

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50122970 (3-Benzo[1,3]dioxol-5-yl-2-(5-pyridin-3-yl-furan-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C. Curated by ChEMBL | Assay Description Inhibitory activity against Phosphodiesterase 5 (PDE5) was evaluated | J Med Chem 46: 441-4 (2003) Article DOI: 10.1021/jm0202573 BindingDB Entry DOI: 10.7270/Q2QR4WHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50122969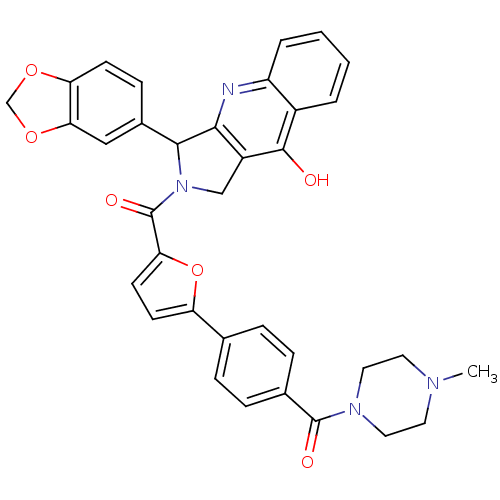 (3-Benzo[1,3]dioxol-5-yl-2-{5-[4-(4-methyl-piperazi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C. Curated by ChEMBL | Assay Description Inhibitory activity against Phosphodiesterase 5 (PDE5) was evaluated | J Med Chem 46: 441-4 (2003) Article DOI: 10.1021/jm0202573 BindingDB Entry DOI: 10.7270/Q2QR4WHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM276786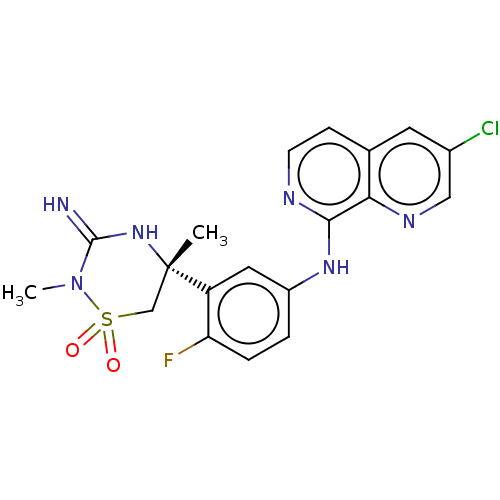 (3-chloro-N-{4-fluoro-3-[(5R)-3-imino-2,5-dimethyl-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | 5.0 | n/a |
TBA US Patent | Assay Description Inhibitor IC50s, at purified human autoBACE-2 are determined in a time-resolved endpoint proteolysis assay that measures hydrolysis of the QSY7-EISEV... | US Patent US10071998 (2018) BindingDB Entry DOI: 10.7270/Q2C82CBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM276793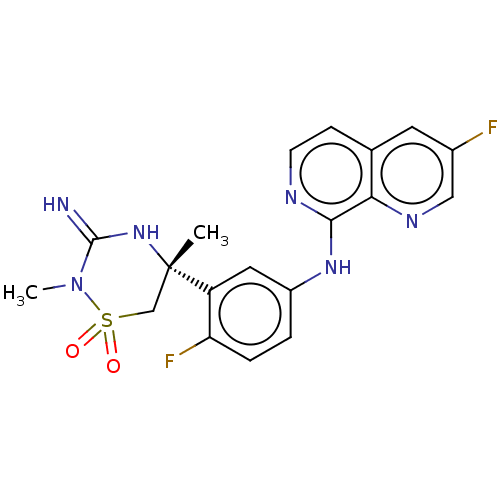 (3-fluoro-N-{4-fluoro-3-[(5R)-3-imino-2,5-dimethyl-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | 5.0 | n/a |
TBA US Patent | Assay Description Inhibitor IC50s, at purified human autoBACE-2 are determined in a time-resolved endpoint proteolysis assay that measures hydrolysis of the QSY7-EISEV... | US Patent US10071998 (2018) BindingDB Entry DOI: 10.7270/Q2C82CBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50122990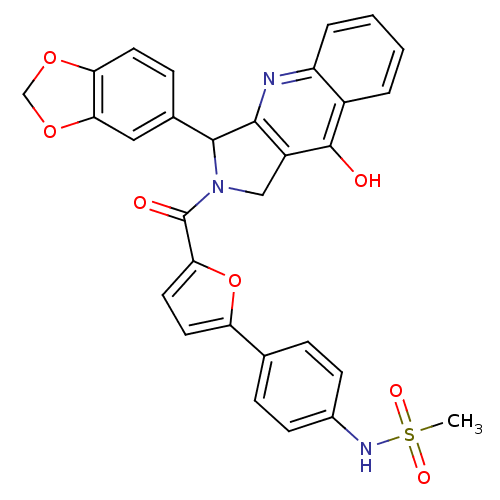 (CHEMBL342159 | N-{4-[5-(3-Benzo[1,3]dioxol-5-yl-9-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C. Curated by ChEMBL | Assay Description Inhibitory activity against Phosphodiesterase 5 (PDE5) was evaluated | J Med Chem 46: 441-4 (2003) Article DOI: 10.1021/jm0202573 BindingDB Entry DOI: 10.7270/Q2QR4WHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50122974 (3-Benzo[1,3]dioxol-5-yl-2-(5-pyridin-4-yl-furan-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C. Curated by ChEMBL | Assay Description Inhibitory activity against Phosphodiesterase 5 (PDE5) was evaluated | J Med Chem 46: 441-4 (2003) Article DOI: 10.1021/jm0202573 BindingDB Entry DOI: 10.7270/Q2QR4WHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM335430 ((3R,6S)-5-amino-3-(2-((3- bromo-1,7-naphthyridin-8...