 Found 68 hits with Last Name = 'villadsen' and Initial = 'js'
Found 68 hits with Last Name = 'villadsen' and Initial = 'js' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Nuclear receptor corepressor 1
(Homo sapiens (Human)) | BDBM50354086

(FK-228 | Istodax | ROMIDEPSIN)Show SMILES C\C=C1/NC(=O)[C@@H](CS)NC(=O)[C@H](CC(=O)C[C@H](OC(=O)[C@@H](NC1=O)C(C)C)\C=C\CCS)C(C)C |r| Show InChI InChI=1S/C25H39N3O6S2/c1-6-19-23(31)28-21(15(4)5)25(33)34-17(9-7-8-10-35)11-16(29)12-18(14(2)3)22(30)27-20(13-36)24(32)26-19/h6-7,9,14-15,17-18,20-21,35-36H,8,10-13H2,1-5H3,(H,26,32)(H,27,30)(H,28,31)/b9-7+,19-6-/t17-,18-,20-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Denmark
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged recombinant human HDAC3/NcoR1 enzyme using flurogenic Ac-LeuGlyLys (Ac)-AMC as substrate after 15 to 30 mins |
J Med Chem 56: 6512-20 (2013)
Article DOI: 10.1021/jm4008449
BindingDB Entry DOI: 10.7270/Q27S7Q6R |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Denmark
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC2 using [Ac-Leu-Gly-Lys(Ac)-AMC substrate incubated for 15 to 30 mins |
J Med Chem 57: 9644-57 (2014)
Article DOI: 10.1021/jm501399d
BindingDB Entry DOI: 10.7270/Q2FJ2JCG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Denmark
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 using [Ac-Leu-Gly-Lys(Ac)-AMC substrate incubated for 15 to 30 mins |
J Med Chem 57: 9644-57 (2014)
Article DOI: 10.1021/jm501399d
BindingDB Entry DOI: 10.7270/Q2FJ2JCG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Denmark
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC3 using [Ac-Leu-Gly-Lys(Ac)-AMC substrate incubated for 15 to 30 mins |
J Med Chem 57: 9644-57 (2014)
Article DOI: 10.1021/jm501399d
BindingDB Entry DOI: 10.7270/Q2FJ2JCG |
More data for this
Ligand-Target Pair | |
Nuclear receptor corepressor 1
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Denmark
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged recombinant human HDAC3/NcoR1 enzyme using flurogenic Ac-LeuGlyLys (Ac)-AMC as substrate after 15 to 30 mins |
J Med Chem 56: 6512-20 (2013)
Article DOI: 10.1021/jm4008449
BindingDB Entry DOI: 10.7270/Q27S7Q6R |
More data for this
Ligand-Target Pair | |
Histone deacetylase 11
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Denmark
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC11 using [Ac-Leu-Gly-Lys(Ac)-AMC substrate incubated for 15 to 30 mins |
J Med Chem 57: 9644-57 (2014)
Article DOI: 10.1021/jm501399d
BindingDB Entry DOI: 10.7270/Q2FJ2JCG |
More data for this
Ligand-Target Pair | |
Nuclear receptor corepressor 1
(Homo sapiens (Human)) | BDBM50377384
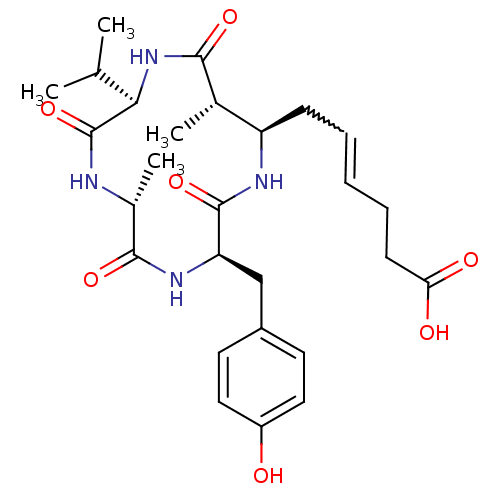
(AZUMAMIDE C)Show SMILES CC(C)[C@H]1NC(=O)[C@@H](C)[C@@H](CC=CCCC(O)=O)NC(=O)[C@@H](Cc2ccc(O)cc2)NC(=O)[C@@H](C)NC1=O |w:11.10| Show InChI InChI=1S/C27H38N4O7/c1-15(2)23-27(38)28-17(4)25(36)30-21(14-18-10-12-19(32)13-11-18)26(37)29-20(16(3)24(35)31-23)8-6-5-7-9-22(33)34/h5-6,10-13,15-17,20-21,23,32H,7-9,14H2,1-4H3,(H,28,38)(H,29,37)(H,30,36)(H,31,35)(H,33,34)/t16-,17+,20+,21+,23+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Denmark
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged recombinant human HDAC3/NcoR1 enzyme using flurogenic Ac-LeuGlyLys (Ac)-AMC as substrate after 15 to 30 mins |
J Med Chem 56: 6512-20 (2013)
Article DOI: 10.1021/jm4008449
BindingDB Entry DOI: 10.7270/Q27S7Q6R |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Denmark
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC6 using [Ac-Leu-Gly-Lys(Ac)-AMC substrate incubated for 15 to 30 mins |
J Med Chem 57: 9644-57 (2014)
Article DOI: 10.1021/jm501399d
BindingDB Entry DOI: 10.7270/Q2FJ2JCG |
More data for this
Ligand-Target Pair | |
Nuclear receptor corepressor 1
(Homo sapiens (Human)) | BDBM50372469
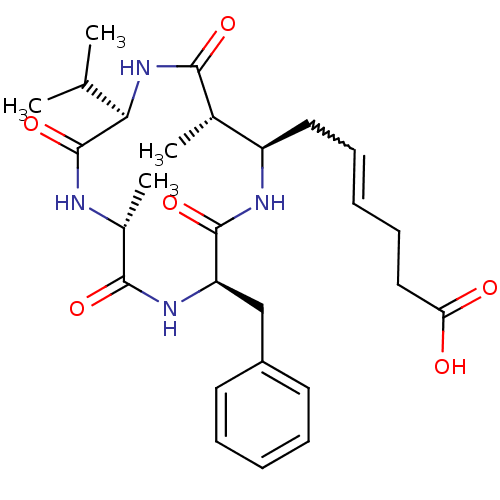
(AZUMAMIDE E)Show SMILES CC(C)[C@H]1NC(=O)[C@@H](C)[C@@H](CC=CCCC(O)=O)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@@H](C)NC1=O |w:11.10| Show InChI InChI=1S/C27H38N4O6/c1-16(2)23-27(37)28-18(4)25(35)30-21(15-19-11-7-5-8-12-19)26(36)29-20(17(3)24(34)31-23)13-9-6-10-14-22(32)33/h5-9,11-12,16-18,20-21,23H,10,13-15H2,1-4H3,(H,28,37)(H,29,36)(H,30,35)(H,31,34)(H,32,33)/t17-,18+,20+,21+,23+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Denmark
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged recombinant human HDAC3/NcoR1 enzyme using flurogenic Ac-LeuGlyLys (Ac)-AMC as substrate after 15 to 30 mins |
J Med Chem 56: 6512-20 (2013)
Article DOI: 10.1021/jm4008449
BindingDB Entry DOI: 10.7270/Q27S7Q6R |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Denmark
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC10 using Ac-Arg-His-Lys(Ac)-Lys(Ac)-AMC substrate incubated for 15 to 30 mins |
J Med Chem 57: 9644-57 (2014)
Article DOI: 10.1021/jm501399d
BindingDB Entry DOI: 10.7270/Q2FJ2JCG |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM50032270
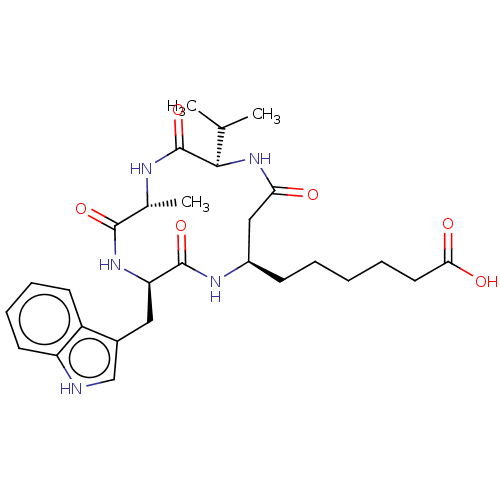
(CHEMBL3352995)Show SMILES CC(C)[C@H]1NC(=O)C[C@@H](CCCCCC(O)=O)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](C)NC1=O |r| Show InChI InChI=1S/C28H39N5O6/c1-16(2)25-28(39)30-17(3)26(37)32-22(13-18-15-29-21-11-8-7-10-20(18)21)27(38)31-19(14-23(34)33-25)9-5-4-6-12-24(35)36/h7-8,10-11,15-17,19,22,25,29H,4-6,9,12-14H2,1-3H3,(H,30,39)(H,31,38)(H,32,37)(H,33,34)(H,35,36)/t17-,19-,22-,25-/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Denmark
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC10 using Ac-Arg-His-Lys(Ac)-Lys(Ac)-AMC substrate incubated for 15 to 30 mins |
J Med Chem 57: 9644-57 (2014)
Article DOI: 10.1021/jm501399d
BindingDB Entry DOI: 10.7270/Q2FJ2JCG |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50032267

