 Found 2268 hits with Last Name = 'walker' and Initial = 'n'
Found 2268 hits with Last Name = 'walker' and Initial = 'n' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
5'-nucleotidase
(Homo sapiens (Human)) | BDBM50527134

(CHEMBL4471306 | US20230295213, Compound a)Show SMILES C[C@H](Nc1cc(Cl)nc2n(ncc12)[C@@H]1O[C@H](COP(O)(=O)CP(O)(O)=O)[C@@H](O)[C@H]1O)c1ccccc1F |r| Show InChI InChI=1S/C20H24ClFN4O9P2/c1-10(11-4-2-3-5-13(11)22)24-14-6-16(21)25-19-12(14)7-23-26(19)20-18(28)17(27)15(35-20)8-34-37(32,33)9-36(29,30)31/h2-7,10,15,17-18,20,27-28H,8-9H2,1H3,(H,24,25)(H,32,33)(H2,29,30,31)/t10-,15+,17+,18+,20+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Competitive reversible inhibition of human C-terminal His6-tagged CD73 expressed in HEK293 cells using AMP as substrate preincubated with substrate f... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00525
BindingDB Entry DOI: 10.7270/Q29W0K29 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5'-nucleotidase
(Homo sapiens (Human)) | BDBM50527134

(CHEMBL4471306 | US20230295213, Compound a)Show SMILES C[C@H](Nc1cc(Cl)nc2n(ncc12)[C@@H]1O[C@H](COP(O)(=O)CP(O)(O)=O)[C@@H](O)[C@H]1O)c1ccccc1F |r| Show InChI InChI=1S/C20H24ClFN4O9P2/c1-10(11-4-2-3-5-13(11)22)24-14-6-16(21)25-19-12(14)7-23-26(19)20-18(28)17(27)15(35-20)8-34-37(32,33)9-36(29,30)31/h2-7,10,15,17-18,20,27-28H,8-9H2,1H3,(H,24,25)(H,32,33)(H2,29,30,31)/t10-,15+,17+,18+,20+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arcus Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human C-terminal His-tagged CD73 (27 to 549 residues) expressed in HEK293 cells using AMP as substrate preincubated for 1 h... |
J Med Chem 63: 3935-3955 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01713
BindingDB Entry DOI: 10.7270/Q2G1648T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50245852
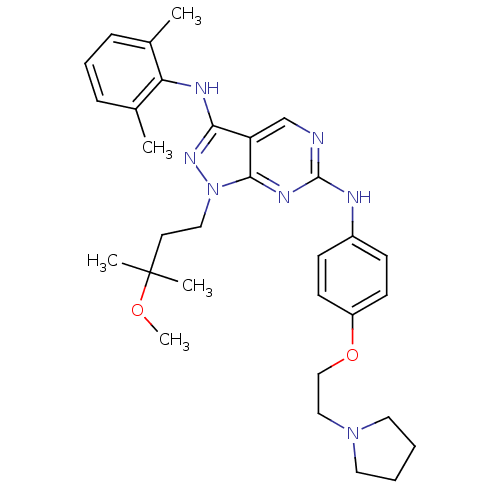
(CHEMBL458333 | N3-(2,6-dimethylphenyl)-1-(3-methox...)Show SMILES COC(C)(C)CCn1nc(Nc2c(C)cccc2C)c2cnc(Nc3ccc(OCCN4CCCC4)cc3)nc12 Show InChI InChI=1S/C31H41N7O2/c1-22-9-8-10-23(2)27(22)34-28-26-21-32-30(35-29(26)38(36-28)18-15-31(3,4)39-5)33-24-11-13-25(14-12-24)40-20-19-37-16-6-7-17-37/h8-14,21H,6-7,15-20H2,1-5H3,(H,34,36)(H,32,33,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LCK (unknown origin) |
Bioorg Med Chem Lett 18: 6352-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.092
BindingDB Entry DOI: 10.7270/Q2B56JKZ |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50050807
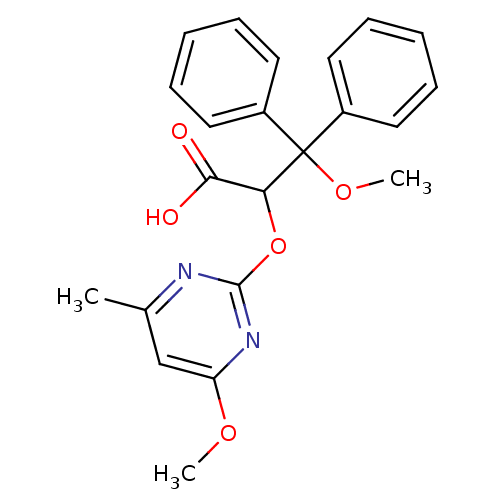
(3-Methoxy-2-(4-methoxy-6-methyl-pyrimidin-2-yloxy)...)Show SMILES COc1cc(C)nc(OC(C(O)=O)C(OC)(c2ccccc2)c2ccccc2)n1 Show InChI InChI=1S/C22H22N2O5/c1-15-14-18(27-2)24-21(23-15)29-19(20(25)26)22(28-3,16-10-6-4-7-11-16)17-12-8-5-9-13-17/h4-14,19H,1-3H3,(H,25,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BASF AG
Curated by ChEMBL
| Assay Description
Binding affinity of the compound towards human Endothelin A receptor by measuring its ability to displace [125I]-ET-1 from CHO cells |
J Med Chem 39: 2123-8 (1996)
Article DOI: 10.1021/jm960274q
BindingDB Entry DOI: 10.7270/Q2G44PDH |
More data for this
Ligand-Target Pair | |
Activated CDC42 kinase 1
(Homo sapiens (Human)) | BDBM50421256
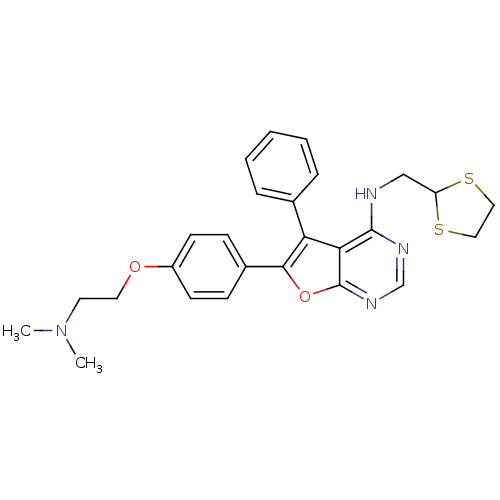
(CHEMBL2087874)Show SMILES CN(C)CCOc1ccc(cc1)-c1oc2ncnc(NCC3SCCS3)c2c1-c1ccccc1 Show InChI InChI=1S/C26H28N4O2S2/c1-30(2)12-13-31-20-10-8-19(9-11-20)24-22(18-6-4-3-5-7-18)23-25(28-17-29-26(23)32-24)27-16-21-33-14-15-34-21/h3-11,17,21H,12-16H2,1-2H3,(H,27,28,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged ACK1 expressed in baculovirus infected Hi5 cells assessed as inhibition of autophosphorylation after 2 hrs by EL... |
Bioorg Med Chem Lett 22: 6212-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.020
BindingDB Entry DOI: 10.7270/Q2VH5Q4X |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50245852

(CHEMBL458333 | N3-(2,6-dimethylphenyl)-1-(3-methox...)Show SMILES COC(C)(C)CCn1nc(Nc2c(C)cccc2C)c2cnc(Nc3ccc(OCCN4CCCC4)cc3)nc12 Show InChI InChI=1S/C31H41N7O2/c1-22-9-8-10-23(2)27(22)34-28-26-21-32-30(35-29(26)38(36-28)18-15-31(3,4)39-5)33-24-11-13-25(14-12-24)40-20-19-37-16-6-7-17-37/h8-14,21H,6-7,15-20H2,1-5H3,(H,34,36)(H,32,33,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BTK (unknown origin) |
Bioorg Med Chem Lett 18: 6352-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.092
BindingDB Entry DOI: 10.7270/Q2B56JKZ |
More data for this
Ligand-Target Pair | |
Activated CDC42 kinase 1
(Homo sapiens (Human)) | BDBM50421257
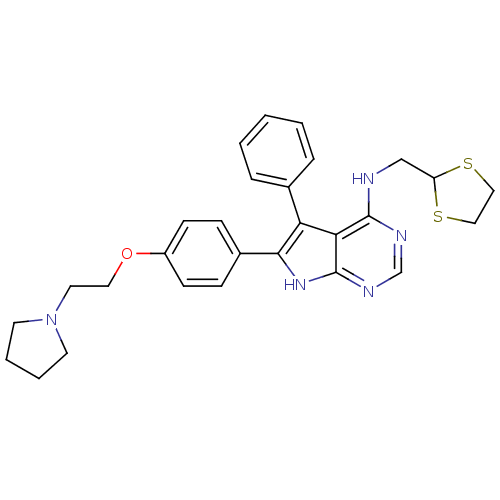
(CHEMBL2087875)Show SMILES C(CN1CCCC1)Oc1ccc(cc1)-c1[nH]c2ncnc(NCC3SCCS3)c2c1-c1ccccc1 Show InChI InChI=1S/C28H31N5OS2/c1-2-6-20(7-3-1)24-25-27(29-18-23-35-16-17-36-23)30-19-31-28(25)32-26(24)21-8-10-22(11-9-21)34-15-14-33-12-4-5-13-33/h1-3,6-11,19,23H,4-5,12-18H2,(H2,29,30,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged ACK1 expressed in baculovirus infected Hi5 cells assessed as inhibition of autophosphorylation after 2 hrs by EL... |
Bioorg Med Chem Lett 22: 6212-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.020
BindingDB Entry DOI: 10.7270/Q2VH5Q4X |
More data for this
Ligand-Target Pair | |
Activated CDC42 kinase 1
(Homo sapiens (Human)) | BDBM50421255
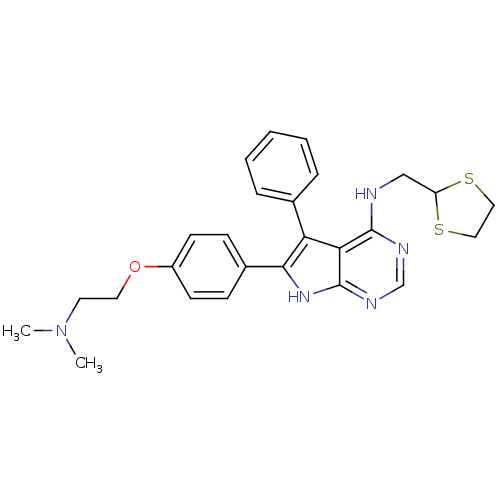
(CHEMBL2087873)Show SMILES CN(C)CCOc1ccc(cc1)-c1[nH]c2ncnc(NCC3SCCS3)c2c1-c1ccccc1 Show InChI InChI=1S/C26H29N5OS2/c1-31(2)12-13-32-20-10-8-19(9-11-20)24-22(18-6-4-3-5-7-18)23-25(28-17-29-26(23)30-24)27-16-21-33-14-15-34-21/h3-11,17,21H,12-16H2,1-2H3,(H2,27,28,29,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged ACK1 expressed in baculovirus infected Hi5 cells assessed as inhibition of autophosphorylation after 2 hrs by EL... |
Bioorg Med Chem Lett 22: 6212-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.020
BindingDB Entry DOI: 10.7270/Q2VH5Q4X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50499195

