 Found 1061 hits with Last Name = 'volpi' and Initial = 'd'
Found 1061 hits with Last Name = 'volpi' and Initial = 'd' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cyclin-A1/Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50307546
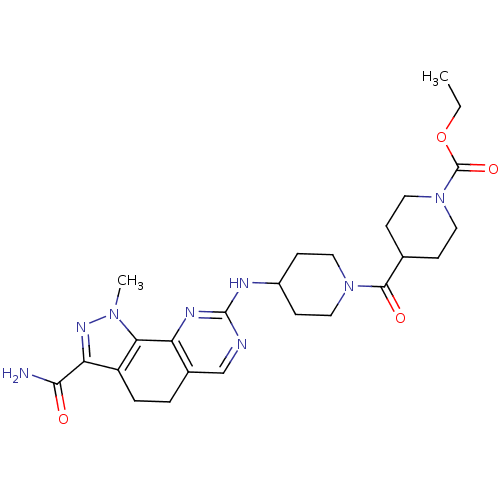
(CHEMBL598401 | Ethyl 4-[(3-Carbamoyl-1-methyl-4,5-...)Show SMILES CCOC(=O)N1CCC(CC1)C(=O)N1CCC(CC1)Nc1ncc2CCc3c(nn(C)c3-c2n1)C(N)=O Show InChI InChI=1S/C25H34N8O4/c1-3-37-25(36)33-10-6-15(7-11-33)23(35)32-12-8-17(9-13-32)28-24-27-14-16-4-5-18-20(22(26)34)30-31(2)21(18)19(16)29-24/h14-15,17H,3-13H2,1-2H3,(H2,26,34)(H,27,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl
Curated by ChEMBL
| Assay Description
Inhibition of CDK2/Cyclin A |
J Med Chem 53: 2171-87 (2010)
Article DOI: 10.1021/jm901710h
BindingDB Entry DOI: 10.7270/Q2N879W9 |
More data for this
Ligand-Target Pair | |
Cyclin-A1/Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50307543
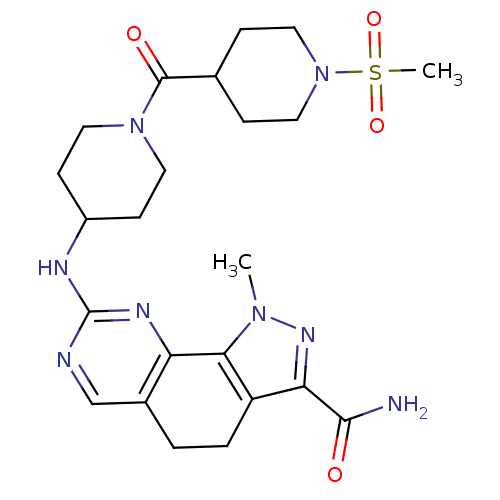
(1-Methyl-8-{[1-(methylsulfonyl)piperidin-4-yl]amin...)Show SMILES Cn1nc(C(N)=O)c2CCc3cnc(NC4CCN(CC4)C(=O)C4CCN(CC4)S(C)(=O)=O)nc3-c12 Show InChI InChI=1S/C23H32N8O4S/c1-29-20-17(19(28-29)21(24)32)4-3-15-13-25-23(27-18(15)20)26-16-7-9-30(10-8-16)22(33)14-5-11-31(12-6-14)36(2,34)35/h13-14,16H,3-12H2,1-2H3,(H2,24,32)(H,25,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl
Curated by ChEMBL
| Assay Description
Inhibition of CDK2/Cyclin A |
J Med Chem 53: 2171-87 (2010)
Article DOI: 10.1021/jm901710h
BindingDB Entry DOI: 10.7270/Q2N879W9 |
More data for this
Ligand-Target Pair | |
Cell division cycle 7-related protein kinase [58-574]/Protein DBF4 homolog A
(Homo sapiens (Human)) | BDBM27359
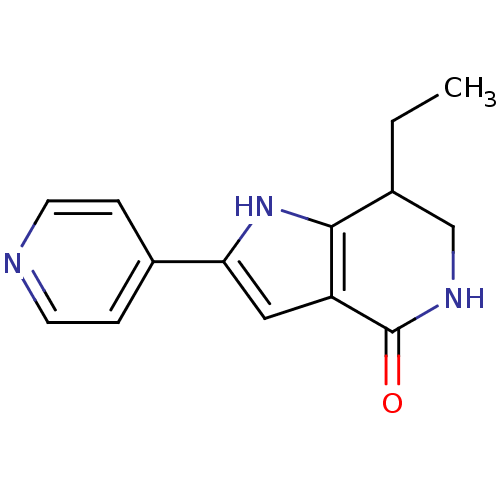
(7-ethyl-2-(pyridin-4-yl)-1H,4H,5H,6H,7H-pyrrolo[3,...)Show InChI InChI=1S/C14H15N3O/c1-2-9-8-16-14(18)11-7-12(17-13(9)11)10-3-5-15-6-4-10/h3-7,9,17H,2,8H2,1H3,(H,16,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.9 | 25 |
Nerviano Medical Sciences Srl
| Assay Description
The potency of the compound toward kinase activity was determined using a Dowex resin-based assay. The substrate was phosphorylated by kinase in the ... |
J Med Chem 52: 293-307 (2009)
Article DOI: 10.1021/jm800977q
BindingDB Entry DOI: 10.7270/Q27W69JX |
More data for this
Ligand-Target Pair | |
Cell division cycle 7-related protein kinase [58-574]/Protein DBF4 homolog A
(Homo sapiens (Human)) | BDBM27380
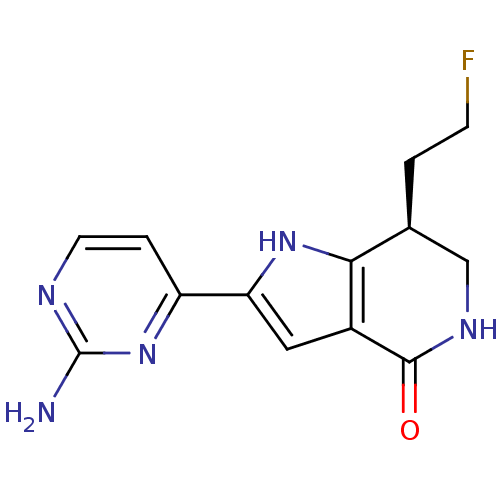
((7S)-2-(2-aminopyrimidin-4-yl)-7-(2-fluoroethyl)-1...)Show SMILES Nc1nccc(n1)-c1cc2c([nH]1)[C@@H](CCF)CNC2=O |r| Show InChI InChI=1S/C13H14FN5O/c14-3-1-7-6-17-12(20)8-5-10(18-11(7)8)9-2-4-16-13(15)19-9/h2,4-5,7,18H,1,3,6H2,(H,17,20)(H2,15,16,19)/t7-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| DrugBank
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl
| Assay Description
The potency of the compound toward kinase activity was determined using a Dowex resin-based assay. The substrate was phosphorylated by kinase in the ... |
J Med Chem 52: 293-307 (2009)
Article DOI: 10.1021/jm800977q
BindingDB Entry DOI: 10.7270/Q27W69JX |
More data for this
Ligand-Target Pair | |
Cell division cycle 7-related protein kinase [58-574]/Protein DBF4 homolog A
(Homo sapiens (Human)) | BDBM27391
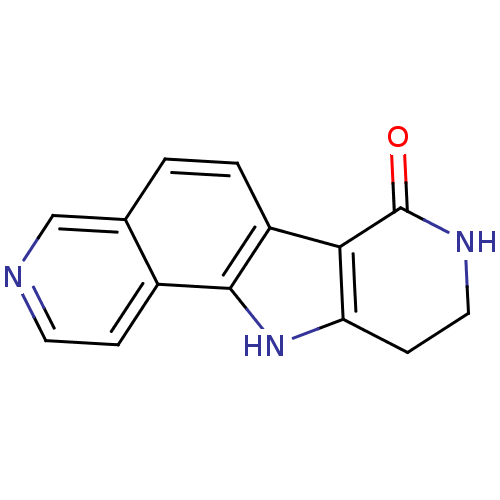
(5,13,17-triazatetracyclo[8.7.0.0^{2,7}.0^{11,16}]h...)Show InChI InChI=1S/C14H11N3O/c18-14-12-10-2-1-8-7-15-5-3-9(8)13(10)17-11(12)4-6-16-14/h1-3,5,7,17H,4,6H2,(H,16,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.9 | 25 |
Nerviano Medical Sciences Srl
| Assay Description
The potency of the compound toward kinase activity was determined using a Dowex resin-based assay. The substrate was phosphorylated by kinase in the ... |
J Med Chem 51: 487-501 (2008)
Article DOI: 10.1021/jm700956r
BindingDB Entry DOI: 10.7270/Q247485B |
More data for this
Ligand-Target Pair | |
Cell division cycle 7-related protein kinase
(Homo sapiens (Human)) | BDBM50329419
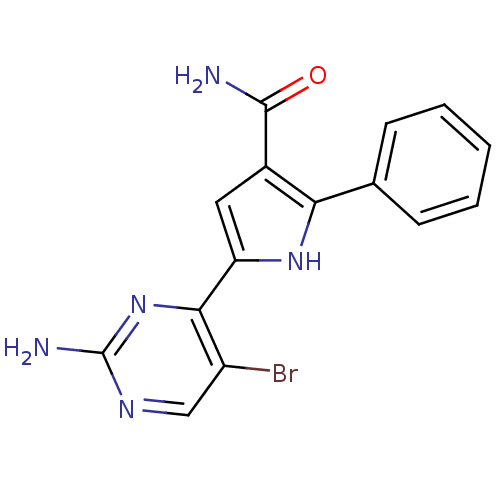
(5-(2-amino-5-bromopyrimidin-4-yl)-2-p-tolyl-1H-pyr...)Show InChI InChI=1S/C15H12BrN5O/c16-10-7-19-15(18)21-13(10)11-6-9(14(17)22)12(20-11)8-4-2-1-3-5-8/h1-7,20H,(H2,17,22)(H2,18,19,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl
Curated by ChEMBL
| Assay Description
Inhibition of Cdc7 |
J Med Chem 53: 7296-315 (2010)
Article DOI: 10.1021/jm100504d