(CHEMBL3352997)Show SMILES CC(C)[C@H]1NC(=O)C[C@@H](C\C=C/CCC(O)=O)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](C)NC1=O |r| Show InChI InChI=1S/C28H37N5O6/c1-16(2)25-28(39)30-17(3)26(37)32-22(13-18-15-29-21-11-8-7-10-20(18)21)27(38)31-19(14-23(34)33-25)9-5-4-6-12-24(35)36/h4-5,7-8,10-11,15-17,19,22,25,29H,6,9,12-14H2,1-3H3,(H,30,39)(H,31,38)(H,32,37)(H,33,34)(H,35,36)/b5-4-/t17-,19-,22-,25-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Denmark
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 using [Ac-Leu-Gly-Lys(Ac)-AMC substrate incubated for 15 to 30 mins |
J Med Chem 57: 9644-57 (2014)
Article DOI: 10.1021/jm501399d
BindingDB Entry DOI: 10.7270/Q2FJ2JCG |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50032272
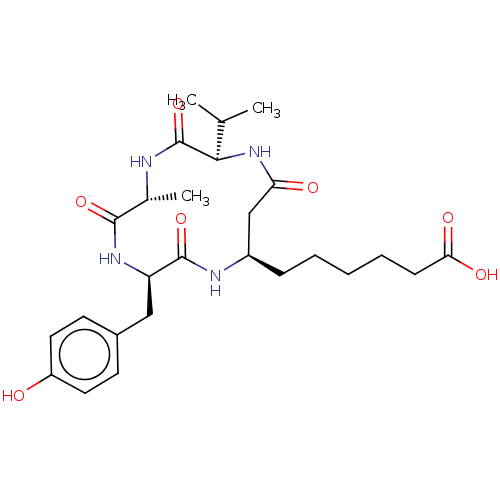
(CHEMBL3352994)Show SMILES CC(C)[C@H]1NC(=O)C[C@@H](CCCCCC(O)=O)NC(=O)[C@@H](Cc2ccc(O)cc2)NC(=O)[C@@H](C)NC1=O |r| Show InChI InChI=1S/C26H38N4O7/c1-15(2)23-26(37)27-16(3)24(35)29-20(13-17-9-11-19(31)12-10-17)25(36)28-18(14-21(32)30-23)7-5-4-6-8-22(33)34/h9-12,15-16,18,20,23,31H,4-8,13-14H2,1-3H3,(H,27,37)(H,28,36)(H,29,35)(H,30,32)(H,33,34)/t16-,18-,20-,23-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Denmark
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC3 using [Ac-Leu-Gly-Lys(Ac)-AMC substrate incubated for 15 to 30 mins |
J Med Chem 57: 9644-57 (2014)
Article DOI: 10.1021/jm501399d
BindingDB Entry DOI: 10.7270/Q2FJ2JCG |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM50032267

(CHEMBL3352997)Show SMILES CC(C)[C@H]1NC(=O)C[C@@H](C\C=C/CCC(O)=O)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](C)NC1=O |r| Show InChI InChI=1S/C28H37N5O6/c1-16(2)25-28(39)30-17(3)26(37)32-22(13-18-15-29-21-11-8-7-10-20(18)21)27(38)31-19(14-23(34)33-25)9-5-4-6-12-24(35)36/h4-5,7-8,10-11,15-17,19,22,25,29H,6,9,12-14H2,1-3H3,(H,30,39)(H,31,38)(H,32,37)(H,33,34)(H,35,36)/b5-4-/t17-,19-,22-,25-/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Denmark
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC10 using Ac-Arg-His-Lys(Ac)-Lys(Ac)-AMC substrate incubated for 15 to 30 mins |
J Med Chem 57: 9644-57 (2014)
Article DOI: 10.1021/jm501399d
BindingDB Entry DOI: 10.7270/Q2FJ2JCG |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50032272

