

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50005159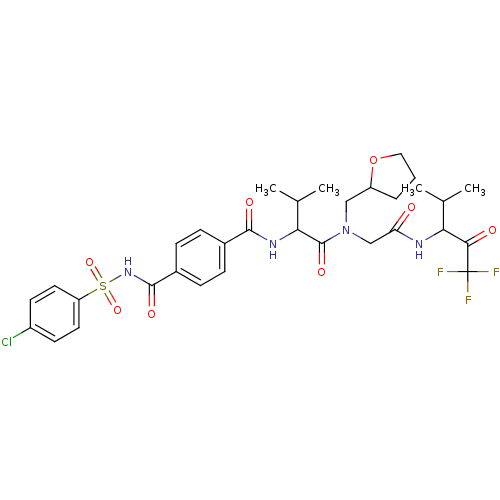 (4-(4-Chloro-benzenesulfonylaminocarbonyl)-N-(2-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against human leukocyte elastase | J Med Chem 35: 641-62 (1992) BindingDB Entry DOI: 10.7270/Q2K074X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [600-1027,R876K]/[600-1155] (Human immunodeficiency virus type 1) | BDBM1517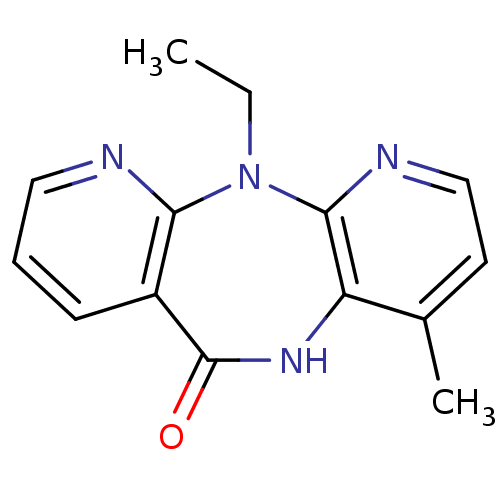 (2-ethyl-7-methyl-2,4,9,15-tetraazatricyclo[9.4.0.0...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 34: 2231-41 (1991) Article DOI: 10.1021/jm00111a045 BindingDB Entry DOI: 10.7270/Q2TT4P4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [600-1027,R876K]/[600-1155] (Human immunodeficiency virus type 1) | BDBM1646 (2-ethyl-10,12,13-trimethyl-2,4,10-triazatricyclo[9...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | 7.8 | 22 |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 34: 2231-41 (1991) Article DOI: 10.1021/jm00111a045 BindingDB Entry DOI: 10.7270/Q2TT4P4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50005147 ((S)-1-{(S)-2-[4-(4-Bromo-benzenesulfonylaminocarbo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against human leukocyte elastase | J Med Chem 35: 641-62 (1992) BindingDB Entry DOI: 10.7270/Q2K074X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [600-1027,R876K]/[600-1155] (Human immunodeficiency virus type 1) | BDBM1639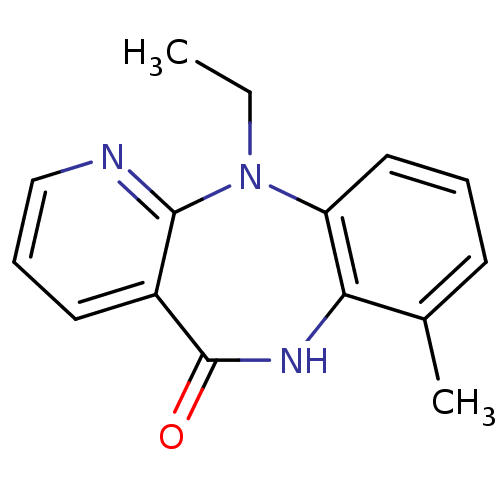 (2-ethyl-12-methyl-2,4,10-triazatricyclo[9.4.0.0^{3...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | 7.8 | 22 |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 34: 2231-41 (1991) Article DOI: 10.1021/jm00111a045 BindingDB Entry DOI: 10.7270/Q2TT4P4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [600-1027,R876K]/[600-1155] (Human immunodeficiency virus type 1) | BDBM1644 (2-ethyl-10,13,14-trimethyl-2,4,10-triazatricyclo[9...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | 7.8 | 22 |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 34: 2231-41 (1991) Article DOI: 10.1021/jm00111a045 BindingDB Entry DOI: 10.7270/Q2TT4P4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50005166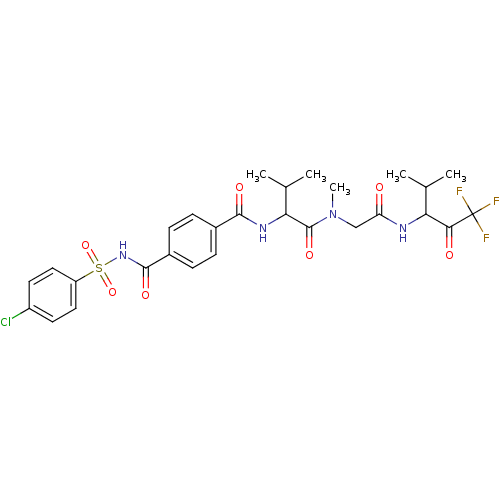 (4-(4-Chloro-benzenesulfonylaminocarbonyl)-N-(2-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against human leukocyte elastase | J Med Chem 35: 641-62 (1992) BindingDB Entry DOI: 10.7270/Q2K074X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50005176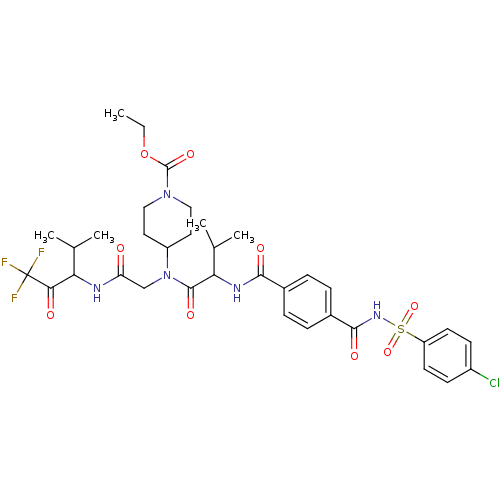 (4-{{2-[4-(4-Chloro-benzenesulfonylaminocarbonyl)-b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against human leukocyte elastase | J Med Chem 35: 641-62 (1992) BindingDB Entry DOI: 10.7270/Q2K074X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50005174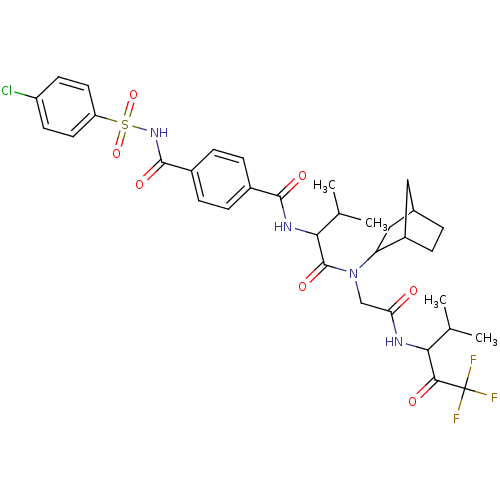 (CHEMBL435649 | N-(1-{Bicyclo[2.2.1]hept-2-yl-[(3,3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against human leukocyte elastase | J Med Chem 35: 641-62 (1992) BindingDB Entry DOI: 10.7270/Q2K074X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [600-1027,R876K]/[600-1155] (Human immunodeficiency virus type 1) | BDBM1647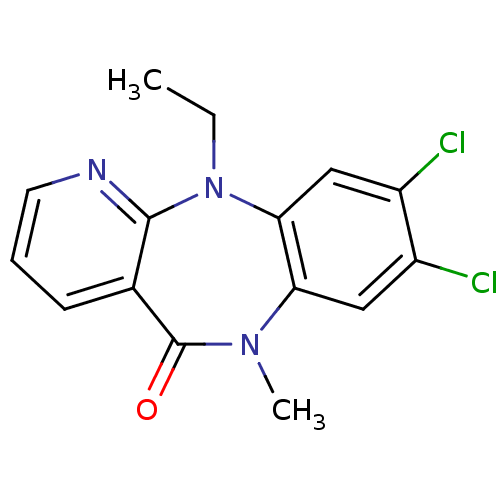 (13,14-dichloro-2-ethyl-10-methyl-2,4,10-triazatric...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 62 | n/a | n/a | n/a | n/a | 7.8 | 22 |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 34: 2231-41 (1991) Article DOI: 10.1021/jm00111a045 BindingDB Entry DOI: 10.7270/Q2TT4P4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50005154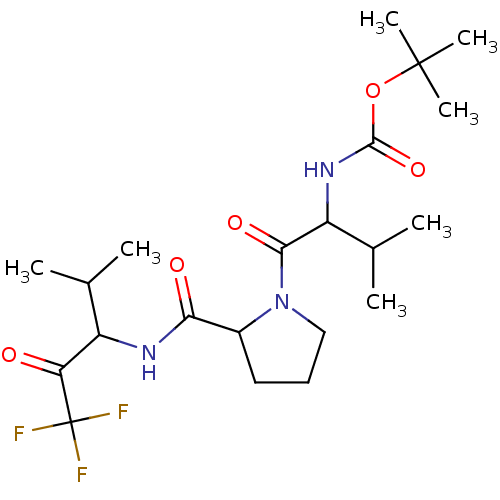 (CHEMBL423067 | {2-Methyl-1-[2-(3,3,3-trifluoro-1-i...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against human leukocyte elastase | J Med Chem 35: 641-62 (1992) BindingDB Entry DOI: 10.7270/Q2K074X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50005156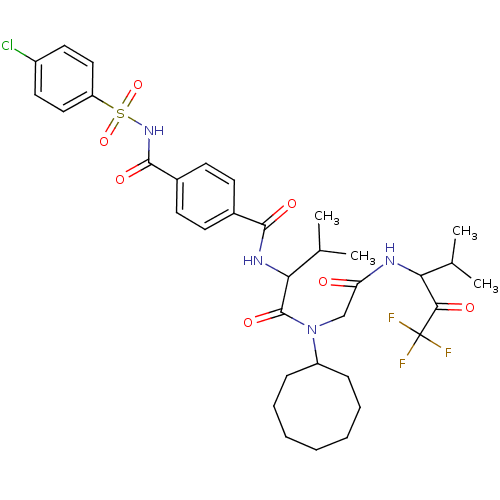 (4-(4-Chloro-benzenesulfonylaminocarbonyl)-N-(1-{cy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against human leukocyte elastase | J Med Chem 35: 641-62 (1992) BindingDB Entry DOI: 10.7270/Q2K074X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50005152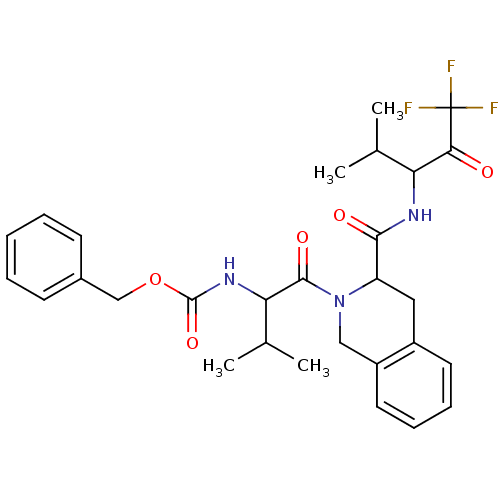 (CHEMBL160365 | {2-Methyl-1-[3-(3,3,3-trifluoro-1-i...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against human leukocyte elastase | J Med Chem 35: 641-62 (1992) BindingDB Entry DOI: 10.7270/Q2K074X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50005152 (CHEMBL160365 | {2-Methyl-1-[3-(3,3,3-trifluoro-1-i...