BindingDB Entry DOI: 10.7270/Q24T6JM3 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK2
(Homo sapiens (Human)) | BDBM50318085
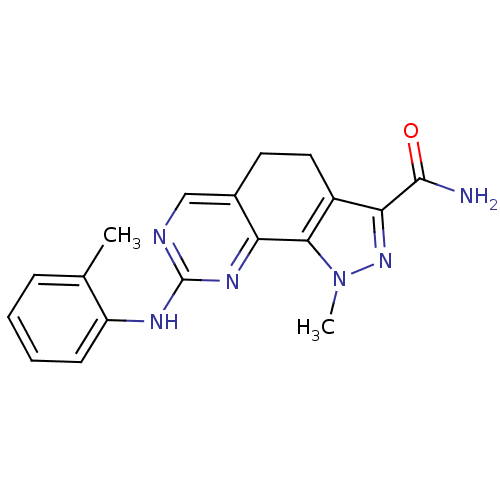
(1-Methyl-8-[(2-methylphenyl)amino]-4,5-dihydro-1H-...)Show InChI InChI=1S/C18H18N6O/c1-10-5-3-4-6-13(10)21-18-20-9-11-7-8-12-15(17(19)25)23-24(2)16(12)14(11)22-18/h3-6,9H,7-8H2,1-2H3,(H2,19,25)(H,20,21,22) | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl
Curated by ChEMBL
| Assay Description
Inhibition of Plk2 assessed as [33P]gamma-ATP incorporation into substrate after 60 mins by gamma counting |
J Med Chem 53: 3532-51 (2010)
Article DOI: 10.1021/jm901713n
BindingDB Entry DOI: 10.7270/Q2RF5V5D |
More data for this
Ligand-Target Pair | |
Cyclin-A1/Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50318089

(8-[(4-Acetylphenyl)amino]-1-methyl-4,5-dihydro-1H-...)Show SMILES CC(=O)c1ccc(Nc2ncc3CCc4c(nn(C)c4-c3n2)C(N)=O)cc1 Show InChI InChI=1S/C19H18N6O2/c1-10(26)11-3-6-13(7-4-11)22-19-21-9-12-5-8-14-16(18(20)27)24-25(2)17(14)15(12)23-19/h3-4,6-7,9H,5,8H2,1-2H3,(H2,20,27)(H,21,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl
Curated by ChEMBL
| Assay Description
Inhibition of CDK2/Cyclin A assessed as [33P]gamma-ATP incorporation into substrate after 60 mins by gamma counting |
J Med Chem 53: 3532-51 (2010)
Article DOI: 10.1021/jm901713n
BindingDB Entry DOI: 10.7270/Q2RF5V5D |
More data for this
Ligand-Target Pair | |
Cell division cycle 7-related protein kinase [58-574]/Protein DBF4 homolog A
(Homo sapiens (Human)) | BDBM27371
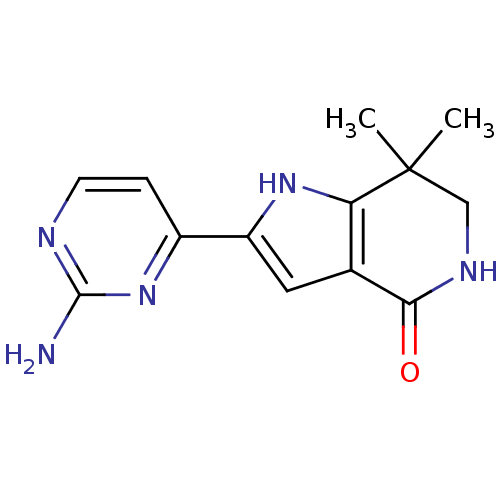
(2-(2-aminopyrimidin-4-yl)-7,7-dimethyl-1H,4H,5H,6H...)Show InChI InChI=1S/C13H15N5O/c1-13(2)6-16-11(19)7-5-9(17-10(7)13)8-3-4-15-12(14)18-8/h3-5,17H,6H2,1-2H3,(H,16,19)(H2,14,15,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.9 | 25 |
Nerviano Medical Sciences Srl
| Assay Description
The potency of the compound toward kinase activity was determined using a Dowex resin-based assay. The substrate was phosphorylated by kinase in the ... |
J Med Chem 52: 293-307 (2009)
Article DOI: 10.1021/jm800977q
BindingDB Entry DOI: 10.7270/Q27W69JX |
More data for this
Ligand-Target Pair | |
Cyclin-A1/Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM31532
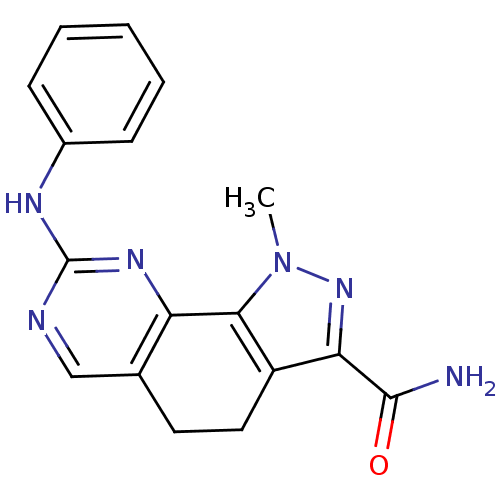
(pyrazolo[4,3-h]quinazoline-3-carboxamide, 1)Show InChI InChI=1S/C17H16N6O/c1-23-15-12(14(22-23)16(18)24)8-7-10-9-19-17(21-13(10)15)20-11-5-3-2-4-6-11/h2-6,9H,7-8H2,1H3,(H2,18,24)(H,19,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl
Curated by ChEMBL
| Assay Description
Inhibition of CDK2/Cyclin A assessed as [33P]gamma-ATP incorporation into substrate after 60 mins by gamma counting |
J Med Chem 53: 3532-51 (2010)
Article DOI: 10.1021/jm901713n
BindingDB Entry DOI: 10.7270/Q2RF5V5D |
More data for this
Ligand-Target Pair | |
Cyclin-A1/Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50318087
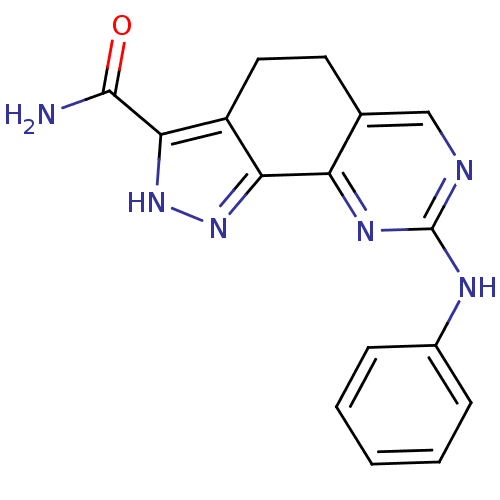
(8-(Phenylamino)-4,5-dihydro-1H-pyrazolo[4,3-h]quin...)Show InChI InChI=1S/C16H14N6O/c17-15(23)14-11-7-6-9-8-18-16(19-10-4-2-1-3-5-10)20-12(9)13(11)21-22-14/h1-5,8H,6-7H2,(H2,17,23)(H,21,22)(H,18,19,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl
Curated by ChEMBL
| Assay Description
Inhibition of CDK2/Cyclin A assessed as [33P]gamma-ATP incorporation into substrate after 60 mins by gamma counting |
J Med Chem 53: 3532-51 (2010)
Article DOI: 10.1021/jm901713n
BindingDB Entry DOI: 10.7270/Q2RF5V5D |
More data for this
Ligand-Target Pair | |
Cyclin-A1/Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50307522
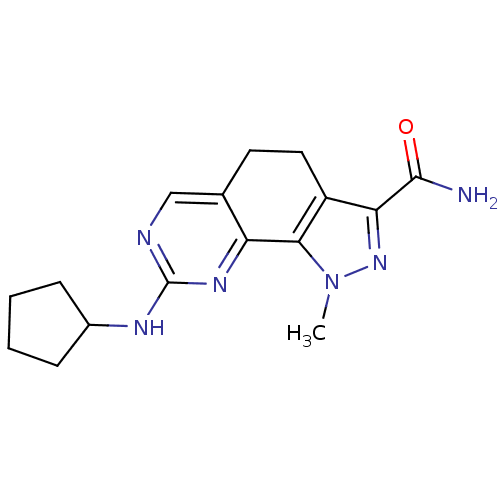
(8-(Cyclopentylamino)-1-methyl-4,5-dihydro-1H-pyraz...)Show InChI InChI=1S/C16H20N6O/c1-22-14-11(13(21-22)15(17)23)7-6-9-8-18-16(20-12(9)14)19-10-4-2-3-5-10/h8,10H,2-7H2,1H3,(H2,17,23)(H,18,19,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl
Curated by ChEMBL
| Assay Description
Inhibition of CDK2/Cyclin A |
J Med Chem 53: 2171-87 (2010)
Article DOI: 10.1021/jm901710h
BindingDB Entry DOI: 10.7270/Q2N879W9 |
More data for this
Ligand-Target Pair | |
Cyclin-A1/Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50307535
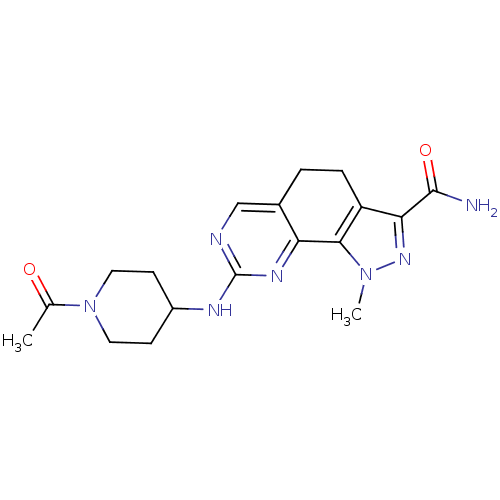
(8-[(1-Acetylpiperidin-4-yl)amino]-1-methyl-4,5-dih...)Show SMILES CC(=O)N1CCC(CC1)Nc1ncc2CCc3c(nn(C)c3-c2n1)C(N)=O Show InChI InChI=1S/C18H23N7O2/c1-10(26)25-7-5-12(6-8-25)21-18-20-9-11-3-4-13-15(17(19)27)23-24(2)16(13)14(11)22-18/h9,12H,3-8H2,1-2H3,(H2,19,27)(H,20,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl
Curated by ChEMBL
| Assay Description
Inhibition of CDK2/Cyclin A |
J Med Chem 53: 2171-87 (2010)
Article DOI: 10.1021/jm901710h
BindingDB Entry DOI: 10.7270/Q2N879W9 |
More data for this
Ligand-Target Pair | |
Cyclin-A1/Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50307507
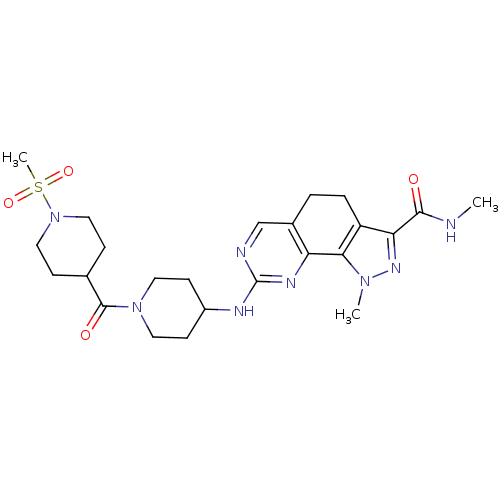
(CHEMBL597754 | N-1-Dimethyl-8-{[1-(methylsulfonyl)...)Show SMILES CNC(=O)c1nn(C)c-2c1CCc1cnc(NC3CCN(CC3)C(=O)C3CCN(CC3)S(C)(=O)=O)nc-21 Show InChI InChI=1S/C24H34N8O4S/c1-25-22(33)20-18-5-4-16-14-26-24(28-19(16)21(18)30(2)29-20)27-17-8-10-31(11-9-17)23(34)15-6-12-32(13-7-15)37(3,35)36/h14-15,17H,4-13H2,1-3H3,(H,25,33)(H,26,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl
Curated by ChEMBL
| Assay Description
Inhibition of CDK2/Cyclin A |
J Med Chem 53: 2171-87 (2010)
Article DOI: 10.1021/jm901710h
BindingDB Entry DOI: 10.7270/Q2N879W9 |
More data for this
Ligand-Target Pair | |
Cell division cycle 7-related protein kinase [58-574]/Protein DBF4 homolog A
(Homo sapiens (Human)) | BDBM27362
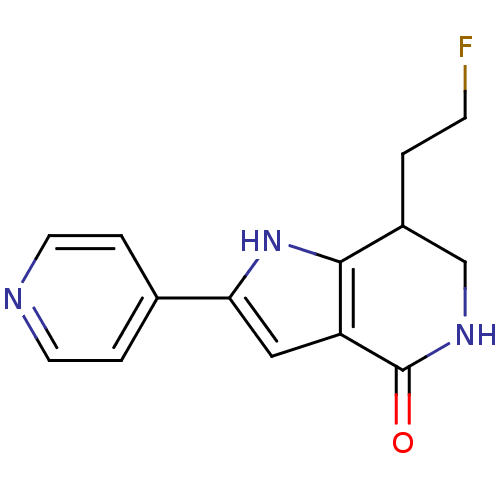
(7-(2-fluoroethyl)-2-(pyridin-4-yl)-1H,4H,5H,6H,7H-...)Show InChI InChI=1S/C14H14FN3O/c15-4-1-10-8-17-14(19)11-7-12(18-13(10)11)9-2-5-16-6-3-9/h2-3,5-7,10,18H,1,4,8H2,(H,17,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.9 | 25 |
Nerviano Medical Sciences Srl
| Assay Description
The potency of the compound toward kinase activity was determined using a Dowex resin-based assay. The substrate was phosphorylated by kinase in the ... |
J Med Chem 52: 293-307 (2009)
Article DOI: 10.1021/jm800977q
BindingDB Entry DOI: 10.7270/Q27W69JX |
More data for this
Ligand-Target Pair | |
Cyclin-A1/Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50318088
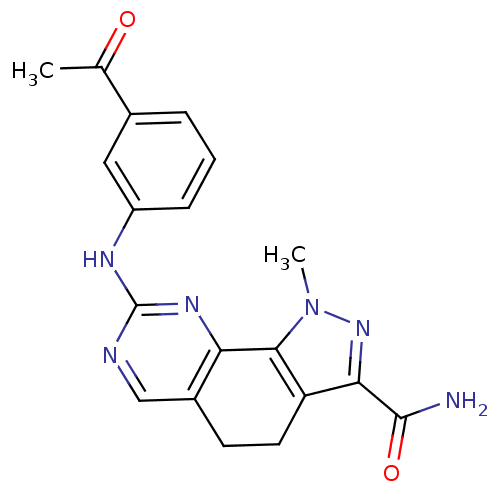
(8-[(3-Acetylphenyl)amino]-1-methyl-4,5-dihydro-1H-...)Show SMILES CC(=O)c1cccc(Nc2ncc3CCc4c(nn(C)c4-c3n2)C(N)=O)c1 Show InChI InChI=1S/C19H18N6O2/c1-10(26)11-4-3-5-13(8-11)22-19-21-9-12-6-7-14-16(18(20)27)24-25(2)17(14)15(12)23-19/h3-5,8-9H,6-7H2,1-2H3,(H2,20,27)(H,21,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl
Curated by ChEMBL
| Assay Description
Inhibition of CDK2/Cyclin A assessed as [33P]gamma-ATP incorporation into substrate after 60 mins by gamma counting |
J Med Chem 53: 3532-51 (2010)
Article DOI: 10.1021/jm901713n
BindingDB Entry DOI: 10.7270/Q2RF5V5D |
More data for this
Ligand-Target Pair | |
Cell division cycle 7-related protein kinase [58-574]/Protein DBF4 homolog A
(Homo sapiens (Human)) | BDBM27361
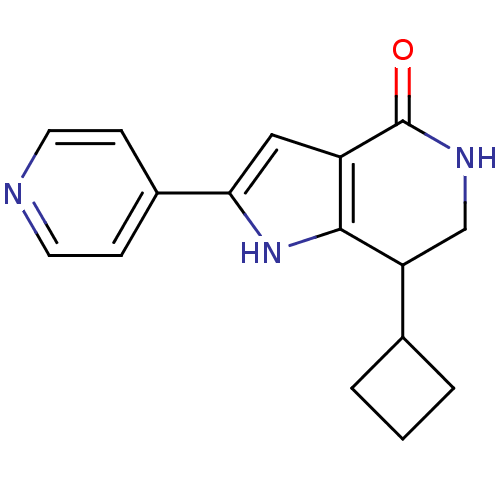
(7-cyclobutyl-2-(pyridin-4-yl)-1H,4H,5H,6H,7H-pyrro...)Show InChI InChI=1S/C16H17N3O/c20-16-12-8-14(11-4-6-17-7-5-11)19-15(12)13(9-18-16)10-2-1-3-10/h4-8,10,13,19H,1-3,9H2,(H,18,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.9 | 25 |
Nerviano Medical Sciences Srl
| Assay Description
The potency of the compound toward kinase activity was determined using a Dowex resin-based assay. The substrate was phosphorylated by kinase in the ... |
J Med Chem 52: 293-307 (2009)
Article DOI: 10.1021/jm800977q
BindingDB Entry DOI: 10.7270/Q27W69JX |
More data for this
Ligand-Target Pair | |
Cell division cycle 7-related protein kinase [58-574]/Protein DBF4 homolog A
(Homo sapiens (Human)) | BDBM27363
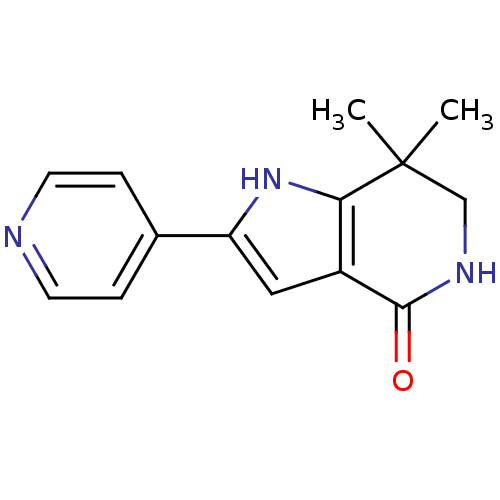
(7,7-dimethyl-2-(pyridin-4-yl)-1H,4H,5H,6H,7H-pyrro...)Show InChI InChI=1S/C14H15N3O/c1-14(2)8-16-13(18)10-7-11(17-12(10)14)9-3-5-15-6-4-9/h3-7,17H,8H2,1-2H3,(H,16,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.9 | 25 |
Nerviano Medical Sciences Srl
| Assay Description
The potency of the compound toward kinase activity was determined using a Dowex resin-based assay. The substrate was phosphorylated by kinase in the ... |
J Med Chem 52: 293-307 (2009)
Article DOI: 10.1021/jm800977q
BindingDB Entry DOI: 10.7270/Q27W69JX |
More data for this
Ligand-Target Pair | |
Cell division cycle 7-related protein kinase [58-574]/Protein DBF4 homolog A
(Homo sapiens (Human)) | BDBM27370
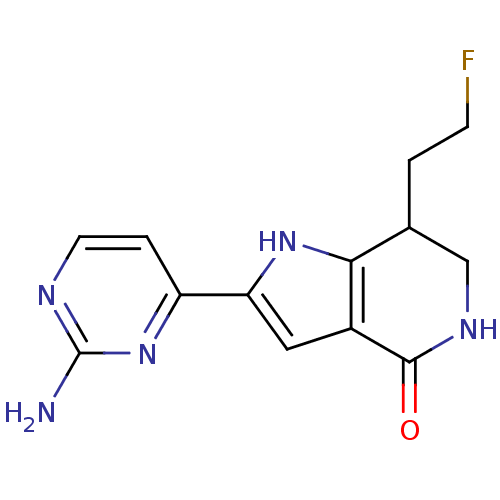
(2-(2-aminopyrimidin-4-yl)-7-(2-fluoroethyl)-1H,4H,...)Show InChI InChI=1S/C13H14FN5O/c14-3-1-7-6-17-12(20)8-5-10(18-11(7)8)9-2-4-16-13(15)19-9/h2,4-5,7,18H,1,3,6H2,(H,17,20)(H2,15,16,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.9 | 25 |
