 Found 124 hits with Last Name = 'viswanath' and Initial = 'an'
Found 124 hits with Last Name = 'viswanath' and Initial = 'an' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Kinesin-like protein KIF11
(Homo sapiens (Human)) | BDBM50427294
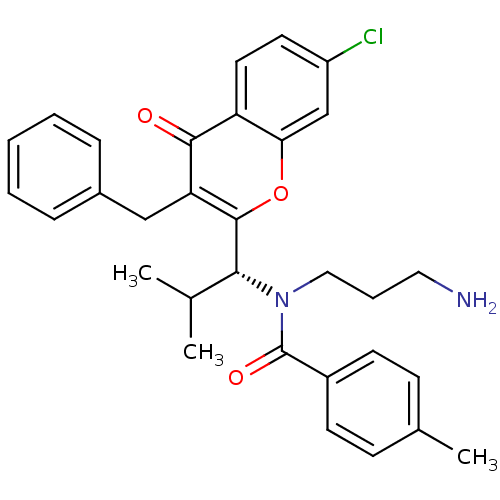
(CHEMBL2325429 | SB-743921)Show SMILES CC(C)[C@@H](N(CCCN)C(=O)c1ccc(C)cc1)c1oc2cc(Cl)ccc2c(=O)c1Cc1ccccc1 |r| Show InChI InChI=1S/C31H33ClN2O3/c1-20(2)28(34(17-7-16-33)31(36)23-12-10-21(3)11-13-23)30-26(18-22-8-5-4-6-9-22)29(35)25-15-14-24(32)19-27(25)37-30/h4-6,8-15,19-20,28H,7,16-18,33H2,1-3H3/t28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of MT-stimulated ATPase activity of wild type Eg5 (unknown origin) |
J Med Chem 56: 6314-6 (2013)
Article DOI: 10.1021/jm401071u
BindingDB Entry DOI: 10.7270/Q2K35W3X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50358430
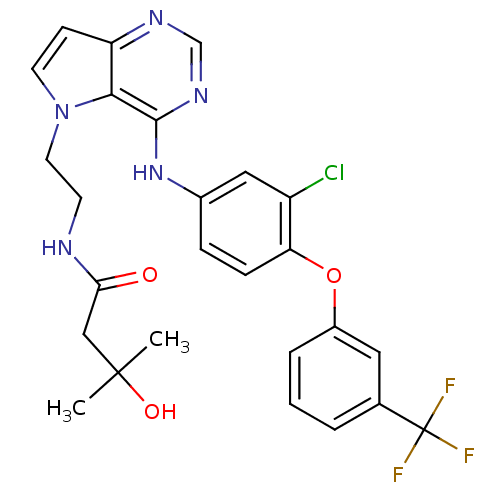
(CHEMBL1614725)Show SMILES CC(C)(O)CC(=O)NCCn1ccc2ncnc(Nc3ccc(Oc4cccc(c4)C(F)(F)F)c(Cl)c3)c12 Show InChI InChI=1S/C26H25ClF3N5O3/c1-25(2,37)14-22(36)31-9-11-35-10-8-20-23(35)24(33-15-32-20)34-17-6-7-21(19(27)13-17)38-18-5-3-4-16(12-18)26(28,29)30/h3-8,10,12-13,15,37H,9,11,14H2,1-2H3,(H,31,36)(H,32,33,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University of Science and Technology (UST)
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-diprenorphine binding to kappa opioid receptor expressed in CHO cells |
Bioorg Med Chem Lett 25: 5147-54 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.003
BindingDB Entry DOI: 10.7270/Q27W6F0D |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50358430

(CHEMBL1614725)Show SMILES CC(C)(O)CC(=O)NCCn1ccc2ncnc(Nc3ccc(Oc4cccc(c4)C(F)(F)F)c(Cl)c3)c12 Show InChI InChI=1S/C26H25ClF3N5O3/c1-25(2,37)14-22(36)31-9-11-35-10-8-20-23(35)24(33-15-32-20)34-17-6-7-21(19(27)13-17)38-18-5-3-4-16(12-18)26(28,29)30/h3-8,10,12-13,15,37H,9,11,14H2,1-2H3,(H,31,36)(H,32,33,34) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University of Science and Technology (UST)
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-diprenorphine binding to kappa opioid receptor expressed in CHO cells |
Bioorg Med Chem Lett 25: 5147-54 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.003
BindingDB Entry DOI: 10.7270/Q27W6F0D |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50132489

(CHEMBL3633929)Show SMILES CC(=O)Nc1ccc2ncnc(Nc3ccc(OCc4ccccn4)c(Cl)c3)c2c1 Show InChI InChI=1S/C22H18ClN5O2/c1-14(29)27-15-5-7-20-18(10-15)22(26-13-25-20)28-16-6-8-21(19(23)11-16)30-12-17-4-2-3-9-24-17/h2-11,13H,12H2,1H3,(H,27,29)(H,25,26,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University of Science and Technology (UST)
Curated by ChEMBL
| Assay Description
Inhibitory activity against metabotropic glutamate receptor 5 (mGluR5) |
Bioorg Med Chem Lett 25: 5147-54 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.003
BindingDB Entry DOI: 10.7270/Q27W6F0D |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50132492

(CHEMBL3633774)Show SMILES NC(=O)Nc1ccc2ncnc(Nc3ccc(Oc4cccc(c4)C(F)(F)F)c(Cl)c3)c2c1 Show InChI InChI=1S/C22H15ClF3N5O2/c23-17-10-14(5-7-19(17)33-15-3-1-2-12(8-15)22(24,25)26)30-20-16-9-13(31-21(27)32)4-6-18(16)28-11-29-20/h1-11H,(H3,27,31,32)(H,28,29,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University of Science and Technology (UST)
Curated by ChEMBL
| Assay Description
Inhibitory concentration of the compound against Thrombin was determined |
Bioorg Med Chem Lett 25: 5147-54 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.003
BindingDB Entry DOI: 10.7270/Q27W6F0D |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM5445

(CHEMBL554 | GW572016 | LAPATINIB DITOSYLATE | Lapa...)Show SMILES CS(=O)(=O)CCNCc1ccc(o1)-c1ccc2ncnc(Nc3ccc(OCc4cccc(F)c4)c(Cl)c3)c2c1 Show InChI InChI=1S/C29H26ClFN4O4S/c1-40(36,37)12-11-32-16-23-7-10-27(39-23)20-5-8-26-24(14-20)29(34-18-33-26)35-22-6-9-28(25(30)15-22)38-17-19-3-2-4-21(31)13-19/h2-10,13-15,18,32H,11-12,16-17H2,1H3,(H,33,34,35) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University of Science and Technology (UST)
Curated by ChEMBL
| Assay Description
Inhibitory constant against Adenosine A2A receptor using [3H]-SCH-58,261 as radio ligand |
Bioorg Med Chem Lett 25: 5147-54 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.003
BindingDB Entry DOI: 10.7270/Q27W6F0D |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50132495
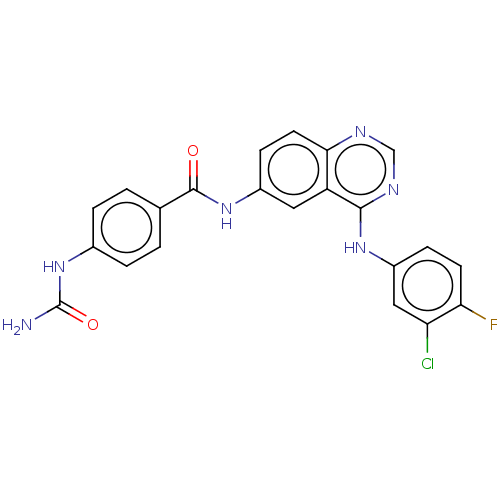
(CHEMBL3633940)Show SMILES NC(=O)Nc1ccc(cc1)C(=O)Nc1ccc2ncnc(Nc3ccc(F)c(Cl)c3)c2c1 Show InChI InChI=1S/C22H16ClFN6O2/c23-17-10-15(5-7-18(17)24)28-20-16-9-14(6-8-19(16)26-11-27-20)29-21(31)12-1-3-13(4-2-12)30-22(25)32/h1-11H,(H,29,31)(H3,25,30,32)(H,26,27,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University of Science and Technology (UST)
Curated by ChEMBL
| Assay Description
Inhibitory activity against metabotropic glutamate receptor 5 (mGluR5) |
Bioorg Med Chem Lett 25: 5147-54 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.003
BindingDB Entry DOI: 10.7270/Q27W6F0D |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM5445