(CHEMBL3352994)Show SMILES CC(C)[C@H]1NC(=O)C[C@@H](CCCCCC(O)=O)NC(=O)[C@@H](Cc2ccc(O)cc2)NC(=O)[C@@H](C)NC1=O |r| Show InChI InChI=1S/C26H38N4O7/c1-15(2)23-26(37)27-16(3)24(35)29-20(13-17-9-11-19(31)12-10-17)25(36)28-18(14-21(32)30-23)7-5-4-6-8-22(33)34/h9-12,15-16,18,20,23,31H,4-8,13-14H2,1-3H3,(H,27,37)(H,28,36)(H,29,35)(H,30,32)(H,33,34)/t16-,18-,20-,23-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Denmark
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC2 using [Ac-Leu-Gly-Lys(Ac)-AMC substrate incubated for 15 to 30 mins |
J Med Chem 57: 9644-57 (2014)
Article DOI: 10.1021/jm501399d
BindingDB Entry DOI: 10.7270/Q2FJ2JCG |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50032270

(CHEMBL3352995)Show SMILES CC(C)[C@H]1NC(=O)C[C@@H](CCCCCC(O)=O)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](C)NC1=O |r| Show InChI InChI=1S/C28H39N5O6/c1-16(2)25-28(39)30-17(3)26(37)32-22(13-18-15-29-21-11-8-7-10-20(18)21)27(38)31-19(14-23(34)33-25)9-5-4-6-12-24(35)36/h7-8,10-11,15-17,19,22,25,29H,4-6,9,12-14H2,1-3H3,(H,30,39)(H,31,38)(H,32,37)(H,33,34)(H,35,36)/t17-,19-,22-,25-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Denmark
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC2 using [Ac-Leu-Gly-Lys(Ac)-AMC substrate incubated for 15 to 30 mins |
J Med Chem 57: 9644-57 (2014)
Article DOI: 10.1021/jm501399d
BindingDB Entry DOI: 10.7270/Q2FJ2JCG |
More data for this
Ligand-Target Pair | |
Histone deacetylase 11
(Homo sapiens (Human)) | BDBM50032270

(CHEMBL3352995)Show SMILES CC(C)[C@H]1NC(=O)C[C@@H](CCCCCC(O)=O)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](C)NC1=O |r| Show InChI InChI=1S/C28H39N5O6/c1-16(2)25-28(39)30-17(3)26(37)32-22(13-18-15-29-21-11-8-7-10-20(18)21)27(38)31-19(14-23(34)33-25)9-5-4-6-12-24(35)36/h7-8,10-11,15-17,19,22,25,29H,4-6,9,12-14H2,1-3H3,(H,30,39)(H,31,38)(H,32,37)(H,33,34)(H,35,36)/t17-,19-,22-,25-/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Denmark
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC11 using [Ac-Leu-Gly-Lys(Ac)-AMC substrate incubated for 15 to 30 mins |
J Med Chem 57: 9644-57 (2014)
Article DOI: 10.1021/jm501399d
BindingDB Entry DOI: 10.7270/Q2FJ2JCG |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50032266
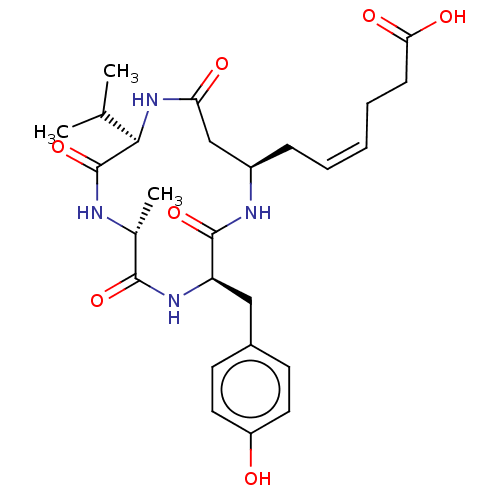
(CHEMBL3352823)Show SMILES CC(C)[C@H]1NC(=O)C[C@@H](C\C=C/CCC(O)=O)NC(=O)[C@@H](Cc2ccc(O)cc2)NC(=O)[C@@H](C)NC1=O |r| Show InChI InChI=1S/C26H36N4O7/c1-15(2)23-26(37)27-16(3)24(35)29-20(13-17-9-11-19(31)12-10-17)25(36)28-18(14-21(32)30-23)7-5-4-6-8-22(33)34/h4-5,9-12,15-16,18,20,23,31H,6-8,13-14H2,1-3H3,(H,27,37)(H,28,36)(H,29,35)(H,30,32)(H,33,34)/b5-4-/t16-,18-,20-,23-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Denmark
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC2 using [Ac-Leu-Gly-Lys(Ac)-AMC substrate incubated for 15 to 30 mins |
J Med Chem 57: 9644-57 (2014)
Article DOI: 10.1021/jm501399d
BindingDB Entry DOI: 10.7270/Q2FJ2JCG |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50032267

(CHEMBL3352997)Show SMILES CC(C)[C@H]1NC(=O)C[C@@H](C\C=C/CCC(O)=O)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](C)NC1=O |r| Show InChI InChI=1S/C28H37N5O6/c1-16(2)25-28(39)30-17(3)26(37)32-22(13-18-15-29-21-11-8-7-10-20(18)21)27(38)31-19(14-23(34)33-25)9-5-4-6-12-24(35)36/h4-5,7-8,10-11,15-17,19,22,25,29H,6,9,12-14H2,1-3H3,(H,30,39)(H,31,38)(H,32,37)(H,33,34)(H,35,36)/b5-4-/t17-,19-,22-,25-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Denmark
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC2 using [Ac-Leu-Gly-Lys(Ac)-AMC substrate incubated for 15 to 30 mins |
J Med Chem 57: 9644-57 (2014)
Article DOI: 10.1021/jm501399d
BindingDB Entry DOI: 10.7270/Q2FJ2JCG |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM50032266

(CHEMBL3352823)Show SMILES CC(C)[C@H]1NC(=O)C[C@@H](C\C=C/CCC(O)=O)NC(=O)[C@@H](Cc2ccc(O)cc2)NC(=O)[C@@H](C)NC1=O |r| Show InChI InChI=1S/C26H36N4O7/c1-15(2)23-26(37)27-16(3)24(35)29-20(13-17-9-11-19(31)12-10-17)25(36)28-18(14-21(32)30-23)7-5-4-6-8-22(33)34/h4-5,9-12,15-16,18,20,23,31H,6-8,13-14H2,1-3H3,(H,27,37)(H,28,36)(H,29,35)(H,30,32)(H,33,34)/b5-4-/t16-,18-,20-,23-/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Denmark
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC10 using Ac-Arg-His-Lys(Ac)-Lys(Ac)-AMC substrate incubated for 15 to 30 mins |
J Med Chem 57: 9644-57 (2014)
Article DOI: 10.1021/jm501399d
BindingDB Entry DOI: 10.7270/Q2FJ2JCG |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM50032272