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention were determined to be potent inhibitors of BACE-2 using the following assay. Inhibitor IC50s at purified human autoBAC... | US Patent US9732088 (2017) BindingDB Entry DOI: 10.7270/Q2XD13S2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50122964 (3-Benzo[1,3]dioxol-5-yl-2-(6-hydroxy-benzofuran-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C. Curated by ChEMBL | Assay Description Inhibitory activity against Phosphodiesterase 5 (PDE5) was evaluated | J Med Chem 46: 441-4 (2003) Article DOI: 10.1021/jm0202573 BindingDB Entry DOI: 10.7270/Q2QR4WHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM276787 (3-bromo-N-{4-fluoro-3-[(5R)-3-imino-2,5-dimethyl-1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | 5.0 | n/a |
TBA US Patent | Assay Description Inhibitor IC50s, at purified human autoBACE-2 are determined in a time-resolved endpoint proteolysis assay that measures hydrolysis of the QSY7-EISEV... | US Patent US10071998 (2018) BindingDB Entry DOI: 10.7270/Q2C82CBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM335458 (8-((3-((3R,6S)-5-amino-6- cyclopropyl-3,6-dimethyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention were determined to be potent inhibitors of BACE-2 using the following assay. Inhibitor IC50s at purified human autoBAC... | US Patent US9732088 (2017) BindingDB Entry DOI: 10.7270/Q2XD13S2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM276791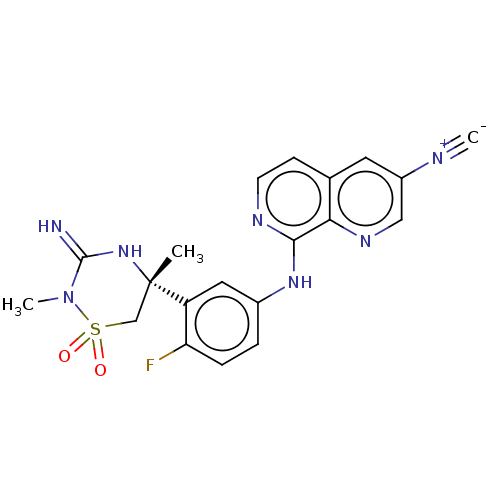 (8-({4-fluoro-3-[(5R)-3-imino-2,5-dimethyl-1,1-diox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | 5.0 | n/a |
TBA US Patent | Assay Description Inhibitor IC50s, at purified human autoBACE-2 are determined in a time-resolved endpoint proteolysis assay that measures hydrolysis of the QSY7-EISEV... | US Patent US10071998 (2018) BindingDB Entry DOI: 10.7270/Q2C82CBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50122966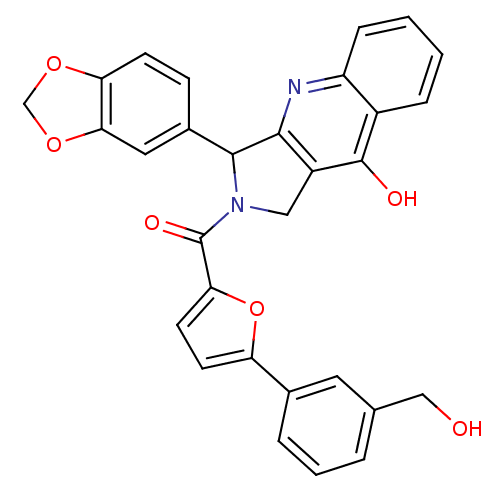 (3-Benzo[1,3]dioxol-5-yl-2-[5-(3-hydroxymethyl-phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C. Curated by ChEMBL | Assay Description Inhibitory activity against Phosphodiesterase 5 (PDE5) was evaluated | J Med Chem 46: 441-4 (2003) Article DOI: 10.1021/jm0202573 BindingDB Entry DOI: 10.7270/Q2QR4WHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM402431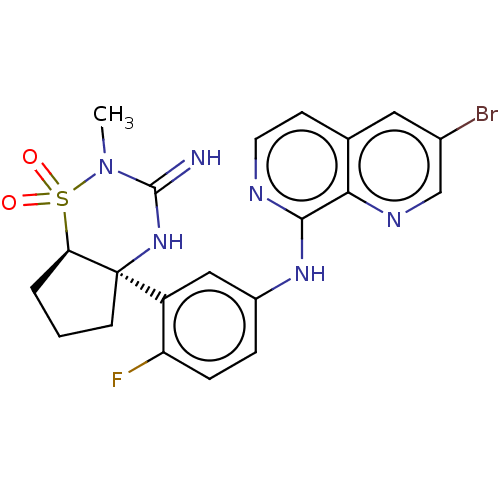 (US10329291, Example 22) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd | Assay Description The compounds of the invention were assessed for their ability to inhibit BACE-1 using the following assay. The resulting values are reported in the ... | Bioorg Med Chem 17: 6590-605 (2009) BindingDB Entry DOI: 10.7270/Q2ZW1P72 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50122971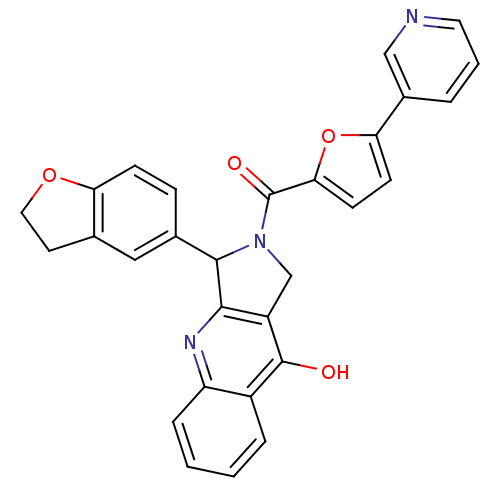 (3-(2,3-Dihydro-benzofuran-5-yl)-2-(5-pyridin-3-yl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C. Curated by ChEMBL | Assay Description Inhibitory activity against Phosphodiesterase 5 (PDE5) was evaluated | J Med Chem 46: 441-4 (2003) Article DOI: 10.1021/jm0202573 BindingDB Entry DOI: 10.7270/Q2QR4WHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM335434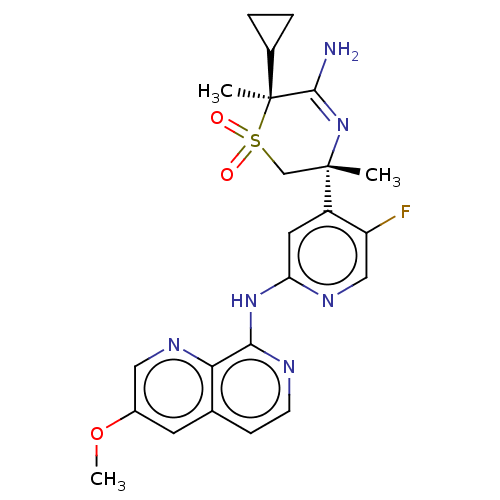 ((3R,6S)-5-amino-6- cyclopropyl-3-(5-fluoro-2-((3- ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention were determined to be potent inhibitors of BACE-2 using the following assay. Inhibitor IC50s at purified human autoBAC... | US Patent US9732088 (2017) BindingDB Entry DOI: 10.7270/Q2XD13S2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM335466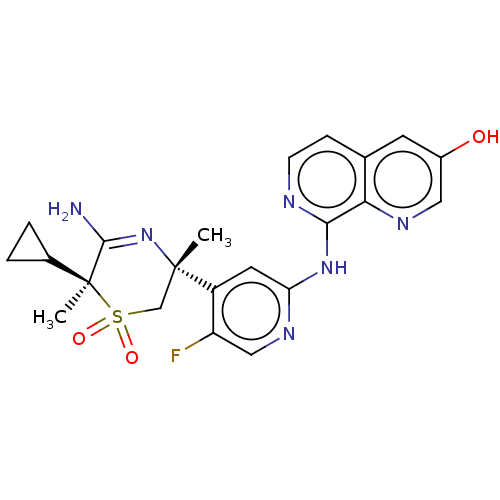 ((3R,6S)-5-amino-6- cyclopropyl-3-(5-fluoro-2-((3- ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention were determined to be potent inhibitors of BACE-2 using the following assay. Inhibitor IC50s at purified human autoBAC... | US Patent US9732088 (2017) BindingDB Entry DOI: 10.7270/Q2XD13S2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM335424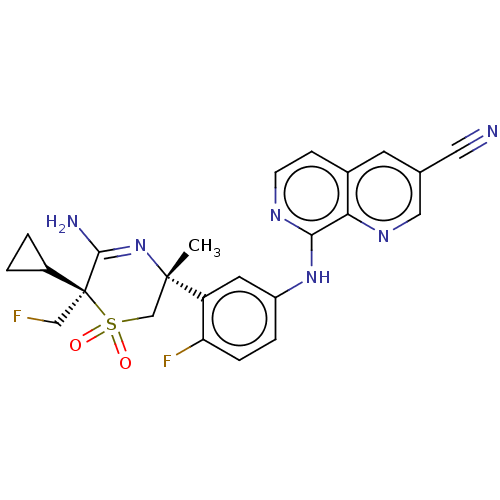 (8-((3-((3R,6S)-5-amino-6- cyclopropyl-6-(fluoromet...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention were determined to be potent inhibitors of BACE-2 using the following assay. Inhibitor IC50s at purified human autoBAC... | US Patent US9732088 (2017) BindingDB Entry DOI: 10.7270/Q2XD13S2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50122973 (3-Benzo[1,3]dioxol-5-yl-2-[5-(4-hydroxymethyl-phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C. Curated by ChEMBL | Assay Description Inhibitory activity against Phosphodiesterase 5 (PDE5) was evaluated | J Med Chem 46: 441-4 (2003) Article DOI: 10.1021/jm0202573 BindingDB Entry DOI: 10.7270/Q2QR4WHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50122983 (3-Benzo[1,3]dioxol-5-yl-2-[5-(4-nitro-phenyl)-fura...