(CHEMBL554 | GW572016 | LAPATINIB DITOSYLATE | Lapa...)Show SMILES CS(=O)(=O)CCNCc1ccc(o1)-c1ccc2ncnc(Nc3ccc(OCc4cccc(F)c4)c(Cl)c3)c2c1 Show InChI InChI=1S/C29H26ClFN4O4S/c1-40(36,37)12-11-32-16-23-7-10-27(39-23)20-5-8-26-24(14-20)29(34-18-33-26)35-22-6-9-28(25(30)15-22)38-17-19-3-2-4-21(31)13-19/h2-10,13-15,18,32H,11-12,16-17H2,1H3,(H,33,34,35) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University of Science and Technology (UST)
Curated by ChEMBL
| Assay Description
Inhibitory activity against metabotropic glutamate receptor 5 (mGluR5) |
Bioorg Med Chem Lett 25: 5147-54 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.003
BindingDB Entry DOI: 10.7270/Q27W6F0D |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Translocator protein
(Rattus norvegicus (rat)) | BDBM22032
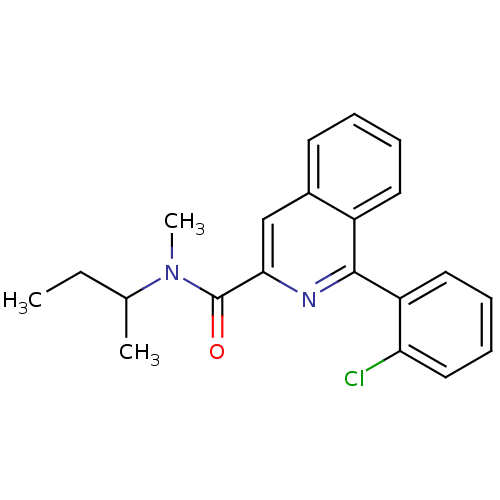
(1-(2-chlorophenyl)-N-methyl-N-(1-methylpropyl)isoq...)Show InChI InChI=1S/C21H21ClN2O/c1-4-14(2)24(3)21(25)19-13-15-9-5-6-10-16(15)20(23-19)17-11-7-8-12-18(17)22/h5-14H,4H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University of Science and Technology (UST)
Curated by ChEMBL
| Assay Description
Displacement of [3H]PK11195 from TSPO receptor in Sprague-Dawley rat cerebral cortex membrane by radiometric competitive assay |
Eur J Med Chem 103: 210-22 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.001
BindingDB Entry DOI: 10.7270/Q2DR2XBB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50132489

(CHEMBL3633929)Show SMILES CC(=O)Nc1ccc2ncnc(Nc3ccc(OCc4ccccn4)c(Cl)c3)c2c1 Show InChI InChI=1S/C22H18ClN5O2/c1-14(29)27-15-5-7-20-18(10-15)22(26-13-25-20)28-16-6-8-21(19(23)11-16)30-12-17-4-2-3-9-24-17/h2-11,13H,12H2,1H3,(H,27,29)(H,25,26,28) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University of Science and Technology (UST)
Curated by ChEMBL
| Assay Description
Inhibitory activity against metabotropic glutamate receptor 5 (mGluR5) |
Bioorg Med Chem Lett 25: 5147-54 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.003
BindingDB Entry DOI: 10.7270/Q27W6F0D |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50132502
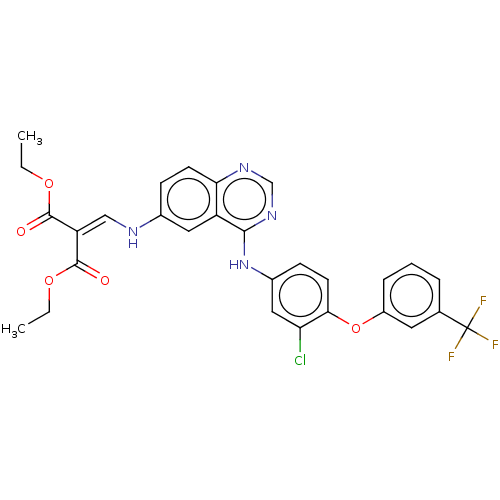
(CHEMBL3633934)Show SMILES [#6]-[#6]-[#8]-[#6](=O)-[#6](=[#6]/[#7]-c1ccc2ncnc(-[#7]-c3ccc(-[#8]-c4cccc(c4)C(F)(F)F)c(Cl)c3)c2c1)\[#6](=O)-[#8]-[#6]-[#6] Show InChI InChI=1S/C29H24ClF3N4O5/c1-3-40-27(38)22(28(39)41-4-2)15-34-18-8-10-24-21(13-18)26(36-16-35-24)37-19-9-11-25(23(30)14-19)42-20-7-5-6-17(12-20)29(31,32)33/h5-16,34H,3-4H2,1-2H3,(H,35,36,37) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University of Science and Technology (UST)
Curated by ChEMBL
| Assay Description
Inhibitory activity against metabotropic glutamate receptor 5 (mGluR5) |
Bioorg Med Chem Lett 25: 5147-54 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.003
BindingDB Entry DOI: 10.7270/Q27W6F0D |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50132506
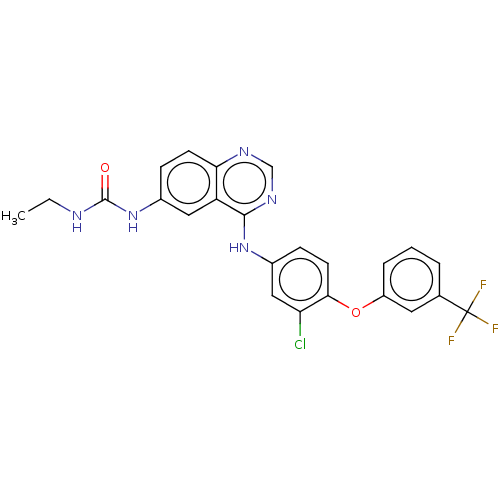
(CHEMBL3633930)Show SMILES CCNC(=O)Nc1ccc2ncnc(Nc3ccc(Oc4cccc(c4)C(F)(F)F)c(Cl)c3)c2c1 Show InChI InChI=1S/C24H19ClF3N5O2/c1-2-29-23(34)33-15-6-8-20-18(11-15)22(31-13-30-20)32-16-7-9-21(19(25)12-16)35-17-5-3-4-14(10-17)24(26,27)28/h3-13H,2H2,1H3,(H2,29,33,34)(H,30,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University of Science and Technology (UST)
Curated by ChEMBL
| Assay Description
Inhibitory activity against metabotropic glutamate receptor 5 (mGluR5) |
Bioorg Med Chem Lett 25: 5147-54 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.003
BindingDB Entry DOI: 10.7270/Q27W6F0D |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50121975