(CHEMBL3735504)Show SMILES CC(C)(C)C(=O)Nc1ccc2n([C@@H]3CC[C@H](CO)CC3)c(NC(=O)c3cccc(c3)C(F)(F)F)nc2c1 |r,wD:12.11,15.15,(-7.45,.88,;-6.39,1.5,;-7.46,2.11,;-6.4,2.74,;-5.05,.74,;-5.04,-.49,;-3.72,1.53,;-2.38,.77,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,;1.76,-1.24,;2.24,-2.7,;3.72,-3.12,;4.09,-4.62,;2.98,-5.68,;3.36,-7.18,;4.54,-7.52,;1.5,-5.26,;1.13,-3.76,;2.66,.02,;4.2,.04,;4.95,1.38,;4.32,2.44,;6.49,1.4,;7.24,2.75,;8.78,2.76,;9.57,1.44,;8.82,.1,;7.28,.08,;9.6,-1.23,;10.83,-1.22,;9,-2.3,;10.23,-2.29,;1.76,1.24,;.3,.77,;-1.03,1.55,)| Show InChI InChI=1S/C27H31F3N4O3/c1-26(2,3)24(37)31-19-9-12-22-21(14-19)32-25(34(22)20-10-7-16(15-35)8-11-20)33-23(36)17-5-4-6-18(13-17)27(28,29)30/h4-6,9,12-14,16,20,35H,7-8,10-11,15H2,1-3H3,(H,31,37)(H,32,33,36)/t16-,20+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length IRAK-4 (unknown origin) using the biotinylated substrate RRRVTSPARRS by chemiluminescent ELISA |
Bioorg Med Chem Lett 25: 5546-50 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.060
BindingDB Entry DOI: 10.7270/Q29W0JG8 |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50050793
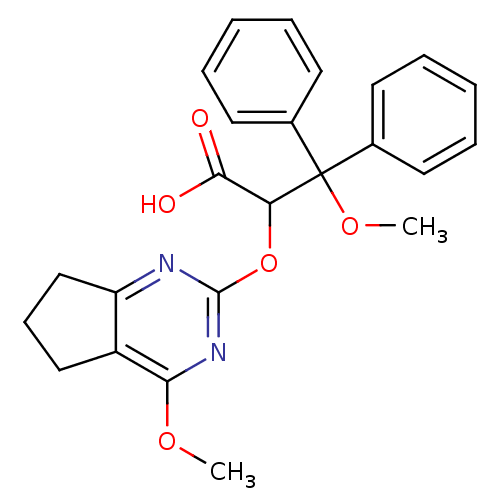
(3-Methoxy-2-(4-methoxy-6,7-dihydro-5H-cyclopentapy...)Show SMILES COc1nc(OC(C(O)=O)C(OC)(c2ccccc2)c2ccccc2)nc2CCCc12 Show InChI InChI=1S/C24H24N2O5/c1-29-21-18-14-9-15-19(18)25-23(26-21)31-20(22(27)28)24(30-2,16-10-5-3-6-11-16)17-12-7-4-8-13-17/h3-8,10-13,20H,9,14-15H2,1-2H3,(H,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BASF AG
Curated by ChEMBL
| Assay Description
Binding affinity of the compound towards human Endothelin A receptor by measuring its ability to displace [125I]-ET-1 from CHO cells |
J Med Chem 39: 2123-8 (1996)
Article DOI: 10.1021/jm960274q
BindingDB Entry DOI: 10.7270/Q2G44PDH |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50499203
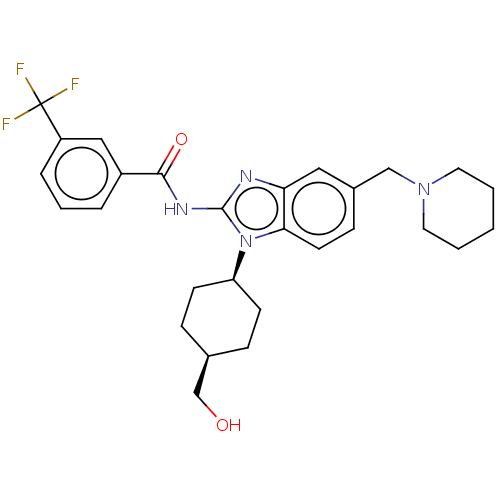
(CHEMBL3736036)Show SMILES OC[C@H]1CC[C@H](CC1)n1c(NC(=O)c2cccc(c2)C(F)(F)F)nc2cc(CN3CCCCC3)ccc12 |r,wD:5.8,2.1,(4.54,-7.52,;3.36,-7.18,;2.98,-5.68,;4.09,-4.62,;3.72,-3.12,;2.24,-2.7,;1.13,-3.76,;1.5,-5.26,;1.76,-1.24,;2.66,.02,;4.2,.04,;4.95,1.38,;4.32,2.44,;6.49,1.4,;7.24,2.75,;8.78,2.76,;9.57,1.44,;8.82,.1,;7.28,.08,;9.6,-1.23,;10.83,-1.22,;9,-2.3,;10.23,-2.29,;1.76,1.24,;.3,.77,;-1.03,1.55,;-2.38,.77,;-3.72,1.53,;-5.05,.74,;-6.39,1.5,;-7.71,.72,;-7.7,-.82,;-6.36,-1.58,;-5.03,-.8,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,)| Show InChI InChI=1S/C28H33F3N4O2/c29-28(30,31)22-6-4-5-21(16-22)26(37)33-27-32-24-15-20(17-34-13-2-1-3-14-34)9-12-25(24)35(27)23-10-7-19(18-36)8-11-23/h4-6,9,12,15-16,19,23,36H,1-3,7-8,10-11,13-14,17-18H2,(H,32,33,37)/t19-,23+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length IRAK-4 (unknown origin) using the biotinylated substrate RRRVTSPARRS by chemiluminescent ELISA |
Bioorg Med Chem Lett 25: 5546-50 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.060
BindingDB Entry DOI: 10.7270/Q29W0JG8 |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50499205
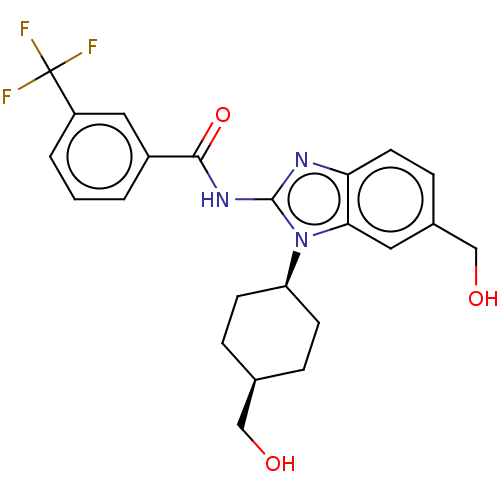
(CHEMBL3734814)Show SMILES OC[C@H]1CC[C@H](CC1)n1c(NC(=O)c2cccc(c2)C(F)(F)F)nc2ccc(CO)cc12 |r,wD:5.8,2.1,(4.54,-7.52,;3.36,-7.18,;2.98,-5.68,;4.09,-4.62,;3.72,-3.12,;2.24,-2.7,;1.13,-3.76,;1.5,-5.26,;1.76,-1.24,;2.66,.02,;4.2,.04,;4.95,1.38,;4.32,2.44,;6.49,1.4,;7.24,2.75,;8.78,2.76,;9.57,1.44,;8.81,.1,;7.28,.08,;9.6,-1.23,;10.83,-1.22,;9,-2.3,;10.23,-2.29,;1.76,1.24,;.3,.77,;-1.03,1.55,;-2.38,.77,;-2.38,-.77,;-3.72,-1.53,;-3.72,-2.76,;-1.03,-1.55,;.3,-.77,)| Show InChI InChI=1S/C23H24F3N3O3/c24-23(25,26)17-3-1-2-16(11-17)21(32)28-22-27-19-9-6-15(13-31)10-20(19)29(22)18-7-4-14(12-30)5-8-18/h1-3,6,9-11,14,18,30-31H,4-5,7-8,12-13H2,(H,27,28,32)/t14-,18+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length IRAK-4 (unknown origin) using the biotinylated substrate RRRVTSPARRS by chemiluminescent ELISA |
Bioorg Med Chem Lett 25: 5546-50 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.060
BindingDB Entry DOI: 10.7270/Q29W0JG8 |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50499208
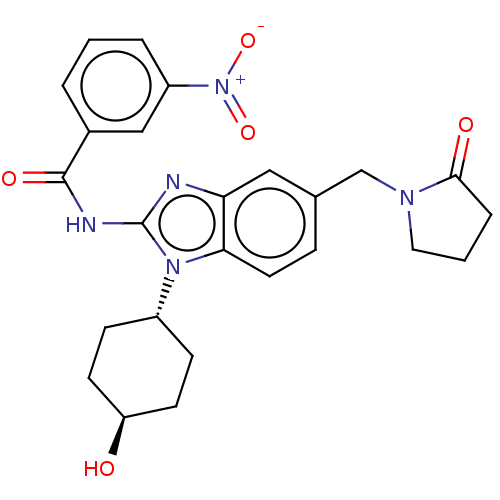
(CHEMBL3734872)Show SMILES O[C@H]1CC[C@@H](CC1)n1c(NC(=O)c2cccc(c2)[N+]([O-])=O)nc2cc(CN3CCCC3=O)ccc12 |r,wU:4.7,wD:1.0,(3.28,-6.88,;2.98,-5.68,;4.09,-4.62,;3.72,-3.12,;2.24,-2.7,;1.13,-3.76,;1.5,-5.26,;1.76,-1.24,;2.66,.02,;4.2,.04,;4.95,1.38,;4.32,2.44,;6.49,1.4,;7.24,2.75,;8.78,2.76,;9.57,1.44,;8.82,.1,;7.28,.08,;9.6,-1.23,;10.83,-1.22,;8.99,-2.3,;1.76,1.24,;.3,.77,;-1.03,1.55,;-2.38,.77,;-3.72,1.53,;-5.05,.74,;-5.17,-.78,;-6.68,-1.11,;-7.46,.21,;-6.44,1.37,;-6.7,2.57,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,)| Show InChI InChI=1S/C25H27N5O5/c31-20-9-7-18(8-10-20)29-22-11-6-16(15-28-12-2-5-23(28)32)13-21(22)26-25(29)27-24(33)17-3-1-4-19(14-17)30(34)35/h1,3-4,6,11,13-14,18,20,31H,2,5,7-10,12,15H2,(H,26,27,33)/t18-,20- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length IRAK-4 (unknown origin) using the biotinylated substrate RRRVTSPARRS by chemiluminescent ELISA |
Bioorg Med Chem Lett 25: 5546-50 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.060
BindingDB Entry DOI: 10.7270/Q29W0JG8 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM29882
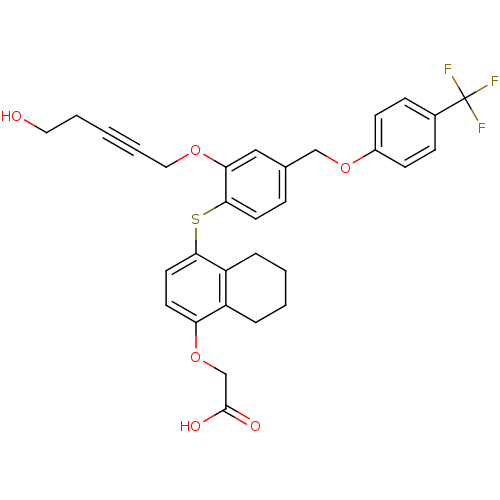
(alkynyl ether, 24)Show SMILES OCCC#CCOc1cc(COc2ccc(cc2)C(F)(F)F)ccc1Sc1ccc(OCC(O)=O)c2CCCCc12 Show InChI InChI=1S/C31H29F3O6S/c32-31(33,34)22-9-11-23(12-10-22)39-19-21-8-14-29(27(18-21)38-17-5-1-4-16-35)41-28-15-13-26(40-20-30(36)37)24-6-2-3-7-25(24)28/h8-15,18,35H,2-4,6-7,16-17,19-20H2,(H,36,37) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a |
Amgen
| Assay Description
The human PPARdelta ligand binding was directly measured using a scintillation proximity assay. Plates were read on a Packard TopCount. IC50 values f... |
Bioorg Med Chem Lett 19: 3550-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.151
BindingDB Entry DOI: 10.7270/Q2NK3CC0 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50602541
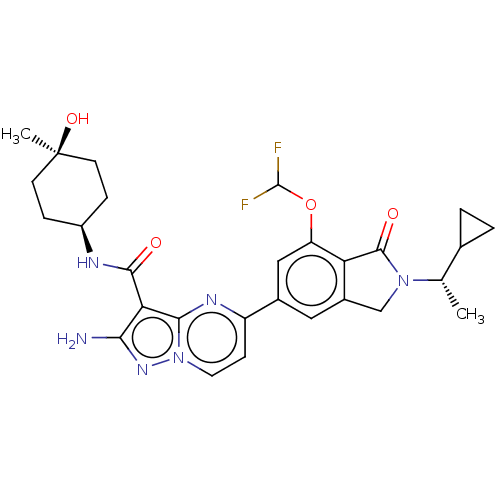
(CHEMBL5209268)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(OC(F)F)c2C1=O)-c1ccn2nc(N)c(C(=O)N[C@H]3CC[C@@](C)(O)CC3)c2n1 |r,wU:30.32,1.1,33.37,(7.66,2.89,;6.89,1.55,;7.66,.22,;7.66,-1.32,;8.99,-.55,;5.35,1.55,;4.44,2.8,;2.98,2.32,;1.65,3.09,;.31,2.32,;.31,.78,;1.65,.01,;1.65,-1.53,;.32,-2.3,;.32,-3.84,;-1.02,-1.53,;2.98,.78,;4.44,.31,;4.84,-1.18,;-1.02,3.09,;-1.02,4.63,;-2.34,5.39,;-3.67,4.63,;-5.14,5.1,;-6.04,3.86,;-7.58,3.86,;-5.14,2.61,;-5.91,1.28,;-7.45,1.28,;-5.14,-.06,;-5.91,-1.39,;-5.14,-2.72,;-5.91,-4.06,;-7.45,-4.06,;-8.22,-5.39,;-8.99,-4.06,;-8.22,-2.72,;-7.45,-1.39,;-3.67,3.09,;-2.34,2.33,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01153
BindingDB Entry DOI: 10.7270/Q2KH0SCH |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50050789
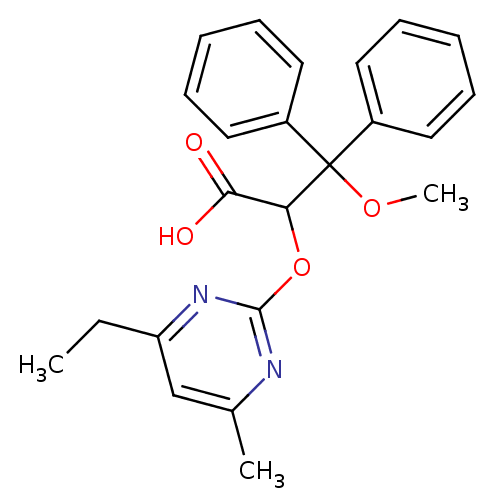
(2-(4-Ethyl-6-methyl-pyrimidin-2-yloxy)-3-methoxy-3...)Show SMILES CCc1cc(C)nc(OC(C(O)=O)C(OC)(c2ccccc2)c2ccccc2)n1 Show InChI InChI=1S/C23H24N2O4/c1-4-19-15-16(2)24-22(25-19)29-20(21(26)27)23(28-3,17-11-7-5-8-12-17)18-13-9-6-10-14-18/h5-15,20H,4H2,1-3H3,(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BASF AG
Curated by ChEMBL
| Assay Description
Binding affinity of the compound towards human Endothelin A receptor by measuring its ability to displace [125I]-ET-1 from CHO cells |
J Med Chem 39: 2123-8 (1996)
Article DOI: 10.1021/jm960274q
BindingDB Entry DOI: 10.7270/Q2G44PDH |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50499194