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C. Curated by ChEMBL | Assay Description Inhibitory activity against Phosphodiesterase 5 (PDE5) was evaluated | J Med Chem 46: 441-4 (2003) Article DOI: 10.1021/jm0202573 BindingDB Entry DOI: 10.7270/Q2QR4WHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM335447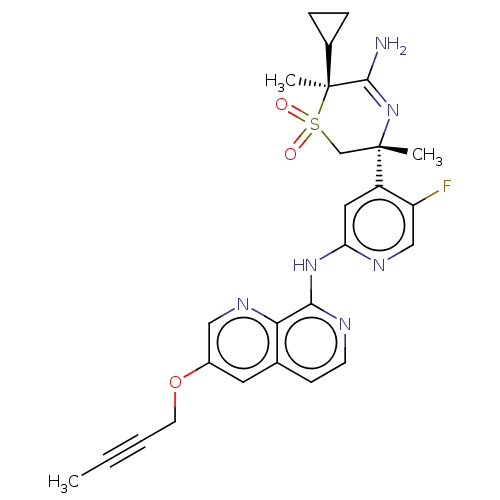 ((3R,6S)-5-amino-3-(2-((3-(but- 2-yn-1-yloxy)-1,7-n...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention were determined to be potent inhibitors of BACE-2 using the following assay. Inhibitor IC50s at purified human autoBAC... | US Patent US9732088 (2017) BindingDB Entry DOI: 10.7270/Q2XD13S2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM335459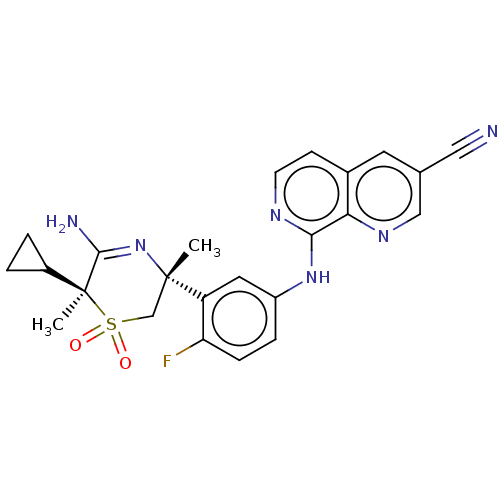 (8-((3-((3R,6S)-5-amino-6- cyclopropyl-3,6-dimethyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention were determined to be potent inhibitors of BACE-2 using the following assay. Inhibitor IC50s at purified human autoBAC... | US Patent US9732088 (2017) BindingDB Entry DOI: 10.7270/Q2XD13S2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM335454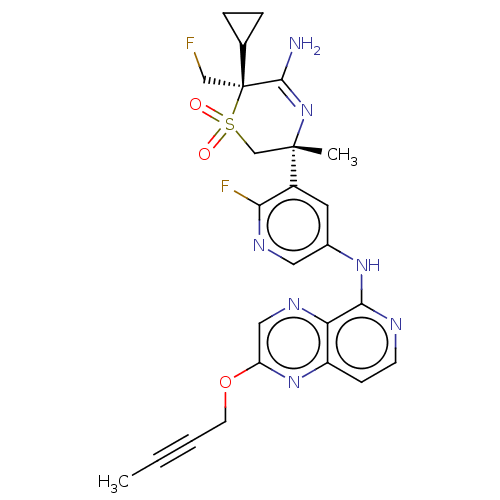 ((3R,6S)-5-amino-3-(5-((2-(but- 2-yn-1-yloxy)pyrido...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention were determined to be potent inhibitors of BACE-1 using the following assay.The following reagents were used in this a... | US Patent US9732088 (2017) BindingDB Entry DOI: 10.7270/Q2XD13S2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50122980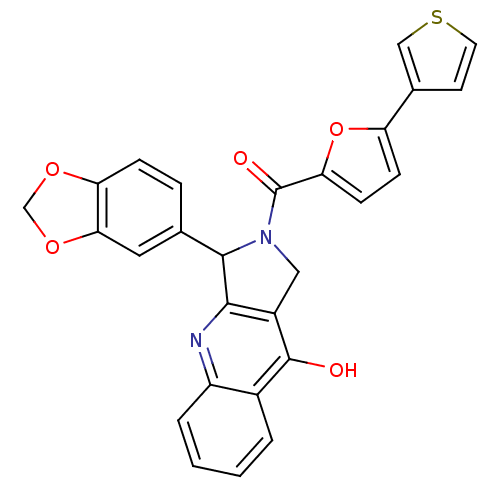 (3-Benzo[1,3]dioxol-5-yl-2-(5-thiophen-3-yl-furan-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C. Curated by ChEMBL | Assay Description Inhibitory activity against Phosphodiesterase 5 (PDE5) was evaluated | J Med Chem 46: 441-4 (2003) Article DOI: 10.1021/jm0202573 BindingDB Entry DOI: 10.7270/Q2QR4WHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50122981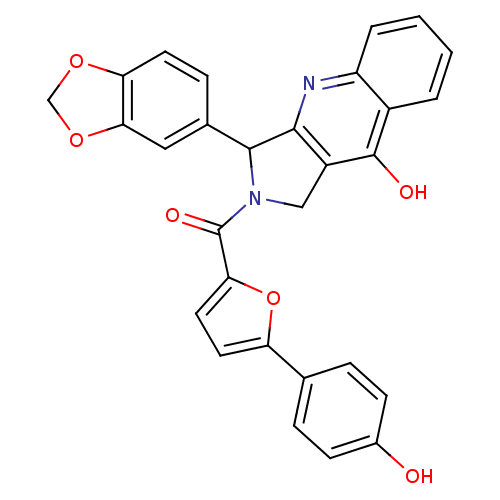 (3-Benzo[1,3]dioxol-5-yl-2-[5-(4-hydroxy-phenyl)-fu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C. Curated by ChEMBL | Assay Description Inhibitory activity against Phosphodiesterase 5 (PDE5) was evaluated | J Med Chem 46: 441-4 (2003) Article DOI: 10.1021/jm0202573 BindingDB Entry DOI: 10.7270/Q2QR4WHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM335456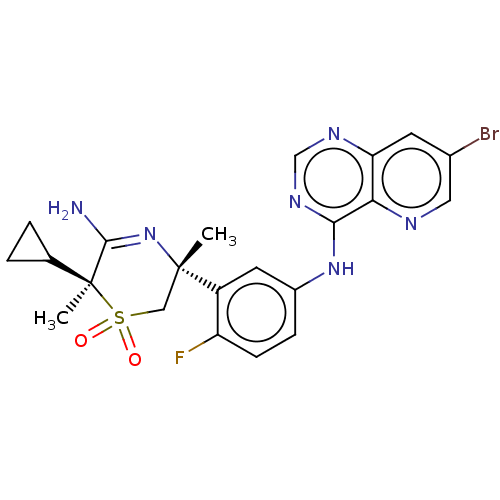 ((3R,6S)-5-amino-3-(5-((7- bromopyrido[3,2-d]pyrimi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention were determined to be potent inhibitors of BACE-2 using the following assay. Inhibitor IC50s at purified human autoBAC... | US Patent US9732088 (2017) BindingDB Entry