((6-Methoxy-quinolin-4-yl)-(5-vinyl-1-aza-bicyclo[2...)Show SMILES COc1ccc2nccc([C@H](O)[C@H]3C[C@@H]4CCN3C[C@@H]4C=C)c2c1 |r,THB:20:19:12.13:16.15,10:12:18.19:16.15| Show InChI InChI=1S/C20H24N2O2/c1-3-13-12-22-9-7-14(13)10-19(22)20(23)16-6-8-21-18-5-4-15(24-2)11-17(16)18/h3-6,8,11,13-14,19-20,23H,1,7,9-10,12H2,2H3/t13-,14-,19+,20-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University of Science and Technology (UST)
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) using luciferin tagged substrate preincubated for 10 mins before substrate addition |
Eur J Med Chem 103: 210-22 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.001
BindingDB Entry DOI: 10.7270/Q2DR2XBB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50132494

(CHEMBL3633941)Show SMILES CCNC(=O)Nc1ccc(cc1)C(=O)Nc1ccc2ncnc(Nc3ccc(F)c(Cl)c3)c2c1 Show InChI InChI=1S/C24H20ClFN6O2/c1-2-27-24(34)32-15-5-3-14(4-6-15)23(33)31-16-8-10-21-18(11-16)22(29-13-28-21)30-17-7-9-20(26)19(25)12-17/h3-13H,2H2,1H3,(H,31,33)(H2,27,32,34)(H,28,29,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University of Science and Technology (UST)
Curated by ChEMBL
| Assay Description
Inhibitory activity against metabotropic glutamate receptor 5 (mGluR5) |
Bioorg Med Chem Lett 25: 5147-54 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.003
BindingDB Entry DOI: 10.7270/Q27W6F0D |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50132504

(CHEMBL3633932)Show SMILES CCNC(=O)Nc1ccc2ncnc(Nc3ccc(OCc4cccc(F)c4)c(Cl)c3)c2c1 Show InChI InChI=1S/C24H21ClFN5O2/c1-2-27-24(32)31-17-6-8-21-19(11-17)23(29-14-28-21)30-18-7-9-22(20(25)12-18)33-13-15-4-3-5-16(26)10-15/h3-12,14H,2,13H2,1H3,(H2,27,31,32)(H,28,29,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University of Science and Technology (UST)
Curated by ChEMBL
| Assay Description
Inhibitory activity against metabotropic glutamate receptor 5 (mGluR5) |
Bioorg Med Chem Lett 25: 5147-54 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.003
BindingDB Entry DOI: 10.7270/Q27W6F0D |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM30130

(CHEMBL1201082 | CHEMBL41 | Fluoxetin | Fluoxetine ...)Show InChI InChI=1S/C17H18F3NO/c1-21-12-11-16(13-5-3-2-4-6-13)22-15-9-7-14(8-10-15)17(18,19)20/h2-10,16,21H,11-12H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University of Science and Technology (UST)
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 (unknown origin) using luciferin tagged substrate preincubated for 10 mins before substrate addition |
Eur J Med Chem 103: 210-22 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.001
BindingDB Entry DOI: 10.7270/Q2DR2XBB |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50161957
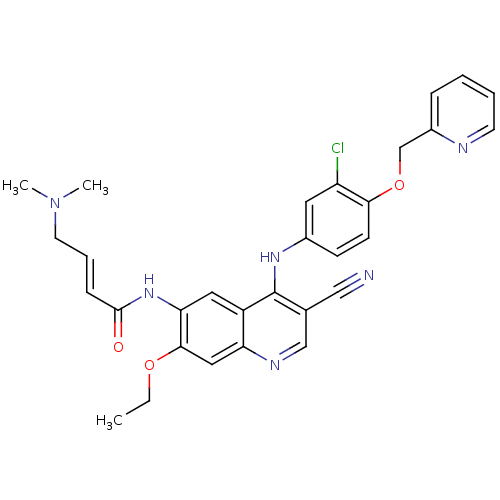
(4-Dimethylamino-but-2-enoic acid {4-[3-chloro-4-(p...)Show SMILES CCOc1cc2ncc(C#N)c(Nc3ccc(OCc4ccccn4)c(Cl)c3)c2cc1NC(=O)\C=C\CN(C)C Show InChI InChI=1S/C30H29ClN6O3/c1-4-39-28-16-25-23(15-26(28)36-29(38)9-7-13-37(2)3)30(20(17-32)18-34-25)35-21-10-11-27(24(31)14-21)40-19-22-8-5-6-12-33-22/h5-12,14-16,18H,4,13,19H2,1-3H3,(H,34,35)(H,36,38)/b9-7+ | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University of Science and Technology (UST)
Curated by ChEMBL
| Assay Description
Effective concentration against retinoic acid receptor alpha in COS-7 cells co-expressing DR5-tk-CAT reporter; value range (4.6-8.9) |
Bioorg Med Chem Lett 25: 5147-54 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.003
BindingDB Entry DOI: 10.7270/Q27W6F0D |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM31768

(CHEMBL295698 | Ketoconazole | Nizoral | Panfungol)Show SMILES CC(=O)N1CCN(CC1)c1ccc(OC[C@@H]2CO[C@](Cn3ccnc3)(O2)c2ccc(Cl)cc2Cl)cc1 |r| Show InChI InChI=1S/C26H28Cl2N4O4/c1-19(33)31-10-12-32(13-11-31)21-3-5-22(6-4-21)34-15-23-16-35-26(36-23,17-30-9-8-29-18-30)24-7-2-20(27)14-25(24)28/h2-9,14,18,23H,10-13,15-17H2,1H3/t23-,26-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University of Science and Technology (UST)
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) using luciferin tagged substrate preincubated for 10 mins before substrate addition |
Eur J Med Chem 103: 210-22 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.001
BindingDB Entry DOI: 10.7270/Q2DR2XBB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50132492

(CHEMBL3633774)Show SMILES NC(=O)Nc1ccc2ncnc(Nc3ccc(Oc4cccc(c4)C(F)(F)F)c(Cl)c3)c2c1 Show InChI InChI=1S/C22H15ClF3N5O2/c23-17-10-14(5-7-19(17)33-15-3-1-2-12(8-15)22(24,25)26)30-20-16-9-13(31-21(27)32)4-6-18(16)28-11-29-20/h1-11H,(H3,27,31,32)(H,28,29,30) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University of Science and Technology (UST)
Curated by ChEMBL
| Assay Description
Inhibitory activity against metabotropic glutamate receptor 5 (mGluR5) |
Bioorg Med Chem Lett 25: 5147-54 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.003
BindingDB Entry DOI: 10.7270/Q27W6F0D |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50090677
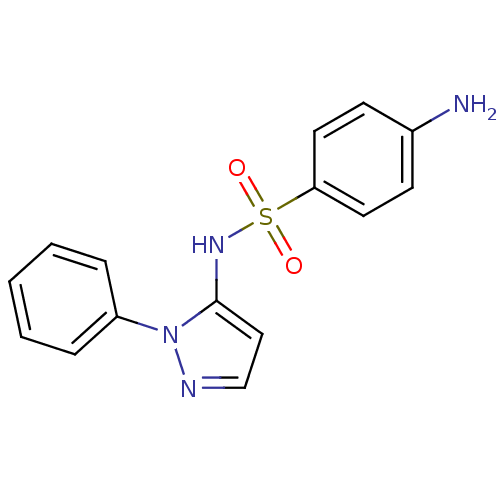
(4-Amino-N-(2-phenyl-2H-pyrazol-3-yl)-benzenesulfon...)Show InChI InChI=1S/C15H14N4O2S/c16-12-6-8-14(9-7-12)22(20,21)18-15-10-11-17-19(15)13-4-2-1-3-5-13/h1-11,18H,16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University of Science and Technology (UST)
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) using luciferin tagged substrate preincubated for 10 mins before substrate addition |
Eur J Med Chem 103: 210-22 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.001
BindingDB Entry DOI: 10.7270/Q2DR2XBB |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50161957