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against human leukocyte elastase | J Med Chem 35: 641-62 (1992) BindingDB Entry DOI: 10.7270/Q2K074X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50005178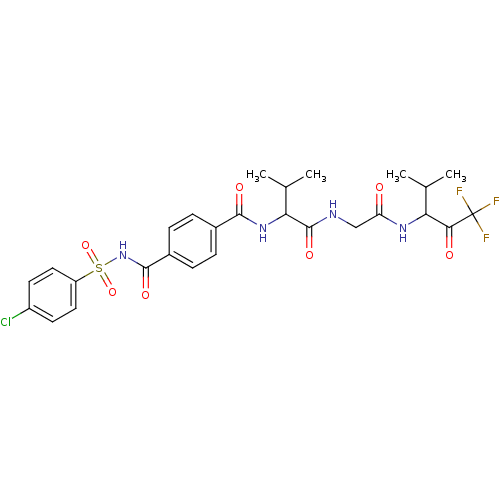 (4-(4-Chloro-benzenesulfonylaminocarbonyl)-N-(2-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against human leukocyte elastase | J Med Chem 35: 641-62 (1992) BindingDB Entry DOI: 10.7270/Q2K074X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [600-1027,R876K]/[600-1155] (Human immunodeficiency virus type 1) | BDBM1434 (11-cyclopropyl-5,11-dihydro-4-methyl-6H-dipyrido[3...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 84 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 34: 2231-41 (1991) Article DOI: 10.1021/jm00111a045 BindingDB Entry DOI: 10.7270/Q2TT4P4X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50004184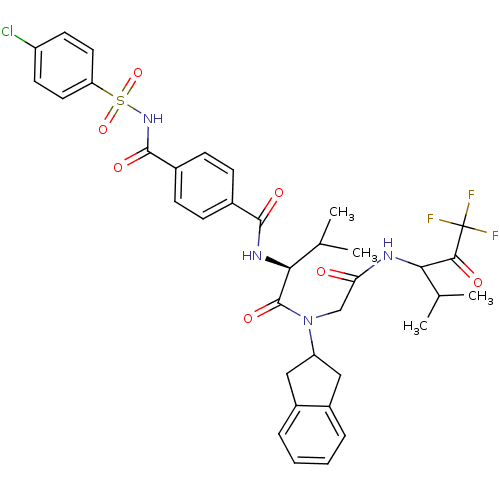 (4-(4-Chloro-benzenesulfonylaminocarbonyl)-N-((S)-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 84 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against human leukocyte elastase | J Med Chem 35: 641-62 (1992) BindingDB Entry DOI: 10.7270/Q2K074X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50005164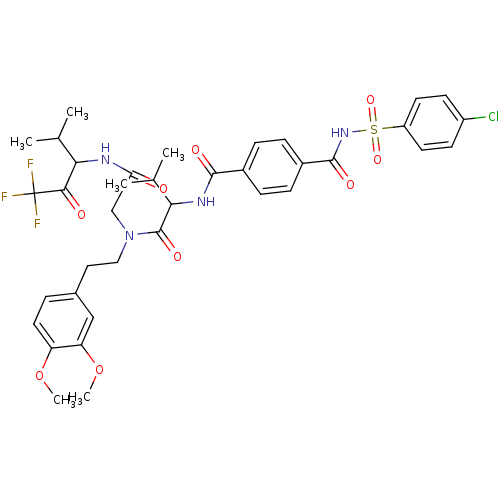 (4-(4-Chloro-benzenesulfonylaminocarbonyl)-N-(1-{[2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 84 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against human leukocyte elastase | J Med Chem 35: 641-62 (1992) BindingDB Entry DOI: 10.7270/Q2K074X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50005153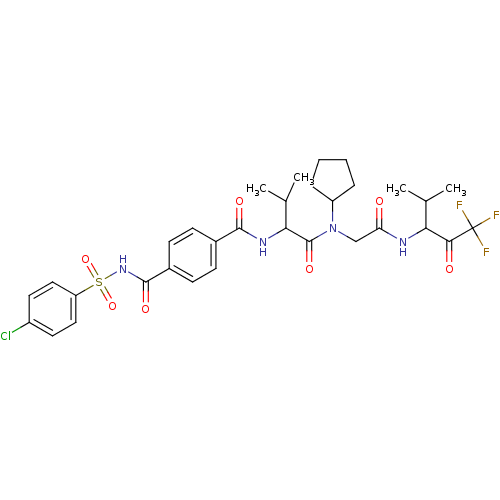 (4-(4-Chloro-benzenesulfonylaminocarbonyl)-N-(1-{cy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 92 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against human leukocyte elastase | J Med Chem 35: 641-62 (1992) BindingDB Entry DOI: 10.7270/Q2K074X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [600-1027,R876K]/[600-1155] (Human immunodeficiency virus type 1) | BDBM1666 (7-chloro-2-ethyl-2,4,9,15-tetraazatricyclo[9.4.0.0...