Nerviano Medical Sciences Srl
| Assay Description
The potency of the compound toward kinase activity was determined using a Dowex resin-based assay. The substrate was phosphorylated by kinase in the ... |
J Med Chem 52: 293-307 (2009)
Article DOI: 10.1021/jm800977q
BindingDB Entry DOI: 10.7270/Q27W69JX |
More data for this
Ligand-Target Pair | |
Cyclin-A1/Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50318090
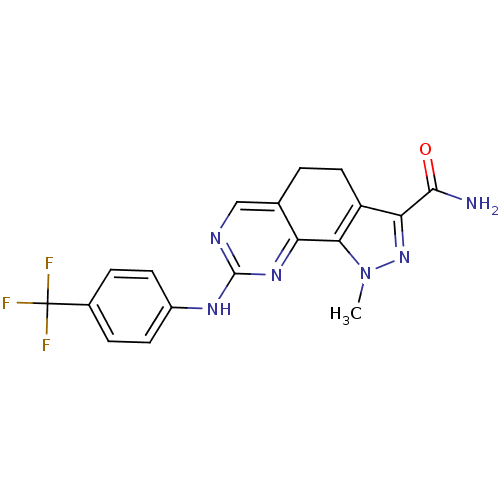
(1-Methyl-8-{[4-(trifluoromethyl)phenyl]amino}-4,5-...)Show SMILES Cn1nc(C(N)=O)c2CCc3cnc(Nc4ccc(cc4)C(F)(F)F)nc3-c12 Show InChI InChI=1S/C18H15F3N6O/c1-27-15-12(14(26-27)16(22)28)7-2-9-8-23-17(25-13(9)15)24-11-5-3-10(4-6-11)18(19,20)21/h3-6,8H,2,7H2,1H3,(H2,22,28)(H,23,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl
Curated by ChEMBL
| Assay Description
Inhibition of CDK2/Cyclin A assessed as [33P]gamma-ATP incorporation into substrate after 60 mins by gamma counting |
J Med Chem 53: 3532-51 (2010)
Article DOI: 10.1021/jm901713n
BindingDB Entry DOI: 10.7270/Q2RF5V5D |
More data for this
Ligand-Target Pair | |
Cyclin-A1/Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50318085

(1-Methyl-8-[(2-methylphenyl)amino]-4,5-dihydro-1H-...)Show InChI InChI=1S/C18H18N6O/c1-10-5-3-4-6-13(10)21-18-20-9-11-7-8-12-15(17(19)25)23-24(2)16(12)14(11)22-18/h3-6,9H,7-8H2,1-2H3,(H2,19,25)(H,20,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl
Curated by ChEMBL
| Assay Description
Inhibition of CDK2/Cyclin A assessed as [33P]gamma-ATP incorporation into substrate after 60 mins by gamma counting |
J Med Chem 53: 3532-51 (2010)
Article DOI: 10.1021/jm901713n
BindingDB Entry DOI: 10.7270/Q2RF5V5D |
More data for this
Ligand-Target Pair | |
Cyclin-A1/Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50307506
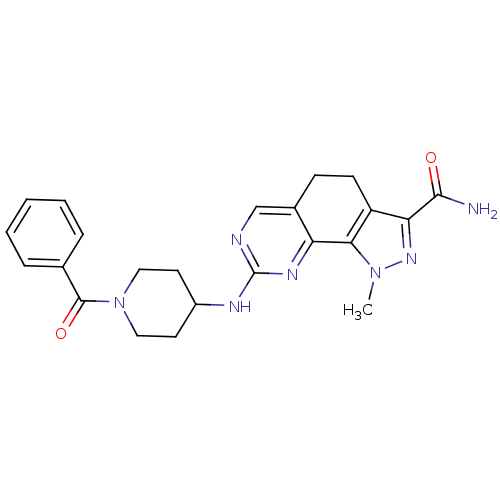
(1-Methyl-8-{[1-(phenylcarbonyl)piperidin-4-yl]amin...)Show SMILES Cn1nc(C(N)=O)c2CCc3cnc(NC4CCN(CC4)C(=O)c4ccccc4)nc3-c12 Show InChI InChI=1S/C23H25N7O2/c1-29-20-17(19(28-29)21(24)31)8-7-15-13-25-23(27-18(15)20)26-16-9-11-30(12-10-16)22(32)14-5-3-2-4-6-14/h2-6,13,16H,7-12H2,1H3,(H2,24,31)(H,25,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl
Curated by ChEMBL
| Assay Description
Inhibition of CDK2/Cyclin A |
J Med Chem 53: 2171-87 (2010)
Article DOI: 10.1021/jm901710h
BindingDB Entry DOI: 10.7270/Q2N879W9 |
More data for this
Ligand-Target Pair | |
Cell division cycle 7-related protein kinase [58-574]/Protein DBF4 homolog A
(Homo sapiens (Human)) | BDBM27348
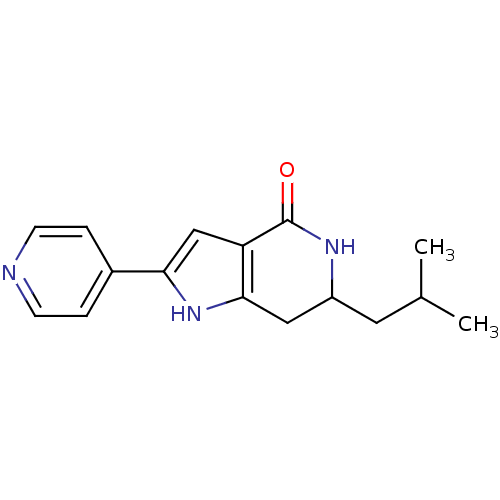
(6-(2-methylpropyl)-2-(pyridin-4-yl)-1H,4H,5H,6H,7H...)Show InChI InChI=1S/C16H19N3O/c1-10(2)7-12-8-15-13(16(20)18-12)9-14(19-15)11-3-5-17-6-4-11/h3-6,9-10,12,19H,7-8H2,1-2H3,(H,18,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.9 | 25 |
Nerviano Medical Sciences Srl
| Assay Description
The potency of the compound toward kinase activity was determined using a Dowex resin-based assay. The substrate was phosphorylated by kinase in the ... |
J Med Chem 52: 293-307 (2009)
Article DOI: 10.1021/jm800977q
BindingDB Entry DOI: 10.7270/Q27W69JX |
More data for this
Ligand-Target Pair | |
Cell division cycle 7-related protein kinase [58-574]/Protein DBF4 homolog A
(Homo sapiens (Human)) | BDBM27413
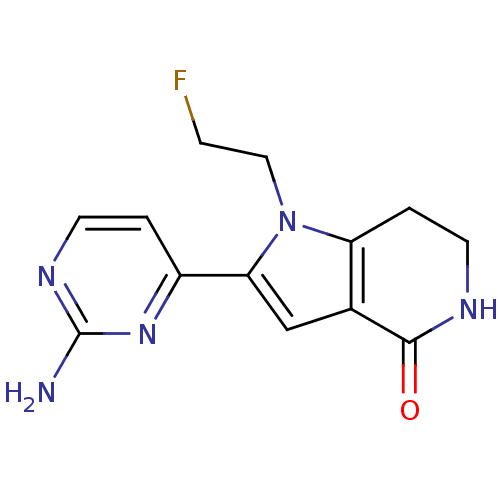
(2-(2-aminopyrimidin-4-yl)-1-(2-fluoroethyl)-1H,4H,...)Show InChI InChI=1S/C13H14FN5O/c14-3-6-19-10-2-5-16-12(20)8(10)7-11(19)9-1-4-17-13(15)18-9/h1,4,7H,2-3,5-6H2,(H,16,20)(H2,15,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl
| Assay Description
The potency of the compound toward kinase activity was determined using a Dowex resin-based assay. The substrate was phosphorylated by kinase in the ... |
J Med Chem 51: 487-501 (2008)
Article DOI: 10.1021/jm700956r
BindingDB Entry DOI: 10.7270/Q247485B |
More data for this
Ligand-Target Pair | |
Cyclin-A1/Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50318080

(1-methyl-8-(phenylamino)-4,5-dihydro-1H-pyrazolo[4...)Show InChI InChI=1S/C17H15N5O2/c1-22-15-12(14(21-22)16(23)24)8-7-10-9-18-17(20-13(10)15)19-11-5-3-2-4-6-11/h2-6,9H,7-8H2,1H3,(H,23,24)(H,18,19,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl
Curated by ChEMBL
| Assay Description
Inhibition of CDK2/Cyclin A assessed as [33P]gamma-ATP incorporation into substrate after 60 mins by gamma counting |
J Med Chem 53: 3532-51 (2010)
Article DOI: 10.1021/jm901713n