(4-Dimethylamino-but-2-enoic acid {4-[3-chloro-4-(p...)Show SMILES CCOc1cc2ncc(C#N)c(Nc3ccc(OCc4ccccn4)c(Cl)c3)c2cc1NC(=O)\C=C\CN(C)C Show InChI InChI=1S/C30H29ClN6O3/c1-4-39-28-16-25-23(15-26(28)36-29(38)9-7-13-37(2)3)30(20(17-32)18-34-25)35-21-10-11-27(24(31)14-21)40-19-22-8-5-6-12-33-22/h5-12,14-16,18H,4,13,19H2,1-3H3,(H,34,35)(H,36,38)/b9-7+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 92 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University of Science and Technology (UST)
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-diprenorphine binding to kappa opioid receptor expressed in CHO cells |
Bioorg Med Chem Lett 25: 5147-54 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.003
BindingDB Entry DOI: 10.7270/Q27W6F0D |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50132490

(CHEMBL3633928)Show SMILES NC(=O)Nc1ccc2ncnc(Nc3ccc(OCc4cccc(F)c4)c(Cl)c3)c2c1 Show InChI InChI=1S/C22H17ClFN5O2/c23-18-10-16(5-7-20(18)31-11-13-2-1-3-14(24)8-13)28-21-17-9-15(29-22(25)30)4-6-19(17)26-12-27-21/h1-10,12H,11H2,(H3,25,29,30)(H,26,27,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 99 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University of Science and Technology (UST)
Curated by ChEMBL
| Assay Description
Inhibitory activity against metabotropic glutamate receptor 5 (mGluR5) |
Bioorg Med Chem Lett 25: 5147-54 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.003
BindingDB Entry DOI: 10.7270/Q27W6F0D |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50132504

(CHEMBL3633932)Show SMILES CCNC(=O)Nc1ccc2ncnc(Nc3ccc(OCc4cccc(F)c4)c(Cl)c3)c2c1 Show InChI InChI=1S/C24H21ClFN5O2/c1-2-27-24(32)31-17-6-8-21-19(11-17)23(29-14-28-21)30-18-7-9-22(20(25)12-18)33-13-15-4-3-5-16(26)10-15/h3-12,14H,2,13H2,1H3,(H2,27,31,32)(H,28,29,30) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 126 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University of Science and Technology (UST)
Curated by ChEMBL
| Assay Description
Inhibition concentration of the compound against beta tryptase was determined |
Bioorg Med Chem Lett 25: 5147-54 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.003
BindingDB Entry DOI: 10.7270/Q27W6F0D |
More data for this
Ligand-Target Pair | |
Translocator protein
(Rattus norvegicus (rat)) | BDBM50000766

(CHEMBL12 | DIAZEPAM | US9271961, Diazepam)Show InChI InChI=1S/C16H13ClN2O/c1-19-14-8-7-12(17)9-13(14)16(18-10-15(19)20)11-5-3-2-4-6-11/h2-9H,10H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 194 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University of Science and Technology (UST)
Curated by ChEMBL
| Assay Description
Displacement of [3H]PK11195 from TSPO receptor in Sprague-Dawley rat cerebral cortex membrane by radiometric competitive assay |
Eur J Med Chem 103: 210-22 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.001
BindingDB Entry DOI: 10.7270/Q2DR2XBB |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50063062
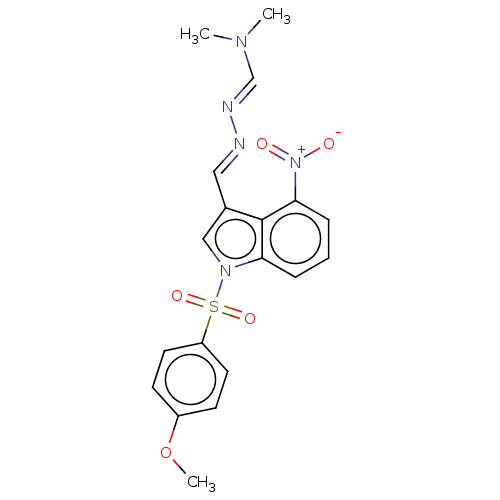
(CHEMBL3398394)Show SMILES COc1ccc(cc1)S(=O)(=O)n1cc(\C=N\N=C\N(C)C)c2c(cccc12)[N+]([O-])=O Show InChI InChI=1S/C19H19N5O5S/c1-22(2)13-21-20-11-14-12-23(17-5-4-6-18(19(14)17)24(25)26)30(27,28)16-9-7-15(29-3)8-10-16/h4-13H,1-3H3/b20-11+,21-13+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 205 | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University
Curated by ChEMBL
| Assay Description
Displacement of [3H]Ketanserin from human recombinant 5-HT2A receptor expressed in CHO-K1 cells after 15 mins |
Bioorg Med Chem 23: 1313-20 (2015)
Article DOI: 10.1016/j.bmc.2015.01.032
BindingDB Entry DOI: 10.7270/Q27S7QFW |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50132503
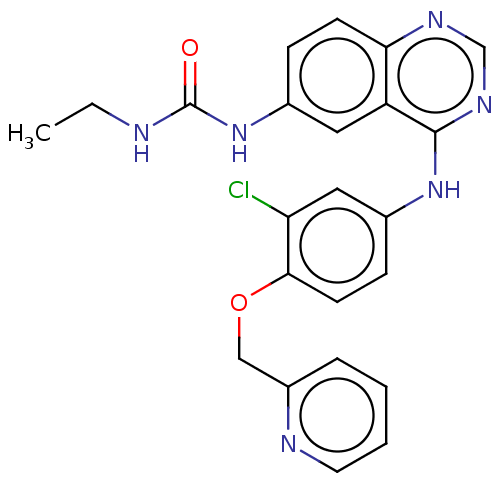
(CHEMBL3633933)Show SMILES CCNC(=O)Nc1ccc2ncnc(Nc3ccc(OCc4ccccn4)c(Cl)c3)c2c1 Show InChI InChI=1S/C23H21ClN6O2/c1-2-25-23(31)30-15-6-8-20-18(11-15)22(28-14-27-20)29-16-7-9-21(19(24)12-16)32-13-17-5-3-4-10-26-17/h3-12,14H,2,13H2,1H3,(H2,25,30,31)(H,27,28,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 227 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University of Science and Technology (UST)
Curated by ChEMBL
| Assay Description
Inhibitory activity against metabotropic glutamate receptor 5 (mGluR5) |
Bioorg Med Chem Lett 25: 5147-54 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.003
BindingDB Entry DOI: 10.7270/Q27W6F0D |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50063060
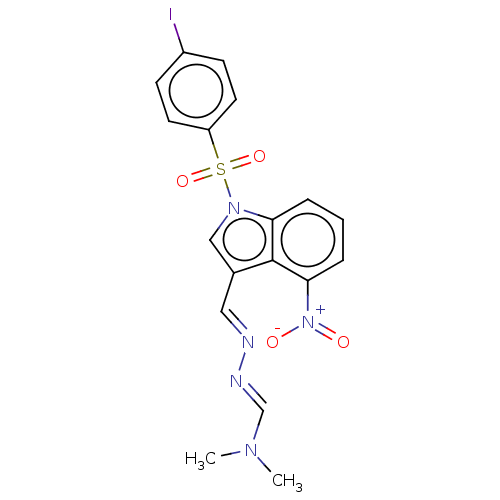
(CHEMBL3398391)Show SMILES CN(C)\C=N\N=C\c1cn(c2cccc([N+]([O-])=O)c12)S(=O)(=O)c1ccc(I)cc1 Show InChI InChI=1S/C18H16IN5O4S/c1-22(2)12-21-20-10-13-11-23(16-4-3-5-17(18(13)16)24(25)26)29(27,28)15-8-6-14(19)7-9-15/h3-12H,1-2H3/b20-10+,21-12+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 247 | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University
Curated by ChEMBL
| Assay Description
Displacement of [3H]Ketanserin from human recombinant 5-HT2A receptor expressed in CHO-K1 cells after 15 mins |
Bioorg Med Chem 23: 1313-20 (2015)
Article DOI: 10.1016/j.bmc.2015.01.032
BindingDB Entry DOI: 10.7270/Q27S7QFW |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50063061
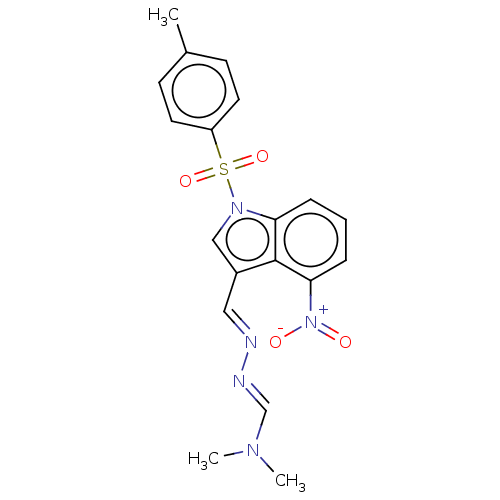
(CHEMBL3398392)Show SMILES CN(C)\C=N\N=C\c1cn(c2cccc([N+]([O-])=O)c12)S(=O)(=O)c1ccc(C)cc1 Show InChI InChI=1S/C19H19N5O4S/c1-14-7-9-16(10-8-14)29(27,28)23-12-15(11-20-21-13-22(2)3)19-17(23)5-4-6-18(19)24(25)26/h4-13H,1-3H3/b20-11+,21-13+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 253 | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University
Curated by ChEMBL
| Assay Description
Displacement of [3H]Ketanserin from human recombinant 5-HT2A receptor expressed in CHO-K1 cells after 15 mins |
Bioorg Med Chem 23: 1313-20 (2015)
Article DOI: 10.1016/j.bmc.2015.01.032
BindingDB Entry DOI: 10.7270/Q27S7QFW |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50132495