(CHEMBL3734854)Show SMILES OC[C@H]1CC[C@H](CC1)n1c(NC(=O)c2cccc(c2)[N+]([O-])=O)nc2cc(CN3CCCCC3)ccc12 |r,wD:5.8,2.1,(4.54,-7.52,;3.36,-7.18,;2.98,-5.68,;4.09,-4.62,;3.72,-3.12,;2.24,-2.7,;1.13,-3.76,;1.5,-5.26,;1.76,-1.24,;2.66,.02,;4.2,.04,;4.95,1.38,;4.32,2.44,;6.49,1.4,;7.24,2.75,;8.78,2.76,;9.57,1.44,;8.82,.1,;7.28,.08,;9.6,-1.23,;10.83,-1.22,;8.99,-2.3,;1.76,1.24,;.3,.77,;-1.03,1.55,;-2.38,.77,;-3.72,1.53,;-5.05,.74,;-6.39,1.5,;-7.71,.72,;-7.7,-.82,;-6.36,-1.58,;-5.03,-.8,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,)| Show InChI InChI=1S/C27H33N5O4/c33-18-19-7-10-22(11-8-19)31-25-12-9-20(17-30-13-2-1-3-14-30)15-24(25)28-27(31)29-26(34)21-5-4-6-23(16-21)32(35)36/h4-6,9,12,15-16,19,22,33H,1-3,7-8,10-11,13-14,17-18H2,(H,28,29,34)/t19-,22+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length IRAK-4 (unknown origin) using the biotinylated substrate RRRVTSPARRS by chemiluminescent ELISA |
Bioorg Med Chem Lett 25: 5546-50 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.060
BindingDB Entry DOI: 10.7270/Q29W0JG8 |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50050800
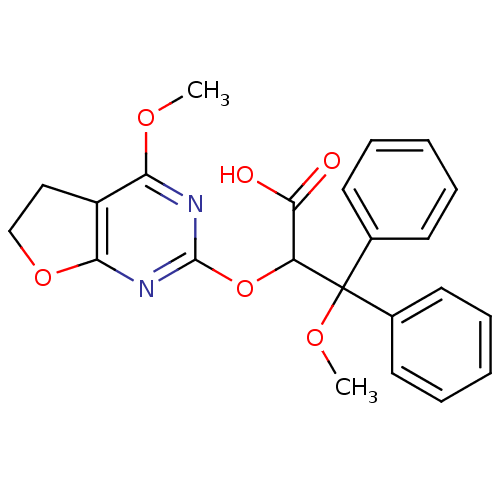
(3-Methoxy-2-(4-methoxy-5,6-dihydro-furo[2,3-d]pyri...)Show SMILES COc1nc(OC(C(O)=O)C(OC)(c2ccccc2)c2ccccc2)nc2OCCc12 Show InChI InChI=1S/C23H22N2O6/c1-28-19-17-13-14-30-20(17)25-22(24-19)31-18(21(26)27)23(29-2,15-9-5-3-6-10-15)16-11-7-4-8-12-16/h3-12,18H,13-14H2,1-2H3,(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BASF AG
Curated by ChEMBL
| Assay Description
Binding affinity of the compound towards human Endothelin A receptor by measuring its ability to displace [125I]-ET-1 from CHO cells |
J Med Chem 39: 2123-8 (1996)
Article DOI: 10.1021/jm960274q
BindingDB Entry DOI: 10.7270/Q2G44PDH |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50050796
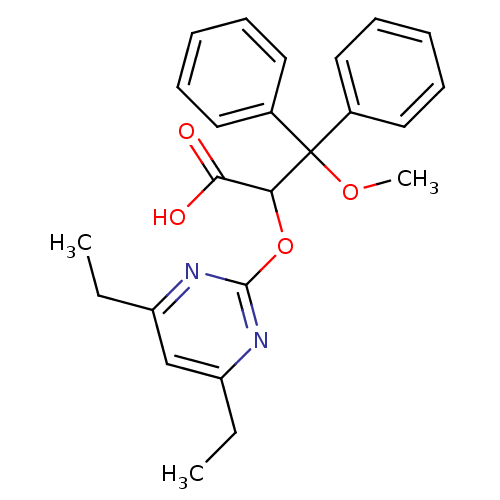
(2-(4,6-Diethyl-pyrimidin-2-yloxy)-3-methoxy-3,3-di...)Show SMILES CCc1cc(CC)nc(OC(C(O)=O)C(OC)(c2ccccc2)c2ccccc2)n1 Show InChI InChI=1S/C24H26N2O4/c1-4-19-16-20(5-2)26-23(25-19)30-21(22(27)28)24(29-3,17-12-8-6-9-13-17)18-14-10-7-11-15-18/h6-16,21H,4-5H2,1-3H3,(H,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BASF AG
Curated by ChEMBL
| Assay Description
Binding affinity of the compound towards human Endothelin A receptor by measuring its ability to displace [125I]-ET-1 from CHO cells |
J Med Chem 39: 2123-8 (1996)
Article DOI: 10.1021/jm960274q
BindingDB Entry DOI: 10.7270/Q2G44PDH |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50325241