BindingDB Entry DOI: 10.7270/Q2RF5V5D |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cyclin-A1/Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50318091
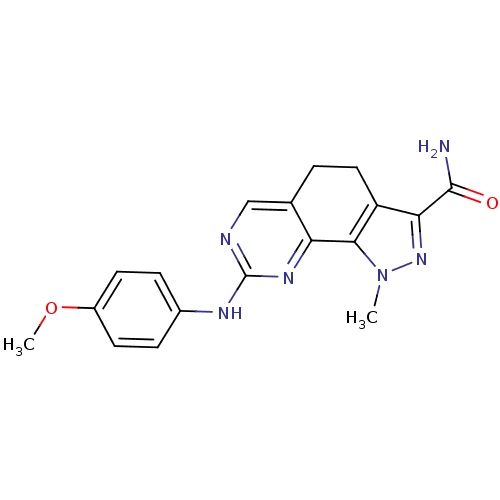
(8-[(4-Methoxyphenyl)amino]-1-methyl-4,5-dihydro-1H...)Show SMILES COc1ccc(Nc2ncc3CCc4c(nn(C)c4-c3n2)C(N)=O)cc1 Show InChI InChI=1S/C18H18N6O2/c1-24-16-13(15(23-24)17(19)25)8-3-10-9-20-18(22-14(10)16)21-11-4-6-12(26-2)7-5-11/h4-7,9H,3,8H2,1-2H3,(H2,19,25)(H,20,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl
Curated by ChEMBL
| Assay Description
Inhibition of CDK2/Cyclin A assessed as [33P]gamma-ATP incorporation into substrate after 60 mins by gamma counting |
J Med Chem 53: 3532-51 (2010)
Article DOI: 10.1021/jm901713n
BindingDB Entry DOI: 10.7270/Q2RF5V5D |
More data for this
Ligand-Target Pair | |
Cell division cycle 7-related protein kinase [58-574]/Protein DBF4 homolog A
(Homo sapiens (Human)) | BDBM27412

(2-(2-aminopyrimidin-4-yl)-1-(cyclopropylmethyl)-1H...)Show InChI InChI=1S/C15H17N5O/c16-15-18-5-3-11(19-15)13-7-10-12(4-6-17-14(10)21)20(13)8-9-1-2-9/h3,5,7,9H,1-2,4,6,8H2,(H,17,21)(H2,16,18,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl
| Assay Description
The potency of the compound toward kinase activity was determined using a Dowex resin-based assay. The substrate was phosphorylated by kinase in the ... |
J Med Chem 51: 487-501 (2008)
Article DOI: 10.1021/jm700956r
BindingDB Entry DOI: 10.7270/Q247485B |
More data for this
Ligand-Target Pair | |
Cell division cycle 7-related protein kinase
(Homo sapiens (Human)) | BDBM50279340

((Z)-2-[(Furan-2-ylmethyl)-amino]-5-[1-(1H-pyrrolo[...)Show SMILES O=C1N=C(NCc2ccco2)SC1=Cc1c[nH]c2ncccc12 |w:13.15,t:2| Show InChI InChI=1S/C16H12N4O2S/c21-15-13(7-10-8-18-14-12(10)4-1-5-17-14)23-16(20-15)19-9-11-3-2-6-22-11/h1-8H,9H2,(H,17,18)(H,19,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of Cdc7 (unknown origin)-mediated phosphorylation of Mcm2 |
J Med Chem 52: 4380-90 (2009)
Article DOI: 10.1021/jm900248g
BindingDB Entry DOI: 10.7270/Q2FF3S8C |
More data for this
Ligand-Target Pair | |
Cell division cycle 7-related protein kinase [58-574]/Protein DBF4 homolog A
(Homo sapiens (Human)) | BDBM27390

(2-(2-amino-5-bromopyrimidin-4-yl)-1H,4H,5H,6H,7H-p...)Show InChI InChI=1S/C11H10BrN5O/c12-6-4-15-11(13)17-9(6)8-3-5-7(16-8)1-2-14-10(5)18/h3-4,16H,1-2H2,(H,14,18)(H2,13,15,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.9 | 25 |
Nerviano Medical Sciences Srl
| Assay Description
The potency of the compound toward kinase activity was determined using a Dowex resin-based assay. The substrate was phosphorylated by kinase in the ... |
J Med Chem 51: 487-501 (2008)
Article DOI: 10.1021/jm700956r
BindingDB Entry DOI: 10.7270/Q247485B |
More data for this
Ligand-Target Pair | |
Cyclin-A1/Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50307545
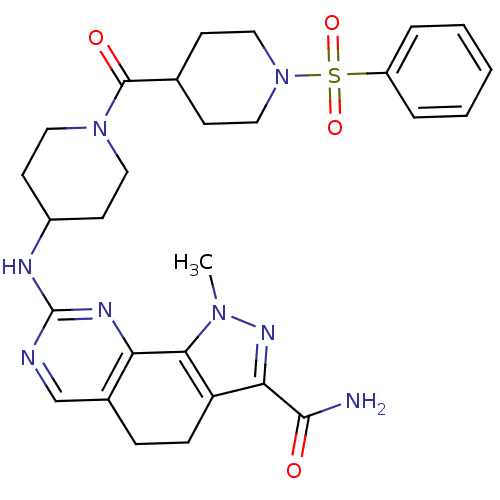
(1-Methyl-8-{[1-(phenylsulfonyl)piperidin-4-yl]amin...)Show SMILES Cn1nc(C(N)=O)c2CCc3cnc(NC4CCN(CC4)C(=O)C4CCN(CC4)S(=O)(=O)c4ccccc4)nc3-c12 Show InChI InChI=1S/C28H34N8O4S/c1-34-25-22(24(33-34)26(29)37)8-7-19-17-30-28(32-23(19)25)31-20-11-13-35(14-12-20)27(38)18-9-15-36(16-10-18)41(39,40)21-5-3-2-4-6-21/h2-6,17-18,20H,7-16H2,1H3,(H2,29,37)(H,30,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl
Curated by ChEMBL
| Assay Description
Inhibition of CDK2/Cyclin A |
J Med Chem 53: 2171-87 (2010)
Article DOI: 10.1021/jm901710h
BindingDB Entry DOI: 10.7270/Q2N879W9 |
More data for this
Ligand-Target Pair | |
Cell division cycle 7-related protein kinase [58-574]/Protein DBF4 homolog A
(Homo sapiens (Human)) | BDBM27368
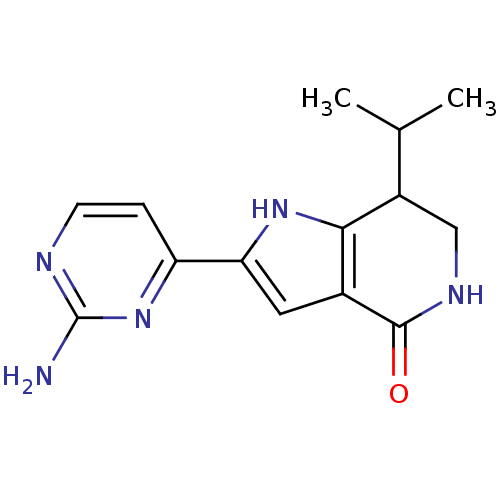
(2-(2-aminopyrimidin-4-yl)-7-(propan-2-yl)-1H,4H,5H...)Show InChI InChI=1S/C14H17N5O/c1-7(2)9-6-17-13(20)8-5-11(18-12(8)9)10-3-4-16-14(15)19-10/h3-5,7,9,18H,6H2,1-2H3,(H,17,20)(H2,15,16,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.9 | 25 |
Nerviano Medical Sciences Srl
| Assay Description
The potency of the compound toward kinase activity was determined using a Dowex resin-based assay. The substrate was phosphorylated by kinase in the ... |
J Med Chem 52: 293-307 (2009)
Article DOI: 10.1021/jm800977q
BindingDB Entry DOI: 10.7270/Q27W69JX |
More data for this
Ligand-Target Pair | |
Cell division cycle 7-related protein kinase [58-574]/Protein DBF4 homolog A
(Homo sapiens (Human)) | BDBM27377