(CHEMBL3633940)Show SMILES NC(=O)Nc1ccc(cc1)C(=O)Nc1ccc2ncnc(Nc3ccc(F)c(Cl)c3)c2c1 Show InChI InChI=1S/C22H16ClFN6O2/c23-17-10-15(5-7-18(17)24)28-20-16-9-14(6-8-19(16)26-11-27-20)29-21(31)12-1-3-13(4-2-12)30-22(25)32/h1-11H,(H,29,31)(H3,25,30,32)(H,26,27,28) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 275 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University of Science and Technology (UST)
Curated by ChEMBL
| Assay Description
Inhibitory constant against Adenosine A2A receptor using [3H]-SCH-58,261 as radio ligand |
Bioorg Med Chem Lett 25: 5147-54 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.003
BindingDB Entry DOI: 10.7270/Q27W6F0D |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50132490

(CHEMBL3633928)Show SMILES NC(=O)Nc1ccc2ncnc(Nc3ccc(OCc4cccc(F)c4)c(Cl)c3)c2c1 Show InChI InChI=1S/C22H17ClFN5O2/c23-18-10-16(5-7-20(18)31-11-13-2-1-3-14(24)8-13)28-21-17-9-15(29-22(25)30)4-6-19(17)26-12-27-21/h1-10,12H,11H2,(H3,25,29,30)(H,26,27,28) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 287 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University of Science and Technology (UST)
Curated by ChEMBL
| Assay Description
Inhibitory activity against metabotropic glutamate receptor 5 (mGluR5) |
Bioorg Med Chem Lett 25: 5147-54 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.003
BindingDB Entry DOI: 10.7270/Q27W6F0D |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50236897
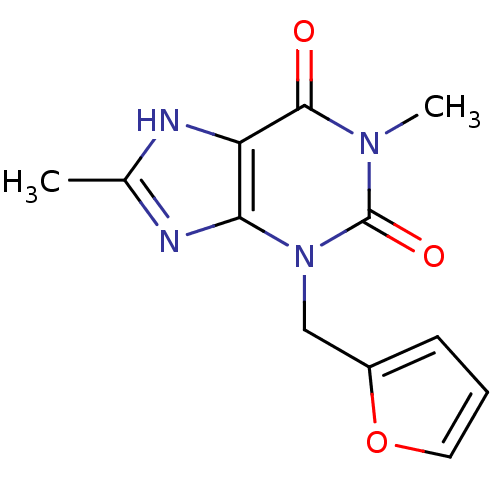
(3-(furan-2-ylmethyl)-1,8-dimethyl-1H-purine-2,6(3H...)Show InChI InChI=1S/C12H12N4O3/c1-7-13-9-10(14-7)16(6-8-4-3-5-19-8)12(18)15(2)11(9)17/h3-5H,6H2,1-2H3,(H,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University of Science and Technology (UST)
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 (unknown origin) using luciferin tagged substrate preincubated for 10 mins before substrate addition |
Eur J Med Chem 103: 210-22 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.001
BindingDB Entry DOI: 10.7270/Q2DR2XBB |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50063058
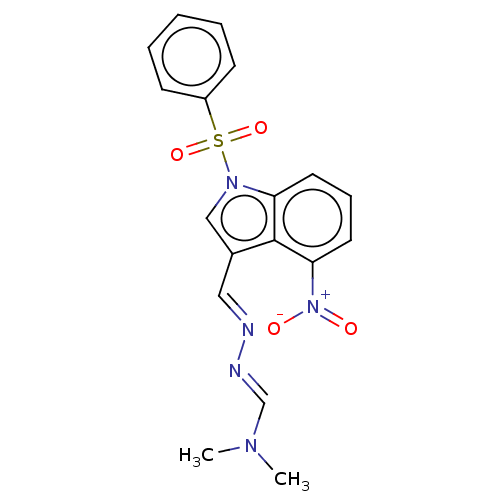
(CHEMBL3398389)Show SMILES CN(C)\C=N\N=C\c1cn(c2cccc([N+]([O-])=O)c12)S(=O)(=O)c1ccccc1 Show InChI InChI=1S/C18H17N5O4S/c1-21(2)13-20-19-11-14-12-22(28(26,27)15-7-4-3-5-8-15)16-9-6-10-17(18(14)16)23(24)25/h3-13H,1-2H3/b19-11+,20-13+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 329 | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University
Curated by ChEMBL
| Assay Description
Displacement of [3H]Ketanserin from human recombinant 5-HT2A receptor expressed in CHO-K1 cells after 15 mins |
Bioorg Med Chem 23: 1313-20 (2015)
Article DOI: 10.1016/j.bmc.2015.01.032
BindingDB Entry DOI: 10.7270/Q27S7QFW |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50380061
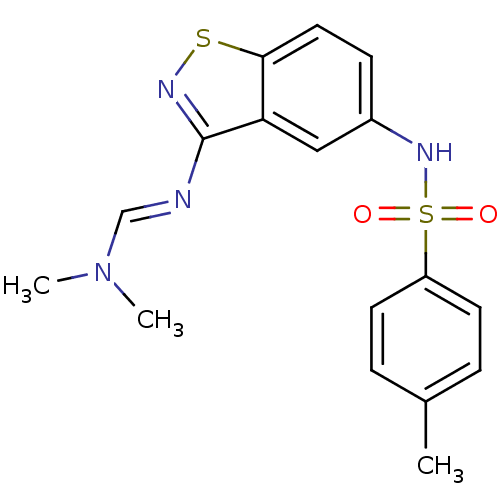
(CHEMBL2012637)Show SMILES CN(C)\C=N\c1nsc2ccc(NS(=O)(=O)c3ccc(C)cc3)cc12 Show InChI InChI=1S/C17H18N4O2S2/c1-12-4-7-14(8-5-12)25(22,23)20-13-6-9-16-15(10-13)17(19-24-16)18-11-21(2)3/h4-11,20H,1-3H3/b18-11+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant 5-HT6 receptor expressed in HEK293 cells assessed as inhibition of 5-HT-induced increase in Ca2+ level pretreated for... |
Bioorg Med Chem 23: 1313-20 (2015)
Article DOI: 10.1016/j.bmc.2015.01.032
BindingDB Entry DOI: 10.7270/Q27S7QFW |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50063055