(CHEMBL1223114)Show SMILES CC(C)[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(O)=O)C(=O)CON1[C@H]2CCCC[C@H]2CC1=O |r| Show InChI InChI=1S/C29H40N4O9/c1-17(2)26(32-29(40)41-15-19-9-5-4-6-10-19)28(39)30-18(3)27(38)31-21(14-25(36)37)23(34)16-42-33-22-12-8-7-11-20(22)13-24(33)35/h4-6,9-10,17-18,20-22,26H,7-8,11-16H2,1-3H3,(H,30,39)(H,31,38)(H,32,40)(H,36,37)/t18-,20-,21-,22-,26-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ICE |
Bioorg Med Chem Lett 20: 5089-94 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.031
BindingDB Entry DOI: 10.7270/Q2SQ90K1 |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50050806

(2-(4,6-Dimethyl-pyrimidin-2-yloxy)-3-methoxy-3,3-d...)Show SMILES COC(C(Oc1nc(C)cc(C)n1)C(O)=O)(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C22H22N2O4/c1-15-14-16(2)24-21(23-15)28-19(20(25)26)22(27-3,17-10-6-4-7-11-17)18-12-8-5-9-13-18/h4-14,19H,1-3H3,(H,25,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BASF AG
Curated by ChEMBL
| Assay Description
Binding affinity of the compound towards human Endothelin A receptor by measuring its ability to displace [125I]-ET-1 from CHO cells |
J Med Chem 39: 2123-8 (1996)
Article DOI: 10.1021/jm960274q
BindingDB Entry DOI: 10.7270/Q2G44PDH |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50325237

((5S,8S,11S)-11-(2-(3,3-diphenylpropanoyloxy)acetyl...)Show SMILES CC(C)[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(O)=O)C(=O)COC(=O)CC(c1ccccc1)c1ccccc1 |r| Show InChI InChI=1S/C36H41N3O9/c1-23(2)33(39-36(46)48-21-25-13-7-4-8-14-25)35(45)37-24(3)34(44)38-29(20-31(41)42)30(40)22-47-32(43)19-28(26-15-9-5-10-16-26)27-17-11-6-12-18-27/h4-18,23-24,28-29,33H,19-22H2,1-3H3,(H,37,45)(H,38,44)(H,39,46)(H,41,42)/t24-,29-,33-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ICE |
Bioorg Med Chem Lett 20: 5089-94 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.031
BindingDB Entry DOI: 10.7270/Q2SQ90K1 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50248569

((1-(((R)-3-methyl-4-(4-((S)-1,1,1-trifluoro-2-hydr...)Show SMILES C[C@@H]1CN(CC2(CC2)C(N)=O)CCN1S(=O)(=O)c1ccc(cc1)[C@](C)(O)C(F)(F)F |r| Show InChI InChI=1S/C19H26F3N3O4S/c1-13-11-24(12-18(7-8-18)16(23)26)9-10-25(13)30(28,29)15-5-3-14(4-6-15)17(2,27)19(20,21)22/h3-6,13,27H,7-12H2,1-2H3,(H2,23,26)/t13-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 using variable cofactor NADPH concentration by Lineweaver burk plot |
Bioorg Med Chem 16: 8922-31 (2008)
Article DOI: 10.1016/j.bmc.2008.08.065
BindingDB Entry DOI: 10.7270/Q22F7N8T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50499197
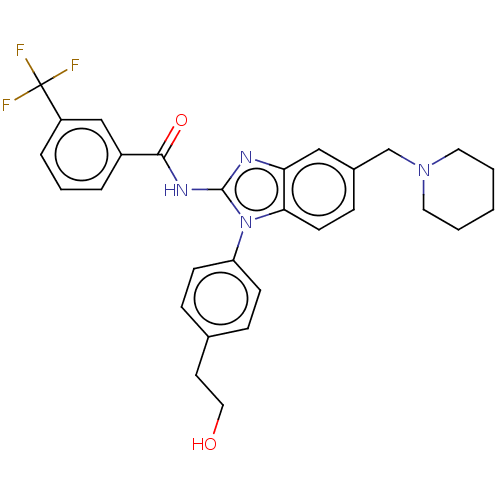
(CHEMBL3736465)Show SMILES OCCc1ccc(cc1)-n1c(NC(=O)c2cccc(c2)C(F)(F)F)nc2cc(CN3CCCCC3)ccc12 Show InChI InChI=1S/C29H29F3N4O2/c30-29(31,32)23-6-4-5-22(18-23)27(38)34-28-33-25-17-21(19-35-14-2-1-3-15-35)9-12-26(25)36(28)24-10-7-20(8-11-24)13-16-37/h4-12,17-18,37H,1-3,13-16,19H2,(H,33,34,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length IRAK-4 (unknown origin) using the biotinylated substrate RRRVTSPARRS by chemiluminescent ELISA |
Bioorg Med Chem Lett 25: 5546-50 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.060
BindingDB Entry DOI: 10.7270/Q29W0JG8 |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50499206