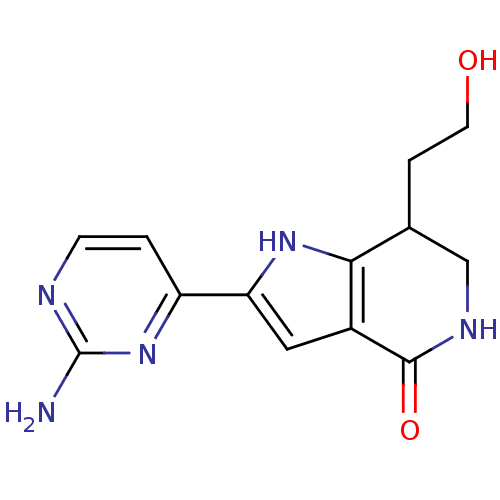
(2-(2-aminopyrimidin-4-yl)-7-(2-hydroxyethyl)-1H,4H...)Show InChI InChI=1S/C13H15N5O2/c14-13-15-3-1-9(18-13)10-5-8-11(17-10)7(2-4-19)6-16-12(8)20/h1,3,5,7,17,19H,2,4,6H2,(H,16,20)(H2,14,15,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl
| Assay Description
The potency of the compound toward kinase activity was determined using a Dowex resin-based assay. The substrate was phosphorylated by kinase in the ... |
J Med Chem 52: 293-307 (2009)
Article DOI: 10.1021/jm800977q
BindingDB Entry DOI: 10.7270/Q27W69JX |
More data for this
Ligand-Target Pair | |
Cyclin-A1/Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50307520
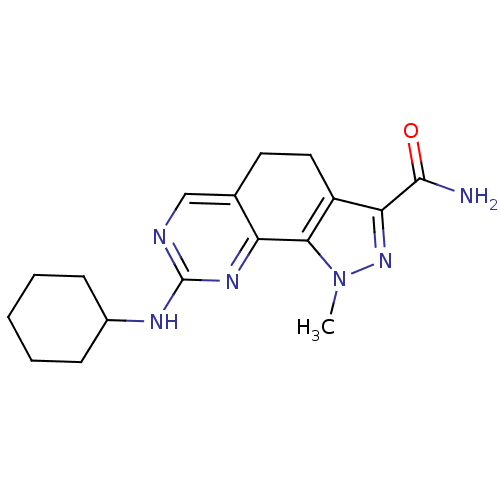
(8-(Cyclohexylamino)-1-methyl-4,5-dihydro-1H-pyrazo...)Show InChI InChI=1S/C17H22N6O/c1-23-15-12(14(22-23)16(18)24)8-7-10-9-19-17(21-13(10)15)20-11-5-3-2-4-6-11/h9,11H,2-8H2,1H3,(H2,18,24)(H,19,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl
Curated by ChEMBL
| Assay Description
Inhibition of CDK2/Cyclin A |
J Med Chem 53: 2171-87 (2010)
Article DOI: 10.1021/jm901710h
BindingDB Entry DOI: 10.7270/Q2N879W9 |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50327930

(3-(4-Morpholin-4-yl-benzoylamino)-1H-thieno[3,2-c]...)Show SMILES C[C@@H](NC(=O)c1cc2n[nH]c(NC(=O)c3ccc(cc3)N3CCOCC3)c2s1)c1ccccc1 |r| Show InChI InChI=1S/C25H25N5O3S/c1-16(17-5-3-2-4-6-17)26-25(32)21-15-20-22(34-21)23(29-28-20)27-24(31)18-7-9-19(10-8-18)30-11-13-33-14-12-30/h2-10,15-16H,11-14H2,1H3,(H,26,32)(H2,27,28,29,31)/t16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences-Oncology
Curated by ChEMBL
| Assay Description
Inhibition of AurA |
Bioorg Med Chem 18: 7113-20 (2010)
Article DOI: 10.1016/j.bmc.2010.07.048
BindingDB Entry DOI: 10.7270/Q2Q240GM |
More data for this
Ligand-Target Pair | |
Cell division cycle 7-related protein kinase [58-574]/Protein DBF4 homolog A
(Homo sapiens (Human)) | BDBM27379
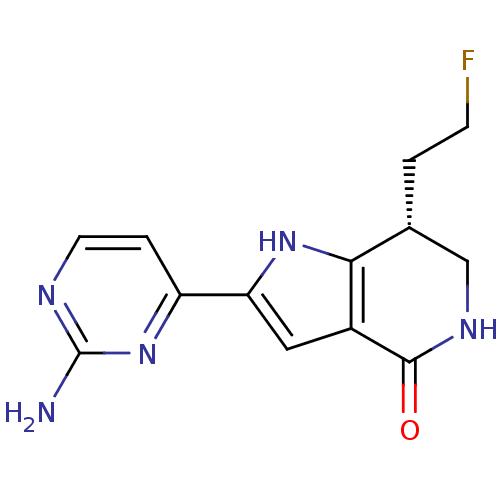
((7R)-2-(2-aminopyrimidin-4-yl)-7-(2-fluoroethyl)-1...)Show SMILES Nc1nccc(n1)-c1cc2c([nH]1)[C@H](CCF)CNC2=O |r| Show InChI InChI=1S/C13H14FN5O/c14-3-1-7-6-17-12(20)8-5-10(18-11(7)8)9-2-4-16-13(15)19-9/h2,4-5,7,18H,1,3,6H2,(H,17,20)(H2,15,16,19)/t7-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl
| Assay Description
The potency of the compound toward kinase activity was determined using a Dowex resin-based assay. The substrate was phosphorylated by kinase in the ... |
J Med Chem 52: 293-307 (2009)
Article DOI: 10.1021/jm800977q
BindingDB Entry DOI: 10.7270/Q27W69JX |
More data for this
Ligand-Target Pair | |
Cell division cycle 7-related protein kinase [58-574]/Protein DBF4 homolog A
(Homo sapiens (Human)) | BDBM27369
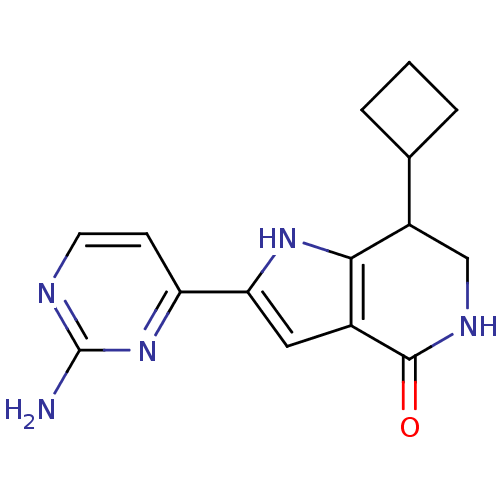
(2-(2-aminopyrimidin-4-yl)-7-cyclobutyl-1H,4H,5H,6H...)Show InChI InChI=1S/C15H17N5O/c16-15-17-5-4-11(20-15)12-6-9-13(19-12)10(7-18-14(9)21)8-2-1-3-8/h4-6,8,10,19H,1-3,7H2,(H,18,21)(H2,16,17,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.9 | 25 |
Nerviano Medical Sciences Srl
| Assay Description
The potency of the compound toward kinase activity was determined using a Dowex resin-based assay. The substrate was phosphorylated by kinase in the ... |
J Med Chem 52: 293-307 (2009)
Article DOI: 10.1021/jm800977q
BindingDB Entry DOI: 10.7270/Q27W69JX |
More data for this
Ligand-Target Pair | |
Cell division cycle 7-related protein kinase [58-574]/Protein DBF4 homolog A
(Homo sapiens (Human)) | BDBM27372
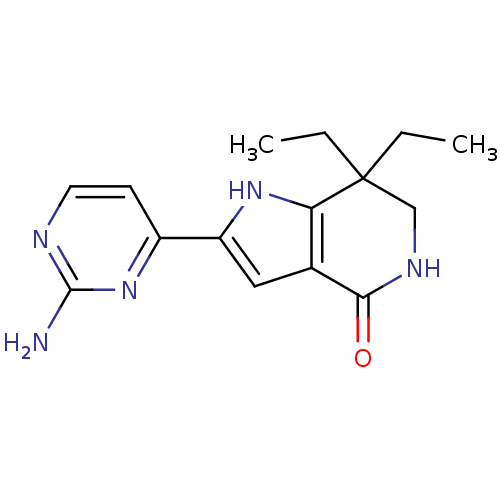
(2-(2-aminopyrimidin-4-yl)-7,7-diethyl-1H,4H,5H,6H,...)Show InChI InChI=1S/C15H19N5O/c1-3-15(4-2)8-18-13(21)9-7-11(19-12(9)15)10-5-6-17-14(16)20-10/h5-7,19H,3-4,8H2,1-2H3,(H,18,21)(H2,16,17,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.9 | 25 |
Nerviano Medical Sciences Srl
| Assay Description
The potency of the compound toward kinase activity was determined using a Dowex resin-based assay. The substrate was phosphorylated by kinase in the ... |
J Med Chem 52: 293-307 (2009)
Article DOI: 10.1021/jm800977q
BindingDB Entry DOI: 10.7270/Q27W69JX |
More data for this
Ligand-Target Pair | |
Cell division cycle 7-related protein kinase [58-574]/Protein DBF4 homolog A
(Homo sapiens (Human)) | BDBM27406
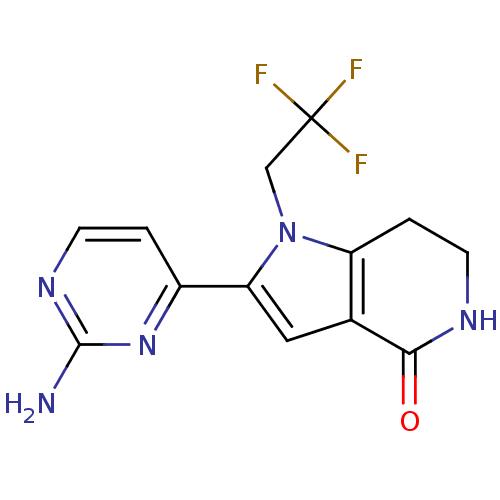