(CHEMBL3352994)Show SMILES CC(C)[C@H]1NC(=O)C[C@@H](CCCCCC(O)=O)NC(=O)[C@@H](Cc2ccc(O)cc2)NC(=O)[C@@H](C)NC1=O |r| Show InChI InChI=1S/C26H38N4O7/c1-15(2)23-26(37)27-16(3)24(35)29-20(13-17-9-11-19(31)12-10-17)25(36)28-18(14-21(32)30-23)7-5-4-6-8-22(33)34/h9-12,15-16,18,20,23,31H,4-8,13-14H2,1-3H3,(H,27,37)(H,28,36)(H,29,35)(H,30,32)(H,33,34)/t16-,18-,20-,23-/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Denmark
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC10 using Ac-Arg-His-Lys(Ac)-Lys(Ac)-AMC substrate incubated for 15 to 30 mins |
J Med Chem 57: 9644-57 (2014)
Article DOI: 10.1021/jm501399d
BindingDB Entry DOI: 10.7270/Q2FJ2JCG |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50032266

(CHEMBL3352823)Show SMILES CC(C)[C@H]1NC(=O)C[C@@H](C\C=C/CCC(O)=O)NC(=O)[C@@H](Cc2ccc(O)cc2)NC(=O)[C@@H](C)NC1=O |r| Show InChI InChI=1S/C26H36N4O7/c1-15(2)23-26(37)27-16(3)24(35)29-20(13-17-9-11-19(31)12-10-17)25(36)28-18(14-21(32)30-23)7-5-4-6-8-22(33)34/h4-5,9-12,15-16,18,20,23,31H,6-8,13-14H2,1-3H3,(H,27,37)(H,28,36)(H,29,35)(H,30,32)(H,33,34)/b5-4-/t16-,18-,20-,23-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Denmark
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC3 using [Ac-Leu-Gly-Lys(Ac)-AMC substrate incubated for 15 to 30 mins |
J Med Chem 57: 9644-57 (2014)
Article DOI: 10.1021/jm501399d
BindingDB Entry DOI: 10.7270/Q2FJ2JCG |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50032266

(CHEMBL3352823)Show SMILES CC(C)[C@H]1NC(=O)C[C@@H](C\C=C/CCC(O)=O)NC(=O)[C@@H](Cc2ccc(O)cc2)NC(=O)[C@@H](C)NC1=O |r| Show InChI InChI=1S/C26H36N4O7/c1-15(2)23-26(37)27-16(3)24(35)29-20(13-17-9-11-19(31)12-10-17)25(36)28-18(14-21(32)30-23)7-5-4-6-8-22(33)34/h4-5,9-12,15-16,18,20,23,31H,6-8,13-14H2,1-3H3,(H,27,37)(H,28,36)(H,29,35)(H,30,32)(H,33,34)/b5-4-/t16-,18-,20-,23-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Denmark
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 using [Ac-Leu-Gly-Lys(Ac)-AMC substrate incubated for 15 to 30 mins |
J Med Chem 57: 9644-57 (2014)
Article DOI: 10.1021/jm501399d
BindingDB Entry DOI: 10.7270/Q2FJ2JCG |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50032269
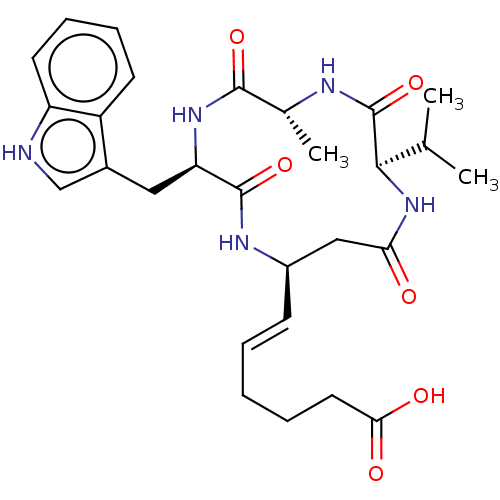
(CHEMBL3352992)Show SMILES CC(C)[C@H]1NC(=O)C[C@H](NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](C)NC1=O)\C=C\CCCC(O)=O |r| Show InChI InChI=1S/C28H37N5O6/c1-16(2)25-28(39)30-17(3)26(37)32-22(13-18-15-29-21-11-8-7-10-20(18)21)27(38)31-19(14-23(34)33-25)9-5-4-6-12-24(35)36/h5,7-11,15-17,19,22,25,29H,4,6,12-14H2,1-3H3,(H,30,39)(H,31,38)(H,32,37)(H,33,34)(H,35,36)/b9-5+/t17-,19-,22-,25-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Denmark
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC2 using [Ac-Leu-Gly-Lys(Ac)-AMC substrate incubated for 15 to 30 mins |
J Med Chem 57: 9644-57 (2014)
Article DOI: 10.1021/jm501399d
BindingDB Entry DOI: 10.7270/Q2FJ2JCG |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50032269

(CHEMBL3352992)Show SMILES CC(C)[C@H]1NC(=O)C[C@H](NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](C)NC1=O)\C=C\CCCC(O)=O |r| Show InChI InChI=1S/C28H37N5O6/c1-16(2)25-28(39)30-17(3)26(37)32-22(13-18-15-29-21-11-8-7-10-20(18)21)27(38)31-19(14-23(34)33-25)9-5-4-6-12-24(35)36/h5,7-11,15-17,19,22,25,29H,4,6,12-14H2,1-3H3,(H,30,39)(H,31,38)(H,32,37)(H,33,34)(H,35,36)/b9-5+/t17-,19-,22-,25-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Denmark
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 using [Ac-Leu-Gly-Lys(Ac)-AMC substrate incubated for 15 to 30 mins |
J Med Chem 57: 9644-57 (2014)
Article DOI: 10.1021/jm501399d
BindingDB Entry DOI: 10.7270/Q2FJ2JCG |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50032267

(CHEMBL3352997)Show SMILES CC(C)[C@H]1NC(=O)C[C@@H](C\C=C/CCC(O)=O)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](C)NC1=O |r| Show InChI InChI=1S/C28H37N5O6/c1-16(2)25-28(39)30-17(3)26(37)32-22(13-18-15-29-21-11-8-7-10-20(18)21)27(38)31-19(14-23(34)33-25)9-5-4-6-12-24(35)36/h4-5,7-8,10-11,15-17,19,22,25,29H,6,9,12-14H2,1-3H3,(H,30,39)(H,31,38)(H,32,37)(H,33,34)(H,35,36)/b5-4-/t17-,19-,22-,25-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Denmark
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC3 using [Ac-Leu-Gly-Lys(Ac)-AMC substrate incubated for 15 to 30 mins |
J Med Chem 57: 9644-57 (2014)
Article DOI: 10.1021/jm501399d
BindingDB Entry DOI: 10.7270/Q2FJ2JCG |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50032270