(CHEMBL3735949)Show SMILES OC[C@H]1CC[C@H](CC1)n1c(NC(=O)c2cccc(c2)[N+]([O-])=O)nc2cc(F)ccc12 |r,wD:5.8,2.1,(4.54,-7.52,;3.36,-7.18,;2.98,-5.68,;4.09,-4.62,;3.72,-3.12,;2.24,-2.7,;1.13,-3.76,;1.5,-5.26,;1.76,-1.24,;2.66,.02,;4.2,.04,;4.95,1.38,;4.32,2.44,;6.49,1.4,;7.24,2.75,;8.78,2.76,;9.57,1.44,;8.82,.1,;7.28,.08,;9.6,-1.23,;10.83,-1.22,;8.99,-2.3,;1.76,1.24,;.3,.77,;-1.03,1.55,;-2.38,.77,;-3.45,1.38,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,)| Show InChI InChI=1S/C21H21FN4O4/c22-15-6-9-19-18(11-15)23-21(25(19)16-7-4-13(12-27)5-8-16)24-20(28)14-2-1-3-17(10-14)26(29)30/h1-3,6,9-11,13,16,27H,4-5,7-8,12H2,(H,23,24,28)/t13-,16+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length IRAK-4 (unknown origin) using the biotinylated substrate RRRVTSPARRS by chemiluminescent ELISA |
Bioorg Med Chem Lett 25: 5546-50 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.060
BindingDB Entry DOI: 10.7270/Q29W0JG8 |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50325240
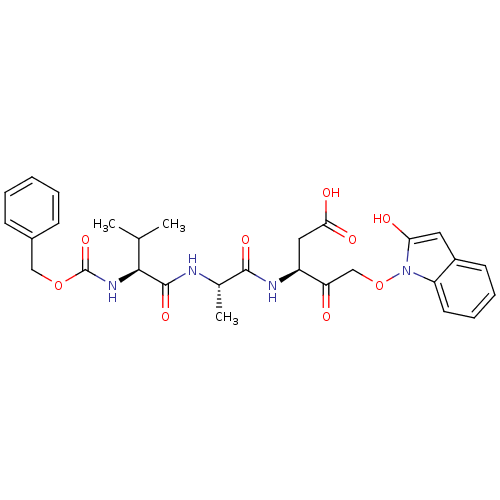
(CHEMBL1223113)Show SMILES CC(C)[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(O)=O)C(=O)COn1c(O)cc2ccccc12 |r| Show InChI InChI=1S/C29H34N4O9/c1-17(2)26(32-29(40)41-15-19-9-5-4-6-10-19)28(39)30-18(3)27(38)31-21(14-25(36)37)23(34)16-42-33-22-12-8-7-11-20(22)13-24(33)35/h4-13,17-18,21,26,35H,14-16H2,1-3H3,(H,30,39)(H,31,38)(H,32,40)(H,36,37)/t18-,21-,26-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ICE |
Bioorg Med Chem Lett 20: 5089-94 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.031
BindingDB Entry DOI: 10.7270/Q2SQ90K1 |
More data for this
Ligand-Target Pair | |
Activated CDC42 kinase 1
(Homo sapiens (Human)) | BDBM50421286
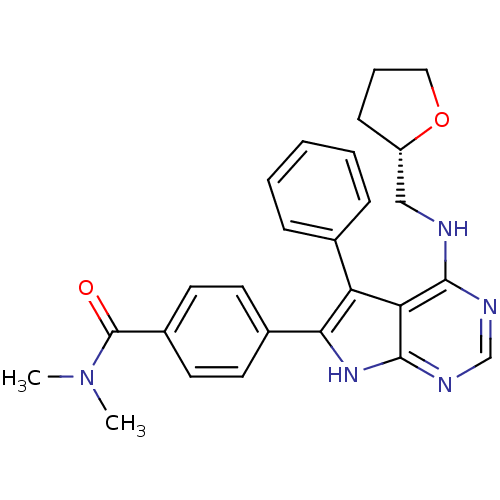
(CHEMBL2087662)Show SMILES CN(C)C(=O)c1ccc(cc1)-c1[nH]c2ncnc(NC[C@@H]3CCCO3)c2c1-c1ccccc1 |r| Show InChI InChI=1S/C26H27N5O2/c1-31(2)26(32)19-12-10-18(11-13-19)23-21(17-7-4-3-5-8-17)22-24(28-16-29-25(22)30-23)27-15-20-9-6-14-33-20/h3-5,7-8,10-13,16,20H,6,9,14-15H2,1-2H3,(H2,27,28,29,30)/t20-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged ACK1 expressed in baculovirus infected Hi5 cells assessed as inhibition of autophosphorylation after 2 hrs by EL... |
Bioorg Med Chem Lett 22: 6212-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.020
BindingDB Entry DOI: 10.7270/Q2VH5Q4X |
More data for this
Ligand-Target Pair | |
Activated CDC42 kinase 1
(Homo sapiens (Human)) | BDBM50421276
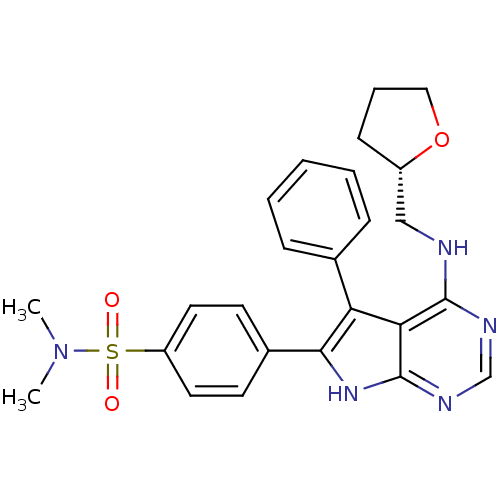
(CHEMBL2087652)Show SMILES CN(C)S(=O)(=O)c1ccc(cc1)-c1[nH]c2ncnc(NC[C@@H]3CCCO3)c2c1-c1ccccc1 |r| Show InChI InChI=1S/C25H27N5O3S/c1-30(2)34(31,32)20-12-10-18(11-13-20)23-21(17-7-4-3-5-8-17)22-24(27-16-28-25(22)29-23)26-15-19-9-6-14-33-19/h3-5,7-8,10-13,16,19H,6,9,14-15H2,1-2H3,(H2,26,27,28,29)/t19-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged ACK1 expressed in baculovirus infected Hi5 cells assessed as inhibition of autophosphorylation after 2 hrs by EL... |
Bioorg Med Chem Lett 22: 6212-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.020
BindingDB Entry DOI: 10.7270/Q2VH5Q4X |
More data for this
Ligand-Target Pair | |
Activated CDC42 kinase 1
(Homo sapiens (Human)) | BDBM50421278
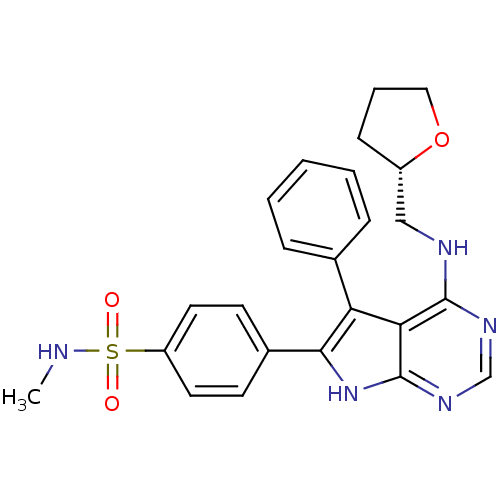
(CHEMBL2087654)Show SMILES CNS(=O)(=O)c1ccc(cc1)-c1[nH]c2ncnc(NC[C@@H]3CCCO3)c2c1-c1ccccc1 |r| Show InChI InChI=1S/C24H25N5O3S/c1-25-33(30,31)19-11-9-17(10-12-19)22-20(16-6-3-2-4-7-16)21-23(27-15-28-24(21)29-22)26-14-18-8-5-13-32-18/h2-4,6-7,9-12,15,18,25H,5,8,13-14H2,1H3,(H2,26,27,28,29)/t18-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged ACK1 expressed in baculovirus infected Hi5 cells assessed as inhibition of autophosphorylation after 2 hrs by EL... |
Bioorg Med Chem Lett 22: 6212-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.020
BindingDB Entry DOI: 10.7270/Q2VH5Q4X |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM29881

(alkynyl ether, 23)Show SMILES OCC#CCOc1cc(COc2ccc(cc2)C(F)(F)F)ccc1Sc1ccc(OCC(O)=O)c2CCCCc12 Show InChI InChI=1S/C30H27F3O6S/c31-30(32,33)21-8-10-22(11-9-21)38-18-20-7-13-28(26(17-20)37-16-4-3-15-34)40-27-14-12-25(39-19-29(35)36)23-5-1-2-6-24(23)27/h7-14,17,34H,1-2,5-6,15-16,18-19H2,(H,35,36) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | 220 | n/a | n/a | n/a | n/a |
Amgen
| Assay Description
The human PPARdelta ligand binding was directly measured using a scintillation proximity assay. Plates were read on a Packard TopCount. IC50 values f... |
Bioorg Med Chem Lett 19: 3550-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.151
BindingDB Entry DOI: 10.7270/Q2NK3CC0 |
More data for this
Ligand-Target Pair | |
Activated CDC42 kinase 1
(Homo sapiens (Human)) | BDBM50246214
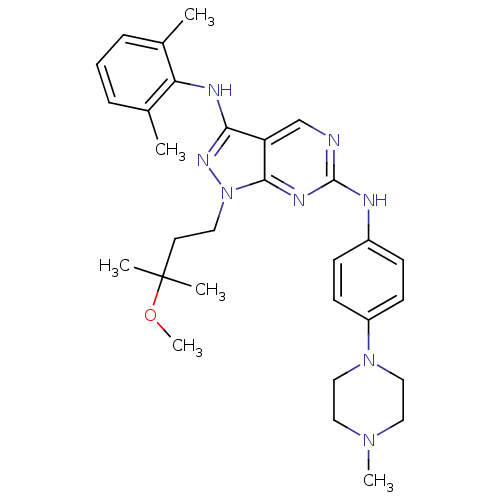
(CHEMBL487897 | N3-(2,6-dimethylphenyl)-1-(3-methox...)Show SMILES COC(C)(C)CCn1nc(Nc2c(C)cccc2C)c2cnc(Nc3ccc(cc3)N3CCN(C)CC3)nc12 Show InChI InChI=1S/C30H40N8O/c1-21-8-7-9-22(2)26(21)33-27-25-20-31-29(34-28(25)38(35-27)15-14-30(3,4)39-6)32-23-10-12-24(13-11-23)37-18-16-36(5)17-19-37/h7-13,20H,14-19H2,1-6H3,(H,33,35)(H,31,32,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of histidine-tagged ACK1 (amino acids 117 to 489) (unknown origin) expressed in SF9 cells |
Bioorg Med Chem Lett 18: 6352-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.092
BindingDB Entry DOI: 10.7270/Q2B56JKZ |
More data for this
Ligand-Target Pair | |
Activated CDC42 kinase 1
(Homo sapiens (Human)) | BDBM50245854