(2-(2-aminopyrimidin-4-yl)-1-(2,2,2-trifluoroethyl)...)Show InChI InChI=1S/C13H12F3N5O/c14-13(15,16)6-21-9-2-4-18-11(22)7(9)5-10(21)8-1-3-19-12(17)20-8/h1,3,5H,2,4,6H2,(H,18,22)(H2,17,19,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.9 | 25 |
Nerviano Medical Sciences Srl
| Assay Description
The potency of the compound toward kinase activity was determined using a Dowex resin-based assay. The substrate was phosphorylated by kinase in the ... |
J Med Chem 51: 487-501 (2008)
Article DOI: 10.1021/jm700956r
BindingDB Entry DOI: 10.7270/Q247485B |
More data for this
Ligand-Target Pair | |
Cell division cycle 7-related protein kinase
(Homo sapiens (Human)) | BDBM50329420
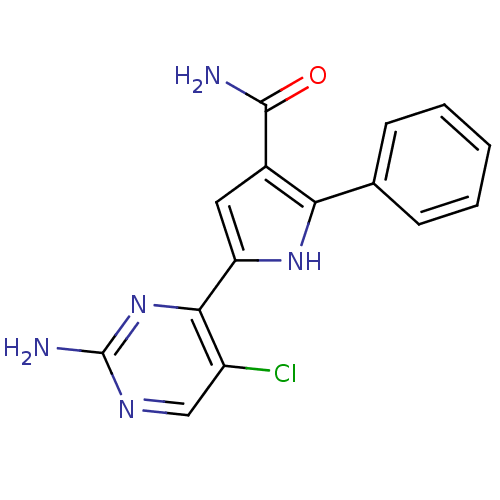
(5-(2-amino-5-chloropyrimidin-4-yl)-2-p-tolyl-1H-py...)Show InChI InChI=1S/C15H12ClN5O/c16-10-7-19-15(18)21-13(10)11-6-9(14(17)22)12(20-11)8-4-2-1-3-5-8/h1-7,20H,(H2,17,22)(H2,18,19,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl
Curated by ChEMBL
| Assay Description
Inhibition of Cdc7 |
J Med Chem 53: 7296-315 (2010)
Article DOI: 10.1021/jm100504d
BindingDB Entry DOI: 10.7270/Q24T6JM3 |
More data for this
Ligand-Target Pair | |
Cyclin-A1/Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50307509
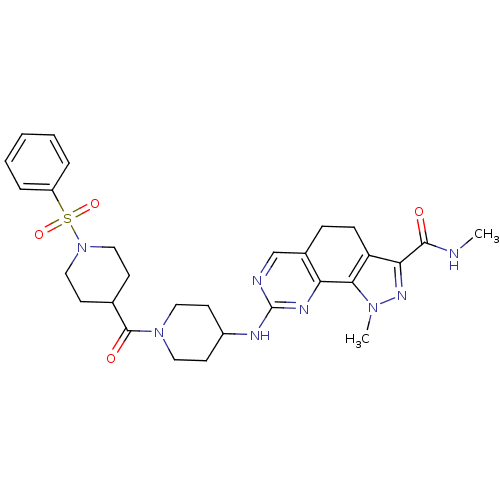
(CHEMBL592272 | N-1-Dimethyl-8-{[1-(phenylsulfonyl)...)Show SMILES CNC(=O)c1nn(C)c-2c1CCc1cnc(NC3CCN(CC3)C(=O)C3CCN(CC3)S(=O)(=O)c3ccccc3)nc-21 Show InChI InChI=1S/C29H36N8O4S/c1-30-27(38)25-23-9-8-20-18-31-29(33-24(20)26(23)35(2)34-25)32-21-12-14-36(15-13-21)28(39)19-10-16-37(17-11-19)42(40,41)22-6-4-3-5-7-22/h3-7,18-19,21H,8-17H2,1-2H3,(H,30,38)(H,31,32,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl
Curated by ChEMBL
| Assay Description
Inhibition of CDK2/Cyclin A |
J Med Chem 53: 2171-87 (2010)
Article DOI: 10.1021/jm901710h
BindingDB Entry DOI: 10.7270/Q2N879W9 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5 activator 1
(Homo sapiens (Human)) | BDBM50378657
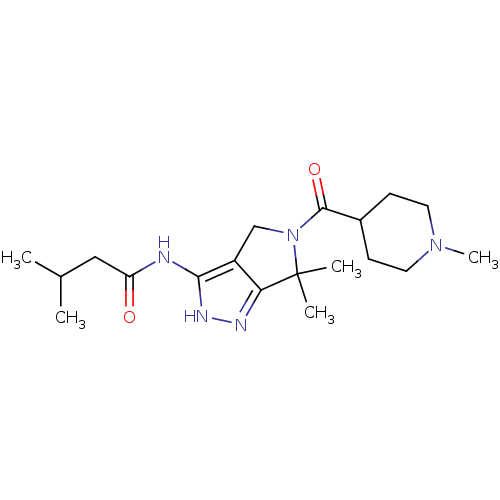
(CHEMBL1230607)Show SMILES CC(C)CC(=O)Nc1[nH]nc2c1CN(C(=O)C1CCN(C)CC1)C2(C)C Show InChI InChI=1S/C19H31N5O2/c1-12(2)10-15(25)20-17-14-11-24(19(3,4)16(14)21-22-17)18(26)13-6-8-23(5)9-7-13/h12-13H,6-11H2,1-5H3,(H2,20,21,22,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl
Curated by ChEMBL
| Assay Description
Inhibition of CDK5/p25 by scintillation proximity assay |
Bioorg Med Chem 18: 1844-53 (2010)
Article DOI: 10.1016/j.bmc.2010.01.042
BindingDB Entry DOI: 10.7270/Q21R6RG5 |
More data for this
Ligand-Target Pair | |
Cyclin-A1/Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50027427

(CHEMBL1744453)Show SMILES CN1CCC(CC1)C(=O)N1Cc2c(NC(=O)c3ccsc3)n[nH]c2C1(C)C Show InChI InChI=1S/C19H25N5O2S/c1-19(2)15-14(10-24(19)18(26)12-4-7-23(3)8-5-12)16(22-21-15)20-17(25)13-6-9-27-11-13/h6,9,11-12H,4-5,7-8,10H2,1-3H3,(H2,20,21,22,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl
Curated by ChEMBL
| Assay Description
Inhibition of human CDK2/cyclin A expressed in Escherichia coli BL21 by scintillation proximity assay |
Bioorg Med Chem 18: 1844-53 (2010)
Article DOI: 10.1016/j.bmc.2010.01.042
BindingDB Entry DOI: 10.7270/Q21R6RG5 |
More data for this
Ligand-Target Pair | |
Cyclin-A1/Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50318094
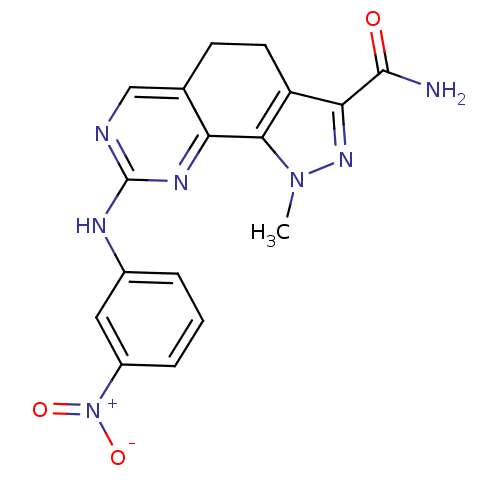
(1-Methyl-8-[(3-nitrophenyl)amino]-4,5-dihydro-1H-p...)Show SMILES Cn1nc(C(N)=O)c2CCc3cnc(Nc4cccc(c4)[N+]([O-])=O)nc3-c12 Show InChI InChI=1S/C17H15N7O3/c1-23-15-12(14(22-23)16(18)25)6-5-9-8-19-17(21-13(9)15)20-10-3-2-4-11(7-10)24(26)27/h2-4,7-8H,5-6H2,1H3,(H2,18,25)(H,19,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl
Curated by ChEMBL
| Assay Description
Inhibition of CDK2/Cyclin A assessed as [33P]gamma-ATP incorporation into substrate after 60 mins by gamma counting |
J Med Chem 53: 3532-51 (2010)
Article DOI: 10.1021/jm901713n
BindingDB Entry DOI: 10.7270/Q2RF5V5D |
More data for this
Ligand-Target Pair | |
Cyclin-A1/Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50318093
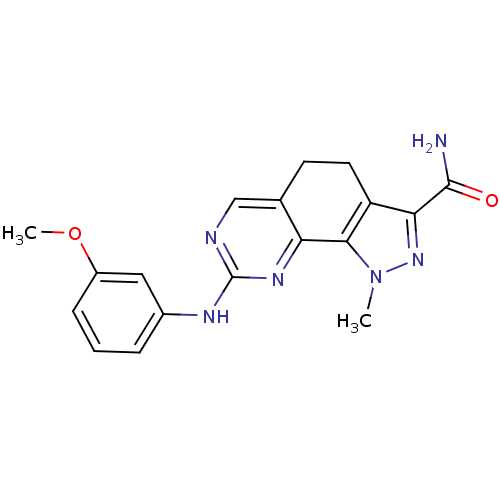
(8-[(3-Methoxyphenyl)amino]-1-methyl-4,5-dihydro-1H...)Show SMILES COc1cccc(Nc2ncc3CCc4c(nn(C)c4-c3n2)C(N)=O)c1 Show InChI InChI=1S/C18H18N6O2/c1-24-16-13(15(23-24)17(19)25)7-6-10-9-20-18(22-14(10)16)21-11-4-3-5-12(8-11)26-2/h3-5,8-9H,6-7H2,1-2H3,(H2,19,25)(H,20,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl
Curated by ChEMBL
| Assay Description
Inhibition of CDK2/Cyclin A assessed as [33P]gamma-ATP incorporation into substrate after 60 mins by gamma counting |
