

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50110796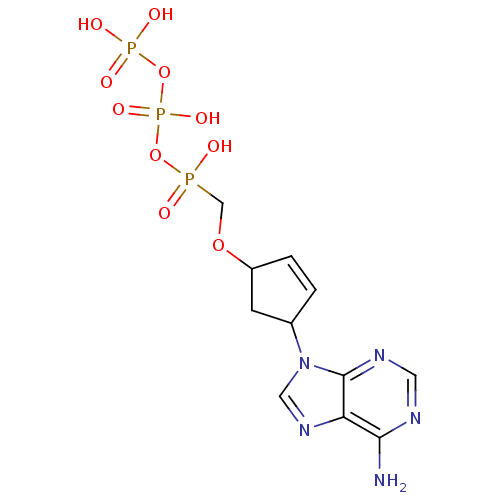 ((4-(6-amino-9H-purin-9-yl)cyclopent-2-enyloxy)meth...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of DNA synthesis by HIV reverse transcriptase | J Med Chem 45: 1284-91 (2002) BindingDB Entry DOI: 10.7270/Q2PR7V9F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50110797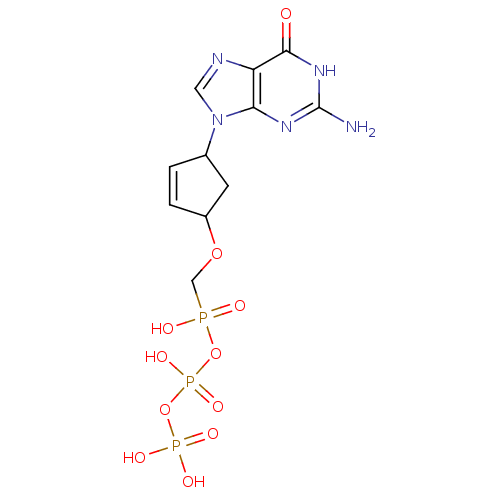 (9-[4-(Diphosphoryloxyphosphonylmethoxy)-cyclopent-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of DNA synthesis by HIV reverse transcriptase | J Med Chem 45: 1284-91 (2002) BindingDB Entry DOI: 10.7270/Q2PR7V9F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50110801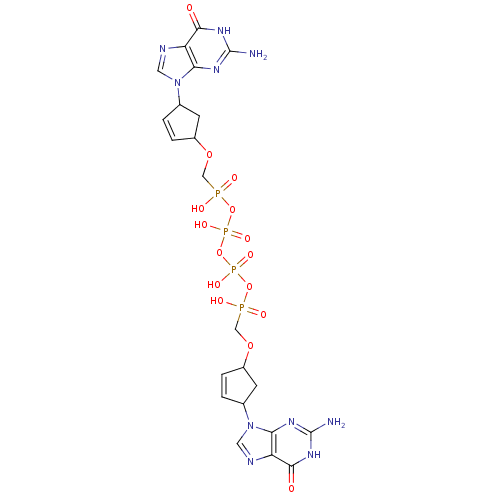 (CHEMBL23361 | P,P'-Bis {[4-(guanine-9-yl)cyclopent...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of DNA synthesis by HIV reverse transcriptase | J Med Chem 45: 1284-91 (2002) BindingDB Entry DOI: 10.7270/Q2PR7V9F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50110804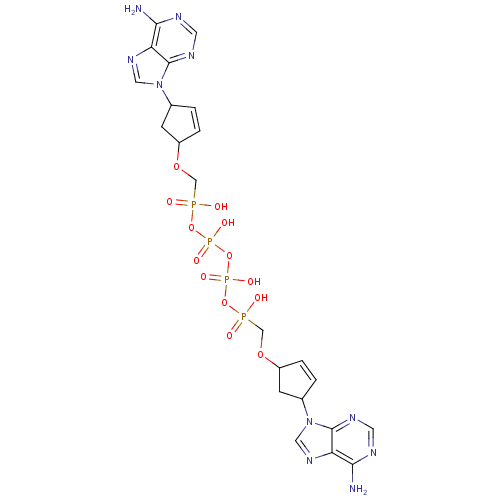 (CHEMBL23358 | P,P'-Bis {[4-(adenine-9-yl)cyclopent...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of DNA synthesis by HIV reverse transcriptase | J Med Chem 45: 1284-91 (2002) BindingDB Entry DOI: 10.7270/Q2PR7V9F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50110800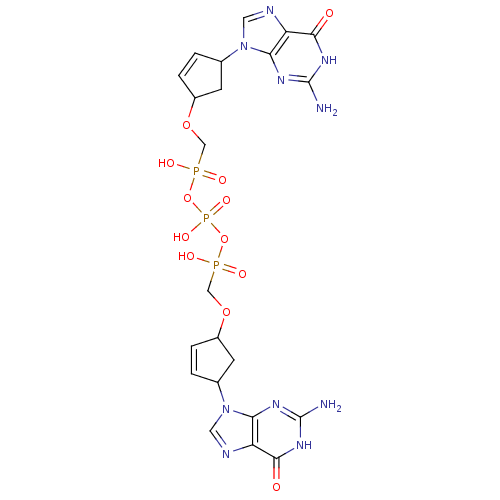 (CHEMBL23886 | P,P'-Bis {[4-(guanine-9-yl)cyclopent...