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 95 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 34: 2231-41 (1991) Article DOI: 10.1021/jm00111a045 BindingDB Entry DOI: 10.7270/Q2TT4P4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50005169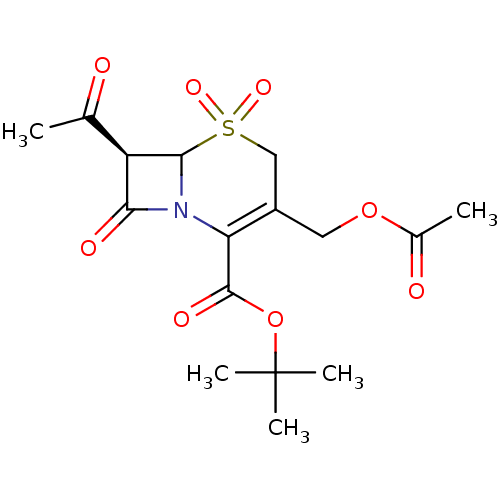 (3-Acetoxymethyl-7-acetyl-5,5-dihydroxy-8-oxo-5lamb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 107 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against human leukocyte elastase | J Med Chem 35: 641-62 (1992) BindingDB Entry DOI: 10.7270/Q2K074X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50005149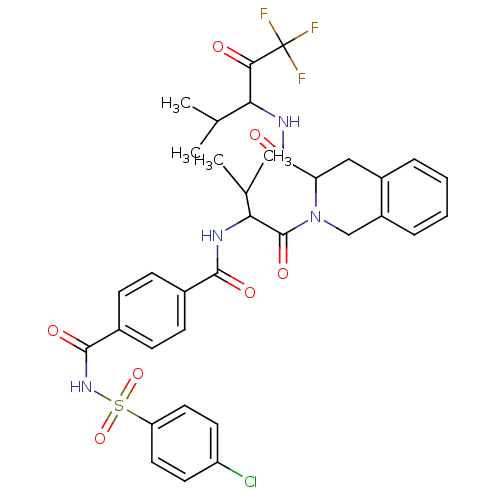 (2-{2-[4-(4-Chloro-benzenesulfonylaminocarbonyl)-be...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against human leukocyte elastase | J Med Chem 35: 641-62 (1992) BindingDB Entry DOI: 10.7270/Q2K074X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [600-1027,R876K]/[600-1155] (Human immunodeficiency virus type 1) | BDBM1667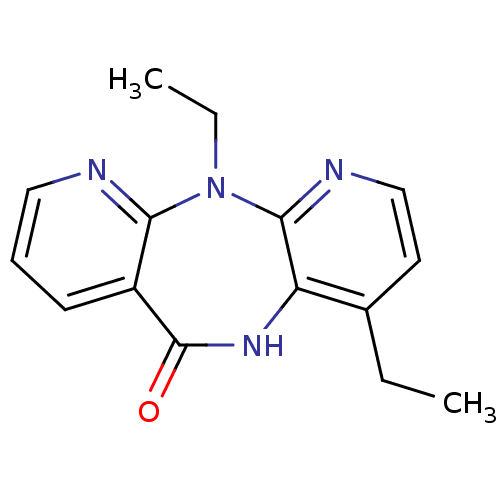 (2,7-diethyl-2,4,9,15-tetraazatricyclo[9.4.0.0^{3,8...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 34: 2231-41 (1991) Article DOI: 10.1021/jm00111a045 BindingDB Entry DOI: 10.7270/Q2TT4P4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [600-1027,R876K]/[600-1155] (Human immunodeficiency virus type 1) | BDBM1518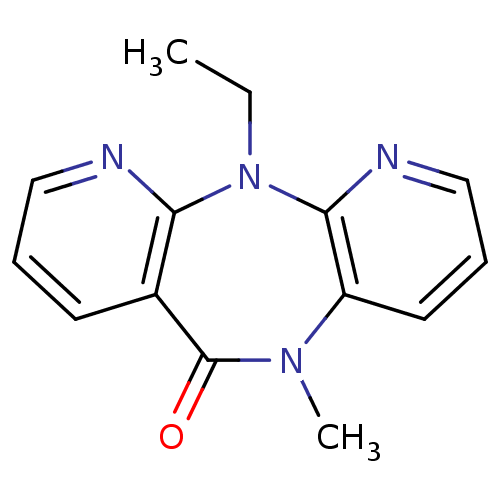 (2-ethyl-9-methyl-2,4,9,15-tetraazatricyclo[9.4.0.0...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 125 | n/a | n/a | n/a | n/a | 7.8 | 22 |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 34: 2231-41 (1991) Article DOI: 10.1021/jm00111a045 BindingDB Entry DOI: 10.7270/Q2TT4P4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50005167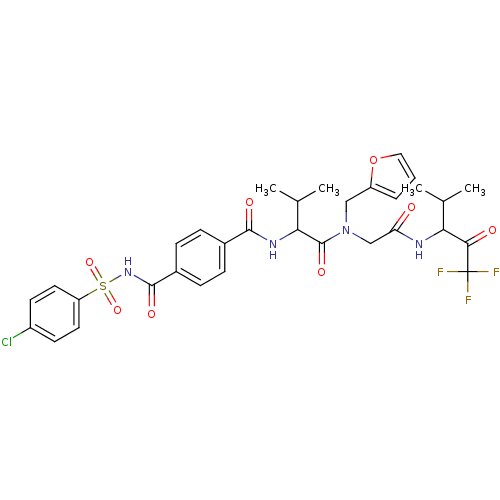 (4-(4-Chloro-benzenesulfonylaminocarbonyl)-N-(1-{fu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 138 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against human leukocyte elastase | J Med Chem 35: 641-62 (1992) BindingDB Entry DOI: 10.7270/Q2K074X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [600-1027,R876K]/[600-1155] (Human immunodeficiency virus type 1) | BDBM1643 (14-azido-2-ethyl-10-methyl-2,4,10-triazatricyclo[9...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | 7.8 | 22 |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 34: 2231-41 (1991) Article DOI: 10.1021/jm00111a045 BindingDB Entry DOI: 10.7270/Q2TT4P4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50005168 (4-(4-Bromo-benzenesulfonylaminocarbonyl)-N-(1-{ind...