(CHEMBL3352995)Show SMILES CC(C)[C@H]1NC(=O)C[C@@H](CCCCCC(O)=O)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](C)NC1=O |r| Show InChI InChI=1S/C28H39N5O6/c1-16(2)25-28(39)30-17(3)26(37)32-22(13-18-15-29-21-11-8-7-10-20(18)21)27(38)31-19(14-23(34)33-25)9-5-4-6-12-24(35)36/h7-8,10-11,15-17,19,22,25,29H,4-6,9,12-14H2,1-3H3,(H,30,39)(H,31,38)(H,32,37)(H,33,34)(H,35,36)/t17-,19-,22-,25-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Denmark
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC3 using [Ac-Leu-Gly-Lys(Ac)-AMC substrate incubated for 15 to 30 mins |
J Med Chem 57: 9644-57 (2014)
Article DOI: 10.1021/jm501399d
BindingDB Entry DOI: 10.7270/Q2FJ2JCG |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50032271
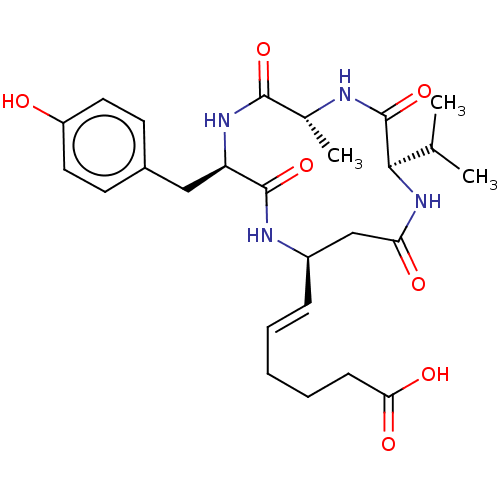
(CHEMBL3352991)Show SMILES CC(C)[C@H]1NC(=O)C[C@H](NC(=O)[C@@H](Cc2ccc(O)cc2)NC(=O)[C@@H](C)NC1=O)\C=C\CCCC(O)=O |r| Show InChI InChI=1S/C26H36N4O7/c1-15(2)23-26(37)27-16(3)24(35)29-20(13-17-9-11-19(31)12-10-17)25(36)28-18(14-21(32)30-23)7-5-4-6-8-22(33)34/h5,7,9-12,15-16,18,20,23,31H,4,6,8,13-14H2,1-3H3,(H,27,37)(H,28,36)(H,29,35)(H,30,32)(H,33,34)/b7-5+/t16-,18-,20-,23-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Denmark
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC3 using [Ac-Leu-Gly-Lys(Ac)-AMC substrate incubated for 15 to 30 mins |
J Med Chem 57: 9644-57 (2014)
Article DOI: 10.1021/jm501399d
BindingDB Entry DOI: 10.7270/Q2FJ2JCG |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50032274
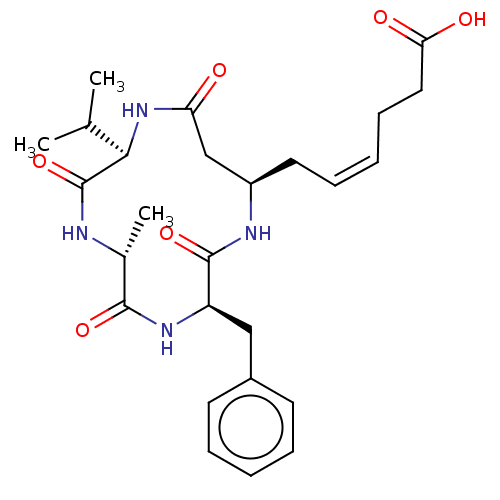
(CHEMBL3352996)Show SMILES CC(C)[C@H]1NC(=O)C[C@@H](C\C=C/CCC(O)=O)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@@H](C)NC1=O |r| Show InChI InChI=1S/C26H36N4O6/c1-16(2)23-26(36)27-17(3)24(34)29-20(14-18-10-6-4-7-11-18)25(35)28-19(15-21(31)30-23)12-8-5-9-13-22(32)33/h4-8,10-11,16-17,19-20,23H,9,12-15H2,1-3H3,(H,27,36)(H,28,35)(H,29,34)(H,30,31)(H,32,33)/b8-5-/t17-,19-,20-,23-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Denmark
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 using [Ac-Leu-Gly-Lys(Ac)-AMC substrate incubated for 15 to 30 mins |
J Med Chem 57: 9644-57 (2014)
Article DOI: 10.1021/jm501399d
BindingDB Entry DOI: 10.7270/Q2FJ2JCG |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50032271

(CHEMBL3352991)Show SMILES CC(C)[C@H]1NC(=O)C[C@H](NC(=O)[C@@H](Cc2ccc(O)cc2)NC(=O)[C@@H](C)NC1=O)\C=C\CCCC(O)=O |r| Show InChI InChI=1S/C26H36N4O7/c1-15(2)23-26(37)27-16(3)24(35)29-20(13-17-9-11-19(31)12-10-17)25(36)28-18(14-21(32)30-23)7-5-4-6-8-22(33)34/h5,7,9-12,15-16,18,20,23,31H,4,6,8,13-14H2,1-3H3,(H,27,37)(H,28,36)(H,29,35)(H,30,32)(H,33,34)/b7-5+/t16-,18-,20-,23-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Denmark
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC2 using [Ac-Leu-Gly-Lys(Ac)-AMC substrate incubated for 15 to 30 mins |
J Med Chem 57: 9644-57 (2014)
Article DOI: 10.1021/jm501399d
BindingDB Entry DOI: 10.7270/Q2FJ2JCG |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM50032269

(CHEMBL3352992)Show SMILES CC(C)[C@H]1NC(=O)C[C@H](NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](C)NC1=O)\C=C\CCCC(O)=O |r| Show InChI InChI=1S/C28H37N5O6/c1-16(2)25-28(39)30-17(3)26(37)32-22(13-18-15-29-21-11-8-7-10-20(18)21)27(38)31-19(14-23(34)33-25)9-5-4-6-12-24(35)36/h5,7-11,15-17,19,22,25,29H,4,6,12-14H2,1-3H3,(H,30,39)(H,31,38)(H,32,37)(H,33,34)(H,35,36)/b9-5+/t17-,19-,22-,25-/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Denmark
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC10 using Ac-Arg-His-Lys(Ac)-Lys(Ac)-AMC substrate incubated for 15 to 30 mins |
J Med Chem 57: 9644-57 (2014)
Article DOI: 10.1021/jm501399d
BindingDB Entry DOI: 10.7270/Q2FJ2JCG |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50032269