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of DNA synthesis by HIV reverse transcriptase | J Med Chem 45: 1284-91 (2002) BindingDB Entry DOI: 10.7270/Q2PR7V9F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50110798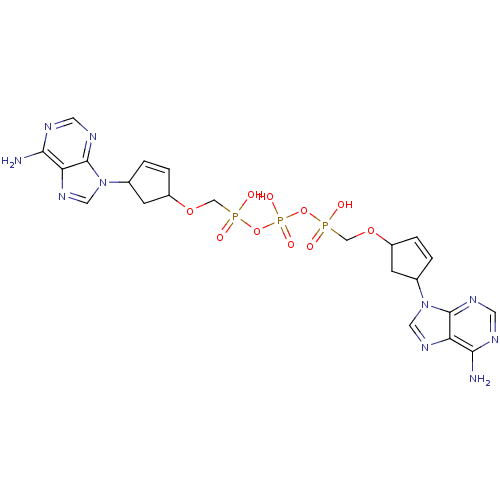 (CHEMBL277903 | P,P'-Bis {[4-(adenine-9-yl)cyclopen...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of DNA synthesis by HIV reverse transcriptase | J Med Chem 45: 1284-91 (2002) BindingDB Entry DOI: 10.7270/Q2PR7V9F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50110799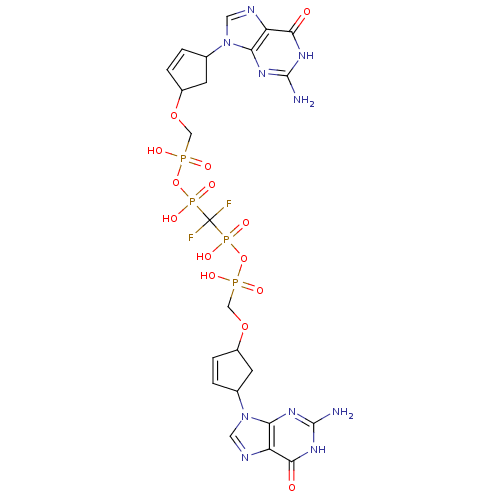 (CHEMBL280273 | P,P'-Bis {[4-(guanine-9-yl)cyclopen...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of DNA synthesis by HIV reverse transcriptase | J Med Chem 45: 1284-91 (2002) BindingDB Entry DOI: 10.7270/Q2PR7V9F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50110795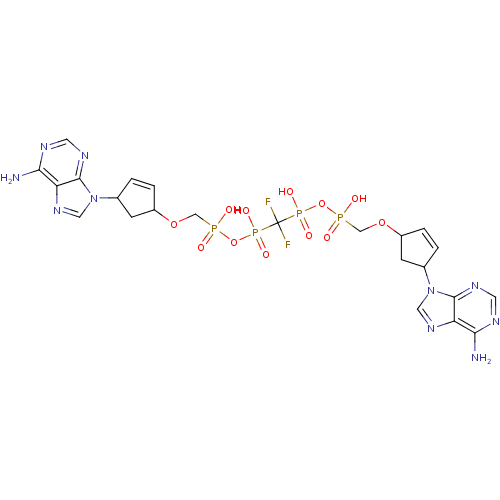 (CHEMBL23428 | NA1 | P,P'-Bis {[4-(adenine-9-yl)cyc...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of DNA synthesis by HIV reverse transcriptase | J Med Chem 45: 1284-91 (2002) BindingDB Entry DOI: 10.7270/Q2PR7V9F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50110802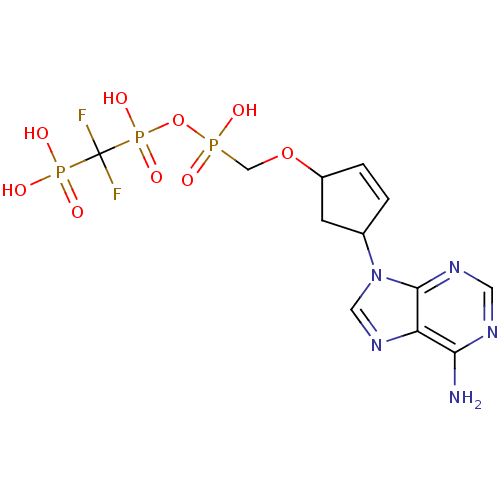 (9-[4-(Difluoromethyldiphosphonyl)oxyphosphonylmeth...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of DNA synthesis by HIV reverse transcriptase | J Med Chem 45: 1284-91 (2002) BindingDB Entry DOI: 10.7270/Q2PR7V9F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50110793 (9-[4-(Difluoromethyldiphosphonyl)oxyphosphonylmeth...