(CHEMBL504331 | N3-(2,6-dichlorophenyl)-N6-(3-fluor...)Show SMILES COC(C)(C)CCn1nc(Nc2c(Cl)cccc2Cl)c2cnc(Nc3ccc(OCCN4CCCC4)c(F)c3)nc12 Show InChI InChI=1S/C29H34Cl2FN7O2/c1-29(2,40-3)11-14-39-27-20(26(37-39)35-25-21(30)7-6-8-22(25)31)18-33-28(36-27)34-19-9-10-24(23(32)17-19)41-16-15-38-12-4-5-13-38/h6-10,17-18H,4-5,11-16H2,1-3H3,(H,35,37)(H,33,34,36) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of histidine-tagged ACK1 (amino acids 117 to 489) (unknown origin) expressed in SF9 cells |
Bioorg Med Chem Lett 18: 6352-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.092
BindingDB Entry DOI: 10.7270/Q2B56JKZ |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50325238

((5S,8S,11S)-11-(2-(2-benzyl-3-phenylpropanoyloxy)a...)Show SMILES CC(C)[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(O)=O)C(=O)COC(=O)C(Cc1ccccc1)Cc1ccccc1 |r| Show InChI InChI=1S/C37H43N3O9/c1-24(2)33(40-37(47)49-22-28-17-11-6-12-18-28)35(45)38-25(3)34(44)39-30(21-32(42)43)31(41)23-48-36(46)29(19-26-13-7-4-8-14-26)20-27-15-9-5-10-16-27/h4-18,24-25,29-30,33H,19-23H2,1-3H3,(H,38,45)(H,39,44)(H,40,47)(H,42,43)/t25-,30-,33-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ICE |
Bioorg Med Chem Lett 20: 5089-94 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.031
BindingDB Entry DOI: 10.7270/Q2SQ90K1 |
More data for this
Ligand-Target Pair | |
Activated CDC42 kinase 1
(Homo sapiens (Human)) | BDBM50246162
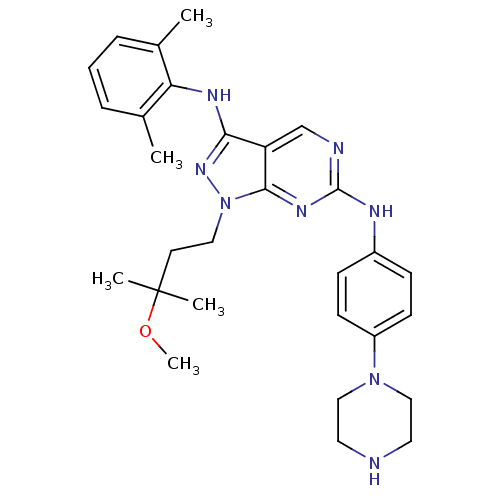
(CHEMBL472392 | N3-(2,6-dimethylphenyl)-1-(3-methox...)Show SMILES COC(C)(C)CCn1nc(Nc2c(C)cccc2C)c2cnc(Nc3ccc(cc3)N3CCNCC3)nc12 Show InChI InChI=1S/C29H38N8O/c1-20-7-6-8-21(2)25(20)33-26-24-19-31-28(34-27(24)37(35-26)16-13-29(3,4)38-5)32-22-9-11-23(12-10-22)36-17-14-30-15-18-36/h6-12,19,30H,13-18H2,1-5H3,(H,33,35)(H,31,32,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of histidine-tagged ACK1 (amino acids 117 to 489) (unknown origin) expressed in SF9 cells |
Bioorg Med Chem Lett 18: 6352-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.092
BindingDB Entry DOI: 10.7270/Q2B56JKZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50248569

((1-(((R)-3-methyl-4-(4-((S)-1,1,1-trifluoro-2-hydr...)Show SMILES C[C@@H]1CN(CC2(CC2)C(N)=O)CCN1S(=O)(=O)c1ccc(cc1)[C@](C)(O)C(F)(F)F |r| Show InChI InChI=1S/C19H26F3N3O4S/c1-13-11-24(12-18(7-8-18)16(23)26)9-10-25(13)30(28,29)15-5-3-14(4-6-15)17(2,27)19(20,21)22/h3-6,13,27H,7-12H2,1-2H3,(H2,23,26)/t13-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 using variable substrate cortisol concentration by Lineweaver burk plot |
Bioorg Med Chem 16: 8922-31 (2008)
Article DOI: 10.1016/j.bmc.2008.08.065
BindingDB Entry DOI: 10.7270/Q22F7N8T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50499198
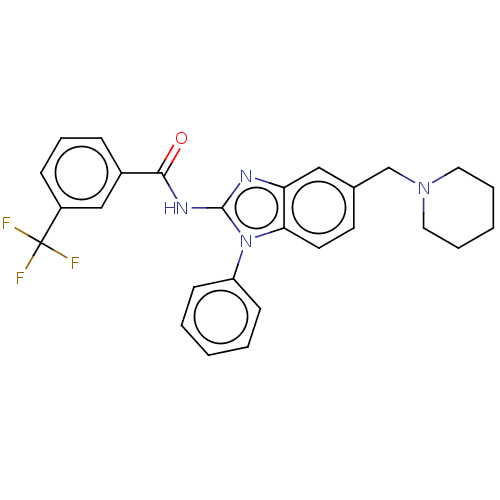
(CHEMBL3736278)Show SMILES FC(F)(F)c1cccc(c1)C(=O)Nc1nc2cc(CN3CCCCC3)ccc2n1-c1ccccc1 Show InChI InChI=1S/C27H25F3N4O/c28-27(29,30)21-9-7-8-20(17-21)25(35)32-26-31-23-16-19(18-33-14-5-2-6-15-33)12-13-24(23)34(26)22-10-3-1-4-11-22/h1,3-4,7-13,16-17H,2,5-6,14-15,18H2,(H,31,32,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length IRAK-4 (unknown origin) using the biotinylated substrate RRRVTSPARRS by chemiluminescent ELISA |
Bioorg Med Chem Lett 25: 5546-50 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.060
BindingDB Entry DOI: 10.7270/Q29W0JG8 |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50499199

(CHEMBL3735673)Show SMILES O[C@H]1CC[C@@H](CC1)n1c(NC(=O)c2cccc(c2)[N+]([O-])=O)nc2cc(CN3CCCCC3)ccc12 |r,wU:4.7,wD:1.0,(3.28,-6.88,;2.98,-5.68,;4.09,-4.62,;3.72,-3.12,;2.24,-2.7,;1.13,-3.76,;1.5,-5.26,;1.76,-1.24,;2.66,.02,;4.2,.04,;4.95,1.38,;4.32,2.44,;6.49,1.4,;7.24,2.75,;8.78,2.76,;9.57,1.44,;8.82,.1,;7.28,.08,;9.6,-1.23,;10.83,-1.22,;8.99,-2.3,;1.76,1.24,;.3,.77,;-1.03,1.55,;-2.38,.77,;-3.72,1.53,;-5.05,.74,;-6.39,1.5,;-7.71,.72,;-7.7,-.82,;-6.36,-1.58,;-5.03,-.8,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,)| Show InChI InChI=1S/C26H31N5O4/c32-22-10-8-20(9-11-22)30-24-12-7-18(17-29-13-2-1-3-14-29)15-23(24)27-26(30)28-25(33)19-5-4-6-21(16-19)31(34)35/h4-7,12,15-16,20,22,32H,1-3,8-11,13-14,17H2,(H,27,28,33)/t20-,22- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length IRAK-4 (unknown origin) using the biotinylated substrate RRRVTSPARRS by chemiluminescent ELISA |
Bioorg Med Chem Lett 25: 5546-50 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.060
BindingDB Entry DOI: 10.7270/Q29W0JG8 |
More data for this
Ligand-Target Pair | |
Activated CDC42 kinase 1
(Homo sapiens (Human)) | BDBM50245855
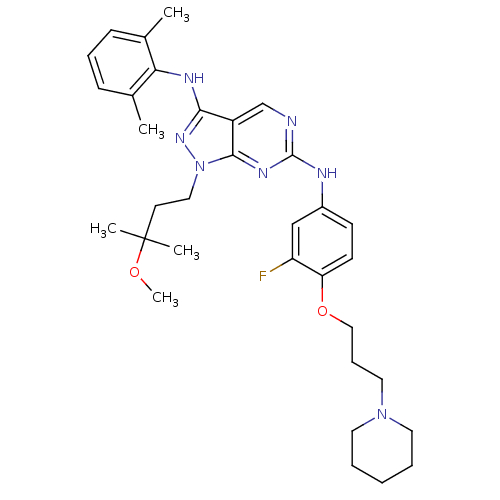
(CHEMBL450918 | N3-(2,6-dimethylphenyl)-N6-(3-fluor...)Show SMILES COC(C)(C)CCn1nc(Nc2c(C)cccc2C)c2cnc(Nc3ccc(OCCCN4CCCCC4)c(F)c3)nc12 Show InChI InChI=1S/C33H44FN7O2/c1-23-11-9-12-24(2)29(23)37-30-26-22-35-32(38-31(26)41(39-30)19-15-33(3,4)42-5)36-25-13-14-28(27(34)21-25)43-20-10-18-40-16-7-6-8-17-40/h9,11-14,21-22H,6-8,10,15-20H2,1-5H3,(H,37,39)(H,35,36,38) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of histidine-tagged ACK1 (amino acids 117 to 489) (unknown origin) expressed in SF9 cells |
Bioorg Med Chem Lett 18: 6352-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.092
BindingDB Entry DOI: 10.7270/Q2B56JKZ |
More data for this
Ligand-Target Pair | |
Activated CDC42 kinase 1
(Homo sapiens (Human)) | BDBM50245853
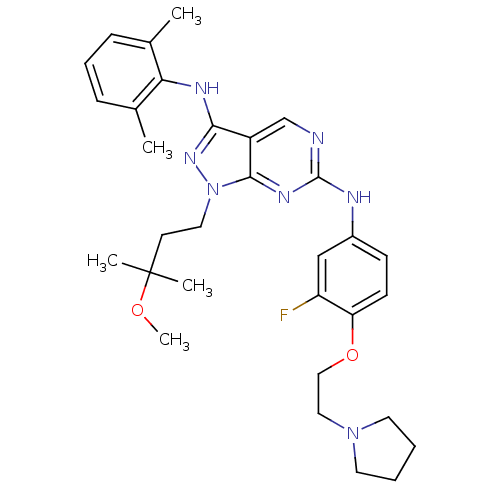
(CHEMBL502156 | N3-(2,6-dimethylphenyl)-N6-(3-fluor...)Show SMILES COC(C)(C)CCn1nc(Nc2c(C)cccc2C)c2cnc(Nc3ccc(OCCN4CCCC4)c(F)c3)nc12 Show InChI InChI=1S/C31H40FN7O2/c1-21-9-8-10-22(2)27(21)35-28-24-20-33-30(36-29(24)39(37-28)16-13-31(3,4)40-5)34-23-11-12-26(25(32)19-23)41-18-17-38-14-6-7-15-38/h8-12,19-20H,6-7,13-18H2,1-5H3,(H,35,37)(H,33,34,36) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of histidine-tagged ACK1 (amino acids 117 to 489) (unknown origin) expressed in SF9 cells |
Bioorg Med Chem Lett 18: 6352-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.092
BindingDB Entry DOI: 10.7270/Q2B56JKZ |
More data for this
Ligand-Target Pair | |
Activated CDC42 kinase 1
(Homo sapiens (Human)) | BDBM50245852