(CHEMBL3398398)Show SMILES CN(C)\C=N\N=C\c1cn(c2cccc([N+]([O-])=O)c12)S(=O)(=O)c1ccc2ccccc2c1 Show InChI InChI=1S/C22H19N5O4S/c1-25(2)15-24-23-13-18-14-26(20-8-5-9-21(22(18)20)27(28)29)32(30,31)19-11-10-16-6-3-4-7-17(16)12-19/h3-15H,1-2H3/b23-13+,24-15+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 433 | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University
Curated by ChEMBL
| Assay Description
Displacement of [3H]Ketanserin from human recombinant 5-HT2A receptor expressed in CHO-K1 cells after 15 mins |
Bioorg Med Chem 23: 1313-20 (2015)
Article DOI: 10.1016/j.bmc.2015.01.032
BindingDB Entry DOI: 10.7270/Q27S7QFW |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50132497
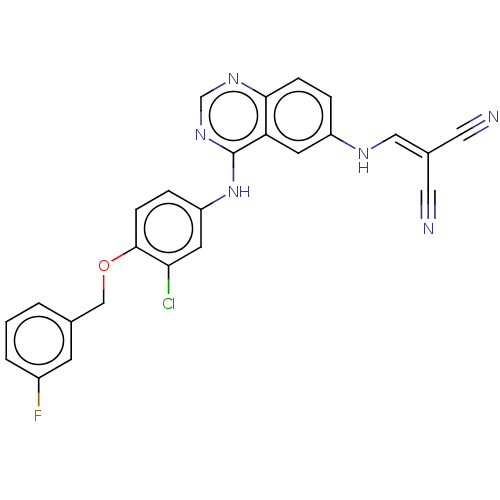
(CHEMBL3633938)Show SMILES Fc1cccc(-[#6]-[#8]-c2ccc(-[#7]-c3ncnc4ccc(-[#7]\[#6]=[#6](\C#N)C#N)cc34)cc2Cl)c1 Show InChI InChI=1S/C25H16ClFN6O/c26-22-10-20(5-7-24(22)34-14-16-2-1-3-18(27)8-16)33-25-21-9-19(30-13-17(11-28)12-29)4-6-23(21)31-15-32-25/h1-10,13,15,30H,14H2,(H,31,32,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 445 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University of Science and Technology (UST)
Curated by ChEMBL
| Assay Description
Inhibitory activity against metabotropic glutamate receptor 5 (mGluR5) |
Bioorg Med Chem Lett 25: 5147-54 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.003
BindingDB Entry DOI: 10.7270/Q27W6F0D |
More data for this
Ligand-Target Pair | |
Translocator protein
(Rattus norvegicus (rat)) | BDBM50130509
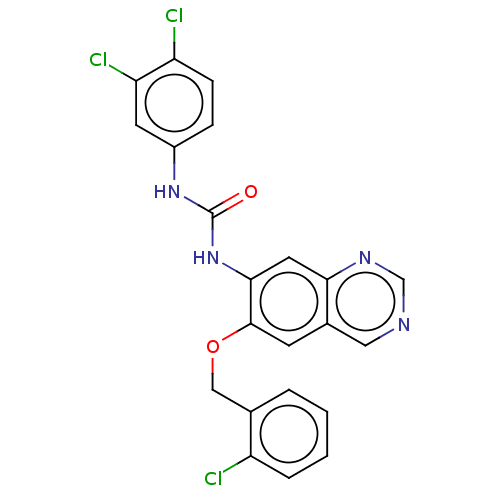
(CHEMBL3634097)Show SMILES Clc1ccc(NC(=O)Nc2cc3ncncc3cc2OCc2ccccc2Cl)cc1Cl Show InChI InChI=1S/C22H15Cl3N4O2/c23-16-4-2-1-3-13(16)11-31-21-7-14-10-26-12-27-19(14)9-20(21)29-22(30)28-15-5-6-17(24)18(25)8-15/h1-10,12H,11H2,(H2,28,29,30) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 447 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University of Science and Technology (UST)
Curated by ChEMBL
| Assay Description
Displacement of [3H]PK11195 from TSPO receptor in Sprague-Dawley rat cerebral cortex membrane by radiometric competitive assay |
Eur J Med Chem 103: 210-22 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.001
BindingDB Entry DOI: 10.7270/Q2DR2XBB |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50063055

(CHEMBL3398398)Show SMILES CN(C)\C=N\N=C\c1cn(c2cccc([N+]([O-])=O)c12)S(=O)(=O)c1ccc2ccccc2c1 Show InChI InChI=1S/C22H19N5O4S/c1-25(2)15-24-23-13-18-14-26(20-8-5-9-21(22(18)20)27(28)29)32(30,31)19-11-10-16-6-3-4-7-17(16)12-19/h3-15H,1-2H3/b23-13+,24-15+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 463 | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University
Curated by ChEMBL
| Assay Description
Displacement of [3H]Mesulergine from human recombinant 5-HT2C receptor expressed in CHO-K1 cells after 15 mins |
Bioorg Med Chem 23: 1313-20 (2015)
Article DOI: 10.1016/j.bmc.2015.01.032
BindingDB Entry DOI: 10.7270/Q27S7QFW |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50063063