(CHEMBL3352992)Show SMILES CC(C)[C@H]1NC(=O)C[C@H](NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](C)NC1=O)\C=C\CCCC(O)=O |r| Show InChI InChI=1S/C28H37N5O6/c1-16(2)25-28(39)30-17(3)26(37)32-22(13-18-15-29-21-11-8-7-10-20(18)21)27(38)31-19(14-23(34)33-25)9-5-4-6-12-24(35)36/h5,7-11,15-17,19,22,25,29H,4,6,12-14H2,1-3H3,(H,30,39)(H,31,38)(H,32,37)(H,33,34)(H,35,36)/b9-5+/t17-,19-,22-,25-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Denmark
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC3 using [Ac-Leu-Gly-Lys(Ac)-AMC substrate incubated for 15 to 30 mins |
J Med Chem 57: 9644-57 (2014)
Article DOI: 10.1021/jm501399d
BindingDB Entry DOI: 10.7270/Q2FJ2JCG |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50032271

(CHEMBL3352991)Show SMILES CC(C)[C@H]1NC(=O)C[C@H](NC(=O)[C@@H](Cc2ccc(O)cc2)NC(=O)[C@@H](C)NC1=O)\C=C\CCCC(O)=O |r| Show InChI InChI=1S/C26H36N4O7/c1-15(2)23-26(37)27-16(3)24(35)29-20(13-17-9-11-19(31)12-10-17)25(36)28-18(14-21(32)30-23)7-5-4-6-8-22(33)34/h5,7,9-12,15-16,18,20,23,31H,4,6,8,13-14H2,1-3H3,(H,27,37)(H,28,36)(H,29,35)(H,30,32)(H,33,34)/b7-5+/t16-,18-,20-,23-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Denmark
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 using [Ac-Leu-Gly-Lys(Ac)-AMC substrate incubated for 15 to 30 mins |
J Med Chem 57: 9644-57 (2014)
Article DOI: 10.1021/jm501399d
BindingDB Entry DOI: 10.7270/Q2FJ2JCG |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM50032274

(CHEMBL3352996)Show SMILES CC(C)[C@H]1NC(=O)C[C@@H](C\C=C/CCC(O)=O)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@@H](C)NC1=O |r| Show InChI InChI=1S/C26H36N4O6/c1-16(2)23-26(36)27-17(3)24(34)29-20(14-18-10-6-4-7-11-18)25(35)28-19(15-21(31)30-23)12-8-5-9-13-22(32)33/h4-8,10-11,16-17,19-20,23H,9,12-15H2,1-3H3,(H,27,36)(H,28,35)(H,29,34)(H,30,31)(H,32,33)/b8-5-/t17-,19-,20-,23-/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Denmark
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC10 using Ac-Arg-His-Lys(Ac)-Lys(Ac)-AMC substrate incubated for 15 to 30 mins |
J Med Chem 57: 9644-57 (2014)
Article DOI: 10.1021/jm501399d
BindingDB Entry DOI: 10.7270/Q2FJ2JCG |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Denmark
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC8 using [Ac-Leu-Gly-Lys(Ac)-AMC substrate incubated for 15 to 30 mins |
J Med Chem 57: 9644-57 (2014)
Article DOI: 10.1021/jm501399d
BindingDB Entry DOI: 10.7270/Q2FJ2JCG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50032273
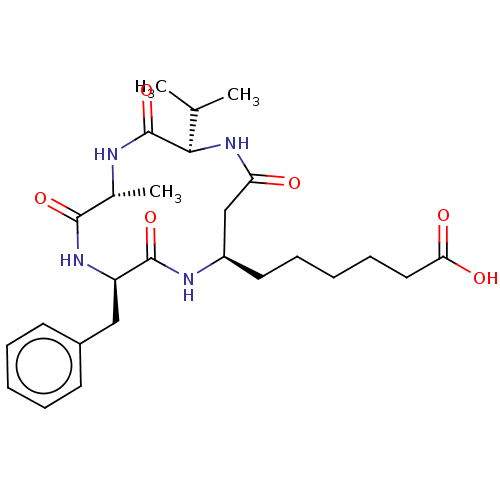
(CHEMBL3352993)Show SMILES CC(C)[C@H]1NC(=O)C[C@@H](CCCCCC(O)=O)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@@H](C)NC1=O |r| Show InChI InChI=1S/C26H38N4O6/c1-16(2)23-26(36)27-17(3)24(34)29-20(14-18-10-6-4-7-11-18)25(35)28-19(15-21(31)30-23)12-8-5-9-13-22(32)33/h4,6-7,10-11,16-17,19-20,23H,5,8-9,12-15H2,1-3H3,(H,27,36)(H,28,35)(H,29,34)(H,30,31)(H,32,33)/t17-,19-,20-,23-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Denmark
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC3 using [Ac-Leu-Gly-Lys(Ac)-AMC substrate incubated for 15 to 30 mins |
J Med Chem 57: 9644-57 (2014)
Article DOI: 10.1021/jm501399d
BindingDB Entry DOI: 10.7270/Q2FJ2JCG |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM50032271

(CHEMBL3352991)Show SMILES CC(C)[C@H]1NC(=O)C[C@H](NC(=O)[C@@H](Cc2ccc(O)cc2)NC(=O)[C@@H](C)NC1=O)\C=C\CCCC(O)=O |r| Show InChI InChI=1S/C26H36N4O7/c1-15(2)23-26(37)27-16(3)24(35)29-20(13-17-9-11-19(31)12-10-17)25(36)28-18(14-21(32)30-23)7-5-4-6-8-22(33)34/h5,7,9-12,15-16,18,20,23,31H,4,6,8,13-14H2,1-3H3,(H,27,37)(H,28,36)(H,29,35)(H,30,32)(H,33,34)/b7-5+/t16-,18-,20-,23-/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Denmark
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC10 using Ac-Arg-His-Lys(Ac)-Lys(Ac)-AMC substrate incubated for 15 to 30 mins |
J Med Chem 57: 9644-57 (2014)
Article DOI: 10.1021/jm501399d
BindingDB Entry DOI: 10.7270/Q2FJ2JCG |
More data for this
Ligand-Target Pair | |
Histone deacetylase 11
(Homo sapiens (Human)) | BDBM50032266

(CHEMBL3352823)Show SMILES CC(C)[C@H]1NC(=O)C[C@@H](C\C=C/CCC(O)=O)NC(=O)[C@@H](Cc2ccc(O)cc2)NC(=O)[C@@H](C)NC1=O |r| Show InChI InChI=1S/C26H36N4O7/c1-15(2)23-26(37)27-16(3)24(35)29-20(13-17-9-11-19(31)12-10-17)25(36)28-18(14-21(32)30-23)7-5-4-6-8-22(33)34/h4-5,9-12,15-16,18,20,23,31H,6-8,13-14H2,1-3H3,(H,27,37)(H,28,36)(H,29,35)(H,30,32)(H,33,34)/b5-4-/t16-,18-,20-,23-/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Denmark
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC11 using [Ac-Leu-Gly-Lys(Ac)-AMC substrate incubated for 15 to 30 mins |
J Med Chem 57: 9644-57 (2014)
Article DOI: 10.1021/jm501399d
BindingDB Entry DOI: 10.7270/Q2FJ2JCG |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50032274