(CHEMBL458333 | N3-(2,6-dimethylphenyl)-1-(3-methox...)Show SMILES COC(C)(C)CCn1nc(Nc2c(C)cccc2C)c2cnc(Nc3ccc(OCCN4CCCC4)cc3)nc12 Show InChI InChI=1S/C31H41N7O2/c1-22-9-8-10-23(2)27(22)34-28-26-21-32-30(35-29(26)38(36-28)18-15-31(3,4)39-5)33-24-11-13-25(14-12-24)40-20-19-37-16-6-7-17-37/h8-14,21H,6-7,15-20H2,1-5H3,(H,34,36)(H,32,33,35) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of histidine-tagged ACK1 (amino acids 117 to 489) (unknown origin) expressed in SF9 cells |
Bioorg Med Chem Lett 18: 6352-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.092
BindingDB Entry DOI: 10.7270/Q2B56JKZ |
More data for this
Ligand-Target Pair | |
Activated CDC42 kinase 1
(Homo sapiens (Human)) | BDBM50246164
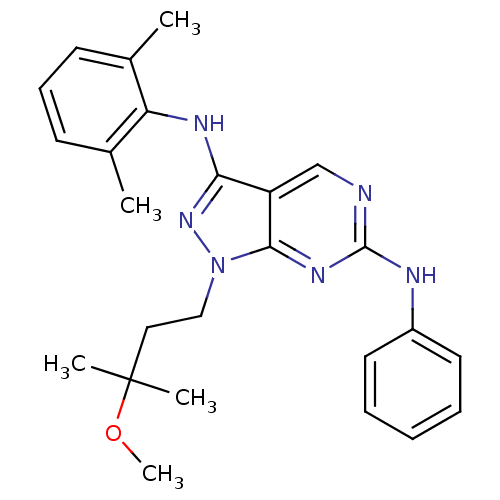
(CHEMBL487242 | N3-(2,6-dimethylphenyl)-1-(3-methox...)Show SMILES COC(C)(C)CCn1nc(Nc2c(C)cccc2C)c2cnc(Nc3ccccc3)nc12 Show InChI InChI=1S/C25H30N6O/c1-17-10-9-11-18(2)21(17)28-22-20-16-26-24(27-19-12-7-6-8-13-19)29-23(20)31(30-22)15-14-25(3,4)32-5/h6-13,16H,14-15H2,1-5H3,(H,28,30)(H,26,27,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of histidine-tagged ACK1 (amino acids 117 to 489) (unknown origin) expressed in SF9 cells |
Bioorg Med Chem Lett 18: 6352-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.092
BindingDB Entry DOI: 10.7270/Q2B56JKZ |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50325236

((5S,8S,11S)-5-isopropyl-8-methyl-11-(2-(2-(naphtha...)Show SMILES CC(C)[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(O)=O)C(=O)COC(=O)Cc1cccc2ccccc12 |r| Show InChI InChI=1S/C33H37N3O9/c1-20(2)30(36-33(43)45-18-22-10-5-4-6-11-22)32(42)34-21(3)31(41)35-26(17-28(38)39)27(37)19-44-29(40)16-24-14-9-13-23-12-7-8-15-25(23)24/h4-15,20-21,26,30H,16-19H2,1-3H3,(H,34,42)(H,35,41)(H,36,43)(H,38,39)/t21-,26-,30-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ICE |
Bioorg Med Chem Lett 20: 5089-94 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.031
BindingDB Entry DOI: 10.7270/Q2SQ90K1 |
More data for this
Ligand-Target Pair | |
Activated CDC42 kinase 1
(Homo sapiens (Human)) | BDBM50421287
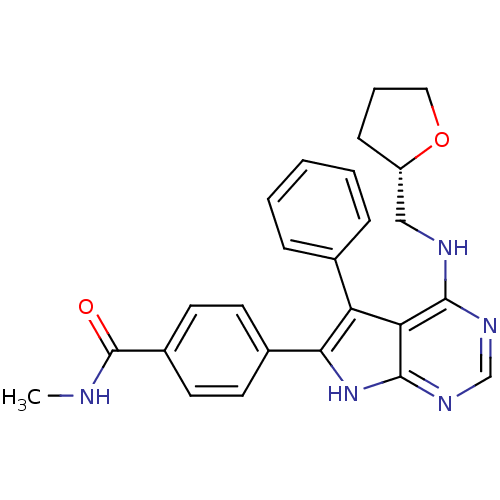
(CHEMBL2087664)Show SMILES CNC(=O)c1ccc(cc1)-c1[nH]c2ncnc(NC[C@@H]3CCCO3)c2c1-c1ccccc1 |r| Show InChI InChI=1S/C25H25N5O2/c1-26-25(31)18-11-9-17(10-12-18)22-20(16-6-3-2-4-7-16)21-23(28-15-29-24(21)30-22)27-14-19-8-5-13-32-19/h2-4,6-7,9-12,15,19H,5,8,13-14H2,1H3,(H,26,31)(H2,27,28,29,30)/t19-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged ACK1 expressed in baculovirus infected Hi5 cells assessed as inhibition of autophosphorylation after 2 hrs by EL... |
Bioorg Med Chem Lett 22: 6212-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.020
BindingDB Entry DOI: 10.7270/Q2VH5Q4X |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50499202