DOI: 10.7270/Q2XD13S2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50122987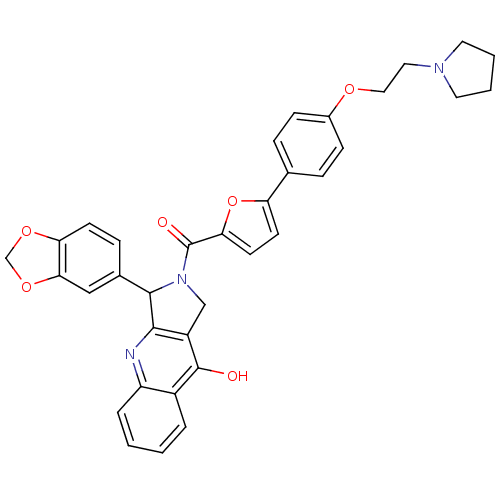 (3-Benzo[1,3]dioxol-5-yl-2-{5-[4-(2-pyrrolidin-1-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C. Curated by ChEMBL | Assay Description Inhibitory activity against Phosphodiesterase 5 (PDE5) was evaluated | J Med Chem 46: 441-4 (2003) Article DOI: 10.1021/jm0202573 BindingDB Entry DOI: 10.7270/Q2QR4WHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM335447 ((3R,6S)-5-amino-3-(2-((3-(but- 2-yn-1-yloxy)-1,7-n...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention were determined to be potent inhibitors of BACE-1 using the following assay.The following reagents were used in this a... | US Patent US9732088 (2017) BindingDB Entry DOI: 10.7270/Q2XD13S2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM276788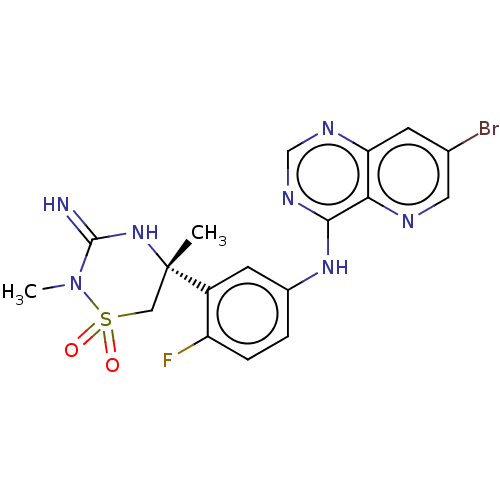 (7-bromo-N-{4-fluoro-3-[(5R)-3-imino-2,5-dimethyl-1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.810 | n/a | n/a | n/a | n/a | n/a | n/a | 5.0 | n/a |
TBA US Patent | Assay Description Inhibitor IC50s, at purified human autoBACE-2 are determined in a time-resolved endpoint proteolysis assay that measures hydrolysis of the QSY7-EISEV... | US Patent US10071998 (2018) BindingDB Entry DOI: 10.7270/Q2C82CBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM335474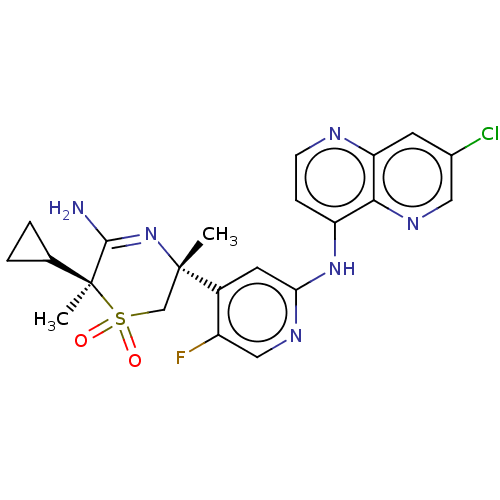 ((3R,6S)-5-amino-3-(2-((7- chloro-1,5-naphthyridin-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention were determined to be potent inhibitors of BACE-2 using the following assay. Inhibitor IC50s at purified human autoBAC... | US Patent US9732088 (2017) BindingDB Entry DOI: 10.7270/Q2XD13S2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM335466 ((3R,6S)-5-amino-6- cyclopropyl-3-(5-fluoro-2-((3- ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention were determined to be potent inhibitors of BACE-1 using the following assay.The following reagents were used in this a... | US Patent US9732088 (2017) BindingDB Entry DOI: 10.7270/Q2XD13S2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM335448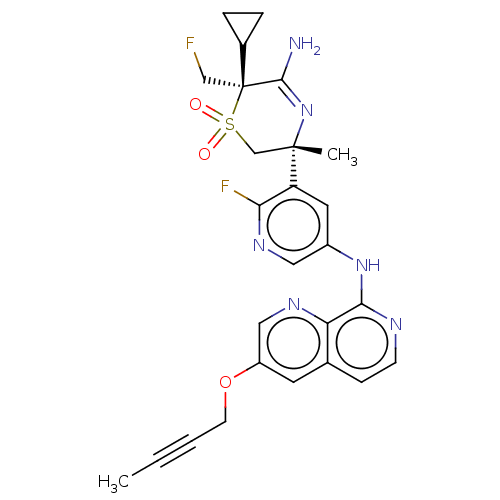 ((3R,6S)-5-amino-3-(5-((3-(but- 2-yn-1-yloxy)-1,7-n...