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 149 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against human leukocyte elastase | J Med Chem 35: 641-62 (1992) BindingDB Entry DOI: 10.7270/Q2K074X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [600-1027,R876K]/[600-1155] (Human immunodeficiency virus type 1) | BDBM1529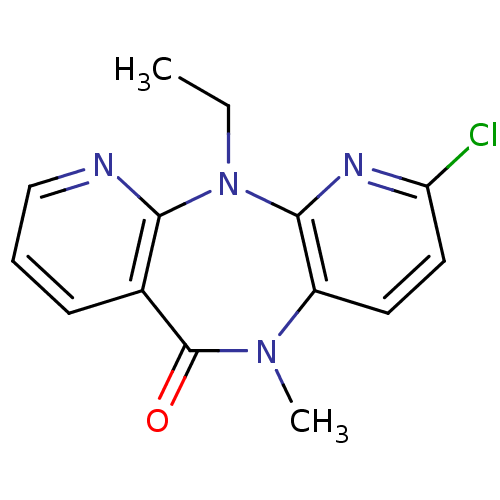 (5-chloro-2-ethyl-9-methyl-2,4,9,15-tetraazatricycl...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 34: 2231-41 (1991) Article DOI: 10.1021/jm00111a045 BindingDB Entry DOI: 10.7270/Q2TT4P4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50005144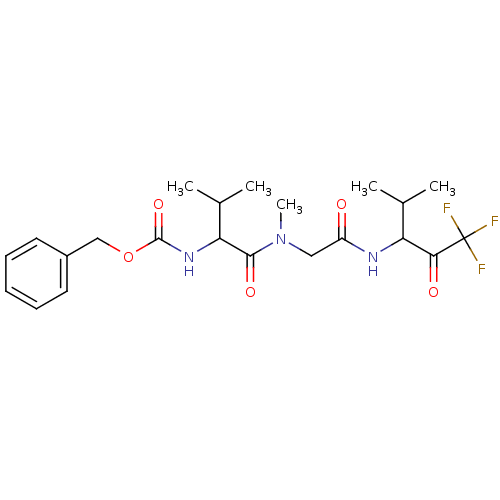 ((2-Methyl-1-{methyl-[(3,3,3-trifluoro-1-isopropyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 153 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against human leukocyte elastase | J Med Chem 35: 641-62 (1992) BindingDB Entry DOI: 10.7270/Q2K074X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50005148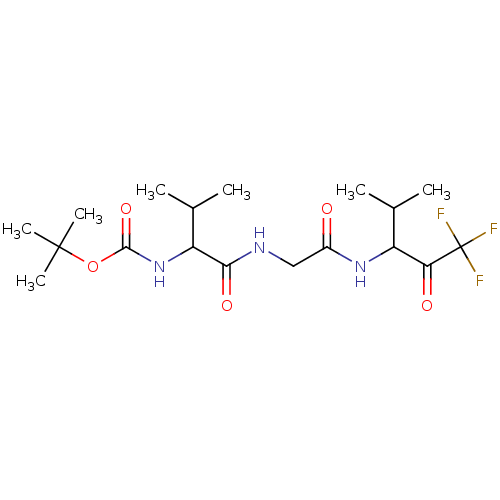 ((2-Methyl-1-{[(3,3,3-trifluoro-1-isopropyl-2-oxo-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 153 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against human leukocyte elastase | J Med Chem 35: 641-62 (1992) BindingDB Entry DOI: 10.7270/Q2K074X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50005155 ((1-{Cyclopentyl-[(3,3,3-trifluoro-1-isopropyl-2-ox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 156 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against human leukocyte elastase | J Med Chem 35: 641-62 (1992) BindingDB Entry DOI: 10.7270/Q2K074X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [600-1027,R876K]/[600-1155] (Human immunodeficiency virus type 1) | BDBM1521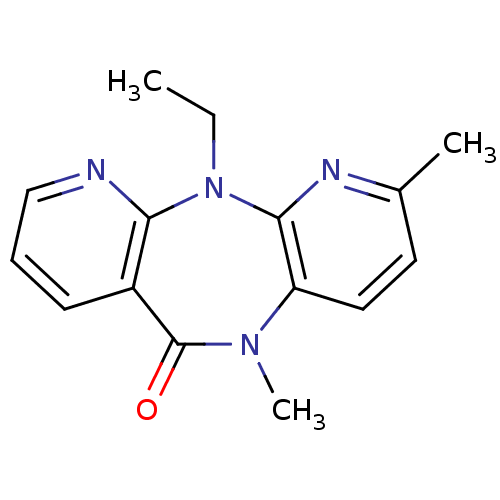 (2-ethyl-5,9-dimethyl-2,4,9,15-tetraazatricyclo[9.4...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 34: 2231-41 (1991) Article DOI: 10.1021/jm00111a045 BindingDB Entry DOI: 10.7270/Q2TT4P4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [600-1027,R876K]/[600-1155] (Human immunodeficiency virus type 1) | BDBM1649 (14-chloro-2-ethyl-10-methyl-2,4,10-triazatricyclo[...