(CHEMBL3398395)Show SMILES COc1ccc(OC)c(c1)S(=O)(=O)n1cc(\C=N\N=C\N(C)C)c2c(cccc12)[N+]([O-])=O Show InChI InChI=1S/C20H21N5O6S/c1-23(2)13-22-21-11-14-12-24(16-6-5-7-17(20(14)16)25(26)27)32(28,29)19-10-15(30-3)8-9-18(19)31-4/h5-13H,1-4H3/b21-11+,22-13+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 479 | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University
Curated by ChEMBL
| Assay Description
Displacement of [3H]Ketanserin from human recombinant 5-HT2A receptor expressed in CHO-K1 cells after 15 mins |
Bioorg Med Chem 23: 1313-20 (2015)
Article DOI: 10.1016/j.bmc.2015.01.032
BindingDB Entry DOI: 10.7270/Q27S7QFW |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50063061

(CHEMBL3398392)Show SMILES CN(C)\C=N\N=C\c1cn(c2cccc([N+]([O-])=O)c12)S(=O)(=O)c1ccc(C)cc1 Show InChI InChI=1S/C19H19N5O4S/c1-14-7-9-16(10-8-14)29(27,28)23-12-15(11-20-21-13-22(2)3)19-17(23)5-4-6-18(19)24(25)26/h4-13H,1-3H3/b20-11+,21-13+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University
Curated by ChEMBL
| Assay Description
Displacement of [3H]Mesulergine from human recombinant 5-HT2C receptor expressed in CHO-K1 cells after 15 mins |
Bioorg Med Chem 23: 1313-20 (2015)
Article DOI: 10.1016/j.bmc.2015.01.032
BindingDB Entry DOI: 10.7270/Q27S7QFW |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50132491
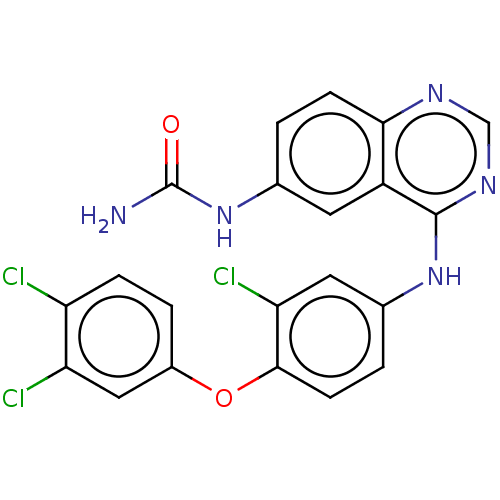
(CHEMBL3633775)Show SMILES NC(=O)Nc1ccc2ncnc(Nc3ccc(Oc4ccc(Cl)c(Cl)c4)c(Cl)c3)c2c1 Show InChI InChI=1S/C21H14Cl3N5O2/c22-15-4-3-13(9-16(15)23)31-19-6-2-12(8-17(19)24)28-20-14-7-11(29-21(25)30)1-5-18(14)26-10-27-20/h1-10H,(H3,25,29,30)(H,26,27,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 543 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University of Science and Technology (UST)
Curated by ChEMBL
| Assay Description
Inhibitory activity against metabotropic glutamate receptor 5 (mGluR5) |
Bioorg Med Chem Lett 25: 5147-54 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.003
BindingDB Entry DOI: 10.7270/Q27W6F0D |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50063065

(CHEMBL3398397)Show SMILES CN(C)\C=N\N=C\c1cn(c2cccc([N+]([O-])=O)c12)S(=O)(=O)c1cccc2ccccc12 Show InChI InChI=1S/C22H19N5O4S/c1-25(2)15-24-23-13-17-14-26(19-10-6-11-20(22(17)19)27(28)29)32(30,31)21-12-5-8-16-7-3-4-9-18(16)21/h3-15H,1-2H3/b23-13+,24-15+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 555 | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University
Curated by ChEMBL
| Assay Description
Displacement of [3H]Ketanserin from human recombinant 5-HT2A receptor expressed in CHO-K1 cells after 15 mins |
Bioorg Med Chem 23: 1313-20 (2015)
Article DOI: 10.1016/j.bmc.2015.01.032
BindingDB Entry DOI: 10.7270/Q27S7QFW |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50132506

(CHEMBL3633930)Show SMILES CCNC(=O)Nc1ccc2ncnc(Nc3ccc(Oc4cccc(c4)C(F)(F)F)c(Cl)c3)c2c1 Show InChI InChI=1S/C24H19ClF3N5O2/c1-2-29-23(34)33-15-6-8-20-18(11-15)22(31-13-30-20)32-16-7-9-21(19(25)12-16)35-17-5-3-4-14(10-17)24(26,27)28/h3-13H,2H2,1H3,(H2,29,33,34)(H,30,31,32) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 625 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University of Science and Technology (UST)
Curated by ChEMBL
| Assay Description
Inhibitory concentration of the compound against Factor Xa was determined |
Bioorg Med Chem Lett 25: 5147-54 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.003
BindingDB Entry DOI: 10.7270/Q27W6F0D |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50063059

(CHEMBL3398390)Show SMILES CN(C)\C=N\N=C\c1cn(c2cccc([N+]([O-])=O)c12)S(=O)(=O)c1ccc(F)cc1 Show InChI InChI=1S/C18H16FN5O4S/c1-22(2)12-21-20-10-13-11-23(16-4-3-5-17(18(13)16)24(25)26)29(27,28)15-8-6-14(19)7-9-15/h3-12H,1-2H3/b20-10+,21-12+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 702 | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University
Curated by ChEMBL
| Assay Description
Displacement of [3H]Ketanserin from human recombinant 5-HT2A receptor expressed in CHO-K1 cells after 15 mins |
Bioorg Med Chem 23: 1313-20 (2015)
Article DOI: 10.1016/j.bmc.2015.01.032
BindingDB Entry DOI: 10.7270/Q27S7QFW |
More data for this
Ligand-Target Pair | |
Translocator protein
(Rattus norvegicus (rat)) | BDBM50130506
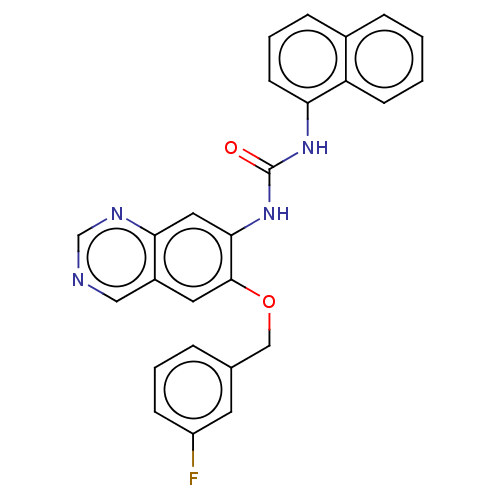
(CHEMBL3634110)Show SMILES Fc1cccc(COc2cc3cncnc3cc2NC(=O)Nc2cccc3ccccc23)c1 Show InChI InChI=1S/C26H19FN4O2/c27-20-8-3-5-17(11-20)15-33-25-12-19-14-28-16-29-23(19)13-24(25)31-26(32)30-22-10-4-7-18-6-1-2-9-21(18)22/h1-14,16H,15H2,(H2,30,31,32) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 703 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University of Science and Technology (UST)
Curated by ChEMBL
| Assay Description
Displacement of [3H]PK11195 from TSPO receptor in Sprague-Dawley rat cerebral cortex membrane by radiometric competitive assay |
Eur J Med Chem 103: 210-22 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.001
BindingDB Entry DOI: 10.7270/Q2DR2XBB |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50132491