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention were determined to be potent inhibitors of BACE-1 using the following assay.The following reagents were used in this a... | US Patent US9732088 (2017) BindingDB Entry DOI: 10.7270/Q2XD13S2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50122989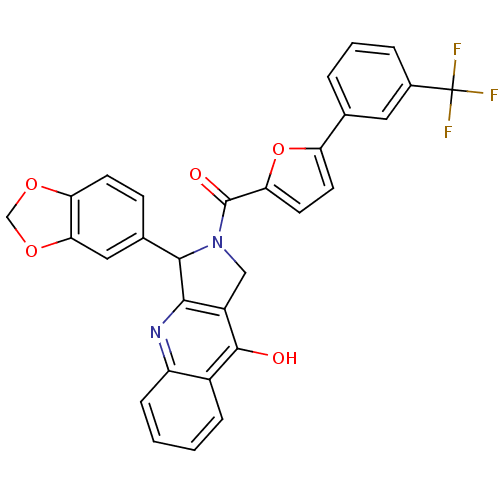 (3-Benzo[1,3]dioxol-5-yl-2-[5-(3-trifluoromethyl-ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C. Curated by ChEMBL | Assay Description Inhibitory activity against Phosphodiesterase 5 (PDE5) was evaluated | J Med Chem 46: 441-4 (2003) Article DOI: 10.1021/jm0202573 BindingDB Entry DOI: 10.7270/Q2QR4WHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50122967 (4-[5-(3-Benzo[1,3]dioxol-5-yl-9-oxo-1,3,4,9-tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.990 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C. Curated by ChEMBL | Assay Description Inhibitory activity against Phosphodiesterase 5 (PDE5) was evaluated | J Med Chem 46: 441-4 (2003) Article DOI: 10.1021/jm0202573 BindingDB Entry DOI: 10.7270/Q2QR4WHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50122976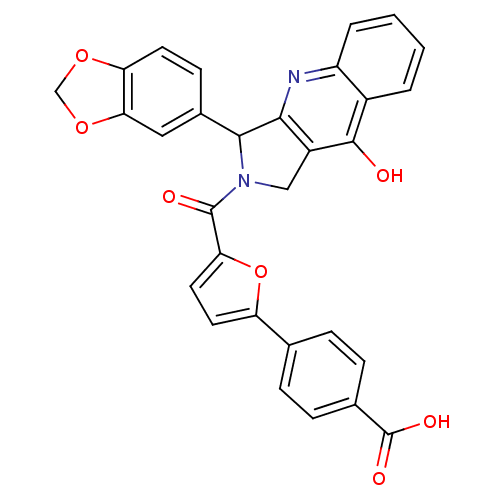 (4-[5-(3-Benzo[1,3]dioxol-5-yl-9-oxo-1,3,4,9-tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C. Curated by ChEMBL | Assay Description Inhibitory activity against Phosphodiesterase 5 (PDE5) was evaluated | J Med Chem 46: 441-4 (2003) Article DOI: 10.1021/jm0202573 BindingDB Entry DOI: 10.7270/Q2QR4WHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM402345 (US10329291, Example 1) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd | Assay Description The compounds of the invention were assessed for their ability to inhibit BACE-1 using the following assay. The resulting values are reported in the ... | Bioorg Med Chem 17: 6590-605 (2009) BindingDB Entry DOI: 10.7270/Q2ZW1P72 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM50271172 (CHEMBL4127711) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd | Assay Description The compounds of the invention were assessed for their ability to inhibit BACE-1 using the following assay. The resulting values are reported in the ... | Bioorg Med Chem 17: 6590-605 (2009) BindingDB Entry DOI: 10.7270/Q2ZW1P72 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM50271163 (CHEMBL4129654) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd | Assay Description The compounds of the invention were assessed for their ability to inhibit BACE-1 using the following assay. The resulting values are reported in the ... | Bioorg Med Chem 17: 6590-605 (2009) BindingDB Entry DOI: 10.7270/Q2ZW1P72 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM335467 ((3R,6S)-5-amino-6- cyclopropyl-3-(5-fluoro-2-((3- ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention were determined to