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | 7.8 | 22 |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 34: 2231-41 (1991) Article DOI: 10.1021/jm00111a045 BindingDB Entry DOI: 10.7270/Q2TT4P4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [600-1027,R876K]/[600-1155] (Human immunodeficiency virus type 1) | BDBM1653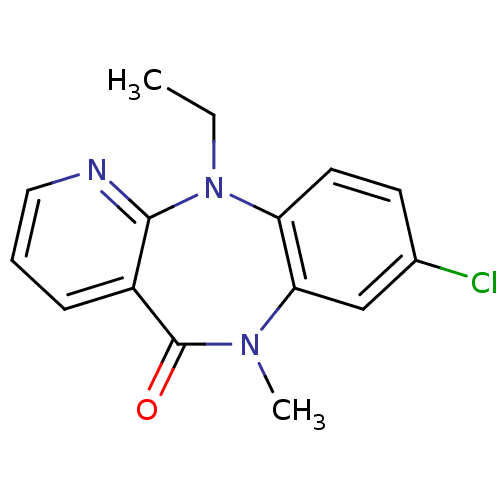 (13-chloro-2-ethyl-10-methyl-2,4,10-triazatricyclo[...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | 7.8 | 22 |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 34: 2231-41 (1991) Article DOI: 10.1021/jm00111a045 BindingDB Entry DOI: 10.7270/Q2TT4P4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50005154 (CHEMBL423067 | {2-Methyl-1-[2-(3,3,3-trifluoro-1-i...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 172 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against human leukocyte elastase | J Med Chem 35: 641-62 (1992) BindingDB Entry DOI: 10.7270/Q2K074X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50005145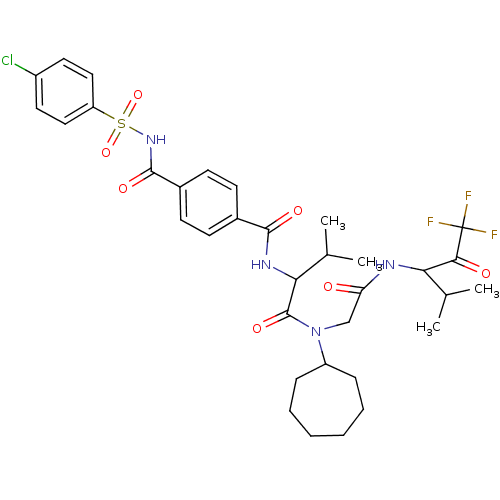 (4-(4-Chloro-benzenesulfonylaminocarbonyl)-N-(1-{cy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 175 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against human leukocyte elastase | J Med Chem 35: 641-62 (1992) BindingDB Entry DOI: 10.7270/Q2K074X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [600-1027,R876K]/[600-1155] (Human immunodeficiency virus type 1) | BDBM1640 (2-ethyl-10,13-dimethyl-2,4,10-triazatricyclo[9.4.0...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | 7.8 | 22 |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 34: 2231-41 (1991) Article DOI: 10.1021/jm00111a045 BindingDB Entry DOI: 10.7270/Q2TT4P4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [600-1027,R876K]/[600-1155] (Human immunodeficiency virus type 1) | BDBM1636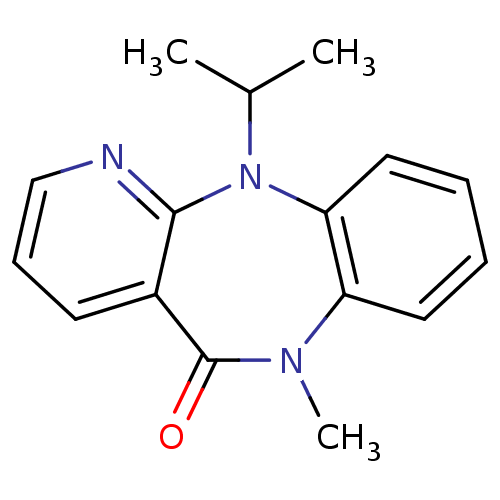 (10-methyl-2-(propan-2-yl)-2,4,10-triazatricyclo[9....) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | 7.8 | 22 |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 34: 2231-41 (1991) Article DOI: 10.1021/jm00111a045 BindingDB Entry DOI: 10.7270/Q2TT4P4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50005175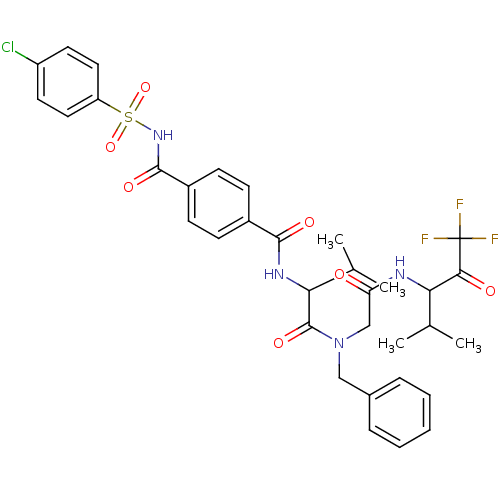 (CHEMBL159116 | N-(1-{Benzyl-[(3,3,3-trifluoro-1-is...