(CHEMBL3633775)Show SMILES NC(=O)Nc1ccc2ncnc(Nc3ccc(Oc4ccc(Cl)c(Cl)c4)c(Cl)c3)c2c1 Show InChI InChI=1S/C21H14Cl3N5O2/c22-15-4-3-13(9-16(15)23)31-19-6-2-12(8-17(19)24)28-20-14-7-11(29-21(25)30)1-5-18(14)26-10-27-20/h1-10H,(H3,25,29,30)(H,26,27,28) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 706 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University of Science and Technology (UST)
Curated by ChEMBL
| Assay Description
Inhibitory activity against metabotropic glutamate receptor 5 (mGluR5) |
Bioorg Med Chem Lett 25: 5147-54 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.003
BindingDB Entry DOI: 10.7270/Q27W6F0D |
More data for this
Ligand-Target Pair | |
Translocator protein
(Rattus norvegicus (rat)) | BDBM50130505
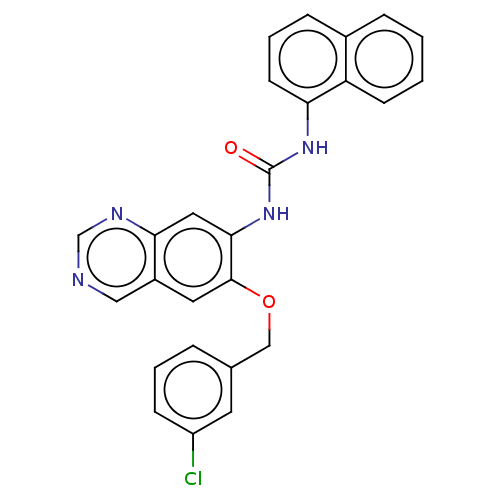
(CHEMBL3634101)Show SMILES Clc1cccc(COc2cc3cncnc3cc2NC(=O)Nc2cccc3ccccc23)c1 Show InChI InChI=1S/C26H19ClN4O2/c27-20-8-3-5-17(11-20)15-33-25-12-19-14-28-16-29-23(19)13-24(25)31-26(32)30-22-10-4-7-18-6-1-2-9-21(18)22/h1-14,16H,15H2,(H2,30,31,32) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 722 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University of Science and Technology (UST)
Curated by ChEMBL
| Assay Description
Displacement of [3H]PK11195 from TSPO receptor in Sprague-Dawley rat cerebral cortex membrane by radiometric competitive assay |
Eur J Med Chem 103: 210-22 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.001
BindingDB Entry DOI: 10.7270/Q2DR2XBB |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50063062

(CHEMBL3398394)Show SMILES COc1ccc(cc1)S(=O)(=O)n1cc(\C=N\N=C\N(C)C)c2c(cccc12)[N+]([O-])=O Show InChI InChI=1S/C19H19N5O5S/c1-22(2)13-21-20-11-14-12-23(17-5-4-6-18(19(14)17)24(25)26)30(27,28)16-9-7-15(29-3)8-10-16/h4-13H,1-3H3/b20-11+,21-13+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 740 | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant 5-HT6 receptor expressed in HEK293 cells assessed as inhibition of 5-HT-induced increase in Ca2+ level pretreated for... |
Bioorg Med Chem 23: 1313-20 (2015)
Article DOI: 10.1016/j.bmc.2015.01.032
BindingDB Entry DOI: 10.7270/Q27S7QFW |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50132500
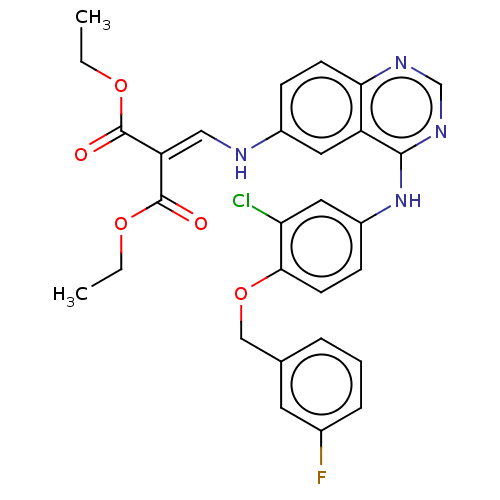
(CHEMBL3633936)Show SMILES [#6]-[#6]-[#8]-[#6](=O)-[#6](=[#6]/[#7]-c1ccc2ncnc(-[#7]-c3ccc(-[#8]-[#6]-c4cccc(F)c4)c(Cl)c3)c2c1)\[#6](=O)-[#8]-[#6]-[#6] Show InChI InChI=1S/C29H26ClFN4O5/c1-3-38-28(36)23(29(37)39-4-2)15-32-20-8-10-25-22(13-20)27(34-17-33-25)35-21-9-11-26(24(30)14-21)40-16-18-6-5-7-19(31)12-18/h5-15,17,32H,3-4,16H2,1-2H3,(H,33,34,35) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 812 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University of Science and Technology (UST)
Curated by ChEMBL
| Assay Description
Inhibitory activity against metabotropic glutamate receptor 5 (mGluR5) |
Bioorg Med Chem Lett 25: 5147-54 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.003
BindingDB Entry DOI: 10.7270/Q27W6F0D |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50132494

(CHEMBL3633941)Show SMILES CCNC(=O)Nc1ccc(cc1)C(=O)Nc1ccc2ncnc(Nc3ccc(F)c(Cl)c3)c2c1 Show InChI InChI=1S/C24H20ClFN6O2/c1-2-27-24(34)32-15-5-3-14(4-6-15)23(33)31-16-8-10-21-18(11-16)22(29-13-28-21)30-17-7-9-20(26)19(25)12-17/h3-13H,2H2,1H3,(H,31,33)(H2,27,32,34)(H,28,29,30) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 855 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University of Science and Technology (UST)
Curated by ChEMBL
| Assay Description
Inhibitory constant against Adenosine A2A receptor using [3H]-SCH-58,261 as radio ligand |
Bioorg Med Chem Lett 25: 5147-54 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.003
BindingDB Entry DOI: 10.7270/Q27W6F0D |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50132502

(CHEMBL3633934)Show SMILES [#6]-[#6]-[#8]-[#6](=O)-[#6](=[#6]/[#7]-c1ccc2ncnc(-[#7]-c3ccc(-[#8]-c4cccc(c4)C(F)(F)F)c(Cl)c3)c2c1)\[#6](=O)-[#8]-[#6]-[#6] Show InChI InChI=1S/C29H24ClF3N4O5/c1-3-40-27(38)22(28(39)41-4-2)15-34-18-8-10-24-21(13-18)26(36-16-35-24)37-19-9-11-25(23(30)14-19)42-20-7-5-6-17(12-20)29(31,32)33/h5-16,34H,3-4H2,1-2H3,(H,35,36,37) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University of Science and Technology (UST)
Curated by ChEMBL
| Assay Description
Inhibitory constant against Adenosine A2A receptor using [3H]-SCH-58,261 as radio ligand |
Bioorg Med Chem Lett 25: 5147-54 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.003
BindingDB Entry DOI: 10.7270/Q27W6F0D |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data