(CHEMBL3735523)Show SMILES O[C@H]1CC[C@@H](CC1)n1c(NC(=O)c2cccc(c2)[N+]([O-])=O)nc2cc(CN3CCOCC3)ccc12 |r,wU:4.7,wD:1.0,(3.28,-6.88,;2.98,-5.68,;4.09,-4.62,;3.72,-3.12,;2.24,-2.7,;1.13,-3.76,;1.5,-5.26,;1.76,-1.24,;2.66,.02,;4.2,.04,;4.95,1.38,;4.32,2.44,;6.49,1.4,;7.24,2.75,;8.78,2.76,;9.57,1.44,;8.82,.1,;7.28,.08,;9.6,-1.23,;10.83,-1.22,;8.99,-2.3,;1.76,1.24,;.3,.77,;-1.03,1.55,;-2.38,.77,;-3.72,1.53,;-5.05,.74,;-6.39,1.5,;-7.71,.72,;-7.7,-.82,;-6.36,-1.58,;-5.03,-.8,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,)| Show InChI InChI=1S/C25H29N5O5/c31-21-7-5-19(6-8-21)29-23-9-4-17(16-28-10-12-35-13-11-28)14-22(23)26-25(29)27-24(32)18-2-1-3-20(15-18)30(33)34/h1-4,9,14-15,19,21,31H,5-8,10-13,16H2,(H,26,27,32)/t19-,21- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length IRAK-4 (unknown origin) using the biotinylated substrate RRRVTSPARRS by chemiluminescent ELISA |
Bioorg Med Chem Lett 25: 5546-50 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.060
BindingDB Entry DOI: 10.7270/Q29W0JG8 |
More data for this
Ligand-Target Pair | |
Activated CDC42 kinase 1
(Homo sapiens (Human)) | BDBM50421277
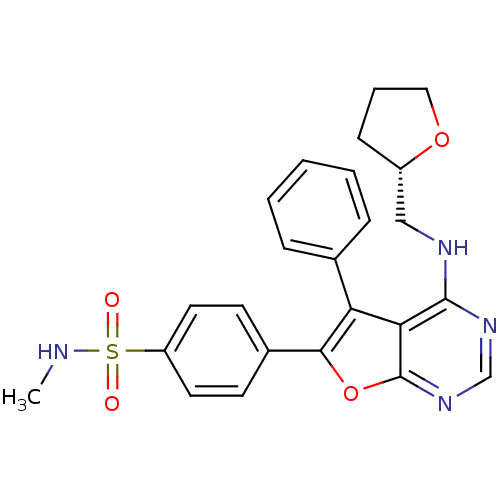
(CHEMBL2087653)Show SMILES CNS(=O)(=O)c1ccc(cc1)-c1oc2ncnc(NC[C@@H]3CCCO3)c2c1-c1ccccc1 |r| Show InChI InChI=1S/C24H24N4O4S/c1-25-33(29,30)19-11-9-17(10-12-19)22-20(16-6-3-2-4-7-16)21-23(27-15-28-24(21)32-22)26-14-18-8-5-13-31-18/h2-4,6-7,9-12,15,18,25H,5,8,13-14H2,1H3,(H,26,27,28)/t18-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged ACK1 expressed in baculovirus infected Hi5 cells assessed as inhibition of autophosphorylation after 2 hrs by EL... |
Bioorg Med Chem Lett 22: 6212-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.020
BindingDB Entry DOI: 10.7270/Q2VH5Q4X |
More data for this
Ligand-Target Pair | |
Activated CDC42 kinase 1
(Homo sapiens (Human)) | BDBM50421261
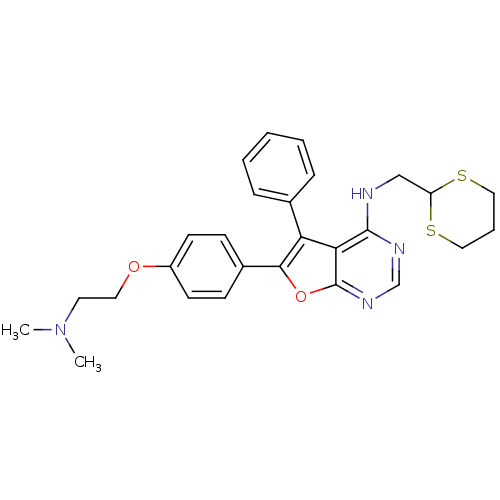
(CHEMBL2087879)Show SMILES CN(C)CCOc1ccc(cc1)-c1oc2ncnc(NCC3SCCCS3)c2c1-c1ccccc1 Show InChI InChI=1S/C27H30N4O2S2/c1-31(2)13-14-32-21-11-9-20(10-12-21)25-23(19-7-4-3-5-8-19)24-26(29-18-30-27(24)33-25)28-17-22-34-15-6-16-35-22/h3-5,7-12,18,22H,6,13-17H2,1-2H3,(H,28,29,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged ACK1 expressed in baculovirus infected Hi5 cells assessed as inhibition of autophosphorylation after 2 hrs by EL... |
Bioorg Med Chem Lett 22: 6212-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.020
BindingDB Entry DOI: 10.7270/Q2VH5Q4X |
More data for this
Ligand-Target Pair | |
Activated CDC42 kinase 1
(Homo sapiens (Human)) | BDBM50421254
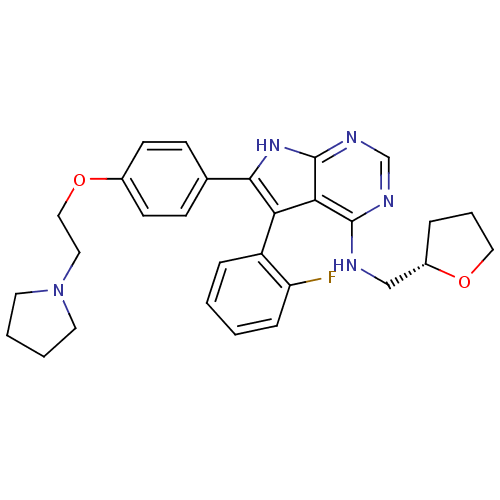
(CHEMBL2087872)Show SMILES Fc1ccccc1-c1c([nH]c2ncnc(NC[C@@H]3CCCO3)c12)-c1ccc(OCCN2CCCC2)cc1 |r,wU:17.17,(1.31,-33.66,;.28,-32.51,;.76,-31.05,;-.28,-29.91,;-1.79,-30.23,;-2.26,-31.7,;-1.23,-32.83,;-1.7,-34.3,;-.79,-35.55,;-1.7,-36.81,;-3.18,-36.33,;-4.51,-37.1,;-5.85,-36.33,;-5.84,-34.78,;-4.51,-34.01,;-4.52,-32.47,;-5.86,-31.71,;-7.19,-32.48,;-8.59,-31.86,;-9.61,-33.01,;-8.84,-34.34,;-7.34,-34.01,;-3.18,-34.78,;.74,-35.55,;1.51,-36.88,;3.05,-36.88,;3.82,-35.55,;5.36,-35.55,;6.13,-36.88,;7.67,-36.88,;8.44,-35.54,;9.97,-35.37,;10.29,-33.87,;8.95,-33.1,;7.81,-34.13,;3.04,-34.21,;1.51,-34.22,)| Show InChI InChI=1S/C29H32FN5O2/c30-24-8-2-1-7-23(24)25-26-28(31-18-22-6-5-16-36-22)32-19-33-29(26)34-27(25)20-9-11-21(12-10-20)37-17-15-35-13-3-4-14-35/h1-2,7-12,19,22H,3-6,13-18H2,(H2,31,32,33,34)/t22-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged ACK1 expressed in baculovirus infected Hi5 cells assessed as inhibition of autophosphorylation after 2 hrs by EL... |
Bioorg Med Chem Lett 22: 6212-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.020
BindingDB Entry DOI: 10.7270/Q2VH5Q4X |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM29885
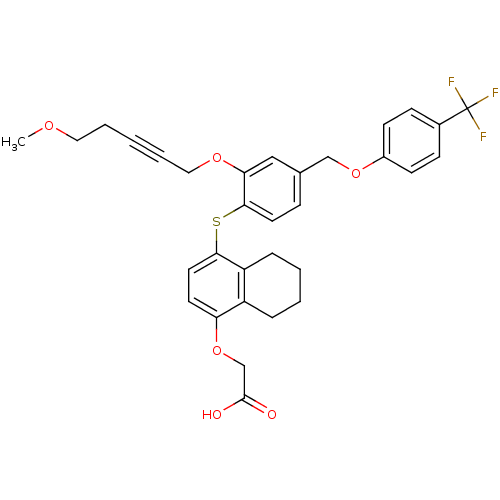
(alkynyl ether, 27)Show SMILES COCCC#CCOc1cc(COc2ccc(cc2)C(F)(F)F)ccc1Sc1ccc(OCC(O)=O)c2CCCCc12 Show InChI InChI=1S/C32H31F3O6S/c1-38-17-5-2-6-18-39-28-19-22(20-40-24-12-10-23(11-13-24)32(33,34)35)9-15-30(28)42-29-16-14-27(41-21-31(36)37)25-7-3-4-8-26(25)29/h9-16,19H,3-5,7-8,17-18,20-21H2,1H3,(H,36,37) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | 470 | n/a | n/a | n/a | n/a |
Amgen
| Assay Description
The human PPARdelta ligand binding was directly measured using a scintillation proximity assay. Plates were read on a Packard TopCount. IC50 values f... |
Bioorg Med Chem Lett 19: 3550-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.151
BindingDB Entry DOI: 10.7270/Q2NK3CC0 |
More data for this
Ligand-Target Pair | |
Activated CDC42 kinase 1
(Homo sapiens (Human)) | BDBM50421284
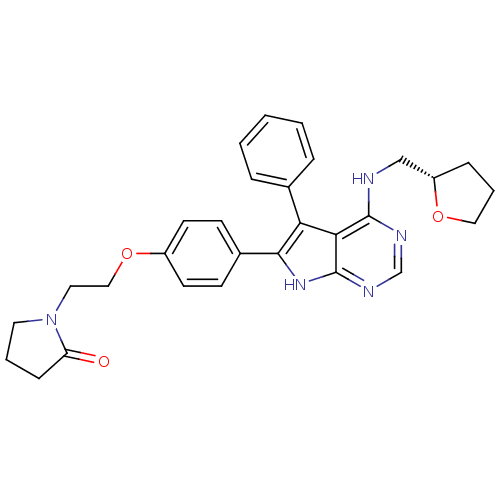
(CHEMBL2087660)Show SMILES O=C1CCCN1CCOc1ccc(cc1)-c1[nH]c2ncnc(NC[C@@H]3CCCO3)c2c1-c1ccccc1 |r| Show InChI InChI=1S/C29H31N5O3/c35-24-9-4-14-34(24)15-17-37-22-12-10-21(11-13-22)27-25(20-6-2-1-3-7-20)26-28(31-19-32-29(26)33-27)30-18-23-8-5-16-36-23/h1-3,6-7,10-13,19,23H,4-5,8-9,14-18H2,(H2,30,31,32,33)/t23-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged ACK1 expressed in baculovirus infected Hi5 cells assessed as inhibition of autophosphorylation after 2 hrs by EL... |
Bioorg Med Chem Lett 22: 6212-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.020
BindingDB Entry DOI: 10.7270/Q2VH5Q4X |
More data for this
Ligand-Target Pair | |
Activated CDC42 kinase 1
(Homo sapiens (Human)) | BDBM50421283
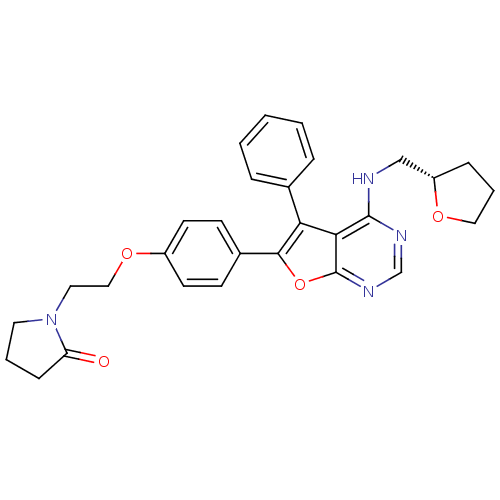
(CHEMBL2087659)Show SMILES O=C1CCCN1CCOc1ccc(cc1)-c1oc2ncnc(NC[C@@H]3CCCO3)c2c1-c1ccccc1 |r| Show InChI InChI=1S/C29H30N4O4/c34-24-9-4-14-33(24)15-17-36-22-12-10-21(11-13-22)27-25(20-6-2-1-3-7-20)26-28(31-19-32-29(26)37-27)30-18-23-8-5-16-35-23/h1-3,6-7,10-13,19,23H,4-5,8-9,14-18H2,(H,30,31,32)/t23-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged ACK1 expressed in baculovirus infected Hi5 cells assessed as inhibition of autophosphorylation after 2 hrs by EL... |
Bioorg Med Chem Lett 22: 6212-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.020
BindingDB Entry DOI: 10.7270/Q2VH5Q4X |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data