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of DNA synthesis by HIV reverse transcriptase | J Med Chem 45: 1284-91 (2002) BindingDB Entry DOI: 10.7270/Q2PR7V9F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50110794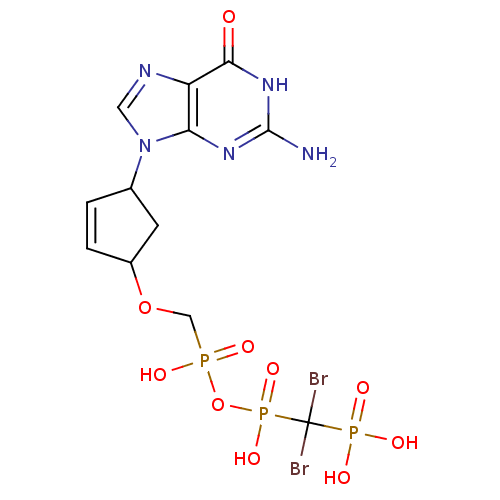 (9-[4-(Dibromomethyldiphosphonyl)oxyphosphonylmetho...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of DNA synthesis by HIV reverse transcriptase | J Med Chem 45: 1284-91 (2002) BindingDB Entry DOI: 10.7270/Q2PR7V9F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50110792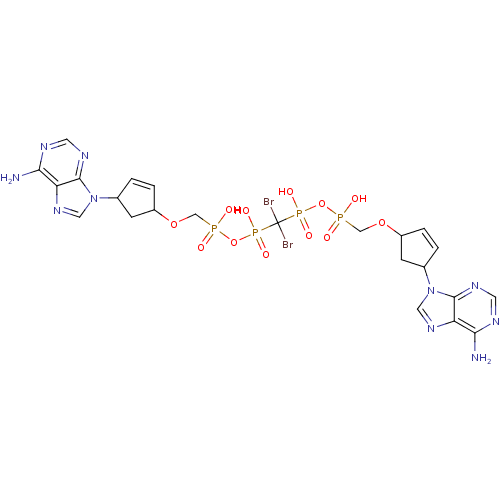 (CHEMBL24006 | NA2 | P,P'-Bis {[4-(ade nine-9-yl)cy...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of DNA synthesis by HIV reverse transcriptase | J Med Chem 45: 1284-91 (2002) BindingDB Entry DOI: 10.7270/Q2PR7V9F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50110791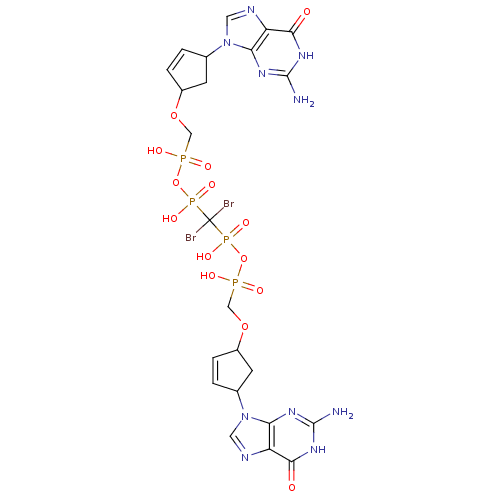 (CHEMBL23522 | P,P'-Bis {[4-(guanine-9-yl)cyclopent...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >2.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of DNA synthesis by HIV reverse transcriptase | J Med Chem 45: 1284-91 (2002) BindingDB Entry DOI: 10.7270/Q2PR7V9F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50110803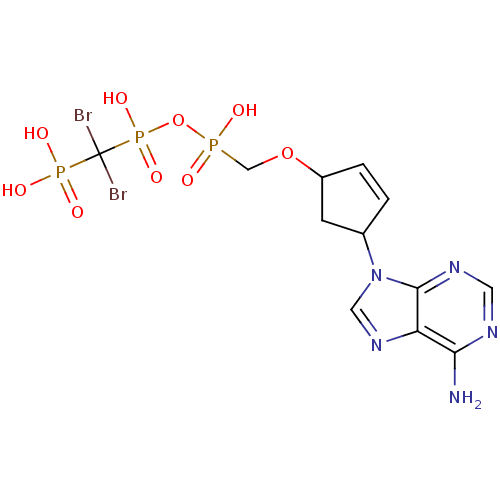 ((4-(6-amino-9H-purin-9-yl)cyclopent-2-enyloxy)meth...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of DNA synthesis by HIV reverse transcriptase | J Med Chem 45: 1284-91 (2002) BindingDB Entry DOI: 10.7270/Q2PR7V9F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||