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 217 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against human leukocyte elastase | J Med Chem 35: 641-62 (1992) BindingDB Entry DOI: 10.7270/Q2K074X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [600-1027,R876K]/[600-1155] (Human immunodeficiency virus type 1) | BDBM1641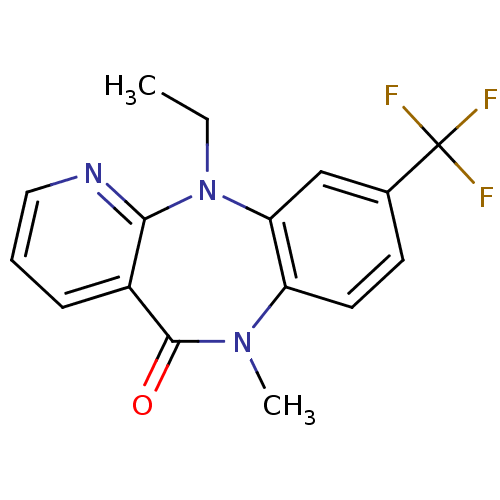 (2-ethyl-10-methyl-14-(trifluoromethyl)-2,4,10-tria...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | 7.8 | 22 |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 34: 2231-41 (1991) Article DOI: 10.1021/jm00111a045 BindingDB Entry DOI: 10.7270/Q2TT4P4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [600-1027,R876K]/[600-1155] (Human immunodeficiency virus type 1) | BDBM1635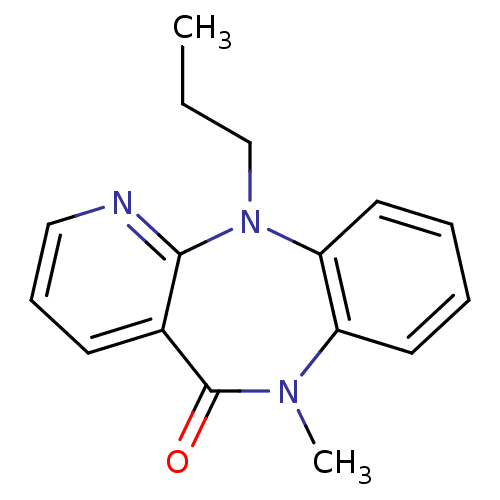 (10-methyl-2-propyl-2,4,10-triazatricyclo[9.4.0.0^{...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | 7.8 | 22 |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 34: 2231-41 (1991) Article DOI: 10.1021/jm00111a045 BindingDB Entry DOI: 10.7270/Q2TT4P4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [600-1027,R876K]/[600-1155] (Human immunodeficiency virus type 1) | BDBM1669 (2-ethyl-5,6,9-trimethyl-2,4,9,15-tetraazatricyclo[...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 34: 2231-41 (1991) Article DOI: 10.1021/jm00111a045 BindingDB Entry DOI: 10.7270/Q2TT4P4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [600-1027,R876K]/[600-1155] (Human immunodeficiency virus type 1) | BDBM1638 (2-ethyl-10,12-dimethyl-2,4,10-triazatricyclo[9.4.0...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | 7.8 | 22 |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 34: 2231-41 (1991) Article DOI: 10.1021/jm00111a045 BindingDB Entry DOI: 10.7270/Q2TT4P4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [600-1027,R876K]/[600-1155] (Human immunodeficiency virus type 1) | BDBM1648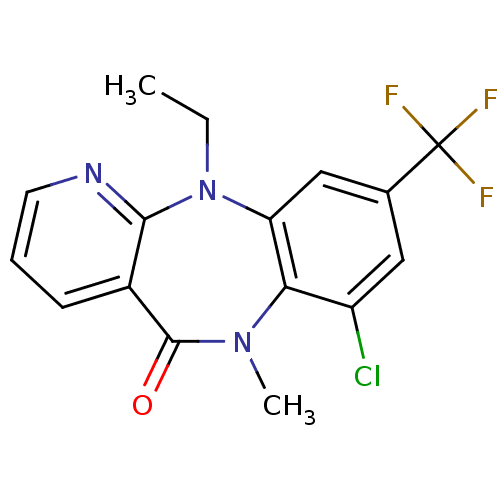 (12-chloro-2-ethyl-10-methyl-14-(trifluoromethyl)-2...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 290 | n/a | n/a | n/a | n/a | 7.8 | 22 |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 34: 2231-41 (1991) Article DOI: 10.1021/jm00111a045 BindingDB Entry DOI: 10.7270/Q2TT4P4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [600-1027,R876K]/[600-1155] (Human immunodeficiency virus type 1) | BDBM1633 (2-ethyl-10-methyl-2,4,10-triazatricyclo[9.4.0.0^{3...