be potent inhibitors of BACE-2 using the following assay. Inhibitor IC50s at purified human autoBAC... | US Patent US9732088 (2017) BindingDB Entry DOI: 10.7270/Q2XD13S2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM402432 (US10329291, Example 23) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd | Assay Description The compounds of the invention were assessed for their ability to inhibit BACE-1 using the following assay. The resulting values are reported in the ... | Bioorg Med Chem 17: 6590-605 (2009) BindingDB Entry DOI: 10.7270/Q2ZW1P72 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM402422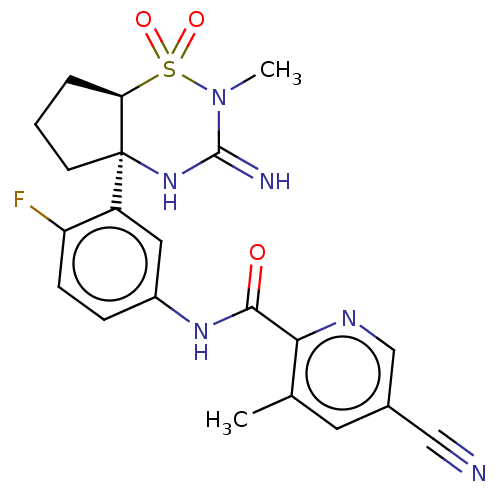 (US10329291, Example 13) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd | Assay Description The compounds of the invention were assessed for their ability to inhibit BACE-1 using the following assay. The resulting values are reported in the ... | Bioorg Med Chem 17: 6590-605 (2009) BindingDB Entry DOI: 10.7270/Q2ZW1P72 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM402422 (US10329291, Example 13) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd | Assay Description The compounds of the invention were assessed for their ability to inhibit BACE-1 using the following assay. The resulting values are reported in the ... | Bioorg Med Chem 17: 6590-605 (2009) BindingDB Entry DOI: 10.7270/Q2ZW1P72 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM402420 (US10329291, Example 11) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd | Assay Description The compounds of the invention were assessed for their ability to inhibit BACE-1 using the following assay. The resulting values are reported in the ... | Bioorg Med Chem 17: 6590-605 (2009) BindingDB Entry DOI: 10.7270/Q2ZW1P72 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM50271168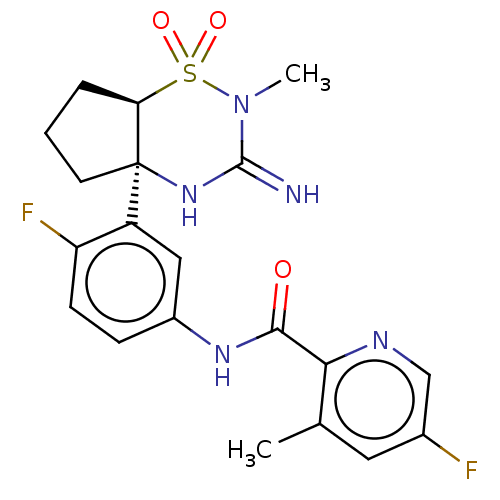 (CHEMBL4127283) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd | Assay Description The compounds of the invention were assessed for their ability to inhibit BACE-1 using the following assay. The resulting values are reported in the ... | Bioorg Med Chem 17: 6590-605 (2009) BindingDB Entry DOI: 10.7270/Q2ZW1P72 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM50271175 (CHEMBL4126365) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd | Assay Description The compounds of the invention were assessed for their ability to inhibit BACE-1 using the following assay. The resulting values are reported in the ... | Bioorg Med Chem 17: 6590-605 (2009) BindingDB Entry DOI: 10.7270/Q2ZW1P72 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM335454 ((3R,6S)-5-amino-3-(5-((2-(but- 2-yn-1-yloxy)pyrido...