(CHEMBL3352996)Show SMILES CC(C)[C@H]1NC(=O)C[C@@H](C\C=C/CCC(O)=O)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@@H](C)NC1=O |r| Show InChI InChI=1S/C26H36N4O6/c1-16(2)23-26(36)27-17(3)24(34)29-20(14-18-10-6-4-7-11-18)25(35)28-19(15-21(31)30-23)12-8-5-9-13-22(32)33/h4-8,10-11,16-17,19-20,23H,9,12-15H2,1-3H3,(H,27,36)(H,28,35)(H,29,34)(H,30,31)(H,32,33)/b8-5-/t17-,19-,20-,23-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Denmark
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC3 using [Ac-Leu-Gly-Lys(Ac)-AMC substrate incubated for 15 to 30 mins |
J Med Chem 57: 9644-57 (2014)
Article DOI: 10.1021/jm501399d
BindingDB Entry DOI: 10.7270/Q2FJ2JCG |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50032273

(CHEMBL3352993)Show SMILES CC(C)[C@H]1NC(=O)C[C@@H](CCCCCC(O)=O)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@@H](C)NC1=O |r| Show InChI InChI=1S/C26H38N4O6/c1-16(2)23-26(36)27-17(3)24(34)29-20(14-18-10-6-4-7-11-18)25(35)28-19(15-21(31)30-23)12-8-5-9-13-22(32)33/h4,6-7,10-11,16-17,19-20,23H,5,8-9,12-15H2,1-3H3,(H,27,36)(H,28,35)(H,29,34)(H,30,31)(H,32,33)/t17-,19-,20-,23-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Denmark
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC2 using [Ac-Leu-Gly-Lys(Ac)-AMC substrate incubated for 15 to 30 mins |
J Med Chem 57: 9644-57 (2014)
Article DOI: 10.1021/jm501399d
BindingDB Entry DOI: 10.7270/Q2FJ2JCG |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50032273

(CHEMBL3352993)Show SMILES CC(C)[C@H]1NC(=O)C[C@@H](CCCCCC(O)=O)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@@H](C)NC1=O |r| Show InChI InChI=1S/C26H38N4O6/c1-16(2)23-26(36)27-17(3)24(34)29-20(14-18-10-6-4-7-11-18)25(35)28-19(15-21(31)30-23)12-8-5-9-13-22(32)33/h4,6-7,10-11,16-17,19-20,23H,5,8-9,12-15H2,1-3H3,(H,27,36)(H,28,35)(H,29,34)(H,30,31)(H,32,33)/t17-,19-,20-,23-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Denmark
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 using [Ac-Leu-Gly-Lys(Ac)-AMC substrate incubated for 15 to 30 mins |
J Med Chem 57: 9644-57 (2014)
Article DOI: 10.1021/jm501399d
BindingDB Entry DOI: 10.7270/Q2FJ2JCG |
More data for this
Ligand-Target Pair | |
Histone deacetylase 11
(Homo sapiens (Human)) | BDBM50032267

(CHEMBL3352997)Show SMILES CC(C)[C@H]1NC(=O)C[C@@H](C\C=C/CCC(O)=O)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](C)NC1=O |r| Show InChI InChI=1S/C28H37N5O6/c1-16(2)25-28(39)30-17(3)26(37)32-22(13-18-15-29-21-11-8-7-10-20(18)21)27(38)31-19(14-23(34)33-25)9-5-4-6-12-24(35)36/h4-5,7-8,10-11,15-17,19,22,25,29H,6,9,12-14H2,1-3H3,(H,30,39)(H,31,38)(H,32,37)(H,33,34)(H,35,36)/b5-4-/t17-,19-,22-,25-/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Denmark
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC11 using [Ac-Leu-Gly-Lys(Ac)-AMC substrate incubated for 15 to 30 mins |
J Med Chem 57: 9644-57 (2014)
Article DOI: 10.1021/jm501399d
BindingDB Entry DOI: 10.7270/Q2FJ2JCG |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50032270

(CHEMBL3352995)Show SMILES CC(C)[C@H]1NC(=O)C[C@@H](CCCCCC(O)=O)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](C)NC1=O |r| Show InChI InChI=1S/C28H39N5O6/c1-16(2)25-28(39)30-17(3)26(37)32-22(13-18-15-29-21-11-8-7-10-20(18)21)27(38)31-19(14-23(34)33-25)9-5-4-6-12-24(35)36/h7-8,10-11,15-17,19,22,25,29H,4-6,9,12-14H2,1-3H3,(H,30,39)(H,31,38)(H,32,37)(H,33,34)(H,35,36)/t17-,19-,22-,25-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Denmark
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 using [Ac-Leu-Gly-Lys(Ac)-AMC substrate incubated for 15 to 30 mins |
J Med Chem 57: 9644-57 (2014)
Article DOI: 10.1021/jm501399d
BindingDB Entry DOI: 10.7270/Q2FJ2JCG |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50032274

(CHEMBL3352996)Show SMILES CC(C)[C@H]1NC(=O)C[C@@H](C\C=C/CCC(O)=O)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@@H](C)NC1=O |r| Show InChI InChI=1S/C26H36N4O6/c1-16(2)23-26(36)27-17(3)24(34)29-20(14-18-10-6-4-7-11-18)25(35)28-19(15-21(31)30-23)12-8-5-9-13-22(32)33/h4-8,10-11,16-17,19-20,23H,9,12-15H2,1-3H3,(H,27,36)(H,28,35)(H,29,34)(H,30,31)(H,32,33)/b8-5-/t17-,19-,20-,23-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Denmark
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC2 using [Ac-Leu-Gly-Lys(Ac)-AMC substrate incubated for 15 to 30 mins |
J Med Chem 57: 9644-57 (2014)
Article DOI: 10.1021/jm501399d
BindingDB Entry DOI: 10.7270/Q2FJ2JCG |
More data for this
Ligand-Target Pair | |
Histone deacetylase 11
(Homo sapiens (Human)) | BDBM50032272