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | 7.8 | 22 |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 34: 2231-41 (1991) Article DOI: 10.1021/jm00111a045 BindingDB Entry DOI: 10.7270/Q2TT4P4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [600-1027,R876K]/[600-1155] (Human immunodeficiency virus type 1) | BDBM1659 (9-acetyl-2-ethyl-2,4,9,15-tetraazatricyclo[9.4.0.0...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 34: 2231-41 (1991) Article DOI: 10.1021/jm00111a045 BindingDB Entry DOI: 10.7270/Q2TT4P4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50004187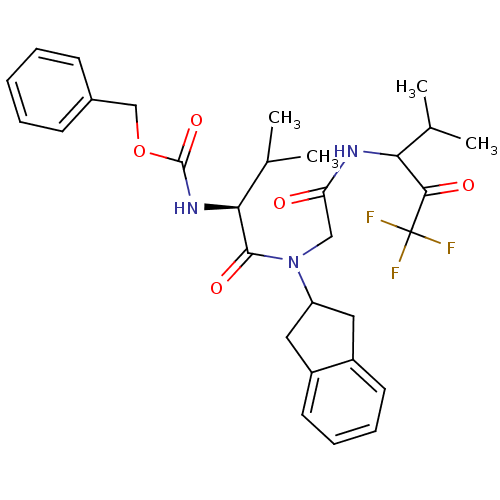 (((S)-1-{Indan-2-yl-[(3,3,3-trifluoro-1-isopropyl-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 365 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against human leukocyte elastase | J Med Chem 35: 641-62 (1992) BindingDB Entry DOI: 10.7270/Q2K074X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [600-1027,R876K]/[600-1155] (Human immunodeficiency virus type 1) | BDBM1634 (2,10-diethyl-2,4,10-triazatricyclo[9.4.0.0^{3,8}]p...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 380 | n/a | n/a | n/a | n/a | 7.8 | 22 |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 34: 2231-41 (1991) Article DOI: 10.1021/jm00111a045 BindingDB Entry DOI: 10.7270/Q2TT4P4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [600-1027,R876K]/[600-1155] (Human immunodeficiency virus type 1) | BDBM1632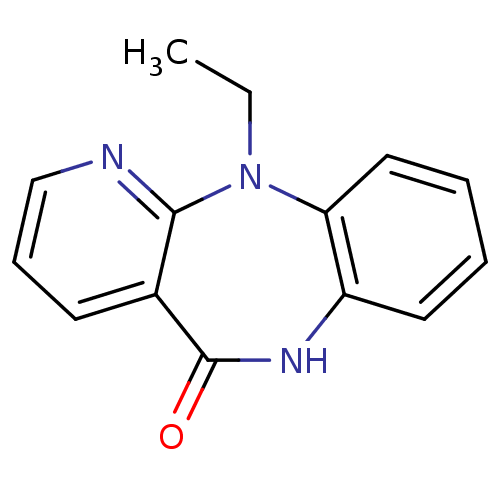 (2-ethyl-2,4,10-triazatricyclo[9.4.0.0^{3,8}]pentad...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 380 | n/a | n/a | n/a | n/a | 7.8 | 22 |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 34: 2231-41 (1991) Article DOI: 10.1021/jm00111a045 BindingDB Entry DOI: 10.7270/Q2TT4P4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [600-1027,R876K]/[600-1155] (Human immunodeficiency virus type 1) | BDBM1642 (2-ethyl-10-methyl-14-nitro-2,4,10-triazatricyclo[9...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | 7.8 | 22 |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 34: 2231-41 (1991) Article DOI: 10.1021/jm00111a045 BindingDB Entry DOI: 10.7270/Q2TT4P4X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Displayed 1 to 50 (of 81 total ) | Next | Last >> |