(CHEMBL1940252)Show SMILES CC(C)n1cc(C(=O)c2cncc(N[C@@H]3CCN([C@@H]3c3ccc(F)cc3)C(C)=O)n2)c2c(N)ncnc12 |r| Show InChI InChI=1S/C26H27FN8O2/c1-14(2)35-12-18(22-25(28)30-13-31-26(22)35)24(37)20-10-29-11-21(33-20)32-19-8-9-34(15(3)36)23(19)16-4-6-17(27)7-5-16/h4-7,10-14,19,23H,8-9H2,1-3H3,(H,32,33)(H2,28,30,31)/t19-,23-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mouse PI3Kalpha after 30 mins by fluorescence polarization assay |
J Med Chem 54: 8490-500 (2011)
Article DOI: 10.1021/jm201019k
BindingDB Entry DOI: 10.7270/Q23N23TV |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50159360

(CHEMBL3786098)Show SMILES CO[C@H]1CN(C[C@@H]1COc1nc(Nc2cnn(C)c2)nc2[nH]cc(Cl)c12)C(=O)C=C |r| Show InChI InChI=1S/C19H22ClN7O3/c1-4-15(28)27-7-11(14(9-27)29-3)10-30-18-16-13(20)6-21-17(16)24-19(25-18)23-12-5-22-26(2)8-12/h4-6,8,11,14H,1,7,9-10H2,2-3H3,(H2,21,23,24,25)/t11-,14+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Reversible binding affinity to human wild type EGFR expressed in baculovirus by fluorometric analysis |
J Med Chem 59: 2005-24 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01633
BindingDB Entry DOI: 10.7270/Q2KS6TDD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Mus musculus (Mouse)) | BDBM50361644

(CHEMBL1940253)Show SMILES CC(C)n1cc(C(=O)c2cncc(N[C@@H]3CCN([C@@H]3c3ccccc3)C(C)=O)n2)c2c(N)ncnc12 |r| Show InChI InChI=1S/C26H28N8O2/c1-15(2)34-13-18(22-25(27)29-14-30-26(22)34)24(36)20-11-28-12-21(32-20)31-19-9-10-33(16(3)35)23(19)17-7-5-4-6-8-17/h4-8,11-15,19,23H,9-10H2,1-3H3,(H,31,32)(H2,27,29,30)/t19-,23-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mouse PI3Kalpha after 30 mins by fluorescence polarization assay |
J Med Chem 54: 8490-500 (2011)
Article DOI: 10.1021/jm201019k
BindingDB Entry DOI: 10.7270/Q23N23TV |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data