J Med Chem 53: 3532-51 (2010)
Article DOI: 10.1021/jm901713n
BindingDB Entry DOI: 10.7270/Q2RF5V5D |
More data for this
Ligand-Target Pair | |
Cyclin-A1/Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50318092
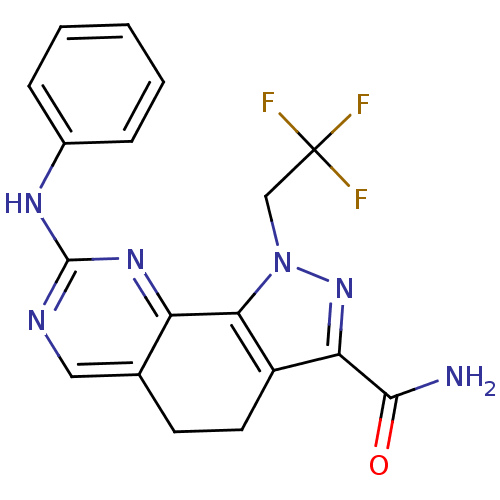
(8-(Phenylamino)-1-(2,2,2-trifluoroethyl)-4,5-dihyd...)Show SMILES NC(=O)c1nn(CC(F)(F)F)c-2c1CCc1cnc(Nc3ccccc3)nc-21 Show InChI InChI=1S/C18H15F3N6O/c19-18(20,21)9-27-15-12(14(26-27)16(22)28)7-6-10-8-23-17(25-13(10)15)24-11-4-2-1-3-5-11/h1-5,8H,6-7,9H2,(H2,22,28)(H,23,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl
Curated by ChEMBL
| Assay Description
Inhibition of CDK2/Cyclin A assessed as [33P]gamma-ATP incorporation into substrate after 60 mins by gamma counting |
J Med Chem 53: 3532-51 (2010)
Article DOI: 10.1021/jm901713n
BindingDB Entry DOI: 10.7270/Q2RF5V5D |
More data for this
Ligand-Target Pair | |
Cyclin-A1/Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50307538
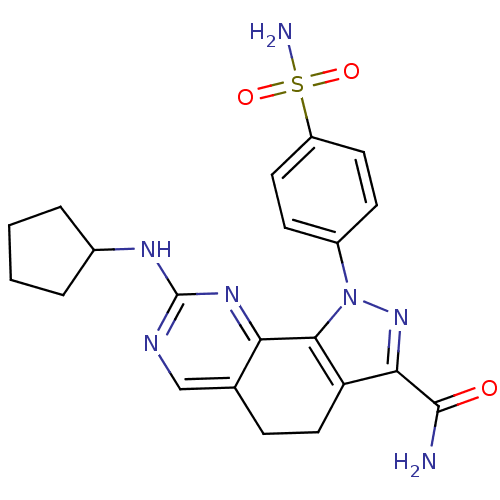
(8-(Cyclopentylamino)-1-(4-sulfamoylphenyl)-4,5-dih...)Show SMILES NC(=O)c1nn(c-2c1CCc1cnc(NC3CCCC3)nc-21)-c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C21H23N7O3S/c22-20(29)18-16-10-5-12-11-24-21(25-13-3-1-2-4-13)26-17(12)19(16)28(27-18)14-6-8-15(9-7-14)32(23,30)31/h6-9,11,13H,1-5,10H2,(H2,22,29)(H2,23,30,31)(H,24,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl
Curated by ChEMBL
| Assay Description
Inhibition of CDK2/Cyclin A |
J Med Chem 53: 2171-87 (2010)
Article DOI: 10.1021/jm901710h
BindingDB Entry DOI: 10.7270/Q2N879W9 |
More data for this
Ligand-Target Pair | |
Cyclin-A1/Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50307529
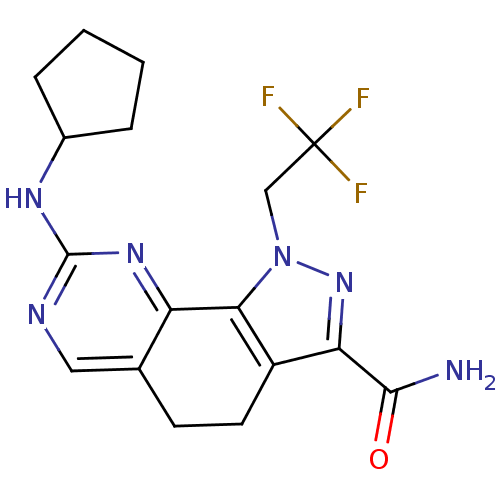
(8-(Cyclopentylamino)-1-(2,2,2-trifluoroethyl)-4,5-...)Show SMILES NC(=O)c1nn(CC(F)(F)F)c-2c1CCc1cnc(NC3CCCC3)nc-21 Show InChI InChI=1S/C17H19F3N6O/c18-17(19,20)8-26-14-11(13(25-26)15(21)27)6-5-9-7-22-16(24-12(9)14)23-10-3-1-2-4-10/h7,10H,1-6,8H2,(H2,21,27)(H,22,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl
Curated by ChEMBL
| Assay Description
Inhibition of CDK2/Cyclin A |
J Med Chem 53: 2171-87 (2010)
Article DOI: 10.1021/jm901710h
BindingDB Entry DOI: 10.7270/Q2N879W9 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase/G2/mitotic-specific cyclin- 1
(Homo sapiens (Human)) | BDBM50307535

(8-[(1-Acetylpiperidin-4-yl)amino]-1-methyl-4,5-dih...)Show SMILES CC(=O)N1CCC(CC1)Nc1ncc2CCc3c(nn(C)c3-c2n1)C(N)=O Show InChI InChI=1S/C18H23N7O2/c1-10(26)25-7-5-12(6-8-25)21-18-20-9-11-3-4-13-15(17(19)27)23-24(2)16(13)14(11)22-18/h9,12H,3-8H2,1-2H3,(H2,19,27)(H,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl
Curated by ChEMBL
| Assay Description
Inhibition of CDK1/Cyclin B |
J Med Chem 53: 2171-87 (2010)
Article DOI: 10.1021/jm901710h
BindingDB Entry DOI: 10.7270/Q2N879W9 |
More data for this
Ligand-Target Pair | |
Cell division cycle 7-related protein kinase [58-574]/Protein DBF4 homolog A
(Homo sapiens (Human)) | BDBM27347
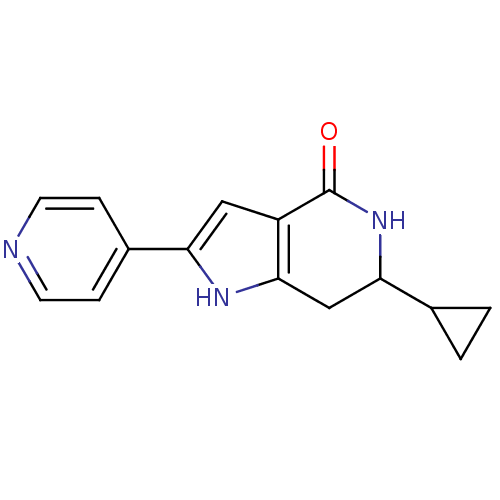
(6-cyclopropyl-2-(pyridin-4-yl)-1H,4H,5H,6H,7H-pyrr...)Show InChI InChI=1S/C15H15N3O/c19-15-11-7-12(10-3-5-16-6-4-10)17-14(11)8-13(18-15)9-1-2-9/h3-7,9,13,17H,1-2,8H2,(H,18,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.9 | 25 |
Nerviano Medical Sciences Srl
| Assay Description
The potency of the compound toward kinase activity was determined using a Dowex resin-based assay. The substrate was phosphorylated by kinase in the ... |
J Med Chem 52: 293-307 (2009)
Article DOI: 10.1021/jm800977q
BindingDB Entry DOI: 10.7270/Q27W69JX |
More data for this
Ligand-Target Pair | |
Cell division cycle 7-related protein kinase [58-574]/Protein DBF4 homolog A
(Homo sapiens (Human)) | BDBM27367
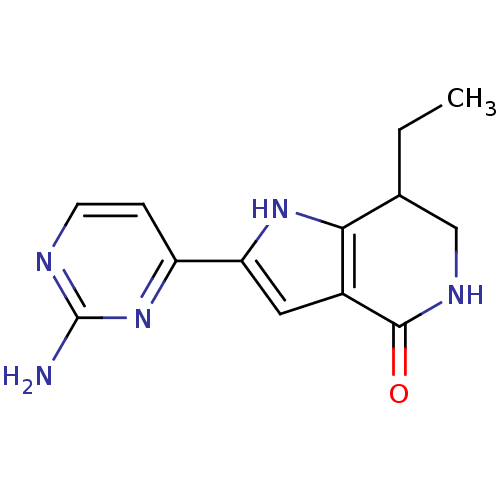
(2-(2-aminopyrimidin-4-yl)-7-ethyl-1H,4H,5H,6H,7H-p...)Show InChI InChI=1S/C13H15N5O/c1-2-7-6-16-12(19)8-5-10(17-11(7)8)9-3-4-15-13(14)18-9/h3-5,7,17H,2,6H2,1H3,(H,16,19)(H2,14,15,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.9 | 25 |
Nerviano Medical Sciences Srl
| Assay Description
The potency of the compound toward kinase activity was determined using a Dowex resin-based assay. The substrate was phosphorylated by kinase in the ... |
J Med Chem 52: 293-307 (2009)
Article DOI: 10.1021/jm800977q
BindingDB Entry DOI: 10.7270/Q27W69JX |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data