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention were determined to be potent inhibitors of BACE-2 using the following assay. Inhibitor IC50s at purified human autoBAC... | US Patent US9732088 (2017) BindingDB Entry DOI: 10.7270/Q2XD13S2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM276796 (8-({2,4-difluoro-3-[(5R)-3-imino-2,5-dimethyl-1,1-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | 5.0 | n/a |
TBA US Patent | Assay Description Inhibitor IC50s, at purified human autoBACE-2 are determined in a time-resolved endpoint proteolysis assay that measures hydrolysis of the QSY7-EISEV... | US Patent US10071998 (2018) BindingDB Entry DOI: 10.7270/Q2C82CBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM276786 (3-chloro-N-{4-fluoro-3-[(5R)-3-imino-2,5-dimethyl-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | 5.0 | n/a |
TBA US Patent | Assay Description The following reagents were used in this assay. Na+-Acetate pH 5.0; 1% Brij-35; Glycerol; Dimethyl Sulfoxide (DMSO); Recombinant human soluble BACE-1... | US Patent US10071998 (2018) BindingDB Entry DOI: 10.7270/Q2C82CBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM335469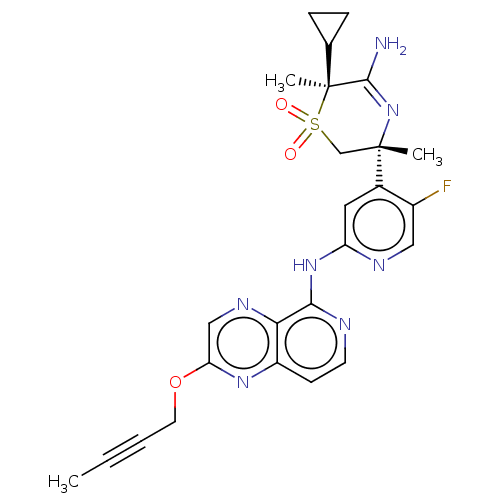 ((3R,6S)-5-amino-3-(2-((2-(but- 2-yn-1-yloxy)pyrido...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention were determined to be potent inhibitors of BACE-2 using the following assay. Inhibitor IC50s at purified human autoBAC... | US Patent US9732088 (2017) BindingDB Entry DOI: 10.7270/Q2XD13S2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50122982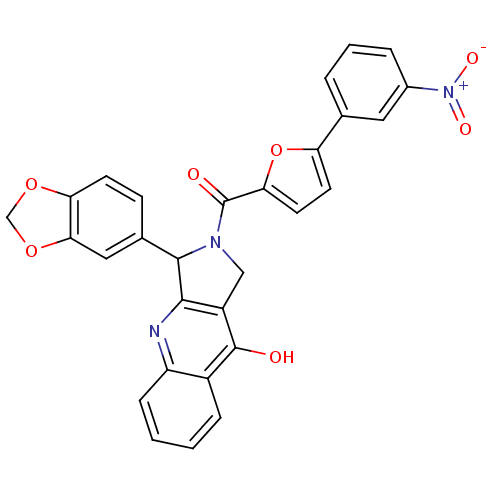 (3-Benzo[1,3]dioxol-5-yl-2-[5-(3-nitro-phenyl)-fura...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C. Curated by ChEMBL | Assay Description Inhibitory activity against Phosphodiesterase 5 (PDE5) was evaluated | J Med Chem 46: 441-4 (2003) Article DOI: 10.1021/jm0202573 BindingDB Entry DOI: 10.7270/Q2QR4WHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM335444 ((3R,6S)-5-amino-6- cyclopropyl-3-(5-fluoro-2-((2- ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention were determined to be potent inhibitors of BACE-2 using the following assay. Inhibitor IC50s at purified human autoBAC... | US Patent US9732088 (2017) BindingDB Entry DOI: 10.7270/Q2XD13S2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1297 total ) | Next | Last >> |