(CHEMBL3352994)Show SMILES CC(C)[C@H]1NC(=O)C[C@@H](CCCCCC(O)=O)NC(=O)[C@@H](Cc2ccc(O)cc2)NC(=O)[C@@H](C)NC1=O |r| Show InChI InChI=1S/C26H38N4O7/c1-15(2)23-26(37)27-16(3)24(35)29-20(13-17-9-11-19(31)12-10-17)25(36)28-18(14-21(32)30-23)7-5-4-6-8-22(33)34/h9-12,15-16,18,20,23,31H,4-8,13-14H2,1-3H3,(H,27,37)(H,28,36)(H,29,35)(H,30,32)(H,33,34)/t16-,18-,20-,23-/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Denmark
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC11 using [Ac-Leu-Gly-Lys(Ac)-AMC substrate incubated for 15 to 30 mins |
J Med Chem 57: 9644-57 (2014)
Article DOI: 10.1021/jm501399d
BindingDB Entry DOI: 10.7270/Q2FJ2JCG |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50032272

(CHEMBL3352994)Show SMILES CC(C)[C@H]1NC(=O)C[C@@H](CCCCCC(O)=O)NC(=O)[C@@H](Cc2ccc(O)cc2)NC(=O)[C@@H](C)NC1=O |r| Show InChI InChI=1S/C26H38N4O7/c1-15(2)23-26(37)27-16(3)24(35)29-20(13-17-9-11-19(31)12-10-17)25(36)28-18(14-21(32)30-23)7-5-4-6-8-22(33)34/h9-12,15-16,18,20,23,31H,4-8,13-14H2,1-3H3,(H,27,37)(H,28,36)(H,29,35)(H,30,32)(H,33,34)/t16-,18-,20-,23-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Denmark
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 using [Ac-Leu-Gly-Lys(Ac)-AMC substrate incubated for 15 to 30 mins |
J Med Chem 57: 9644-57 (2014)
Article DOI: 10.1021/jm501399d
BindingDB Entry DOI: 10.7270/Q2FJ2JCG |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM50032273

(CHEMBL3352993)Show SMILES CC(C)[C@H]1NC(=O)C[C@@H](CCCCCC(O)=O)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@@H](C)NC1=O |r| Show InChI InChI=1S/C26H38N4O6/c1-16(2)23-26(36)27-17(3)24(34)29-20(14-18-10-6-4-7-11-18)25(35)28-19(15-21(31)30-23)12-8-5-9-13-22(32)33/h4,6-7,10-11,16-17,19-20,23H,5,8-9,12-15H2,1-3H3,(H,27,36)(H,28,35)(H,29,34)(H,30,31)(H,32,33)/t17-,19-,20-,23-/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Denmark
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC10 using Ac-Arg-His-Lys(Ac)-Lys(Ac)-AMC substrate incubated for 15 to 30 mins |
J Med Chem 57: 9644-57 (2014)
Article DOI: 10.1021/jm501399d
BindingDB Entry DOI: 10.7270/Q2FJ2JCG |
More data for this
Ligand-Target Pair | |
Histone deacetylase 11
(Homo sapiens (Human)) | BDBM50032269

(CHEMBL3352992)Show SMILES CC(C)[C@H]1NC(=O)C[C@H](NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](C)NC1=O)\C=C\CCCC(O)=O |r| Show InChI InChI=1S/C28H37N5O6/c1-16(2)25-28(39)30-17(3)26(37)32-22(13-18-15-29-21-11-8-7-10-20(18)21)27(38)31-19(14-23(34)33-25)9-5-4-6-12-24(35)36/h5,7-11,15-17,19,22,25,29H,4,6,12-14H2,1-3H3,(H,30,39)(H,31,38)(H,32,37)(H,33,34)(H,35,36)/b9-5+/t17-,19-,22-,25-/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Denmark
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC11 using [Ac-Leu-Gly-Lys(Ac)-AMC substrate incubated for 15 to 30 mins |
J Med Chem 57: 9644-57 (2014)
Article DOI: 10.1021/jm501399d
BindingDB Entry DOI: 10.7270/Q2FJ2JCG |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50032268
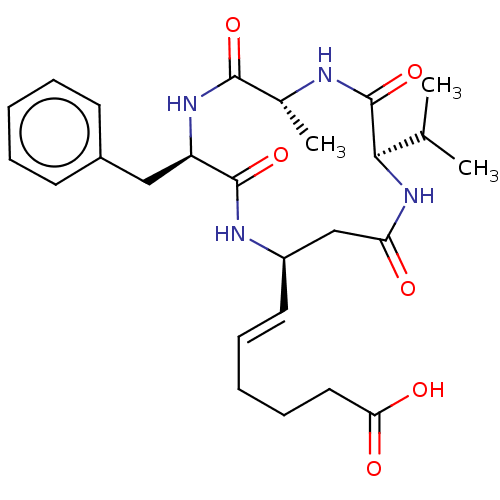
(CHEMBL3352990)Show SMILES CC(C)[C@H]1NC(=O)C[C@H](NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@@H](C)NC1=O)\C=C\CCCC(O)=O |r| Show InChI InChI=1S/C26H36N4O6/c1-16(2)23-26(36)27-17(3)24(34)29-20(14-18-10-6-4-7-11-18)25(35)28-19(15-21(31)30-23)12-8-5-9-13-22(32)33/h4,6-8,10-12,16-17,19-20,23H,5,9,13-15H2,1-3H3,(H,27,36)(H,28,35)(H,29,34)(H,30,31)(H,32,33)/b12-8+/t17-,19-,20-,23-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Denmark
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 using [Ac-Leu-Gly-Lys(Ac)-AMC substrate incubated for 15 to 30 mins |
J Med Chem 57: 9644-57 (2014)
Article DOI: 10.1021/jm501399d
BindingDB Entry DOI: 10.7270/Q2FJ2JCG |
More data for this
Ligand-Target Pair | |
Histone deacetylase 11
(Homo sapiens (Human)) | BDBM50032271

(CHEMBL3352991)Show SMILES CC(C)[C@H]1NC(=O)C[C@H](NC(=O)[C@@H](Cc2ccc(O)cc2)NC(=O)[C@@H](C)NC1=O)\C=C\CCCC(O)=O |r| Show InChI InChI=1S/C26H36N4O7/c1-15(2)23-26(37)27-16(3)24(35)29-20(13-17-9-11-19(31)12-10-17)25(36)28-18(14-21(32)30-23)7-5-4-6-8-22(33)34/h5,7,9-12,15-16,18,20,23,31H,4,6,8,13-14H2,1-3H3,(H,27,37)(H,28,36)(H,29,35)(H,30,32)(H,33,34)/b7-5+/t16-,18-,20-,23-/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Denmark
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC11 using [Ac-Leu-Gly-Lys(Ac)-AMC substrate incubated for 15 to 30 mins |
J Med Chem 57: 9644-57 (2014)
Article DOI: 10.1021/jm501399d
BindingDB Entry DOI: 10.7270/Q2FJ2JCG |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data